Machine Learning-Driven Prediction of Reactive Oxygen Species Dynamics for Assessing Nanomaterials’ Cytotoxicity
Abstract
1. Introduction
2. Literature Survey
3. Machine Learning Aids in the Investigation of ROS Induction in Primary Types of NMs
3.1. Metal Oxide (MeOx) NMs
3.2. Metal-Based NMs
3.3. Carbon Nanomaterials (CNMs)
3.4. Mixed Types of NMs
4. Machine Learning Aids in the Investigation of ROS Scavenging in Primary Types of NMs
5. Discussion
5.1. Data Deficiency and Lack of Standardized, Curated Datasets
5.2. Algorithm Enhancement
5.3. Translational Barriers to Real-World Application
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| R2 | coefficient of determination |
| MSE | mean squared error |
| RMSE | root mean square error |
| MAE | mean absolute error |
| MC-PLS | Monte Carlo partial least squares |
| PLSR | partial least squares regression |
| PLS | partial least squares |
| RF | random forest |
| DT | decision tree |
| GB | gradient boosting |
| SVM | support vector machine |
| NB | naive bayes |
| ETs | extremely random trees |
| EN | elastic net |
| MLP | multilayer perceptron |
| ANN | artificial neural network |
| LR | linear regression |
| KNN | k-nearest neighbor |
| SVR | support vector regressor |
| XGB | extreme gradient boosting |
| LightGBM | light gradient boosting machine |
| AdaBoost | adaptive boosting |
| CNN | convolutional neural networks |
| NEM | neural network models |
| TC | texture coefficient |
| BG | bandgap |
| SSA | specific surface area |
| fP | zeta potential |
| MW | molecular weight |
| TPSA | topological polar surface area |
Appendix A
| RF | GB | ANN | DT | KNN | SVM | PLS | ETs | LR | NB | Adaboost | Logistic Regression | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 14 | 11 | 9 | 8 | 8 | 5 | 5 | 4 | 3 | 3 | 2 | 7 |
| Dosage | Exposure Regime | Cell/Tissue Type | Experiment Method | Plant Extracts for Synthesis |
|---|---|---|---|---|
| 9 | 4 | 3 | 2 | 1 |
References
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1689–1694. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-Based Nanomaterials for Energy Applications. Small 2017, 13, 1702240. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Env. Health Perspect 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Society, R. Nanoscience and Nanotechnologies: Opportunities and Uncertainties: Summary and Recomendations; Royal Society: London, UK, 2004. [Google Scholar]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Makhdoumi, P.; Karimi, H.; Khazaei, M. Review on Metal-Based Nanoparticles: Role of Reactive Oxygen Species in Renal Toxicity. Chem. Res. Toxicol. 2020, 33, 2503–2514. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Achawi, S.; Feneon, B.; Pourchez, J.; Forest, V. Assessing biological oxidative damage induced by graphene-based materials: An asset for grouping approaches using the FRAS assay. Regul. Toxicol. Pharmacol. 2021, 127, 105067. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-J.; Muneeswaran, T.; Choi, J.S.; Kim, S.; Han, J.H.; Cho, W.-S.; Park, J.-W. Modified toxic potential of multi-walled carbon nanotubes to zebrafish (Danio rerio) following a two-year incubation in water. J. Hazard. Mater. 2024, 462, 132763. [Google Scholar] [CrossRef] [PubMed]
- Pikula, K.; Johari, S.A.; Santos-Oliveira, R.; Golokhvast, K. Toxicity and Biotransformation of Carbon-Based Nanomaterials in Marine Microalgae Heterosigma akashiwo. Int. J. Mol. Sci. 2023, 24, 10020. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Zhu, X.; Hondroulis, E.; Liu, W.; Li, C.-z. Biosensing Approaches for Rapid Genotoxicity and Cytotoxicity Assays upon Nanomaterial Exposure. Small 2013, 9, 1821–1830. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ Res 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.-F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew. Chem. 2017, 129, 14455–14459. [Google Scholar] [CrossRef]
