Bio-Membrane-Based Nanofiber Scaffolds: Targeted and Controlled Carriers for Drug Delivery—An Experimental In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Functionalization
2.2. Animal Experimentation
2.3. Surgical Procedure
2.4. Histology
2.5. Statistical Analysis
3. Results
3.1. General Healing and Membrane Integration
3.2. Cellular Distribution in Si-M Membranes
3.3. Cellular Distribution in Zn-M Membranes
3.4. Cellular Distribution in Dox-M Membranes
3.5. Quantitative Comparison Among Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| Dox | Doxycycline |
| Dox-M | SiO2-NP-doped membrane functionalized with Doxycycline |
| ECM | Extracellular Matrix |
| FESEM | Field Emission Scanning Electron Microscopy |
| GBR | Guided Bone Regeneration |
| GTR | Guided Tissue Regeneration |
| HEA | Hydroxyethyl acrylate |
| HEMA | Hydroxyethyl methacrylate |
| IL-6 | Interleukin 6 |
| MA | Methyl acrylate |
| MMA | Methyl methacrylate |
| MMPs | Matrix Metalloproteinases |
| MSCs | Mesenchymal Stem Cells |
| NB | New Formed Bone |
| NPs | Nanoparticles |
| OB | Old Bone |
| OsB | Osteoid Bone |
| SD | Standard Deviation |
| Si-M | silicon oxide nanoparticle-doped membrane |
| SiO2-NPs | silicon oxide nanoparticles |
| TB | Toluidine Blue |
| TNF-α | Tumor Necrosis Factor-α |
| VEGF | Vascular Endothelial Growth Factor |
| Zn | Zinc |
| Zn-M | SiO2-NP-doped membrane functionalized with Zinc |
References
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier Membranes: More than the Barrier Effect? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 103–123. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.-I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier Membranes for Tissue Regeneration in Dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New Attachment Following Surgical Treatment of Human Periodontal Disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and Regenerative Technologies Used in Bone Regeneration in the Craniomaxillofacial Region: Consensus Report of Group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 82–91. [Google Scholar] [CrossRef]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous Asymmetric Collagen/Curcumin Membrane Containing Aspirin-Loaded PLGA Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef]
- Martinez, A.; Balboa, O.; Gasamans, I.; Otero-Cepeda, X.L.; Guitian, F. Deproteinated Bovine Bone vs. Beta-Tricalcium Phosphate as Bone Graft Substitutes: Histomorphometric Longitudinal Study in the Rabbit Cranial Vault. Clin. Oral. Implants Res. 2015, 26, 623–632. [Google Scholar] [CrossRef]
