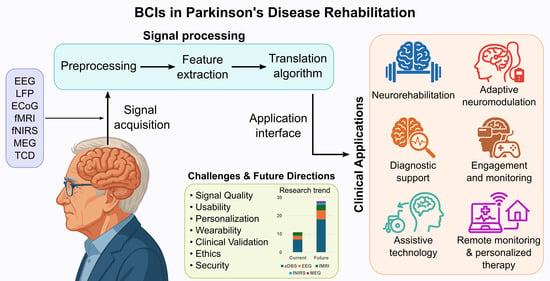
Brain–Computer Interfaces in Parkinson’s Disease Rehabilitation
Abstract
1. Introduction
2. Search Strategy
3. Principles of Brain–Computer Interfaces
3.1. Signal Acquisition
3.2. Non-Invasive Electrode Technologies for EEG-Based BCIs
3.3. Signal Processing
3.4. Feedback Mechanisms in BCI-Based Rehabilitation
3.5. Common eBCI Paradigms
3.6. Toward Clinical Integration
4. Neurophysiological Correlates of Parkinson’s Disease Relevant to BCI
5. Current and Emerging Applications of BCIs in Parkinson’s Disease
5.1. Neurorehabilitation and Therapeutic Modulation
| Study | Intervention | Time | Sample Size | Main Findings |
|---|---|---|---|---|
| Turconi et al., 2014 [64] | EEG-BCI neurofeedback (motor imagery) for motor and cognitive rehabilitation. | 15 sessions, 2–3 times per week | 3 PD | Decrease in severity of gait freezing, improvement in mobility, increase in alpha and beta EEG bands power, and better performance on attention and executive tasks. |
| Lavermicocca et al., 2018 [63] | EEG-BCI neurofeedback (attentional control) for cognitive rehabilitation. | 24 sessions in 3 months | 10 PD | Cognitive performance increased compared to baseline in all cognitive domains (attention, set shifting, executive functions, verbal fluency, immediate and delayed auditory-verbal memory, and visual–spatial reasoning), with a positive impact on reaction time, processing speed, and overall efficiency. |
| Subramanian et al., 2011 [62] | fMRI-BCI neurofeedback (motor imagery) for hand motor rehabilitation. | 2 BCI sessions and 2 to 6 months of neurofeedback practice at home | 5 PD | Improvement in motor speed (finger tapping) and clinical ratings of motor symptoms (37% in UPDRS part III). |
| Buyukturkoglu et al., 2013 [54] | fMRI-BCI neurofeedback (motor imagery), plus a motor task for hand motor rehabilitation. | 1 session | 1 PD 3 HS | Hand motor responses slowed down. |
| Little et al., 2013 [56] | BCI-controlled adaptive DBS (unilateral). | 640 s | 8 PD | Improved motor scores (UPDRS) and reduction in stimulation time and energy requirements compared to those of conventional DBS. |
| Little et al., 2016 [57] | BCI-controlled adaptive DBS (bilateral). | 15 min | 4 PD | Motor scores showed improvement compared to those in the absence of stimulation. |
| Arlotti et al., 2018 [58] | BCI-controlled adaptive DBS (unilateral). | 8 h | 11 PD | Motor scores showed improvement compared to those in the absence of stimulation. |
| Swann et al., 2018 [60] | BCI-controlled adaptive DBS (multisite brain recordings, bilateral stimulation). | – | 5 PD | Four of the five patients showed improved motor function 1 year postoperatively. |
| Velisar et al., 2019 [61] | BCI-controlled adaptive DBS (bilateral, Activa™ PC + S-NexusD3). | 21 min | 13 PD | Closed-loop DBS was feasible, well-tolerated, and improved tremor and bradykinesia, reducing energy requirements. |
| Arlotti et al., 2021 [44] | BCI-controlled adaptive DBS (AlphaDBS System). | 24 h; then 2 weeks | 3 PD | The implanted BCI was viable for adaptive DBS with artifact-free and long-term recordings. |
| Dold et al., 2025 [59] | BCI-controlled adaptive DBS (Dareplane) for research. | 23 min | 1 PD | The system was viable for adaptive DBS. |
5.2. Diagnostic Potential and Disease Monitoring
5.3. Domotic Control and Daily Function
5.4. Education, Engagement, and Neurocognitive Stimulation
6. Design Considerations for BCIs in Parkinson’s Disease
6.1. Electrode Technology and Signal Acquisition
6.2. Signal Processing and Feature Extraction
6.3. Adaptive and Inclusive System Design
6.4. Paradigm Selection and Feedback Integration
6.5. Commercial Wearable EEG Systems: Opportunities and Limitations
7. Current Research and Future Perspectives on BCIs for Parkinson’s Disease Rehabilitation
7.1. Emerging Roles of Non-Invasive BCIs in PD
7.2. The Future of eBCIs: Wearability, Accessibility, and Personalization
7.3. Ethical, Equity, and Security Considerations
7.4. Ten Challenges in Parkinson’s Disease Treatment and Opportunities for BCIs
7.5. Barriers to Clinical Translation of BCIs in Parkinson’s Disease
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| AI | Artificial Intelligence |
| BCI | Brain–Computer Interface |
| CCA | Canonical Correlation Analysis |
| CNN | Convolutional Neural Network |
| CSP | Common Spatial Pattern |
| DBS | Deep Brain Stimulation |
| DL | Deep Learning |
| ECoG | Electrocorticography |
| EEG | Electroencephalography |
| EMG | Electromyography |
| EOG | Electrooculography |
| GAN | Generative Adversarial Network |
| ICA | Independent Component Analysis |
| LFP | Local Field Potential |
| MCI | Mild Cognitive Impairment |
| MEG | Magnetoencephalography |