- Moglianetti, M.; De Luca, E.; Pedone, D.; Marotta, R.; Catelani, T.; Sartori, B.; Amenitsch, H.; Retta, S.F.; Pompa, P.P. Platinum nanozymes recover cellular ROS homeostasis in an oxidative stress-mediated disease model. Nanoscale 2016, 8, 3739–3752. [Google Scholar] [CrossRef]
- Liao, S.; Liu, G.; Tan, B.; Qi, M.; Li, J.; Li, X.; Zhu, C.; Huang, J.; Yin, Y.; Tang, Y. Fullerene C60 protects against intestinal injury from deoxynivalenol toxicity by improving antioxidant capacity. Life 2021, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Meng, L.; Xu, L.; Bai, R.; Du, J.; Zhang, L.; Li, Y.; Chang, Y.; Zhao, Y.; Chen, C. Acute pulmonary and moderate cardiovascular responses of spontaneously hypertensive rats after exposure to single-wall carbon nanotubes. Nanotoxicology 2012, 6, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.-D.; Zhou, D.-M.; Dionysiou, D.D. Superoxide mediated production of hydroxyl radicals by magnetite nanoparticles: Demonstration in the degradation of 2-chlorobiphenyl. J. Hazard. Mater. 2013, 250, 68–75. [Google Scholar] [CrossRef]
- Choi, A.O.; Cho, S.J.; Desbarats, J.; Lovrić, J.; Maysinger, D. Quantum dot-induced cell death involves Fas upregulation and lipid peroxidation in human neuroblastoma cells. J. Nanobiotechnol. 2007, 5, 1. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Han, S.; Zheng, P.; Chen, Z.; Jia, G. Titanium dioxide nanoparticles induced reactive oxygen species (ROS) related changes of metabolomics signatures in human normal bronchial epithelial (BEAS-2B) cells. Toxicol. Appl. Pharmacol. 2022, 444, 116020. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Hsieh, S.-F.; Bello, D.; Schmidt, D.F.; Pal, A.K.; Stella, A.; Isaacs, J.A.; Rogers, E.J. Mapping the Biological Oxidative Damage of Engineered Nanomaterials. Small 2013, 9, 1853–1865. [Google Scholar] [CrossRef]
- Barnard, A.S. How can ab initio simulations address risks in nanotech? Nat. Nanotechnol. 2009, 4, 332–335. [Google Scholar] [CrossRef]
- Singh, A.V.; Rosenkranz, D.; Ansari, M.H.D.; Singh, R.; Kanase, A.; Singh, S.P.; Johnston, B.; Tentschert, J.; Laux, P.; Luch, A. Artificial Intelligence and Machine Learning Empower Advanced Biomedical Material Design to Toxicity Prediction. Adv. Intell. Syst. 2020, 2, 2000084. [Google Scholar] [CrossRef]
- Braham, E.J.; Cho, J.; Forlano, K.M.; Watson, D.F.; Arròyave, R.; Banerjee, S. Machine Learning-Directed Navigation of Synthetic Design Space: A Statistical Learning Approach to Controlling the Synthesis of Perovskite Halide Nanoplatelets in the Quantum-Confined Regime. Chem. Mater. 2019, 31, 3281–3292. [Google Scholar] [CrossRef]
- Epps, R.W.; Bowen, M.S.; Volk, A.A.; Abdel-Latif, K.; Han, S.; Reyes, K.G.; Amassian, A.; Abolhasani, M. Artificial Chemist: An Autonomous Quantum Dot Synthesis Bot. Adv. Mater. 2020, 32, 2001626. [Google Scholar] [PubMed]
- Wang, R.; Liu, C.; Wei, Y.; Wu, P.; Su, Y.; Zhang, Z. Inverse design of metal nanoparticles based on deep learning. Results Opt. 2021, 5, 100134. [Google Scholar] [CrossRef]
- He, J.; He, C.; Zheng, C.; Wang, Q.; Ye, J. Plasmonic nanoparticle simulations and inverse design using machine learning. Nanoscale 2019, 11, 17444–17459. [Google Scholar] [CrossRef]
- Li, S.; Barnard, A.S. Inverse Design of Nanoparticles Using Multi-Target Machine Learning. Adv. Theory Simul. 2022, 5, 2100414. [Google Scholar] [CrossRef]
- Gousiadou, C.; Marchese Robinson, R.; Kotzabasaki, M.; Doganis, P.; Wilkins, T.; Jia, X.; Sarimveis, H.; Harper, S. Machine learning predictions of concentration-specific aggregate hazard scores of inorganic nanomaterials in embryonic zebrafish. Nanotoxicology 2021, 15, 446–476. [Google Scholar] [CrossRef]
