The Unilateral Jumping Structures of the Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae): A Highly Functional and Integrated Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anatomy
2.3. High-Speed Recordings of Jumping Actions
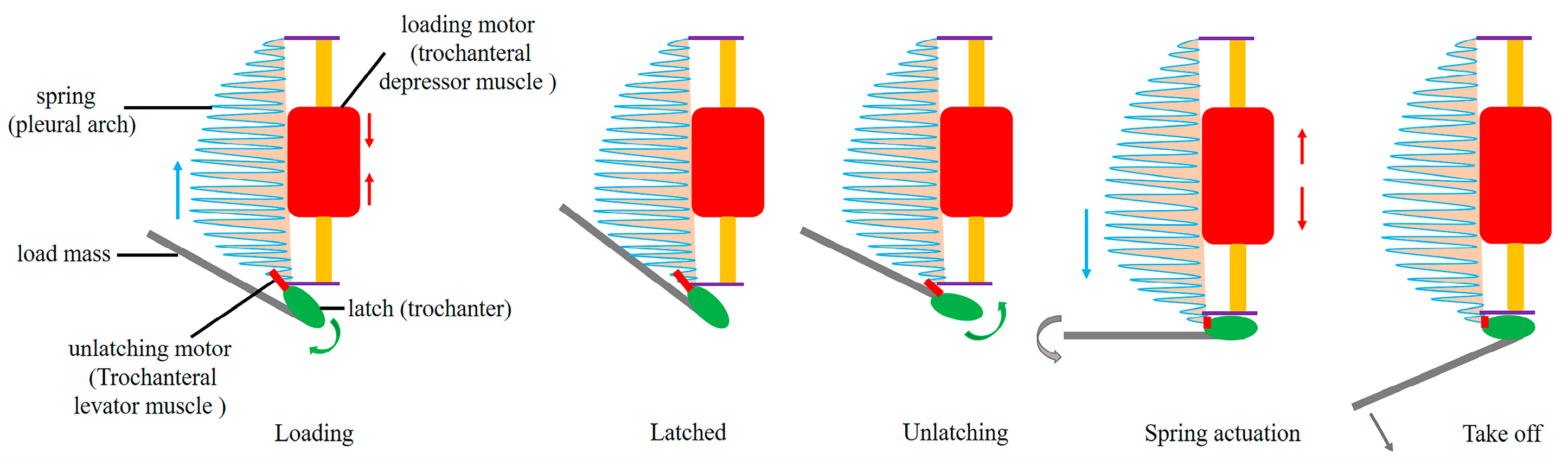
3. Results
3.1. Morphology
3.1.1. Jumping Components
3.1.2. Energy Store Component
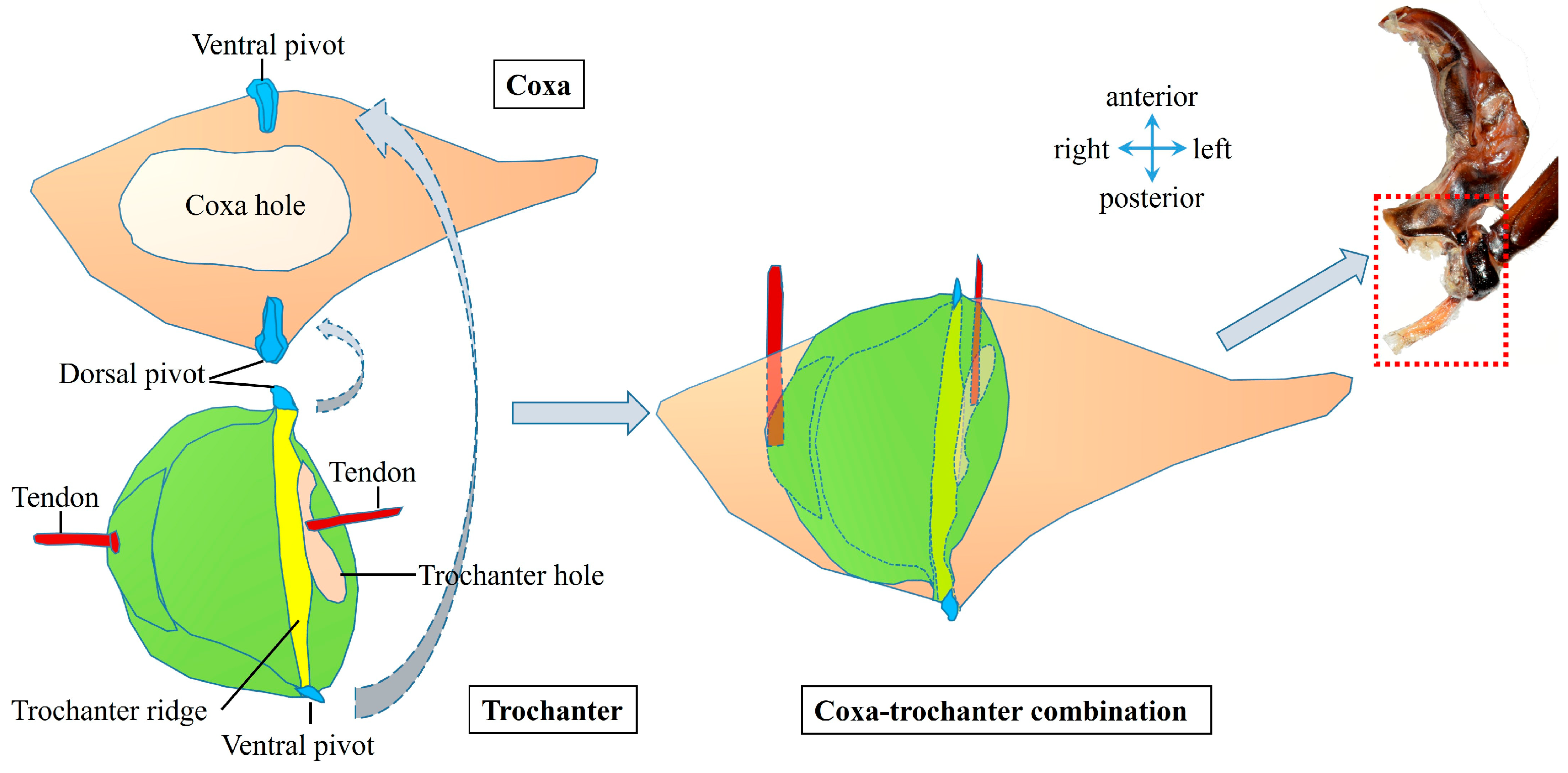
3.1.3. Coupling Component
3.1.4. Lever Component
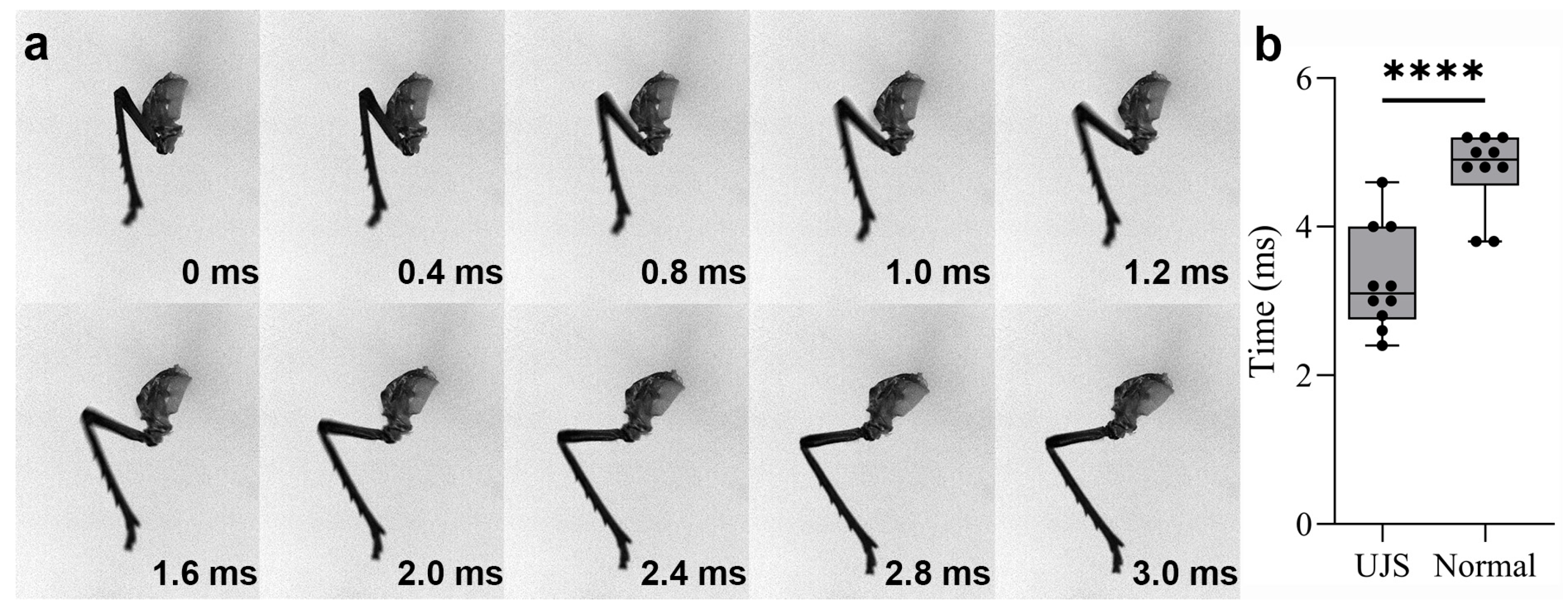
3.2. Kinetics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Glossary of Technical Terms in Jumping Structures of L. delicatula
| Jargon | Definition |
| Unilateral jumping structures (UJS) | A functional structural unit of L. delicatula that can relatively independently perform jumping actions. It consists of the pleural arch, coxa, and hind leg and can be divided into an energy storage component, a coupling component, and a lever component. |
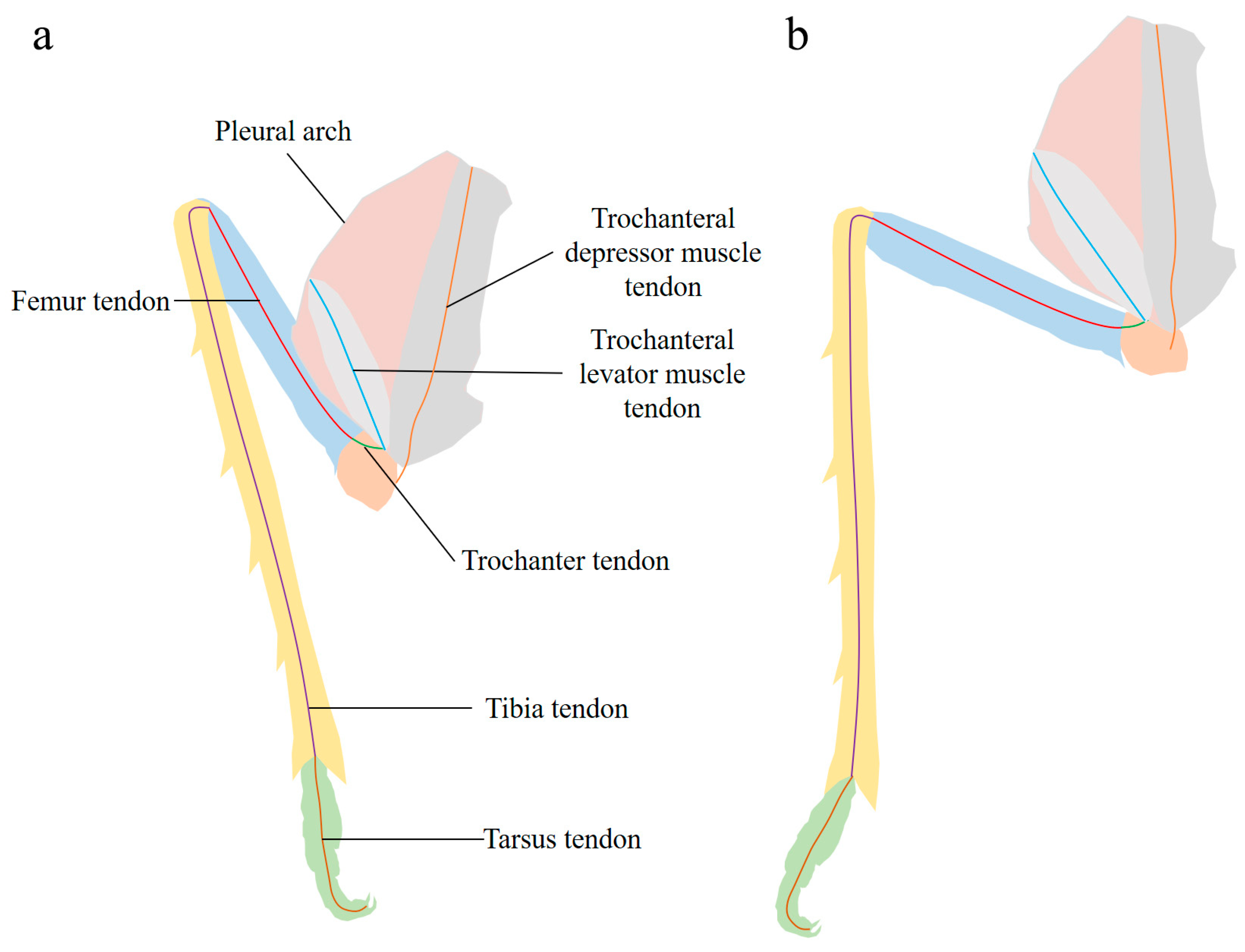
| Pleural arch | A part of the internal skeleton of the thorax of L. delicatula, which is bow-shaped, located on both sides of the metathorax, and fused with the coxa. It deforms while jumping to store and release energy. |
| Trochanteral depressor muscles | The muscles mainly consist of peripheral muscle fibers and a core part. They contract to bend the pleural arch for energy storage, being crucial muscles for the storage and release of energy in jumping. |
| Coxa | One segment at the base of the hind leg, which is closely connected to the pleural arch. It is part of the coupling component and plays a role in connecting and transmitting forces in the jumping mechanism. |
| Trochanter | Located between the coxa and the femur, it interacts with the coxa through protuberances and pivots. Its structure and movement affect the realization of jumping actions. |