| MEMD | Multivariate Empirical Mode Decomposition |
| MI | Motor Imagery |
| NclDBS | Neural Closed-Loop Deep Brain Stimulation |
| PAC | Phase–Amplitude Coupling |
| PD | Parkinson’s Disease |
| SCP | Slow Cortical Potential |
| SMA | Supplementary Motor Area |
| STN | Subthalamic Nucleus |
| TCD | Transcranial Doppler Ultrasonography |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| WT | Wavelet Transform |
| aDBS | Adaptive Deep Brain Stimulation |
| eBCI | EEG-based Brain–Computer Interface |
| fMRI | Functional Magnetic Resonance Imaging |
| fNIRS | Functional Near-Infrared Spectroscopy |
Appendix A
| NCT Number | Study Title | Type of BCI | Purpose | Enrollment |
|---|---|---|---|---|
| NCT03422757 | Safety and Efficacy of Adaptive DBS vs Conventional DBS in Patients With Parkinson’s Disease | aDBS | To assess safety and efficacy (in motor impairment and dyskinesia). | 6 * |
| NCT02154724 | Clinical Study for Adaptive Deep Brain Stimulation (aDBS) Controlled by Intracerebral Activity in Parkinson’s Disease | aDBS | To assess safety and efficacy (in motor impairment). | 20 † |
| NCT03724734 | Trial of Adaptive Deep Brain Stimulation | aDBS | To assess safety and efficacy (in motor impairment during the day and night). | 15 † |
| NCT02384421 | Adaptive Closed-Loop Neuromodulation and Neural Signatures of Parkinson’s Disease | aDBS | To assess efficacy (in motor symptoms, tremor, freezing of gait, bradykinesia). | 22 * |
| NCT06891781 | Investigating Adaptive Deep Brain Stimulation in Parkinson’s Disease Management | aDBS | To assess safety and efficacy (in motor and non-motor symptoms and quality of life). | 72 † |
| NCT04681534 | Safety and Efficacy of Adaptive Deep Brain Stimulation | aDBS | To assess safety and efficacy (in motor impairment and dyskinesia). | 15 † |
| NCT05262348 | An Open-Label Clinical Trial to Compare the Safety and Effectiveness of Adaptive versus Conventional Deep Brain Stimulation | aDBS | To assess safety and efficacy (in motor impairment). | 0 * |
| NCT05402163 | CANadian Adaptive DBS TriAl | aDBS | To assess efficacy (motor fluctuations, speech, gait impairment, and falls). | 10 † |
| NCT06909045 | Adaptive vs. Continuous Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease | aDBS | To assess safety and efficacy (in motor and non-motor symptoms and quality of life). | 130 † |
| NCT06791902 | Study on Preliminary Safety and Efficacy of Adaptive DBS Aligned to Locomotor States to Improve Locomotor Functions in Parkinson’s Patients | aDBS | To assess safety and efficacy (in locomotor function). | 10 † |
| NCT04547712 | Adaptive DBS Algorithm for Personalized Therapy in Parkinson’s Disease | aDBS | To assess safety and efficacy (in motor impairment). | 85 * |
| NCT04675398 | Adaptive Deep Brain Stimulation to Improve Motor and Gait Functions in Parkinson’s Disease | aDBS | To assess safety and efficacy (in motor learning and gait function). | 10 † |
| NCT05070013 | Adaptive Neurostimulation to Restore Sleep in Parkinson’s Disease (Aim 2) | aDBS | To assess efficacy (in sleep efficiency and quality). | 20 † |
| NCT02318927 | A Responsive Closed-Loop Approach to Treat Freezing of Gait in Parkinson’s Disease | aDBS | To assess safety and efficacy (in motor function and freezing of gait). | 8 * |
| NCT04620551 | Adaptive Neurostimulation to Restore Sleep in Parkinson’s Disease | aDBS | To assess efficacy (in sleep efficiency and quality). | 20 * |
| NCT06012461 | Closed-Loop DBS in Parkinson’s Disease | aDBS | To assess long-term safety and efficacy (in motor function and sleep). | 10 † |
| NCT06819020 | Adaptive Deep Brain Stimulation for Freezing of Gait in Parkinson’s Disease | aDBS | To assess efficacy (in motor function and freezing of gait). | 20 † |
| NCT03446833 | LFP Beta aDBS Feasibility Study | aDBS | To assess safety and efficacy (in motor function, speech, and dyskinesia). | 1 * |
| NCT06642519 | Brain–Machine Interface for Freezing of Gait (Cortical Stimulation) | ECoG | To assess efficacy (in motor function and freezing of gait). | 10 † |
| NCT05696925 | Effects of Motor Imagery and Action Observation on Upper Limb Motor Changes and Cognitive Changes in Parkinson’s Disease | EEG | To assess efficacy (in upper limb motor function and cognitive changes). | 60 * |
| NCT06690931 | Neurofeedback Rehabilitation With FES and VR for PD | EEG | To assess efficacy (in motor function and quality of life). | 30 † |
| NCT05986643 | Brain Training to Improve Balance in Parkinson’s Disease | EEG | To assess efficacy (in balance and gait function). | 100 † |
| NCT04651478 | Mental Representation Techniques for the Treatment of Parkinson’s Disease-Related Pain | EEG | To assess efficacy (in pain management and motor function). | 32 † |
