Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease marked by pruritus and eczematous lesions that significantly impacts patient quality of life. This review covers the intricate interplay of barrier dysfunction, immune dysregulation, and microbial dysbiosis in the complex pathophysiology of AD. The roles of epigenetic factors and environmental exposures are also examined. The evolving understanding of these factors has revolutionized AD treatment. Beyond foundational topical agents, the landscape for moderate-to-severe AD treatment is now dominated by highly targeted immunotherapies, such as biologics and Janus Kinase (JAK) inhibitors, that precisely block specific inflammatory pathways. Emerging strategies explore microbiome modulation and vitamin D supplementation. This paradigm shift from broad immunosuppression to precision medicine offers improved disease control and reduced systemic toxicities and enables more personalized AD management, significantly benefiting patients.
1. Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin condition characterized by epidermal barrier dysfunction, pruritus, and increased risk to bacterial and viral infections. It has a genetic predisposition and is often the initial step in the so-called “Atopic March,” namely allergic asthma, rhinoconjunctivitis, and food allergy. It is the most common chronic inflammatory skin condition in the population, affecting 15–20% of children and 10% of adults [1,2]. The understanding of AD has evolved from the hypothesis of it being primarily a skin barrier dysfunction or the immune dysregulation hypothesis to a more integrated, complex model involving a multifaceted interplay of factors including microbial dysbiosis and environmental triggers [3,4,5]. Given its complexity, AD is considered a highly heterogeneous disease and is classified not only by its clinical presentation (phenotype) but also by its underlying molecular mechanisms (endotype).
AD Phenotypes and Endotypes
Phenotypes in AD describe the observable clinical characteristics of the disease. Phenotypically, AD is classically divided into extrinsic (IgE-high) and intrinsic (IgE-normal) forms. Extrinsic AD constitutes a major subtype and is associated with barrier impairment, filaggrin mutations, and food/protein allergies. Intrinsic AD, on the other hand, is more common in women, sometimes linked to metal allergy, and is characterized by a deficiency of Suprabasin (SBSN), a suprabasal epidermal protein crucial for cornified envelope formation. This deficiency suggests a distinct barrier abnormality underlies this intrinsic endotype, even in the absence of filaggrin mutations [6,7]. Additional phenotypic classifications include age-related forms (pediatric vs. adult-onset) and ethnic patterns (European American, Asian, African American), each of which reflects unique underlying immunologic and barrier characteristics.
Endotypes in AD are defined by the unique immunologic and molecular mechanisms underlying the disease’s physical characteristics. The most prevalent endotype is type 2 cytokine–high profile (IL-4, IL-13, IL-31), which is associated with barrier dysfunction and elevated IgE. Other endotypes include type 1 (Th1) and type 3 (Th17/Th22) cytokine profiles, whose prevalence varies by age, ethnicity, and chronicity. For example, African American patients often demonstrate a Th2/Th22 skewing with fewer filaggrin mutations, while Asian patients frequently demonstrate mixed Th2/Th17 activation [6,7]. More recently, transcriptomic studies have identified eosinophil-high vs. eosinophil-low endotypes, a classification that can help inform the therapeutic approach for these patients [8]. Table 1 summarizes the key characteristics of AD phenotypes and endotypes.

Table 1.
Phenotypes and endotypes in atopic dermatitis.
To effectively manage AD, it is crucial to understand the interplay between these factors and how they contribute to the unique ways the disease appears in different individuals. This review will summarize the latest research on AD pathophysiology and explore interventions that target these underlying mechanisms.
2. Skin Barrier Dysfunction in AD
Skin barrier dysfunction is recognized as a fundamental and central element in the initiation and perpetuation of AD. The skin, our body’s largest organ, functions as a critical protective interface with the external environment. It is organized into three main layers: the epidermis, dermis, and hypodermis. Of these, the epidermis, the outermost layer, plays the most direct role in barrier function, consisting of five distinct sub-layers. The most superficial of these, the stratum corneum, is particularly vital. Often described as a “brick and mortar” structure, it is composed of flattened, anucleated cells called corneocytes (the “bricks”) embedded in a rich intercellular lipid matrix (the “mortar”). This resilient stratum corneum normally provides robust protection against environmental threats, preventing excessive water loss from within and blocking the entry of allergens, irritants, and microbes from outside.
In individuals with AD, this crucial skin barrier is inherently compromised (Figure 1). This vulnerability stems from a complex interplay of factors, including well-established genetic predispositions, such as loss-of-function mutations in the filaggrin (FLG) gene, which are central to maintaining the integrity of corneocytes. Beyond filaggrin, defects can also arise from impaired assembly or function of other structural proteins, or from the loss of tight junctions in deeper epidermal layers (stratum granulosum), which typically form a seal between cells. Furthermore, an altered lipid composition within the stratum corneum’s extracellular matrix contributes significantly to barrier defects. These underlying issues render the stratum corneum and often the stratum granulosum more permeable. Consequently, the skin becomes abnormally porous, leading to excessive transepidermal water loss (TEWL) and significantly facilitating the penetration of environmental allergens, irritants, and microbial antigens, setting the stage for the characteristic inflammation and immune responses seen in AD [4,5].
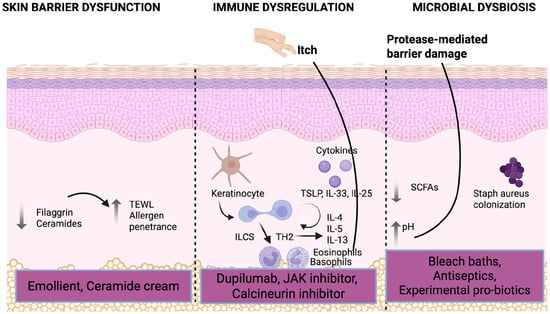
Figure 1.
Pathophysiology of atopic dermatitis and its targeted treatment (created in BioRender. Hansen-Sackey, T. (2025) https://BioRender.com/84ut7ez [9] (accessed on 6 August 2025)).
2.1. Mutations in the Filaggrin (FLG) Gene
Loss-of-function mutations in the gene encoding filaggrin (FLG) are a significant genetic risk factor for AD. The stratum corneum is primarily composed of densely packed corneocytes, held together by keratin fibers, filaggrin (FLG), and an extracellular lipid matrix. Filaggrin is a key component of the cornified envelope, a structure that anchors corneocytes to the surrounding extracellular lipid matrix. A deficiency in filaggrin protein compromises the stratum corneum, leading to cytoskeletal changes. This results in increased TEWL and facilitates the penetration of antigens [5]. These FLG mutations are particularly associated with an earlier onset and more persistent course of AD.
2.2. Lipid Abnormalities
Abnormalities in skin lipids play a critical role in AD, impacting both the epidermal lipid barrier and systemic lipid metabolism. The extracellular lamellar layer of the stratum corneum is composed of ceramides, fatty acids, and cholesterol. In AD, dysregulation of key lipid biosynthetic enzymes occurs, partly driven by the inflammation-mediated environment, particularly by type 2 cytokines like Interleukin-4 (IL-4) and Interleukin-13 (IL-13) [10]. This creates a vicious cycle: a reduction in total stratum corneum lipids, shorter chain ceramides, and free fatty acids, along with altered ceramide subclass classification, leads to further skin barrier compromise and increased TEWL [11]. Prior studies have demonstrated that human epidermal equivalents treated with IL-4 show a downregulation of ceramide synthesis enzymes, specifically glucocerebrosidase and sphingomyelinase, resulting in reduced ceramide synthesis and increased TEWL [10]. Early use of emollients helps address these lipid abnormalities by replenishing deficient ceramides, fatty acids, and cholesterol in the stratum corneum, thereby restoring barrier integrity and mitigating inflammation-mediated reductions in lipid biosynthetic enzymes such as glucocerebrosidase and sphingomyelinase [12,13].
