Bioinspired Polyvinyl Alcohol-Based Foam Fabricated via Supercritical Carbon Dioxide Foaming for Atmospheric Water Harvesting
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
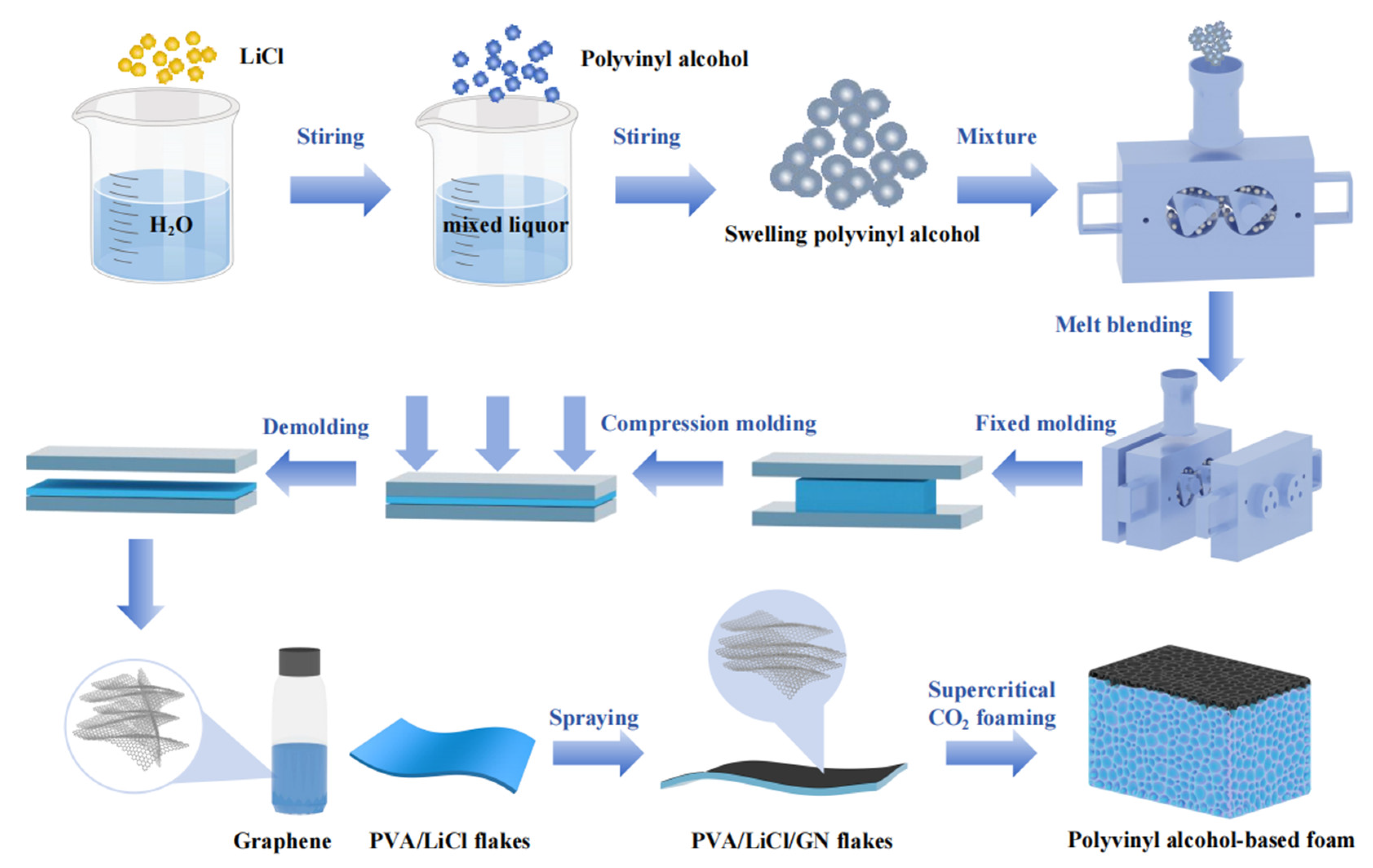
2.2. Sample Preparation
2.3. Material Characterization
3. Results and Discussion
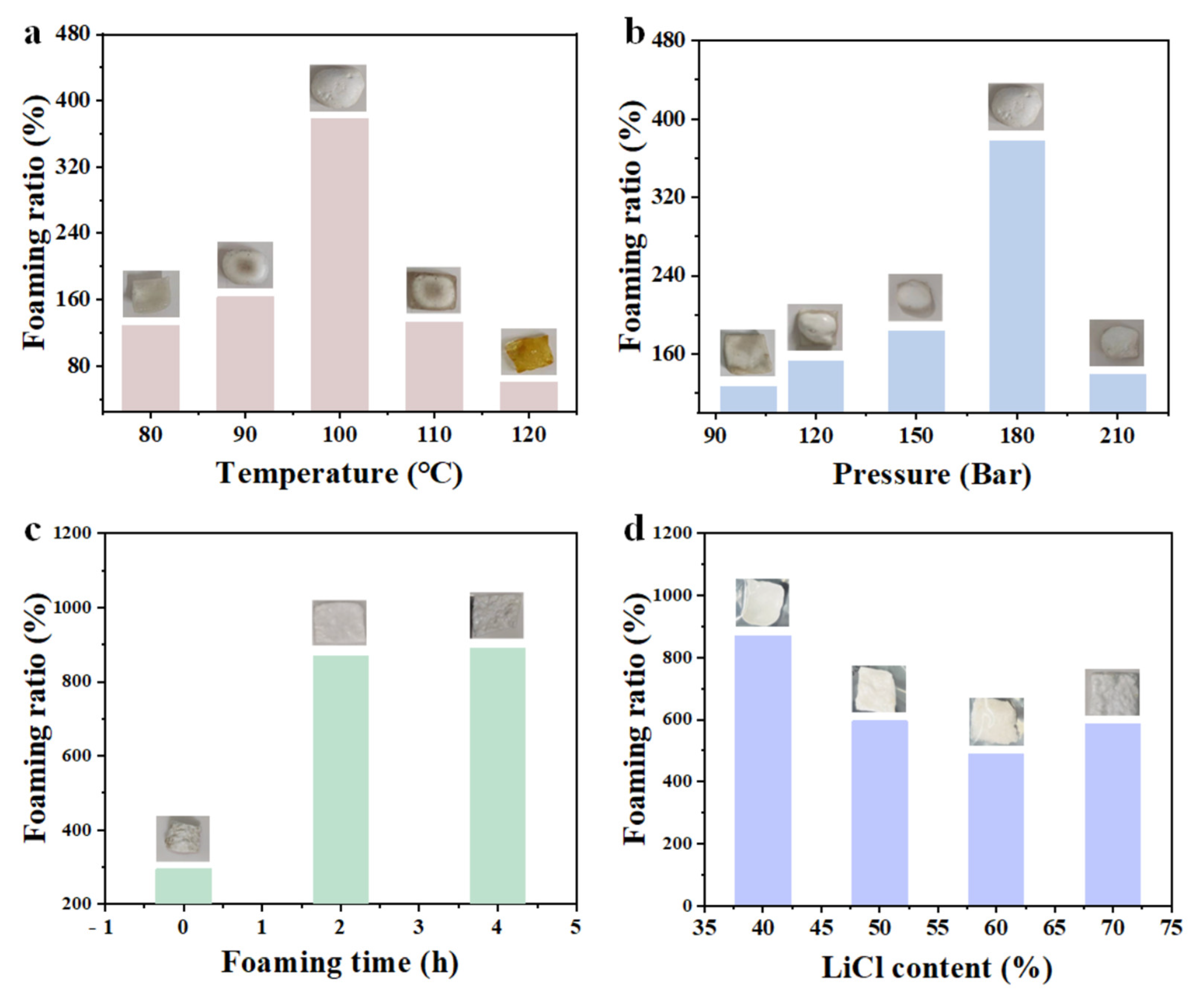
3.1. Exploration of Foaming Process
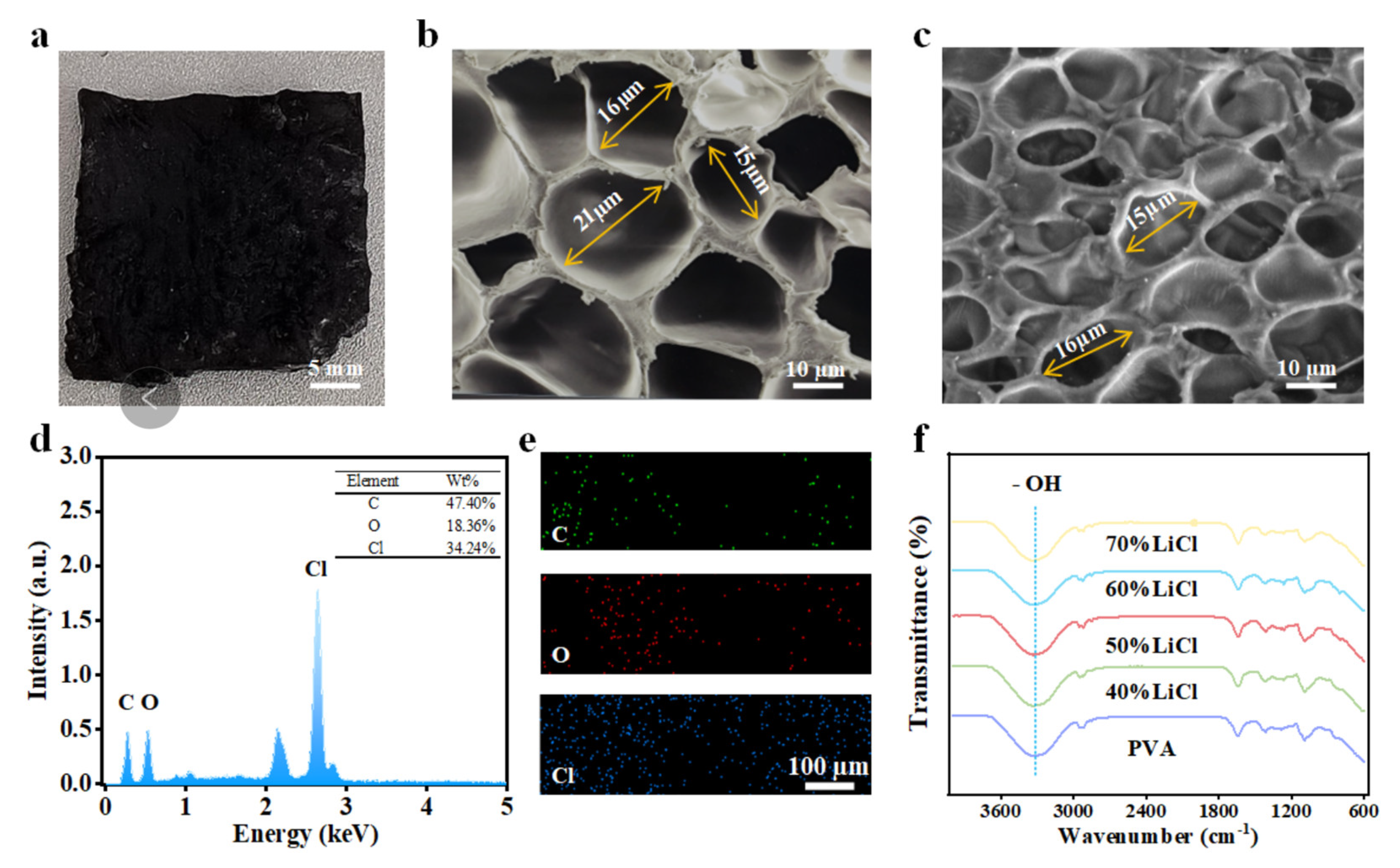
3.2. Characterization of PVAF
3.3. Exploration of Material Adsorption Performance
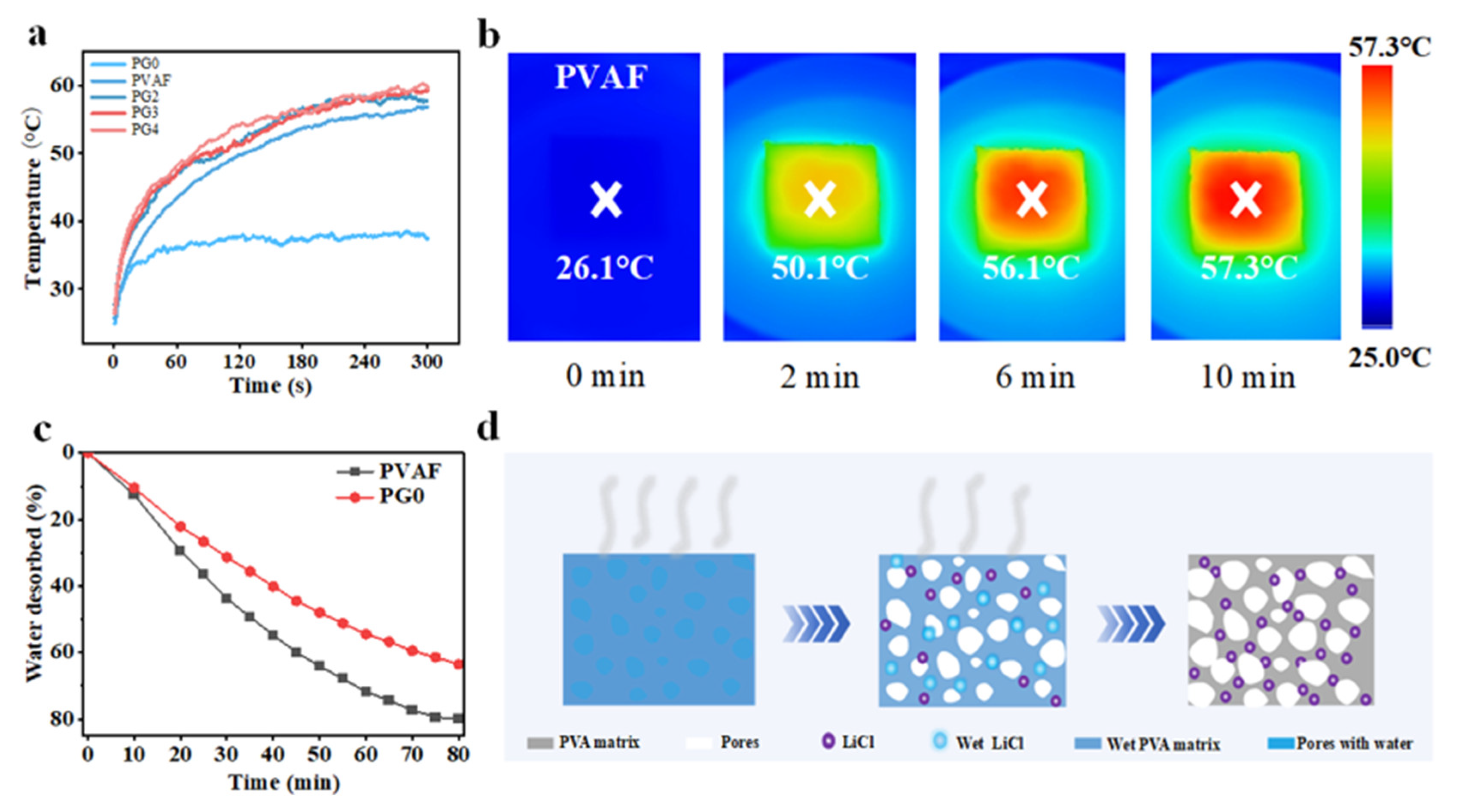
3.4. Exploration of Material Surface Temperature and Desorption Performance
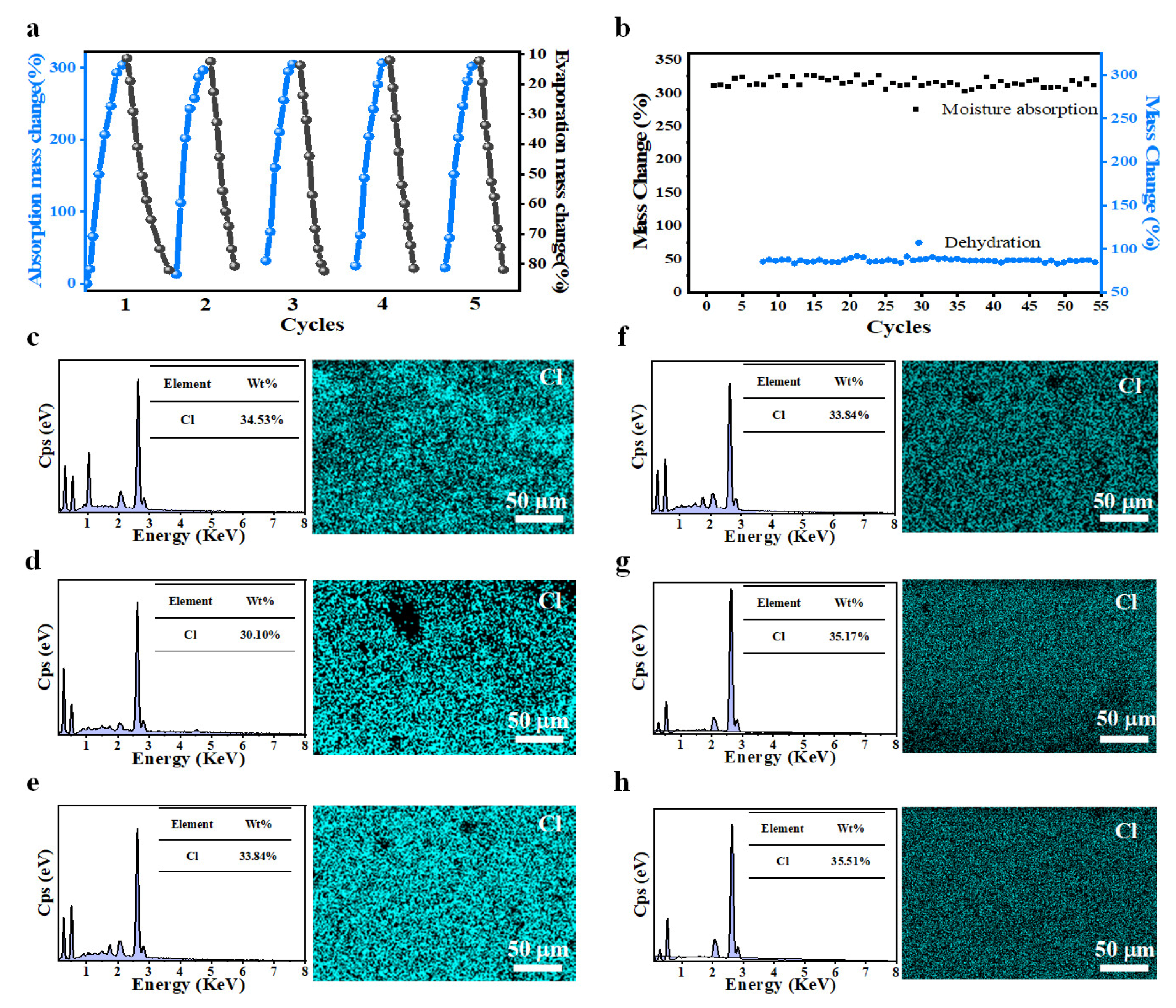
3.5. Exploration of Moisture Absorption-Dehydration Cycle Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future Global Urban Water Scarcity and Potential Solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef]
- Greenhalgh, S.; Samarasinghe, O. Sustainably Managing Freshwater Resources. Ecol. Soc. 2018, 23, 44. [Google Scholar] [CrossRef]
- Bernauer, T.; Böhmelt, T. International Conflict and Cooperation over Freshwater Resources. Nat. Sustain. 2020, 3, 350. [Google Scholar] [CrossRef]
