Toxocara spp. Infection Influences on Eosinophil Levels: An Immunological Indicator of Severe Asthma and Allergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Sample Collection, Total IgE and sIgE Measurements
2.3. Skin Prick Tests (SPTs)
2.4. Sera Absorption with A. lumbricoides Extract
2.5. Detection of IgG Anti-Toxocara spp. Antibodies
2.6. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
3.2. Risk Factors Between Seropositivities and Eosinophils
3.3. Influence of Toxocara spp. Antibody on Eosinophil Numbers
3.4. Association Between Clinical Indicators of Asthma Severity and Toxocara spp. (Under Eosinophilia)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beran, D.; Zar, H.J.; Perrin, C.; Menezes, A.M.; Burney, P.; Forum of International Respiratory Societies working group collaboration. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir. Med. 2015, 3, 159–170. [Google Scholar] [CrossRef]
- The Global Asthma Report. Int. J. Tuberc. Lung Dis. 2022, 26, 1–102. [CrossRef]
- Teixeira, H.M.P.; Cruz, A.A.; Jesus, T.S.; de Santana, M.B.R.; Jesus, M.S.; Tugores, R.; Araujo, W.S.; Reis, R.C.C.; Pinheiro, G.P.; Figueiredo, C.A.; et al. The rs2601796 variant in ADCY9 gene is associated with severe asthma and less bronchodilator response. Gene 2023, 886, 147714. [Google Scholar] [CrossRef]
- Ntontsi, P.; Photiades, A.; Zervas, E.; Xanthou, G.; Samitas, K. Genetics and Epigenetics in Asthma. Int. J. Mol. Sci. 2021, 22, 2412. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.L.; Ribeiro-Silva Rde, C.; Malta, D.C.; Oliveira-Campos, M.; Andreazzi, M.A.; Cruz, A.A. Prevalence of asthma symptoms among adolescents in Brazil: National Adolescent School-based Health Survey (PeNSE 2012). Rev. Bras. Epidemiol. 2014, 17 (Suppl. S1), 106–115. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Acevedo, N. New Allergens of Relevance in Tropical Regions: The Impact of Ascaris lumbricoides Infections. World Allergy Organ. J. 2011, 4, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, O.; Genuneit, J.; Latzin, P.; Buchele, G.; Horak, E.; Loss, G.; Sozanska, B.; Weber, J.; Boznanski, A.; Heederik, D.; et al. Farming environments and childhood atopy, wheeze, lung function, and exhaled nitric oxide. J. Allergy Clin. Immunol. 2012, 130, 382–388 e386. [Google Scholar] [CrossRef]
- Levine, S.J.; Wenzel, S.E. Narrative review: The role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes. Ann. Intern. Med. 2010, 152, 232–237. [Google Scholar] [CrossRef]
- Mkhize-Kwitshana, Z.L.; Naidoo, P.; Nkwanyana, N.M.; Mabaso, M.L.H. Concurrent allergy and helminthiasis in underprivileged urban South African adults previously residing in rural areas. Parasite Immunol. 2022, 44, e12913. [Google Scholar] [CrossRef]
- Dlugosz, E.; Basalaj, K.; Zawistowska-Deniziak, A. Cytokine production and signalling in human THP-1 macrophages is dependent on Toxocara canis glycans. Parasitol. Res. 2019, 118, 2925–2933. [Google Scholar] [CrossRef]
