Design and Performance of a Neurosurgery Assisting Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Requirements
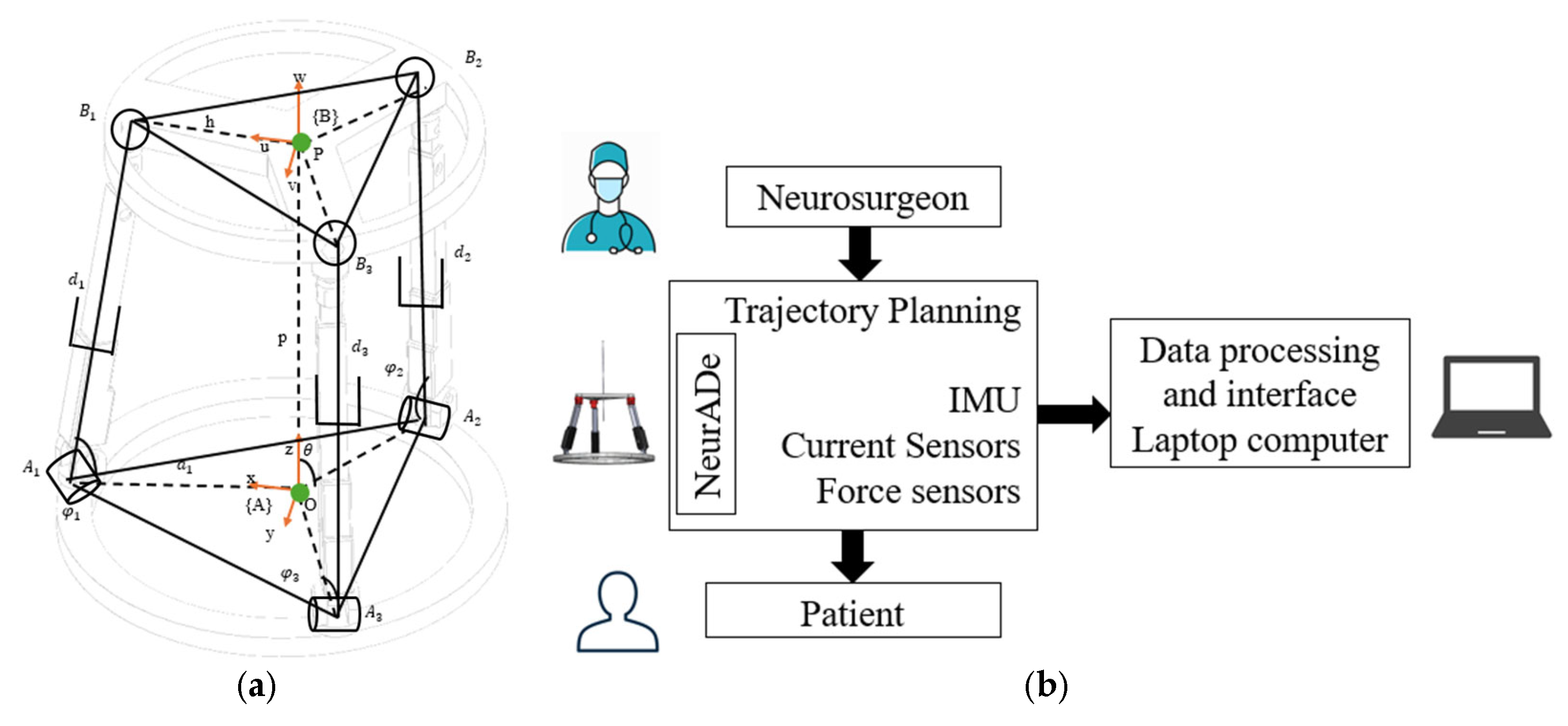
2.2. Conceptual Design
2.3. CAD Design and Modeling
3. Results
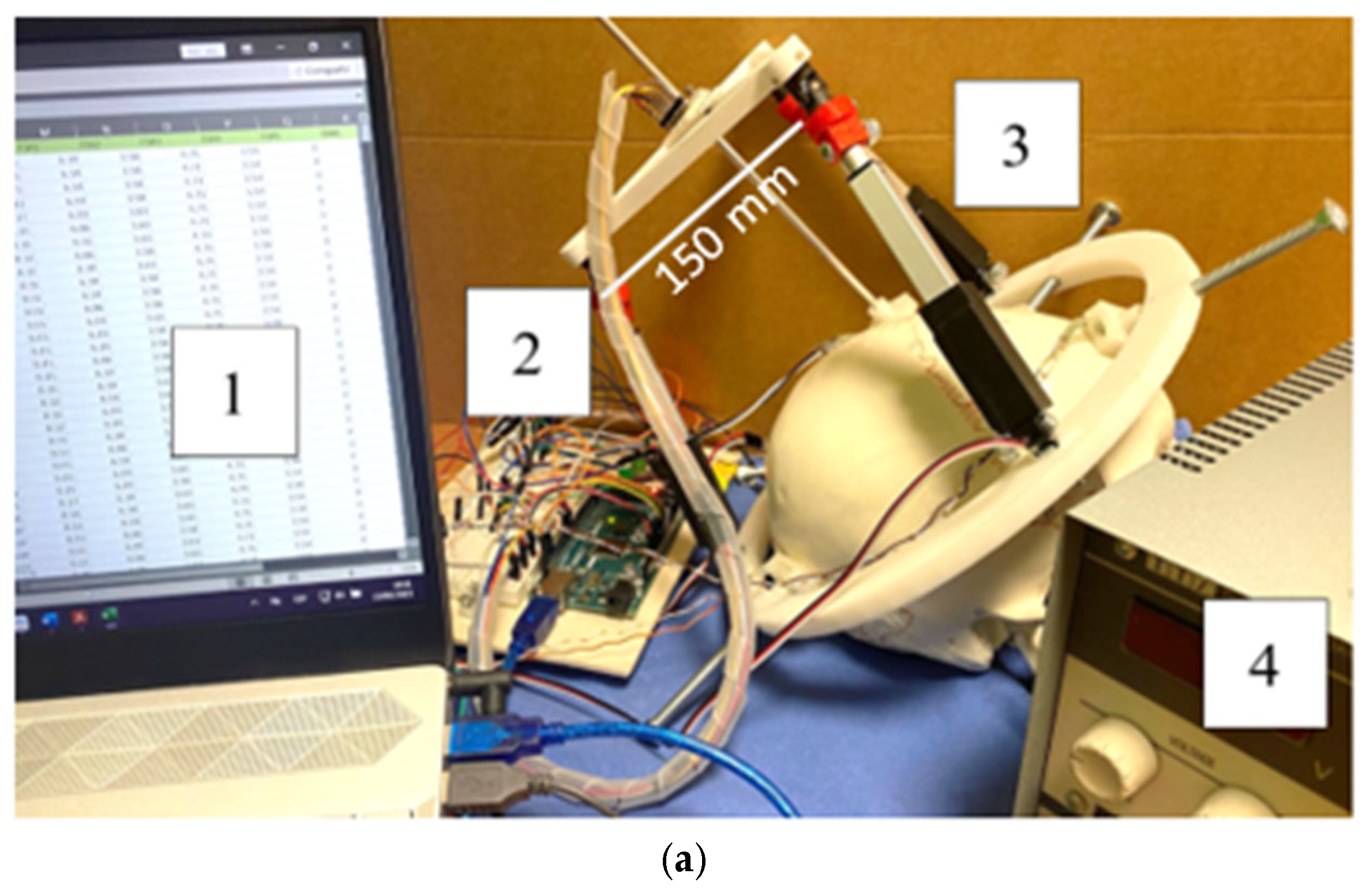
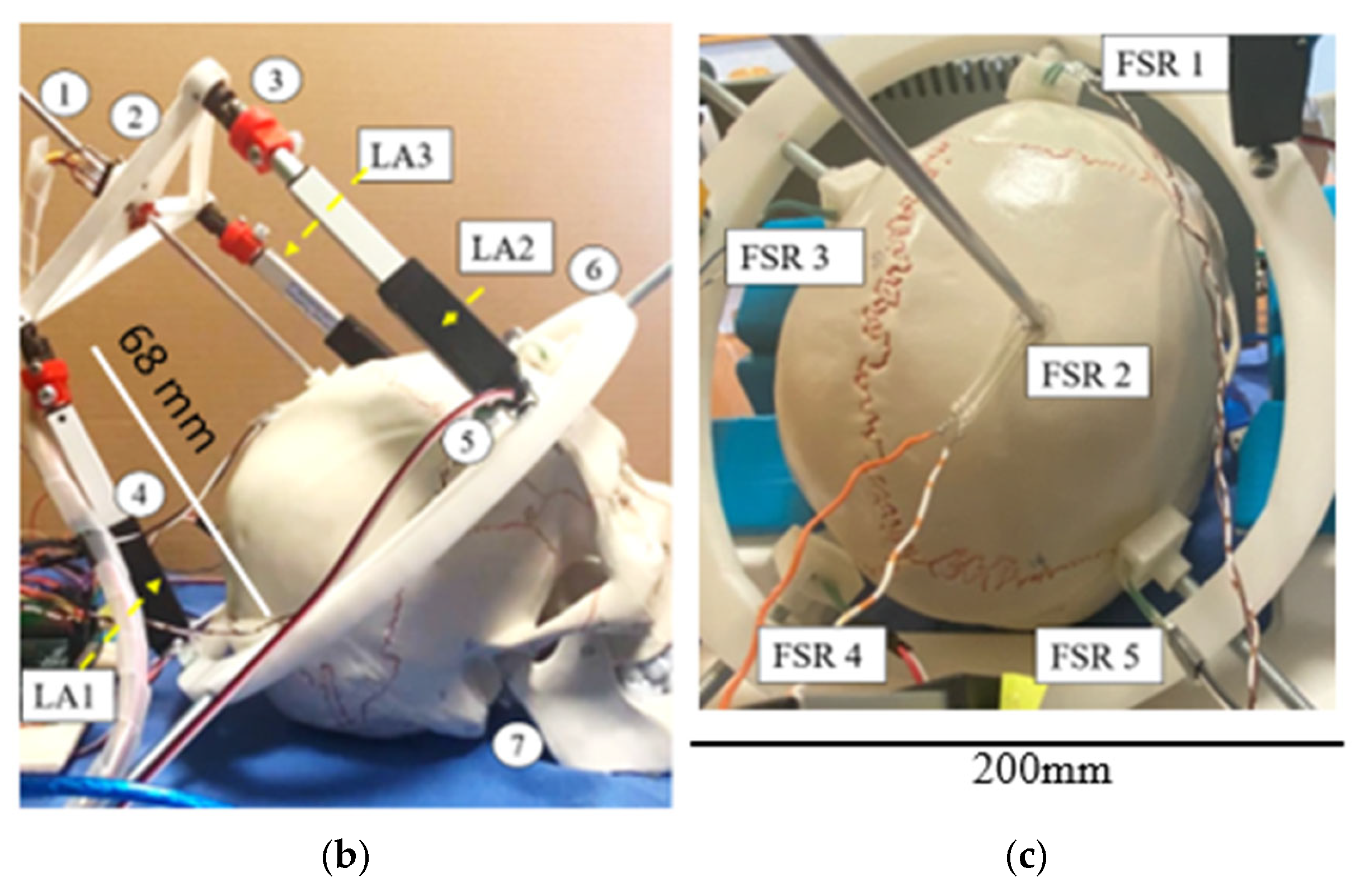
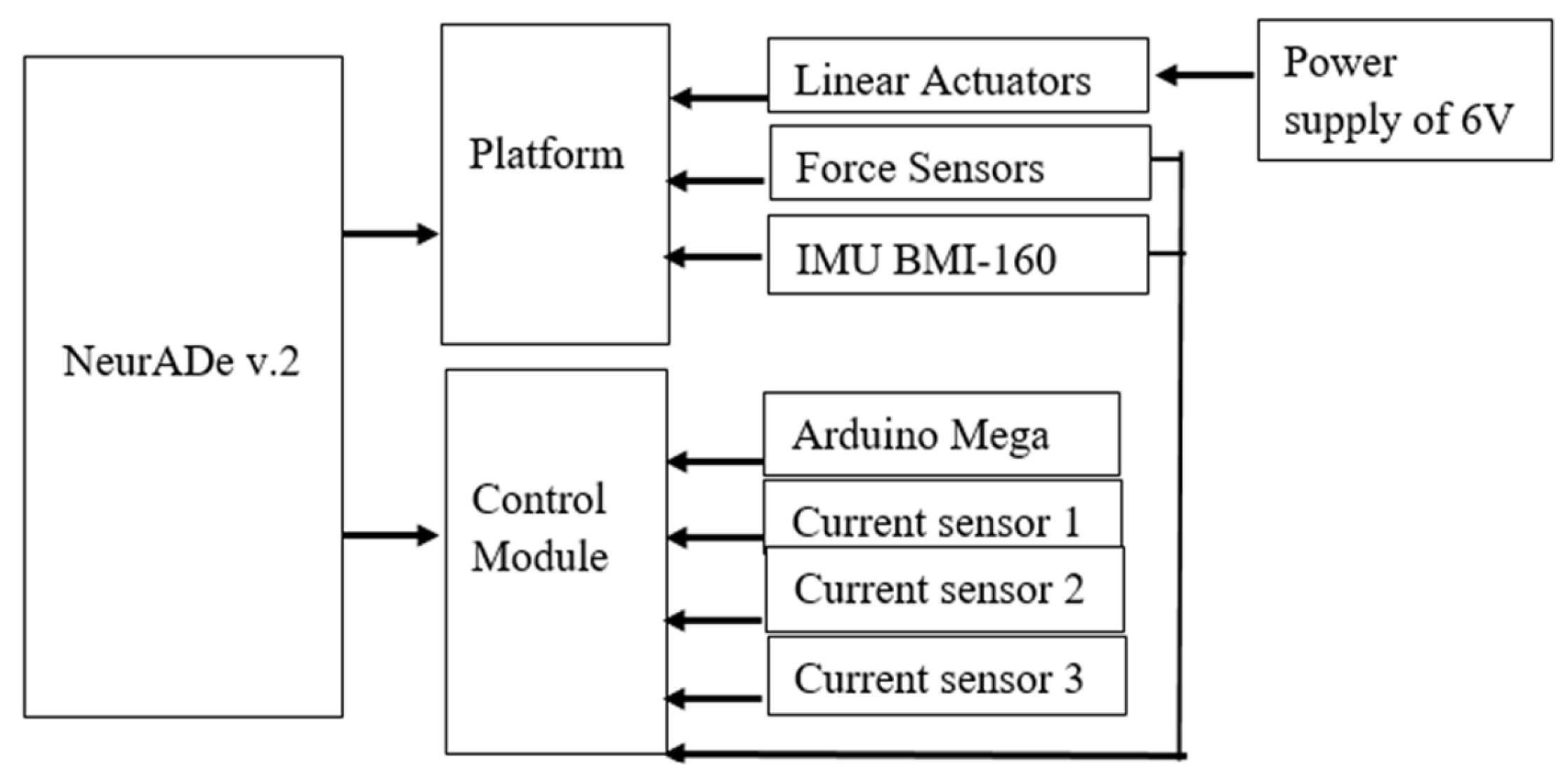
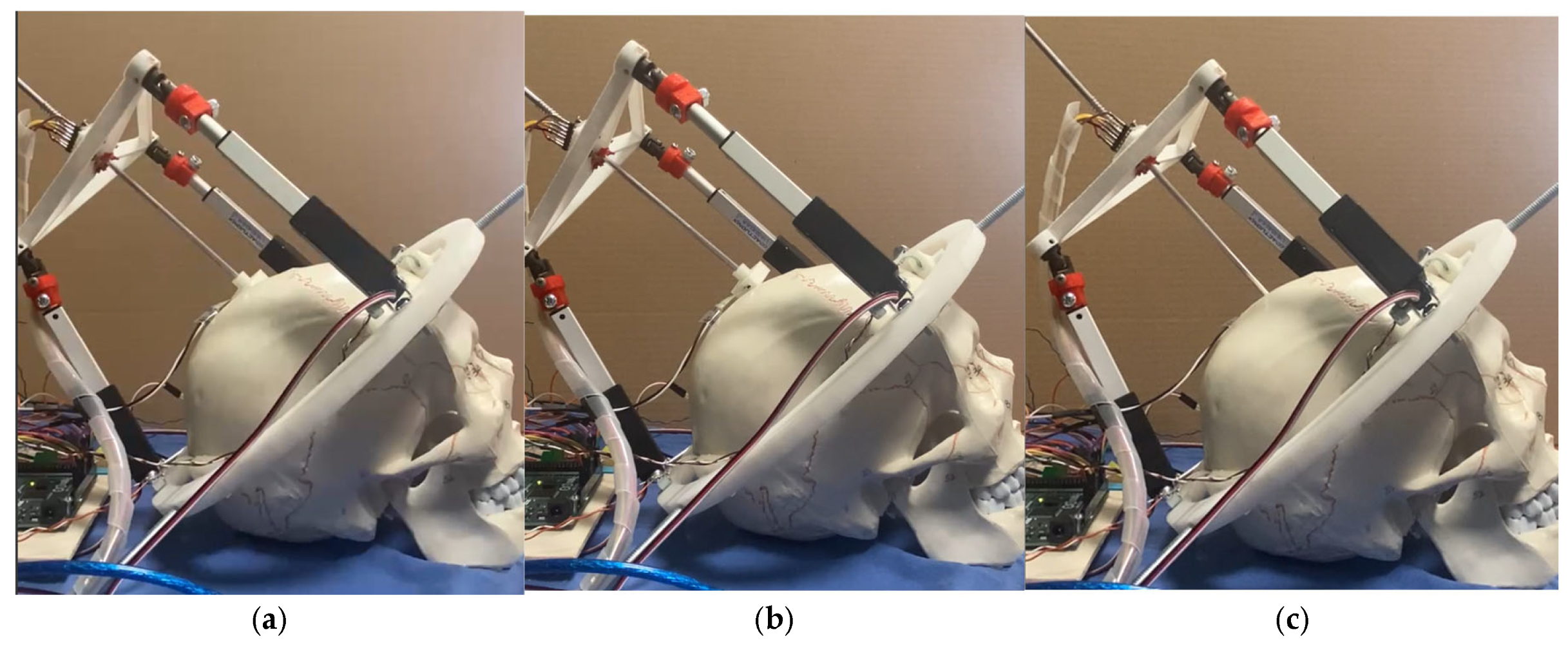
3.1. Prototype Assembly
3.2. Testing Layout and Model
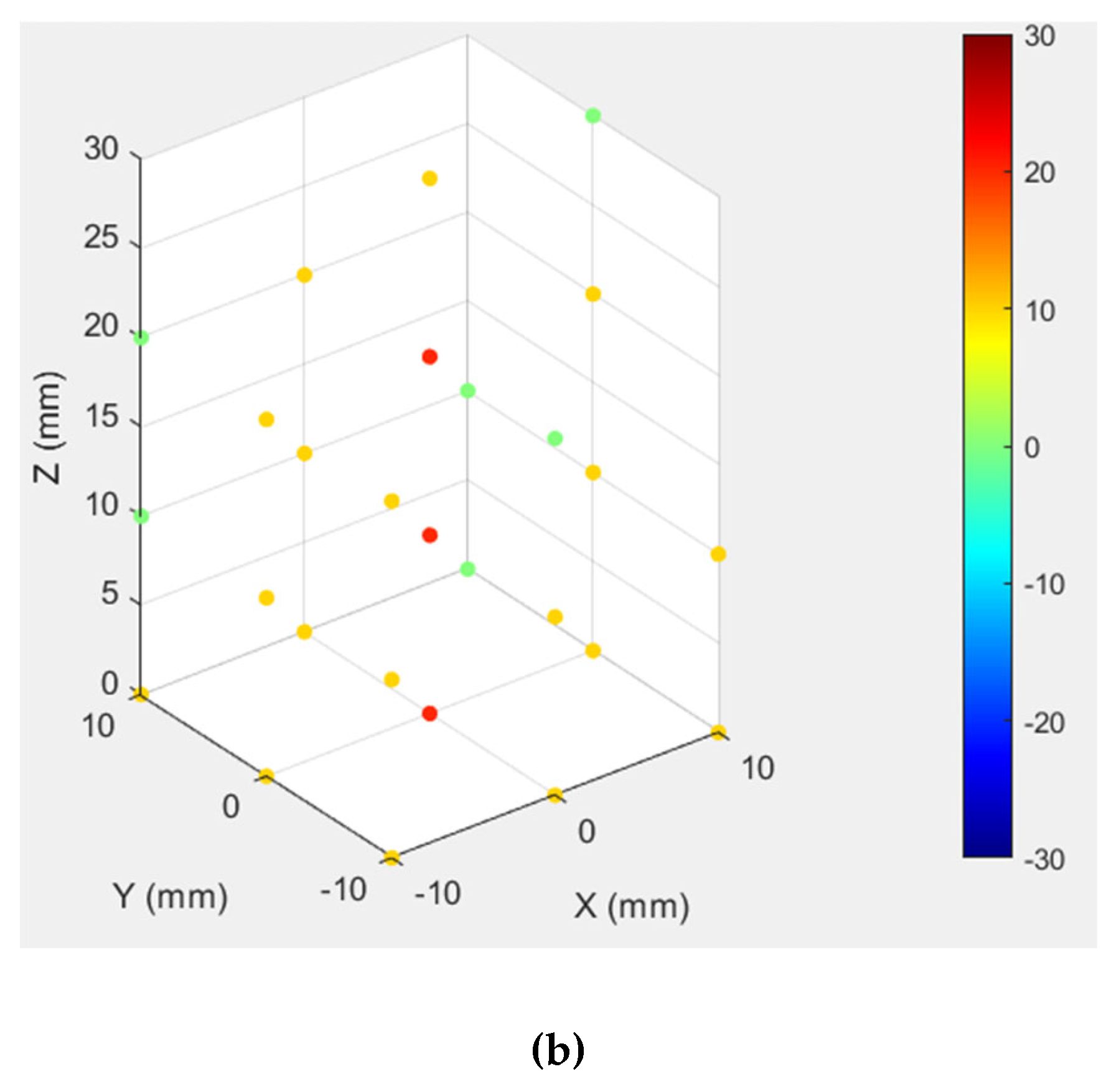
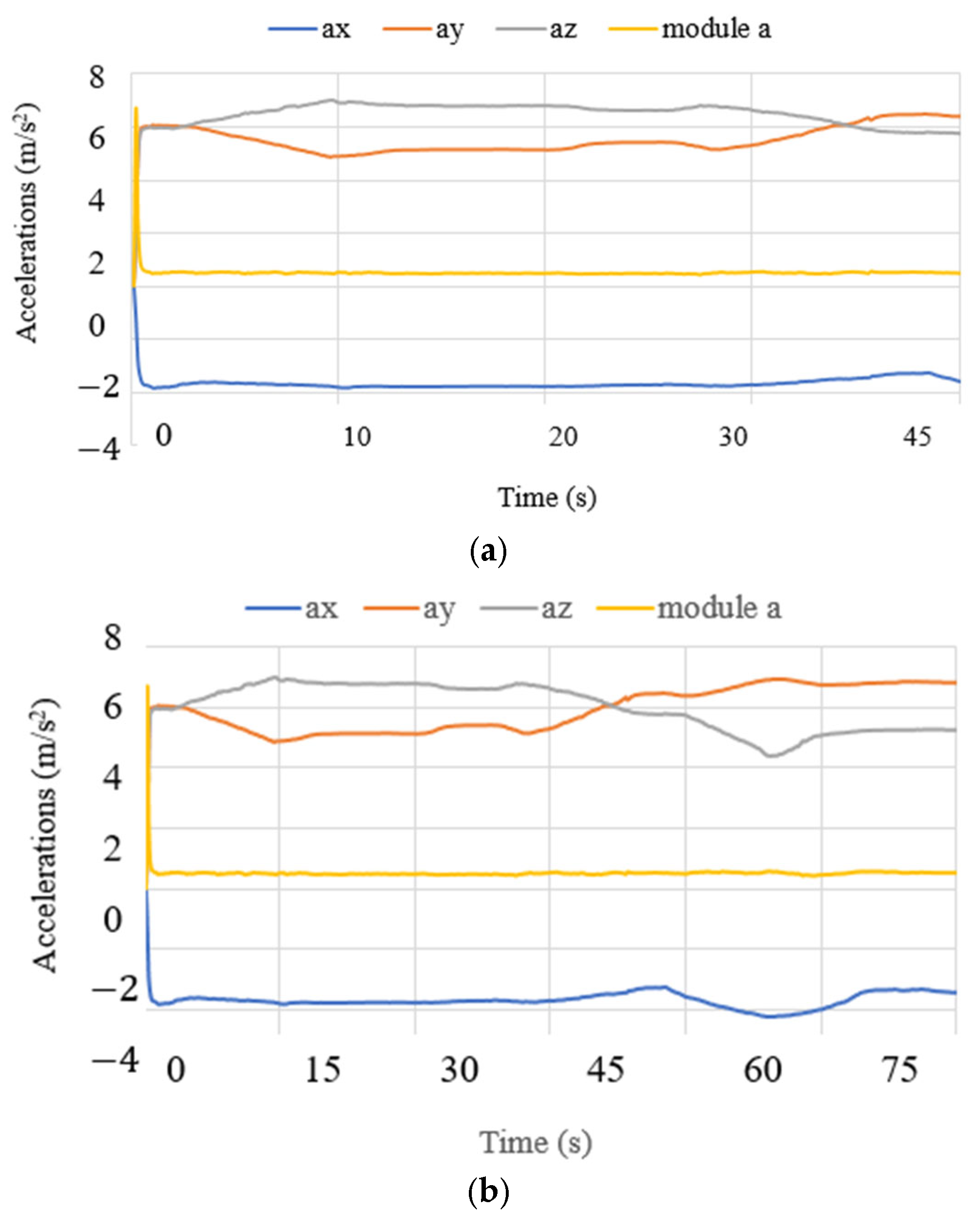
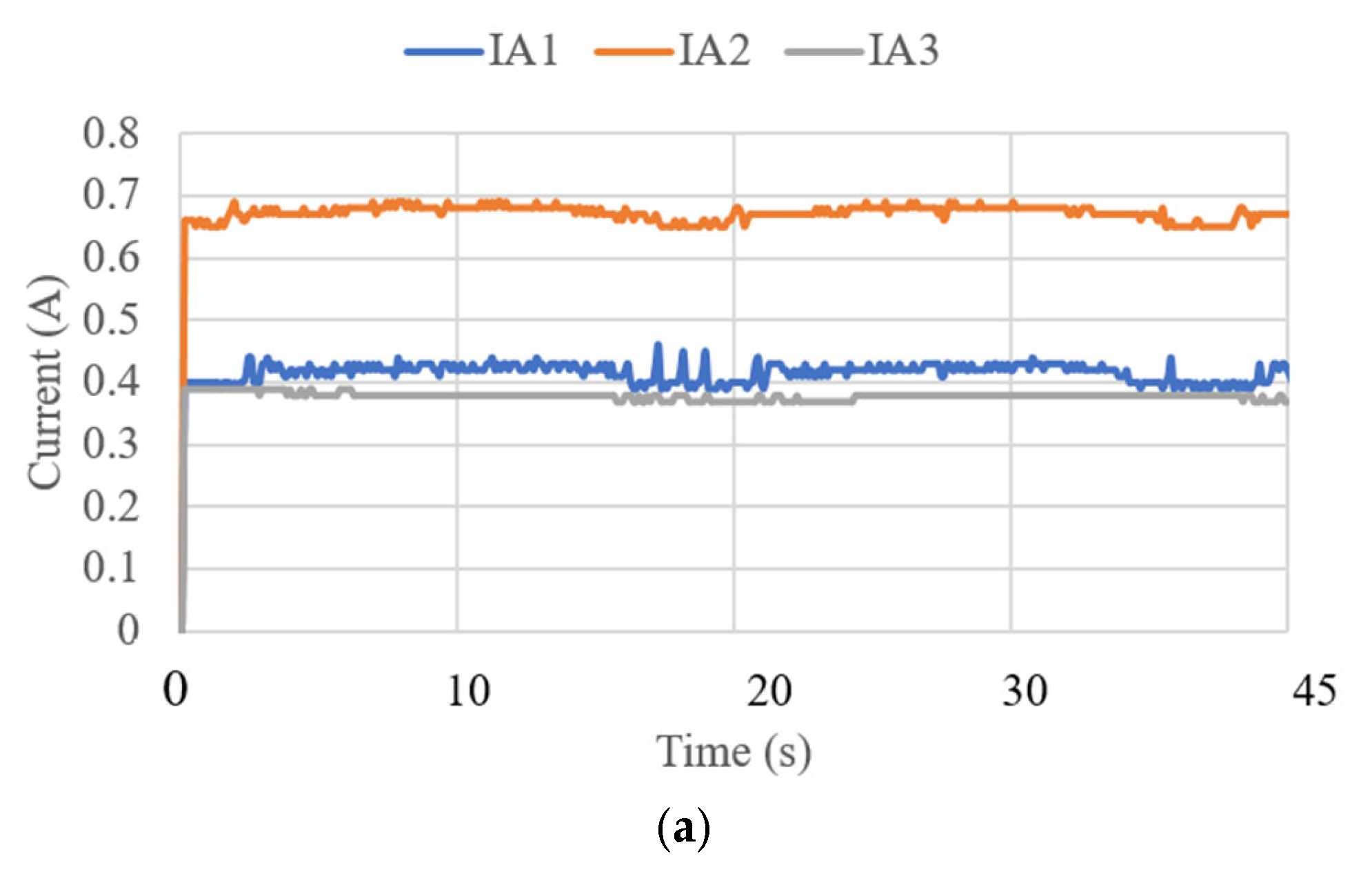
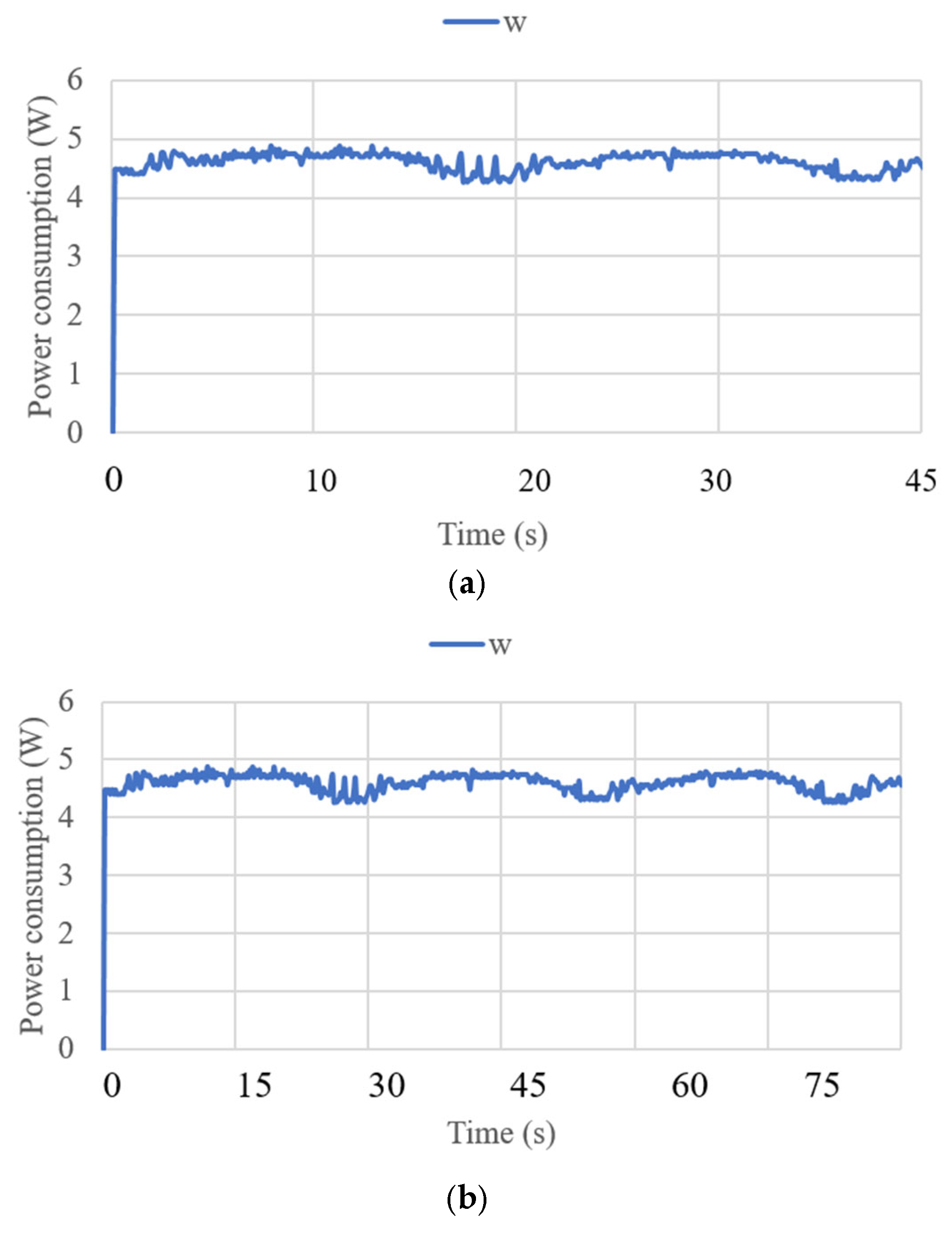
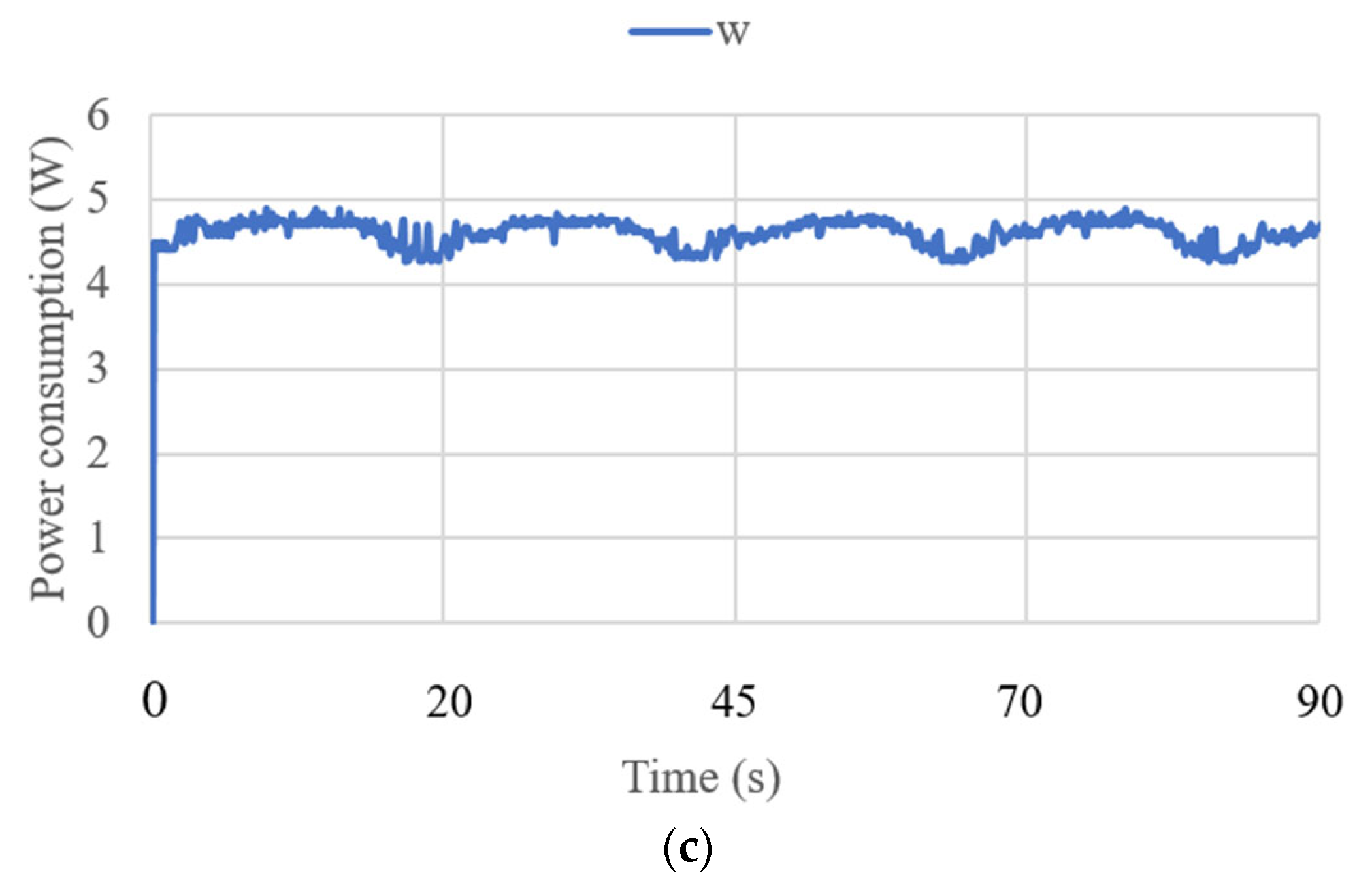
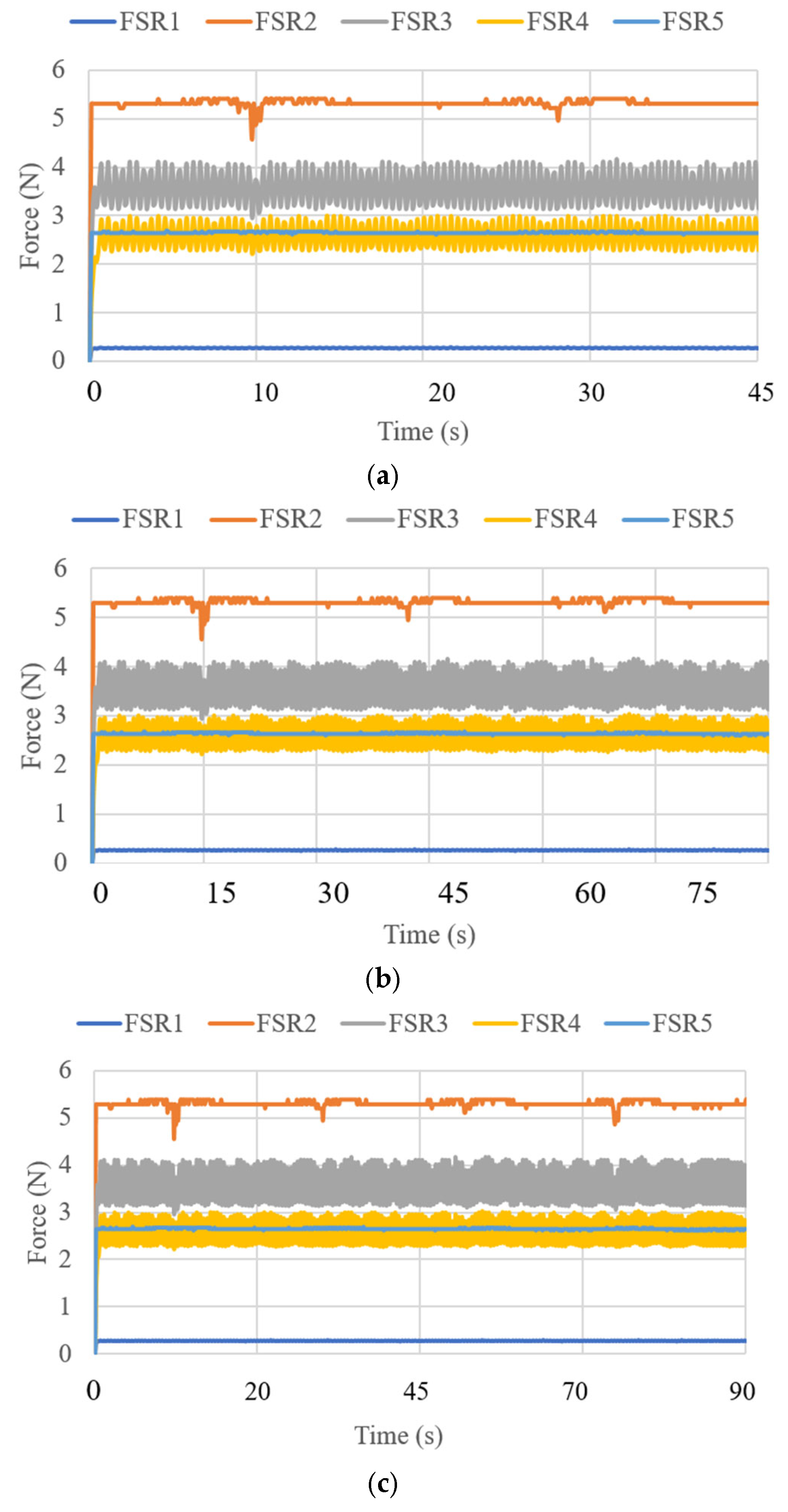
3.3. Test Results
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NeurADe | Neurosurgery-Assisting Device |
| 3-RPS | 3- Revolute Prismatic Spherical Joint |
| DoF | Degrees of Freedom |
References
- Picozzi, P.; Nocco, U.; Labate, C.; Gambini, I.; Puleo, G.; Silvi, F.; Pezzillo, A.; Mantione, R.; Cimolin, V. Advances in Robotic Surgery: A Review of New Surgical Platforms. Electronics 2024, 13, 4675. [Google Scholar] [CrossRef]
- Gamal, A.; Moschovas, M.C.; Jaber, A.R.; Saikali, S.; Perera, R.; Headley, C.; Patel, E.; Rogers, T.; Roche, M.W.; Leveillee, R.J.; et al. Clinical applications of robotic surgery platforms: A comprehensive review. J. Robot. Surg. 2024, 18, 29. [Google Scholar] [CrossRef]
- Chatterjee, S.; Das, S.; Ganguly, K.; Mandal, D. Advancements in robotic surgery: Innovations, challenges and future prospects. J. Robot. Surg. 2024, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Günkan, A.; Ferreira, M.Y.; Bocanegra-Becerra, J.E.; Pehlivan, U.A.; Vilardo, M.; Semione, G.; Batista, S.; Ferreira, C.; Serulle, Y.; Yardimcioglu, I.; et al. In the era of transition from fiction to reality: Robotic-assisted neurointervention-a systematic review and meta-analysis. Neurosurg. Rev. 2025, 48, 7. [Google Scholar] [CrossRef]