- Wang, M.; Liu, E.; Jin, T.; Zafar, S.; Mei, X.; Fauconnier, M.-L.; De Clerck, C. Towards a Better Understanding of Atmospheric Water Harvesting (AWH) Technology. Water Res. 2024, 250, 121052. [Google Scholar] [CrossRef] [PubMed]
- Ejeian, M.; Wang, R.Z. Adsorption-Based Atmospheric Water Harvesting. Joule 2021, 5, 1678. [Google Scholar] [CrossRef]
- Bai, Q.; Zhou, W.; Cui, W.; Qi, Z. Research Progress on Hygroscopic Agents for Atmospheric Water Harvesting Systems. Materials 2024, 17, 722. [Google Scholar] [CrossRef]
- Liu, X.; Beysens, D.; Bourouina, T. Water Harvesting from Air: Current Passive Approaches and Outlook. ACS Mater. Lett. 2022, 4, 1003–1024. [Google Scholar] [CrossRef]
- Lu, H.; Shi, W.; Zhang, J.H.; Chen, A.C.; Guan, W.; Lei, C.; Greer, J.R.; Boriskina, S.V.; Yu, G. Tailoring the Desorption Behavior of Hygroscopic Gels for Atmospheric Water Harvesting in Arid Climates. Adv. Mater. 2022, 34, 2203198–2203208. [Google Scholar] [CrossRef]
- Feng, A.; Akther, N.; Duan, X.; Peng, S.; Onggowarsito, C.; Mao, S.; Fu, Q.; Kolev, S.D. Recent Development of Atmospheric Water Harvesting Materials: A Review. ACS Mater. Au 2022, 2, 576–595. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, H.; Zhao, F.; Yu, G. Atmospheric Water Harvesting: A Review of Material and Structural Designs. ACS Mater. Lett. 2020, 2, 671–684. [Google Scholar] [CrossRef]
