Occupational Exposure to Biological Agents in a Typical Restaurant Setting: Is a Photocatalytic Air Purifier Helpful?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Timeline of the Study
2.3. Microbiological Monitoring
2.4. Sanitizing Device
2.5. Sample Collection and Incubation
2.6. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLV | Threshold Limit Values |
| COPD | Chronic Obstructive Pulmonary Disease |
| CFU | Colony Forming Units |
| INAIL | Istituto Nazionale Assicurazione Infortuni sul Lavoro |
| PPE | Protective Personal Equipment |
| HVAC | Heating, Ventilation, and Air Conditioning |
| HEPA | High Efficiency Particulate Air |
| PCO | Photocatalytic Oxidation |
| VOC | Volatile Organic Compounds |
References
- Alija-Martínez, B.; Becerro de Bengoa Vallejo, R.; Sevillano Fernández, D.; González, N.; Losa Iglesias, M.E.; Collado, L.; Espinosa-Rubio, R.; Alou, L. Fungal bioaerosol as an occupational hazard in the podiatrist’s workplace. Int. J. Environ. Health Res. 2023, 33, 180–191. [Google Scholar] [CrossRef]
- On the interpretation of bioaerosol exposure measurements and impacts on health. J. Air Waste Manag. Assoc. 2019, 69, 789–804. [CrossRef]
- Szymańska, J. Dental bioaerosol as an occupational hazard in a dentist’s workplace. Ann. Agric. Environ. Med. AAEM 2007, 14, 203–207. [Google Scholar]
- Liebers, V.; Brüning, T.; Raulf, M. Occupational endotoxin exposure and health effects. Arch. Toxicol. 2020, 94, 3629–3644. [Google Scholar] [CrossRef]
- Kennedy, S.M.; Christiani, D.C.; Eisen, E.A.; Wegman, D.H.; Greaves, I.A.; Olenchock, S.A.; Ye, T.T.; Lu, P.L. Cotton dust and endotoxin exposure-response relationships in cotton textile workers. Am. Rev. Respir. Dis. 1987, 135, 194–200. [Google Scholar]
- Milton, D.K.; Wypij, D.; Kriebel, D.; Walters, M.D.; Hammond, S.K.; Evans, J.S. Endotoxin exposure-response in a fiberglass manufacturing facility. Am. J. Ind. Med. 1996, 29, 3–13. [Google Scholar] [CrossRef]
- Douwes, J.; Mannetje, A.; Heederik, D. Work-related symptoms in sewage treatment workers. Ann. Agric. Environ. Med. 2001, 8, 39–45. [Google Scholar]
- Europe Union. Directive 2000/54/Ec—On the Protection of Workers from Risks Related to Exposure to Biological Agents at Work. Off. J. Eur. Communities 2000, 3, 25. [Google Scholar]
- European Collaborative Action. Indoor Air Quality & Its Impact on Man—Report n° 12—Biological Particles in Indoor Environments. 1993. Available online: https://op.europa.eu/it/publication-detail/-/publication/859b1f78-ea84-44a1-a045-c230c2283c9e (accessed on 2 June 2021).
- Górny, R.L.; Dutkiewicz, J. Bacterial and fungal aerosols in indoor environment in Central and Eastern European countries. Ann. Agric. Environ. Med. AAEM 2002, 9, 17–23. [Google Scholar]
- Kolk, A. Verfahren zur Bestimmung der Bakterienkonzentration in der Luft am Arbeitsplatz (#9430); Institut für Arbeitsschutz der DGUV: Sankt Augustin, Germany, 2004. [Google Scholar]
- Nevalainen, A. Bacterial Aerosols in Indoor Air; National Public Health Institute: Helsinki, Finland, 1988. [Google Scholar]
- Neto, F.R.A.; Siqueira, L.F. Guidelines for Indoor Air Quality in Offices in Brazil. 2000. Available online: https://api.semanticscholar.org/CorpusID:130418683 (accessed on 28 June 2021).
- Inail—National Institute for Insurance against Accidents at Work. Il Monitoraggio Microbiologico Negli Ambienti di Lavoro—Campionamento e Analisi (Microbiological Montioring at Workplace—Contarp Lineguides). 2010. Available online: https://www.inail.it/cs/internet/comunicazione/pubblicazioni/catalogo-generale/il-monitoraggio-microbiologico-negli-ambienti-di-lavoro.html (accessed on 28 June 2021).