- Mir-Mari, J.; Wui, H.; Jung, R.E.; Hämmerle, C.H.F.; Benic, G.I. Influence of Blinded Wound Closure on the Volume Stability of Different GBR Materials: An in Vitro Cone-Beam Computed Tomographic Examination. Clin. Oral. Implants Res. 2016, 27, 258–265. [Google Scholar] [CrossRef]
- Bueno, J.; Sánchez, M.C.; Toledano-Osorio, M.; Figuero, E.; Toledano, M.; Medina-Castillo, A.L.; Osorio, R.; Herrera, D.; Sanz, M. Antimicrobial Effect of Nanostructured Membranes for Guided Tissue Regeneration: An in Vitro Study. Dent. Mater. 2020, 36, 1566–1577. [Google Scholar] [CrossRef]
- Toledano, M.; Gutierrez-Pérez, J.L.; Gutierrez-Corrales, A.; Serrera-Figallo, M.A.; Toledano-Osorio, M.; Rosales-Leal, J.I.; Aguilar, M.; Osorio, R.; Torres-Lagares, D. Novel Non-Resorbable Polymeric-Nanostructured Scaffolds for Guided Bone Regeneration. Clin. Oral. Investig. 2020, 24, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Toledano-Osorio, M.; Osorio, R.; Carrasco-Carmona, Á.; Gutiérrez-Pérez, J.-L.; Gutiérrez-Corrales, A.; Serrera-Figallo, M.-A.; Lynch, C.D.; Torres-Lagares, D. Doxycycline and Zinc Loaded Silica-Nanofibrous Polymers as Biomaterials for Bone Regeneration. Polymers 2020, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef]
- de Souza Araújo, I.J.; Ferreira, J.A.; Daghrery, A.; Ribeiro, J.S.; Castilho, M.; Puppin-Rontani, R.M.; Bottino, M.C. Self-Assembling Peptide-Laden Electrospun Scaffolds for Guided Mineralized Tissue Regeneration. Dent. Mater. 2022, 38, 1749–1762. [Google Scholar] [CrossRef]
- Kouhi, M.; Jayarama Reddy, V.; Fathi, M.; Shamanian, M.; Valipouri, A.; Ramakrishna, S. Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Fibrinogen/Bredigite Nanofibrous Membranes and Their Integration with Osteoblasts for Guided Bone Regeneration. J. Biomed. Mater. Res. A 2019, 107, 1154–1165. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Osorio, R.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C. Doxycycline-Doped Membranes Induced Osteogenic Gene Expression on Osteoblastic Cells. J. Dent. 2021, 109, 103676. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early Osseointegration to Hydrophilic and Hydrophobic Implant Surfaces in Humans. Clin. Oral. Implants Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Terriza, A.; Saffar, J.-L.; Batista-Cruzado, A.; Lynch, C.D.; Sloan, A.J.; Gutiérrez-Pérez, J.-L.; Torres-Lagares, D. Pre-Prosthetic Use of Poly(Lactic-Co-Glycolic Acid) Membranes Treated with Oxygen Plasma and TiO2 Nanocomposite Particles for Guided Bone Regeneration Processes. J. Dent. 2016, 47, 71–79. [Google Scholar] [CrossRef]