| NCT05987865 | Neurofeedback Training for PD | EEG/ LFPs | To assess efficacy (in motor symptoms and quality of life). | 40 † |
| NCT01867827 | Real-Time fMRI Neurofeedback for Treatment of Parkinson’s Disease | fMRI | To assess efficacy (in motor function and brain activity modulation). | 30 * |
| NCT06582355 | FMRI-neurofeedback in Parkinson’s Disease | fMRI | To assess efficacy (in motor function and neuroplasticity). | 60 † |
| NCT03623386 | Effect of Mental Imagery Training on Brain Plasticity and Motor Function in Individuals With Parkinson’s Disease | fMRI | To assess efficacy (in brain plasticity and motor function). | 63 * |
| NCT05800470 | The Effects of fNIRS-based Neurofeedback Training on Balance and Gait in Parkinson’s Disease | fNIRS | To assess efficacy (in balance and gait function). | 48 † |
| NCT03837548 | A Study of Neurofeedback for the Treatment of Parkinson’s Disease | MEG | To assess efficacy (in motor function and brain activity modulation). | 20 † |
References
- Chaudhary, U.; Birbaumer, N.; Ramos-Murguialday, A. Brain-computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 2016, 12, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.J.; Krusienski, D.J.; Wolpaw, J.R. Brain-computer interfaces in medicine. Mayo Clin. Proc. 2012, 87, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mamun, K.A.; Ahmed, K.; Mostafa, R.; Naik, G.R.; Darvishi, S.; Khandoker, A.H.; Baumert, M. Progress in Brain Computer Interface: Challenges and Opportunities. Front. Syst. Neurosci. 2021, 15, 578875. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, W.L.; Liu, M.; Chen, M.Y.; Pereira, T.; Doda, D.Y.; Ke, Y.F.; Wang, S.Y.; Wen, D.; Tong, X.G.; et al. Recent applications of EEG-based brain-computer-interface in the medical field. Mil. Med. Res. 2025, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.V.; Jamal, S.; Sethuraman, S.C. A Comprehensive Survey of Brain-Computer Interface Technology in Health care: Research Perspectives. J. Med. Signals Sens. 2025, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Khorev, V.; Kurkin, S.; Badarin, A.; Antipov, V.; Pitsik, E.; Andreev, A.; Grubov, V.; Drapkina, O.; Kiselev, A.; Hramov, A. Review on the Use of Brain Computer Interface Rehabilitation Methods for Treating Mental and Neurological Conditions. J. Integr. Neurosci. 2024, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.J.R.; Lo, J.Y.T.; Tan, Y.Y.; Lin, H.Y.; Wang, Y.; Tan, D.; Wang, E.; Naing Ma, Y.Y.; Wei Ng, J.J.; Jefree, R.A.; et al. The state-of-the-art of invasive brain-computer interfaces in humans: A systematic review and individual patient meta-analysis. J. Neural Eng. 2025, 22, 026013. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.C.; Akpan, A.; Tang, K.T.; Lakany, H. Brain computer interfaces for cognitive enhancement in older people—Challenges and applications: A systematic review. BMC Geriatr. 2025, 25, 36. [Google Scholar] [CrossRef] [PubMed]
- Foltynie, T.; Bruno, V.; Fox, S.; Kühn, A.A.; Lindop, F.; Lees, A.J. Medical, surgical, and physical treatments for Parkinson’s disease. Lancet 2024, 403, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Rawls, A.; Tholanikunnel, T.; Okun, M.S. Clinical Management of Parkinson’s Disease: Features, Diagnosis, and Principles of Treatment. Cold Spring Harb. Perspect. Med. 2025, a041638. [Google Scholar] [CrossRef] [PubMed]
- Riederer, P.; Strobel, S.; Nagatsu, T.; Watanabe, H.; Chen, X.; Löschmann, P.A.; Sian-Hulsmann, J.; Jost, W.H.; Müller, T.; Dijkstra, J.M.; et al. Levodopa treatment: Impacts and mechanisms throughout Parkinson’s disease progression. J. Neural. Transm. 2025, 132, 743–779. [Google Scholar] [CrossRef] [PubMed]
- de Bie, R.M.A.; Katzenschlager, R.; Swinnen, B.; Peball, M.; Lim, S.Y.; Mestre, T.A.; Perez Lloret, S.; Coelho, M.; Aquino, C.; Tan, A.H.; et al. Update on Treatments for Parkinson’s Disease Motor Fluctuations—An International Parkinson and Movement Disorder Society Evidence-Based Medicine Review. Mov. Disord. 2025, 40, 776–794. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, W.; Alabdullkarim, F.A.; Abukaram, T.M.; Gubran, L.; Alsulami, D.S.; Albehairi, S.A.; Alabdulkarim, F.A.; Wadaan, A.M. Comparison of Efficacy and Safety of Device-Based Interventions Versus Pharmacological Therapy in the Management of Patients With Advanced Parkinson’s Disease: A Literature Review. Cureus 2024, 16, e76044. [Google Scholar] [CrossRef] [PubMed]