- Adams, R.I.; Bhangar, S.; Pasut, W.; Arens, E.A.; Taylor, J.W.; Lindow, S.E.; Nazaroff, W.W.; Bruns, T.D. Chamber bioaerosol study: Outdoor air and human occupants as sources of indoor airborne microbes. PLoS ONE 2015, 10, e0128022. [Google Scholar] [CrossRef]
- Heo, K.J.; Lim, C.E.; Kim, H.B.; Lee, B.U. Effects of human activities on concentrations of culturable bioaerosols in indoor air environments. J. Aerosol Sci. 2017, 104, 58–65. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Bai, W.; Hou, J.; Ma, T.; Zeng, X.; Zhang, L.; An, T. The source and transport of bioaerosols in the air: A review. Front. Environ. Sci. Eng. 2021, 15, 44. [Google Scholar] [CrossRef]
- Kwan, S.E.; Shaughnessy, R.; Haverinen-Shaughnessy, U.; Kwan, T.A.; Peccia, J. The impact of ventilation rate on the fungal and bacterial ecology of home indoor air. Build. Environ. 2020, 177, 106800. [Google Scholar] [CrossRef]
- Almeida, A.G.C.D.S.; Bruna, C.Q.d.M.; Moriya, G.A.d.A.; Navarini, A.; Sasagawa, S.M.; Mimica, L.M.J.; Gambale, V.; Graziano, K.U. Impact of negative pressure system on microbiological air quality in a Central Sterile Supply Department. J. Occup. Health 2021, 63, e12234. [Google Scholar] [CrossRef]
- Yan, C.; Leng, Y.L.; Wu, J.T. Quantitative microbial risk assessment for occupational health of temporary entrants and staffs equipped with various grade PPE and exposed to microbial bioaerosols in two WWTPs. Int. Arch. Occup. Environ. Health 2021, 94, 1327–1343. [Google Scholar] [CrossRef]
- Valdez-Castillo, M.; Arriaga, S. Response of bioaerosol cells to photocatalytic inactivation with ZnO and TiO(2) impregnated onto Perlite and Poraver carriers. Front. Environ. Sci. Eng. 2021, 15, 43. [Google Scholar] [CrossRef]
- Cao, R.; Qiu, P.; Xu, B.; Lin, J.; Chu, D.; Fan, Z. Effectiveness of interventions to reduce aerosol generation in dental environments: A systematic review. Prev. Med. Rep. 2023, 35, 102383. [Google Scholar] [CrossRef]
- Uhde, E.; Salthammer, T.; Wientzek, S.; Springorum, A.; Schulz, J. Effectiveness of air-purifying devices and measures to reduce the exposure to bioaerosols in school classrooms. Indoor Air 2022, 32, e13087. [Google Scholar] [CrossRef]
- Dai, R.; Liu, S.; Li, Q.; Wu, H.; Wu, L.; Ji, C. A systematic review and meta-analysis of indoor bioaerosols in hospitals: The influence of heating, ventilation, and air conditioning. PLoS ONE 2021, 16, e0259996. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Negishi, N.; Yamano, R.; Hori, T.; Koura, S.; Maekawa, Y.; Sato, T. Development of a high-speed bioaerosol elimination system for treatment of indoor air. Build. Environ. 2023, 227, 109800. [Google Scholar] [CrossRef]
- Matsuura, R.; Lo, C.W.; Wada, S.; Somei, J.; Ochiai, H.; Murakami, T.; Saito, N.; Ogawa, T.; Shinjo, A.; Benno, Y.; et al. SARS-CoV-2 disinfection of air and surface contamination by TiO2 photocatalyst-mediated damage to viral morphology, rna, and protein. Viruses 2021, 13, 942. [Google Scholar] [CrossRef]
- Agenzia Delle Entrate (Internal Revenue Agency). Nota Tecnica e Metodologica Studio di Settore VG36U—Servizi Di Ristorazione Commerciale (Sectoral Fiscal Study about Restaurant Services). Available online: https://www1.agenziaentrate.gov.it/settore/studiapprovati/note_tecniche_2012/Nota_tecnica_VG36U.pdf (accessed on 14 January 2022).