- Gosain, A.K.; Song, L.; Yu, P.; Mehrara, B.J.; Maeda, C.Y.; Gold, L.I.; Longaker, M.T. Osteogenesis in Cranial Defects: Reassessment of the Concept of Critical Size and the Expression of TGF-Beta Isoforms. Plast. Reconstr. Surg. 2000, 106, 360–371; discussion 372. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Tang, D.; Liu, X.; Meng, J.; Wei, W.; Gong, R.H.; Li, J. Heterogeneous Porous PLLA/PCL Fibrous Scaffold for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2023, 235, 123781. [Google Scholar] [CrossRef] [PubMed]
- de Silva, L.; Bernal, P.N.; Rosenberg, A.; Malda, J.; Levato, R.; Gawlitta, D. Biofabricating the Vascular Tree in Engineered Bone Tissue. Acta Biomater. 2023, 156, 250–268. [Google Scholar] [CrossRef]
- Alfayez, E.; Veschini, L.; Dettin, M.; Zamuner, A.; Gaetani, M.; Carreca, A.P.; Najman, S.; Ghanaati, S.; Coward, T.; Di Silvio, L. DAR 16-II Primes Endothelial Cells for Angiogenesis Improving Bone Ingrowth in 3D-Printed BCP Scaffolds and Regeneration of Critically Sized Bone Defects. Biomolecules 2022, 12, 1619. [Google Scholar] [CrossRef]
- Turri, A.; Elgali, I.; Vazirisani, F.; Johansson, A.; Emanuelsson, L.; Dahlin, C.; Thomsen, P.; Omar, O. Guided Bone Regeneration Is Promoted by the Molecular Events in the Membrane Compartment. Biomaterials 2016, 84, 167–183. [Google Scholar] [CrossRef]
- Elgali, I.; Turri, A.; Xia, W.; Norlindh, B.; Johansson, A.; Dahlin, C.; Thomsen, P.; Omar, O. Guided Bone Regeneration Using Resorbable Membrane and Different Bone Substitutes: Early Histological and Molecular Events. Acta Biomater. 2016, 29, 409–423. [Google Scholar] [CrossRef]
- Jin, S.; Yang, R.; Hu, C.; Xiao, S.; Zuo, Y.; Man, Y.; Li, Y.; Li, J. Plant-Derived Polyphenol and LL-37 Peptide-Modified Nanofibrous Scaffolds for Promotion of Antibacterial Activity, Anti-Inflammation, and Type-H Vascularized Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 7804–7820. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Zhou, Y. Strontium Functionalized in Biomaterials for Bone Tissue Engineering: A Prominent Role in Osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022, 10, 928799. [Google Scholar] [CrossRef]
- Huang, X.; Lan, Y.; Shen, J.; Chen, Z.; Xie, Z. Extracellular Vesicles in Bone Homeostasis: Emerging Mediators of Osteoimmune Interactions and Promising Therapeutic Targets. Int. J. Biol. Sci. 2022, 18, 4088–4100. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Y.; Luo, J.; Liu, X.; Yang, Q.; Shi, X.; Wang, Y. Black Phosphorus Nanosheets-Enabled DNA Hydrogel Integrating 3D-Printed Scaffold for Promoting Vascularized Bone Regeneration. Bioact. Mater. 2023, 21, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Tsiklin, I.L.; Shabunin, A.V.; Kolsanov, A.V.; Volova, L.T. In Vivo Bone Tissue Engineering Strategies: Advances and Prospects. Polymers 2022, 14, 3222. [Google Scholar] [CrossRef] [PubMed]
