Abstract
Purpose: This study aimed to evaluate the immunological response to the 23-valent pneumococcal polysaccharide vaccine (PPV23) in patients investigated for immunodeficiencies due to recurrent infections at EPM-UNIFESP Clinical Immunology outpatient clinic. Methods: This is a longitudinal retrospective study. Data were collected from the medical records of patients between 2012 and 2020. The analyses were developed in two stages: before and after administration of the PPV23 vaccine. Results: A total of 390 patients who received the PPV23 vaccine were selected. Among those who demonstrated an adequate serological response (63.6%), there was a notable decrease in the risk of upper respiratory tract infections (URTI) by 66%, tonsillitis by 74%, otitis by 76%, sinusitis by 49%, and uncomplicated pneumonia (PNM) by 77%. For invasive infections, the risk reduction was 95% for pneumonia with parapneumonic effusion and 93% for meningitis. Conclusions: The study demonstrated a significant decrease in the risk of bacterial infections following the administration of the PPV23 vaccine in this population. Therefore, we recommend including PPV23 in the vaccination schedule following pneumococcal conjugated vaccines for patients with recurrent pneumococcal infections to enhance protection and avoid complications.
1. Introduction
Streptococcus pneumoniae (pneumococcus) infections contribute to increase morbidity and mortality among individuals with infectious diseases, particularly in children under 5 years old and adults over 60 years old [1]. Globally, pneumococcus accounts for approximately 15 cases of invasive pneumococcal disease per 100,000 individuals annually and is responsible for over one million deaths each year. The World Health Organization (WHO) estimates that approximately 300,000 children under five die annually from Streptococcus pneumoniae infections [2].
The bacterium’s polysaccharide capsule is a key virulence factor, enabling it to evade phagocytosis. Variations in the capsule’s polysaccharide structure impact the pneumococcus’s ability to colonize the nasopharynx and cause either localized or invasive infections [3]. Following infection, the production of serotype-specific antibodies signals the onset of convalescence, as these antibodies are highly opsonizing and provide protection against subsequent exposures to the same pneumococcal serotype [4].
Over the past two decades, the widespread administration of pneumococcal conjugate vaccines (PCVs) in children as part of public health programs has dramatically reduced both nasopharyngeal colonization by pneumococcus and the incidence of invasive disease across various age groups [5]. In Brazil, the introduction of the 10-valent Conjugate Vaccine (PCV10) into the National Immunization Program in March 2010 marked a significant progress in controlling pneumococcal disease, positioning the country as a leader in implementing those vaccines in its public health system [6].
Pneumococcal vaccines, including PCVs and the 23-valent pneumococcal polysaccharide vaccine (PPV23), are crucial for diagnosing and managing primary immunodeficiencies, now known as inborn errors of immunity (IEI). For patients suspected to present with immunodeficiencies due to recurrent infections, specific antibody responses are essential for diagnosing and evaluating IEI [7]. In 2012, the American Academy of Asthma, Allergy, and Immunology established that a normal response to the pneumococcal vaccine should result in antibody titers above 1.3 μg/mL for more than 50% of the serotypes present in the vaccine for children under six years old and for more than 70% of the serotypes for patients over six years old [8].
In the immunocompetent population, the use of the PPV23 vaccine, alongside PCVs, has been shown to enhance the immune response to pneumococcus, thereby reducing the incidence of infections, particularly invasive ones, as demonstrated in individuals over 60 years old [9]. However, data on the use of PPV23 in children and adolescents suspected to present with immunodeficiencies due to recurrent infections remain limited. Therefore, additional studies are necessary to determine the actual need for immunization with PPV23 in this group of patients.
2. Materials and Methods
Data were collected from the medical records of patients followed at the Clinical Immunology Outpatient Clinic of the Federal University of São Paulo (EPM-UNIFESP). Patients of both sexes with a history suspected to present with immunodeficiencies due to recurrent infections were included in the study and followed between 2012 to 2020. A laboratory investigation to assess the response to polysaccharide antigens was documented in the medical record, and the patient had to be at least two years of age at the time of the investigation.