- 10339:1995; Impianti Aeraulici al Fini di Benessere. Generalitá, Classificazione e Requisiti. Regole per la Richiesta d’Offerta, l’Offerta, l’Ordine e la Fornitura. UNI Ente Italiano di Normazione—Italian Standardization Institute: Milano, Italy. 1995. Available online: https://store.uni.com/uni-10339-1995 (accessed on 9 August 2023).
- Hill, A.R.; Kethireddipalli, P. Dairy Products: Cheese and Yogurt. In Biochemistry of Foods; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Schiraldi, C.; De Rosa, M. Mesophilic Organisms. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Denham, S.T.; Wambaugh, M.A.; Brown, J.C. How Environmental Fungi Cause a Range of Clinical Outcomes in Susceptible Hosts. J. Mol. Biol. 2019, 431, 2982–3009. [Google Scholar] [CrossRef]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi that Infect Humans. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Khan, A.H.; Karuppayil, S.M. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Niu, R.; Xu, L.; Lu, Y.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef]
- Martínez-Montelongo, J.H.; Medina-Ramírez, I.E.; Romo-Lozano, Y.; Zapien, J.A. Development of a sustainable photocatalytic process for air purification. Chemosphere 2020, 257, 127236. [Google Scholar] [CrossRef]
- Grignani, E.; Mansi, A.; Cabella, R.; Castellano, P.; Tirabasso, A.; Sisto, R.; Spagnoli, M.; Fabrizi, G.; Frigerio, F.; Tranfo, G. Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of COVID-19. Gases 2021, 1, 19–32. [Google Scholar] [CrossRef]
- CDC National Institute for Occupational Safety and Health. NIOSH Pocket Guide to Chemical Hazard—Ozone. 2019. Available online: https://www.cdc.gov/niosh/npg/npgd0476.html (accessed on 15 July 2021).