2.3. Protease Activation and pH Imbalance
An elevated stratum corneum pH, signifying an alkaline environment, disrupts the skin’s normal acidic state. This disruption leads to heightened activity of serine proteases, notably kallikrein-related peptidases [14]. Such protease activation degrades structural proteins and lipid-processing enzymes, further compromising the skin barrier. This degradation facilitates the entry of allergens and microbes and triggers inflammation and pruritus through the activation of protease-activated receptor 2 (PAR2) signaling [15,16]. Both elevated skin pH and enhanced protease activity are observed in lesional and non-lesional skin of AD patients, correlating with disease biomarkers and severity [15]. Environmental factors, such as the use of alkaline soaps and detergents, can further raise skin pH, exacerbating this protease-mediated barrier damage. This compromised barrier also promotes microbial dysbiosis, particularly favoring colonization by Staphylococcus aureus in AD.
3. Immune Dysregulation in AD
Immune dysregulation is a fundamental aspect of AD pathophysiology, involving both innate and adaptive immune responses that contribute to chronic inflammation, barrier dysfunction, and intense pruritus.
3.1. Innate Immune Dysregulation
Innate immune dysfunction acts as a critical early driver in the pathogenesis of AD, particularly in response to epidermal barrier disruption. Keratinocytes, the primary cells of the epidermis, are not merely structural components; they also serve as crucial immune sentinels [17]. When the skin barrier is compromised, often due to genetic factors like filaggrin mutations or external insults, these keratinocytes swiftly react by releasing a suite of “alarmins” or epithelial-derived cytokines. Key alarmins include Thymic Stromal Lymphopoietin (TSLP), Interleukin-33 (IL-33), and Interleukin-25 (IL-25). The release of these alarmins triggers a cascade of events. They directly activate various innate immune cells, including Type 2 Innate Lymphoid Cells (ILC2s), dendritic cells, and basophils. This activation initiates a rapid type 2-skewed immune response, characterized by the robust production and secretion of central pro-inflammatory cytokines such as IL-4, IL-13, and IL-31 [18,19]. These cytokines are direct drivers of the cardinal symptoms of AD, namely inflammation, intense itch (pruritus), and further impairment of the skin barrier, thus establishing a self-perpetuating cycle.
Beyond alarmins, Toll-like Receptors (TLRs) on keratinocytes and other innate immune cells play a significant role [20]. These receptors detect various microbial components (e.g., from Staphylococcus aureus) and endogenous danger signals released from damaged cells. Their activation further amplifies inflammation and serves as a critical bridge to the adaptive immune response. Furthermore, this innate immune dysregulation often impairs the production of essential antimicrobial peptides (AMPs) by keratinocytes, leading to a disrupted cutaneous microbiome (dysbiosis) and increased susceptibility to skin infections, which in turn reinforces the inflammatory cycle. Leading allergy and immunology societies consistently highlight innate immune activation as a key initiating event in AD, establishing the groundwork for chronic inflammation and subsequent adaptive immune responses.
3.2. Type 2 (Th2) Pathway
The type 2 immune pathway, predominantly mediated by T-helper 2 (Th2) cells and ILC2s, plays a core and persistent role in immune dysregulation in AD. These cells are prolific secretors of the key cytokines: IL-4, IL-13, and IL-31. These cytokines, upon binding to their respective receptors, activate crucial intracellular signaling pathways, most notably the Janus Kinase (JAK) pathway [17], which then transduces the inflammatory signals within target cells. The profound impact of IL-4 and IL-13 extends to multiple aspects of AD pathophysiology. They directly disrupt epidermal barrier integrity by downregulating the expression of vital structural proteins like filaggrin and loricrin. This leads to increased TEWL and significantly facilitates the entry of allergens, irritants, and microbes into the skin [16]. These cytokines also strongly stimulate B cells to undergo class switching and produce Immunoglobulin E (IgE) antibodies, reinforcing the allergic component of AD inflammation [17]. Furthermore, IL-4 and IL-13 also impair proper keratinocyte differentiation and function, preventing the skin from effectively repairing its barrier [18]. This creates a vicious cycle where barrier disruption fuels immune activation, and immune activation further compromises the barrier. The epithelial-derived cytokines (alarmins) mentioned previously—TSLP, IL-33, and IL-25—act synergistically to further amplify this type 2 response by strongly activating both ILC2s and Th2 cells [19], solidifying the central role of these pathways in the chronic inflammation of AD.
3.3. Type 1 (Th1) and Type 3 (Th17/22) Pathways
T helper 1 (Th1) and T helper 17 (Th17) cells contribute to immune dysregulation in atopic dermatitis (AD), particularly during the chronic phase and in phenotypic variants such as pediatric and intrinsic AD [21,22,23]. Whereas acute AD is characterized by Th2-driven inflammation, the chronic stage is marked by a relative shift toward increased Th1 and Th17 activity. Th1 cells secrete interferon-γ and interleukin-12, which sustain inflammation, induce keratinocyte apoptosis, and impair barrier function [16,24]. Th17 cells produce IL-17 and IL-22, which are variably upregulated in AD, with higher activity observed in intrinsic AD, Asian patients, and children [21,22]. Compared to psoriasis, AD demonstrates relatively low IL-17 but more prominent IL-22 expression. Reduced IL-17 limits antimicrobial peptide production, increasing susceptibility to bacterial infections, while elevated IL-22 drives epidermal hyperplasia, barrier disruption, and chronic inflammation. Clinical trials targeting the IL-12/23 p40 pathway, intended to suppress Th17 activity, have not been successful in AD, reinforcing the conclusion that Th17 cytokines are not the primary drivers of the disease [23,25].
Despite their presence in chronic AD lesions, the precise pathogenic roles of Th1 and Th17 cells remain incompletely defined, and current therapies primarily target Th2 pathways [26]. The interplay between the Th1, Th17, and Th2 axes highlights the heterogeneity of AD and may influence both clinical phenotype and therapeutic responses [21,26].
3.4. Neuroimmune Dysregulation
The neuroimmune axis in AD describes the bidirectional interactions between immune cells and sensory neurons that underlies both acute and chronic itch. Skin barrier dysfunction enables allergens and irritants to penetrate the epidermis, prompting keratinocytes to release epithelial-derived cytokines such as TSLP, IL-33, and IL-25. These cytokines recruit and activate type 2 innate lymphoid cells (ILC2s), dendritic cells, and basophils, which subsequently stimulate Th2 cells to secrete IL-4, IL-13, and IL-31—key mediators of cutaneous itch and inflammation that represent a major concern for patients with AD [27].
Acute itch in AD is believed to be mediated in part by a basophil–neuronal axis [27]. In normal skin, itch is primarily mediated by the histaminergic pathway, where environmental triggers (e.g., insect bites) activate mast cells to release histamine. This activates a small subset of histamine-sensitive C-nerve fibers via H1 and H4 receptors, leading to transient itch that is typically responsive to antihistamines [28]. In contrast, in AD-inflamed skin, basophils infiltrate the dermis and release leukotrienes that directly stimulate sensory neurons, circumventing the classical mast cell–histamine pathway. This mechanism helps explain why antihistamines are frequently ineffective in AD and highlights the importance of basophil-derived mediators in acute flares [27].
Chronic itch, on the other hand, is sustained through persistent neuroimmune signaling. Th2 cytokines (IL-4, IL-13, IL-31) not only promote inflammation but also drive hyperinnervation and enhance the excitability of cutaneous sensory nerves, thereby sustaining itch. IL-31, produced by Th2 cells, basophils, and macrophages, acts directly on IL-31RA receptors on sensory neurons to drive ongoing itch. The resulting itch–scratch cycle further damages the epidermal barrier, amplifies immune activation and neuronal sensitization. Targeting IL-31 with systemic therapy such as nemolizumab has emerged as a significant advancement in mitigating the itch pathway in AD.
3.5. Biomarkers in AD
Biomarker research in AD has identified multiple blood- and skin-based biomarkers associated with disease diagnosis, severity, and phenotypes. In addition to blood-based biomarkers, recent skin stripping studies have enabled minimally invasive, repeated sampling of the stratum corneum, capturing immune and barrier-related biomarkers directly at the site of the lesions in comparison to the non-lesional skin. Biomarkers such as TARC (CCL17), periostin, IL-13, and IL-22 correlate strongly with disease severity and treatment outcomes, while inflammatory chemokines like CXCL9, CXCL10, and CXCL11 reflect immune activation and systemic involvement, especially in adult AD, and can direct more targeted therapy [29,30].