- Zhang, C.; Hallbeck, M.S.; Salehinejad, H.; Thiels, C. The integration of artificial intelligence in robotic surgery: A narrative review. Surgery 2024, 176, 552–557. [Google Scholar] [CrossRef]
- Shaik, S.T.; Dwarakanath, T.A.; Moiyadi, A.V. Evolution of Robotics in Neurosurgery. Asian J Neurosurg. 2024, 19, 598–609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baghdadi, A.; Lama, S.; Singh, R.; Sutherland, G.R. Tool-tissue force segmentation and pattern recognition for evaluating neurosurgical performance. Sci. Rep. 2023, 13, 9591. [Google Scholar] [CrossRef]
- Layard Horsfall, H.; Salvadores Fernandez, C.; Bagchi, B.; Datta, P.; Gupta, P.; Koh, C.H.; Khan, D.; Muirhead, W.; Desjardins, A.; Tiwari, M.K.; et al. A Sensorised Surgical Glove to Analyze Forces During Neurosurgery. Neurosurgery 2023, 92, 639–646. [Google Scholar] [CrossRef]
- Kazemzadeh, K.; Akhlaghdoust, M.; Zali, A. Advances in artificial intelligence, robotics, augmented and virtual reality in neurosurgery. Front. Surg. 2023, 10, 1241923. [Google Scholar] [CrossRef]
- Ahmed, F.A.; Yousef, M.; Ahmed, M.A.; Ali, H.O.; Mahboob, A.; Ali, H.; Shah, Z.; Aboumarzouk, O.; Al Ansari, A.; Balakrishnan, A. Deep learning for surgical instrument recognition and segmentation in robotic-assisted surgeries: A systematic review. Artif. Intell. Rev. 2025, 58, 1. [Google Scholar] [CrossRef]
- Chuck, C.; Ali, R.; Lee, C.K.; Malik, A.N.; Svokos, K.A.; Cielo, D.; Doberstein, C.E.; Rosenberg, H.J.; Boxerman, J.L.; Rajasekaran, J.; et al. Neuro-oncology application of next-generation, optically tracked robotic stereotaxis with intraoperative computed tomography: A pilot experience. Neurosurg. Focus 2024, 57, E4. [Google Scholar] [CrossRef]
