Beyond Staphylococcus: The Cutaneous Microbiome in Itch Pathobiology
Abstract
1. Introduction
2. Materials and Methods
3. Biological Mechanisms of Pruritus Relevant to the Microbiome
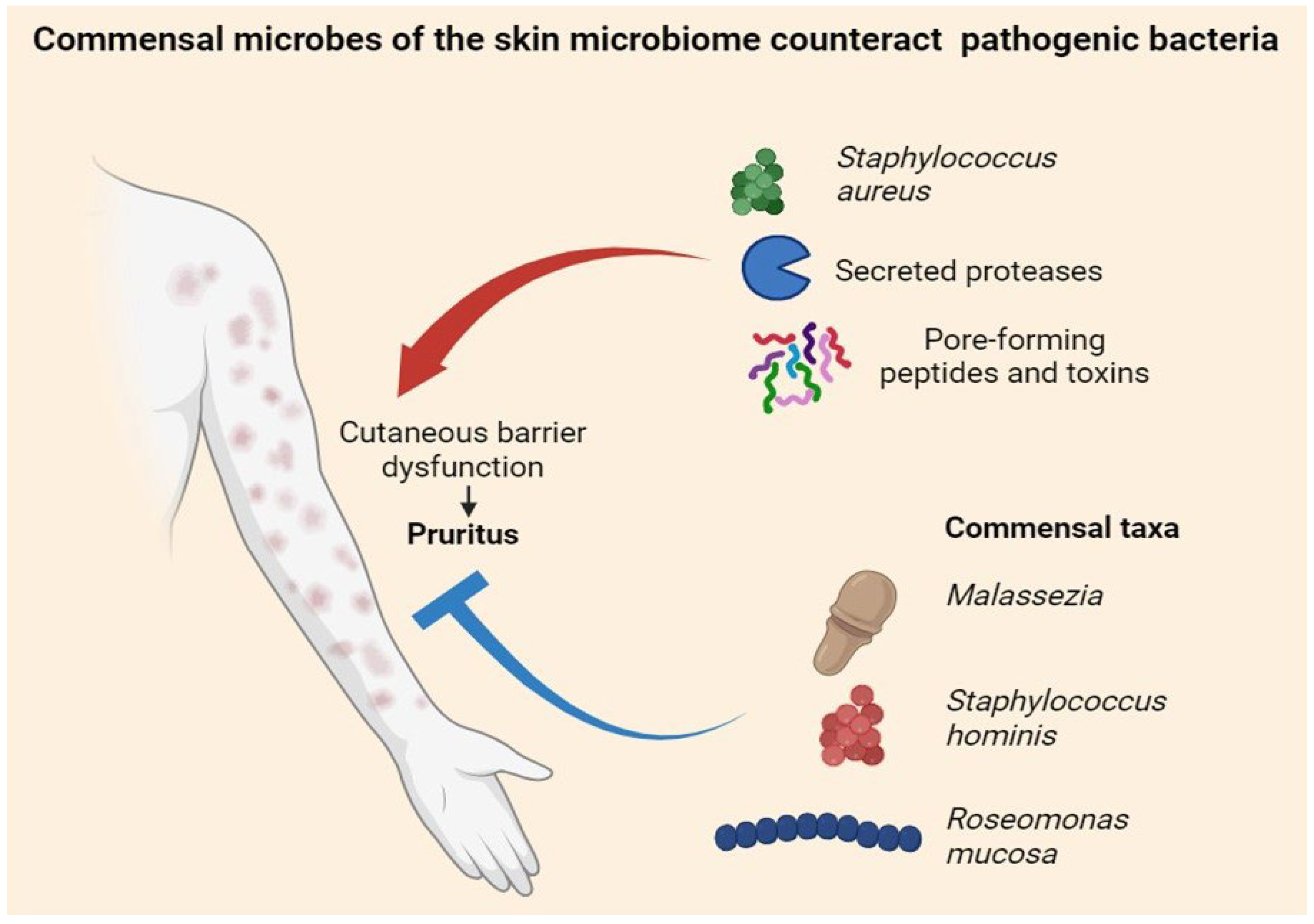
4. Cutaneous Microbiome and Itch
5. Gut Microbiome and Itch (The “Gut–Skin–Liver–Kidney Axis”)
6. Therapeutic Implications of Microbiome Targeting
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, D.C.; Berger, T.; Elmariah, S.; Kim, B.; Chisolm, S.; Kwatra, S.G.; Mollanazar, N.; Yosipovitch, G. Chronic Pruritus: A Review. JAMA 2024, 331, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune communication regulating pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1875–1898. [Google Scholar] [CrossRef] [PubMed]
- Biazus Soares, G.; Hashimoto, T.; Yosipovitch, G. Atopic Dermatitis Itch: Scratching for an Explanation. J. Investig. Dermatol. 2024, 144, 978–988. [Google Scholar] [CrossRef]
- Balaji, S.K.; Khuwaja, W.M.; Hossain, M.L.; Fernando, L.G.B.; Dong, X. Neuroimmune interactions between itch neurons and skin microbes. Semin. Immunol. 2025, 78, 101933. [Google Scholar] [CrossRef]
- Deng, L.; Costa, F.; Blake, K.J.; Choi, S.; Chandrabalan, A.; Yousuf, M.S.; Shiers, S.; Dubreuil, D.; Vega-Mendoza, D.; Rolland, C.; et al. S. aureus drives itch and scratch-induced skin damage through a V8 protease-PAR1 axis. Cell 2023, 186, 5375–5393.e25. [Google Scholar] [CrossRef]
- Gallo, R.L.; Horswill, A.R. Staphylococcus aureus: The Bug Behind the Itch in Atopic Dermatitis. J. Investig. Dermatol. 2024, 144, 950–953. [Google Scholar] [CrossRef]
- Misery, L.; Pierre, O.; Le Gall-Ianotto, C.; Lebonvallet, N.; Chernyshov, P.V.; Le Garrec, R.; Talagas, M. Basic mechanisms of itch. J. Allergy Clin. Immunol. 2023, 152, 11–23. [Google Scholar] [CrossRef]
- Labib, A.; Ju, T.; Yosipovitch, G. Emerging treatments for itch in atopic dermatitis: A review. J. Am. Acad. Dermatol. 2023, 89, 338–344. [Google Scholar] [CrossRef]
- Oh, J.; Voigt, A.Y. The human skin microbiome: From metagenomes to therapeutics. Nat. Rev. Microbiol. 2025, 23, 771–787. [Google Scholar] [CrossRef]
- Lunjani, N.; Hlela, C.; O’Mahony, L. Microbiome and skin biology. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 328–333. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef]
- Kengmo Tchoupa, A.; Kretschmer, D.; Schittek, B.; Peschel, A. The epidermal lipid barrier in microbiome–skin interaction. Trends Microbiol. 2023, 31, 723–734. [Google Scholar] [CrossRef]
- Hülpüsch, C.; Rohayem, R.; Reiger, M.; Traidl-Hoffmann, C. Exploring the skin microbiome in atopic dermatitis pathogenesis and disease modification. J. Allergy Clin. Immunol. 2024, 154, 31–41. [Google Scholar] [CrossRef]