- Crowell, A.L.; Ryapolova-Webb, E.S.; Ostrem, J.L.; Galifianakis, N.B.; Shimamoto, S.; Lim, D.A.; Starr, P.A. Oscillations in sensorimotor cortex in movement disorders: An electrocorticography study. Brain 2012, 135, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Polverino, P.; Ajčević, M.; Catalan, M.; Mazzon, G.; Bertolotti, C.; Manganotti, P. Brain oscillatory patterns in mild cognitive impairment due to Alzheimer’s and Parkinson’s disease: An exploratory high-density EEG study. Clin. Neurophysiol. 2022, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maitin, A.M.; Romero Muñoz, J.P.; García-Tejedor, Á.J. Survey of Machine Learning Techniques in the Analysis of EEG Signals for Parkinson’s Disease: A Systematic Review. Appl. Sci. 2022, 12, 6967. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Millán, J.D.R.; Ramsey, N.F. Brain-computer interfaces: Definitions and principles. Handb. Clin. Neurol. 2020, 168, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Soekadar, S.R.; Haagen, K.; Birbaumer, N. Brain-Computer Interfaces (BCI): Restoration of Movement and Thought from Neuroelectric and Metabolic Brain Activity. In Coordination: Neural, Behavioral and Social Dynamics; Fuchs, A., Jirsa, V.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 229–252. [Google Scholar] [CrossRef]
- Livanis, E.; Voultsos, P.; Vadikolias, K.; Pantazakos, P.; Tsaroucha, A. Understanding the Ethical Issues of Brain-Computer Interfaces (BCIs): A Blessing or the Beginning of a Dystopian Future? Cureus 2024, 16, e58243. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Sun, H.Q.; Si, J.Y.; Lin, Z.Y.; Zhai, X.M.; Lu, L. Challenges and Suggestions of Ethical Review on Clinical Research Involving Brain-Computer Interfaces. Chin. Med. Sci. J. 2024, 39, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Moran, D. Evolution of brain-computer interface: Action potentials, local field potentials and electrocorticograms. Curr. Opin. Neurobiol. 2010, 20, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Kim, S.M.; Ryu, R.H.; Kim, S.P.; Sohn, J.W. Implantable Neural Probes for Brain-Machine Interfaces—Current Developments and Future Prospects. Exp. Neurobiol. 2018, 27, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Im, C.; Seo, J.-M. A review of electrodes for the electrical brain signal recording. Biomed. Eng. Lett. 2016, 6, 104–112. [Google Scholar] [CrossRef]
- Marceglia, S.; Guidetti, M.; Harmsen, I.E.; Loh, A.; Meoni, S.; Foffani, G.; Lozano, A.M.; Volkmann, J.; Moro, E.; Priori, A. Deep brain stimulation: Is it time to change gears by closing the loop? J. Neural Eng. 2021, 18, 061001. [Google Scholar] [CrossRef] [PubMed]
- Peksa, J.; Mamchur, D. State-of-the-Art on Brain-Computer Interface Technology. Sensors 2023, 23, 6001. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Fan, M.; Feng, Y.; Li, Y.; Yang, C.; Zheng, J.; Wang, C.; Zhou, J. Advancements in dry and semi-dry EEG electrodes: Design, interface characteristics, and performance evaluation. AIP Adv. 2025, 15, 040703. [Google Scholar] [CrossRef]
- Lin, S.; Jiang, J.; Huang, K.; Li, L.; He, X.; Du, P.; Wu, Y.; Liu, J.; Li, X.; Huang, Z.; et al. Advanced Electrode Technologies for Noninvasive Brain-Computer Interfaces. ACS Nano 2023, 17, 24487–24513. [Google Scholar] [CrossRef] [PubMed]
- Janapati, R.; Dalal, V.; Sengupta, R. Advances in modern EEG-BCI signal processing: A review. Mater. Today Proc. 2023, 80, 2563–2566. [Google Scholar] [CrossRef]
- Hramov, A.E.; Maksimenko, V.A.; Pisarchik, A.N. Physical principles of brain–computer interfaces and their applications for rehabilitation, robotics and control of human brain states. Phys. Rep. 2021, 918, 1–133. [Google Scholar] [CrossRef]
- Gudikandula, N.; Janapati, R.; Sengupta, R.; Chintala, S. Recent Advancements in Online Ocular Artifacts Removal in EEG based BCI: A Review. In Proceedings of the 2024 15th International Conference on Computing Communication and Networking Technologies (ICCCNT), Kamand, India, 24–28 June 2024; pp. 1–6. [Google Scholar]
- Aggarwal, S.; Chugh, N. Review of Machine Learning Techniques for EEG Based Brain Computer Interface. Arch. Comput. Methods Eng. 2022, 29, 3001–3020. [Google Scholar] [CrossRef]
- Lotte, F.; Congedo, M.; Lécuyer, A.; Lamarche, F.; Arnaldi, B. A review of classification algorithms for EEG-based brain–computer interfaces. J. Neural Eng. 2007, 4, R1. [Google Scholar] [CrossRef] [PubMed]
- Rainio, O.; Teuho, J.; Klén, R. Evaluation metrics and statistical tests for machine learning. Sci. Rep. 2024, 14, 6086. [Google Scholar] [CrossRef] [PubMed]
- Riascos, J.; Molinas, M.; Lotte, F. Machine Learning Methods for BCI: Challenges, pitfalls and promises. In Proceedings of the ESANN 2024—European Symposium on Artificial Neural Networks, Computational Intelligence and Machine Learning, Bruges, Belgium, 9–11 October 2024. [Google Scholar]
- Kim, D.-H.; Yeom, H.G.; Kim, M.; Kim, S.H.; Yang, T.-W.; Kwon, O.-Y.; Kim, Y.-S. Introduction of brain computer interface to neurologists. ACN 2021, 23, 92–98. [Google Scholar] [CrossRef]