- Lyu, R.; Chen, Y.; Shuai, Y.; Wang, J.; Lu, L.; Cheng, Q.; Cai, J.; Mao, C.; Yang, M. Novel Biomaterial-Binding/Osteogenic Bi-Functional Peptide Binds to Silk Fibroin Membranes to Effectively Induce Osteogenesis In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2023, 15, 7673–7685. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Vig, S.; Fernandes, M.H. Bone Cell Exosomes and Emerging Strategies in Bone Engineering. Biomedicines 2022, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Mahajan, A.; Shah, N.; Dadhania, A.P. Preemptive Analgesia in Third Molar Impaction Surgery. Natl. J. Maxillofac. Surg. 2012, 3, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Meza, R.; Lieber, R.L. Skeletal Muscle Fibroblasts in Health and Disease. Differentiation 2016, 92, 108–115. [Google Scholar] [CrossRef]
- Xu, Z.; You, W.; Chen, W.; Zhou, Y.; Nong, Q.; Valencak, T.G.; Wang, Y.; Shan, T. Single-Cell RNA Sequencing and Lipidomics Reveal Cell and Lipid Dynamics of Fat Infiltration in Skeletal Muscle. J. Cachexia Sarcopenia Muscle 2021, 12, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, J.; Li, T.; Sun, X.; Lin, R.; He, Y.; Sun, K.; Han, J.; Yang, G.; Li, X.; et al. CD34+ Cell-Derived Fibroblast-Macrophage Cross-Talk Drives Limb Ischemia Recovery through the OSM-ANGPTL Signaling Axis. Sci. Adv. 2023, 9, eadd2632. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, Definitions, and Functions in Health and Disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Wei, K.; Nguyen, H.N.; Brenner, M.B. Fibroblast Pathology in Inflammatory Diseases. J. Clin. Invest. 2021, 131, e149538. [Google Scholar] [CrossRef]
- Punet, X.; Mauchauffé, R.; Rodríguez-Cabello, J.C.; Alonso, M.; Engel, E.; Mateos-Timoneda, M.A. Biomolecular Functionalization for Enhanced Cell-Material Interactions of Poly(Methyl Methacrylate) Surfaces. Regen. Biomater. 2015, 2, 167–175. [Google Scholar] [CrossRef]
- Osorio, R.; Carrasco-Carmona, Á.; Toledano, M.; Osorio, E.; Medina-Castillo, A.L.; Iskandar, L.; Marques, A.; Deb, S.; Toledano-Osorio, M. Ex Vivo Investigations on Bioinspired Electrospun Membranes as Potential Biomaterials for Bone Regeneration. J. Dent. 2020, 98, 103359. [Google Scholar] [CrossRef]
- Toledano, M.; Carrasco-Carmona, Á.; Medina-Castillo, A.L.; Toledano-Osorio, M.; Osorio, R. Protein Adsorption and Bioactivity of Functionalized Electrospun Membranes for Bone Regeneration. J. Dent. 2020, 102, 103473. [Google Scholar] [CrossRef]