- Feng, Y.; Tang, W.; Zhang, Y.; Zhang, T.; Shang, Y.; Chi, Q.; Chen, Q.; Lei, Q. Machine learning and microstructure design of polymer nanocomposites for energy storage application. High Volt. 2022, 7, 242–250. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Cao, J.; Wei, X.; Li, Y.; Wang, Z.; Cai, X.; Li, R.; Chen, J. Use of dissociation degree in lysosomes to predict metal oxide nanoparticle toxicity in immune cells: Machine learning boosts nano-safety assessment. Environ. Int. 2022, 164, 107258. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, Y.; Zhang, H.; Li, X.; Li, F. Machine learning models for quantitatively prediction of toxicity in macrophages induced by metal oxide nanoparticles. Chemosphere 2025, 370, 143923. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, H.; Yang, L. Machine Learning-Driven Multidomain Nanomaterial Design: From Bibliometric Analysis to Applications. ACS Appl. Nano Mater. 2024, 7, 26579–26600. [Google Scholar] [CrossRef]
- Gao, X.J.; Ciura, K.; Ma, Y.; Mikolajczyk, A.; Jagiello, K.; Wan, Y.; Gao, Y.; Zheng, J.; Zhong, S.; Puzyn, T. Toward the integration of machine learning and molecular modeling for designing drug delivery nanocarriers. Adv. Mater. 2024, 36, 2407793. [Google Scholar] [CrossRef]
- He, Y.; Liu, F.; Min, W.; Liu, G.; Wu, Y.; Wang, Y.; Yan, X.; Yan, B. De novo design of biocompatible nanomaterials using quasi-SMILES and recurrent neural networks. ACS Appl. Mater. Interfaces 2024, 16, 66367–66376. [Google Scholar] [CrossRef]
- Hosni, Z.; Achour, S.; Saadi, F.; Chen, Y.; Al Qaraghuli, M. Machine learning-driven nanoparticle toxicity. Ecotoxicol. Environ. Saf. 2025, 299, 118340. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Xu, S.; Zheng, H.; Zhang, L.; Chen, J.; Hong, H.; Kusko, R.; Li, R. Quantitative Structure–Activity Relationship Models for Predicting Inflammatory Potential of Metal Oxide Nanoparticles. Environ. Health Perspect. 2020, 128, 067010. [Google Scholar] [CrossRef]
- Ji, Z.; Guo, W.; Sakkiah, S.; Liu, J.; Patterson, T.A.; Hong, H. Nanomaterial Databases: Data Sources for Promoting Design and Risk Assessment of Nanomaterials. Nanomaterials 2021, 11, 1599. [Google Scholar] [CrossRef]
- Sizochenko, N.; Leszczynski, J. Review of Current and Emerging Approaches for Quantitative Nanostructure-Activity Relationship Modeling: The Case of Inorganic Nanoparticles. J. Nanotoxicol. Nanomed. 2016, 1, 18. [Google Scholar] [CrossRef]
- Tantra, R.; Oksel, C.; Puzyn, T.; Wang, J.; Robinson, K.N.; Wang, X.Z.; Ma, C.Y.; Wilkins, T. Nano(Q)SAR: Challenges, pitfalls and perspectives. Nanotoxicology 2015, 9, 636–642. [Google Scholar] [CrossRef]
- Pang, Y.; Li, R.; Zhang, Z.; Ying, J.; Li, M.; Li, F.; Zhang, T. Based on the Nano-QSAR model: Prediction of factors influencing damage to C. elegans caused by metal oxide nanomaterials and validation of toxic effects. Nano Today 2023, 52, 101967. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Teng, Y.; Ji, C.; Wu, H. Characterization of oxidative damage induced by nanoparticles via mechanism-driven machine learning approaches. Sci. Total Environ. 2023, 871, 162103. [Google Scholar] [CrossRef]
- Sang, L.; Wang, Y.; Zong, C.; Wang, P.; Zhang, H.; Guo, D.; Yuan, B.; Pan, Y. Machine Learning for Evaluating the Cytotoxicity of Mixtures of Nano-TiO2 and Heavy Metals: QSAR Model Apply Random Forest Algorithm after Clustering Analysis. Molecules 2022, 27, 6125. [Google Scholar] [CrossRef]
- Le, T.C.; Yin, H.; Chen, R.; Chen, Y.; Zhao, L.; Casey, P.S.; Chen, C.; Winkler, D.A. An Experimental and Computational Approach to the Development of ZnO Nanoparticles that are Safe by Design. Small 2016, 12, 3568–3577. [Google Scholar] [CrossRef]
- Nedkyalkova, M.; Vasighi, M.; Lattuada, M. Integrating surface chemistry properties and machine learning to map the toxicity landscape of superparamagnetic iron oxide nanoparticles. Chemosphere 2025, 378, 144381. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Mane, R.S.; Min, B.K.; Hwang, Y.J.; Joo, O.-s. D-sorbitol-induced phase control of TiO2 nanoparticles and its application for dye-sensitized solar cells. Sci. Rep. 2016, 6, 20103. [Google Scholar] [CrossRef]
- Banerjee, K.; Das, S.; Choudhury, P.; Ghosh, S.; Baral, R.; Choudhuri, S.K. A novel approach of synthesizing and evaluating the anticancer potential of silver oxide nanoparticles in vitro. Chemotherapy 2017, 62, 279–289. [Google Scholar] [CrossRef]
