Pneumococcal Vaccine in Patients with Recurrent Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Recurrent Infections
2.2. Laboratory Evaluation
2.3. Polysaccharide Vaccine Response Evaluation
3. Statistical Analysis
4. Ethical Approval
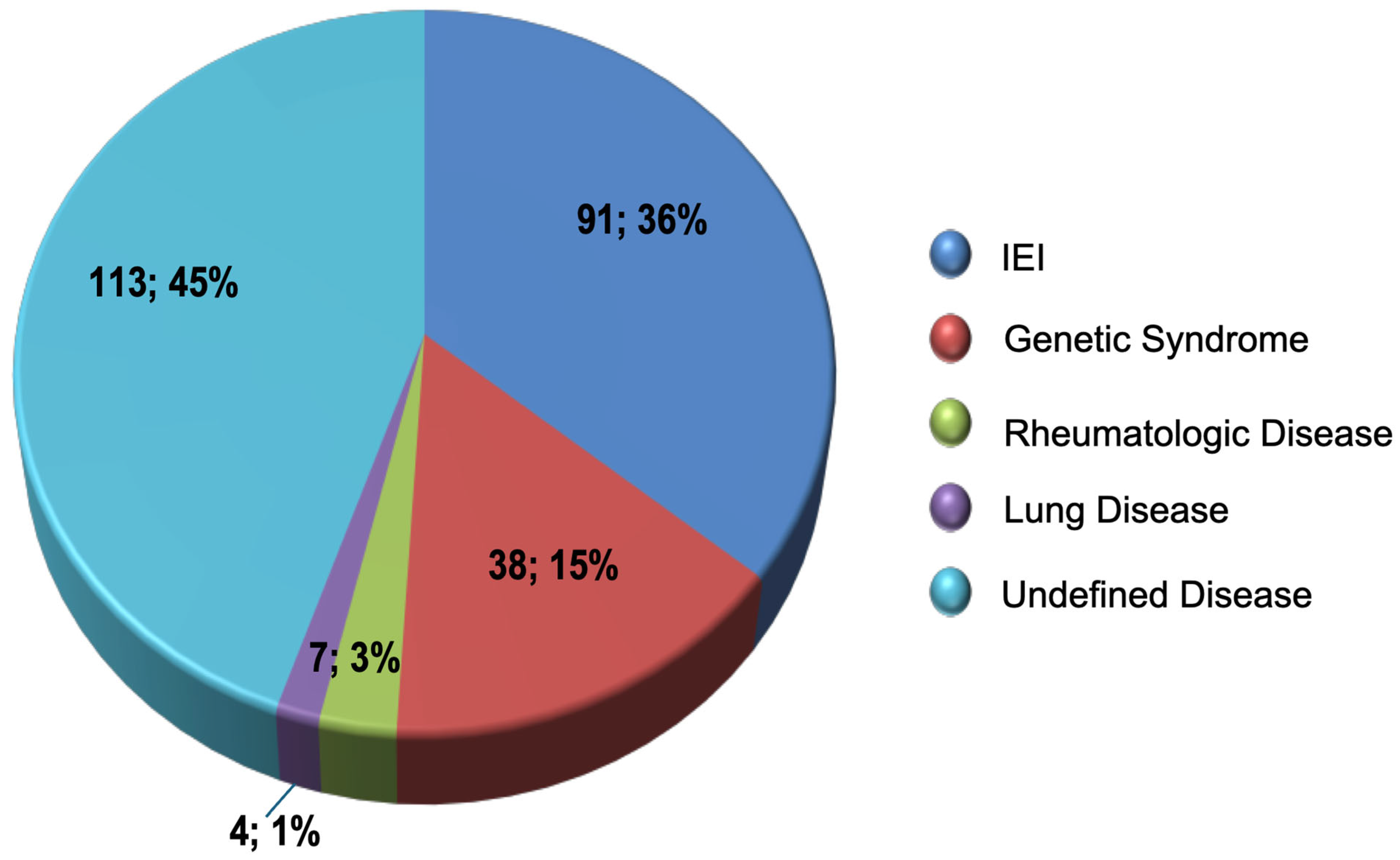
5. Results
Patients with an Adequate Response to PPV23
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malchrzak, W.; Mastalerz-Migas, A. Epidemiologic Benefits of Pneumococcal Vaccine Introduction into Preventive Vaccination Programs. In Medical Research and Innovation; Pokorski, M., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; Volume 1324, pp. 11–19. [Google Scholar]
- CDC—Centers for Disease Control and Prevention. Global Pneumococcal Disease and Vaccine. Pneumococcal Disease Surveillance and Trends. September 2024. Available online: https://www.cdc.gov/pneumococcal/php/surveillance/index.html#:~:text=Global%20trends,deaths%20occur%20in%20developing%20countries (accessed on 17 June 2025).