- Kanobana, K.; Vereecken, K.; Junco Diaz, R.; Sariego, I.; Rojas, L.; Bonet Gorbea, M.; Polman, K. Toxocara seropositivity, atopy and asthma: A study in Cuban schoolchildren. Trop. Med. Int. Health 2013, 18, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Riahi, S.M.; Holland, C.V.; Taghipour, A.; Khalili-Fomeshi, M.; Fakhri, Y.; Omrani, V.F.; Hotez, P.J.; Gasser, R.B. Seroprevalence estimates for toxocariasis in people worldwide: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007809. [Google Scholar] [CrossRef] [PubMed]
- Woodhall, D.M.; Eberhard, M.L.; Parise, M.E. Neglected parasitic infections in the United States: Toxocariasis. Am. J. Trop. Med. Hyg. 2014, 90, 810–813. [Google Scholar] [CrossRef]
- Buendia, E.; Zakzuk, J.; Mercado, D.; Alvarez, A.; Caraballo, L. The IgE response to Ascaris molecular components is associated with clinical indicators of asthma severity. World Allergy Organ. J. 2015, 8, 8. [Google Scholar] [CrossRef]
- Ma, G.; Holland, C.V.; Wang, T.; Hofmann, A.; Fan, C.K.; Maizels, R.M.; Hotez, P.J.; Gasser, R.B. Human toxocariasis. Lancet Infect. Dis. 2018, 18, e14–e24. [Google Scholar] [CrossRef]
- Alcantara-Neves, N.M.; dos Santos, A.B.; Mendonca, L.R.; Figueiredo, C.A.; Pontes-de-Carvalho, L. An improved method to obtain antigen-excreting Toxocara canis larvae. Exp. Parasitol. 2008, 119, 349–351. [Google Scholar] [CrossRef]
- McDonnell, J.M.; Dhaliwal, B.; Sutton, B.J.; Gould, H.J. IgE, IgE Receptors and Anti-IgE Biologics: Protein Structures and Mechanisms of Action. Annu. Rev. Immunol. 2023, 41, 255–275. [Google Scholar] [CrossRef]
- Darlan, D.M.; Tala, Z.Z.; Amanta, C.; Warli, S.M.; Arrasyid, N.K. Correlation between Soil Transmitted Helminth Infection and Eosinophil Levels among Primary School Children in Medan. Open Access Maced. J. Med. Sci. 2017, 5, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Rodolpho, J.M.A.; Camillo, L.; Araujo, M.S.S.; Speziali, E.; Coelho-Dos-Reis, J.G.; Correia, R.O.; Neris, D.M.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Anibal, F.F. Robust Phenotypic Activation of Eosinophils during Experimental Toxocara canis Infection. Front. Immunol. 2018, 9, 64. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.; Woodfolk, J.A.; Erwin, E.A.; Aalberse, R. Mechanisms of tolerance to inhalant allergens: The relevance of a modified Th2 response to allergens from domestic animals. Springer Semin. Immunopathol. 2004, 25, 271–279. [Google Scholar] [CrossRef]
- Global Strategy for Asthma Management and Prevention. 2006. Available online: https://www.ginasthma.org/wp-content/uploads/2019/01/2007-GINA.pdf (accessed on 20 January 2025).
- Global Strategy for Asthma Management and Prevention. 2012. Available online: https://ginasthma.org/wp-content/uploads/2019/01/2012-GINA.pdf (accessed on 20 January 2025).