2.1. Recurrent Infections
The following outcomes were evaluated in two stages (before and after PPV23): the number of hospitalizations, the number of admissions to the intensive care unit (ICU), and the number of infections (both absolute and per year) related to upper respiratory tract infections (URTI), tonsillitis, otitis, sinusitis, uncomplicated pneumonia, pneumonia with parapneumonic effusion, post-septal cellulitis, skin infection, osteomyelitis, joint infections, organ abscess, endocarditis, and sepsis.
These outcomes were also compared at two time points (before and after PPV23) within groups: sex, age at onset of symptoms, age at the time of vaccination, family history of (IEI), early deaths in the family, consanguinity, and other comorbidities such as prematurity, heart disease, pneumopathy/bronchiectasis, and gastroesophageal reflux disease (GERD). Additionally, allergy disease like atopic dermatitis, asthma, allergic rhinitis, and allergic conjunctivitis were also considered.
Recurrent infections were defined as four or more URTIs treated with antibiotics per year, four or more episodes of acute otitis media treated with antibiotics per year, and two or more episodes of sinusitis or pneumonia per year. Sepsis was characterized by bacterial generalized infection with organ dysfunction and inflammation. Otitis was defined as acute otitis media, and skin infections included cellulitis and impetigo. Episodes of pneumococcal invasive disease, such as pneumonia with parapneumonic effusion, meningitis, or sepsis, did not need to recur to be included in the study.
2.2. Laboratory Evaluation
For the laboratory evaluation of patients, information was obtained from medical records for the following tests: complete blood count, measurement of immunoglobulins A, G, and M, counts of T lymphocytes (CD3, CD4, and CD8), CD19 cells, natural killer (NK) cells, and complement system analysis. The evaluation of previous vaccine responses was conducted as an integral component of the investigation for diagnosing inborn errors of immunity (IEI).
2.3. Polysaccharide Vaccine Response Evaluation
Our group of patients was heterogeneous, encompassing various age ranges and having received different vaccination regimens for pneumococcal vaccines in accordance with the Brazilian national vaccination schedule. Therefore, regardless of previous pneumococcal vaccination, infections were evaluated both before and after PPV23.
Adequate Response to the PPV23 Vaccine: An adequate response to the PPV23 vaccine was defined as antibody levels equal to or greater than 1.3 µg/mL for 50% or more of the serotypes tested in children under six years of age and for at least 70% of the serotypes tested in individuals over six years of age, or post-vaccination levels at least four times higher than pre-vaccination levels across all ages [8]. We measured specific antibodies to pneumococcal antigens (serotypes PS1, PS5, PS6, PS9, PS14, PS18, and PS23) before and after the PPV23 vaccine.
Patients were categorized into two groups: those who exhibited an adequate response to the PPV23 vaccine and those who did not.
3. Statistical Analysis
Statistical analyses were performed using SPSS 20.0 and STATA 12 statistical software. The existence of an association between two categorical variables was assessed using the chi-square test or Fisher’s exact test in cases of small sample sizes. Comparisons of percentages between evaluation time points were performed using McNemar’s test. Due to the lack of normality in data distribution, mean comparisons between two evaluation time points and between two groups were carried out using the non-parametric Wilcoxon and Mann–Whitney tests, respectively. Normality in distribution was tested using the Kolmogorov–Smirnov test. Associations between numerical variables were assessed using Spearman’s correlation.
To evaluate the effect of the vaccine on the number of admissions, ICU admissions, upper respiratory tract infections (URTI), tonsillitis, otitis, sinusitis, uncomplicated pneumonia, pneumonia with parapneumonic effusion, cutaneous infections, meningitis, endocarditis, and sepsis—adjusted for exposure time and patient characteristics (predictive variables)—negative binomial regression models were utilized. The appropriateness of using the negative binomial model was verified through the likelihood ratio test. A significance level of 5% was established for all statistical tests.
4. Ethical Approval
The Research Ethics Committee of UNIFESP approved this protocol under No. 4.458.922.
5. Results
A total of 390 medical records of patients who received PPV23 and were followed up at the Clinical Immunology Outpatient Clinic at EPM-UNIFESP were evaluated. Among these patients, 63.6% (248) (95% CI: 58.6-68.4) demonstrated an adequate response.