- Whyte, W.; Green, G.; Albisu, A. Collection efficiency and design of microbial air samplers. J. Aerosol Sci. 2007, 38, 97–110. [Google Scholar] [CrossRef][Green Version]
- Foschi, C.; Giorgi, B.; Ambretti, S.; Lazzarotto, T.; Violante, F.S. Real-life assessment of the ability of an ultraviolet C lamp (SanificaAria 200, Beghelli) to inactivate airborne microorganisms in a healthcare environment. Life 2023, 13, 1221. [Google Scholar] [CrossRef]
- Aadland, E.; Kvalheim, O.M.; Rajalahti, T.; Skrede, T.; Resaland, G.K. Aerobic fitness and metabolic health in children: A clinical validation of directly measured maximal oxygen consumption versus performance measures as markers of health. Prev. Med. Rep. 2017, 7, 74–76. [Google Scholar] [CrossRef]
- Mehta, S.K.; Bell-Robinson, D.M.; Groves, T.O.; Stetzenbach, L.D.; Pierson, D.L. Evaluation of portable air samplers for monitoring airborne culturable bacteria. AIHA J. 2000, 61, 850–854. [Google Scholar] [CrossRef]
- Macher, J.M. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am. Ind. Hyg. Assoc. J. 1989, 50, 561–568. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 2 May 2022).
- RStudio Team. RStudio: Integrated Development Environment for R. 2020. Available online: http://www.rstudio.com/ (accessed on 2 May 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; Francois, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Bogdal, C. Interactions between indoor and outdoor air pollution—Trends and scientific challenges. Environ. Pollut. 2012, 169, 150–151. [Google Scholar] [CrossRef]
- González-Martín, J.; Kraakman, N.J.R.; Pérez, C.; Lebrero, R.; Muñoz, R. A state-of-the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef]