- Barreto, M.L.; Cunha, S.S.; Alcantara-Neves, N.; Carvalho, L.P.; Cruz, A.A.; Stein, R.T.; Genser, B.; Cooper, P.J.; Rodrigues, L.C. Risk factors and immunological pathways for asthma and other allergic diseases in children: Background and methodology of a longitudinal study in a large urban center in Northeastern Brazil (Salvador-SCAALA study). BMC Pulm. Med. 2006, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.D.; Zhao, J.W.; Sun, X.X.; Wu, F.; Jiang, Z.Y.; Cheng, Z.; Zheng, L.; Xue, H.K.; Yang, J.J.; Ming, L. Prevalence of allergen sensitization among 1,091 patients with urticaria. Exp. Ther. Med. 2020, 19, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Weinmayr, G.; Weiland, S.K.; Bjorksten, B.; Brunekreef, B.; Buchele, G.; Cookson, W.O.; Garcia-Marcos, L.; Gotua, M.; Gratziou, C.; van Hage, M.; et al. Atopic sensitization and the international variation of asthma symptom prevalence in children. Am. J. Respir. Crit. Care Med. 2007, 176, 565–574. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Savigny, D.H. In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigen for use in serodiagnostic tests for visceral larva migrans. J. Parasitol. 1975, 61, 781–782. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry; the Principles and Practice of Statistics in Biological Research; W. H. Freeman: San Francisco, CA, USA, 1969; p. xxi. 776p. [Google Scholar]
- Kim, B.S.; Wojno, E.D.; Artis, D. Innate lymphoid cells and allergic inflammation. Curr. Opin. Immunol. 2013, 25, 738–744. [Google Scholar] [CrossRef]
- Li, L.; Gao, W.; Yang, X.; Wu, D.; Bi, H.; Zhang, S.; Huang, M.; Yao, X. Asthma and toxocariasis. Ann. Allergy Asthma Immunol. 2014, 113, 187–192. [Google Scholar] [CrossRef]
- Rubinsky-Elefant, G.; Hirata, C.E.; Yamamoto, J.H.; Ferreira, M.U. Human toxocariasis: Diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann. Trop. Med. Parasitol. 2010, 104, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, C.N. The epidemiology and public health importance of toxocariasis: A zoonosis of global importance. Int. J. Parasitol. 2013, 43, 999–1008. [Google Scholar] [CrossRef]
- Silva, R.C.; Silva, J.W.d.; Fernandes, A.M.S.; Figueiredo, C.A.V.d.; Coneglian, N.G.d.M.; Alcântara Neves, N.M.; Pinheiro, C.d.S. A Roadmap to Toxocariasis Infection Control: A Comprehensive Study on Its Impact, Seroprevalence, and Allergic Implications in Latin America. Allergies 2024, 4, 124–137. [Google Scholar] [CrossRef]
- Despommier, D. Toxocariasis: Clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin. Microbiol. Rev. 2003, 16, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh Afshar, M.J.; Zahabiun, F.; Heydarian, P.; Mozafar Saadati, H.; Mohtasebi, S.; Khodamoradi, F.; Raissi, V. A Systematic Review and Meta-analysis of Toxocariasis in Iran: Is it Time to Take it Seriously? Acta Parasitol. 2020, 65, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Anibal, F.F.; Rogerio, A.P.; Malheiro, A.; Machado, E.R.; Martins-Filho, O.A.; Andrade, M.C.; Soares, E.G.; Medeiros, A.I.; Faccioli, L.H. Impact of MK886 on eosinophil counts and phenotypic features in toxocariasis. Scand. J. Immunol. 2007, 65, 344–352. [Google Scholar] [CrossRef]