4. Microbial Dysbiosis in AD
Microbial dysbiosis is increasingly recognized as a significant factor in the pathogenesis of AD, particularly in children. It is characterized by a detrimental imbalance between beneficial commensal microorganisms and potentially harmful pathogenic species. This microbial dysregulation is not confined to a single site but manifests distinctly in both the skin and the gut, collectively disrupting barrier functions and exacerbating immune dysregulation.
4.1. Skin Microbiome Alterations
On the skin, AD is consistently associated with reduced microbial diversity. This includes a notable decrease in beneficial skin flora, such as Cutibacterium species, Corynebacterium, and Streptococcus. Concurrently, there is a significant overgrowth of Staphylococcus aureus (S. aureus). S. aureus acts as a major exacerbating factor in AD. It produces various virulence factors, including proteases and toxins, which directly compromise the integrity of the epidermal skin barrier. Furthermore, S. aureus superantigens can promote a pronounced T-helper 2 (Th2)-skewed immune response, leading to the release of pro-inflammatory cytokines such as IL-4 and IL-13 [20]. This perpetuates a vicious cycle of inflammation, intense pruritus (itch), and increased disease severity.
4.2. Gut Microbiome Dysbiosis and the Gut–Skin Axis
The microbial disruption observed in AD extends beyond the skin to the gut microbiome. Here, dysbiosis often presents as an overgrowth of pathogenic microbes, including species like Escherichia coli, Klebsiella pneumoniae, and Clostridium difficile, which disrupt the delicate intestinal ecological balance [31]. This imbalance leads to a reduced abundance of bacteria that produce short-chain fatty acids (SCFAs), while increasing the proportion of pro-inflammatory species.
An altered gut microbiota can profoundly modulate both systemic and cutaneous immune responses through the intricate gut–skin axis, thereby contributing significantly to AD susceptibility and severity. SCFAs, such as butyrate, propionate, and acetate, play a crucial protective role. They modulate Th2 immune responses and suppress the production of key AD-associated cytokines, including IL-4, IL-13, and IL-31.
4.3. Factors Influencing Early-Life Microbiome and AD Risk
In children, several early-life factors that can shape the developing microbiome and potentially influence AD risk have been identified:
- Vaginal delivery promotes the initial colonization of diverse beneficial microbiota in newborns [32].
- Breastfeeding provides human milk oligosaccharides (HMOs), which are prebiotics that nourish beneficial gut bacteria. HMOs contribute to the maturation of gut immunity by promoting SCFA production, fostering IgA secretion (a key mucosal antibody), and supporting the growth of beneficial microbes [33].
- A rural upbringing has also been associated with a more diverse microbiome and reduced AD risk, likely due to increased environmental microbial exposure [34].
Furthermore, recent research highlights the role of specific microbial metabolites. Probiotics, for instance, can metabolize dietary tryptophan into bioactive compounds like indole-3-lactic acid (ILA) and indole-3-carbaldehyde (I3C). ILA has been shown to inhibit lipopolysaccharide (LPS) production, thereby dampening Toll-like Receptor (TLR)-mediated inflammatory signaling. I3C, on the other hand, activates the aryl hydrocarbon receptor, which can suppress TSLP, consequently reducing Th2-driven inflammation [33]. Conversely, early-life antibiotic exposure is a significant disruptor of gut microbiota composition, hindering the establishment of beneficial probiotic strains and increasing susceptibility to AD [35].
5. Role of Epigenetic Factors and the Exposome in the Development of AD
Beyond classical genetics, epigenetic factors and the comprehensive “external exposome” (representing the sum of all environmental exposures throughout a lifetime), often combined with dietary influences, are gaining significant recognition for their profound role in facilitating the development and progression of AD.
5.1. Epigenetics
Epigenetics refers to heritable changes in gene expression that occur without altering the underlying DNA sequence itself. These modifications act as crucial regulators, effectively turning genes “on” or “off.” Key epigenetic mechanisms implicated in AD include DNA methylation, histone modification, and non-coding RNA.
DNA methylation involves the addition of a methyl group to DNA, typically leading to gene silencing. Studies have revealed altered DNA methylation patterns in AD patients, with changes linked to disease severity and susceptibility. For instance, hypomethylation of the FLG gene (filaggrin) can increase AD risk by reducing its expression and compromising the skin barrier [36,37]. Conversely, changes in methylation can impact genes involved in immune function, such as IL-13, where hypomethylation can lead to increased expression and enhanced allergic responses [37].
Histone modifications involve chemical changes to histone proteins around which DNA is wrapped. Such modifications can either relax or compact the chromatin structure, thereby influencing gene accessibility and expression. In AD, histone modifications have been shown to regulate genes critical for inflammation and immune response, with increased levels of certain histone marks (e.g., H3K4me3) found in promoter regions of pro-inflammatory cytokine genes like IL-4 and IL-13 in AD patients [38]. Non-coding RNAs (ncRNAs), including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), regulate gene expression post-transcriptionally. Their dysregulation has also been observed in AD, affecting the expression of pro-inflammatory cytokines and other genes central to the disease’s pathogenesis [37,39].
5.2. Exposome
The exposome refers to the sum of environmental exposures, encompassing physical, chemical, and biological factors, that an individual encounters from conception onward. The exposomes interacts with the body’s biological and epigenetic landscape, thus modulating AD susceptibility and severity. Environmental pollutants are a key component of the exposome and play an important role in both the development and exacerbation of AD. Exposure to fine particulate matter, polycyclic aromatic hydrocarbons (PAHs), and volatile organic compounds have been shown to disrupt the epidermal barrier, induce oxidative stress, and trigger epigenetic alterations in immune-regulating genes such as IFN-γ, thereby increasing AD susceptibility [40,41,42,43]. Household exposure to BTEX (benzene, toluene, ethylbenzene, xylenes) compounds is strongly linked to childhood AD, with studies highlighting geographic disparities in exposure [41]. These volatile compounds promote Staphylococcus aureus survival on synthetic textiles, alter commensal metabolism, and activate the TRPA1 itch receptor, linking pollutants to both the onset and exacerbation of AD [41]. Other compounds such as isocyanates have been found to impair the protective lipid metabolism of beneficial commensals such as Roseomonas mucosa and Staphylococcus epidermidis, reducing barrier resilience and diminishing the efficacy of microbiome-based therapies [40]. This phenomenon is consistent with the concept of the “dysbiotic march,” where pollutants disrupt the skin microbiome in ways that perpetuate barrier dysfunction and chronic inflammation. This framework illustrates the role of pollutants as both triggers for disease onset in genetically susceptible individuals and as key contributors to disease persistence and exacerbations. Chronic exposure to dust mites, pollen, soaps, and detergents can trigger and exacerbate AD, potentially by inducing epigenetic changes that perpetuate inflammation and barrier dysfunction [44]. Early-life microbial diversity, both cutaneous and in the gut, as part of the exposome, plays a crucial role. Disturbances can lead to epigenetic changes affecting immune programming [37,44].
Dietary factors, as a significant part of the exposome, also exert a profound influence on AD development. Unhealthy dietary patterns, such as those high in processed foods or low in essential nutrients (e.g., folate, vitamin D), can contribute to systemic inflammation and gut dysbiosis and have been shown to be associated with increasing AD susceptibility [45]. Additionally, a diet lacking in specific micronutrients can alter DNA methylation patterns, impacting genes involved in anti-inflammatory pathways or barrier integrity. Protein consumption, especially protein derived from cereal, nuts, fish, and seafood, is linked with decreased AD in adolescents aged 13–14 years [46]. Supplementation with vitamin A, vitamin C, vitamin D, and vitamin E has also been shown to help reduce AD symptoms [47,48,49].
Recognition of the capacity of environmental exposures to induce stable epigenetic changes provides compelling insights into the rising incidence of AD in industrialized nations and the marked heterogeneity observed in disease expression, even among genetically similar individuals. This complex gene–environment interplay points toward novel approaches to prevention and the development of targeted therapies aimed at reversing aberrant epigenetic modifications in AD [44].