- Yan, X.; Sedykh, A.; Wang, W.; Zhao, X.; Yan, B.; Zhu, H. In silico profiling nanoparticles: Predictive nanomodeling using universal nanodescriptors and various machine learning approaches. Nanoscale 2019, 11, 8352–8362. [Google Scholar] [CrossRef]
- Wang, W.; Sedykh, A.; Sun, H.; Zhao, L.; Russo, D.P.; Zhou, H.; Yan, B.; Zhu, H. Predicting Nano–Bio Interactions by Integrating Nanoparticle Libraries and Quantitative Nanostructure Activity Relationship Modeling. ACS Nano 2017, 11, 12641–12649. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Cao, L.; Xiong, Z.; Tang, Y.; Pan, Y. Cytotoxicity of phytosynthesized silver nanoparticles: A meta-analysis by machine learning algorithms. Sustain. Chem. Pharm. 2021, 21, 100425. [Google Scholar] [CrossRef]
- Marchwiany, M.E.; Birowska, M.; Popielski, M.; Majewski, J.A.; Jastrzębska, A.M. Surface-Related Features Responsible for Cytotoxic Behavior of MXenes Layered Materials Predicted with Machine Learning Approach. Materials 2020, 13, 3083. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Tepliakov, N.V.; Kundelev, E.V.; Khavlyuk, P.D.; Xiong, Y.; Leonov, M.Y.; Zhu, W.; Baranov, A.V.; Fedorov, A.V.; Rogach, A.L.; Rukhlenko, I.D. sp2–sp3-Hybridized Atomic Domains Determine Optical Features of Carbon Dots. ACS Nano 2019, 13, 10737–10744. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Yin, Z.; Jia, Z.; Wei, J. Carbon Nanodots Derived from Urea and Citric Acid in Living Cells: Cellular Uptake and Antioxidation Effect. Langmuir 2020, 36, 8632–8640. [Google Scholar] [CrossRef]
- Ji, Z.; Arvapalli, D.M.; Zhang, W.; Yin, Z.; Wei, J. Nitrogen and sulfur co-doped carbon nanodots in living EA.hy926 and A549 cells: Oxidative stress effect and mitochondria targeting. J. Mater. Sci. 2020, 55, 6093–6104. [Google Scholar] [CrossRef]
- Awere, C.O.; Sneha, A.; Rakkammal, K.; Muthui, M.M.; Govindan, S.; Çolak, A.B.; Bayrak, M.; Muthuramalingam, P.; Anadebe, V.C.; Archana, P. Carbon dot unravels accumulation of triterpenoid in Evolvulus alsinoides hairy roots culture by stimulating growth, redox reactions and ANN machine learning model prediction of metabolic stress response. Plant Physiol. Biochem. 2024, 216, 109142. [Google Scholar] [CrossRef]
- Wang, T.; Russo, D.P.; Bitounis, D.; Demokritou, P.; Jia, X.; Huang, H.; Zhu, H. Integrating structure annotation and machine learning approaches to develop graphene toxicity models. Carbon 2023, 204, 484–494. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, Z. Integrating Machine Learning and Nano-QSAR Models to Predict the Oxidative Stress Potential Caused by Single and Mixed Carbon Nanomaterials in Algal Cells. Environ. Toxicol. Chem. 2025, 44, 2535–2544. [Google Scholar] [CrossRef]
- Gajewicz, A.; Puzyn, T.; Odziomek, K.; Urbaszek, P.; Haase, A.; Riebeling, C.; Luch, A.; Irfan, M.A.; Landsiedel, R.; van der Zande, M. Decision tree models to classify nanomaterials according to the DF4nanoGrouping scheme. Nanotoxicology 2018, 12, 1–17. [Google Scholar] [CrossRef]
- Sizochenko, N.; Mikolajczyk, A.; Jagiello, K.; Puzyn, T.; Leszczynski, J.; Rasulev, B. How the toxicity of nanomaterials towards different species could be simultaneously evaluated: A novel multi-nano-read-across approach. Nanoscale 2018, 10, 582–591. [Google Scholar] [CrossRef] [PubMed]
- El Yamani, N.; Mariussen, E.; Gromelski, M.; Wyrzykowska, E.; Grabarek, D.; Puzyn, T.; Tanasescu, S.; Dusinska, M.; Rundén-Pran, E. Hazard identification of nanomaterials: In silico unraveling of descriptors for cytotoxicity and genotoxicity. Nano Today 2022, 46, 101581. [Google Scholar] [CrossRef]
- Shirokii, N.; Din, Y.; Petrov, I.; Seregin, Y.; Sirotenko, S.; Razlivina, J.; Serov, N.; Vinogradov, V. Quantitative Prediction of Inorganic Nanomaterial Cellular Toxicity via Machine Learning. Small 2023, 19, 2207106. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorpeh, F. Toxicity prediction of nanoparticles using machine learning approaches. Toxicology 2024, 501, 153697. [Google Scholar] [CrossRef] [PubMed]