- Cooper, P.J.; Chico, M.E.; Sandoval, C.; Espinel, I.; Guevara, A.; Kennedy, M.W.; Urban, J.F., Jr.; Griffin, G.E.; Nutman, T.B. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J. Infect. Dis. 2000, 182, 1207–1213. [Google Scholar] [CrossRef]
- Dana, D.; Vlaminck, J.; Ayana, M.; Tadege, B.; Mekonnen, Z.; Geldhof, P.; Levecke, B. Evaluation of copromicroscopy and serology to measure the exposure to Ascaris infections across age groups and to assess the impact of 3 years of biannual mass drug administration in Jimma Town, Ethiopia. PLoS Negl. Trop. Dis. 2020, 14, e0008037. [Google Scholar] [CrossRef] [PubMed]
- Elefant, G.R.; Shimizu, S.H.; Sanchez, M.C.; Jacob, C.M.; Ferreira, A.W. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA, and IgE antibodies by enzyme-linked immunosorbent assay. J. Clin. Lab. Anal. 2006, 20, 164–172. [Google Scholar] [CrossRef]
- Dold, C.; Holland, C.V. Investigating the underlying mechanism of resistance to Ascaris infection. Microbes Infect. 2011, 13, 624–631. [Google Scholar] [CrossRef]
- Figueiredo, S.D.; Taddei, J.A.; Menezes, J.J.; Novo, N.F.; Silva, E.O.; Cristóvão, H.L.; Cury, M.C. Estudo clínico-epidemiológico da toxocaríase em população infantil. J. Pediatr. 2005, 81, 126–132. [Google Scholar] [CrossRef]
- Carvalho, E.A.; Rocha, R.L. Toxocariasis: Visceral larva migrans in children. J. Pediatr. 2011, 87, 100–110. [Google Scholar] [CrossRef]
- Fan, C.K.; Liao, C.W.; Cheng, Y.C. Factors affecting disease manifestation of toxocarosis in humans: Genetics and environment. Vet. Parasitol. 2013, 193, 342–352. [Google Scholar] [CrossRef]
- Fica, A.; Jercic, M.I.; Navarrete, C. Brain lesions associated with eosinophilia. A useful clue for neurotoxocariasis. Report of one case. Rev. Med. Chil. 2021, 149, 1673–1678. [Google Scholar] [CrossRef]
- Lin, S.M.; Liao, C.W.; Lin, Y.H.; Lee, C.C.; Kao, T.C.; Fan, C.K. Inducible nitric oxide synthase inhibition influenced granuloma formation with suppressed collagen expression in myositis caused by Toxocara canis in mice. Parasitol. Res. 2008, 102, 577–585. [Google Scholar] [CrossRef]
- Wu, M.S.; Liao, C.W.; Du, W.Y.; Kao, T.C.; Su, K.E.; Lin, Y.H.; Chang, C.C.; Fan, C.K. Enhanced expression of transforming growth factor-beta 1 in inflammatory cells, alpha-smooth muscle actin in stellate cells, and collagen accumulation in experimental granulomatous hepatitis caused by Toxocara canis in mice. Acta Trop. 2008, 105, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, J.; Yang, L.; Yang, D.; Wang, D.; Liu, B.; Huang, T.; Wang, X.; Liang, B.; Liu, C. Efficacy and safety of antagonists for chemoattractant receptor-homologous molecule expressed on Th2 cells in adult patients with asthma: A meta-analysis and systematic review. Respir. Res. 2018, 19, 217. [Google Scholar] [CrossRef]