| Femur | A part of the hind leg and an important component of the lever component. During the preparation and execution of jumping, it collaborates with other leg structures to help the insect complete the jumping movement. |
| Tibia | There are four spines on the hind leg. The ventral side of its distal margin has sclerotized spines. It participates in leg movement through extension and contraction during jumping, playing an important role in the propulsion and stability of jumping. |
| Tarsus | It consists of two tarsal segments and a pretarsus. The pretarsus includes a pair of claws and an arolium. It is used to stabilize the body and prevent slipping during jumping. |
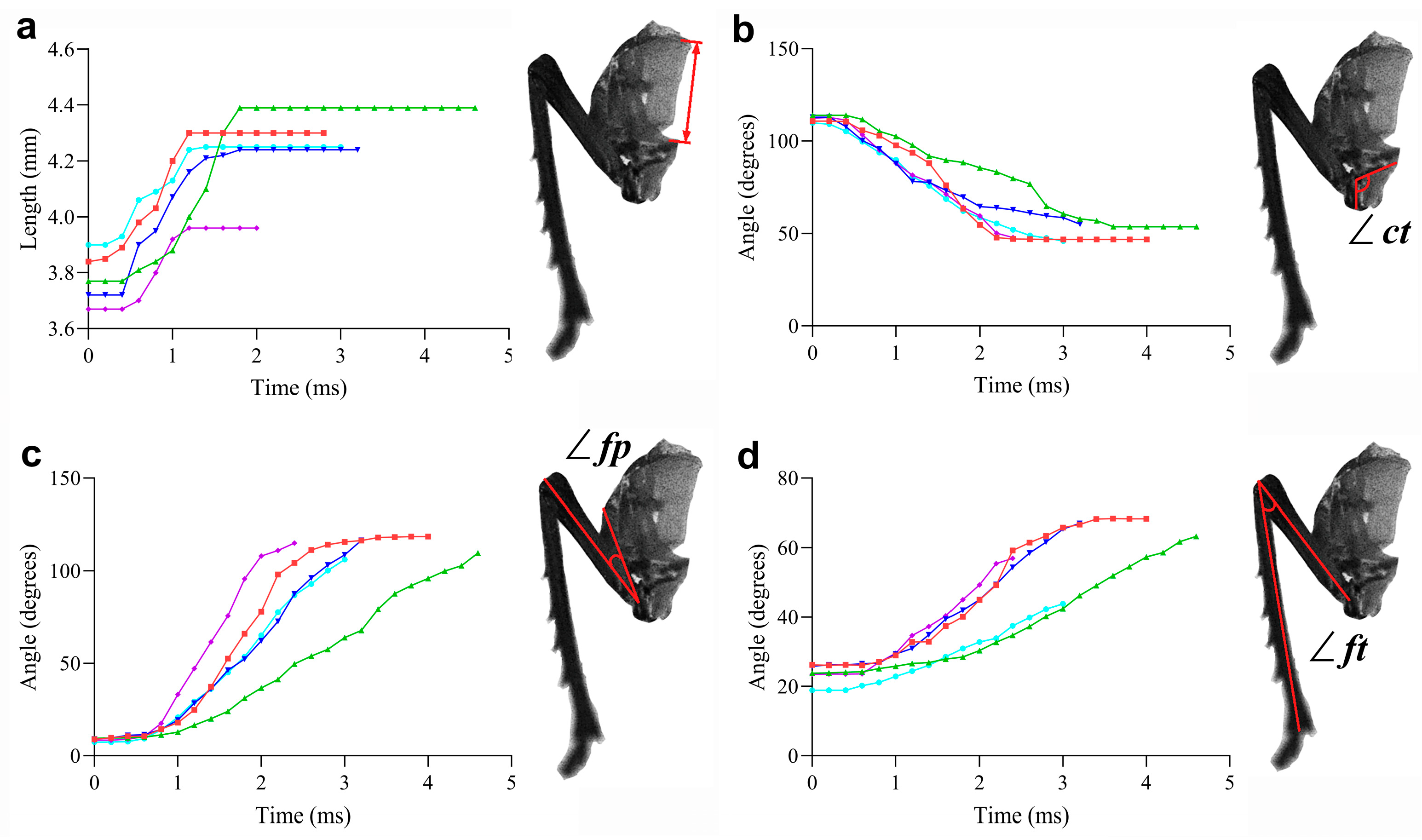
| V-notch, U-notch | Structures on the pleural arch. The deformation zone extends from the V-notch to the U-notch, accounting for approximately two-thirds of the pleural arch and playing an important role in the deformation process of the pleural arch. |
| ∠ct | The angle between the coxa and the trochanter. It changes significantly during the preparation and execution of jumping, reflecting the movement state of the leg joint and the mechanical changes of the jumping mechanism. |
| ∠fp | The angle between the femur and the pleural arch. The angle changes obviously before and after jumping, which is an important indicator to measure the relative movement between the leg and the energy- storing structure during the jumping process. |
| ∠ft | The angle between the femur and the tibia. The angle increases during jumping, and its change has an important impact on the leg extension and the generation of jumping propulsion force. |
References
- Hawkes, E.W.; Xiao, C.; Peloquin, R.-A.; Keeley, C.; Begley, M.R.; Pope, M.T.; Niemeyer, G. Engineered Jumpers Overcome Biological Limits via Work Multiplication. Nature 2022, 604, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M. Jumping Performance of Planthoppers (Hemiptera, Issidae). J. Exp. Biol. 2009, 212, 2844–2855. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Ge, W.; Miraglia, M.; Inglese, F.; Zhao, D.; Stefanini, C.; Romano, D. Jumping Locomotion Strategies: From Animals to Bioinspired Robots. Appl. Sci. 2020, 10, 8607. [Google Scholar] [CrossRef]