- Mudgal, S.K.; Sharma, S.K.; Chaturvedi, J.; Sharma, A. Brain computer interface advancement in neurosciences: Applications and issues. Interdiscip. Neurosurg. 2020, 20, 100694. [Google Scholar] [CrossRef]
- Besharat, A.; Samadzadehaghdam, N.; Afghan, R. A Comparative Review of Detection Methods in SSVEP-Based Brain-Computer Interfaces. IEEE Access 2024, 12, 181232–181270. [Google Scholar] [CrossRef]
- Al-Qaysi, Z.T.; Albahri, A.S.; Ahmed, M.A.; Hamid, R.A.; Alsalem, M.A.; Albahri, O.S.; Alamoodi, A.H.; Homod, R.Z.; Shayea, G.G.; Duhaim, A.M. A comprehensive review of deep learning power in steady-state visual evoked potentials. Neural Comput. Appl. 2024, 36, 16683–16706. [Google Scholar] [CrossRef]
- Kalra, J.; Mittal, P.; Mittal, N.; Arora, A.; Tewari, U.; Chharia, A.; Upadhyay, R.; Kumar, V.; Longo, L. How Visual Stimuli Evoked P300 is Transforming the Brain–Computer Interface Landscape: A PRISMA Compliant Systematic Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Wu, Q.; Wan, F.; Hu, Y. State-of-the-art non-invasive brain–computer interface for neural rehabilitation: A review. J. Neurorestoratology 2020, 8, 12–25. [Google Scholar] [CrossRef]
- Cantillo-Negrete, J.; Carino-Escobar, R.I.; Ortega-Robles, E.; Arias-Carrión, O. A comprehensive guide to BCI-based stroke neurorehabilitation interventions. MethodsX 2023, 11, 102452. [Google Scholar] [CrossRef] [PubMed]
- Cantillo-Negrete, J.; Carino-Escobar, R.I.; Leyva-Martinez, I.; Barrera-Ortiz, A.; Rodriguez-Barragan, M.A.; Mendoza-Montoya, O.; Antelis, J.M. Upper Limb Recovery in Cervical Spinal Cord Injury After a Brain-Computer Interface Controlled Functional Electrical Stimulation Intervention. J. Med. Biol. Eng. 2023, 43, 522–531. [Google Scholar] [CrossRef]
- Rezvani, S.; Hosseini-Zahraei, S.H.; Tootchi, A.; Guger, C.; Chaibakhsh, Y.; Saberi, A.; Chaibakhsh, A. A review on the performance of brain-computer interface systems used for patients with locked-in and completely locked-in syndrome. Cogn. Neurodyn 2024, 18, 1419–1443. [Google Scholar] [CrossRef] [PubMed]
- Arlotti, M.; Colombo, M.; Bonfanti, A.; Mandat, T.; Lanotte, M.M.; Pirola, E.; Borellini, L.; Rampini, P.; Eleopra, R.; Rinaldo, S.; et al. A New Implantable Closed-Loop Clinical Neural Interface: First Application in Parkinson’s Disease. Front. Neurosci. 2021, 15, 763235. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, A.; Gross, J. Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 2005, 6, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Özkurt, T.E. Abnormally low sensorimotor α band nonlinearity serves as an effective EEG biomarker of Parkinson’s disease. J. Neurophysiol. 2024, 131, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, J.; Özkurt, T.E.; Butz, M.; Homburger, M.; Elben, S.; Hartmann, C.J.; Vesper, J.; Wojtecki, L.; Schnitzler, A. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson’s disease. Neuroimage 2011, 55, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Foffani, G.; Rossi, L.; Marceglia, S. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp. Neurol. 2013, 245, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Vinding, M.C.; Tsitsi, P.; Waldthaler, J.; Oostenveld, R.; Ingvar, M.; Svenningsson, P.; Lundqvist, D. Reduction of spontaneous cortical beta bursts in Parkinson’s disease is linked to symptom severity. Brain Commun. 2020, 2, fcaa052. [Google Scholar] [CrossRef] [PubMed]
- de Hemptinne, C.; Ryapolova-Webb, E.S.; Air, E.L.; Garcia, P.A.; Miller, K.J.; Ojemann, J.G.; Ostrem, J.L.; Galifianakis, N.B.; Starr, P.A. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc. Natl. Acad. Sci. USA 2013, 110, 4780–4785. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.R.; van der Meij, R.; Peterson, E.J.; de Hemptinne, C.; Starr, P.A.; Voytek, B. Nonsinusoidal Beta Oscillations Reflect Cortical Pathophysiology in Parkinson’s Disease. J. Neurosci. 2017, 37, 4830–4840. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, H.; Kwon, I.; An, K.O.; Kim, H.; Park, G.; Hyung, W.; Im, C.H.; Shin, J.H. Efficacy of brain-computer interface training with motor imagery-contingent feedback in improving upper limb function and neuroplasticity among persons with chronic stroke: A double-blinded, parallel-group, randomized controlled trial. J. Neuroeng. Rehabil. 2025, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Fahey, P.; Liu, K.P.Y. Effectiveness of Motor Imagery in the Rehabilitation of People With Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neurorehabil. Neural Repair. 2024, 38, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Buyukturkoglu, K.; Rana, M.; Ruiz, S.; Hackley, S.A.; Soekadar, S.R.; Birbaumer, N.; Sitaram, R. Volitional regulation of the supplementary motor area with fMRI-BCI neurofeedback in Parkinson’s disease: A pilot study. In Proceedings of the 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER), San Diego, CA, USA, 6–8 November 2013; pp. 677–681. [Google Scholar]