In Table 1, we compared the characteristics of patients in terms of adequate response to PPV23 versus those without an adequate response. Significant differences were observed in the average age at onset of symptoms and the age at the time of vaccination, with those demonstrating an adequate response being younger. Regarding sex, there were no differences in the proportion of males and females across all samples.

Table 1.
Characterization of the groups regarding clinical and demographic variables.
In terms of additional clinical occurrences, the group with an adequate response to the vaccine exhibited lower percentages of heart disease (4.4% versus 14.8%, respectively) and delays related to developmental neurological problems (DNPM) (18.5% versus 28.2%, respectively). When comparing allergic diseases, there were no differences in the proportion of patients with asthma, rhinitis, atopic dermatitis, or allergic conjunctivitis between the two groups (Table 1).
Patients with an Adequate Response to PPV23
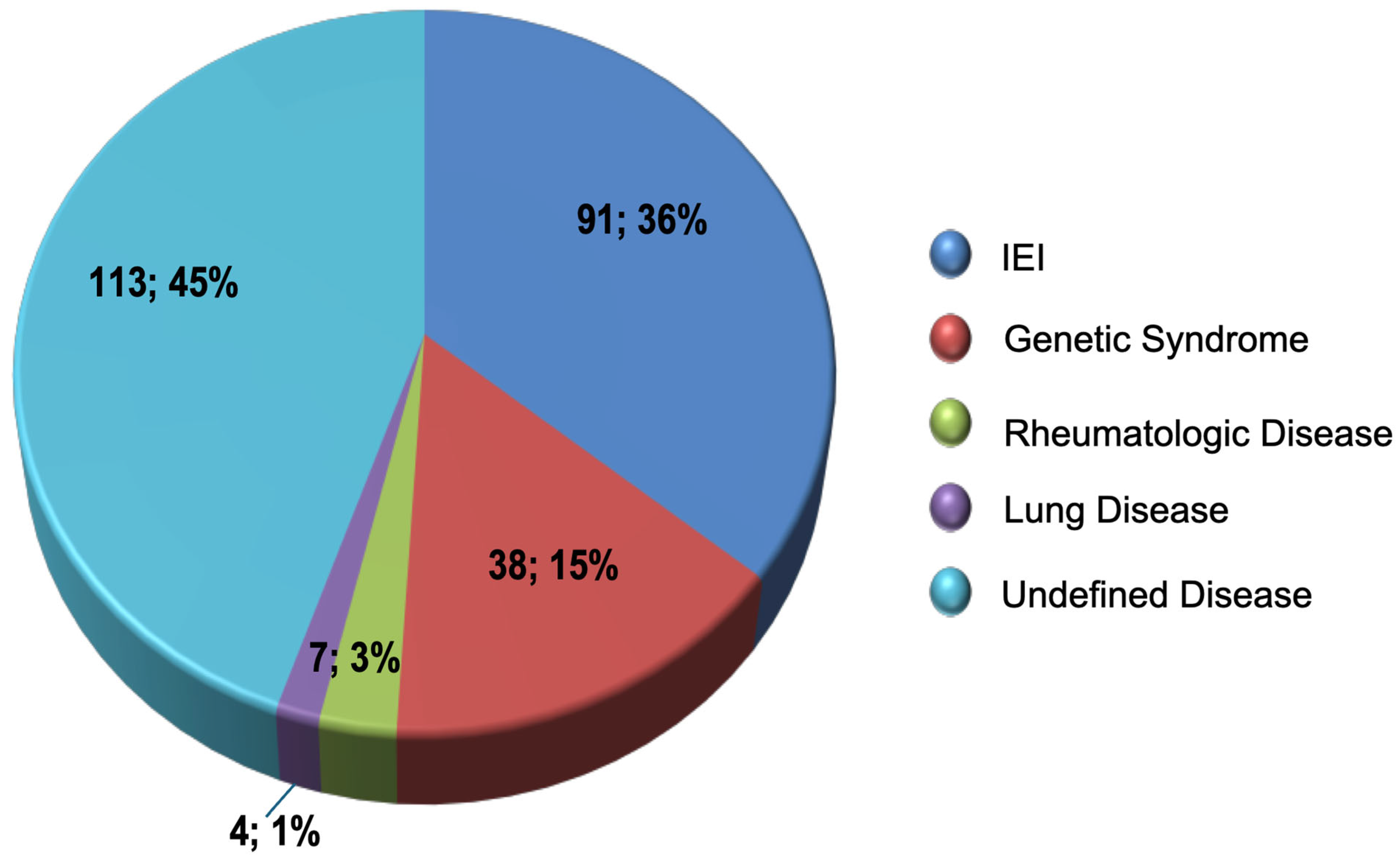
The 248 patients with an adequate response to vaccination with PPV23 presented a significant reduction in the rates of infectious events, except for skin infection and post-septal cellulitis (Table 2). The underlying disease diagnoses described in these patients’ medical records are shown in Figure 1.