- Wah, J.N.K. Revolutionizing surgery: AI and robotics for precision, risk reduction, and innovation. J. Robot. Surg. 2025, 19, 47. [Google Scholar] [CrossRef]
- Abarca, V.E.; Elias, D.A. A Review of Parallel Robots: Rehabilitation, Assistance, and Humanoid Applications for Neck, Shoulder, Wrist, Hip, and Ankle Joints. Robotics 2023, 12, 131. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Jin, L.; Wu, J. Evolution of Neurosurgical Robots: Historical Progress and Future Direction. World Neurosurg. 2024, 191, 49–57. [Google Scholar] [CrossRef]
- Łajczak, P.; Łajczak, A. Hydrocephalus Surgery 2.0: A Systematic Review of the Robotic Effectiveness in Neurosurgical Interventions. Res. Sq. 2024; preprint. [Google Scholar] [CrossRef]
- Huang, X.; Rendon-Morales, E.; Aviles-Espinosa, R. A High-Precision Robotic System Design for Microsurgical Applications. Eng. Proc. 2023, 58, 66. [Google Scholar] [CrossRef]
- Truckenmueller, P.; Früh, A.; Kissner, J.F.; Moser, N.K.; Misch, M.; Faust, K.; Onken, J.; Vajkoczy, P.; Xu, R. Integration of a lightweight and table-mounted robotic alignment tool with automated patient-to-image registration using robotic cone-beam CT for intracranial biopsies and stereotactic electroencephalography. Neurosurg. Focus 2024, 57, E2. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liang, C.; Gao, F.; Qi, C.; He, B.; Liu, R.; Chen, L. Design of a Novel Macro-Micro Integrated Brain Surgery Robot Based on Modular Parallel Mechanisms. Chin. J. Mech. Eng. 2024, 37, 130. [Google Scholar] [CrossRef]
- Vaids, C.; Birlescu, I.; Herman, B.; Condurache, D.; Chablat, D.; Pisla, D. An analysis of higher-order kinematics formalisms for an innovative surgical parallel robot. Mech. Mach. Theory 2025, 209, 105986. [Google Scholar] [CrossRef]
