Task-Related EEG as a Biomarker for Preclinical Alzheimer’s Disease: An Explainable Deep Learning Approach
Abstract
1. Introduction
- This study introduces an interpretable framework for identifying task-related EEG features indicative of Alzheimer’s disease risk, providing insights into the underlying neural mechanisms.
- Theta and alpha oscillatory features in the parietal and temporal regions are identified as key biomarkers for early AD risk, aligned with established neurodegenerative patterns.
- This study highlights the potential of developing cost-effective assessment tools and biomarkers that are highly sensitive to cognitive decline and neurological dysfunction in clinically healthy populations at the preclinical stage.
2. Materials and Methods
2.1. Data Preparation
2.2. InterpretableCNN
2.3. Implementation Details
3. Results
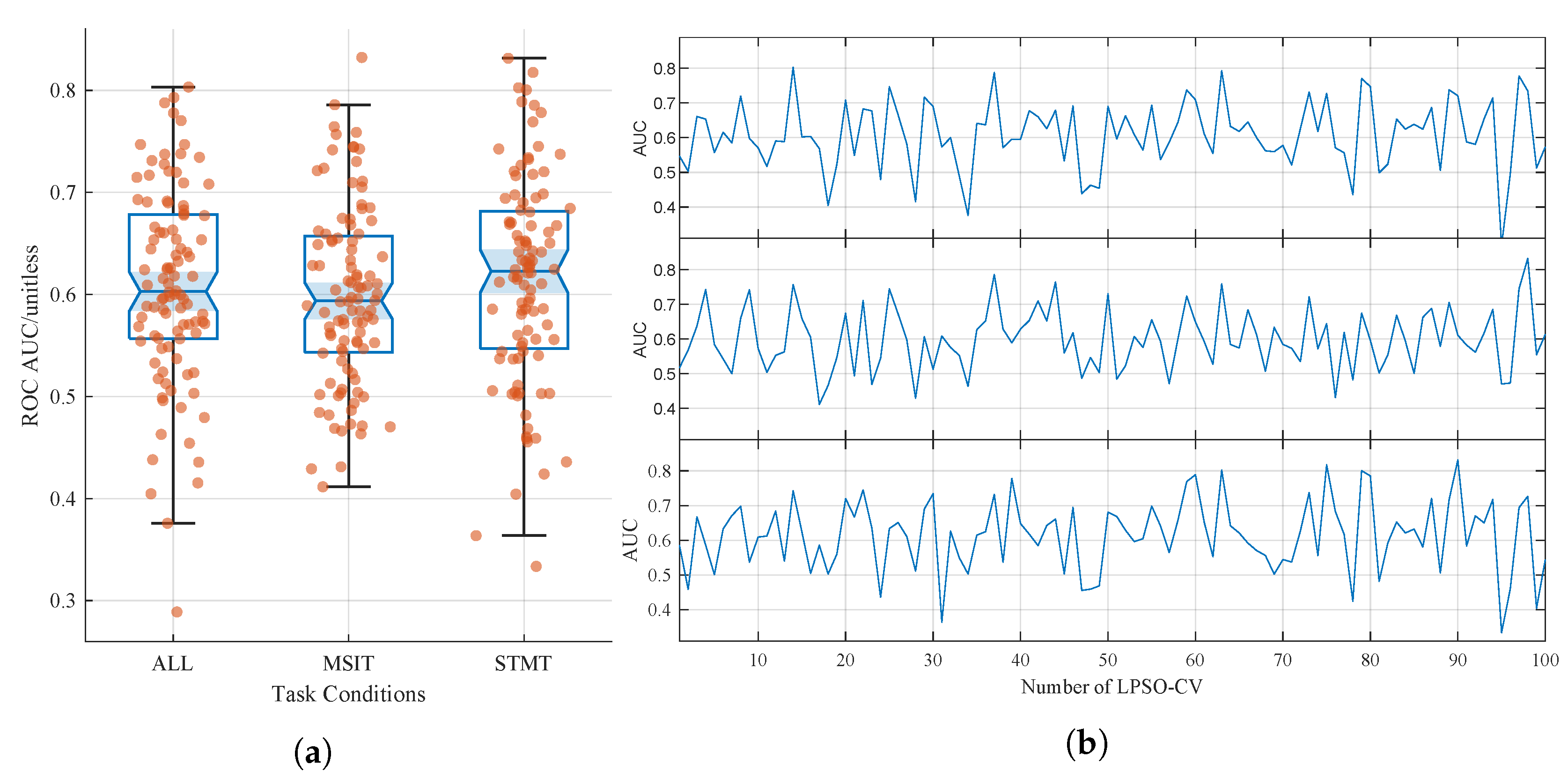
3.1. Classification Performance
3.2. Interpretation of the Learned Characteristics
3.3. Unclear and Unexplained Results
4. Discussion
- Various mechanisms beyond genetic risk.While this study focuses on individuals stratified by APOE and PICALM genotypes, it is important to acknowledge that Alzheimer’s disease (AD) is a multifactorial condition, and genetic predisposition represents only one aspect of its etiology. Various biological mechanisms beyond genetic risk—such as neuroinflammation, metabolic dysfunction, and, notably, cholinergic deficits—may contribute to early AD-related brain changes, even in individuals without known genetic risk alleles. For example, individuals approaching the age of 60 may begin to experience early cholinergic dysfunction—an aspect of AD pathology [53]. Consequently, some misclassified subjects in the “N” (non-carrier) group may have exhibited preclinical AD driven by cholinergic deficits rather than genetic risk factors.