Table 2.
Annual rate of infectious events before and after PPV23 vaccination.
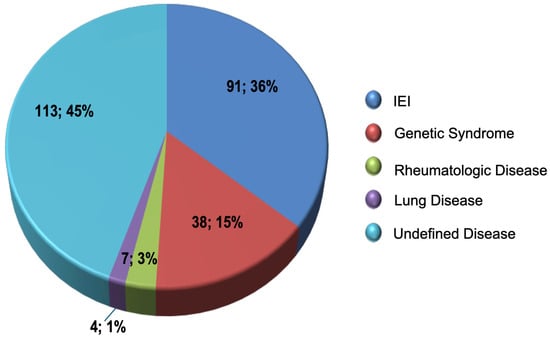
Figure 1.
Distribution of diagnoses in patients with an adequate PPV23 response.
Negative binomial regression models were employed to evaluate the effect of the vaccine on the number of hospitalizations, ICU admissions, and various infections, such as upper respiratory tract infections (URTI), tonsillitis, otitis, sinusitis, uncomplicated pneumonia, pneumonia with parapneumonic effusion, meningitis, and sepsis, adjusted by exposure time and patient characteristics. Annual rates or occurrences of infectious events were assessed before and after PPV23 vaccination.
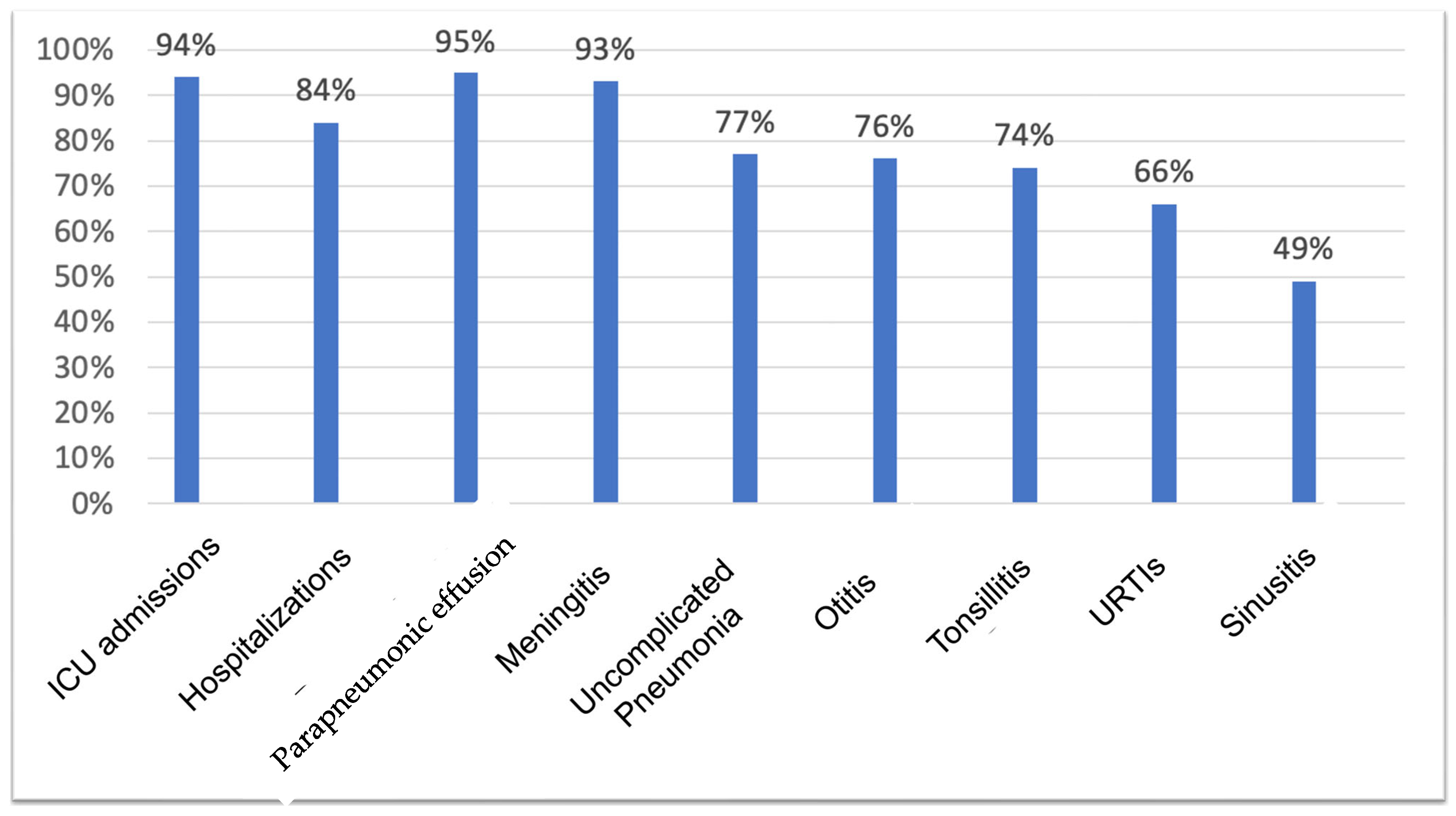
After vaccination, the risk of hospitalization reduced 84% compared to the period before the vaccine. Regarding ICU admissions, a 94% risk reduction was observed after using the vaccine. In terms of infectious episodes, adjusted individually for each of the characteristics, a reduction in the risk of 66% in URTIs was observed after the PPV23 vaccine. For non-invasive infections, the risk reduction of contracting the infection was 74% for tonsillitis, 76% for otitis, 49% for sinusitis, and 77% for uncomplicated pneumonia. For invasive infections, the risk reduction after PPV23 was 95% for pneumonia with parapneumonic effusion and 93% for meningitis (Figure 2). For sepsis, there were no reductions in risk after vaccination, likely due to the low number of occurrences described for the patients evaluated.