- Chen, M.W.; Zhang, Y.L.; Sun, M. Wing and Body Motion and Aerodynamic and Leg Forces during Take-off in Droneflies. J. R. Soc. Interface 2013, 10, 20130808. [Google Scholar] [CrossRef]
- Burrows, M.; Shaw, S.R.; Sutton, G.P. Resilin and Chitinous Cuticle Form a Composite Structure for Energy Storage in Jumping by Froghopper Insects. BMC Biol. 2008, 6, 41. [Google Scholar] [CrossRef]
- Gorb, S.N. The Jumping Mechanism of Cicada Cercopis Vulnerata (Auchenorrhyncha, Cercopidae): Skeleton–Muscle Organisation, Frictional Surfaces, and Inverse-Kinematic Model of Leg Movements. Arthropod Struct. Dev. 2004, 33, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M. Energy Storage and Synchronisation of Hind Leg Movements during Jumping in Planthopper Insects (Hemiptera, Issidae). J. Exp. Biol. 2010, 213, 469–478. [Google Scholar] [CrossRef]
- Burrows, M. Froghopper Insects Leap to New Heights. Nature 2003, 424, 509. [Google Scholar] [CrossRef]
- Burrows, M. Jumping Performance of Froghopper Insects. J. Exp. Biol. 2006, 209, 4607–4621. [Google Scholar] [CrossRef]
- Burrows, M. Neural Control and Coordination of Jumping in Froghopper Insects. J. Neurophysiol. 2007, 97, 320–330. [Google Scholar] [CrossRef]
- Clemente, C.J.; Goetzke, H.H.; Bullock, J.M.R.; Sutton, G.P.; Burrows, M.; Federle, W. Jumping without Slipping: Leafhoppers (Hemiptera: Cicadellidae) Possess Special Tarsal Structures for Jumping from Smooth Surfaces. J. R. Soc. Interface 2017, 14, 20170022. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M. Jumping Mechanisms in Gum Treehopper Insects (Hemiptera, Eurymelinae). J. Exp. Biol. 2013, 216, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M.; Bräunig, P. Actions of Motor Neurons and Leg Muscles in Jumping by Planthopper Insects (Hemiptera, Issidae). J. Comp. Neurol. 2010, 518, 1349–1369. [Google Scholar] [CrossRef]