- Moderate classification performance. Although the model outperforms previous handcrafted feature methods [22], its ROC AUC remains around 60–61%, indicating only fair agreement. This reflects the challenge of identifying subtle EEG alterations in cognitively normal individuals. Future work should increase the sample size and diversity to enhance signal robustness and improve model generalizability.
- Lack of external validation. The model’s generalization was only tested on the A+P+ group, which was not used in training, and no independent external dataset was used. This limits the broader applicability of the results. Future studies should validate findings using external cohorts to assess reproducibility across sites and populations.
- Limited interpretability. Although post hoc visualization provided insights into the model’s decisions, the interpretations remain qualitative and lack biological confirmation. Future research should integrate EEG with multimodal imaging (e.g., MRI and PET) and explore explainability-guided training strategies to enhance the neuroscientific interpretability of learned features. While the interpretability analysis provides some visual explanations, there is still a lack of biological validation, making it unclear whether these features are truly associated with the pathological mechanisms of AD.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| EEG | Electroencephalography |
| MSIT | Multi-Source Interference Task |
| STMT | Sternberg Memory Task |
| ROC AUC | Receiver Operating Characteristic Area Under the Curve |
| APOE | Apolipoprotein E |
| PICALM | Phosphatidylinositol Binding Clathrin Assembly Protein |
| LPSO-CV | Leave-p%-Subjects-Out Cross-Validation |
| InterpretableCNN | Interpretable Deep Learning Framework |
| MCI | Mild Cognitive Impairment |
| CAM | Class Activation Map |
References
- Rayathala, J.; Kumar, K. Review on Alzheimer’s disease: Past, present and future. J. Innov. Appl. Pharm. Sci. (JIAPS) 2022, 7, 28–31. [Google Scholar] [CrossRef]
- Hampel, H.; Lista, S.; Khachaturian, Z.S. Development of biomarkers to chart all Alzheimer’s disease stages: The royal road to cutting the therapeutic Gordian Knot. Alzheimer’s Dement. 2012, 8, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Modir, A.; Shamekhi, S.; Ghaderyan, P. A systematic review and methodological analysis of EEG-based biomarkers of Alzheimer’s disease. Measurement 2023, 220, 113274. [Google Scholar] [CrossRef]
- Bradfield, N.I. Mild cognitive impairment: Diagnosis and subtypes. Clin. EEG Neurosci. 2023, 54, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Tang, E.; Taylor, J.P. Dementia: Timely diagnosis and early intervention. BMJ 2015, 350, h3029. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef]
- Portet, F.; Ousset, P.J.; Visser, P.J.; Frisoni, G.B.; Nobili, F.; Scheltens, P.; Vellas, B.; Touchon, J.; MCI Working Group of the European Consortium on Alzheimer’s Disease (EADC). Mild cognitive impairment (MCI) in medical practice: A critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 714–718. [Google Scholar] [CrossRef]
- Göschel, L.; Dell’Orco, A.; Fillmer, A.; Aydin, S.; Ittermann, B.; Riemann, L.; Lehmann, S.; Cano, S.; Melin, J.; Pendrill, L.; et al. Plasma p-tau181 and GFAP reflect 7T MR-derived changes in Alzheimer’s disease: A longitudinal study of structural and functional MRI and MRS. Alzheimer’s Dement. 2024, 20, 8684–8699. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; Van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; Van Der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Sethi, P.; Bhaskar, R.; Singh, K.K.; Gupta, S.; Han, S.S.; Avinash, D.; Abomughaid, M.M.; Koul, A.; Rani, B.; Ghosh, S.; et al. Exploring advancements in early detection of Alzheimer’s disease with molecular assays and animal models. Ageing Res. Rev. 2024, 100, 102411. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Mapstone, M.E.; Cheema, A.K.; Federoff, H.J. The critical need for defining preclinical biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, S196–S212. [Google Scholar] [CrossRef]