- Toledano, M.; Vallecillo, C.; Serrera-Figallo, M.-A.; Vallecillo-Rivas, M.; Gutierrez-Corrales, A.; Lynch, C.D.; Toledano-Osorio, M. Doped Electrospinned Material-Guides High Efficiency Regional Bone Regeneration. Polymers 2023, 15, 1726. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Alfonso-Rodríguez, C.A.; Osorio, E.; Medina-Castillo, A.L.; Alaminos, M.; Toledano-Osorio, M.; Toledano, M. Novel Potential Scaffold for Periodontal Tissue Engineering. Clin. Oral. Investig. 2017, 21, 2695–2707. [Google Scholar] [CrossRef]
- Ma, S.-F.; Chen, Y.-J.; Zhang, J.-X.; Shen, L.; Wang, R.; Zhou, J.-S.; Hu, J.-G.; Lü, H.-Z. Adoptive Transfer of M2 Macrophages Promotes Locomotor Recovery in Adult Rats after Spinal Cord Injury. Brain Behav. Immun. 2015, 45, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Buchacher, T.; Ohradanova-Repic, A.; Stockinger, H.; Fischer, M.B.; Weber, V. M2 Polarization of Human Macrophages Favors Survival of the Intracellular Pathogen Chlamydia Pneumoniae. PLoS ONE 2015, 10, e0143593. [Google Scholar] [CrossRef]
- Turri, A.; Omar, O.; Trobos, M.; Thomsen, P.; Dahlin, C. Modulation of Gene Expression and Bone Formation by Expanded and Dense Polytetrafluoroethylene Membranes during Guided Bone Regeneration: An Experimental Study. Clin. Implant. Dent. Relat. Res. 2023, 26, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-H.; Guo, Y.; Zhu, J.-Y.; Tang, C.-Y.; Zhao, Y.-Q.; Zhou, H.-D. Spheroid Co-Culture of BMSCs with Osteocytes Yields Ring-Shaped Bone-like Tissue That Enhances Alveolar Bone Regeneration. Sci. Rep. 2022, 12, 14636. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface Treatments of Titanium Dental Implants for Rapid Osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific Evidence on the Links between Periodontal Diseases and Diabetes: Consensus Report and Guidelines of the Joint Workshop on Periodontal Diseases and Diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes-Different Cell Effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological Principle and Therapeutic Applications. Clin. Oral. Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.; Khalid, A.W.; Pae, H.-C.; Cha, J.-K.; Lee, J.-S.; Paik, J.-W.; Jung, U.-W.; Choi, S.-H. Distinctive Bone Regeneration of Calvarial Defects Using Biphasic Calcium Phosphate Supplemented Ultraviolet-Crosslinked Collagen Membrane. J. Periodontal Implant. Sci. 2020, 50, 14–27. [Google Scholar] [CrossRef]
- Kameo, Y.; Ozasa, M.; Adachi, T. Computational Framework for Analyzing Flow-Induced Strain on Osteocyte as Modulated by Microenvironment. J. Mech. Behav. Biomed. Mater. 2022, 126, 105027. [Google Scholar] [CrossRef]
- Parmentier, L.; Riffault, M.; Hoey, D.A. Utilizing Osteocyte Derived Factors to Enhance Cell Viability and Osteogenic Matrix Deposition within IPN Hydrogels. Materials 2020, 13, 1690. [Google Scholar] [CrossRef]