- Saluja, A.; Goyal, V.; Dhamija, R.K. Multi-Modal Rehabilitation Therapy in Parkinson’s Disease and Related Disorders. Ann. Indian. Acad. Neurol. 2023, 26, S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Pogosyan, A.; Neal, S.; Zavala, B.; Zrinzo, L.; Hariz, M.; Foltynie, T.; Limousin, P.; Ashkan, K.; FitzGerald, J.; et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 2013, 74, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Beudel, M.; Zrinzo, L.; Foltynie, T.; Limousin, P.; Hariz, M.; Neal, S.; Cheeran, B.; Cagnan, H.; Gratwicke, J.; et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Arlotti, M.; Marceglia, S.; Foffani, G.; Volkmann, J.; Lozano, A.M.; Moro, E.; Cogiamanian, F.; Prenassi, M.; Bocci, T.; Cortese, F.; et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 2018, 90, e971–e976. [Google Scholar] [CrossRef] [PubMed]
- Dold, M.; Pereira, J.; Sajonz, B.; Coenen, V.A.; Thielen, J.; Janssen, M.L.F.; Tangermann, M. Dareplane: A modular open-source software platform for BCI research with application in closed-loop deep brain stimulation. J. Neural Eng. 2025, 22, 026029. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.C.; de Hemptinne, C.; Miocinovic, S.; Qasim, S.; Ostrem, J.L.; Galifianakis, N.B.; Luciano, M.S.; Wang, S.S.; Ziman, N.; Taylor, R.; et al. Chronic multisite brain recordings from a totally implantable bidirectional neural interface: Experience in 5 patients with Parkinson’s disease. J. Neurosurg. 2018, 128, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Velisar, A.; Syrkin-Nikolau, J.; Blumenfeld, Z.; Trager, M.H.; Afzal, M.F.; Prabhakar, V.; Bronte-Stewart, H. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 2019, 12, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, L.; Hindle, J.V.; Johnston, S.; Roberts, M.V.; Husain, M.; Goebel, R.; Linden, D. Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinson’s disease. J. Neurosci. 2011, 31, 16309–16317. [Google Scholar] [CrossRef] [PubMed]
- Lavermicocca, V.; Dellomonaco, A.R.; Tedesco, A.; Notarnicola, M.; Di Fede, R.; Battaglini, P.P. Neurofeedback in Parkinson’s disease: Technologies in speech and language therapy. Recenti Prog. Med. 2018, 109, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Turconi, M.M.; Mezzarobba, S.; Franco, G.; Busan, P.; Fornasa, E.; Jarmolowska, J.; Accardo, A.; Battaglini, P.P. BCI-based neuro-rehabilitation treatment for Parkinson’s disease: Cases report. In Proceedings of the Trieste Symposium on Perception and Cognition (TSPC2014), Trieste, Italia, 27–28 November 2014; pp. 63–65. [Google Scholar]
- Waninger, S.; Berka, C.; Stevanovic Karic, M.; Korszen, S.; Mozley, P.D.; Henchcliffe, C.; Kang, Y.; Hesterman, J.; Mangoubi, T.; Verma, A. Neurophysiological Biomarkers of Parkinson’s Disease. J. Park. Dis. 2020, 10, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kalitin, K.Y.; Nevzorov, A.A.; Spasov, A.A.; Mukha, O.Y. Deep learning analysis of intracranial EEG for recognizing drug effects and mechanisms of action. Comput. Res. Model. 2024, 16, 755–772. [Google Scholar] [CrossRef]
- Parker, K.L.; Lamichhane, D.; Caetano, M.S.; Narayanan, N.S. Executive dysfunction in Parkinson’s disease and timing deficits. Front. Integr. Neurosci. 2013, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Magosso, E.; Borra, D. The strength of anticipated distractors shapes EEG alpha and theta oscillations in a Working Memory task. Neuroimage 2024, 300, 120835. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.J.; Daly, J.; Boulay, C.; Parvaz, M. Therapeutic Applications of BCI Technologies. Brain Comput. Interfaces 2017, 47, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.; Ehrlich, S.K.; Andersen, R.; Brumberg, J.; Guenther, F.; Hallett, M.; Howard, M.A.; Millán, J.D.R.; Reilly, R.B.; Schultz, T.; et al. Brain-Computer Interfaces for Treatment of Focal Dystonia. Mov. Disord. 2022, 37, 1798–1802. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M. Sweating dysfunctions in Parkinson’s disease. J. Neurol. 2006, 253 (Suppl. S7), Vii42–Vii47. [Google Scholar] [CrossRef] [PubMed]
- Niemann, N.; Billnitzer, A.; Jankovic, J. Parkinson’s disease and skin. Park. Relat. Disord. 2021, 82, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, D.; Gambioli, F.; De Bartolo, M.I.; Mancinelli, R.; Biagioni, F.; Carotti, S.; Falato, E.; Leodori, G.; Puglisi-Allegra, S.; Vivacqua, G.; et al. Pain in Parkinson’s disease: A neuroanatomy-based approach. Brain Commun. 2024, 6, fcae210. [Google Scholar] [CrossRef] [PubMed]