- Raskin, J.; Cummings, J.; Hardy, J.; Schuh, K.; A Dean, R. Neurobiology of Alzheimer’s disease: Integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr. Alzheimer Res. 2015, 12, 712–722. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Hu, F. Are you afraid of heights and suitable for working at height? Biomed. Signal Process. Control 2019, 52, 23–31. [Google Scholar] [CrossRef]
- Fu, R.; Wang, H.; Zhao, W. Dynamic driver fatigue detection using hidden Markov model in real driving condition. Expert Syst. Appl. 2016, 63, 397–411. [Google Scholar] [CrossRef]
- Ehteshamzad, S. Assessing the Potential of EEG in Early Detection of Alzheimer’s Disease: A Systematic Comprehensive Review (2000–2023). J. Alzheimer’s Dis. Rep. 2024, 8, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R.; Barbey, F.M.; Cominetti, M.R.; Gillan, C.M.; Rosická, A.M. Developments in scalable strategies for detecting early markers of cognitive decline. Transl. Psychiatry 2022, 12, 473. [Google Scholar] [CrossRef]
- Simmatis, L.E.; Russo, E.E.; Steininger, T.; Riddell, H.; Chen, E.; Chiu, Q.; Lin, M.; Oh, D.; Taheri, P.; Harmsen, I.E.; et al. EEG biomarkers for Alzheimer’s disease: A novel automated pipeline for detecting and monitoring disease progression. J. Alzheimer’s Dis. 2025. [Google Scholar] [CrossRef]
- Moretti, D.V. Theta and alpha EEG frequency interplay in subjects with mild cognitive impairment: Evidence from EEG, MRI, and SPECT brain modifications. Front. Aging Neurosci. 2015, 7, 31. [Google Scholar] [CrossRef]
- Ishii, R.; Canuet, L.; Aoki, Y.; Hata, M.; Iwase, M.; Ikeda, S.; Nishida, K.; Ikeda, M. Healthy and pathological brain aging: From the perspective of oscillations, functional connectivity, and signal complexity. Neuropsychobiology 2018, 75, 151–161. [Google Scholar] [CrossRef]
- Horváth, A.A.; Szucs, A.; Csukly, G.; Sakovics, A.; Stefanics, G.; Kamondi, A. EEG and ERP biomarkers of Alzheimer’s disease: A critical review. Front. Biosci.-Landmark 2018, 23, 183–220. [Google Scholar] [CrossRef]
- Dzianok, P. Imaging Genetics: APOE/PICALM Genes Are Associated with Mild Alterations in Brain Function in Healthy, Middle-Aged Individuals. Ph.D. Thesis, Instytut Biologii Doświadczalnej im, Marcelego Nenckiego PAN, Warsaw, Poland, 2023. [Google Scholar]
- Li, Z.; Wang, H.; Song, J.; Gong, J. Exploring Task-Related EEG for Cross-Subject Early Alzheimer’s Disease Susceptibility Prediction in Middle-Aged Adults Using Multitaper Spectral Analysis. Sensors 2024, 25, 52. [Google Scholar] [CrossRef]
- Dzianok, P.; Kublik, E. Alzheimer’s disease risk alleles of PICALM gene may accelerate cognitive (memory and attention) performance in middle-aged healthy APOE-ε4 carriers. Alzheimer’s Dement. 2023, 19, e067545. [Google Scholar] [CrossRef]
- Gaubert, S.; Houot, M.; Raimondo, F.; Ansart, M.; Corsi, M.C.; Naccache, L.; Sitt, J.D.; Habert, M.O.; Dubois, B.; Fallani, F.D.V.; et al. A machine learning approach to screen for preclinical Alzheimer’s disease. Neurobiol. Aging 2021, 105, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, C.; Lu, J.; Sun, J.; Sun, X.; Yang, J.; Wang, L.; Zuo, C.; Shi, K. Using interpretable deep learning radiomics model to diagnose and predict progression of early AD disease spectrum: A preliminary [18F] FDG PET study. Eur. Radiol. 2025, 35, 2620–2633. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Ito, J.; Kadowaki, T.; Tsuda, K. An interpretable machine learning model for diagnosis of Alzheimer’s disease. PeerJ 2019, 7, e6543. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, B.; Huang, J.; Wang, H.; Xu, R.; Shao, W.; Zhang, D. Deep multi-modal discriminative and interpretability network for alzheimer’s disease diagnosis. IEEE Trans. Med. Imaging 2022, 42, 1472–1483. [Google Scholar] [CrossRef]