- Schaller, B.; Fujioka-Kobayashi, M.; Zihlmann, C.; Schuler, V.C.; Katagiri, H.; Lang, N.P.; Saulacic, N. Effects of Additional Collagen in Biphasic Calcium Phosphates: A Study in a Rabbit Calvaria. Clin. Oral. Investig. 2020, 24, 3093–3103. [Google Scholar] [CrossRef]
- Agarwal, A.; Bhattacharya, H.S.; Srikanth, G.; Singh, A. Comparative Evaluation of Decalcified Freeze Dried Bone Allograft with and without Local Doxycycline in Non-Contained Human Periodontal Infrabony Defects. J. Indian. Soc. Periodontol. 2013, 17, 490–494. [Google Scholar] [CrossRef]
- Su, N.; Villicana, C.; Barati, D.; Freeman, P.; Luo, Y.; Yang, F. Stem Cell Membrane-Coated Microribbon Scaffolds Induce Regenerative Innate and Adaptive Immune Responses in a Critical-Size Cranial Bone Defect Model. Adv. Mater. 2023, 35, e2208781. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.D.; Peres, B.U.; Maezono, H.; Shen, Y.; Haapasalo, M.; Jackson, J.; Carvalho, R.M.; Manso, A.P. Doxycycline Release and Antibacterial Activity from PMMA/PEO Electrospun Fiber Mats. J. Appl. Oral Sci. 2019, 27, e20180663. [Google Scholar] [CrossRef]
- Doyle, M.E.; Dalgarno, K.; Masoero, E.; Ferreira, A.M. Advances in Biomimetic Collagen Mineralisation and Future Approaches to Bone Tissue Engineering. Biopolymers 2023, 114, e23527. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; López-Otín, C. Strategies for MMP Inhibition in Cancer: Innovations for the Post-Trial Era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward Guided Tissue and Bone Regeneration: Morphology, Attachment, Proliferation, and Migration of Cells Cultured on Collagen Barrier Membranes. A Systematic Review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Mukundan, S.; Karaca, E.; Dolatshahi-Pirouz, A.; Patel, A.; Rangarajan, K.; Mihaila, S.M.; Iviglia, G.; Zhang, H.; Khademhosseini, A. Nanoclay-Enriched Poly(ε-Caprolactone) Electrospun Scaffolds for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2014, 20, 2088–2101. [Google Scholar] [CrossRef]
- Saintigny, G.; Bonnard, M.; Damour, O.; Collombel, C. Reconstruction of Epidermis on a Chitosan Cross-Linked Collagen-GAG Lattice: Effect of Fibroblasts. Acta Derm. Venereol. 1993, 73, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, M.; Zhu, Y.; Wang, L.; Tomsia, A.P.; Mao, C. Phage Nanofibers Induce Vascularized Osteogenesis in 3D Printed Bone Scaffolds. Adv. Mater. 2014, 26, 4961–4966. [Google Scholar] [CrossRef]
- Zhai, Y.; Schilling, K.; Wang, T.; El Khatib, M.; Vinogradov, S.; Brown, E.B.; Zhang, X. Spatiotemporal Blood Vessel Specification at the Osteogenesis and Angiogenesis Interface of Biomimetic Nanofiber-Enabled Bone Tissue Engineering. Biomaterials 2021, 276, 121041. [Google Scholar] [CrossRef]