6. Pathophysiology of the Atopic March and Age-Related Differences in AD
6.1. Pathophysiology of the Atopic March
The atopic march refers to the natural progression of allergic diseases that typically begin early in life. It usually starts with AD in infancy and may progress sequentially to IGE-mediated food allergies, allergic rhinitis, and asthma as the individual grows. The current theory postulates that early-life skin barrier dysfunction, often due to filaggrin mutations and other genetic or environmental factors, impairs barrier function and facilitates epicutaneous allergen sensitization. This promotes a type 2 (Th2)-skewed immune response characterized by IL-4, IL-13, and IL-31, in addition to epithelial-derived alarmins (TSLP, IL-33, IL-25). These mediators promote local and systemic inflammation, drive IgE class switching, and increase susceptibility to downstream allergic diseases [50,51,52,53,54,55]. It should be noted, however, that not all children follow the full trajectory; only a subset transition from atopic dermatitis (AD) to allergic rhinitis, asthma, and food allergies [50,51]. This reflects the importance of the genetic and environmental risk factors that drive the immune mechanism of type 2 (Th2) inflammation and underscores the importance of controlling AD early in life to prevent further progression of the atopic march.
6.2. Differences Between Pediatric and Adult AD
Atopic dermatitis demonstrates distinct age-related phenotypes and endotypes as detailed in Table 2. Pediatric AD is mainly characterized by Th2 and Th22 inflammation with some Th17 involvement and exhibits increased epithelial alarmin activity (IL-33, IL-25, TSLP). Barrier dysfunction is evident even in non-lesional skin, increasing the risk for epicutaneous sensitization and progression through the atopic march [56,57]. Clinically, pediatric AD often presents with exudative, erythematous lesions, perifollicular accentuation, and involvement of the face, scalp, and extensor surfaces in infancy, with flexural distribution becoming more prominent later [56].

Table 2.
Distinct characteristics of pediatric vs. adult atopic dermatitis.
Adult AD is more heterogeneous. Although Th2 activity continues in adulthood, there is greater Th1 polarization seen with increased IFN-γ and CXCL9/10/11 alongside chronic epidermal hyperplasia and more pronounced filaggrin deficiency [58,59]. In adults, AD commonly presents as lichenified plaques, hand eczema, and nummular lesions, with less flexural involvement. Adults also demonstrate distinct proteomic profiles, specifically with elevated serum levels of inflammatory chemokines (e.g., CXCL9, CXCL10, CXCL11) and vascular adhesion molecules. These are features that overlap with pathways implicated in atherosclerosis and metabolic syndrome, indicating a broader systemic inflammatory burden beyond the skin [59]. These immunologic and phenotypic differences underscore the importance of age-tailored diagnostic criteria and therapeutic strategies.
7. Targeted Therapeutic Approaches for AD
The therapeutic landscape for AD has evolved significantly towards more targeted and pathophysiology-driven interventions. These approaches aim to address the multifaceted nature of the disease, from barrier dysfunction to immune dysregulation and microbial imbalances.
7.1. Topical Agents
Topical therapies remain the first-line and foundational treatment for managing mild-to-moderate AD and serve as adjuncts in severe cases. Emollients and moisturizers are the cornerstone of AD management. This includes lipid-replenishing moisturizers that contain essential components of the skin barrier, such as ceramides, fatty acids, and cholesterol. Their application aims to directly restore the lamellar structure of the stratum corneum, reinforcing its protective function. Additionally, pH-balanced emollients are crucial as they help to re-acidify the stratum corneum, thereby inhibiting the overactivity of serine proteases (like kallikreins), which contribute to barrier degradation [60]. The regular and consistent use of bland emollients is foundational for both preventing flares and maintaining remission in AD. Recent research is actively investigating novel topical agents specifically designed to target protease inhibition and enhance tight junction integrity, with some promising candidates currently under preclinical investigation [60].
Topical corticosteroids (TCSs) remain a mainstay of AD treatment. Examples include hydrocortisone, triamcinolone, and mometasone, available in various potencies. TCSs work by reducing inflammation, pruritus, and erythema by suppressing the immune response. They achieve this by inhibiting the synthesis and release of various inflammatory mediators. Their appropriate use, often in a “proactive” regimen (e.g., twice weekly on previously affected areas), can help prevent flares. Topical calcineurin inhibitors (TCIs) like tacrolimus and pimecrolimus offer steroid-sparing anti-inflammatory effects by inhibiting T cell activation and cytokine release. They are particularly useful for sensitive areas like the face and skin folds. Phosphodiesterase 4 (PDE4) inhibitors work by inhibiting the enzyme PDE4, which leads to an increase in intracellular cyclic adenosine monophosphate (cAMP) levels. Elevated cAMP can suppress the release of pro-inflammatory cytokines (e.g., TNF-α, IL-23, IL-17) and enhance the production of anti-inflammatory mediators. Crisaborole ointment is approved for mild-to-moderate AD, while Roflumilast cream, a newer PDE4 inhibitor, offers a once-daily application for its anti-inflammatory effects [61].
7.2. Systemic Biologics
Targeted biologics, particularly monoclonal antibodies, have truly revolutionized the treatment landscape for moderate-to-severe AD, offering precise immunomodulation with significantly improved efficacy and safety profiles compared to traditional systemic immunosuppressants. Currently, there are three different classes of biologics based on the pathways that they inhibit: IL4/IL13 inhibitors, IL31 inhibitors, and JAK inhibitors.
7.2.1. Dupilumab: Cornerstone of AD Treatment and Implications for Th-17
Dupilumab, the first biologic approved for AD, is a monoclonal antibody that specifically blocks the IL-4 receptor alpha (IL-4Rα) subunit. Since IL-4Rα is a shared receptor component for both IL-4 and IL-13 signaling pathways, dupilumab effectively inhibits the downstream effects of both these pivotal cytokines. This leads to significant improvement in inflammation, pruritus, and restoration of skin barrier function across various age groups. Dupilumab is associated with normalization of Th2 inflammatory molecule expression and reverses epidermal barrier abnormalities, for example, by increasing the expression of differentiation genes such as FLG [62]. Skin stripping studies following dupilumab treatment shows significant decreased in several molecules, including MMP12, CCL13, CCL17, IL12b, CXCL1, and cytokines involved in innate immunity (IL-6, IL8, and IL17C) [63,64].
Dupilumab has shown efficacy and safety in treating AD across different age groups, but some age-related differences in response have been noted. Younger children (under 6 years) tend to respond as well or slightly better compared to older children [65,66]. In adults and older adults, dupilumab also maintains clinical effectiveness over long-term treatment (up to 5 years observed), with similar reductions in disease severity measures. Although studies show adults may have slightly more filaggrin deficiency and Th1 polarization, overall response to dupilumab is comparable among children, adults, and older adults, though discontinuation due to side effects or inefficacy occurs in about 20–25% of adult patients [67]. Overall, differences in immune profiles by age may exist but do not substantially alter dupilumab’s efficacy. Safety profiles are favorable across all populations, with common adverse events including conjunctivitis and paradoxical eczema flares, particularly reported in younger children [66].
While dupilumab suppresses Th2-driven inflammation, it may result in relative skewing toward the Th17 pathway in a subset of patients. This phenomenon is most evident in psoriasiform eruptions and other Th17-mediated conditions, with transcriptomic and protein analyses showing increased IL-17 and IL-36 activity alongside decreased Th2 gene expression [68,69,70]. Large pharmacological studies and case reports have linked dupilumab to emergent Th17-type diseases, including psoriasis and seronegative arthritis, suggesting that IL-4/IL-13 blockade can unmask IL-23/IL-17 axis activity in predisposed individuals [68,70].
In most patients with AD, dupilumab selectively suppresses Th2 and Th22 cytokines without altering overall T helper cell proportions [71,72,73]. Rarely, particularly in those with low Th2/IL-13 expression, Th17 and Th1 pathways may become more prominent, leading to paradoxical inflammatory manifestations such as psoriasiform disease, arthritis, or ocular inflammation [74,75]. In summary, dupilumab remains a cornerstone of AD therapy, but clinicians should monitor for rare Th17-driven adverse events in susceptible patients.
7.2.2. Other Systemic Therapies
Tralokinumab is a monoclonal antibody that specifically neutralizes IL-13 and prevents its binding to receptors. It offers a targeted approach to inhibiting a key cytokine in the Th2 pathway, demonstrating favorable efficacy and safety profiles. Lebrikizumab is another monoclonal antibody that specifically targets and neutralizes IL-13. Similarly, to tralokinumab, it aims to reduce IL-13-driven inflammation and symptoms in AD patients. Cendakimab is also an anti-IL-13 agent currently undergoing phase 2 clinical trials, indicating continued development in this highly effective therapeutic area [76].