- Štrumbelj, E.; Kononenko, I. Explaining prediction models and individual predictions with feature contributions. Knowl. Inf. Syst. 2014, 41, 647–665. [Google Scholar] [CrossRef]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. “Why should i trust you?” Explaining the predictions of any classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 1135–1144. [Google Scholar]
- Jones, C.; Thornton, J.; Wyatt, J.C. Enhancing trust in clinical decision support systems: A framework for developers. BMJ Health Care Inform. 2021, 28, e100247. [Google Scholar] [CrossRef]
- Chan, Y.H.; Girish, D.; Gupta, S.; Xia, J.; Kasi, C.; He, Y.; Wang, C.; Rajapakse, J.C. Discovering robust biomarkers of psychiatric disorders from resting-state functional MRI via graph neural networks: A systematic review. arXiv 2024, arXiv:2405.00577. [Google Scholar]
- Borra, D.; Bossi, F.; Rivolta, D.; Magosso, E. Deep learning applied to EEG source-data reveals both ventral and dorsal visual stream involvement in holistic processing of social stimuli. Sci. Rep. 2023, 13, 7365. [Google Scholar] [CrossRef]
- Rajpura, P.; Cecotti, H.; Meena, Y.K. Explainable artificial intelligence approaches for brain-computer interfaces: A review and design space. J. Neural Eng. 2024, 21, 041003. [Google Scholar] [CrossRef]
- Khosravi, M.; Parsaei, H.; Rezaee, K.; Helfroush, M.S. Fusing convolutional learning and attention-based Bi-LSTM networks for early Alzheimer’s diagnosis from EEG signals towards IoMT. Sci. Rep. 2024, 14, 26002. [Google Scholar] [CrossRef]
- Cui, J.; Lan, Z.; Sourina, O.; Müller-Wittig, W. EEG-based cross-subject driver drowsiness recognition with an interpretable convolutional neural network. IEEE Trans. Neural Netw. Learn. Syst. 2022, 34, 7921–7933. [Google Scholar] [CrossRef]
- Jiang, P.T.; Zhang, C.B.; Hou, Q.; Cheng, M.M.; Wei, Y. Layercam: Exploring hierarchical class activation maps for localization. IEEE Trans. Image Process. 2021, 30, 5875–5888. [Google Scholar] [CrossRef]
- Jung, H.; Oh, Y. Towards better explanations of class activation mapping. In Proceedings of the IEEE/CVF International Conference on Computer Vision, Montreal, QC, Canada, 10–17 October 2021; pp. 1336–1344. [Google Scholar]
- Patrycja, D.; Ewa, K. A Polish Electroencephalography, Alzheimer’s Risk-Genes, Lifestyle and Neuroimaging (PRERL-Neuro) Database. Available online: https://openneuro.org/datasets/ds004796/versions/1.0.9 (accessed on 6 August 2004).
- Howard, A.G. Mobilenets: Efficient convolutional neural networks for mobile vision applications. arXiv 2017, arXiv:1704.04861. [Google Scholar]
- Seigel, D.G.; Podgo, M.J.; Remaley, N.A. Acceptable values of kappa for comparison of two groups. Am. J. Epidemiol. 1992, 135, 571–578. [Google Scholar] [CrossRef]
- Davatzikos, C. Machine learning in neuroimaging: Progress and challenges. Neuroimage 2019, 197, 652–656. [Google Scholar] [CrossRef]
- Snoek, L.; Miletić, S.; Scholte, H.S. How to control for confounds in decoding analyses of neuroimaging data. Neuroimage 2019, 184, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Mohammadi, M.R.; Shahi, K.; Nasrabadi, A.M. Computational neuroscience approach to psychiatry: A review on theory-driven approaches. Clin. Psychopharmacol. Neurosci. 2022, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Arbabshirani, M.R.; Plis, S.; Sui, J.; Calhoun, V.D. Single subject prediction of brain disorders in neuroimaging: Promises and pitfalls. Neuroimage 2017, 145, 137–165. [Google Scholar] [CrossRef] [PubMed]