- Jyakhwo, S.; Serov, N.; Dmitrenko, A.; Vinogradov, V.V. Machine Learning Reinforced Genetic Algorithm for Massive Targeted Discovery of Selectively Cytotoxic Inorganic Nanoparticles. Small 2024, 20, 2305375. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Méndez, J.L.; Navarro-López, D.E.; Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Garcia-Amezquita, L.E.; Tiwari, N.; Juarez-Moreno, K.; Sanchez-Ante, G.; López-Mena, E.R. Lanthanide-Doped ZnO Nanoparticles: Unraveling Their Role in Cytotoxicity, Antioxidant Capacity, and Nanotoxicology. Antioxidants 2024, 13, 213. [Google Scholar] [CrossRef]
- Mejia-Mendez, J.L.; Reza-Zaldívar, E.E.; Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Navarro-López, D.E.; Marcelo Lozano, L.; Armendariz-Borunda, J.; Tiwari, N.; Jacobo-Velázquez, D.A.; Sanchez-Ante, G. Exploring the cytotoxic and antioxidant properties of lanthanide-doped ZnO nanoparticles: A study with machine learning interpretation. J. Nanobiotechnol. 2024, 22, 687. [Google Scholar] [CrossRef]
- Ceballos-Sanchez, O.; Navarro-López, D.E.; Mejía-Méndez, J.L.; Sanchez-Ante, G.; Rodríguez-González, V.; Sánchez-López, A.L.; Sanchez-Martinez, A.; Duron-Torres, S.M.; Juarez-Moreno, K.; Tiwari, N.; et al. Enhancing antioxidant properties of CeO2 nanoparticles with Nd3+ doping: Structural, biological, and machine learning insights. Biomater. Sci. 2024, 12, 2108–2120. [Google Scholar] [CrossRef]
- Mirzaei, M.; Furxhi, I.; Murphy, F.; Mullins, M. Employing Supervised Algorithms for the Prediction of Nanomaterial’s Antioxidant Efficiency. Int. J. Mol. Sci. 2023, 24, 2972. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Rao, L.; Yuan, Y.; Shen, X.; Yu, G.; Chen, X. Designing nanotheranostics with machine learning. Nat. Nanotechnol. 2024, 19, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, J.; Wu, Y.; Liu, H.; Meng, F.; Liu, Q.; Midgley, A.C.; Zhang, X.; Qi, T.; Kang, H. Prediction and design of nanozymes using explainable machine learning. Adv. Mater. 2022, 34, 2201736. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.J.; Yan, J.; Zheng, J.J.; Zhong, S.; Gao, X. Clear-Box Machine Learning for Virtual Screening of 2D Nanozymes to Target Tumor Hydrogen Peroxide. Adv Heal. Mater 2023, 12, e2202925. [Google Scholar] [CrossRef]
- Wan, K.; Wang, H.; Shi, X. Machine Learning-Accelerated High-Throughput Computational Screening: Unveiling Bimetallic Nanoparticles with Peroxidase-Like Activity. ACS Nano 2024, 18, 12367–12376. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Zhang, T.; Wu, Y.; Hu, K.; Wu, C.; Dai, X.; Feng, W.; Chen, Y. Driving Multifunctional Nanomedicine Design for Non-Inflammatory Tumor Therapy with Integrated Machine Learning and Density Functional Theory. Adv. Mater. 2025, 37, 2503576. [Google Scholar] [CrossRef]
- Gao, X.J.; Zhao, Y.; Gao, X. Catalytic Signal Transduction Theory Enabled Virtual Screening of Nanomaterials for Medical Functions. Acc. Chem. Res. 2023, 56, 2366–2377. [Google Scholar] [CrossRef]
- Aasim, M.; Katırcı, R.; Akgur, O.; Yildirim, B.; Mustafa, Z.; Nadeem, M.A.; Baloch, F.S.; Karakoy, T.; Yılmaz, G. Machine learning (ML) algorithms and artificial neural network for optimizing in vitro germination and growth indices of industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2022, 181, 114801. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Monthony, A.S.; Baiton, A.; Phineas Jones, A.M. Modeling and optimizing in vitro seed germination of industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2021, 170, 113753. [Google Scholar] [CrossRef]
- Giusti, A.; Atluri, R.; Tsekovska, R.; Gajewicz, A.; Apostolova, M.D.; Battistelli, C.L.; Bleeker, E.A.; Bossa, C.; Bouillard, J.; Dusinska, M. Nanomaterial grouping: Existing approaches and future recommendations. NanoImpact 2019, 16, 100182. [Google Scholar] [CrossRef]