- Blanchard, C.; Rothenberg, M.E. Biology of the eosinophil. Adv. Immunol. 2009, 101, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G.M. Profile of reslizumab in eosinophilic disease and its potential in the treatment of poorly controlled eosinophilic asthma. Biologics 2013, 7, 7–11. [Google Scholar] [CrossRef]
- Pinelli, E.; Brandes, S.; Dormans, J.; Gremmer, E.; van Loveren, H. Infection with the roundworm Toxocara canis leads to exacerbation of experimental allergic airway inflammation. Clin. Exp. Allergy 2008, 38, 649–658. [Google Scholar] [CrossRef]
- Fischer, N.; Rostaher, A.; Zwickl, L.; Deplazes, P.; Olivry, T.; Favrot, C. A Toxocara canis infection influences the immune response to house dust mite allergens in dogs. Vet. Immunol. Immunopathol. 2018, 202, 11–17. [Google Scholar] [CrossRef]
- Rujeni, N.; Nausch, N.; Midzi, N.; Gwisai, R.; Mduluza, T.; Taylor, D.W.; Mutapi, F. Soluble CD23 levels are inversely associated with atopy and parasite-specific IgE levels but not with polyclonal IgE levels in people exposed to helminth infection. Int. Arch. Allergy Immunol. 2013, 161, 333–341. [Google Scholar] [CrossRef]
- Scrivener, S.; Yemaneberhan, H.; Zebenigus, M.; Tilahun, D.; Girma, S.; Ali, S.; McElroy, P.; Custovic, A.; Woodcock, A.; Pritchard, D.; et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: A nested case-control study. Lancet 2001, 358, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Magnaval, J.F.; Faufingue, J.H.; Morassin, B.; Fabre, R. Eosinophil cationic protein, specific IgE and IgG4 in human toxocariasis. J. Helminthol. 2006, 80, 417–423. [Google Scholar] [CrossRef]
- Silva, M.B.; Amor, A.L.M.; Santos, L.N.; Galvao, A.A.; Oviedo Vera, A.V.; Silva, E.S.; Barbosa, C.G.; Goncalves, M.S.; Cooper, P.J.; Figueiredo, C.A.; et al. Risk factors for Toxocara spp. seroprevalence and its association with atopy and asthma phenotypes in school-age children in a small town and semi-rural areas of Northeast Brazil. Acta Trop. 2017, 174, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Acevedo, N. Allergy in the tropics: The impact of cross-reactivity between mites and ascaris. Front. Biosci. (Elite Ed.) 2011, 3, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, R.P.; Monteiro, M.B.; Lemos, E.M.; Pereira, F.E. Anti-Toxocara antibodies detected in children attending elementary school in Vitoria, State of Espirito Santo, Brazil: Prevalence and associated factors. Rev. Soc. Bras. Med. Trop. 2011, 44, 461–466. [Google Scholar] [CrossRef]
- Nagy, D.; Bede, O.; Danka, J.; Szenasi, Z.; Sipka, S. Analysis of serum cytokine levels in children with chronic cough associated with Toxocara canis infection. Parasite Immunol. 2012, 34, 581–588. [Google Scholar] [CrossRef]