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Loughran, A.J.; Orihuela, C.J.; Tuomanen, E.I. Streptococcus pneumoniae: Invasion and Inflammation. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.L.; Rosen, J.; Peterson, J.T.; Williams-Diaz, A.; Gakhar, V.; Sterling, T.M.; Acosta, C.J.; Nolan, K.M.; Li, J.; Pedley, A.; et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum. Vaccin. Immunother. 2019, 15, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Kupek, E.; Vieira, I.L. O impacto da vacina pneumocócica PCV10 na redução da mortalidade por pneumonia em crianças menores de um ano em Santa Catarina, Brasil. Cad. Saúde Pública 2016, 32, e00131414. [Google Scholar] [CrossRef]
- Erman, B.; Demirtas, D.; Bildik, H.N.; Çağdaş-Ayvaz, D.; Sanal, O.; Tezcan, I. Defective pneumococcal antibody response in patients with recurrent respiratory tract infections. Turk. J. Pediatr. 2017, 59, 555–560. [Google Scholar] [CrossRef]
- Orange, J.S.; Ballow, M.; Stiehm, E.R.; Ballas, Z.K.; Chinen, J.; De La Morena, M.; Kumararatne, D.; Harville, T.O.; Hesterberg, P.; Koleilat, M.; et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the basic and clinical immunology interest section of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2012, 130 (Suppl. S3), S1–S24. [Google Scholar]
- Wang, Y.; Lib, J.; Wang, Y.; Gu, W.; Zhu, F. Effectiveness and practical uses of 23-valent pneumococcal polysaccharide vaccine in healthy and special populations. Hum. Vaccines Immunother. 2018, 14, 1003–1012. [Google Scholar] [CrossRef]
- Dagan, R.; Givon-Lavi, N.; Greenberg, D.; Fritzell, B.; Siegrist, C.A. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J. Infect. Dis. 2010, 201, 1570–1579. [Google Scholar] [CrossRef]
- Parker, A.R.; Park, M.A.; Harding, S.; Abraham, R.S. The total IgM, IgA and IgG antibody responses to pneumococcal polysaccharide vaccination (Pneumovax®23) in a healthy adult population and patients diagnosed with primary immunodeficiencies. Vaccine 2019, 37, 1350–1355. [Google Scholar] [CrossRef]
- Pasternak, G.; Lewandowicz-Uszyńska, A.; Królak-Olejnik, B. Recurrent respiratory tract infections in children. Pol. Merkur. Lek. 2020, 49, 260–266. [Google Scholar]
- Suzuki, M.; Dhoubhadel, G.; Ishifuji, T.; Yasunami, M.; Yaegashi, M.; Asoh, N.; Ishida, M.; Hamaguchi, S.; Aoshima, M.; Ariyoshi, K.; et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: A multicentre, prospective, test-negative design study. Lancet 2017, 17, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Berical, A.C.; Harris, D.; Dela Cruz, C.S.; Possick, J.D. Pneumococcal Vaccination Strategies An Update and Perspective. AnnalsATS 2016, 13, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Niederman, M.S.; Folaramni, T.; Buchwald, U.K.; Musey, L.; Cripps, A.W.; Johnson, K.D. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and noninvasive pneumococcal disease and related outcomes: A review of available evidence. Expert Rev. Vaccines 2021, 20, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Heathb, P.T.; Siegristc, C.A. Use of pneumococcal polysaccharide vaccine in children: What is the evidence? Curr. Opin. Infect. Dis. 2012, 25, 292–303. [Google Scholar] [CrossRef]