- Mascia, A.; Collu, R.; Spanu, A.; Fraschini, M.; Barbaro, M.; Cosseddu, P. Wearable System Based on Ultra-Thin Parylene C Tattoo Electrodes for EEG Recording. Sensors 2023, 23, 766. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.M.; Wang, Y.T.; Wang, Y.; Maier, C.; Jung, T.P.; Cauwenberghs, G. Dry and noncontact EEG sensors for mobile brain-computer interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bayin, C.; Li, H.; Shu, X.; Deng, J.; Yuan, H.; Shen, H.; Liang, Z.; Li, Y. A flexible, stable, semi-dry electrode with low impedance for electroencephalography recording. RSC Adv. 2024, 14, 34415–34427. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, A.M.; Croce, P.; Merla, A.; Zappasodi, F. Deep learning for hybrid EEG-fNIRS brain-computer interface: Application to motor imagery classification. J. Neural Eng. 2018, 15, 036028. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Li, H.; Yang, H.; Lv, X.; Li, P.; Liu, T.; Yao, D.; Xu, P. The extraction of motion-onset VEP BCI features based on deep learning and compressed sensing. J. Neurosci. Methods 2017, 275, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Allison, B.Z.; Wolpaw, E.W.; Wolpaw, J.R. Brain-computer interface systems: Progress and prospects. Expert. Rev. Med. Devices 2007, 4, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ke, Y.; Liu, P.; Liu, W.; Kong, L.; Wang, N.; Xu, M.; An, X.; Ming, D. A two-step idle-state detection method for SSVEP BCI. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 3095–3098. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Xu, G.; Li, M.; Lin, F. An idle state-detecting method based on transient visual evoked potentials for an asynchronous ERP-based BCI. J. Neurosci. Methods 2020, 337, 108670. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, T.; Li, Z.; Zhao, J.; Li, X. Using oscillatory and aperiodic neural activity features for identifying idle state in SSVEP-based BCIs reduces false triggers. J. Neural Eng. 2023, 20, 066032. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-H.; Müller, K.-R.; Hwang, H.-J. Brain-Switches for Asynchronous Brain–Computer Interfaces: A Systematic Review. Electronics 2020, 9, 422. [Google Scholar] [CrossRef]
- Diez, P.F.; Garcés Correa, A.; Orosco, L.; Laciar, E.; Mut, V. Attention-level transitory response: A novel hybrid BCI approach. J. Neural Eng. 2015, 12, 056007. [Google Scholar] [CrossRef] [PubMed]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A comprehensive review of EEG-based brain-computer interface paradigms. J. Neural Eng. 2019, 16, 011001. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Song, M.; Jang, S.H.; Kim, J. Investigating the cortical effect of false positive feedback on motor learning in motor imagery based rehabilitative BCI training. J. Neuroeng. Rehabil. 2025, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Peng, Y.; Wang, L.; Song, S.; Chen, S.; Yang, L.; Liu, H.; Wang, H.; Shi, G.; Han, C.; et al. Effect of BCI-Controlled Pedaling Training System With Multiple Modalities of Feedback on Motor and Cognitive Function Rehabilitation of Early Subacute Stroke Patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Higdon, L.M.; Sperling, M.R. Long-Term Home EEG Recording: Wearable and Implantable Devices. J. Clin. Neurophysiol. 2024, 41, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Mohamed, N.; Kim, J.G. Advancements in Wearable EEG Technology for Improved Home-Based Sleep Monitoring and Assessment: A Review. Biosensors 2023, 13, 1019. [Google Scholar] [CrossRef] [PubMed]
- Kaongoen, N.; Choi, J.; Woo Choi, J.; Kwon, H.; Hwang, C.; Hwang, G.; Kim, B.H.; Jo, S. The future of wearable EEG: A review of ear-EEG technology and its applications. J. Neural Eng. 2023, 20, 051002. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Sánchez, M.R.; Moreno-Verdú, M.; Atín-Arratibel, M.; Martín-Casas, P. Differences in Motor Imagery Ability between People with Parkinson’s Disease and Healthy Controls, and Its Relationship with Functionality, Independence and Quality of Life. Healthcare 2023, 11, 2898. [Google Scholar] [CrossRef] [PubMed]
- Ubeda Matzilevich, E.; Daniel, P.L.; Little, S. Towards therapeutic electrophysiological neurofeedback in Parkinson’s disease. Park. Relat. Disord. 2024, 121, 106010. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.; Hindle, J.; Lawrence, C.; Bellomo, E.; Pritchard, A.W.; MacLeod, C.A.; Martin-Forbes, P.; Jones, S.; Bracewell, M.; Linden, D.E.J.; et al. Effects of home-based EEG neurofeedback training as a non-pharmacological intervention for Parkinson’s disease. Neurophysiol. Clin. 2024, 54, 102997. [Google Scholar] [CrossRef] [PubMed]
- IDC. Worldwide Quarterly Wearable Device Tracker. Available online: https://www.idc.com/promo/wearablevendor/ (accessed on 25 May 2025).