- Buhl, T.; Ikoma, A.; Kempkes, C.; Cevikbas, F.; Sulk, M.; Buddenkotte, J.; Akiyama, T.; Crumrine, D.; Camerer, E.; Carstens, E.; et al. Protease-Activated Receptor-2 Regulates Neuro-Epidermal Communication in Atopic Dermatitis. Front. Immunol. 2020, 11, 1740. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Lerner, E.A.; Carstens, E. Protease-activated receptors and itch. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 226, pp. 219–235. [Google Scholar] [CrossRef]
- Meixiong, J.; Anderson, M.; Limjunyawong, N.; Sabbagh, M.F.; Hu, E.; Mack, M.R.; Oetjen, L.K.; Wang, F.; Kim, B.S.; Dong, X. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity 2019, 50, 1163–1171.e5. [Google Scholar] [CrossRef]
- Steinhoff, M.; Buddenkotte, J.; Lerner, E.A. Role of mast cells and basophils in pruritus. Immunol. Rev. 2018, 282, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Corbière, A.; Loste, A.; Gaudenzio, N. MRGPRX2 sensing of cationic compounds—A bridge between nociception and skin diseases? Exp. Dermatol. 2021, 30, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Serhan, N.; Basso, L.; Sibilano, R.; Petitfils, C.; Meixiong, J.; Bonnart, C.; Reber, L.L.; Marichal, T.; Starkl, P.; Cenac, N.; et al. House dust mites activate nociceptor–mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 2019, 20, 1435–1443. [Google Scholar] [CrossRef]
- Jha, M.K.; Han, Y.; Liu, Z.; Hara, Y.; Langohr, I.M.; Morel, C.; Maloney, C.L.; Piepenhagen, P.; Xing, H.; Bodea, C.A.; et al. Type 2 cytokines pleiotropically modulate sensory nerve architecture and neuroimmune interactions to mediate itch. J. Allergy Clin. Immunol. 2025, 156, 1066–1081.e12. [Google Scholar] [CrossRef]
- Mack, M.R.; Miron, Y.; Chen, F.; Miller, P.E.; Zhang, A.; Korotzer, A.; Richman, D.; Bryce, P.J. Type 2 cytokines sensitize human sensory neurons to itch-associated stimuli. Front. Mol. Neurosci. 2023, 16, 1258823. [Google Scholar] [CrossRef]
- Wiegmann, H.; Renkhold, L.; Zeidler, C.; Agelopoulos, K.; Ständer, S. Interleukin Profiling in Atopic Dermatitis and Chronic Nodular Prurigo. Int. J. Mol. Sci. 2024, 25, 8445. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Vafaeian, A.; Rajabi, F.; Rezaei, N. Toll-like receptors in atopic dermatitis: Pathogenesis and therapeutic implications. Heliyon 2025, 11, e42226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, Y.; Hu, Q.; Wang, X.; Zhang, L.; Liu, T.; Zhang, J.; Yang, X.; Fu, Q.; Fu, D.; et al. Keratinocyte TLR2 and TLR7 contribute to chronic itch through pruritic cytokines and chemokines in mice. J. Cell. Physiol. 2023, 238, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa-Mineoka, R. Toll-like receptors: Their roles in pathomechanisms of atopic dermatitis. Front. Immunol. 2023, 14, 1239244. [Google Scholar] [CrossRef]
- Kuo, I.H.; Yoshida, T.; De Benedetto, A.; Beck, L.A. The cutaneous innate immune response in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 266–278. [Google Scholar] [CrossRef]