- Ochoa-gondar, O.; Vila-corcoles, A.; Rodriguez-blanco, T.; GomezBertomeu, F.; Figuerola-Massana, E.; Raga-Luria, X.; Hospital-Guardiola, I. Effectiveness of the 23-Valent Pneumococcal Polysaccharide vaccine against community-acquired pneumonia in the general population Aged >60 Years: 3 years of follow-up in the CAPAMIS study. Clin. Infect. Dis. 2014, 58, 909–917. [Google Scholar] [CrossRef]
- Moberley, S.; Holden, J.; Tatham, D.P.; Andrews, R.M. Cochrane Acute Respiratory Infections Group. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 2013, 2013, CD000422. [Google Scholar]
- Reda, S.M.; El-Ghoneimy, D.H.; Afifi, H.M. Clinical Predictors of Primary Immunodeficiency Diseases in Children. Allergy Asthma Immunol. Res. 2013, 5, 88–95. [Google Scholar] [CrossRef]
- Huwyler, C.; Lin, S.Y.; Liang, J. Primary Immunodeficiency and Rhinosinusitis. Immunol. Allergy Clin. N. Am. 2020, 40, 233–249. [Google Scholar] [CrossRef]
- Moraes Lui, C.; Oliveira, L.C.; Diogo, C.L.; Kirschfink, M.; Grumach, A.S. Immunoglobulin G subclass concentrations and infections in children and adolescents with severe asthma. Pediatr. Allergy Immunol. 2002, 13, 195–202. [Google Scholar] [CrossRef]
- Castro-Rodrigues, J.A.; Abarca, K.; Forno, E. Asthma and the Risk of Invasive Pneumococcal Disease: A Meta-analysis. Pediatrics 2020, 145, e20191200. [Google Scholar] [CrossRef]
- Ingels, H.A.S.; Kantsø, B.; Slotved, H.C. Serologic response to pneumococcal vaccination in children experiencing recurrent invasive pneumococcal disease. BMC Infect. Dis. 2018, 18, 366. [Google Scholar] [CrossRef]
- Estrada, J.; Najera, M.; Pounds, N.; Catano, G.; Infante, A.J. Clinical and Serologic Response to the 23-valent Polysaccharide Pneumococcal Vaccine in Children and Teens with Recurrent Upper Respiratory Tract Infections and Selective Antibody Deficiency. Pediatr. Infect. Dis. J. 2016, 35, 205–208. [Google Scholar] [CrossRef]

| Variable | Group | n | Mean ± SD | Median (Min–Max) | Total (n) | p Value | |
|---|---|---|---|---|---|---|---|
| Onset of symptoms (years) | No response | 142 | 8.5 ± 14.9 | 2.2 (0–75.4) | 390 | 0.002 b | |
| Good response | 248 | 4.6 ± 9.7 | 1.0 (0–64.8) | ||||
| Total | 6.0 ± 12.0 | 1.2 (0–75.4) | |||||
| Age at vaccination (years) | No response | 142 | 16.0 ± 15.6 | 11.1 (2.3–77.7) | 390 | 0.001 b | |
| Good response | 248 | 10.3 ± 11.2 | 6.3 (2.3–69.5) | ||||
| Total | 12.4 ± 13.2 | 8.0 (2.3–77.7) | |||||
| Categorical clinical and demographic characteristics (N = 390) | |||||||
| Variable | No response (n, %) | Good response (n, %) | Total (n, %) | p value | |||
| Male | 77 (54.2%) | 136 (54.8%) | 213 (54.6%) | 0.907 | |||
| IEI family history | 8 (5.8%) | 19 (7.8%) | 27 (7.0%) | 0.461 | |||
| Early deaths in the family | 20 (14.4%) | 20 (8.2%) | 40 (10.4%) | 0.055 | |||
| Consanguinity | 15 (10.8%) | 21 (8.6%) | 36 (9.4%) | 0.473 | |||
| Prematurity | 17 (12.0%) | 48 (19.4%) | 65 (16.7%) | 0.060 | |||
| Heart disease | 21 (14.8%) | 11 (4.4%) | 32 (8.2%) | <0.001 | |||
| Pneumopathy/Bronchiectasis | 14 (9.9%) | 25 (10.1%) | 39 (10.0%) | 0.944 | |||
| GERD | 13 (9.2%) | 20 (8.1%) | 33 (8.5%) | 0.710 | |||
| Developmental delay/Neurological | 40 (28.2%) | 46 (18.5%) | 86 (22.1%) | 0.027 | |||
| Atopic dermatitis | 23 (16.3%) | 28 (11.3%) | 51 (13.1%) | 0.158 | |||
| Asthma | 81 (57.0%) | 144 (58.1%) | 225 (57.7%) | 0.844 | |||
| Allergic rhinitis | 93 (66.0%) | 174 (70.2%) | 267 (68.6%) | 0.844 | |||
| Allergic conjunctivitis | 12 (8.6%) | 13 (5.2%) | 25 (6.4%) | 0.200 | |||