- Heilig, S.; Sander, K. Zahnradsektoren Zur Koordination Der Sprungbeine—Eine Larvale Synapomorphie Der Fulgoromorphen Zikaden (Homoptera, Cicadina, Fulgoroidea). Zool. Jahrbucher Abt. Syst. Okologie Geogr. Tiere 1986, 113, 307–317. [Google Scholar]
- Sander, K. Bau Und Funktion Des Sprungapparates von Pyrilla Perpusilla Walker (Homoptera–Fulgoridae). Zool. Jb. Jena Anat. 1957, 75, 383–388. [Google Scholar]
- Siwanowicz, I.; Burrows, M. Three Dimensional Reconstruction of Energy Stores for Jumping in Planthoppers and Froghoppers from Confocal Laser Scanning Microscopy. eLife 2017, 6, e23824. [Google Scholar] [CrossRef]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.S.; Blua, M.J.; Mizell Iii, R.F.; Andersen, P.C. The Biology of Xylem Fluid-Feeding Insect Vectors of Xylella Fastidiosa and Their Relation to Disease Epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef]
- Pompon, J.; Quiring, D.; Goyer, C.; Giordanengo, P.; Pelletier, Y. A Phloem-Sap Feeder Mixes Phloem and Xylem Sap to Regulate Osmotic Potential. J. Insect Physiol. 2011, 57, 1317–1322. [Google Scholar] [CrossRef]
- Novotny, V.; Wilson, M.R. Why Are There No Small Species among Xylem-Sucking Insects? Evol. Ecol. 1997, 11, 419–437. [Google Scholar] [CrossRef]
- Burrows, M. Jumping Mechanisms of Treehopper Insects (Hemiptera, Auchenorrhyncha, Membracidae). J. Exp. Biol. 2013, 216, 788–799. [Google Scholar] [CrossRef]
- Bräunig, P.; Burrows, M. Neurons Controlling Jumping in Froghopper Insects. J. Comp. Neurol. 2008, 507, 1065–1075. [Google Scholar] [CrossRef]