- Huang, X.; Rendon-Morales, E.; Aviles-Espinosa, R. A Biomedical Robotic Platform Combined with an Application-Specific Laser-Based End-Effector for Achieving High Precision Neurosurgery. In Proceedings of the 2024 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Eindhoven, The Netherlands, 26–28 June 2024; pp. 1–6. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.; Ma, Z.; Sun, X.; Zhao, J. An MRI-guided stereotactic neurosurgical robotic system for semi-enclosed head coils. J. Robot. Surg. 2025, 19, 35. [Google Scholar] [CrossRef]
- Varas, J.; Coronel, B.V.; Villagrán, I.; Escalona, G.; Hernandez, R.; Schuit, G.; Durán, V.; Lagos-Villaseca, A.; Jarry, C.; Neyem, A.; et al. Innovations in surgical training: Exploring the role of artificial intelligence and large language models (LLM). Rev. Col. Bras. Cir. 2023, 50, e20233605. [Google Scholar] [CrossRef]
- Awad, M.M.; Raynor, M.C.; Padmanabhan-Kabana, M.; Schumacher, L.Y.; Blatnik, J.A. Evaluation of forces applied to tissues during robotic-assisted surgical tasks using a novel force feedback technology. Surg. Endosc. 2024, 38, 6193–6202. [Google Scholar] [CrossRef] [PubMed]
- Silva-Garces, K.N.; Torres-San Miguel, C.R.; Jimenez-Ponce, F.; Ceccarelli, M. Design of Medical Robot for Stereotactic Surgery Based on a 3-RPS Parallel Mechanism. In Advances in Mechanism and Machine Science; Okada, M., Ed.; IFToMM WC 2023; Springer: Cham, Switzerland, 2023; Volume 147. [Google Scholar] [CrossRef]
- Silva-Garces, K.N.; Russo, M.; Torres-SanMiguel, C.R.; Guerrero-Hernández, L.A.; Ceccarelli, M. A PK Platform Lab Test for a Brain Neurosurgery. In Mechanism Design for Robotics; Lovasz, E.C., Ceccarelli, M., Ciupe, V., Eds.; MEDER 2024; Springer: Cham, Switzerland, 2024; Volume 166. [Google Scholar] [CrossRef]