- Pourgholaminejad, A.; Razipour, H.; Heydarian, P.; Ashrafi, K.; Roushan, Z.A.; Sharifdini, M. A survey on the seroprevalence of toxocariasis and related risk factors in Eosinophilic children of Northwest Iran. Afr. Health Sci. 2022, 22, 617–625. [Google Scholar] [CrossRef]
- Takamoto, M.; Wang, Z.X.; Watanabe, N.; Matsuzawa, A.; Nariuchi, H.; Sugane, K. Eosinophilia, IgE production, and cytokine production by lung T cells in surface CD4-deficient mutant mice infected with Toxocara canis. Immunology 1998, 95, 97–104. [Google Scholar] [CrossRef]
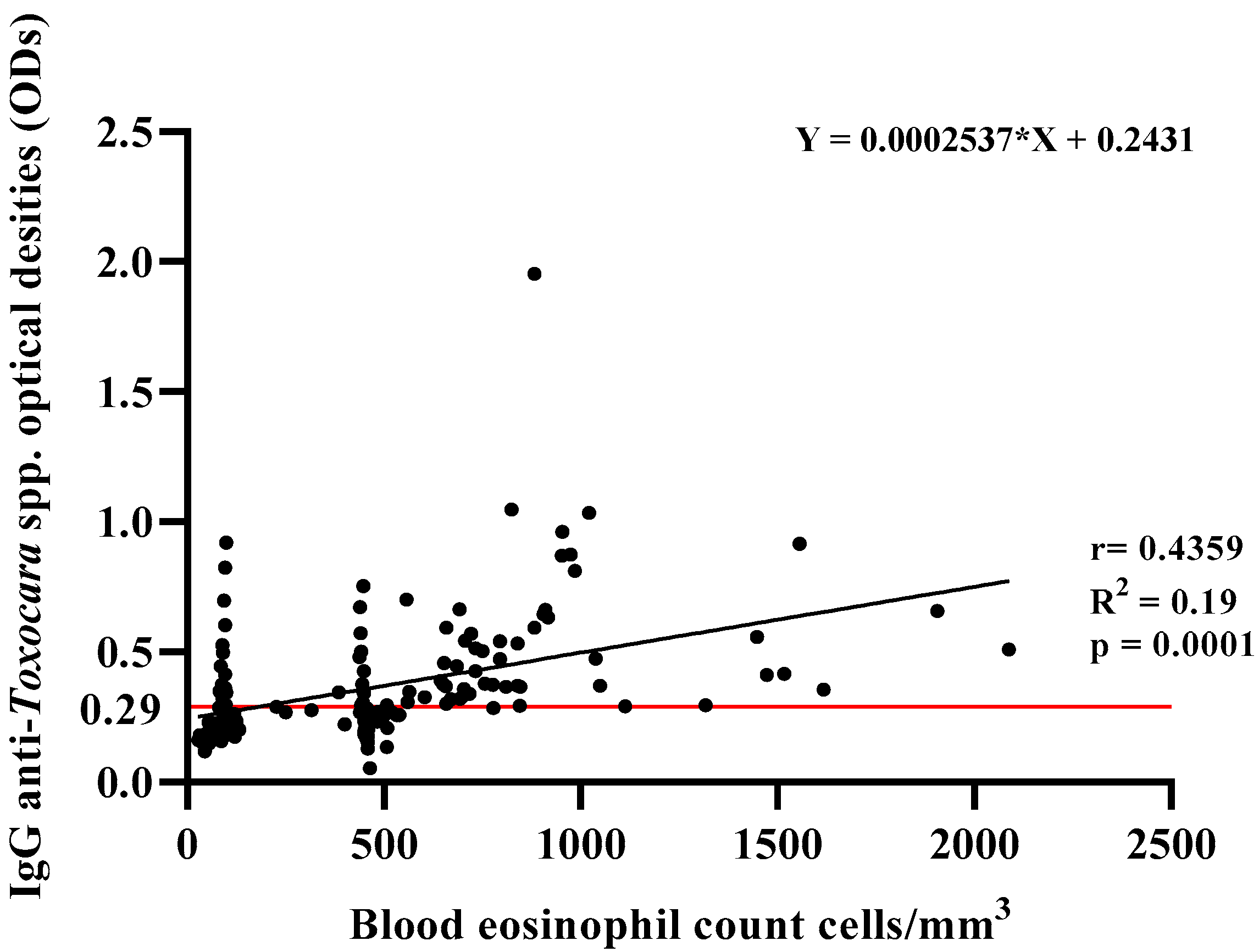
| Variables | IgG Anti-Toxocara spp. Positives (n = 89) | IgG Anti-Toxocara spp. Negatives (n = 87) |
|---|---|---|
| Male–N (%) | 22 (25) | 19 (21.8) |
| Female–N (%) | 67 (75) | 68 (78.2) |
| Age (years)–median/SD a (IQ range) b | 50/1.32 (19–82) | 49/1.4 (20–78) |
| Total IgE UI/dl–median/SD a (IQ range) b | 360/65.6 (8.86–3.000) | 257/77.7 (3.32–3.000) |
| IgE > 160 UI/dl–N (%) | 66 (74) | 54 (62) |
| Positive SPT c–N (%) | 60 (67) | 43 (49) |
| Blood eosinophil count cells/mm3 -median/SD a (IQ range) b | 659/46.98 (83.0–2.088) | 132/21.16 (29–778) |
| Blood eosinophils < 100 cells/mm3–N (%) | 23 (27) | 35 (40) |
| Blood eosinophils ≥ 100 ≤ 450 cells/mm3–N (%) | 12 (13) | 14 (16) |
| Blood eosinophils ≥ 450 cells/mm3–N (%) | 54 (60) | 38 (44) |
| FEV1 percent of predicted value post-BD–median (IQ range) b | 86% (43–86) | 86% (55–62) |
| FVC percent of predicted value post-BD–median (IQ range) b | 69% (32–74) | 74% (38–72) |
| FVE1/FVC post-BD–median (IQ range) b | 0.6% (0.42–0.89) | 0.72% (0.36–0.95) |
| Age of asthma onset–median/SD b (IQ range) b | 5/1.4 (1–71) | 12/1.5 (1–55) |
| Use of > 880 μg of inhaled Fluticasone/day–N (%) | 37 (39) | 31 (35) |
| Use of inhaled Beta-2 agonists/day–N (%) | 83 (93) | 80 (92) |
| Exacerbation/year (one or more times)–N (%) | 62 (70) | 51 (58) |
| Hospitalization/year (one or more times)–N (%) | 7 (7) | 2 (2) |
| Uncontrolled asthma d–N (%) | 55 (62) | 40 (46) |
| Variables (N) | IgG Anti-Toxocara spp. | ||||
|---|---|---|---|---|---|
| Negative (n = 87) | Positive (n = 89) n/N (%) | OR crude | OR adjusted | ||
| n/N (%) | |||||
| Gender | |||||
| Female | 68/135 (51) | 67/135 (49) | 1 | 1 | |
| Male | 19/41 (46) | 22/41 (54) | 1.17 (0.58–2.36) | 0.99 (0.47–2.07) | |
| Age | |||||
| ≤44 | 35/57 (61) | 22/57 (39) | 1 | 1 | |
| ≥45 | 52/119 (44) | 67/119 (56) | 2.05 (1.07–3.90) * | 2.35 (1.11–4.96) * | |
| Rural area | |||||
| No | 33/78 (42) | 45/78 (58) | 1 | 1 | |
| Yes | 53/97 (55) | 44/97 (45) | 0.62 (0.34–1.14) | 0.50 (0.26–0.95) * | |
| Salary | |||||
| ≤1 salary | 45/88 (51) | 43/88 (49) | 1 | 1 | |
| >1 salary | 42/88 (48) | 46/88 (52) | 1.14 (0.63–2.07) | 1.09 (0.57–2.07) | |
| Scholarity | |||||
| 1st grade incomplete | 21/48 (44) | 27/48 (56) | 1 | 1 | |
| 1st grade to 2nd complete | 60/116 (52) | 56/116 (48) | 0.76 (0.40–1.42) | 0.85 (0.61–1.92) | |
| Superior complete | 6/12 (50) | 6/12 (50) | 0.97 (0.30–3.15) | 1.09 (0.71–1.71) | |