- Burrows, M. Kinematics of Jumping in Leafhopper Insects (Hemiptera, Auchenorrhyncha, Cicadellidae). J. Exp. Biol. 2007, 210, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.P.; St Pierre, R.; Kuo, C.-Y.; Summers, A.P.; Bergbreiter, S.; Cox, S.; Patek, S.N. Dual Spring Force Couples Yield Multifunctionality and Ultrafast, Precision Rotation in Tiny Biomechanical Systems. J. Exp. Biol. 2022, 225, jeb244077. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.E.; Ray, W.J.; Santhanakrishnan, A.; Patek, S.N. Mantis Shrimp Locomotion: Coordination and Variation of Hybrid Metachronal Swimming. Integr. Org. Biol. 2023, 5, obad019. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, A.J.; Beery, E.; Hsieh, S.T.; Kane, S.A. Putting a New Spin on Insect Jumping Performance Using 3D Modeling and Computer Simulations of Spotted Lanternfly Nymphs. J. Exp. Biol. 2023, 226, jeb246340. [Google Scholar] [CrossRef]
- Burrows, M. Morphology and Action of the Hind Leg Joints Controlling Jumping in Froghopper Insects. J. Exp. Biol. 2006, 209, 4622–4637. [Google Scholar] [CrossRef]
- Kane, S.A.; Bien, T.; Contreras-Orendain, L.; Ochs, M.F.; Tonia Hsieh, S. Many Ways to Land Upright: Novel Righting Strategies Allow Spotted Lanternfly Nymphs to Land on Diverse Substrates. J. R. Soc. Interface 2021, 18, 20210367. [Google Scholar] [CrossRef]
- Kang, C.; Moon, H.; Sherratt, T.N.; Lee, S.-I.; Jablonski, P.G. Multiple Lines of Anti-Predator Defence in the Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae). Biol. J. Linn. Soc. 2017, 120, 115–124. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, Y.; Wang, X.; Cao, L.; Hoelmer, K.A.; Broadley, H.J.; Gould, J.R. Exploratory Survey of Spotted Lanternfly (Hemiptera: Fulgoridae) and Its Natural Enemies in China. Environ. Entomol. 2021, 50, 36–45. [Google Scholar] [CrossRef]
- Urban, J.M.; Leach, H. Biology and Management of the Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae), in the United States. Annu. Rev. Entomol. 2023, 68, 151–167. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, Y.-L.; Leskey, T.C. A Review of Biology and Management of Lycorma delicatula (Hemiptera: Fulgoridae), an Emerging Global Invasive Species. J. Asia-Pac. Entomol. 2019, 22, 589–596. [Google Scholar] [CrossRef]