- Tsagareli, M.G.; Follansbee, T.; Iodi Carstens, M.; Carstens, E. Targeting Transient Receptor Potential (TRP) Channels, Mas-Related G-Protein-Coupled Receptors (Mrgprs), and Protease-Activated Receptors (PARs) to Relieve Itch. Pharmaceuticals 2023, 16, 1707. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Ständer, S. Chronic Pruritus: Current and Emerging Treatment Options. Drugs 2017, 77, 999–1007. [Google Scholar] [CrossRef]
- Mollanazar, N.K.; Smith, P.K.; Yosipovitch, G. Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clin. Rev. Allergy Immunol. 2016, 51, 263–292. [Google Scholar] [CrossRef]
- Uberoi, A.; Murga-Garrido, S.M.; Bhanap, P.; Campbell, A.E.; Knight, S.A.; Wei, M.; Chan, A.; Senay, T.; Tegegne, S.; White, E.K.; et al. Commensal-derived tryptophan metabolites fortify the skin barrier: Insights from a 50-species gnotobiotic model of human skin microbiome. Cell. Chem. Biol. 2025, 32, 111–125.e6. [Google Scholar] [CrossRef]
- Celoria, V.; Rosset, F.; Pala, V.; Dapavo, P.; Ribero, S.; Quaglino, P.; Mastorino, L. The Skin Microbiome and Its Role in Psoriasis: A Review. Psoriasis Targets Ther. 2023, 13, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Cau, L.; Williams, M.R.; Butcher, A.M.; Nakatsuji, T.; Kavanaugh, J.S.; Cheng, J.Y.; Shafiq, F.; Higbee, K.; Hata, T.R.; Horswill, A.R.; et al. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 955–966.e16. [Google Scholar] [CrossRef]
- Moniaga, C.S.; Tominaga, M.; Takamori, K. An Altered Skin and Gut Microbiota Are Involved in the Modulation of Itch in Atopic Dermatitis. Cells 2022, 11, 3930. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Rangel, S.M.; Paller, A.S. Bacterial colonization, overgrowth, and superinfection in atopic dermatitis. Clin. Dermatol. 2018, 36, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yosipovitch, G. The Role of the Microbiome and Microbiome-Derived Metabolites in Atopic Dermatitis and Non-Histaminergic Itch. Am. J. Clin. Dermatol. 2020, 21 (Suppl. S1), 44–50. [Google Scholar] [CrossRef]
- Kim, J.-C.; Shim, W.-S.; Kwak, I.-S.; Lee, D.-H.; Park, J.-S.; Lee, S.-Y.; Kang, S.-Y.; Chung, B.-Y.; Park, C.-W.; Kim, H.-O. Pathogenesis and Treatment of Pruritus Associated with Chronic Kidney Disease and Cholestasis. Int. J. Mol. Sci. 2023, 24, 1559. [Google Scholar] [CrossRef]
- Vander Does, A.; Levy, C.; Yosipovitch, G. Cholestatic Itch: Our Current Understanding of Pathophysiology and Treatments. Am. J. Clin. Dermatol. 2022, 23, 647–659. [Google Scholar] [CrossRef]
- Beuers, U.; Wolters, F.; Oude Elferink, R.P.J. Mechanisms of pruritus in cholestasis: Understanding and treating the itch. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 26–36. [Google Scholar] [CrossRef]