Nemolizumab is a monoclonal antibody that acts as an IL-31 receptor alpha antagonist. Given IL-31’s direct role in stimulating sensory neurons and driving pruritus, nemolizumab primarily targets the debilitating itch pathway in AD, offering relief often where other treatments fall short.
Janus Kinase (JAK) inhibitors are small-molecule drugs that inhibit the intracellular JAK-STAT signaling pathway, which is crucial for the signaling of numerous cytokines involved in AD inflammation (including IL-4, IL-13, IL-31, IFN-γ, etc.). They are available in both oral and topical formulations. Oral JAK inhibitors such as Baricitinib (a JAK1/2 inhibitor), Upadacitinib, and Abrocitinib (both are selective JAK1 inhibitors), are approved for moderate-to-severe AD and have demonstrated rapid and robust efficacy in clinical trials. Currently Ruxolitinib is the only topical JAK inhibitor approved for mild-to-moderate AD in the USA. Delgocitinib, a pan-JAK inhibitor approved in Japan for moderate-to-severe AD, is expected to gain FDA approval for chronic hand eczema in the US. Other topical JAK inhibitors such as brepocitinib, ATI-1777, jaktinib, ifidancitinib, and ivarmacitinib are in various stages of clinical development, highlighting intense research in this class [77].
7.2.3. Emerging Systemic Therapies and Ongoing Trials
Beyond established biologics such as dupilumab and tralokinumab, the therapeutic pipeline for atopic dermatitis (AD) is rapidly expanding, with several agents in late-stage development. As of 2025, multiple phase 3 clinical trials are underway evaluating novel biologics and small molecules for moderate-to-severe AD. These include Jaktinib (NCT05526222), a pan-JAK inhibitor targeting JAK1, JAK2, JAK3, and TYK2. Its phase 3 program enrolls adults with moderate-to-severe AD in a randomized, double-blind, placebo-controlled design, assessing both efficacy and safety. Another candidate, CM310 (Stapokibart) (NCT05265923), is a humanized monoclonal antibody against IL-4Rα that blocks both IL-4 and IL-13 signaling [78]. In its phase 2b trial, CM310 achieved EASI-75 in up to 70% of patients at 16 weeks with significant improvements in pruritus, quality of life, and inflammatory biomarkers [79]. The ongoing phase 3 trial is enrolling patients aged 18–75 years with moderate-to-severe AD. Collectively, these phase 3 trials reflect a shift toward expanding therapeutic targets while broadening inclusion to adolescent and adult populations to ensure safe and effective long-term options across the age spectrum.
7.3. Emerging Treatments in Microbiome-Targeted Approaches and Nutritional Supplementation
Therapeutic strategies aimed at correcting microbial dysbiosis have garnered considerable attention in recent years, recognizing the critical interplay between the microbiome and AD pathophysiology. A key area of focus is the reduction in Staphylococcus aureus colonization and the restoration of healthy microbial diversity on the skin and in the gut.
Topical probiotics derived from commensal or exogenous bacterial strains, specifically those demonstrated to inhibit S. aureus growth in in vivo and in vitro studies, have been evaluated in multiple clinical trials. These studies have reported promising results including clinical improvement and a decrease in S. aureus colonization, generally with minimal side effects [80]. However, it is important to note that some systematic reviews suggest that while S. aureus burden is consistently reduced, the objective clinical improvement in moderate-to-severe AD, particularly as a monotherapy, may still be limited. Therefore, larger, well-designed clinical trials are needed to further clarify their consistent efficacy and optimal integration into treatment regimens [80,81].
Oral probiotics have shown mixed results in AD, with inconsistent outcomes observed across various studies, suggesting that strain-specific effects, patient populations, and study designs significantly influence efficacy. More robust research is needed to identify optimal strains and treatment protocols. Newer, more advanced approaches, such as microbiome transplantation (e.g., fecal microbiota transplantation for gut dysbiosis or targeted skin microbiome transfer) are also under investigation [82].
Vitamin D supplementation has shown potential benefits in reducing the severity of AD, particularly in individuals with vitamin D deficiency or insufficiency. Systematic reviews and meta-analyses report clinically meaningful improvements, especially in standardized severity scores such as SCORAD (Scoring Atopic Dermatitis) and EASI (Eczema Area and Severity Index). SCORAD incorporates both objective signs (extent and intensity of lesions) and subjective symptoms (itch and sleep disturbance), while EASI provides an objective assessment based on lesion severity and body surface area affected. Clinical trials have shown improvements with vitamin D doses of 1000–2000 IU/day over 1–3 months [83,84]. Mechanistically, vitamin D may enhance skin barrier function and reduce Staphylococcus aureus colonization by upregulating LL-37, a key antimicrobial peptide involved in innate immunity that also modulates inflammation and wound healing [83,84,85]. However, results across studies are mixed, with some randomized controlled trials showing no significant benefit [49,85]. Therefore, while vitamin D may be a useful adjunct in patients with confirmed deficiency, routine supplementation for all individuals with AD is not currently supported by consistent evidence.
7.4. Doubtful Role of Antibiotics
Antibiotics have a limited and highly specific role in the management of atopic dermatitis (AD). They are indicated only when there is clear clinical evidence of secondary bacterial infection, such as pustules, purulent exudate, or systemic signs of infection. In such cases, systemic antibiotics may be prescribed alongside standard anti-inflammatory therapies such as topical corticosteroids [86,87]. Recent clinical trials and systematic reviews have confirmed that antibiotics do not improve outcomes in AD flares without overt infection. Their use in this context may instead promote antimicrobial resistance and disrupt the skin microbiome [88,89]. Colonization with Staphylococcus aureus is common in AD; however, eradication of colonization alone has not demonstrated sustained improvement in disease severity [89]. Instead, management should hinge on the importance of early barrier repair, patient education, and topical anti-inflammatory therapy while recommending biologics and JAK inhibitors in severe disease [77,89]. When antibiotics are required for secondary infection, their selection should be guided by local antimicrobial susceptibility patterns. Empiric therapy should be tailored to local resistance thresholds and adjusted according to clinical severity. Topical antibiotics are generally not recommended in uninfected AD, as they do not improve disease outcomes compared to topical corticosteroids alone and may increase the risk of contact dermatitis and antimicrobial resistance [87,89]. In recognition of the limitations of antibiotics, non-antibiotic strategies—such as dilute bleach baths and topical antiseptics like chlorhexidine—may be considered in patients with recurrent infections or severe phenotypes prone to colonization [89,90]. These adjunctive approaches provide options for reducing bacterial load without exposing patients to the risks of systemic antibiotic use.
7.5. Comparison of International Guidelines on AD Management
International guidelines for atopic dermatitis (AD) management hinge on the importance of early barrier repair, patient education, and topical anti-inflammatory therapy, with some differences in diagnostic frameworks, escalation strategies, and access considerations. The World Health Organization (WHO) emphasizes practical, resource-adapted approaches prioritizing emollients and topical corticosteroids (TCS) [91]. The European Academy of Allergy and Clinical Immunology (EAACI) in collaboration with European Task Force on Atopic Dermatitis (ETFAD)/European Academy of Dermatology and Venerology (EADV) advocates for validated diagnostic criteria, proactive maintenance strategies, and structured patient education while recommending biologics and JAK inhibitors in severe disease [92,93,94]. The American Academy of Allergy, Asthma, and Immunology (AAAAI) apply a GRADE-based framework, incorporating patient values, shared decision-making, and specific recommendations for biologics and JAK inhibitors stratified by age [61].
In summary, these guidelines share a common consensus: AD should be clinically diagnosed, treated first with emollients and topical anti-inflammatory agents, with an emphasis on patient education (Table 3). WHO guidance prioritizes feasibility in resource-limited settings [91]. EAACI/ETFAD/EADV highlight proactive anti-inflammatory regimens and structured education programs [92,93], while the AAAAI emphasizes individualized care through shared decision-making and recommendations for advanced therapies across pediatric and adult populations [61].

Table 3.
Comparative summary of international guidelines on AD management.