- Yanamala, N.; Desai, I.C.; Miller, W.; Kodali, V.K.; Syamlal, G.; Roberts, J.R.; Erdely, A.D. Grouping of carbonaceous nanomaterials based on association of patterns of inflammatory markers in BAL fluid with adverse outcomes in lungs. Nanotoxicology 2019, 13, 1102–1116. [Google Scholar] [CrossRef]
- Billing, A.M.; Knudsen, K.B.; Chetwynd, A.J.; Ellis, L.-J.A.; Tang, S.V.Y.; Berthing, T.; Wallin, H.; Lynch, I.; Vogel, U.; Kjeldsen, F. Fast and Robust Proteome Screening Platform Identifies Neutrophil Extracellular Trap Formation in the Lung in Response to Cobalt Ferrite Nanoparticles. ACS Nano 2020, 14, 4096–4110. [Google Scholar] [CrossRef]
- Bahl, A.; Halappanavar, S.; Wohlleben, W.; Nymark, P.; Kohonen, P.; Wallin, H.; Vogel, U.; Haase, A. Bioinformatics and machine learning to support nanomaterial grouping. Nanotoxicology 2024, 18, 373–400. [Google Scholar] [CrossRef]
- Khosla, C.; Saini, B.S. Enhancing performance of deep learning models with different data augmentation techniques: A survey. In Proceedings of the 2020 International Conference on Intelligent Engineering and Management (ICIEM), London, UK, 17–19 June 2020; IEEE: New York, NY, USA; pp. 79–85. [Google Scholar]
- Lin, B.; Emami, N.; Santos, D.A.; Luo, Y.; Banerjee, S.; Xu, B.-X. A deep learned nanowire segmentation model using synthetic data augmentation. npj Comput. Mater. 2022, 8, 88. [Google Scholar] [CrossRef]
- Basei, G.; Rauscher, H.; Jeliazkova, N.; Hristozov, D. A methodology for the automatic evaluation of data quality and completeness of nanomaterials for risk assessment purposes. Nanotoxicology 2022, 16, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Beronius, A.; Nymark, P. SciRAPnano: A pragmatic and harmonized approach for quality evaluation of in vitro toxicity data to support risk assessment of nanomaterials. Front. Toxicol. 2023, 5, 1319985. [Google Scholar] [CrossRef] [PubMed]
- Bendre, A.; Bhat, M.P.; Lee, K.-H.; Altalhi, T.; Alruqi, M.A.; Kurkuri, M. Recent developments in microfluidic technology for synthesis and toxicity-efficiency studies of biomedical nanomaterials. Mater. Today Adv. 2022, 13, 100205. [Google Scholar] [CrossRef]
- Karatzas, P.; Melagraki, G.; Ellis, L.-J.A.; Lynch, I.; Varsou, D.-D.; Afantitis, A.; Tsoumanis, A.; Doganis, P.; Sarimveis, H. Development of Deep Learning Models for Predicting the Effects of Exposure to Engineered Nanomaterials on Daphnia magna. Small 2020, 16, 2001080. [Google Scholar] [CrossRef]
- Aversa, R.; Modarres, M.H.; Cozzini, S.; Ciancio, R.; Chiusole, A. The first annotated set of scanning electron microscopy images for nanoscience. Sci. Data 2018, 5, 180172. [Google Scholar] [CrossRef] [PubMed]
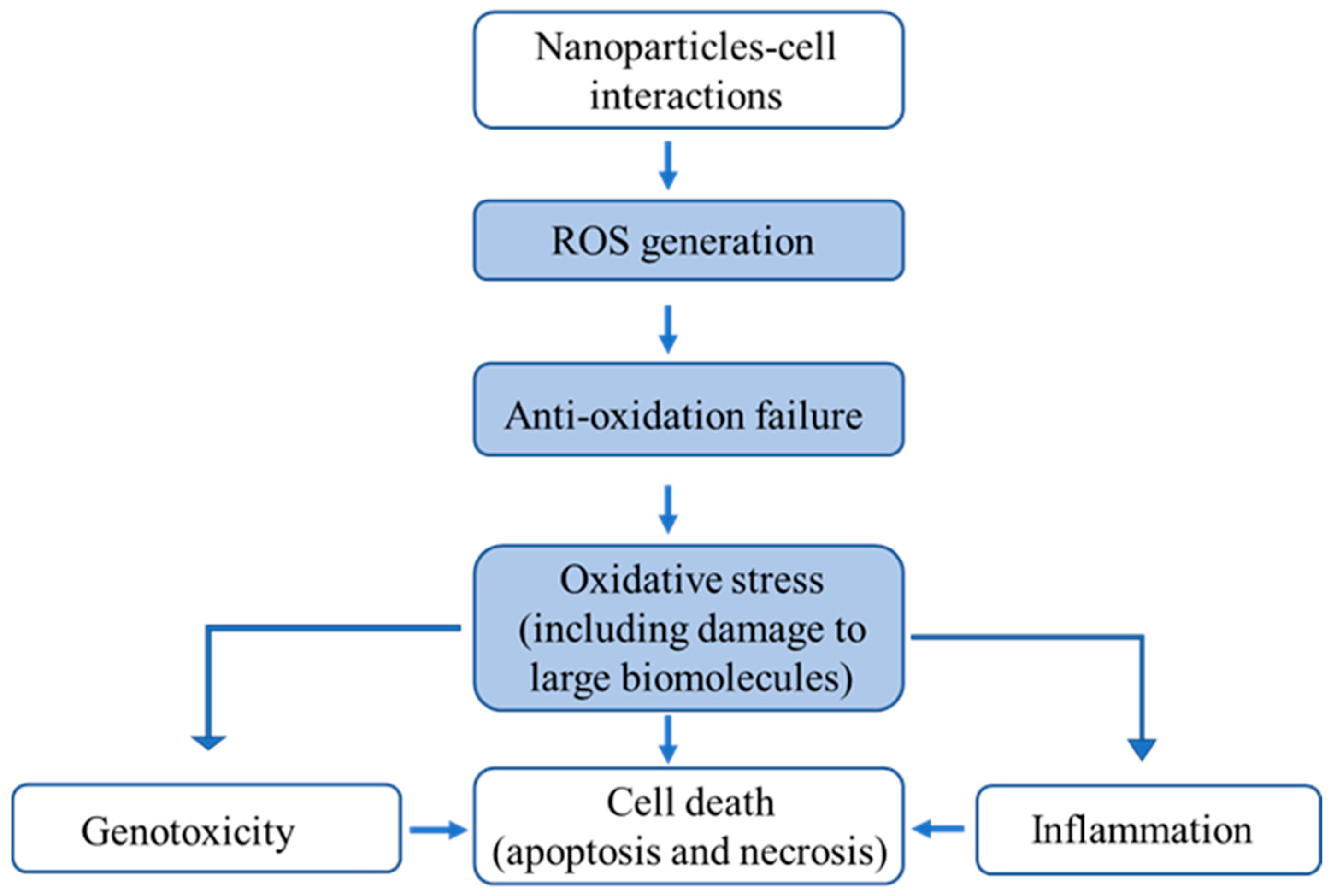
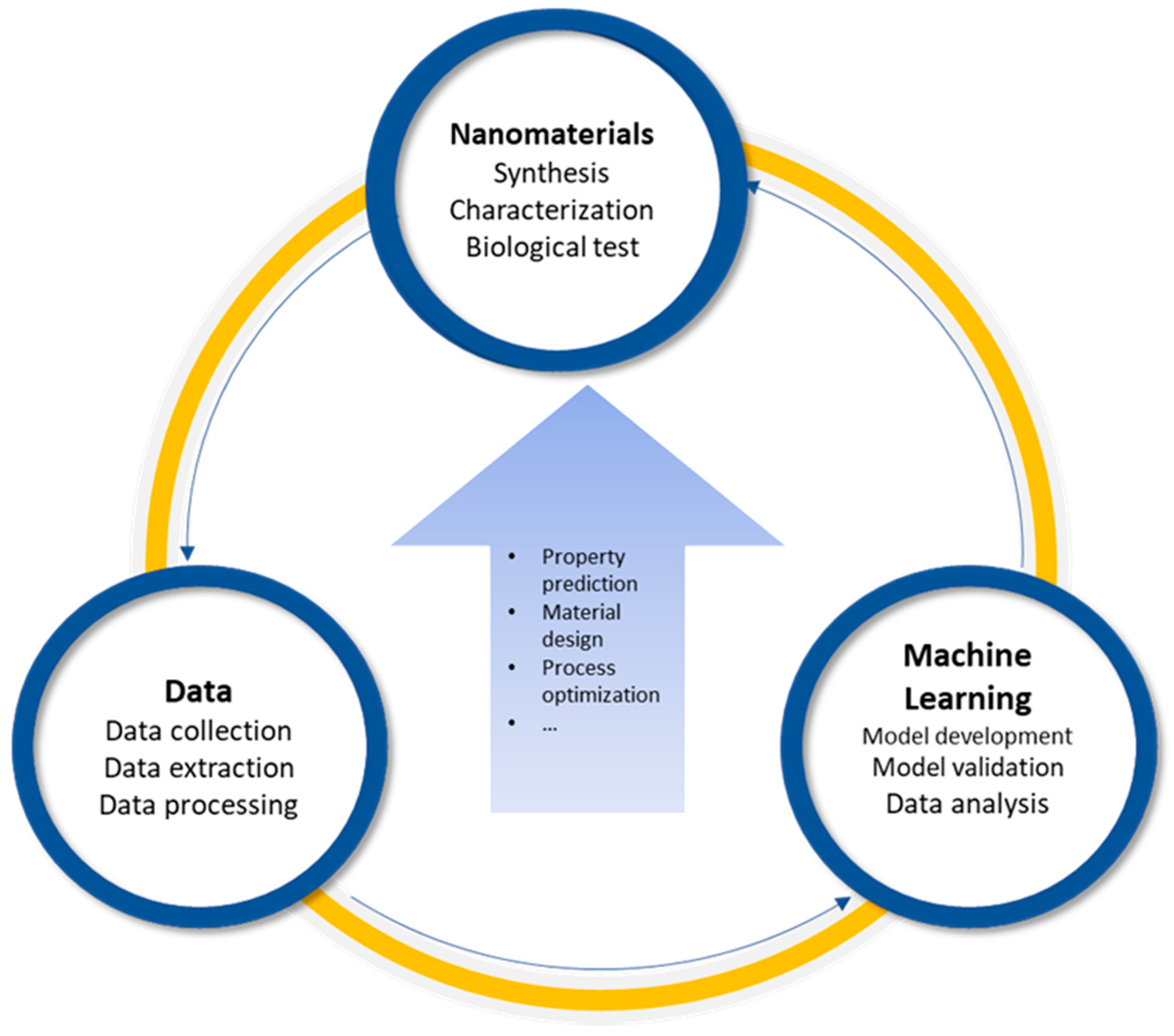
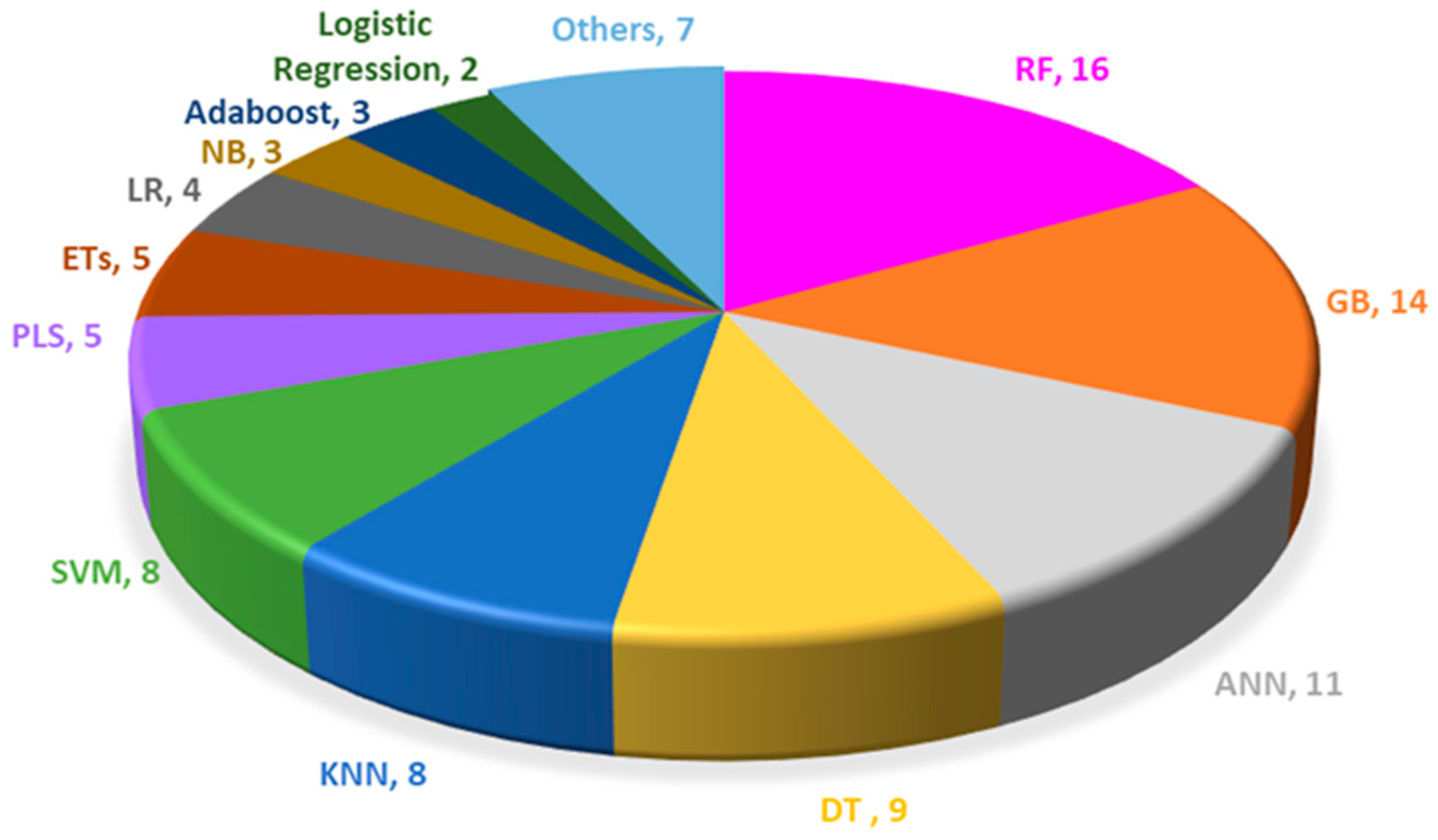
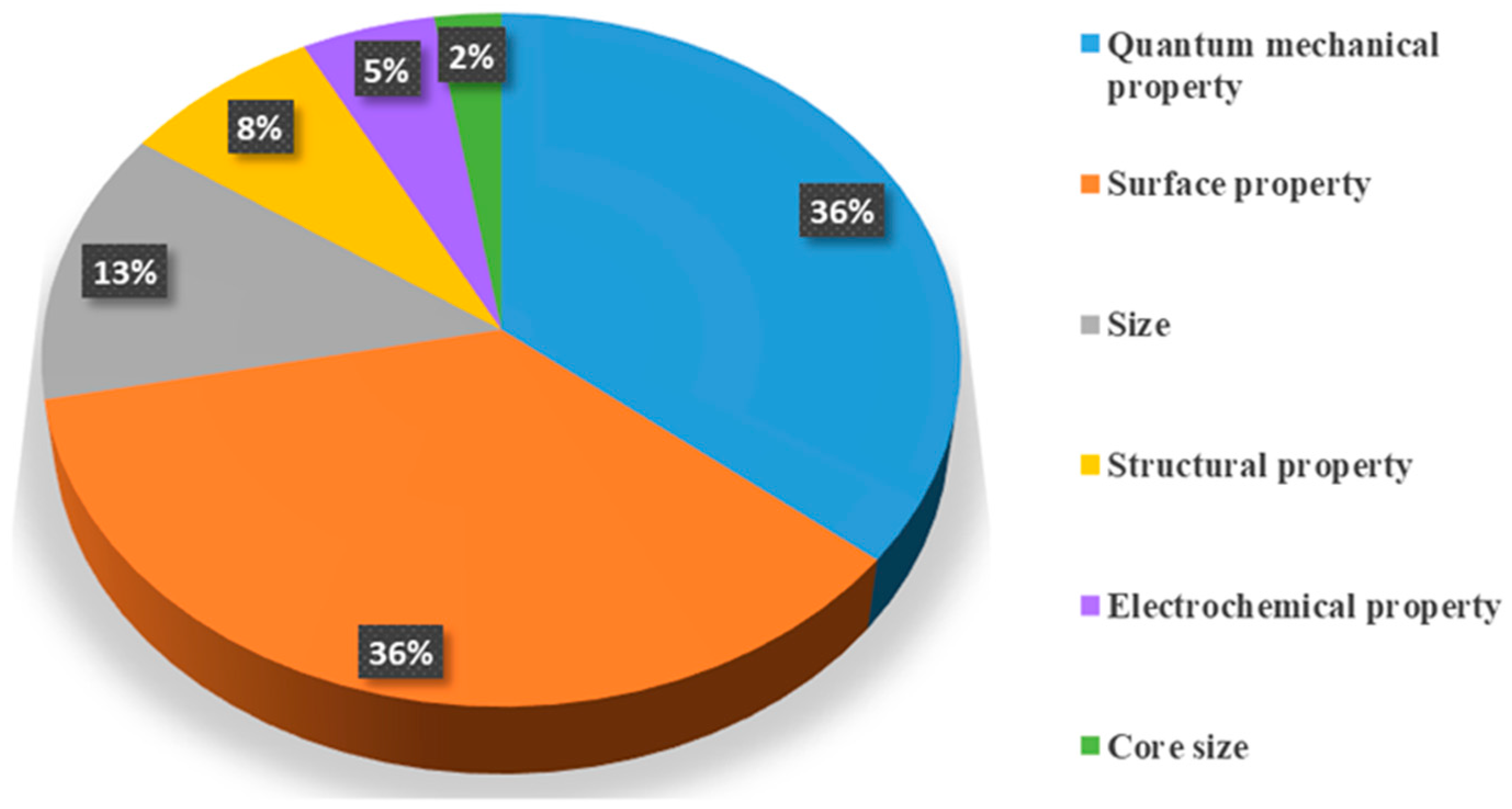
| ENMs | Endpoints | In Vitro/ In Vivo | Algorithms | Data Size | Descriptors | Performance | Ref. |
|---|---|---|---|---|---|---|---|
| MeOx | DCFH-DA assay | C. elegans | MC-PLS | 16 MeOx NMs | quasi-SMILES descriptors (mass percentage of metal elements, cationic charge and MW, Initial size, aggregation size, fP) | N/A | [48] |
| nTiO2 | Oxidative stress biomarkers (CAT, GPx, GST, MDA, ROS, and SOD) | bivalves | RF, ANN, KNN, XGB, SVC, Gaussian NB | 32 relevant references | physicochemical descriptors and experimental variables (NP size, exposure conc., intervals of time exposed to NPs, dissimilar assay organisms/tissues/organs) | accuracy varied from 0.75 to 1.00 (training sets) and 0.60 to 1.00 (external validation sets) | [49] |
| Nano-TiO2-heavy metals (CdCl2, ZnCl2, MnCl2, CoCl2, CuSO4, NiCl2, Pb(NO3)2, SbCl3) | CCK-8 assay | HK-2 human renal cells | PLS, RF, AdaBoost, K-means clustering | 72 total samples (8 heavy metals and 9 concentrations mixed with nanoTiO2) | orbital energies, ionization potential, electron affinity, electronegativity, hardness, molecular and adsorption energy | 0.31 < R2 < 0.95 with clustered RF performing best | [50] |