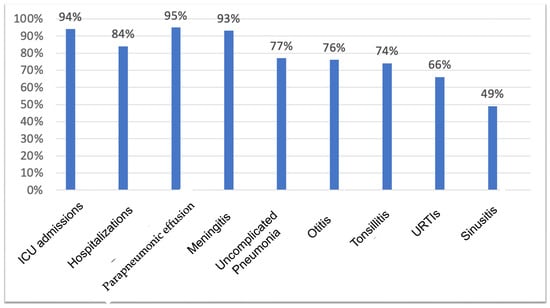
Figure 2.
Risk reduction of presenting an occurrence after PPV23.
6. Discussion
Conjugate vaccines have undoubtedly reduced the burden of pneumococcal disease worldwide; however, pneumococcal infections continue to significantly impact global morbidity and mortality. That certain serotypes are not included in the vaccines raises considerable concern and underscores the need to identify the optimal vaccination schedule for pneumococcus [10].
Recent studies indicate that for the healthy population over two years of age, PPV23 is effective in preventing invasive pneumococcal diseases. It has been demonstrated that mean antibody titers for all serotypes tested increased dramatically after PPV23 vaccination. However, demonstrating a decrease in nasopharyngeal colonization has proven more challenging, as reflected in the literature [9].
Parker et al. evaluated IgG levels specific to PPV23 serotypes in a healthy population that had not previously received a pneumococcal vaccine at the following time points: pre-vaccination, four to six weeks post-vaccination, and six years after PPV23 vaccination. The authors observed that antibody responses remained two to four times pre-vaccination levels after six years, even in adults over 60 years of age. This demonstrates a sustained serotype-specific response following PPV23 vaccination [11].