- Lei, C.; Guan, W.; Zhao, Y.; Yu, G. Chemistries and Materials for Atmospheric Water Harvesting. Chem. Soc. Rev. 2024, 53, 7328–7362. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Chen, Z.; Xiang, C.; Wang, C.; Wang, R. Sorption-Based Atmospheric Water Harvesting: Filling the Gap between Material and System. Resour. Conserv. Recycl. 2022, 185, 106521. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, H.; Du, Y.; Wu, T.; Wang, Z.; Qu, J. Bioinspired Heterogeneous Surface for Radiative Cooling Enhanced Power-free Moisture Harvesting in Unsaturated Atmosphere. Adv. Mater. 2025, 37, 2414389. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water Harvesting from Air with Metal-Organic Frameworks Powered by Natural Sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, W.; Chen, W.; Wu, H.; Wang, R.; Xiao, T.; Jiang, L.; Tan, X.; Lei, Y. Superhydrophobic COFs@PVDF Film for Ultraefficient Atmospheric Water Harvesting. ACS Sustain. Chem. Eng. 2025, 13, 2641–2648. [Google Scholar] [CrossRef]
- Graeber, G.; Díaz-Marín, C.D.; Gaugler, L.C.; Zhong, Y.; El Fil, B.; Liu, X.; Wang, E.N. Extreme Water Uptake of Hygroscopic Hydrogels through Maximized Swelling-induced Salt Loading. Adv. Mater. 2024, 36, 2308456–2308465. [Google Scholar] [CrossRef]
- Komarala, E.P.; Mariyappan, K.; Park, S.; Park, S.H. DNA Foams Constructed by Freeze Drying and Their Optoelectronic Characteristics. Colloids Surf. B Biointerfaces 2022, 217, 112648. [Google Scholar] [CrossRef]
- Touzet, A.; Pfefferlé, F.; Der Wel, P.V.; Lamprecht, A.; Pellequer, Y. Active Freeze Drying for Production of Nanocrystal-Based Powder: A Pilot Study. Int. J. Pharm. 2018, 536, 222–230. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Li, Y.; Li, S.; Chen, P.; Guo, T. Fabrication of Novel BPO4 Ceramic Foams Using the Combination of the Direct Foaming Method and Freeze-drying Techniques. Int. J. Appl. Ceram. Technol. 2023, 20, 3565–3575. [Google Scholar] [CrossRef]
- Ding, Y.; Ying, S.; Xiao, Z.; Wu, X. Cell Structure of Microcellular Combustible Object Foamed by Supercritical Carbon Dioxide. Def. Technol. 2019, 15, 419–425. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, L.; Li, J.; Chen, S.; Yi, Q. Recent Advances in Supercritical Carbon Dioxide Foaming Technology for Thermoplastic Polyurethane Foams: Process Optimization, Property Modulation, and Emerging Applications. J. Polym. Sci. 2025, 63, 3047–3064. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, Y.; Peng, X. Applications and Challenges of Supercritical Foaming Technology. Polymers 2023, 15, 402. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, P.; Li, W. Fabrication of Functionally Graded Porous Polymer via Supercritical CO2 Foaming. Compos. Part B Eng. 2011, 42, 318–325. [Google Scholar] [CrossRef]
- He, S.; Chen, D.; Jin, B.; Zhang, B.; Wang, Z.; Shi, Q.; Li, G.; Gong, P. Supercritical Carbon Dioxide Foaming to Fabricate Low Loss Air Material for High Performance Antenna. J. CO2 Util. 2025, 96, 103095. [Google Scholar] [CrossRef]
- Wang, G.; Dong, M.; Deng, H.; Ma, X.; Zhu, B.; Zhou, L.; Zhang, X.; Tan, D.; Algadi, H. Polypropylene Foaming Using Supercritical Carbon Dioxide: A Review on Fundamentals, Technology, and Applications. Adv. Compos. Hybrid Mater. 2025, 8, 84. [Google Scholar] [CrossRef]
- Nikolai, P.; Rabiyat, B.; Aslan, A.; Ilmutdin, A. Supercritical CO2: Properties and Technological Applications—A Review. J. Therm. Sci. 2019, 28, 394–430. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, T.; Hu, D.; Xu, Z.; Zhao, L. Foaming Window for Preparation of Microcellular Rigid Polyurethanes Using Supercritical Carbon Dioxide as Blowing Agent. J. Supercrit. Fluids 2019, 147, 254–262. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, T.; Xi, Z.; Zhao, L. Cell Characteristics of Epoxy Resin Foamed by Step Temperature-Rising Process Using Supercritical Carbon Dioxide as Blowing Agent. J. Cell. Plast. 2018, 54, 359–377. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Liu, P.; Li, L. Facile One-Step Approach to Manufacture Environmentally Friendly Poly (Vinyl Alcohol) Bead Foam Products. Ind. Eng. Chem. Res. 2021, 60, 2962–2970. [Google Scholar] [CrossRef]
- Han, X.; Xue, Y.; Lou, R.; Ding, S.; Wang, S. Facile and Efficient Chitosan-Based Hygroscopic Aerogel for Air Dehumidification. Int. J. Biol. Macromol. 2023, 251, 126191. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, T.; Ju, A.; Xu, Z.; Zhao, Y. Macroporous, Highly Hygroscopic, and Leakage-Free Composites for Efficient Atmospheric Water Harvesting. ACS Appl. Mater. Interfaces 2024, 16, 16893–16902. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, F.; Zhang, Y.; Shi, M.; Zhang, Y.; Yu, H.; Liu, S.; Li, J.; Tan, S.C.; Chen, W. Biopolymers for Hygroscopic Material Development. Adv. Mater. 2024, 36, 2209479. [Google Scholar] [CrossRef]