- Leung, D.Y.C. Outdoor-indoor air pollution in urban environment: Challenges and opportunity. Front. Environ. Sci. 2015, 2, 69. [Google Scholar] [CrossRef]
- Stobnicka-Kupiec, A.; Gołofit-Szymczak, M.; Górny, R. Microbial contamination level and microbial diversity of occupational environmentin commercial and traditional dairy plants. Ann. Agric. Environ. Med. 2019, 26, 555–565. [Google Scholar] [CrossRef]
- Cyprowski, M.; Ławniczek-Wałczyk, A.; Stobnicka-Kupiec, A.; Górny, R.L. Occupational exposure to anaerobic bacteria in a waste sorting plant. J. Air Waste Manag. Assoc. 2021, 71, 1292–1302. [Google Scholar] [CrossRef]
- Lu, R.; Frederiksen, M.W.; Uhrbrand, K.; Li, Y.; Østergaard, C.; Madsen, A.M. Wastewater treatment plant workers’ exposure and methods for risk evaluation of their exposure. Ecotoxicol. Environ. Saf. 2020, 205, 111365. [Google Scholar] [CrossRef]
- Walser, S.M.; Gerstner, D.G.; Brenner, B.; Bünger, J.; Eikmann, T.; Janssen, B.; Kolb, S.; Kolk, A.; Nowak, D.; Raulf, M.; et al. Evaluation of exposure-response relationships for health effects of microbial bioaerosols—A systematic review. Int. J. Hyg. Environ. Health 2015, 218, 577–589. [Google Scholar] [CrossRef]
- Zacarías, S.M.; Pirola, S.; Manassero, A.; Visuara, M.E.; Alfano, O.M.; Satuf, M.L. Photocatalytic inactivation of bioaerosols in a fixed-bed reactor with TiO2-coated glass rings. Photochem. Photobiol. Sci. 2019, 18, 884–890. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; Arriaga, S. Mesoporous TiO2 monoliths impregnated with CdS and CuO nanoparticles for airborne bacteria inactivation under visible light. Catal. Lett. 2022, 152, 629–640. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Miranda, S.M.; Lopes, F.V.S.; Silva, M.; Dezotti, M.; Silva, A.M.T.; Faria, J.L.; Boaventura, R.A.R.; Vilar, V.J.P.; Pinto, E. Bacteria and fungi inactivation by photocatalysis under UVA irradiation: Liquid and gas phase. Environ. Sci. Pollut. Res. 2016, 24, 6372–6381. [Google Scholar] [CrossRef]
- Thabet, S.; Simonet, F.; Lemaire, M.; Guillard, C.; Cotton, P. Impact of Photocatalysis on Fungal Cells: Depiction of Cellular and Molecular Effects on Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2014, 80, 7527–7535. [Google Scholar] [CrossRef]
- Wolfrum, E.J.; Huang, J.; Blake, D.M.; Maness, P.C.; Huang, Z.; Fiest, J.; Jacoby, W.A. Photocatalytic Oxidation of Bacteria, Bacterial and Fungal Spores, and Model Biofilm Components to Carbon Dioxide on Titanium Dioxide-Coated Surfaces. Environ. Sci. Technol. 2002, 36, 3412–3419. [Google Scholar] [CrossRef]
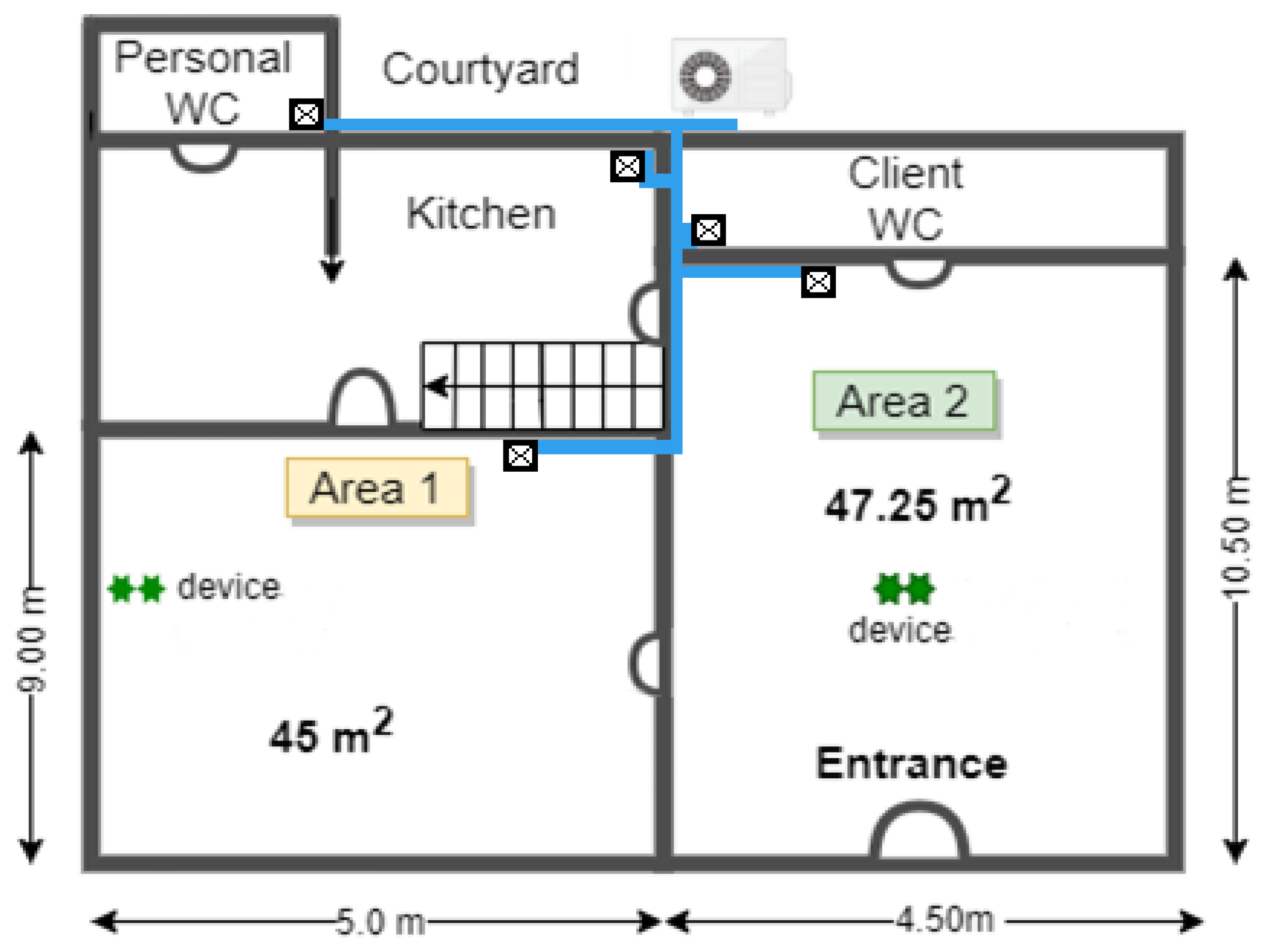
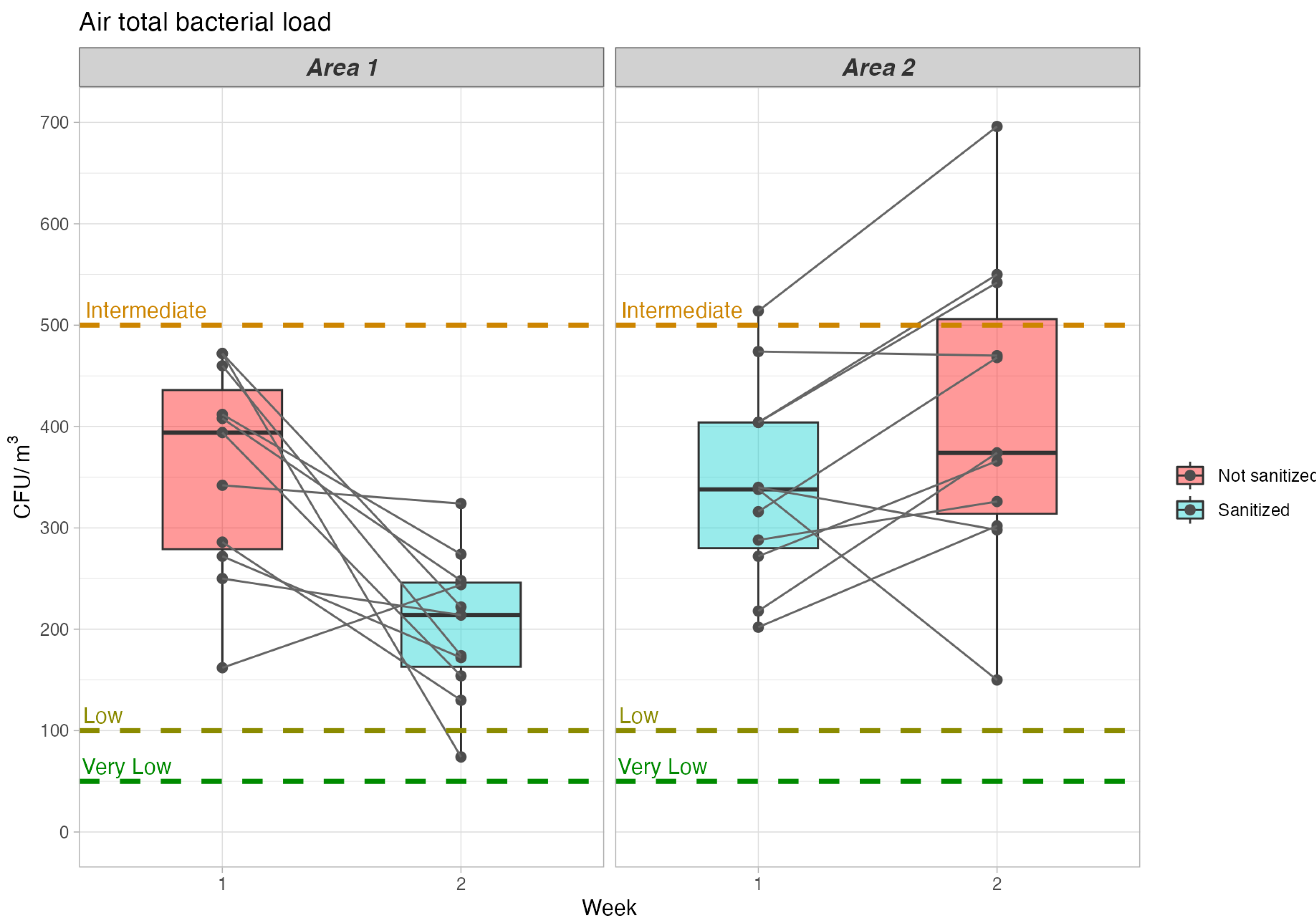
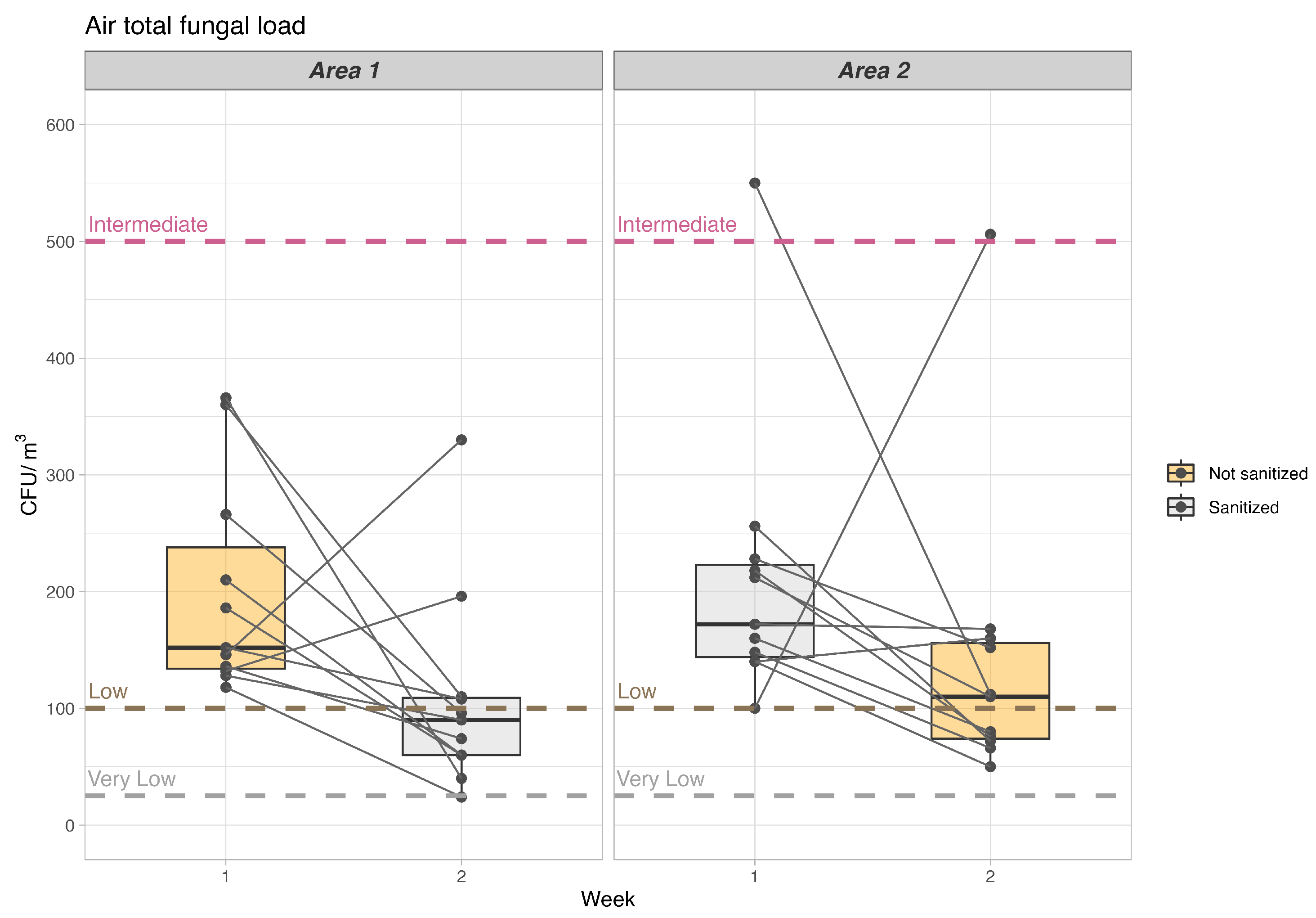
| Area/Charge | Non-Sanitized Measure—Mean (SD) | Sanitized Measure—Mean (SD) | Mean of the Differences in Paired Values (95%CI) | Relative Percentage Change Compared to Unsanitized Measure | p Value |
|---|---|---|---|---|---|
| Area 1 – bacterial | 346.8 (104.6) | 202.7 (70.7) | 154.5 (63.2–245.9) | % | <0.01 |
| of which mesophilic | 183.5 (60.8) | 107.3 (44.3) | |||
| of which psychrophilic | 163.3 (50.7) | 95.5 (35.9) | |||
| Area 1—fungal | 189.7 (94.3) | 108.0 (86.6) | 92 (–186.3) | % | 0.055 |
| of which yeast | 32.7 (15.2) | 21.5 (11.7) | |||
| of which mold | 157 (89.9) | 86.5 (76.2) | |||
| Area 2—bacterial | 412.9 (150.9) | 342.2 (94.5) | 70.2 (–145.0) | % | 0.063 |
| of which mesophilic | 233.1 (102.6) | 186.7 (50.9) | |||