Due to the immaturity of the immune system, children are inferred to have a higher incidence of infections in their early years. Understanding the ontogeny of the immune system aids in comprehending immunization schedules, which consist of repeated doses of certain vaccines to ensure adequate stimulation and consequent production of antibodies. In our study, we found a higher prevalence of patients under the age of 18. Additionally, patients in the group with an adequate response to the PPV23 vaccine exhibited a lower average age at the onset of symptoms and at the time of vaccination compared to those who did not respond to PPV23. This observation prompts us to consider whether patients presenting with recurrent infections and an impaired response to conjugate vaccines administered in infancy respond serologically and clinically to the PPV23 vaccine due to their immune system’s immaturity. In this context, PPV23 may serve as an excellent stimulus for the maturation of the immune system and antibody production. It is imperative to identify those children who may present with IEI [12].
In children at increased risk of pneumococcal invasive diseases, a pertinent question arises regarding the appropriate response to the serotype replacement that occurs after vaccinating the population with conjugate vaccines: should a booster with PPV23 be administered or should additional doses of conjugate vaccines be provided? More studies are needed to address this topic.
It is essential to emphasize that no pneumococcal vaccine offers protection against all existing serotypes. Furthermore, the effectiveness of PPV23 appears to vary based on the evaluated outcomes. Generally, rates of invasive disease decline more significantly compared to milder infections following PPV23 immunization [13].
The efficacy and effectiveness of the PPV23 vaccine have been demonstrated in various clinical trials involving specific populations [13,14]. Its recommended use—often in conjunction with a conjugate vaccine—is for individuals aged 2 to 64 years with conditions that present a higher risk for invasive pneumococcal infections, as well as for adults over 65 years of age, regardless of their medical history [15].
Studies investigating the use of PPV23 in the general population have demonstrated an efficacy of approximately 60%, though the majority of these studies focus on population subgroups at higher risk of pneumococcal diseases. In children with recurrent infections, the scarcity of data in the literature hampers the ability to draw definitive conclusions about the immunogenicity of PPV23 in this group [16].
While this study did not directly evaluate the efficacy of the PPV23 vaccine, it did find serological titers above 1.3 µg/mL in more than 50% of the serotypes tested for patients with recurrent infections, suggesting a positive vaccine response in 63.6% of the evaluated patients who received the polysaccharide vaccine.
The Spanish CAPAMIS study conducted with adults over 60 years old demonstrated that during the first five years after vaccination, PPV23 was effective in preventing hospitalization due to non-bacteremic pneumococcal pneumonia in 48% of cases and in preventing community-acquired pneumonia of all causes by 25% [17]. A Cochrane meta-analysis published in 2013 concluded that vaccination with PPV23 reduced bacteremic pneumococcal pneumonia, non-bacteremic pneumococcal pneumonia, and invasive infections by 74%, 54%, and 80%, respectively. In pneumonia from all causes, a 46% reduction was found in low-income countries [18].
Regarding allergic diseases, a large proportion of our patients had asthma (57%) and allergic rhinitis (66%), similar to what has been reported in the literature [19,20,21]. However, there was no difference between these groups in relation to the response to PPV23. On the other hand, in the “allergic” group with a good response to PPV23, there was a considerable reduction in the number of all infections and hospitalizations, mainly pneumonia, after the vaccine (relative risks greater than 1 with a decline to 0.1).
Children with asthma are at a greater risk of developing pneumonia or pneumococcal invasive infections. Castro-Rodrigues et al. reported a 90% increase in the chance of pneumococcal invasive disease in asthmatic children, even with regular vaccination with conjugate vaccines, compared to children without asthma. It recommends that asthmatic children over two years of age receive PPV23 even after their usual vaccination with conjugate vaccines and regardless of the intensity of their asthma and the use of oral corticosteroids [22].
The evaluation of PPV23 in patients with recurrent infections is notably scarce in the literature. Ingels et al. demonstrated that in children at higher risk for recurrent invasive diseases, the recommendation of vaccination with PPV23 as a complement to the vaccination schedule with conjugate vaccines brings benefits, promoting adequate responses to the vaccine serotypes [23]. Estrada et al. evaluated the response to PPV23 in children and adolescents with recurrent respiratory infections. Of the 72 patients evaluated, 83% (60 patients) exhibited a serological response to the vaccine, and 96% (69 patients) experienced a clinical response to the vaccine, with resolution of infections within two to four years of follow-up [24].