| ZnO | Luciferase assays | cells | sparse MLR (MLREM), Bayesian regularized artificial neural networks (BRANNLP) | 45 types of ZnO NPs | conduction band energy, reduction potential, ionization potential, fP, solubility, percentage of metal oxide dopant, surface coating type, calcination temperature, NP size, surface area, volume, and aspect ratio. | 0.50 < R2 < 0.67 | [51] |
| Superparamagnetic iron oxide NPs | N/A | N/A | PLSR | 74 types of SPIONs | experimentally obtained surface properties (size, shape, surface charge, coating type and functional groups) with molecular descriptors MW, TPSA Wiener Index (W) LogP 3D Autocorrelation) | (R2 = 0.9515) and MSE of prediction (MSECV = 0.6270) | [52] |
| MeONPs (e.g., Cr2O3, CoO, MnO2, CuO, ZnO, Ni2O3, Co3O4, etc.) | MTS assay | THP-1 cells | PR, MPR, GMPR, SVM, RF, kNN, GkNN, DT, BR, NN + RF | 240 observations (30 types of MeONPs and 8 concentrations) | size, charge, ion release, electronegativity, concentration | R2 values ranged from as low as –1.00 (SVM, poor generalization) to as high as 0.90 (GkNN, excellent prediction) | [39] |
| Gold NPs | H2O2 level | cells | RF, kNN | 191 unique GNPs | geometrical nanodescriptors | moderate accuracy: R2 = 0.68 | [56] |
| Gold NPs | HO-1 level | cells | kNN | 34 GNPs | chemical descriptors | R2 = 0.967 and MAE= 0.14 | [57] |
| Silver NPs | MTT/MTS, LDH, SRB, NRU, and trypan blue | cells | DT, RF | 690 data points | particle size, fP, exposure dose, exposure time, cell type, species, plant family, extraction solvent | accuracy improved from ~0.73 (DT1) up to 0.83 (RF2), with AUC ranging 0.815–0.904, especially for RF with biosynthesis features included | [58] |
| MXenes | MTT assay/CCK-8 assay/DCFH-DA | cells | Regularized Logistic Regression, RF, SVM, ERT | 132 records | surface chemistry, modifications, morphology, and synthesis conditions, with MxOy and Li presence as the strongest predictors of cytotoxicity. | SVM-rbf, RF achieved ~0.85–0.93 accuracy, while purely theoretical features performed poorly. | [59] |
| CDs | H2O2 content | Hairy root culture | MLP | N/A | metabolite | MSE = 1.99 × 10−3, R2 = 0.99939 | [64] |
| Graphene | ROS generation | cells | PLSR | 11 2DNMs | geometrical nanodescriptors | R2 = 0.760, RMSE = 0.164 | [65] |
| Fullerene, Graphene Oxide, SWCNTs, MWCNTs, Graphene Nanosheets | DCFH-DA assay | cells | Classification: C4.5 DT, SVM, ANN, NB, kNN; Regression: DT, RF, GB, Adaboost. | N/A | physical and chemical descriptors: fP, DH, and SSA | classification models: performance metrics (Accuracy, Recall, Precision, F1-score) all exceeding 0.600; regression models: 0.850 < R2 < 0.999 on the training set | [66] |
| Organic and inorganic | Ferric reduction ability of serum assay | in human blood serum | DT | 19 NMs | 285 structural descriptors (both experimental and calculated) | 100% of balanced accuracy | [67] |
| Inorganic (metal- and silica oxide) | Viability test | cells, bacteria, algae, and protozoa. | 184 inorganic (30 unique types) NPs | ionic characteristics | [68] | ||
| Inorganic NMs (TiO2, ZnO, silver and silica) | The level of oxidized base lesions (oxidatively damaged DNA) | cells | the supervised PLS method | 17 JRC repository NMs | physicochemical descriptors | N/A | [69] |
| Inorganic NMs (metals and metal oxides) | Cell viability, concentration-dependent toxicity | cells | LightGBM regressor, RF, ET, HGB, Binary Relevance | 3087 samples | atom-based, nanoparticle physicochemical, and experimental condition descriptors | LGBM show s the best overall; Q2 = 0.86, RMSE = 12.2% | [70] |
| Metallic (Au, Ag), metal-oxide (ZnO, TiO2, CuO, CoFe2O4, Fe2O3), polymeric (polystyrene), and SiO2 NPs | N/A | cells | DT, RF, SVM, NB, ANN | 244 records (NanoHub repository) | physicochemical properties, exposure conditions, cell model characteristics | Random Forest showed superior performance (AUC ≈ 0.97, accuracy > 93%), outperforming DT, SVM, NB, and ANN. | [71] |