| Toxocara Infection | n/N =176 (%) | OR adjusted | n/N = 176 (%) | OR adjusted | n/N = 176 (%) | OR adjusted |
|---|---|---|---|---|---|---|
| Eosinophils ≤ 100 mm3 | Eosinophils > 100 < 450 mm3 | Eosinophils ≥ 450 mm3 | ||||
| No | 35/87 (40) | 1 | 14/87 (16) | 1 | 38/87 (43) | 1 |
| Yes | 23/89 (26) | 0.51 (0.27–0.98) * | 12/89 (13) | 0.81 (0.35–1.87) | 54/89 (60) | 1.98 (1.09–3.62) * |
| Variables | Eosinophilia Negative. | Eosinophilia Positive. | ||||
|---|---|---|---|---|---|---|
| Toxocara spp. Infections (n) | Toxocara spp. Infections (n) | |||||
| N | n/N (%) | χ2 (p-Value) | n/N (%) | χ2 (p-Value) | ||
| Skin Prick Test | No | 24/49 (49) | 0.020 (0.88) | No | 19/54 (35) | 4.749 (0.03) * |
| Yes | 25/49 (51) | Yes | 35/54 (65) | |||
| sIgE ≥ 0.70 KU/L to D. farinae | No | 21/38 (55) | 0.421 (0.51) | No | 15/46 (33) | 5.565 (0.01) * |
| Yes | 17/38 (45) | Yes | 31/46 (67) | |||
| sIgE ≥ 0.70 KU/L to D. pteronyssinus | No | 20/37 (54) | 0.243 (0.62) | No | 14/43 (33) | 5.233 (0.02) * |
| Yes | 17/37 (46) | Yes | 29/43 (67) | |||
| sIgE ≥ 0.70 KU/L to B. tropicalis | No | 23/42 (55) | 0.381 (0.53) | No | 17/48 (35) | 4.083 (0.04) * |
| Yes | 19/42 (45) | Yes | 31/48 (65) | |||
| IgE ≥ 160 UI/dl | No | 23/43 (53) | 0.381 (0.53) | No | 26/78 (33) | 4.083 (0.04) * |
| Yes | 20/43 (46) | 0.209 (0.64) | Yes | 52/78 (67) | 8.667 (0.003) * | |
| Uncontrolled asthma | No | 20/40 (50) | 0.00 (1.00) | No | 19/52 (36) | 3.769 (0.05) * |
| Yes | 20/40 (50) | Yes | 33/52 (64) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.C.; da Silva, M.B.; Galvão, A.A.; Fernandes, J.S.; Pinheiro, G.P.; Cruz, Á.A.; Pinheiro, C.d.S.; Alcântara-Neves, N.M. Toxocara spp. Infection Influences on Eosinophil Levels: An Immunological Indicator of Severe Asthma and Allergy. Allergies 2025, 5, 24. https://doi.org/10.3390/allergies5030024
Silva RC, da Silva MB, Galvão AA, Fernandes JS, Pinheiro GP, Cruz ÁA, Pinheiro CdS, Alcântara-Neves NM. Toxocara spp. Infection Influences on Eosinophil Levels: An Immunological Indicator of Severe Asthma and Allergy. Allergies. 2025; 5(3):24. https://doi.org/10.3390/allergies5030024
Chicago/Turabian StyleSilva, Raphael Chagas, Márcia Barbosa da Silva, Alana Alcantara Galvão, Jamile Souza Fernandes, Gabriela Pimentel Pinheiro, Álvaro A. Cruz, Carina da Silva Pinheiro, and Neuza Maria Alcântara-Neves. 2025. "Toxocara spp. Infection Influences on Eosinophil Levels: An Immunological Indicator of Severe Asthma and Allergy" Allergies 5, no. 3: 24. https://doi.org/10.3390/allergies5030024
APA StyleSilva, R. C., da Silva, M. B., Galvão, A. A., Fernandes, J. S., Pinheiro, G. P., Cruz, Á. A., Pinheiro, C. d. S., & Alcântara-Neves, N. M. (2025). Toxocara spp. Infection Influences on Eosinophil Levels: An Immunological Indicator of Severe Asthma and Allergy. Allergies, 5(3), 24. https://doi.org/10.3390/allergies5030024