In agreement with these findings, in this present study, patients with an immunological pneumococcus response after PPV23 showed a clinical decrease in the frequency of most infections. When analyzing the negative binomial model, we found that after receiving the vaccine, in addition to reducing the risk of presenting invasive diseases by 95% for pneumonia with parapneumonic effusion and 93% for meningitis, there were also significant reductions in the risk of presenting non-invasive infections: tonsillitis (74% risk reduction), otitis (76%), sinusitis (49%), and uncomplicated pneumonia (77%). These results are unprecedented and reinforce the recommendation of carrying out a booster with PPV23 after completing the vaccination schedule with conjugated vaccines, as a tool for diagnosing specific antibody deficiency and as a treatment for this group of patients with recurrent infections.
7. Conclusions
Patients with recurrent infections constitute a diverse group, ranging from those with primary immunodeficiencies to those who exhibit immature immune systems or display an impaired response to pneumococcal conjugate vaccines. In this context, the PPV23 vaccine emerges as a crucial tool in both diagnosing and treating these individuals. The response to the PPV23 vaccine is integral to the diagnostic assessment of IEI, facilitating more tailored and effective care for these patients. In particular, those patients who demonstrate an adequate response to the PPV23 vaccine and are merely immunologically immature benefit significantly from vaccination, as evidenced by the observed reductions in both infections and hospitalizations post-vaccination.
Therefore, we recommend administering the PPV23 vaccine following the pneumococcal conjugate vaccine schedule in patients with recurrent infections to enhance overall health outcomes.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by M.d.G.-P.P., C.S.A., R.R.G., E.K.I., D.S. and A.C.-N. The first draft of the manuscript was written by M.d.G.-P.P. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of of UNIFESP approved the project under No. 4.458.922, date of approval: 12 December 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest with respect to research, authorship, and/or publication of this article.
References
- Malchrzak, W.; Mastalerz-Migas, A. Epidemiologic Benefits of Pneumococcal Vaccine Introduction into Preventive Vaccination Programs. In Medical Research and Innovation; Pokorski, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; Volume 1324, pp. 11–19. [Google Scholar]
- CDC—Centers for Disease Control and Prevention. Global Pneumococcal Disease and Vaccine. Pneumococcal Disease Surveillance and Trends. September 2024. Available online: https://www.cdc.gov/pneumococcal/php/surveillance/index.html#:~:text=Global%20trends,deaths%20occur%20in%20developing%20countries (accessed on 17 June 2025).
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Loughran, A.J.; Orihuela, C.J.; Tuomanen, E.I. Streptococcus pneumoniae: Invasion and Inflammation. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.L.; Rosen, J.; Peterson, J.T.; Williams-Diaz, A.; Gakhar, V.; Sterling, T.M.; Acosta, C.J.; Nolan, K.M.; Li, J.; Pedley, A.; et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum. Vaccin. Immunother. 2019, 15, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Kupek, E.; Vieira, I.L. O impacto da vacina pneumocócica PCV10 na redução da mortalidade por pneumonia em crianças menores de um ano em Santa Catarina, Brasil. Cad. Saúde Pública 2016, 32, e00131414. [Google Scholar] [CrossRef]
- Erman, B.; Demirtas, D.; Bildik, H.N.; Çağdaş-Ayvaz, D.; Sanal, O.; Tezcan, I. Defective pneumococcal antibody response in patients with recurrent respiratory tract infections. Turk. J. Pediatr. 2017, 59, 555–560. [Google Scholar] [CrossRef]