- Li, S.; Ou, R.; Li, J.; Li, Z.; Yu, L.; Wang, Z.; Murto, P.; Xu, X. Self-Contained Hygroscopic Elastic Foams for Synergistic Atmospheric Moisture Control and Shock Protection. Chem. Eng. J. 2025, 516, 164217. [Google Scholar] [CrossRef]
- Miao, M.; Lu, Y.; Guo, D.; Wang, X.; Wong, N.H.; Sunarso, J.; Li, N. A Porous Composite Membrane for Spontaneous Moisture Adsorption. Compos. Sci. Technol. 2023, 239, 110027. [Google Scholar] [CrossRef]
- Nan, Q.; Yin, C.; Tian, R.; Zhang, J.; Wang, J.; Yan, C.; Zhang, J.; Wu, J.; Zhang, J. Superhygroscopic Aerogels with Hierarchical String-Bag Structure for Effective Humidity Control. ACS Nano 2025, 19, 16696–16705. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Zhong, G.; Gao, P.; Zhang, H.; Wang, R. An Ultra-Hygroscopic Polymer for High-Efficiency Dehumidification. Cell Rep. Phys. Sci. 2023, 4, 101554. [Google Scholar] [CrossRef]
- Yin, C.; Sun, J.; Ni, W.; Shi, L. Flexible Gelatin/PVA/LNH Salogels for Synergistic Hygroscopic and Thermal Management. J. Appl. Polym. Sci. 2025, 142, 57166. [Google Scholar] [CrossRef]