| Metal and metal oxide NPs (e.g., Ag, TiO2, ZnO, Au, Pt, CuO) | N/A | cells | 41 regression ML models | 3627 samples with 27 features | NPs concentration, fP, hydrodynamic diameter, exposure time, electronegativity of central atom, cell type/line identify | tree-based ensemble models (LGBM, XGB) clearly outperformed all others, with LGBM optimized to the best accuracy (Q2~0.80). | [72] |
| ENMs | Endpoints | In Vitro/ In Vivo | Algorithms | Data Size | Descriptors | Performance | Ref. |
|---|---|---|---|---|---|---|---|
| (La, Sm)-doped ZnO NPs | DCFH2-DA assay | cells | LR, MLP, RF, DTs, ETs, KNNs, GB, SVR | 196 observations | material, grain size, TC, BG, defects, charge, average particle size, method, and Conc. | LR: RMSE = 22.28; RF: RMSE = 13.43; ET: RMSE = 16.12; DT: RMSE = 16.07; MLP: RMSE = 31.4; KNN: RMSE = 33.09; GB: RMSE = 13.19; SVR: RMSE = 32.93. | [73] |
| (Er, Yb)-doped ZnO NPs | DPPH scavenging (%), ABTS scavenging (%), H2O2 scavenging (%). | in vitro | LR, RF, ET, DT, MLP, KNN, GB, SVR | 480 observations | NP Conc., optical BG, SSA, fP, Zn, Er, Yb composition | ET: R2 = 98.92 DT: R2 = 98.21 RF: R2 = 96.89 GB: R2 = 94.76 MLP: R2 = 91.88 KNN: R2 = 84.93 LR: R2 = 78.54 SVR: R2 = 70.83 | [74] |
| Nd-doped CeO2 NPs | DPPH and ABTS assays | cells | RF, GB, logistic regression, MLP | 144 observations | molarity, MW, lattice constant, fP, SSA, Ce3+/Ce4+ ratio, and scavenging activity. | RF: accuracy = 96.35%, GB: accuracy = 96.47%, LR: accuracy = 92.67%, MLP: accuracy = 88.33% | [75] |
| Different inorganic and organic NMs | DPPH assay | In vitro | regression models (RF, ET, LIGHTGBM, DT, KNN, LASSO, EN). | 62 in vitro studies | P-chem properties, exposure conditions and the method of NMs’ synthesis (NMs’ type, core size, shape, dosage, coating, the synthesis process, medium used, absorbance and duration) | RF: R2 = 0.83. ET: R2 = 0.79 LIGHTGBM: R2 = 0.81 DT: R2 = 0.76 KNN: R2 = 0.70 LASSO: R2 = 0.52 EN: R2 = 0.30 | [76] |
| 2D Nanozymes | Peroxidase- and Catalase-like activity | N/A | XGBR, LR, Ridge regression, SVM, KNN, GBR, RF, NEM | 1019 2D materials | atomic number, valence electrons, electronegativity, ionization energy, atomic radius, electron affinity | XGBR achieved the highest accuracy (R2 ≈ 0.85–0.97) | [80] |
| Bimetallic NPs | Catalytic dissociation of H2O2 | N/A | CatBoost regression | 1260 bimetallic alloy structures | structure descriptor | R2 = 0.964, MAE = 0.108, RMSE =0.169. | [81] |
| Nanozymes | CAT-like activity | N/A | RF, XGBoost, AdaBoost, MLP, CNN | over 100 types of NMs | shape, size, metal type, the number of valence electrons for each metal, nonmetallic elements, and surface modification techniques. | RF: accuracy = 62% XGB: Accuracy = 58% AdaBoost: Accuracy = 67% MLP: Accuracy = 78% CNN: Accuracy = 80% | [82] |
| Inorganic NMs | H2O2 activation, H2O2 dismutation, O2 activation, and O2·− dismutation | N/A | XGBoost regression | 1019 materials | adsorption energies, electronic structure, reaction energy and energy barriers | N/A | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.; Yin, Z. Machine Learning-Driven Prediction of Reactive Oxygen Species Dynamics for Assessing Nanomaterials’ Cytotoxicity. Biomimetics 2025, 10, 718. https://doi.org/10.3390/biomimetics10110718
Ji Z, Yin Z. Machine Learning-Driven Prediction of Reactive Oxygen Species Dynamics for Assessing Nanomaterials’ Cytotoxicity. Biomimetics. 2025; 10(11):718. https://doi.org/10.3390/biomimetics10110718
Chicago/Turabian StyleJi, Zuowei, and Ziyu Yin. 2025. "Machine Learning-Driven Prediction of Reactive Oxygen Species Dynamics for Assessing Nanomaterials’ Cytotoxicity" Biomimetics 10, no. 11: 718. https://doi.org/10.3390/biomimetics10110718
APA StyleJi, Z., & Yin, Z. (2025). Machine Learning-Driven Prediction of Reactive Oxygen Species Dynamics for Assessing Nanomaterials’ Cytotoxicity. Biomimetics, 10(11), 718. https://doi.org/10.3390/biomimetics10110718