8. Conclusions
The field of AD is experiencing rapid advancements, shifting towards more precise and personalized treatment strategies. Recent research highlighted in this review underscores the complex and intricate interplay among genetic predispositions, immune system dysregulation, compromised skin barrier function, and various environmental factors. This deeper understanding is directly leading to the development of highly targeted therapies, such as biologics and JAK inhibitors, which represent a significant paradigm shift from broad immunosuppression toward highly targeted immunotherapy (Table 4). This precision approach offers improved disease control, often with a reduced risk of the systemic toxicities associated with older, less specific treatments, thereby offering significant improvements for patients, including those living with moderate-to-severe forms of the disease.

Table 4.
Targeted approach in atopic dermatitis based on pathophysiology of disease.
Author Contributions
Conceptualization, E.B.H.-S. and S.H.; writing—original draft preparation, E.B.H.-S.; writing—review and editing, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Atopic dermatitis |
| TEWL | Transepidermal water loss |
| IL-4 | Interleukin-4 |
| IL-13 | Interleukin-13 |
| TSLP | Thymic Stromal Lymphopoietin |
| IL-33 | Interleukin-33 |
| IL-25 | Interleukin-25 |
| ILC | Innate Lymphoid Cell |
| JAK | Janus Kinase |
| SCFA | Short-chain fatty acid |
| HMO | Human milk oligosaccharide |
| ILA | indole-3-lactic acid |
| I3C | indole-3-carbaldehyde |
| LPS | Lipopolysaccharide |
| SCORAD | Scoring Atopic Dermatitis |
| EASI | Eczema Area and Severity Index |
References
- Silverberg, J.I. Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol. Clin. 2017, 35, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M. Current Understanding of Pathophysiological Mechanisms of Atopic Dermatitis: Interactions among Skin Barrier Dysfunction, Immune Abnormalities and Pruritus. Biol. Pharm. Bull. 2020, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, N.; Irvine, A.D. Filaggrin and beyond: New insights into the skin barrier in atopic dermatitis and allergic diseases, from genetics to therapeutic perspectives. Ann. Allergy Asthma Immunol. 2024, 132, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kabashima, K. Advances in atopic dermatitis in 2019–2020: Endotypes from skin barrier, ethnicity, properties of antigen, cytokine profiles, microbiome, and engagement of immune cells. J. Allergy Clin. Immunol. 2021, 148, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Möbus, L.; Rodriguez, E.; Harder, I.; Boraczynski, N.; Szymczak, S.; Hübenthal, M.; Stölzl, D.; Gerdes, S.; Kleinheinz, A.; Abraham, S.; et al. Blood transcriptome profiling identifies 2 candidate endotypes of atopic dermatitis. J. Allergy Clin. Immunol. 2022, 150, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Sackey, T. Pathophysiology of atopic dermatitis and its targeted treatment. Created in BioRender. 2025. Available online: https://BioRender.com/um1kk7z (accessed on 6 August 2025).
- Hatano, Y.; Katagiri, K.; Arakawa, S.; Fujiwara, S. Interleukin-4 depresses levels of transcripts for acid-sphingomyelinase and glucocerebrosidase and the amount of ceramide in acetone-wounded epidermis, as demonstrated in a living skin equivalent. J. Dermatol. Sci. 2007, 47, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.R.; Seminario-Vidal, L.; Abe, B.; Ghobadi, C.; Sims, J.T. Cytokines and Epidermal Lipid Abnormalities in Atopic Dermatitis: A Systematic Review. Cells 2023, 12, 2793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simpson, E.L.; Chalmers, J.R.; Hanifin, J.M.; Thomas, K.S.; Cork, M.J.; McLean, W.H.I.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. JAMA Pediatr. 2014, 168, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Cork, M.J.; Danby, S.G.; Vasilopoulos, Y.; Hadgraft, J.; Lane, M.E.; Moustafa, M.; Guy, R.H.; MacGowan, A.; Tazi-Ahnini, R.; Ward, S.J. Epidermal barrier dysfunction in atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1892–1908. [Google Scholar] [CrossRef] [PubMed]
- Morizane, S.; Sunagawa, K.; Nomura, H.; Ouchida, M. Aberrant serine protease activities in atopic dermatitis. J. Dermatol. Sci. 2022, 107, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Jeong, S.K.; Lee, S.H. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med. J. 2010, 51, 808–822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nomura, H.; Suganuma, M.; Takeichi, T.; Kono, M.; Isokane, Y.; Sunagawa, K.; Kobashi, M.; Sugihara, S.; Kajita, A.; Miyake, T.; et al. Multifaceted Analyses of Epidermal Serine Protease Activity in Patients with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meesters, L.D.; Roubroeks, J.A.Y.; Gerritsen, A.; Velthuijs, N.; Klijnhout, J.A.; Laberthonnière, C.; van Vlijmen-Willems, I.M.; Hübenthal, M.; Rodijk-Olthuis, D.; Peters, R.H.W.; et al. Dissecting key contributions of TH2 and TH17 cytokines to atopic dermatitis pathophysiology. J. Allergy Clin. Immunol. 2025, 156, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, Y.; Nakashima, C.; Otsuka, A. Interplay of cytokines in the pathophysiology of atopic dermatitis: Insights from Murin models and human. Front. Med. 2024, 11, 1342176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vafaeian, A.; Rajabi, F.; Rezaei, N. Toll-like receptors in atopic dermatitis: Pathogenesis and therapeutic implications. Heliyon 2025, 11, e42226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suárez-Fariñas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is T2 but also T17 polarized in skin. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Czarnowicki, T. The pathogenetic role of Th17 immune response in atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O. Targeting cytokines and signaling molecules related to immune pathways in atopic dermatitis: Therapeutic implications and challenges. Arch. Pharm. Res. 2022, 45, 894–908. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T.; Paller, A.S.; Kabashima, K.; Feely, M.; Rueda, M.J.; Terres, J.A.R.; Wollenberg, A. Atopic dermatitis: Pathomechanisms and lessons learned from novel systemic therapeutic options. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1432–1449. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S. Atopic dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Trier, A.M.; Li, F.; Kim, S.; Chen, Z.; Chai, J.N.; Mack, M.R.; Morrison, S.A.; Hamilton, J.D.; Baek, J.; et al. A basophil-neuronal axis promotes itch. Cell 2021, 184, 422–440.e17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Misery, L.; Pierre, O.; Le Gall-Ianotto, C.; Lebonvallet, N.; Chernyshov, P.V.; Le Garrec, R.; Talagas, M. Basic mechanisms of itch. J. Allergy Clin. Immunol. 2023, 152, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Renert-Yuval, Y.; Pavel, A.B.; Bose, S.; Gómez-Arias, P.J.; Rangel, S.M.; Estrada, Y.D.; Paller, A.S.; Guttman-Yassky, E. Tape strips capture atopic dermatitis-related changes in nonlesional skin throughout maturation. Allergy 2022, 77, 3445–3447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Libon, F.; Caron, J.; Nikkels, A.F. Biomarkers in Atopic Dermatitis. Dermatol. Ther. 2024, 14, 1729–1738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, H.; Li, W.; Xu, Y.; Zhou, Y.; Hamblin, M.R.; Wen, X. Gut microbiota modulation: A key determinant of atopic dermatitis susceptibility in children. Front. Microbiol. 2025, 16, 1549895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Sakanaka, M.; von Burg, N.; Mörbe, U.; Andersen, D.; Moll, J.M.; Pekmez, C.T.; Rivollier, A.; Michaelsen, K.F.; Mølgaard, C.; et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat. Microbiol. 2021, 6, 1367–1382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hülpüsch, C.; Rohayem, R.; Reiger, M.; Traidl-Hoffmann, C. Exploring the skin microbiome in atopic dermatitis pathogenesis and disease modification. J. Allergy Clin. Immunol. 2024, 154, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Hoskinson, C.; Medeleanu, M.V.; Reyna, M.E.; Dai, D.L.Y.; Chowdhury, B.; Moraes, T.J.; Mandhane, P.J.; Simons, E.; Kozyrskyj, A.L.; Azad, M.B.; et al. Antibiotics taken within the first year of life are linked to infant gut microbiome disruption and elevated atopic dermatitis risk. J. Allergy Clin. Immunol. 2024, 154, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.D.; de Guzman Strong, C. Current understanding of epigenetics in atopic dermatitis. Exp. Dermatol. 2021, 30, 1150–1155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- da Silva Duarte, A.J.; Sanabani, S.S. Deciphering epigenetic regulations in the inflammatory pathways of atopic dermatitis. Life Sci. 2024, 348, 122713. [Google Scholar] [CrossRef] [PubMed]