- Silva-Garces, K.N.; Jimenez-Ponce, F.; Miguel, C.R.T.S. ADALINE Neurons Used for Targeting Performance on the Deep Brain Stimulation Platform. In Artificial Intelligence in Healthcare; Xie, X., Styles, I., Powathil, G., Ceccarelli, M., Eds.; AIiH 2024; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2024; Volume 14975. [Google Scholar] [CrossRef]
- BOSCH, Inertial Measurement Unit GY-BMI160, Datasheet, 2025, Germany. Available online: https://www.bosch-sensortec.com/media/boschsensortec/downloads/datasheets/bst-bmi160-ds000.pdf (accessed on 28 January 2025).
- Actuonix, L16-R Linear Actuator with Integrated RC Position Controller, Datasheet. 2025. Available online: https://www.actuonix.com/l16-50-63-6-r (accessed on 28 January 2025).
- Restivo, E.; Peluso, E.; Bloise, N.; Bello, G.L.; Bruni, G.; Giannaccari, M.; Raiteri, R.; Fassina, L.; Visai, L. Surface Properties of a Biocompatible Thermoplastic Polyurethane and Its Anti-Adhesive Effect against E. coli and S. aureus. J. Funct. Biomater. 2024, 15, 24. [Google Scholar] [CrossRef]
- Barchiki, F.; Fracaro, L.; Dominguez, A.C.; Senegaglia, A.C.; Vaz, I.M.; Soares, P.; Moura, S.A.B.; Brofman, P.R.S. Biocompatibility of ABS and PLA Polymers with Dental Pulp Stem Cells Enhance Their Potential Biomedical Applications. Polymers 2023, 15, 4629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Interlink Electronics, FSR, Datasheet. 2025. Available online: https://cdn.sparkfun.com/assets/e/3/b/3/8/force_sensing_resistor_guide.pdf (accessed on 28 January 2025).
- Arduino, Arduino Mega, Datasheet, 2025, Monza, MB. Available online: https://store.arduino.cc/arduino-mega-2560-rev3 (accessed on 28 January 2025).
- Allegro microsystems, ACS712 Current Sensor, Datasheet, 2024, Manchester. Available online: https://www.allegromicro.com/-/media/files/datasheets/acs712-datasheet.ashx (accessed on 28 January 2025).