- Kang, C.-K.; Lee, S.-I.; Jablonski, P.G. Effect of Sex and Bright Coloration on Survival and Predator-Induced Wing Damage in an Aposematic Lantern Fly with Startle Display. Ecol. Entomol. 2011, 36, 709–716. [Google Scholar] [CrossRef]
- Xue, G.; Yuan, S. Separation and Preparation of Indole Alkaloids in Lycorma delicatula White. by HPLC. China J. Chin. Mater. Medica 1996, 21, 554–555. [Google Scholar]
- Burrows, M.; Ghosh, A.; Sutton, G.P.; Yeshwanth, H.M.; Rogers, S.M.; Sane, S.P. Jumping in Lantern Bugs (Hemiptera, Fulgoridae). J. Exp. Biol. 2021, 224, jeb243361. [Google Scholar] [CrossRef]
- Sutton, G.P.; Mendoza, E.; Azizi, E.; Longo, S.J.; Olberding, J.P.; Ilton, M.; Patek, S.N. Why Do Large Animals Never Actuate Their Jumps with Latch-Mediated Springs? Because They Can Jump Higher Without Them. Integr. Comp. Biol. 2019, 59, 1609–1618. [Google Scholar] [CrossRef]
- Pang, A.; Nicol, A.M.; Rutter, A.; Zeeb, B. Improved Methods for Quantifying Soil Invertebrates During Ecotoxicological Tests: Chill Comas and Anesthetics. Heliyon 2023, 9, e12850. [Google Scholar] [CrossRef]
- Findsen, A.; Pedersen, T.H.; Petersen, A.G.; Nielsen, O.B.; Overgaard, J. Why Do Insects Enter and Recover from Chill Coma? Low Temperature and High Extracellular Potassium Compromise Muscle Function in Locusta migratoria. J. Exp. Biol. 2014, 217, 1297–1306. [Google Scholar] [CrossRef]
- Bennet-Clark, H.C. The Energetics of the Jump of the Locust Schistocerca Gregaria. J. Exp. Biol. 1975, 63, 53–83. [Google Scholar] [CrossRef]
- Patek, S.N. The Power of Mantis Shrimp Strikes: Interdisciplinary Impacts of an Extreme Cascade of Energy Release. Integr. Comp. Biol. 2019, 59, 1573–1585. [Google Scholar] [CrossRef]
- Card, G.M. Escape Behaviors in Insects. Curr. Opin. Neurobiol. 2012, 22, 180–186. [Google Scholar] [CrossRef]