- Spiller, K.L.; Koh, T.J. Macrophage-Based Therapeutic Strategies in Regenerative Medicine. Adv. Drug Deliv. Rev. 2017, 122, 74–83. [Google Scholar] [CrossRef]
- Hozain, S.; Cottrell, J. CDllb+ Targeted Depletion of Macrophages Negatively Affects Bone Fracture Healing. Bone 2020, 138, 115479. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Liu, L.; Wang, Y.; Ju, Y.; Zeng, C.; Lu, Z.; Xie, D.; Guo, J. Zinc-Based Tannin-Modified Composite Microparticulate Scaffolds with Balanced Antimicrobial Activity and Osteogenesis for Infected Bone Defect Repair. Adv. Healthc. Mater. 2023, 12, e2300303. [Google Scholar] [CrossRef] [PubMed]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A Critical Review of Multifunctional Titanium Surfaces: New Frontiers for Improving Osseointegration and Host Response, Avoiding Bacteria Contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Morón-Calvente, V.; Romero-Pinedo, S.; Toribio-Castelló, S.; Plaza-Díaz, J.; Abadía-Molina, A.C.; Rojas-Barros, D.I.; Beug, S.T.; LaCasse, E.C.; MacKenzie, A.; Korneluk, R.; et al. Inhibitor of Apoptosis Proteins, NAIP, cIAP1 and cIAP2 Expression during Macrophage Differentiation and M1/M2 Polarization. PLoS ONE 2018, 13, e0193643. [Google Scholar] [CrossRef] [PubMed]
- Galhano, G.Á.P.; Pellizzer, E.P.; Mazaro, J.V.Q. Optical Impression Systems for CAD-CAM Restorations. J. Craniofac. Surg. 2012, 23, e575–e579. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liang, Y.; Liu, X. Biomaterials for Periodontal Regeneration. Dent. Clin. N. Am. 2022, 66, 659–672. [Google Scholar] [CrossRef] [PubMed]
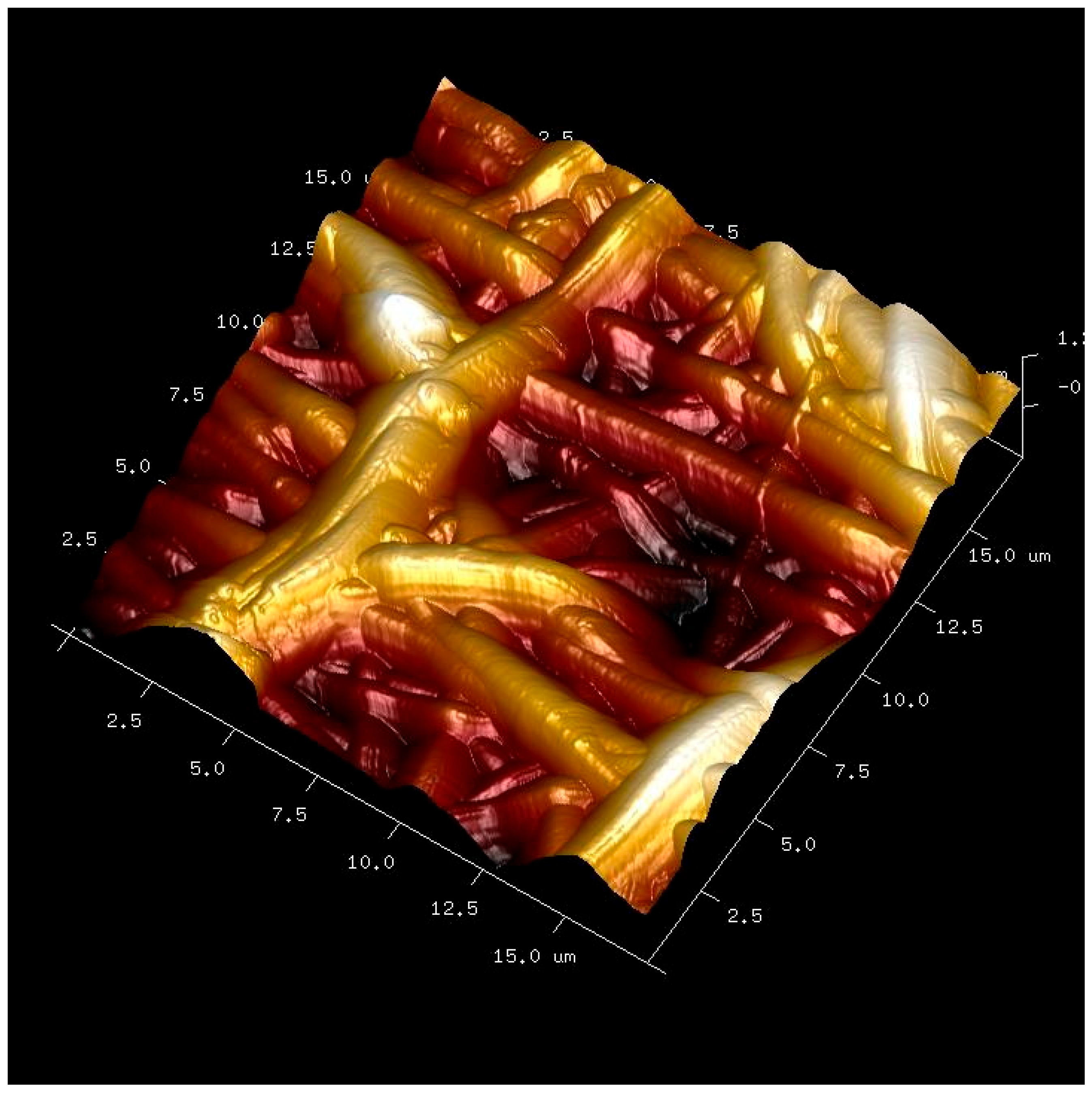
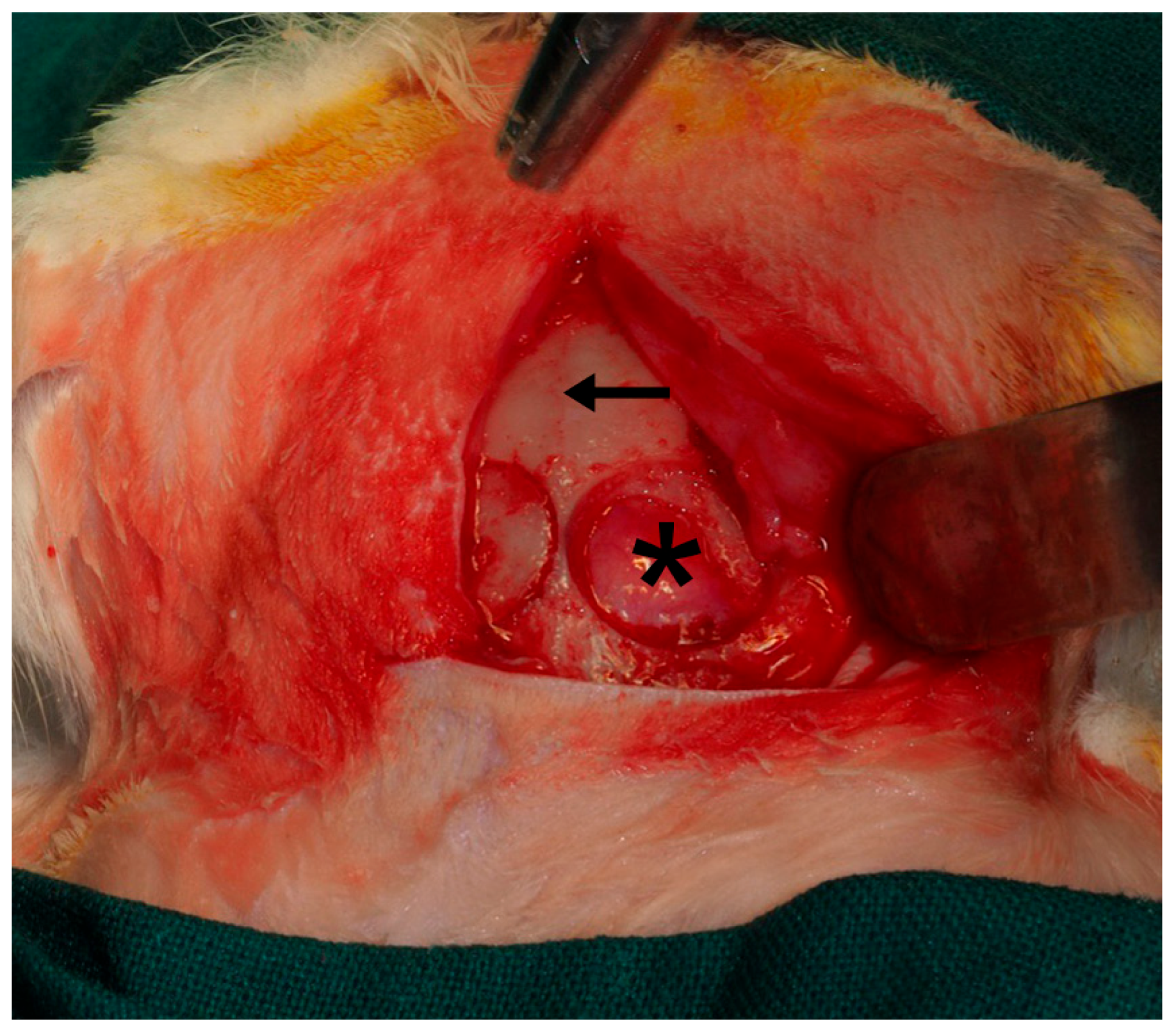


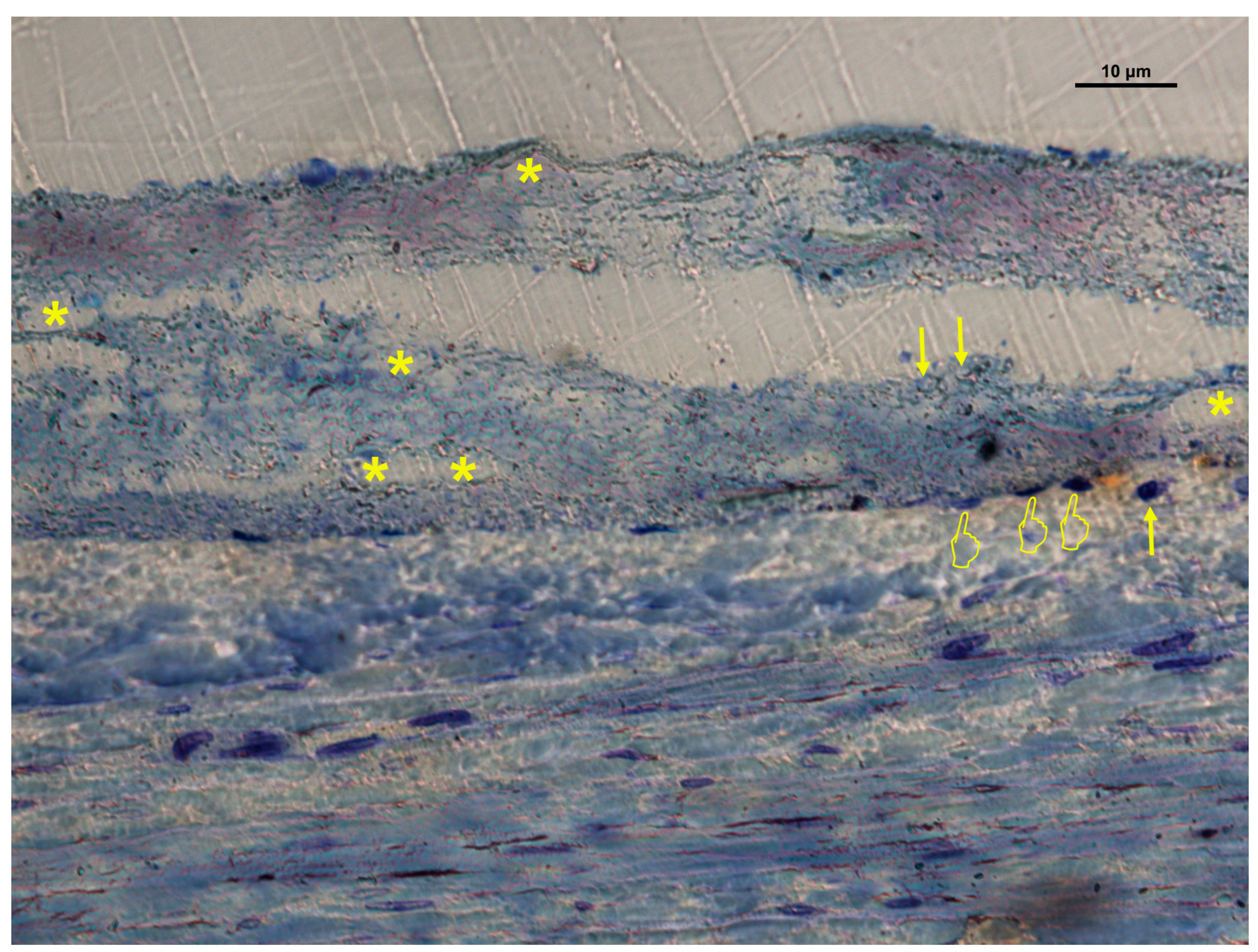
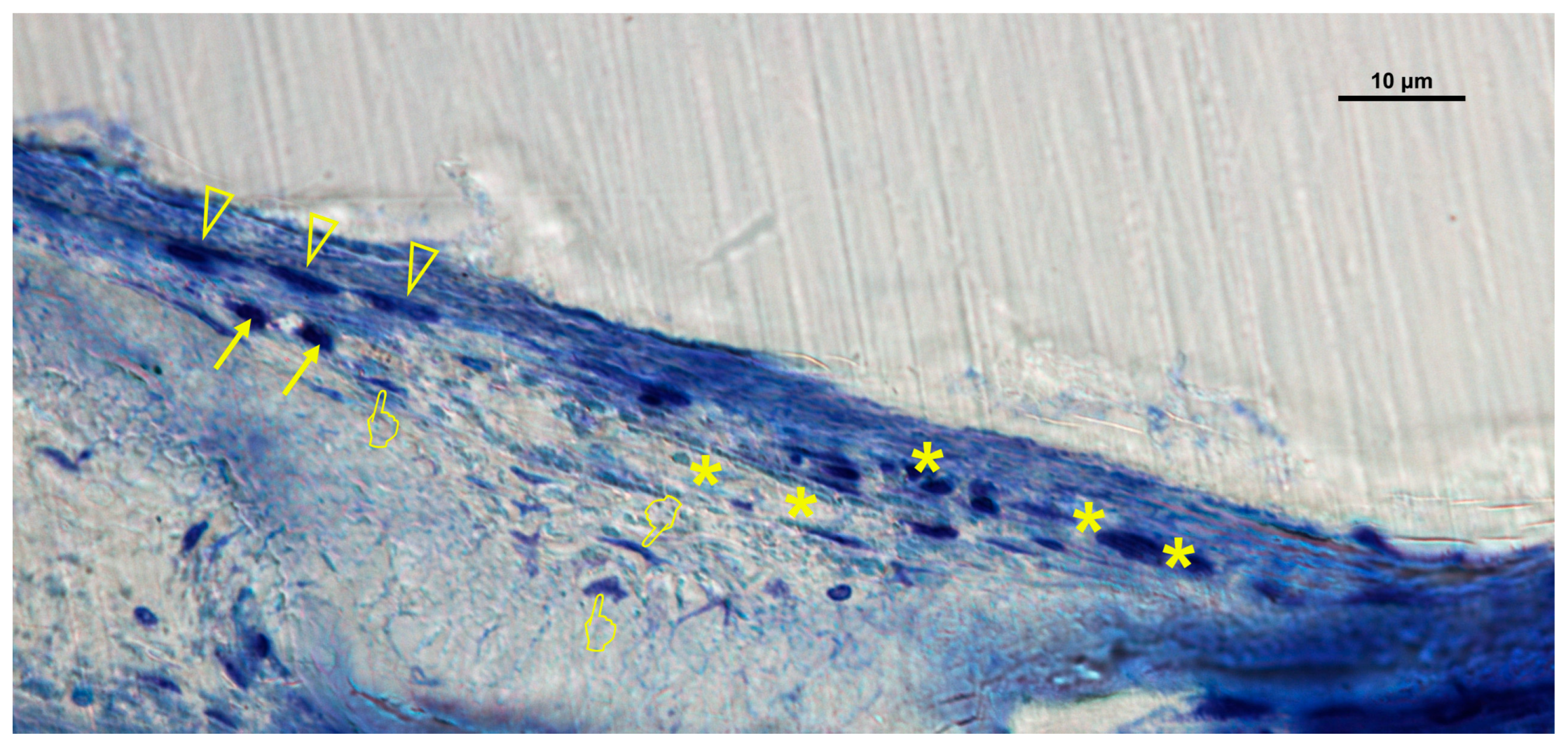


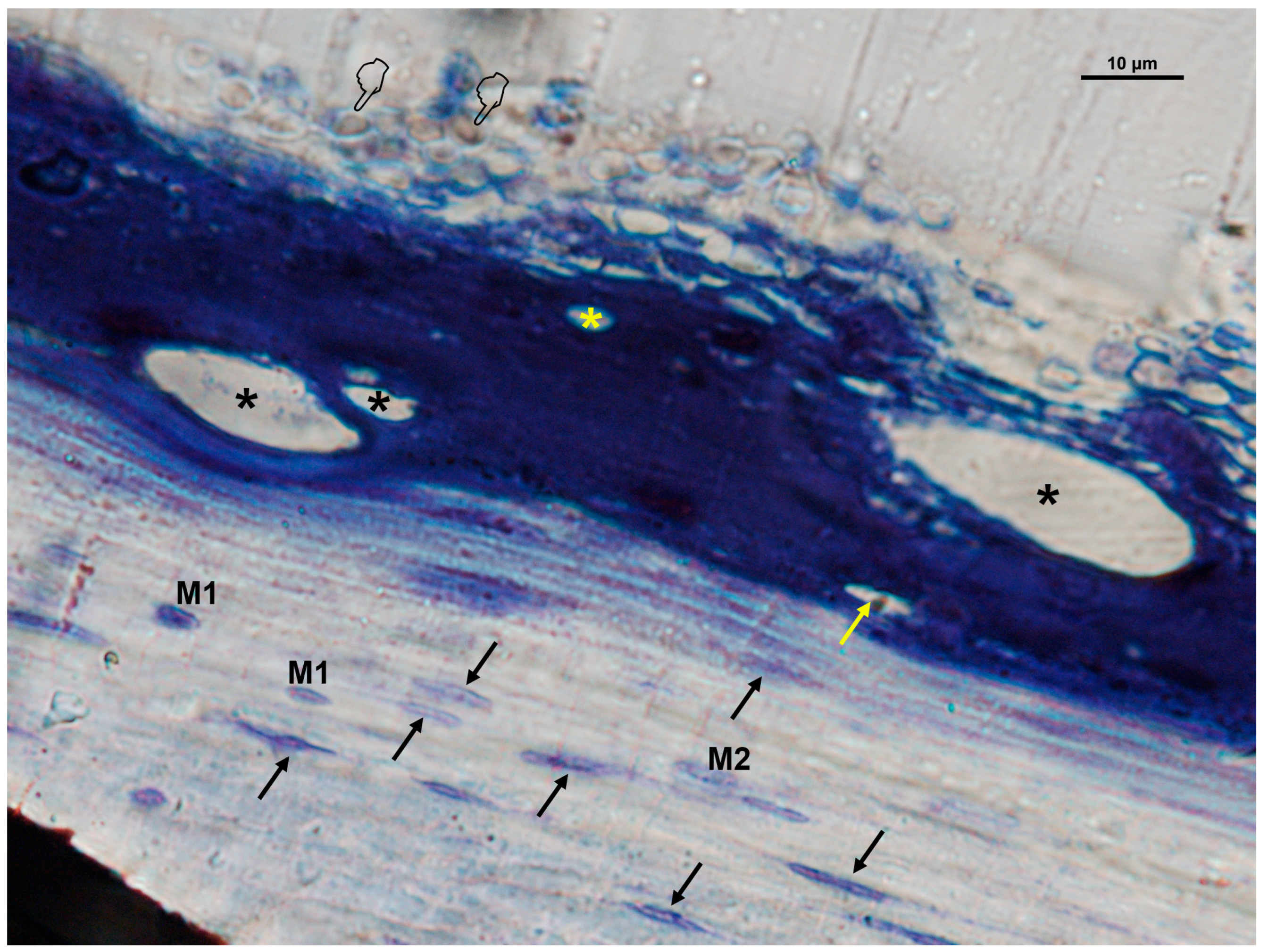
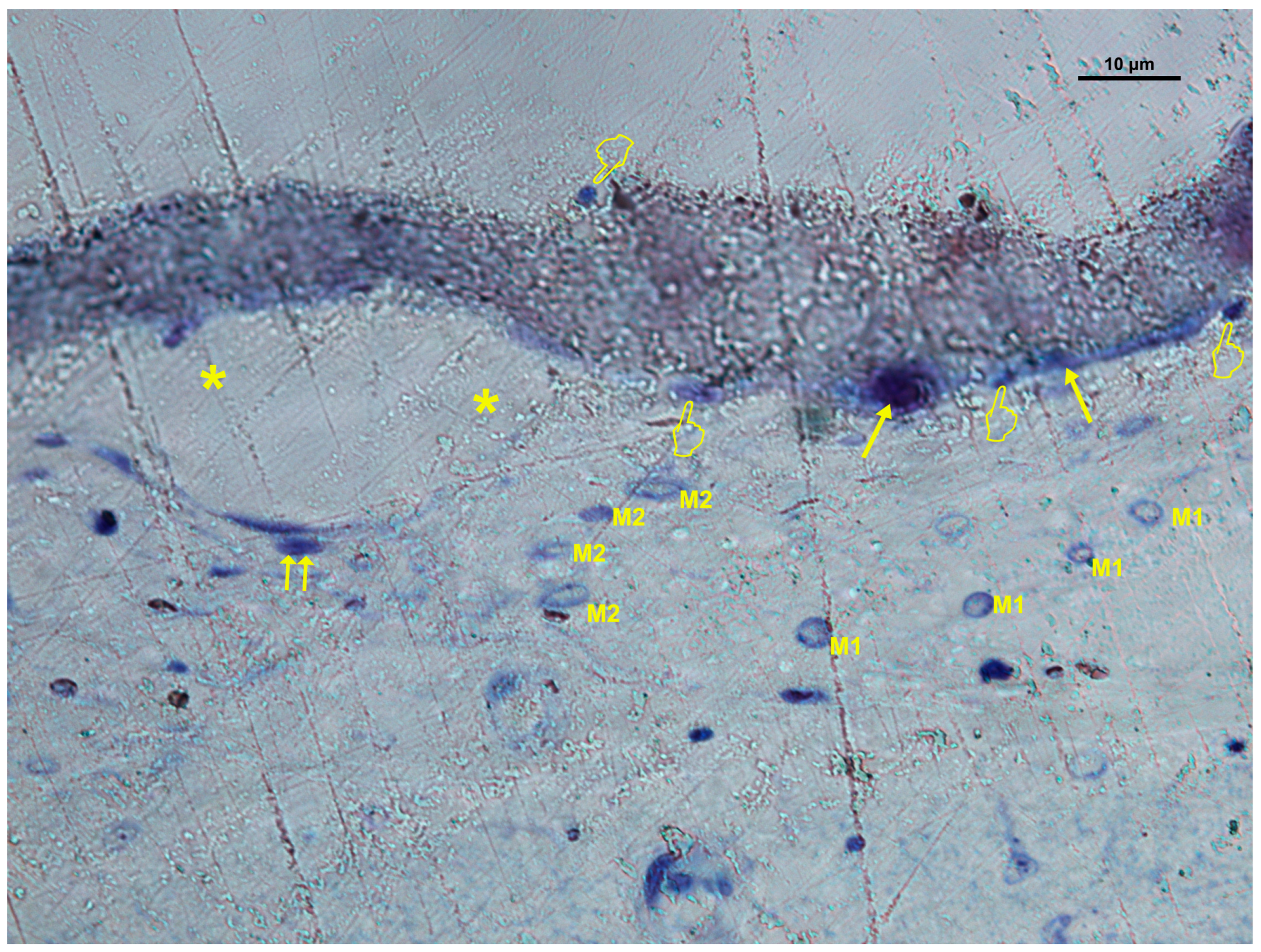
| (A) | ||||||||
| Osteocytes | Osteoblast | Osteoclast | M1 | M2 | M1/M2 | Fibroblast | Blood Vessel | |
| Over | 0.003 ± 0.003 A1 | 0.324 ± 0.064 A1 | 0.014 ± 0.012 A1 | 0.600 ± 0.146 A1 | 0.487 ± 0.129 A1 | 1.134 ± 0.064 A1 | 0.100 ± 0.045 A1 | 0.00 ± 0.000 A1 |
| Inner | 0.000 ± 0.000 A1 | 1.072 ± 0.106 B1 | 0.066 ± 0.033 AB1 | 0.752 ± 0.114 A1 | 0.435 ± 0.075 A1 | 1.321 ± 0.078 AB1 | 1.517 ± 0.125 B1 | 0.041 ± 0.014 A1 |
| Under | 0.041 ± 0.041 A1 | 2.462 ± 0.180 C1 | 0.183 ± 0.072 B1 | 1.686 ± 0.190 B1 | 1.170 ± 0.156 B1 | 1.453 ± 0.102 B1 | 1.541 ± 0.138 B1 | 0.121 ± 0.026 B1 |
| p | 0.400 | 0.00 | 0.030 | 0.000 | 0.000 | 0.024 | 0.000 | 0.000 |
| (B) | ||||||||
| Osteocytes | Osteoblast | Osteoclast | M1 | M2 | M1/M2 | Fibroblast | Blood Vessel | |