- Ceccarelli, M.; Russo, M. Mechanism Design for Robotic Systems; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar]




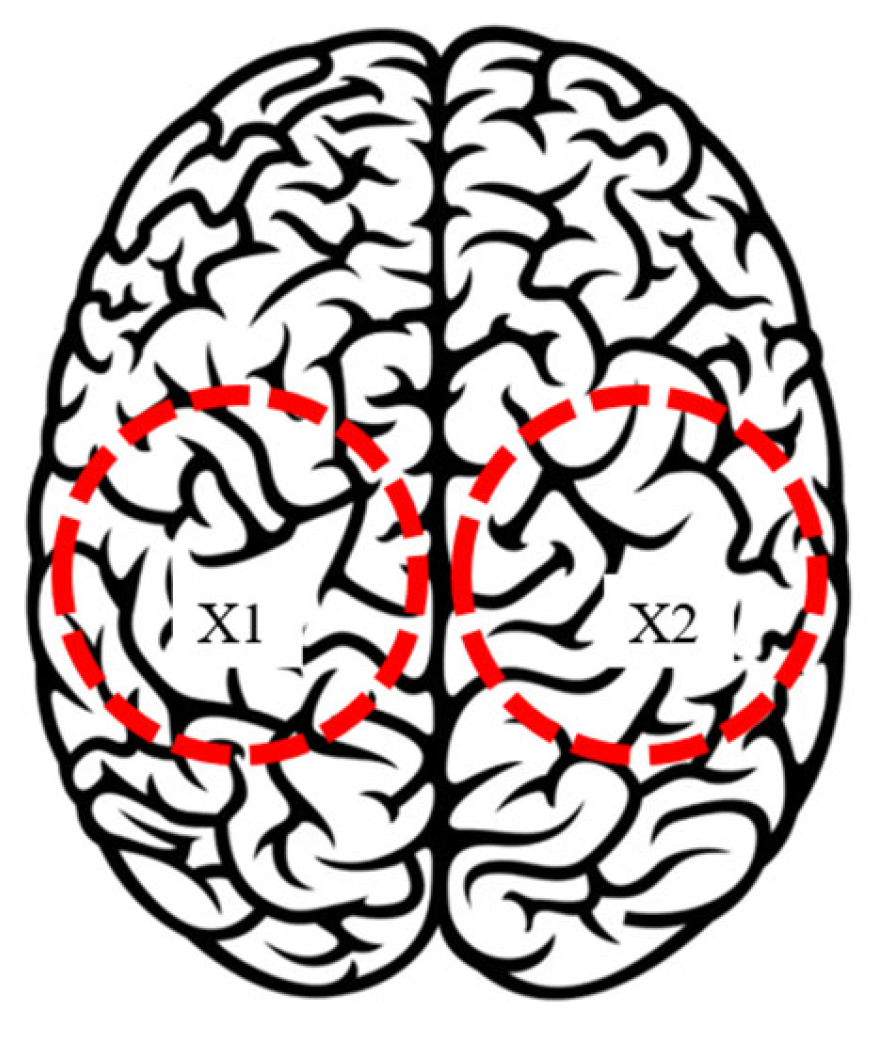
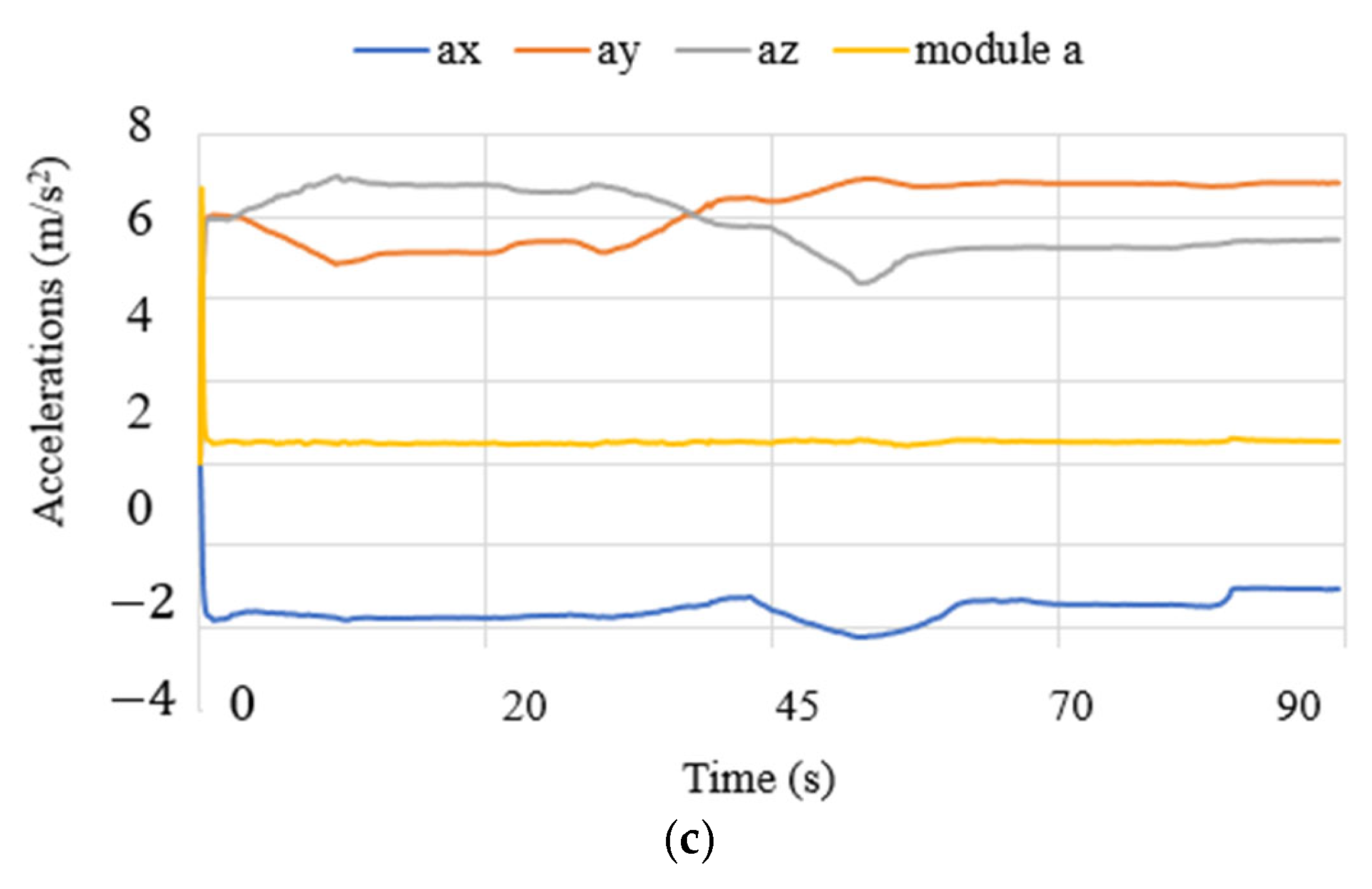
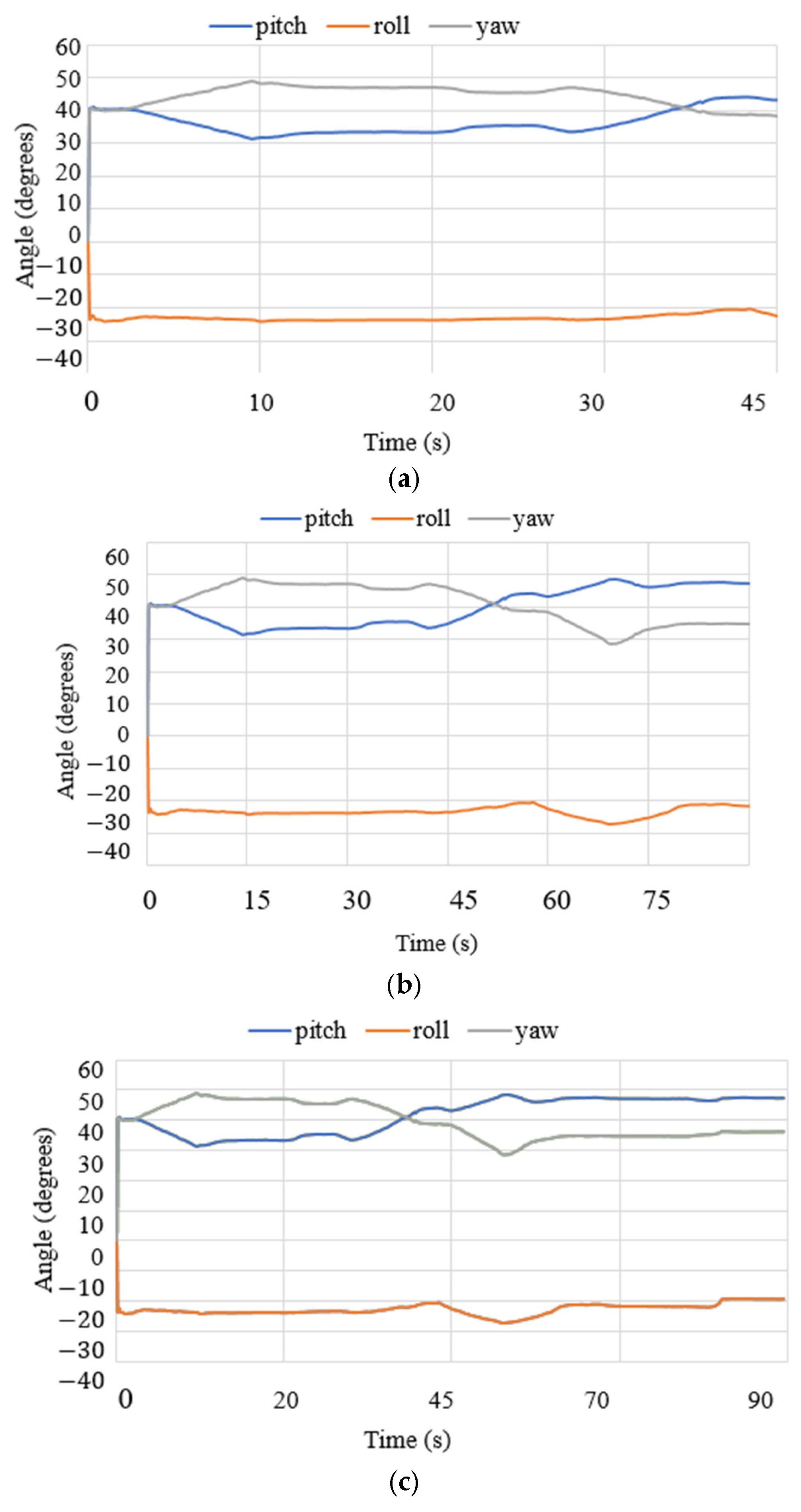
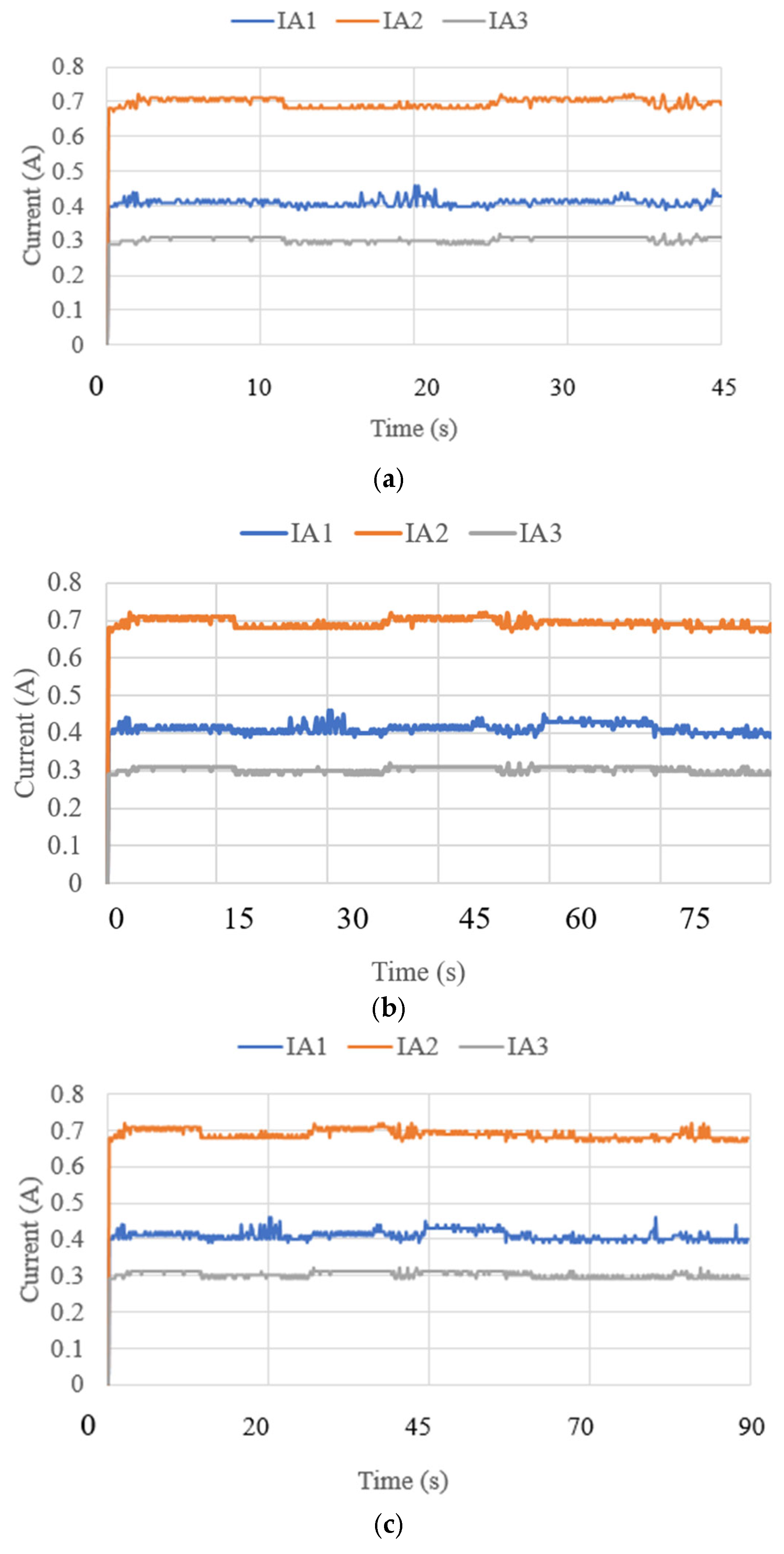
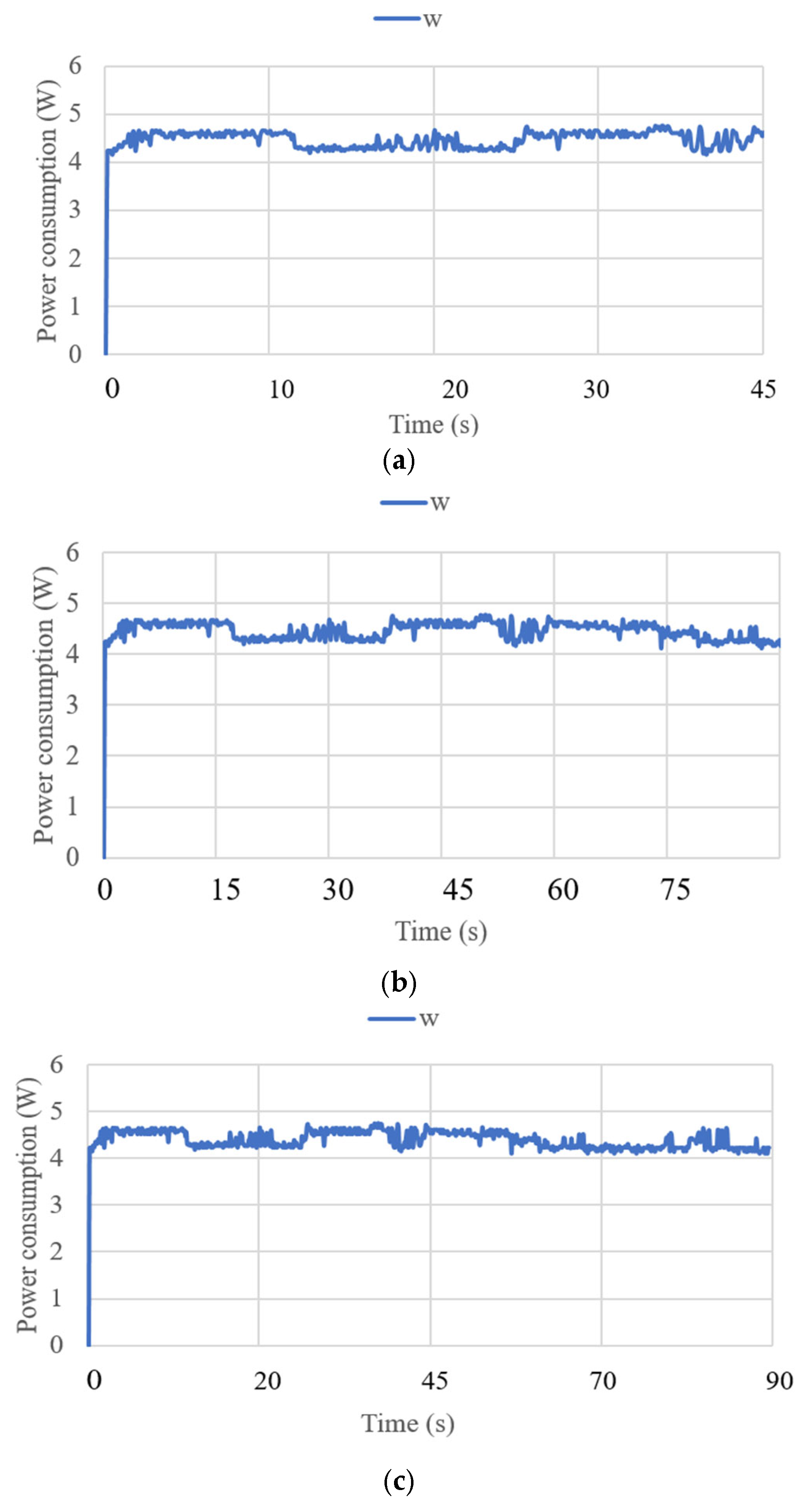
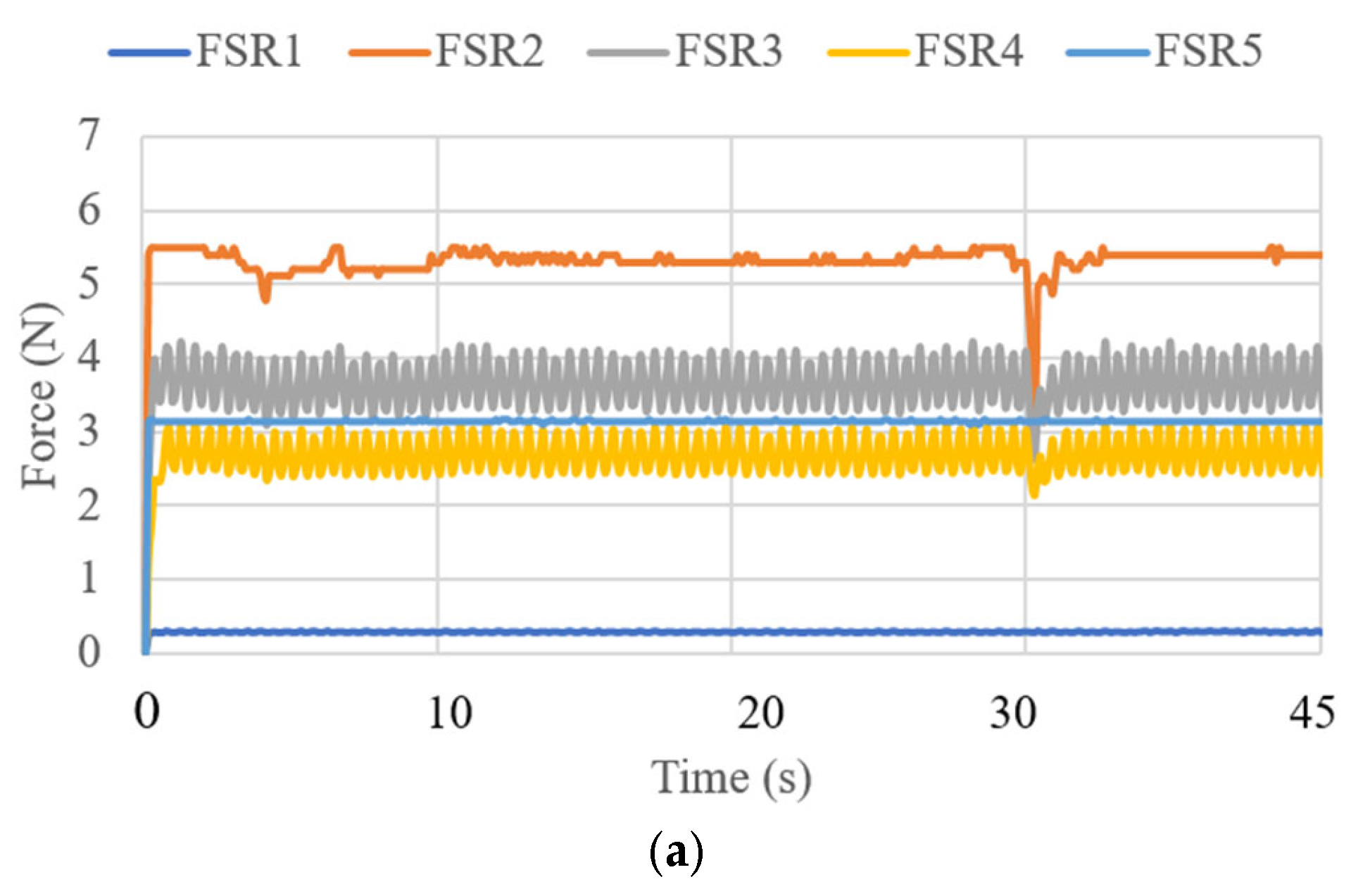
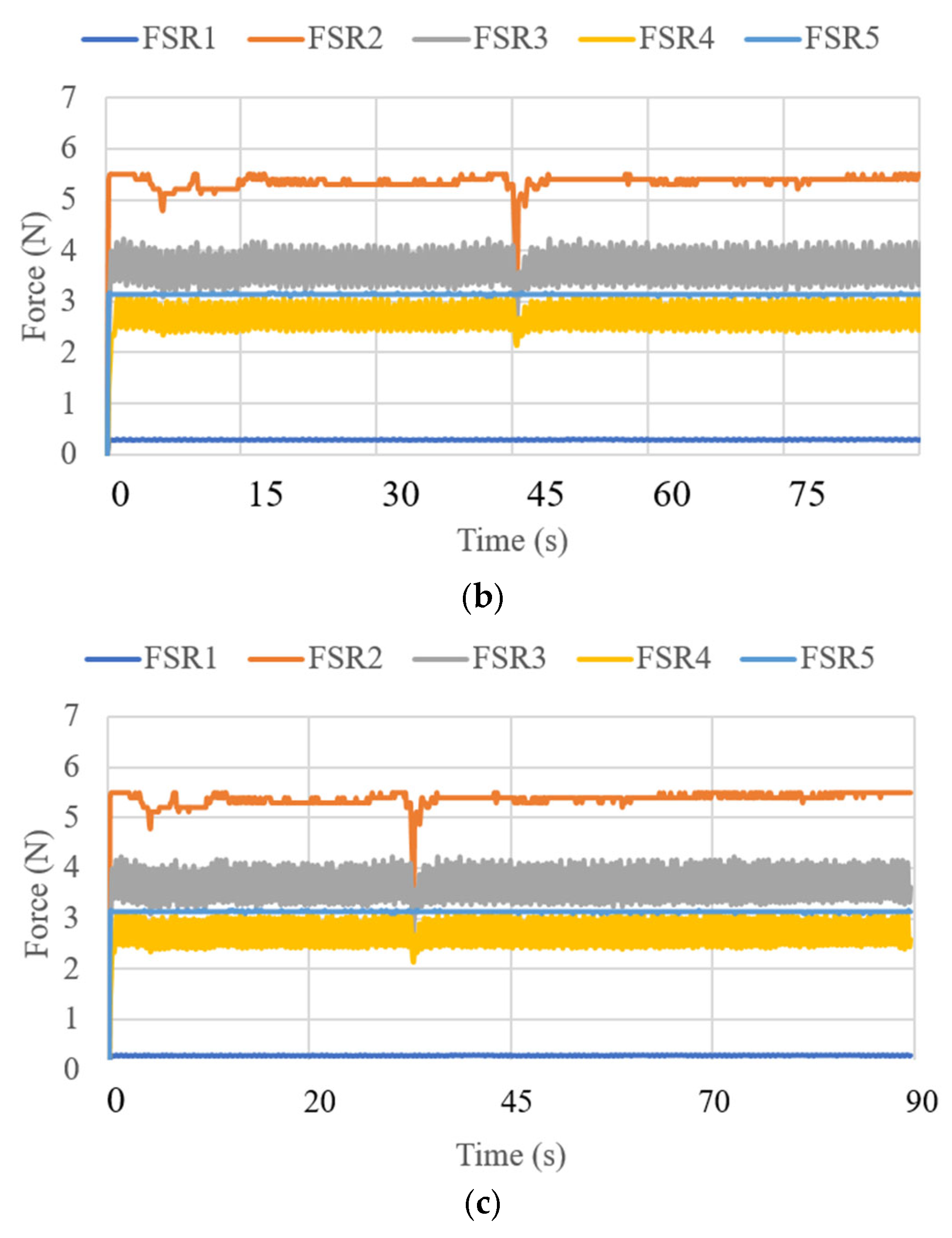
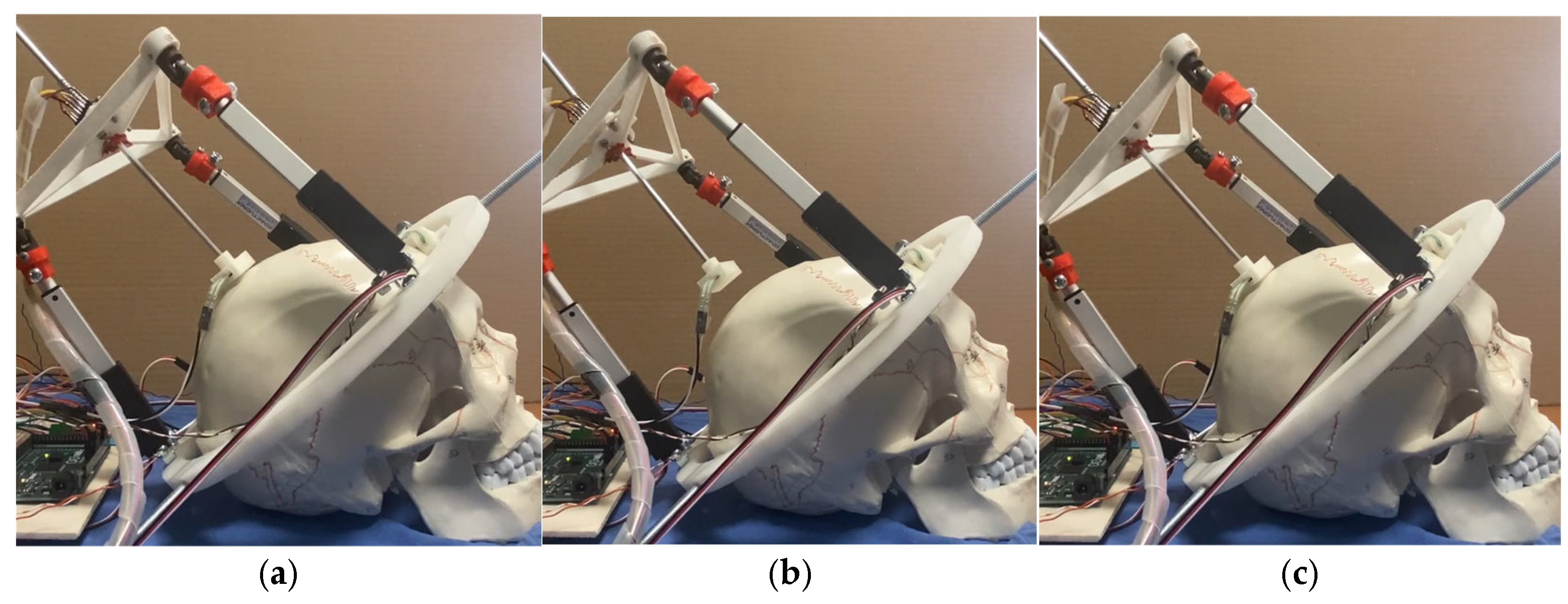

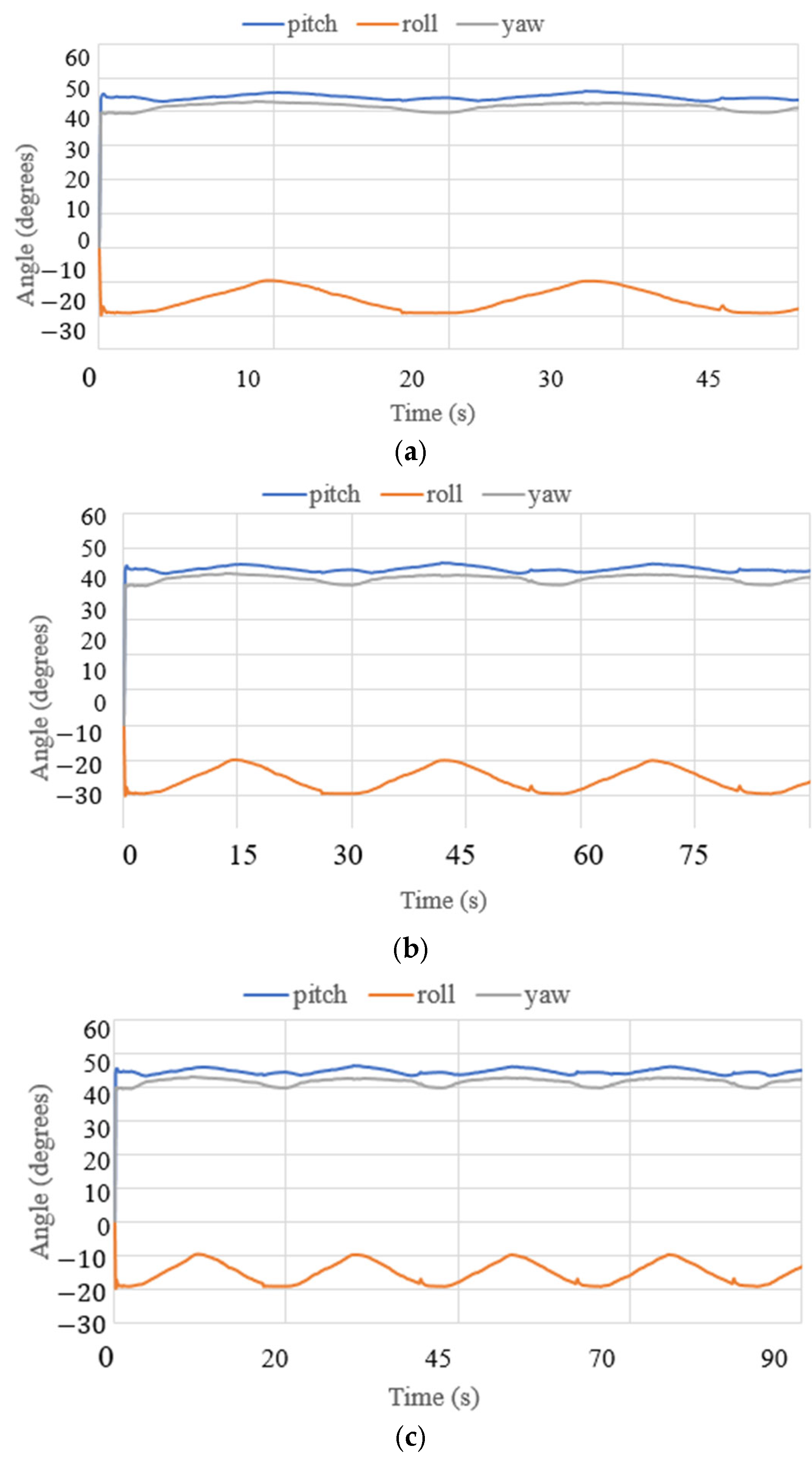

| Test Mode | Input | Output |
|---|---|---|