- Orange, J.S.; Ballow, M.; Stiehm, E.R.; Ballas, Z.K.; Chinen, J.; De La Morena, M.; Kumararatne, D.; Harville, T.O.; Hesterberg, P.; Koleilat, M.; et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the basic and clinical immunology interest section of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2012, 130 (Suppl. S3), S1–S24. [Google Scholar]
- Wang, Y.; Lib, J.; Wang, Y.; Gu, W.; Zhu, F. Effectiveness and practical uses of 23-valent pneumococcal polysaccharide vaccine in healthy and special populations. Hum. Vaccines Immunother. 2018, 14, 1003–1012. [Google Scholar] [CrossRef]
- Dagan, R.; Givon-Lavi, N.; Greenberg, D.; Fritzell, B.; Siegrist, C.A. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J. Infect. Dis. 2010, 201, 1570–1579. [Google Scholar] [CrossRef]
- Parker, A.R.; Park, M.A.; Harding, S.; Abraham, R.S. The total IgM, IgA and IgG antibody responses to pneumococcal polysaccharide vaccination (Pneumovax®23) in a healthy adult population and patients diagnosed with primary immunodeficiencies. Vaccine 2019, 37, 1350–1355. [Google Scholar] [CrossRef]
- Pasternak, G.; Lewandowicz-Uszyńska, A.; Królak-Olejnik, B. Recurrent respiratory tract infections in children. Pol. Merkur. Lek. 2020, 49, 260–266. [Google Scholar]
- Suzuki, M.; Dhoubhadel, G.; Ishifuji, T.; Yasunami, M.; Yaegashi, M.; Asoh, N.; Ishida, M.; Hamaguchi, S.; Aoshima, M.; Ariyoshi, K.; et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: A multicentre, prospective, test-negative design study. Lancet 2017, 17, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Berical, A.C.; Harris, D.; Dela Cruz, C.S.; Possick, J.D. Pneumococcal Vaccination Strategies An Update and Perspective. AnnalsATS 2016, 13, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Niederman, M.S.; Folaramni, T.; Buchwald, U.K.; Musey, L.; Cripps, A.W.; Johnson, K.D. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and noninvasive pneumococcal disease and related outcomes: A review of available evidence. Expert Rev. Vaccines 2021, 20, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Heathb, P.T.; Siegristc, C.A. Use of pneumococcal polysaccharide vaccine in children: What is the evidence? Curr. Opin. Infect. Dis. 2012, 25, 292–303. [Google Scholar] [CrossRef]
- Ochoa-gondar, O.; Vila-corcoles, A.; Rodriguez-blanco, T.; GomezBertomeu, F.; Figuerola-Massana, E.; Raga-Luria, X.; Hospital-Guardiola, I. Effectiveness of the 23-Valent Pneumococcal Polysaccharide vaccine against community-acquired pneumonia in the general population Aged >60 Years: 3 years of follow-up in the CAPAMIS study. Clin. Infect. Dis. 2014, 58, 909–917. [Google Scholar] [CrossRef]
- Moberley, S.; Holden, J.; Tatham, D.P.; Andrews, R.M. Cochrane Acute Respiratory Infections Group. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 2013, 2013, CD000422. [Google Scholar]
- Reda, S.M.; El-Ghoneimy, D.H.; Afifi, H.M. Clinical Predictors of Primary Immunodeficiency Diseases in Children. Allergy Asthma Immunol. Res. 2013, 5, 88–95. [Google Scholar] [CrossRef]
- Huwyler, C.; Lin, S.Y.; Liang, J. Primary Immunodeficiency and Rhinosinusitis. Immunol. Allergy Clin. N. Am. 2020, 40, 233–249. [Google Scholar] [CrossRef]
- Moraes Lui, C.; Oliveira, L.C.; Diogo, C.L.; Kirschfink, M.; Grumach, A.S. Immunoglobulin G subclass concentrations and infections in children and adolescents with severe asthma. Pediatr. Allergy Immunol. 2002, 13, 195–202. [Google Scholar] [CrossRef]
- Castro-Rodrigues, J.A.; Abarca, K.; Forno, E. Asthma and the Risk of Invasive Pneumococcal Disease: A Meta-analysis. Pediatrics 2020, 145, e20191200. [Google Scholar] [CrossRef]
- Ingels, H.A.S.; Kantsø, B.; Slotved, H.C. Serologic response to pneumococcal vaccination in children experiencing recurrent invasive pneumococcal disease. BMC Infect. Dis. 2018, 18, 366. [Google Scholar] [CrossRef]
- Estrada, J.; Najera, M.; Pounds, N.; Catano, G.; Infante, A.J. Clinical and Serologic Response to the 23-valent Polysaccharide Pneumococcal Vaccine in Children and Teens with Recurrent Upper Respiratory Tract Infections and Selective Antibody Deficiency. Pediatr. Infect. Dis. J. 2016, 35, 205–208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).