- Ilton, M.; Bhamla, M.S.; Ma, X.; Cox, S.M.; Fitchett, L.L.; Kim, Y.; Koh, J.; Krishnamurthy, D.; Kuo, C.-Y.; Temel, F.Z.; et al. The Principles of Cascading Power Limits in Small, Fast Biological and Engineered Systems. Science 2018, 360, eaao1082. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Pandhigunta, K.; Acevedo, M.A.; Walker, A.; Didcock, R.L.; Castro, J.T.; O’Neill, D.; Acharya, R.; Bhamla, M.S.; Anderson, P.S.L.; et al. A Tunable, Simplified Model for Biological Latch Mediated Spring Actuated Systems. Integr. Org. Biol. 2022, 4, obac032. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M. Anatomy of the Hind Legs and Actions of Their Muscles during Jumping in Leafhopper Insects. J. Exp. Biol. 2007, 210, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Földvári, M.; Mikó, I.; Ulmer, J.M.; Dos Santos Rolo, T.; Csősz, S.; Pomiankowski, A.; Baumbach, T.; Van De Kamp, T. Jumping and Grasping: Universal Locking Mechanisms in Insect Legs. Insect Syst. Divers. 2019, 3, 3. [Google Scholar] [CrossRef]
- Goetzke, H.H.; Pattrick, J.G.; Federle, W. Froghoppers Jump from Smooth Plant Surfaces by Piercing Them with Sharp Spines. Proc. Natl. Acad. Sci. USA 2019, 116, 3012–3017. [Google Scholar] [CrossRef]
- Dickinson, M.H. Bionics: Biological insight into mechanical design. Proc. Natl. Acad. Sci. USA 1999, 96, 14208–14209. [Google Scholar] [CrossRef]
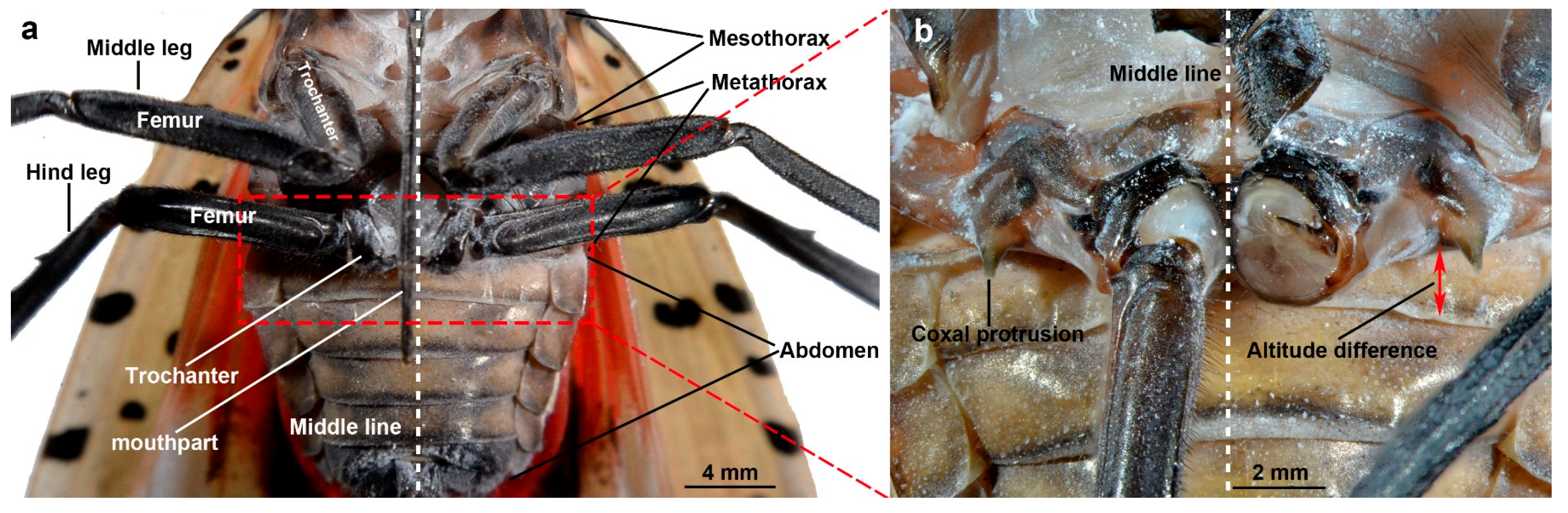
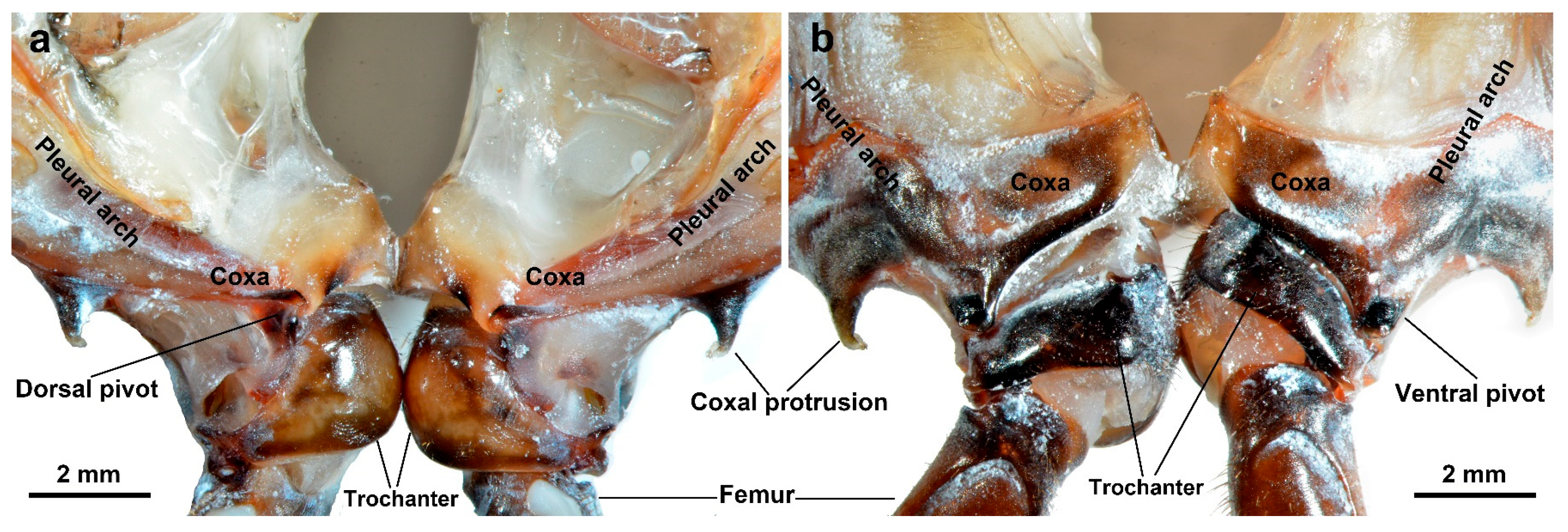
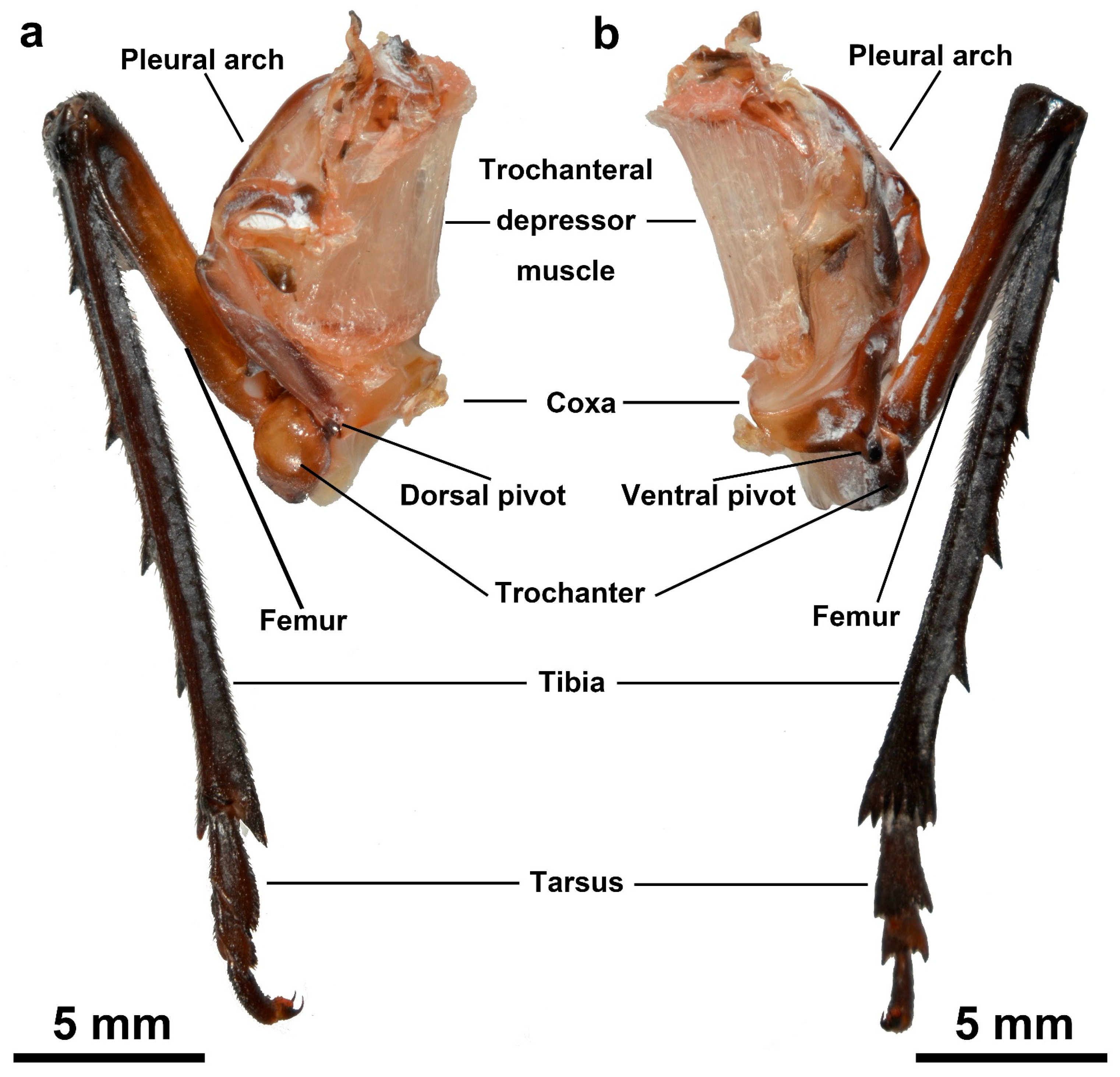
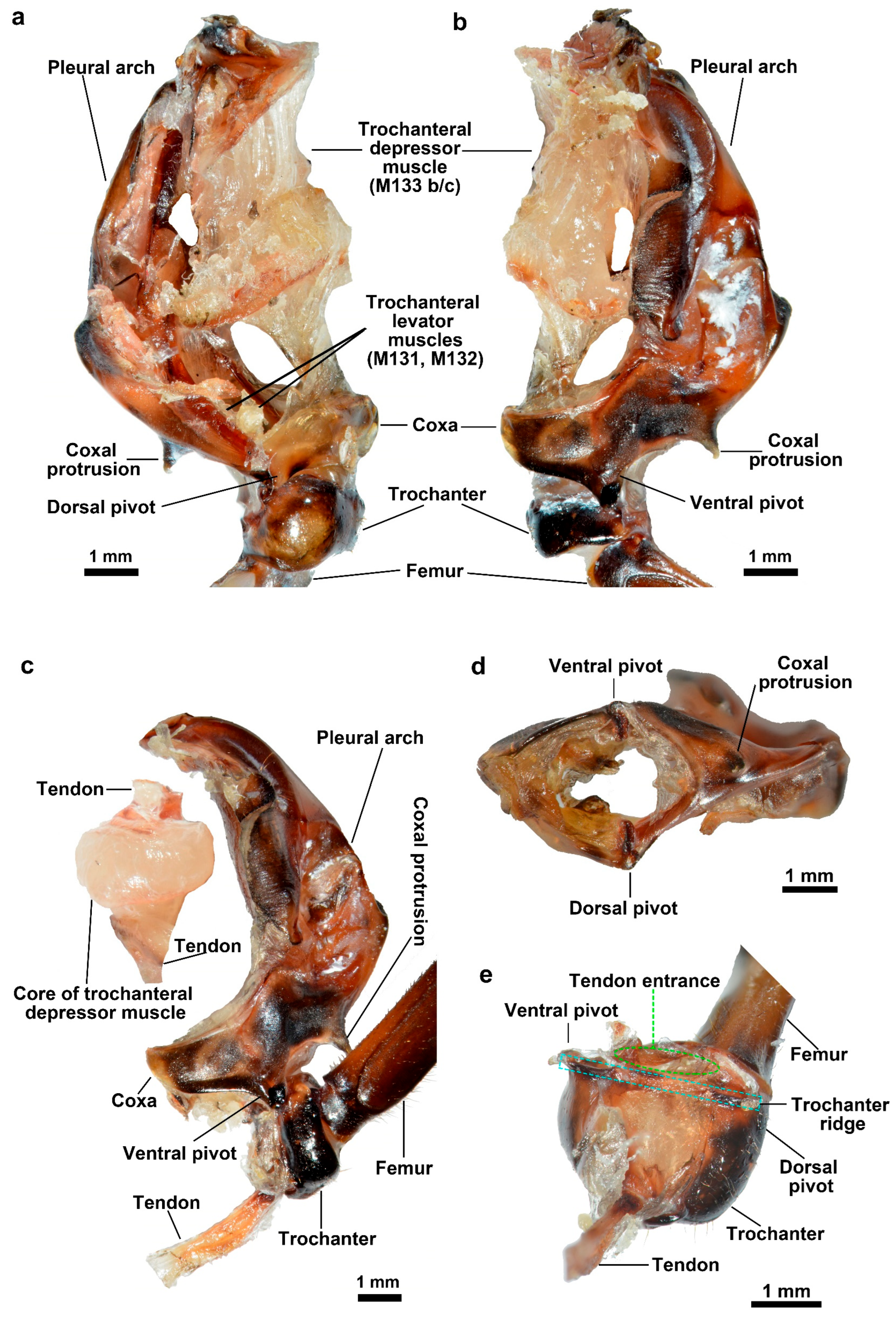


| Structures | Biological Significance | UJS/Normal Jump | Before Jumping | After Jumping | Variation |
|---|---|---|---|---|---|
| N = 10 (Mean ± SE) * | |||||
| Pleural arch length (mm) | The deformation length is crucial for energy storage and release during the jumping-like motion. | UJS | 3.72 ± 0.07 | 4.68 ± 0.06 | 0.96 ± 0.06 |
| Normal | 3.62 ± 0.11 | 4.64 ± 0.11 | 1.02 ± 0.07 | ||