| 1 | Left parietal lobe X1 | Accelerations, angles, currents, power consumption, and forces. |
| 2 | Right parietal lobe X2 | Accelerations, angles, currents, power consumption, and forces. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Garcés, K.N.; Ceccarelli, M.; Russo, M.; Torres-SanMiguel, C.R. Design and Performance of a Neurosurgery Assisting Device. Biomimetics 2025, 10, 345. https://doi.org/10.3390/biomimetics10060345
Silva-Garcés KN, Ceccarelli M, Russo M, Torres-SanMiguel CR. Design and Performance of a Neurosurgery Assisting Device. Biomimetics. 2025; 10(6):345. https://doi.org/10.3390/biomimetics10060345
Chicago/Turabian StyleSilva-Garcés, Karla Nayeli, Marco Ceccarelli, Matteo Russo, and Christopher René Torres-SanMiguel. 2025. "Design and Performance of a Neurosurgery Assisting Device" Biomimetics 10, no. 6: 345. https://doi.org/10.3390/biomimetics10060345
APA StyleSilva-Garcés, K. N., Ceccarelli, M., Russo, M., & Torres-SanMiguel, C. R. (2025). Design and Performance of a Neurosurgery Assisting Device. Biomimetics, 10(6), 345. https://doi.org/10.3390/biomimetics10060345