- Traisaeng, S.; Herr, D.R.; Kao, H.J.; Chuang, T.H.; Huang, C.M. A Derivative of Butyric Acid, the Fermentation Metabolite of Staphylococcus epidermidis, Inhibits the Growth of a Staphylococcus aureus Strain Isolated from Atopic Dermatitis Patients. Toxins 2019, 11, 311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liew, W.C.; Sundaram, G.M.; Quah, S.; Lum, G.G.; Tan, J.S.L.; Ramalingam, R.; Common, J.E.A.; Tang, M.B.Y.; Lane, E.B.; Thng, S.T.G.; et al. Belinostat resolves skin barrier defects in atopic dermatitis by targeting the dysregulated miR-335:SOX6 axis. J. Allergy Clin. Immunol. 2020, 146, 606–620.e12. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, J.; Ratley, G.; Shobnam, N.; Myles, I.A. The clinical, mechanistic, and social impacts of air pollution on atopic dermatitis. J. Allergy Clin. Immunol. 2024, 154, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Ratley, G.; Zeldin, J.; Sun, A.A.; Yadav, M.; Chaudhary, P.P.; Myles, I.A. Spatial modeling connecting childhood atopic dermatitis prevalence with household exposure to pollutants. Commun. Med. 2024, 4, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, B.E.; Kim, J.; Goleva, E.; Berdyshev, E.; Lee, J.; Vang, K.A.; Lee, U.H.; Han, S.; Leung, S.; Hall, C.F.; et al. Particulate matter causes skin barrier dysfunction. JCI Insight 2021, 6, e145185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Y.Y.; Jin, H.; Lu, Q. Effect of polycyclic aromatic hydrocarbons on immunity. J. Transl. Autoimmun. 2022, 5, 100177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grafanaki, K.; Bania, A.; Kaliatsi, E.G.; Vryzaki, E.; Vasilopoulos, Y.; Georgiou, S. The Imprint of Exposome on the Development of Atopic Dermatitis across the Lifespan: A Narrative Review. J. Clin. Med. 2023, 12, 2180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, S.I.; Lee, H.; Lee, D.H.; Kim, K.H. Association of frequent intake of fast foods, energy drinks, or convenience food with atopic dermatitis in adolescents. Eur. J. Nutr. 2020, 59, 3171–3182. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Strachan, D.; Asher, I.; Ellwood, P.; Pearce, N.; Garcia-Marcos, L.; ISAAC Phase III Study Group; ISAAC Phase Three Study Group. Combined impact of healthy lifestyle factors on risk of asthma, rhinoconjunctivitis and eczema in school children: ISAAC phase III. Thorax 2019, 74, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Jaffary, F.; Faghihi, G.; Mokhtarian, A.; Hosseini, S.M. Effects of oral vitamin E on treatment of atopic dermatitis: A randomized controlled trial. J. Res. Med. Sci. 2015, 20, 1053–1057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, J.; Kim, Y.J.; Kwon, O.; Kim, N.I.; Cho, Y. Associations among plasma vitamin C, epidermal ceramide and clinical severity of atopic dermatitis. Nutr. Res. Pract. 2016, 10, 398–403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nielsen, A.Y.; Høj, S.; Thomsen, S.F.; Meteran, H. Vitamin D supplementation for treating atopic dermatitis in children and adults: A systematic review and meta-analysis. Nutrients 2024, 16, 4128. [Google Scholar] [CrossRef]
- Davidson, W.F.; Leung, D.Y.M.; Beck, L.A.; Berin, C.M.; Boguniewicz, M.; Busse, W.W.; Chatila, T.A.; Geha, R.S.; Gern, J.E.; Guttman-Yassky, E.; et al. Report from the National Institute of Allergy and Infectious Diseases Workshop on “Atopic Dermatitis and the Atopic March: Mechanisms and Interventions”. J. Allergy Clin. Immunol. 2019, 143, 894–913. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, A.D. The Atopic March and Atopic Multimorbidity: Many Trajectories, Many Pathways. J. Allergy Clin. Immunol. 2019, 143, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Du Toit, G.; Davis, C.M. Might Biologics Serve to Interrupt the Atopic March? J. Allergy Clin. Immunol. 2023, 151, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Roan, F.; Ziegler, S.F. The Atopic March: Current Insights Into Skin Barrier Dysfunction and Epithelial Cell-Derived Cytokines. Immunol. Rev. 2017, 278, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Novel Concepts of Prevention and Treatment of Atopic Dermatitis Through Barrier and Immune Manipulations with Implications for the Atopic March. J. Allergy Clin. Immunol. 2017, 139, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F. Thymic Stromal Lymphopoietin, Skin Barrier Dysfunction, and the Atopic March. Ann. Allergy Asthma Immunol. 2021, 127, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Marín, H.A.; Silverberg, J.I. Differences Between Pediatric and Adult Atopic Dermatitis. Pediatr. Dermatol. 2022, 39, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Renert-Yuval, Y.; Del Duca, E.; Pavel, A.B.; Fang, M.; Lefferdink, R.; Wu, J.; Diaz, A.; Estrada, Y.D.; Canter, T.; Zhang, N.; et al. The Molecular Features of Normal and Atopic Dermatitis Skin in Infants, Children, Adolescents, and Adults. J. Allergy Clin. Immunol. 2021, 148, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Munayco Maldonado, G.; Foy, V.; Tai, H.; Chiesa Fuxench, Z.C. Variation in Clinical Presentation of Pediatric-Onset and Adult-Onset Atopic Dermatitis: A Retrospective, Single-Center, Chart Review of Adults with Atopic Dermatitis from the United States. Arch. Dermatol. Res. 2024, 316, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Facheris, P.; Da Rosa, J.C.; Pagan, A.D.; Angelov, M.; Del Duca, E.; Rabinowitz, G.; Gómez-Arias, P.J.; Rothenberg-Lausell, C.; Estrada, Y.D.; Bose, S.; et al. Age of Onset Defines Two Distinct Profiles of Atopic Dermatitis in Adults. Allergy 2023, 78, 2202–2214. [Google Scholar] [CrossRef] [PubMed]
- Sala-Cunill, A.; Lazaro, M.; Herráez, L.; Quiñones, M.D.; Moro-Moro, M.; Sanchez, I.; Skin Allergy Committee of Spanish Society of Allergy and Clinical Immunology (SEAIC). Basic Skin Care and Topical Therapies for Atopic Dermatitis: Essential Approaches and Beyond. J. Investig. Allergol. Clin. Immunol. 2018, 28, 379–391. [Google Scholar] [CrossRef] [PubMed]
- AAAAI/ACAAI JTF Atopic Dermatitis Guideline Panel; Chu, D.K.; Schneider, L.; Asiniwasis, R.N.; Boguniewicz, M.; De Benedetto, A.; Ellison, K.; Frazier, W.T.; Greenhawt, M.; Huynh, J.; et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE- and Institute of Medicine-based recommendations. Ann. Allergy Asthma Immunol. 2024, 132, 274–312. [Google Scholar] [CrossRef] [PubMed]