| of which psychrophilic | 179.8 (72.0) | 155.5 (54.0) | |||
| Area 2—fungal | 141.1 (127.6) | 205.0 (117.9) | (5–63.2) | 45.30% | 0.268 |
| of which yeast | 30.4 (19.9) | 32.8 (13.3) | |||
| of which mold | 110.7 (114.3) | 172.2 (114.7) |
| Category | Houses (Bacteria—CFU/m3) | Non Industrial Environment (Bacteria—CFU/m3) | Houses (Fungi—CFU/m3) | Non Industrial Environment (Fungi—CFU/m3) |
|---|---|---|---|---|
| Very Low | <100 | <50 | <50 | <25 |
| Low | <500 | <100 | <500 | <100 |
| Intermediate | <2500 | <500 | <1000 | <500 |
| High | <10,000 | <2000 | <10,000 | <2000 |
| Very High | >10,000 | >2000 | >10,000 | >2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratti, M.; Ceriotti, D.; Bibi, R.; Conti, A.; Panella, M. Occupational Exposure to Biological Agents in a Typical Restaurant Setting: Is a Photocatalytic Air Purifier Helpful? Safety 2023, 9, 81. https://doi.org/10.3390/safety9040081
Ratti M, Ceriotti D, Bibi R, Conti A, Panella M. Occupational Exposure to Biological Agents in a Typical Restaurant Setting: Is a Photocatalytic Air Purifier Helpful? Safety. 2023; 9(4):81. https://doi.org/10.3390/safety9040081
Chicago/Turabian StyleRatti, Matteo, Daniele Ceriotti, Rabia Bibi, Andrea Conti, and Massimiliano Panella. 2023. "Occupational Exposure to Biological Agents in a Typical Restaurant Setting: Is a Photocatalytic Air Purifier Helpful?" Safety 9, no. 4: 81. https://doi.org/10.3390/safety9040081
APA StyleRatti, M., Ceriotti, D., Bibi, R., Conti, A., & Panella, M. (2023). Occupational Exposure to Biological Agents in a Typical Restaurant Setting: Is a Photocatalytic Air Purifier Helpful? Safety, 9(4), 81. https://doi.org/10.3390/safety9040081