- Oakden-Rayner, L.; Dunnmon, J.; Carneiro, G.; Ré, C. Hidden stratification causes clinically meaningful failures in machine learning for medical imaging. In Proceedings of the ACM Conference on Health, Inference, and Learning, Toronto, ON, Canada, 2–4 April 2020; pp. 151–159. [Google Scholar]
- Kavcic, V.; Daugherty, A.M.; Giordani, B. Post-task modulation of resting state EEG differentiates MCI patients from controls. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12153. [Google Scholar] [CrossRef]
- Pulver, R.L.; Kronberg, E.; Medenblik, L.M.; Kheyfets, V.O.; Ramos, A.R.; Holtzman, D.M.; Morris, J.C.; Toedebusch, C.D.; Sillau, S.H.; Bettcher, B.M.; et al. Mapping sleep’s oscillatory events as a biomarker of Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 301–315. [Google Scholar] [CrossRef]
- Gouw, A.A.; Hillebrand, A.; Schoonhoven, D.N.; Demuru, M.; Ris, P.; Scheltens, P.; Stam, C.J. Routine magnetoencephalography in memory clinic patients: A machine learning approach. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12227. [Google Scholar] [CrossRef]
- Varghese, T.; Sheelakumari, R.; James, J.S.; Mathuranath, P.S. A review of neuroimaging biomarkers of Alzheimer’s disease. Neurol. Asia 2013, 18, 239. [Google Scholar]
- Deng, J.; Sun, B.; Kavcic, V.; Liu, M.; Giordani, B.; Li, T. Novel methodology for detection and prediction of mild cognitive impairment using resting-state EEG. Alzheimer’s Dement. 2024, 20, 145–158. [Google Scholar] [CrossRef]
- Jack, C.R.; Holtzman, D.M. Biomarker modeling of Alzheimer’s disease. Neuron 2013, 80, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Aztiria, A.; Basarab, A. On the early diagnosis of Alzheimer’s Disease from multimodal signals: A survey. Artif. Intell. Med. 2016, 71, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Terry, A., Jr.; Buccafusco, J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]




| Groups | Subjects | Count | Age | Female/% | MSIT | STMT | All |
|---|---|---|---|---|---|---|---|
| N | 1 2 6 7 8 10 13 14 15 16 17 18 19 22 23 24 25 26 28 29 31 | 21 | 55.29 ± 2.94 | 49.76 | 3176 | 2442 | 5618 |
| A+P− | 47 53 57 58 59 60 62 63 65 67 70 73 74 75 77 78 79 80 | 18 | 55.17 ± 3.03 | 51.45 | 2720 | 2151 | 4871 |
| A+P+ | 32 34 35 37 39 40 41 42 44 48 52 54 | 12 | 56.67 ± 3.70 | 66.67 | 1738 | 1364 | 3102 |
| Layer | Kernel | Filter | Group | Output Shape |
|---|---|---|---|---|
| Input | (N, 1, 60, 320) | |||
| Conv2D | (60, 1) | 16 | 1 | (N, 16, 1, 320) |
| Conv2D | (1, 64) | 32 | 2 | (N, 32, 1, 257) |
| ReLU | (N, 32, 1, 257) | |||
| BatchNorm2d | (N, 32, 1, 257) | |||
| AvgPool2d | (1, 257) | (N, 32) | ||
| Dense | (N, 2) | |||
| Softmaxt | (N, 2) |
| ALL | MSIT | STMT | ||
|---|---|---|---|---|
| Validation | ROC AUC/% | 60.84 ± 9.65 | 59.90 ± 8.79 | 61.44 ± 10.19 |
| Kappa/ | 21.68 ± 19.29 | 19.80 ± 17.59 | 22.88 ± 20.37 | |
| Sensitivity/% | 49.34 ± 13.63 | 53.61 ± 14.40 | 48.96 ± 16.72 | |
| Test | Sensitivity/% | 47.86 ± 8.75 | 51.05 ± 11.17 | 45.98 ± 13.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, H.; Li, L. Task-Related EEG as a Biomarker for Preclinical Alzheimer’s Disease: An Explainable Deep Learning Approach. Biomimetics 2025, 10, 468. https://doi.org/10.3390/biomimetics10070468
Li Z, Wang H, Li L. Task-Related EEG as a Biomarker for Preclinical Alzheimer’s Disease: An Explainable Deep Learning Approach. Biomimetics. 2025; 10(7):468. https://doi.org/10.3390/biomimetics10070468
Chicago/Turabian StyleLi, Ziyang, Hong Wang, and Lei Li. 2025. "Task-Related EEG as a Biomarker for Preclinical Alzheimer’s Disease: An Explainable Deep Learning Approach" Biomimetics 10, no. 7: 468. https://doi.org/10.3390/biomimetics10070468
APA StyleLi, Z., Wang, H., & Li, L. (2025). Task-Related EEG as a Biomarker for Preclinical Alzheimer’s Disease: An Explainable Deep Learning Approach. Biomimetics, 10(7), 468. https://doi.org/10.3390/biomimetics10070468