| Angle between coxa and trochanter (∠ct) (deg.) | This angle reflects the positioning and movement of the coxa and trochanter, which are key components in the coupling mechanism of the UJS. | UJS | 111.40 ± 1.14 | 54.02 ± 1.01 | 57.42 ± 1.60 |
| Normal | 110.90 ± 1.07 | 54.08 ± 0.95 | 56.78 ± 1.32 | ||
| Angle between femur and pleural arch (∠fp) (deg.) | This angle indicates the bending and recovery of the pleural arch, which is essential for energy storage and release. | UJS | 9.25 ± 0.36 | 110.70 ± 1.52 | 101.40 ± 1.59 |
| Normal | 9.23 ± 0.35 | 110.80 ± 1.41 | 101.60 ± 1.54 | ||
| Angle between femur and tibia (∠ft) (deg.) | This angle represents the movement of the femur and tibia, which are critical for converting stored energy into kinetic energy during the jumping-like motion. | UJS | 23.24 ± 0.90 | 59.31 ± 2.55 a | 36.06 ± 2.41 a |
| Normal | 23.32 ± 0.79 | 107.40 ± 0.98 b | 84.03 ± 0.83 b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liang, A. The Unilateral Jumping Structures of the Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae): A Highly Functional and Integrated Unit. Biomimetics 2025, 10, 444. https://doi.org/10.3390/biomimetics10070444
Chen X, Liang A. The Unilateral Jumping Structures of the Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae): A Highly Functional and Integrated Unit. Biomimetics. 2025; 10(7):444. https://doi.org/10.3390/biomimetics10070444
Chicago/Turabian StyleChen, Xu, and Aiping Liang. 2025. "The Unilateral Jumping Structures of the Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae): A Highly Functional and Integrated Unit" Biomimetics 10, no. 7: 444. https://doi.org/10.3390/biomimetics10070444
APA StyleChen, X., & Liang, A. (2025). The Unilateral Jumping Structures of the Spotted Lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae): A Highly Functional and Integrated Unit. Biomimetics, 10(7), 444. https://doi.org/10.3390/biomimetics10070444