| Over | 0.000 ± 0.000 A1 | 0.596 ± 0.120 A2 | 0.070 ± 0.042 A2 | 1.270 ± 0.218 A1 | 0.908 ± 0.198 A1 | 1.487 ± 0.142 A2 | 0.258 ± 0.063 A2 | 0.000 ± 0.000 A1 |
| Inner | 0.058 ± 0.058 A1 | 1.723 ± 0.183 B2 | 0.212 ± 0.130 A1 | 0.835 ± 0.176 A2 | 0.362 ± 0.096 B2 | 1.425 ± 0.114 A1 | 1.765 ± 0.150 B1 | 0.038 ± 0.14 AB1 |
| Under | 0.050 ± 0.050 A1 | 3.323 ± 0.227 C2 | 0.112 ± 0.035 A1 | 1.942 ± 0.256 B2 | 1.350 ± 0.209 C1 | 1.507 ± 0.128 A1 | 1.439 ± 0.142 B1 | 0.073 ± 0.021 B2 |
| p | 0.604 | 0.000 | 0.445 | 0.002 | 0.000 | 0.895 | 0.000 | 0.002 |
| (C) | ||||||||
| Osteocytes | Osteoblast | Osteoclast | M1 | M2 | M1/M2 | Fibroblast | Blood Vessel | |
| Over | 0.000 ± 0.000 A1 | 0.247 ± 0.051 A1 | 0.000 ± 0.000 A3 | 0.306 ± 0.089 A1 | 0.133 ± 0.058 A1 | 1.123 ± 0.046 A1 | 0.068 ± 0.029 A1 | 0.003 ± 0.003 A1 |
| Inner | 0.033 ± 0.033 A1 | 1.117 ± 0.114 B1 | 0.000 ± 0.000 A2 | 0.656 ± 0.123 A1 | 0.412 ± 0.093 A1 | 1.307 ± 0.083 A1 | 1.428 ± 0.118 B1 | 0.041 ± 0.012 B1 |
| Under | 0.008 ± 0.008 A1 | 2.491 ± 0.161 C1 | 0.041 ± 0.028 A2 | 1.344 ± 0181 B3 | 1.092 ± 0.157 B1 | 1.269 ± 0.042 A2 | 2.726 ± 0.199 C2 | 0.038 ± 0.012 B3 |
| p | 0.466 | 0.000 | 0.122 | 0.000 | 0.000 | 0.164 | 0.000 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledano, M.; Vallecillo-Rivas, M.; Serrera-Figallo, M.-A.; Gutierrez-Corrales, A.; Lynch, C.D.; Torres-Lagares, D.; Vallecillo, C. Bio-Membrane-Based Nanofiber Scaffolds: Targeted and Controlled Carriers for Drug Delivery—An Experimental In Vivo Study. Biomimetics 2025, 10, 726. https://doi.org/10.3390/biomimetics10110726
Toledano M, Vallecillo-Rivas M, Serrera-Figallo M-A, Gutierrez-Corrales A, Lynch CD, Torres-Lagares D, Vallecillo C. Bio-Membrane-Based Nanofiber Scaffolds: Targeted and Controlled Carriers for Drug Delivery—An Experimental In Vivo Study. Biomimetics. 2025; 10(11):726. https://doi.org/10.3390/biomimetics10110726
Chicago/Turabian StyleToledano, Manuel, Marta Vallecillo-Rivas, María-Angeles Serrera-Figallo, Aida Gutierrez-Corrales, Christopher D. Lynch, Daniel Torres-Lagares, and Cristina Vallecillo. 2025. "Bio-Membrane-Based Nanofiber Scaffolds: Targeted and Controlled Carriers for Drug Delivery—An Experimental In Vivo Study" Biomimetics 10, no. 11: 726. https://doi.org/10.3390/biomimetics10110726
APA StyleToledano, M., Vallecillo-Rivas, M., Serrera-Figallo, M.-A., Gutierrez-Corrales, A., Lynch, C. D., Torres-Lagares, D., & Vallecillo, C. (2025). Bio-Membrane-Based Nanofiber Scaffolds: Targeted and Controlled Carriers for Drug Delivery—An Experimental In Vivo Study. Biomimetics, 10(11), 726. https://doi.org/10.3390/biomimetics10110726