- Mastraftsi, S.; Vrioni, G.; Bakakis, M.; Nicolaidou, E.; Rigopoulos, D.; Stratigos, A.J.; Gregoriou, S. Atopic Dermatitis: Striving for Reliable Biomarkers. J. Clin. Med. 2022, 11, 4639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goleva, E.; Berdyshev, E.; Kreimer, S.; Reisz, J.A.; D’Alessandro, A.; Bronova, I.; Lyubchenko, T.; Richers, B.N.; Hall, C.F.; Xiao, O.; et al. Longitudinal integrated proteomic and metabolomic skin changes in patients with atopic dermatitis treated with dupilumab. J. Allergy Clin. Immunol. 2025, 155, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, L. Potential biomarkers of atopic dermatitis. Front. Med. 2022, 9, 1028694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, N.; Ye, Y.; Shao, J.; Wu, H.; Xu, Q.; Zhu, J.; Liu, J.; Li, Z. Efficacy of Dupilumab in Children 6 Months to 11 Years Old with Atopic Dermatitis: A Retrospective Real-World Study in China. Dermatitis 2024, 35 (Suppl. S1), S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, B.; Wang, W. Effectiveness and safety of dupilumab in the treatment of pediatric atopic dermatitis: A real-world study from China. Front. Immunol. 2025, 16, 1644875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boesjes, C.M.; Kamphuis, E.; de Graaf, M.; Spekhorst, L.S.; Haeck, I.; van der Gang, L.F.; Loman, L.; Zuithoff, N.P.A.; Dekkers, C.; van der Rijst, L.P.; et al. Long-Term Effectiveness and Reasons for Discontinuation of Dupilumab in Patients with Atopic Dermatitis. JAMA Dermatol. 2024, 160, 1044–1055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grolleau, C.; Calugareanu, A.; Demouche, S.; Nosbaum, A.; Staumont-Sallé, D.; Aubert, H.; Cassius, C.; Jachiet, M.; Saussine, A.; Bagot, M.; et al. IL-4/IL-13 inhibitors for atopic dermatitis induce psoriatic rash transcriptionally close to pustular psoriasis. J. Invest. Dermatol. 2023, 143, 711–721.e7. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Wu, L.; Qiu, Y.; Li, M. Case report: Clinical and histopathological characteristics of psoriasiform erythema and IL-17A cytokines expression on lesioned skin in atopic dermatitis children treated with dupilumab. Front. Med. 2022, 9, 932766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bridgewood, C.; Wittmann, M.; Macleod, T.; Watad, A.; Newton, D.; Bhan, K.; Amital, H.; Damiani, G.; Giryes, S.; Bragazzi, N.L.; et al. T helper 2 IL-4/IL-13 dual blockade with dupilumab is linked to some emergent T helper 17–type diseases, including seronegative arthritis and enthesitis/enthesopathy, but not to humoral autoimmune diseases. J. Invest. Dermatol. 2022, 142, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, G.; Napolitano, M.; Quaranta, M.; Picone, V.; Fabbrocini, G.; Patruno, C. Phenotype-endotype relationship in elderly atopic dermatitis and effects of dupilumab therapy: Prospective study. Arch. Dermatol. Res. 2025, 317, 575. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Valido, K.; Swallow, M.; Okifo, K.O.; Wang, A.; Cohen, J.M.; Damsky, W. Baseline skin cytokine profiles determined by RNA in situ hybridization correlate with response to dupilumab in patients with eczematous dermatitis. J. Am. Acad. Dermatol. 2023, 88, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Camela, E.; Giampetruzzi, A.R.; De Pità, O.; Pallotta, S.; Russo, F. Dupilumab in real-life settings: A review of adverse events and their pathogenesis. Expert. Opin. Drug Saf. 2024, 23, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Thormann, K.; Lüthi, A.S.; Deniau, F.; Heider, A.; Cazzaniga, S.; Radonjic-Hoesli, S.; Lehmann, M.; Schlapbach, C.; Herzog, E.L.; Kreuzer, M.; et al. Dupilumab-associated ocular surface disease is characterized by a shift from Th2/Th17 toward Th1/Th17 inflammation. Allergy 2024, 79, 937–948. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/search?cond=atopic%20dermatitis (accessed on 2 August 2025).
- ClinicalTrials.gov. A Study of Jaktinib in Patients with Moderate-to-Severe Atopic Dermatitis. Available online: https://clinicaltrials.gov/study/NCT05526222 (accessed on 13 September 2025).
- ClinicalTrials.gov. A Study of CM310 (Stapokibart) in Patients with Moderate-to-Severe Atopic Dermatitis. Available online: https://clinicaltrials.gov/study/NCT05265923 (accessed on 13 September 2025).
- Yang, X.; Li, Z.; Ding, Y.; Li, J.; Ding, Y.; Wu, L.; Zhang, L.; Wang, J.; Zhu, X.; Zhang, F.; et al. Efficacy and safety of CM310, a novel IL-4Rα antagonist, in adults with moderate-to-severe atopic dermatitis: A randomized, double-blind, placebo-controlled, phase 2b trial. Lancet Reg. Health West. Pac. 2023, 39, 100796. [Google Scholar] [CrossRef]
- Chu, C.Y. Treatments for childhood atopic dermatitis: An update on emerging therapies. Clin. Rev. Allergy Immunol. 2021, 61, 114–127. [Google Scholar] [CrossRef]
- Wrześniewska, M.; Wołoszczak, J.; Świrkosz, G.; Szyller, H.; Gomułka, K. The Role of the Microbiota in the Pathogenesis and Treatment of Atopic Dermatitis-A Literature Review. Int. J. Mol. Sci. 2024, 25, 6539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Fang, Y.; Hu, J.; Li, S.; Zeng, L.; Chen, S.; Li, Z.; Meng, R.; Yang, X.; Zhang, F.; et al. Innovative microbial strategies in atopic dermatitis. Front. Immunol. 2025, 16, 1605434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borzutzky, A.; Iturriaga, C.; Pérez-Mateluna, G.; Cristi, F.; Cifuentes, L.; Silva-Valenzuela, S.; Vera-Kellet, C.; Cabalín, C.; Hoyos-Bachiloglu, R.; Navarrete-Dechent, C.; et al. Effect of weekly vitamin D supplementation on the severity of atopic dermatitis and type 2 immunity biomarkers in children: A randomized controlled trial. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Svensson, D.; Nebel, D.; Voss, U.; Ekblad, E.; Nilsson, B.O. Vitamin D-induced up-regulation of human keratinocyte cathelicidin anti-microbial peptide expression involves retinoid X receptor α. Cell Tissue Res. 2016, 366, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, Y.; Huang, H.; Wang, D. Serum Vitamin D Level and Efficacy of Vitamin D Supplementation in Children with Atopic Dermatitis: A Systematic Review and Meta-analysis. Comput. Math. Methods Med. 2022, 2022, 9407888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sidbury, R.; Davis, D.M.; Cohen, D.E.; Cordoro, K.M.; Berger, T.G.; Bergman, J.N.; Chamlin, S.L.; Cooper, K.D.; Feldman, S.R.; Hanifin, J.M.; et al. Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J. Am. Acad. Dermatol. 2014, 71, 327–349. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef]
- Elizalde-Jiménez, I.G.; Ruiz-Hernández, F.G.; Carmona-Cruz, S.A.; Pastrana-Arellano, E.; Aquino-Andrade, A.; Romo-González, C.; la Garza, E.A.-D.; Álvarez-Villalobos, N.A.; García-Romero, M.T. Global antimicrobial susceptibility patterns of Staphylococcus aureus in atopic dermatitis: A systematic review and meta-analysis. JAMA Dermatol. 2024, 160, 1171–1181. [Google Scholar] [CrossRef]
- George, S.M.; Karanovic, S.; Harrison, D.A.; Rani, A.; Birnie, A.J.; Bath-Hextall, F.J.; Ravenscroft, J.C.; Williams, H.C. Interventions to reduce Staphylococcus aureus in the management of eczema. Cochrane Database Syst Rev. 2019, 10, CD003871. [Google Scholar] [CrossRef]
- Schoch, J.J.; Anderson, K.R.; Jones, A.E.; Tollefson, M.M. Atopic dermatitis: Update on skin-directed management. Pediatrics 2025, 155, e2025071812. [Google Scholar] [CrossRef]
- Faye, O.; Flohr, C.; Kabashima, K.; Ma, L.; Paller, A.S.; Rapelanoro, F.R.; Steinhoff, M.; Su, J.C.; Takaoka, R.; Wollenberg, A.; et al. Atopic Dermatitis: A Global Health Perspective. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Christen-Zäch, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; de Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J.; et al. ETFAD/EADV Eczema Task Force 2020 Position Paper on Diagnosis and Treatment of Atopic Dermatitis in Adults and Children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Werfel, T.; Ring, J.; Ott, H.; Gieler, U.; Weidinger, S. Atopic Dermatitis in Children and Adults—Diagnosis and Treatment. Dtsch. Arztebl. Int. 2023, 120, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Maintz, L.; Bieber, T. Treatment of Atopic Dermatitis: Recently Approved Drugs and Advanced Clinical Development Programs. Allergy 2024, 79, 1501–1515. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).