- Kanda, T.; Sasaki-Tanaka, R.; Kimura, N.; Abe, H.; Yoshida, T.; Hayashi, K.; Sakamaki, A.; Yokoo, T.; Kamimura, H.; Tsuchiya, A.; et al. Pruritus in Chronic Cholestatic Liver Diseases, Especially in Primary Biliary Cholangitis: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 1883. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.E.; Bolier, R.; Van Dijk, R.; Oude Elferink, R.P.J.; Beuers, U. Advances in pathogenesis and management of pruritus in cholestasis. Dig. Dis. 2014, 32, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Rušanac, A.; Škibola, Z.; Matijašić, M.; Čipčić Paljetak, H.; Perić, M. Microbiome-Based Products: Therapeutic Potential for Inflammatory Skin Diseases. Int. J. Mol. Sci. 2025, 26, 6745. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fang, Y.; Hu, J.; Li, S.; Zeng, L.; Chen, S.; Li, Z.; Meng, R.; Yang, X.; Zhang, F.; et al. Innovative microbial strategies in atopic dermatitis. Front. Immunol. 2025, 16, 1605434. [Google Scholar] [CrossRef]
- Zeidler, C.; Raap, U.; Witte, F.; Ständer, S. Clinical aspects and management of chronic itch. J. Allergy Clin. Immunol. 2023, 152, 1–10. [Google Scholar] [CrossRef]
- Mastorino, L.; Ribero, S.; Burlando, M.; Mendes-Bastos, P. Editorial: Patients-oriented treatments for chronic inflammatory skin diseases. Front. Med. 2024, 11, 1473753. [Google Scholar] [CrossRef]
- Sutaria, N.; Adawi, W.; Goldberg, R.; Roh, Y.S.; Choi, J.; Kwatra, S.G. Itch: Pathogenesis and treatment. J. Am. Acad. Dermatol. 2022, 86, 17–34. [Google Scholar] [CrossRef]
- Misery, L.; Brenaut, E.; Pierre, O.; Le Garrec, R.; Gouin, O.; Lebonvallet, N.; Abasq-Thomas, C.; Talagas, M.; Le Gall-Ianotto, C.; Besner-Morin, C.; et al. Chronic itch: Emerging treatments following new research concepts. Br. J. Pharmacol. 2021, 178, 4775–4791. [Google Scholar] [CrossRef]
- Erickson, S.; Heul AVer Kim, B.S. New and emerging treatments for inflammatory itch. Ann. Allergy Asthma Immunol. 2021, 126, 13–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosset, F.; Pala, V.; Santaniello, U.; Celoria, V.; Mastorino, L.; Goso, F.; Pucciariello, A.; Bongiovanni, E.; Ribero, S.; Quaglino, P. Beyond Staphylococcus: The Cutaneous Microbiome in Itch Pathobiology. Allergies 2025, 5, 41. https://doi.org/10.3390/allergies5040041
Rosset F, Pala V, Santaniello U, Celoria V, Mastorino L, Goso F, Pucciariello A, Bongiovanni E, Ribero S, Quaglino P. Beyond Staphylococcus: The Cutaneous Microbiome in Itch Pathobiology. Allergies. 2025; 5(4):41. https://doi.org/10.3390/allergies5040041
Chicago/Turabian StyleRosset, Francois, Valentina Pala, Umberto Santaniello, Valentina Celoria, Luca Mastorino, Federico Goso, Andrea Pucciariello, Eleonora Bongiovanni, Simone Ribero, and Pietro Quaglino. 2025. "Beyond Staphylococcus: The Cutaneous Microbiome in Itch Pathobiology" Allergies 5, no. 4: 41. https://doi.org/10.3390/allergies5040041
APA StyleRosset, F., Pala, V., Santaniello, U., Celoria, V., Mastorino, L., Goso, F., Pucciariello, A., Bongiovanni, E., Ribero, S., & Quaglino, P. (2025). Beyond Staphylococcus: The Cutaneous Microbiome in Itch Pathobiology. Allergies, 5(4), 41. https://doi.org/10.3390/allergies5040041