- Moumdjian, R.A. Bioethics of neurotechnologies: A field in effervescence. Neurol. Res. 2025, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Satam, I.A.; Szabolcsi, R. Ethical and Safety Challenges of Implantable Brain-Computer Interface. Interdiscip. Descr. Complex. Syst. INDECS 2025, 23, 82–94. [Google Scholar] [CrossRef]
- Kritika, E. Ethical Frontiers: Navigating the Intersection of Neurotechnology and Cybersecurity. J. Exp. Neurol. 2025, 6, 21–25. [Google Scholar] [CrossRef]
- Marsili, L.; Bologna, M.; Chen, L.Y.; Espay, A.J. Treatment of Motor Symptoms of Parkinson’s Disease. Neurol. Clin. 2025, 43, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Deutschländer, A.; Heckman, M.G.; Ossi, M.; Vargas, E.R.; Strongosky, A.J.; van Gerpen, J.A.; Uitti, R.J.; Ross, O.A.; Wszolek, Z.K. Comparison of clinical features among Parkinson’s disease subtypes: A large retrospective study in a single center. J. Neurol. Sci. 2018, 386, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Alcalay, R.N.; Caspell-Garcia, C.; Coffey, C.; Chan, P.; Duda, J.E.; Facheris, M.F.; Fernández-Santiago, R.; Ruíz-Martínez, J.; Mestre, T.; et al. Motor and nonmotor heterogeneity of LRRK2-related and idiopathic Parkinson’s disease. Mov. Disord. 2016, 31, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Filidei, M.; Marsili, L.; Colosimo, C. Do Parkinson’s Disease clinical subtypes really exist? Neurol. Neurochir. Pol. 2025, 59, 127–143. [Google Scholar] [CrossRef] [PubMed]



| Type | Method | Temporal Resolution | Spatial Resolution | Long-Term Recording * | Portability * | Cost * | Safety * | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|---|
| Invasive | ECoG (incl. μECoG) | ms | mm | +++ | ++ | ++ | − | Better SNR and resolution than EEG; less invasive than Utah probes. | Requires surgery; limited cortical coverage. |
| SEEG | ms | mm | + | + | ++ | − | Records from deep brain structures; stable over time. | Surgical risks. | |
| Multi-single unit action potentials (e.g., Utah array) | µs–ms | µm | − − | + | +++ | − | High-fidelity neuronal recordings; precise decoding. | Tissue damage; signal degradation over time. | |
| LFPs | ms | mm | +++ | +++ | ++ | + | Captures population-level dynamics; less sensitive to noise. | Lower resolution than a single unit; surgical risks. | |
| Non-invasive | EEG | ms | cm | ++ | +++ | − | +++ | Cheap; portable; widely used. | Low spatial resolution; prone to artifacts. |
| fNIRS | s | cm | ++ | +++ | + | +++ | Portable, safe, and valuable in infants and bedside settings. | Poor temporal resolution; limited to superficial cortex. | |
| fMRI | s | mm | − − | − − | +++ | ++ | Excellent spatial resolution; whole-brain imaging. | Bulky; expensive; slow; not real-time. | |
| MEG | ms | mm–cm | + | − | +++ | +++ | Good spatial and temporal resolution. | An expensive, magnetically shielded room required. | |
| TCD | s | cm | + | ++ | + | +++ | Inexpensive, portable, real-time blood flow measure. | Very low spatial resolution; indirect measure of brain activity. |
| Paradigm | Type of Signal | Mental Workload * | Training Time | Possible applications in PD | Limitations |
|---|---|---|---|---|---|
| P300 | Evoked | Moderate | Short (<1 h) | Spelling systems; attention monitoring. | Reduced performance in cases of visual or cognitive decline. |
| SSVEP | Evoked | Low | Short (<1 h) | Smart home control; assistive mobility. | Requires intact vision and gaze control. |
| Motor Imagery | Spontaneous | High | Long (>5 sessions) | Motor rehabilitation; neurofeedback. | High inter-subject variability; BCI illiteracy in some users. |
| Slow Cortical Potentials | Spontaneous | Moderate | Long (>5 sessions) | Binary communication or control in severe disability. | Low information transfer rate. |
| Hybrid (e.g., MI + SSVEP) | Mixed | High | Variable | High-dimensional control. | Complex configuration; risk of mental fatigue. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Robles, E.; Carino-Escobar, R.I.; Cantillo-Negrete, J.; Arias-Carrión, O. Brain–Computer Interfaces in Parkinson’s Disease Rehabilitation. Biomimetics 2025, 10, 488. https://doi.org/10.3390/biomimetics10080488
Ortega-Robles E, Carino-Escobar RI, Cantillo-Negrete J, Arias-Carrión O. Brain–Computer Interfaces in Parkinson’s Disease Rehabilitation. Biomimetics. 2025; 10(8):488. https://doi.org/10.3390/biomimetics10080488
Chicago/Turabian StyleOrtega-Robles, Emmanuel, Ruben I. Carino-Escobar, Jessica Cantillo-Negrete, and Oscar Arias-Carrión. 2025. "Brain–Computer Interfaces in Parkinson’s Disease Rehabilitation" Biomimetics 10, no. 8: 488. https://doi.org/10.3390/biomimetics10080488
APA StyleOrtega-Robles, E., Carino-Escobar, R. I., Cantillo-Negrete, J., & Arias-Carrión, O. (2025). Brain–Computer Interfaces in Parkinson’s Disease Rehabilitation. Biomimetics, 10(8), 488. https://doi.org/10.3390/biomimetics10080488