| Clinical Event | Timepoint | Mean ± SD | Median (Min–Max) | Mean Difference ± SD | Median Difference (Min–Max) | p Value | |
|---|---|---|---|---|---|---|---|
| Hospitalizations | Before | 0.89 ± 1.16 | 0.51 (0.00–7.68) | <0.001 | |||
| After | 0.13 ± 0.53 | 0.00 (0.00–6.67) | −0.76 ± 1.17 | −0.39 (−7.68 to 4.04) | |||
| ICU admissions | Before | 0.18 ± 0.41 | 0.00 (0.00–3.21) | <0.001 | |||
| After | 0.00 ± 0.05 | 0.00 (0.00–0.67) | −0.17 ± 0.40 | 0.00 (−3.21 to 0.19) | |||
| URTIs | Before | 4.11 ± 3.40 | 3.34 (0.00–19.92) | <0.001 | |||
| After | 1.55 ± 1.76 | 1.11 (0.00–13.33) | −2.56 ± 3.27 | −1.90 (−16.22 to 7.96) | |||
| Tonsillitis | Before | 1.61 ± 2.05 | 0.76 (0.00–11.60) | <0.001 | |||
| After | 0.51 ± 1.16 | 0.00 (0.00–10.00) | −1.09 ± 2.23 | −0.44 (−11.60 to 8.32) | |||
| Otitis | Before | 1.28 ± 2.32 | 0.16 (0.00–14.54) | <0.001 | |||
| After | 0.32 ± 1.15 | 0.00 (0.00–12.00) | −0.96 ± 2.08 | −0.07 (−12.94 to 3.79) | |||
| Sinusitis | Before | 0.90 ± 1.58 | 0.12 (0.00–9.96) | <0.001 | |||
| After | 0.53 ± 1.01 | 0.00 (0.00–6.67) | −0.37 ± 1.70 | 0.00 (−9.96 to 6.42) | |||
| Uncomplicated pneumonia | Before | 1.02 ± 1.59 | 0.32 (0.00–11.95) | <0.001 | |||
| After | 0.22 ± 0.65 | 0.00 (0.00–4.67) | −0.79 ± 1.59 | −0.17 (−11.95 to 3.49) | |||
| Pneumonia with parapneumonic effusion | Before | 0.07 ± 0.31 | 0.00 (0.00–3.21) | <0.001 | |||
| After | 0.00 ± 0.01 | 0.00 (0.00–0.18) | −0.07 ± 0.31 | 0.00 (−3.21 to 0.18) | |||
| Skin infections | Before | 0.08 ± 0.52 | 0.00 (0.00–7.50) | 0.078 | |||
| After | 0.04 ± 0.20 | 0.00 (0.00–2.00) | −0.04 ± 0.49 | 0.00 (−6.83 to 1.41) | |||
| Meningitis | Before | 0.03 ± 0.15 | 0.00 (0.00–1.17) | <0.001 | |||
| After | 0.00 ± 0.04 | 0.00 (0.00–0.67) | −0.03 ± 0.14 | 0.00 (−1.17 to 0.00) | |||
| Endocarditis | Before | 0.02 ± 0.11 | 0.00 (0.00–1.00) | 0.018 | |||
| After | 0.00 ± 0.00 | 0.00 (0.00–0.00) | −0.02 ± 0.11 | 0.00 (−1.00 to 0.00) | |||
| Sepsis | Before | 0.06 ± 0.18 | 0.00 (0.00–1.05) | <0.001 | |||
| After | 0.00 ± 0.00 | 0.00 (0.00–0.00) | −0.06 ± 0.18 | 0.00 (−1.05 to 0.00) | |||
| Rare events (N = 240) | |||||||
| Event | Before (n = 240) | After (n = 240) | p value | ||||
| Post-septal cellulitis | 1 (0.4%) | 1 (0.4%) | 1.000 a | ||||
| Osteomyelitis | 1 (0.4%) | 0 (0.0%) | – | ||||
| Joint infections | 2 (0.8%) | 0 (0.0%) | – | ||||
| Organ abscess | 1 (0.4%) | 0 (0.0%) | – | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel, M.d.G.-P.; Aranda, C.S.; Guimarães, R.R.; Ishizuka, E.K.; Solé, D.; Condino-Neto, A. Pneumococcal Vaccine in Patients with Recurrent Infections. Allergies 2025, 5, 21. https://doi.org/10.3390/allergies5020021
Pimentel MdG-P, Aranda CS, Guimarães RR, Ishizuka EK, Solé D, Condino-Neto A. Pneumococcal Vaccine in Patients with Recurrent Infections. Allergies. 2025; 5(2):21. https://doi.org/10.3390/allergies5020021
Chicago/Turabian StylePimentel, Mariana de Gouveia-Pereira, Carolina Sanchez Aranda, Rafaela Rola Guimarães, Edson Kiyotaka Ishizuka, Dirceu Solé, and Antônio Condino-Neto. 2025. "Pneumococcal Vaccine in Patients with Recurrent Infections" Allergies 5, no. 2: 21. https://doi.org/10.3390/allergies5020021
APA StylePimentel, M. d. G.-P., Aranda, C. S., Guimarães, R. R., Ishizuka, E. K., Solé, D., & Condino-Neto, A. (2025). Pneumococcal Vaccine in Patients with Recurrent Infections. Allergies, 5(2), 21. https://doi.org/10.3390/allergies5020021