- Wang, Z.; He, Y.; Wang, C.; Ye, Y.; Wei, Y.; Bi, H.; Wang, P.; Chen, G. A Moisture-Absorbing Cellulose Nanofibril-Based Foam via Ambient Drying for High-Performance Dehumidification. Chem. Eng. J. 2024, 486, 150063. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Guo, C.; Wang, H.; Lu, J.; Xie, H.; Wu, T. Bioinspired Polyvinyl Alcohol-Based Foam Fabricated via Supercritical Carbon Dioxide Foaming for Atmospheric Water Harvesting. Biomimetics 2025, 10, 599. https://doi.org/10.3390/biomimetics10090599
Chen Y, Guo C, Wang H, Lu J, Xie H, Wu T. Bioinspired Polyvinyl Alcohol-Based Foam Fabricated via Supercritical Carbon Dioxide Foaming for Atmospheric Water Harvesting. Biomimetics. 2025; 10(9):599. https://doi.org/10.3390/biomimetics10090599
Chicago/Turabian StyleChen, Yingying, Changjun Guo, Hao Wang, Jiabao Lu, Heng Xie, and Ting Wu. 2025. "Bioinspired Polyvinyl Alcohol-Based Foam Fabricated via Supercritical Carbon Dioxide Foaming for Atmospheric Water Harvesting" Biomimetics 10, no. 9: 599. https://doi.org/10.3390/biomimetics10090599
APA StyleChen, Y., Guo, C., Wang, H., Lu, J., Xie, H., & Wu, T. (2025). Bioinspired Polyvinyl Alcohol-Based Foam Fabricated via Supercritical Carbon Dioxide Foaming for Atmospheric Water Harvesting. Biomimetics, 10(9), 599. https://doi.org/10.3390/biomimetics10090599





