Abstract
Aerogels have recently started to be considered as “advanced materials”; therefore, as a general consideration, aerogels’ toxicity testing should focus on their functionality which resides in their nanoscale open internal porosity. To assess the hazards of organic aerogels, testing at three levels may characterize their biophysical, in vitro and in vivo toxicity, defining distinct categories of aerogels. At the first level of testing, their abiotic characteristics are investigated, and the best aerogel(s) is forwarded to be tested at level 2, wherein in vitro methodologies may mainly evaluate the aerogels’ cellular behavior. Within level 2 of testing, the main characteristics of toxicity are investigated and the selected aerogels are introduced to in vivo animal models at level 3. In the animal model testing, target organs are investigated along with systemic parameters of toxicity. Some study cases are presented for organic or anorganic aerogels. Within this tiered workflow, aerogels-based materials can be tested in terms of human health hazard.
1. Introduction
Aerogels are uncommon materials that stand by themselves as nanosized structures that have a high specific surface area, low density and high porosity. In recent years, aerogels technology has been used to develop innovative tools in the food industry by the microencapsulation of additives or in the health system to incorporate drugs [1,2,3,4]. The raised question is whether aerogels are nanomaterials or not? Under the REACH Annexes, aerogels are explicitly excluded [5,6] as a “nanoform”. In several countries (e.g., Belgium, the USA or Canada), aerogels do not need to be reported to the national nanomaterials product inventories [7]. According to the new definition of nanomaterials, which was published in 2022, if the intended constitute material has at least 50% of nanomaterial, it can be assessed as such. Therefore, one can assume that, although there are no nanoform per se, aerogels can be analyzed using the nanomaterials guidelines [8]. Consequently, the majority of the materials that can be framed as an aerogel have low toxicity, but an enhanced bioactivity that results from their large inner surface area, high surface reactivity and/or an increased dissolution; all these particularities should be taken into account when assessing aerogels [9].
The hazard assessment of aerogels has to take into account the fact that inhalable or ingestible fragments have components that can have toxicity characteristics matching the ones displayed by nanostructures [10]. Moreover, aerogels present a large surface area, so their potency resides in this extended functional surface [11]. Regardless, the testing strategy should follow the future use of the aerogel. For example, in the future oral use of an aerogel-based product, an unintended inhalation of its dusts can be imagined; thus, the aerogel-based compound should be tested for pulmonary deposition as a hazardous route of exposure [12]. When using a controlled drug release, aerogels’ oral bioavailability will depend on several factors, such as dose, solubility, permeability and metabolism [13]. Then, if the drug release is intended in a specific site (e.g., release in the gastrointestinal tract or as wound dressing), testing should also be conducted for drug physicochemical stability and/or permeability in the intended site [14,15]. Furthermore, if the drug is intended for a prolonged time of action, the development of oral formulations should also test a prolonged action and the drug delivery should be regulated at a precise release rate [16].
When manufacturing aerogels (e.g., consumer goods of alginate, chitosan and so on), the powder handling can induce occupational exposures. Almost 60 years ago, it was shown that silica desiccant gels, related to aerogels, could have inhalable fragments, inducing toxicological effects [17,18,19]. There are still very many unknown issues about the hazard potential of most aerogels [20]. Nevertheless, the most studied characteristics focus on the biomedical applications of aerogels [21,22], from implants [23,24] to drugs’ bioavailability, to biocides encompassed in aerogels [25,26,27].
Within this short compendium-like paper, we will describe the main phases to be followed when evaluating a biomedical-intended aerogel.
2. Aerogels’ Particularities as Innovative Materials
This type of material is solid, with a very light weight and displaying a coherent open porous matrix of lightly packed, bonded particles or nanoscale fibers. Their structure is obtained from a gel that is subjected to the removal of the pore fluid without damaging its inherent structure. Due to these particular characteristics, aerogels have a very high specific surface area [28]. Moreover, this special group of materials has distinct properties like high porosity, low bulk density, very good textural properties and, just as important, tunable surface chemistry that endows them with exquisite applicability in various domains [29]. Therefore, the combination of low density and super-nanoporosity with pores of 2–50 nm in diameter was explored for silica aerogels in industrial applications like thermal insulation of building materials and even in aerospace technologies. There are already commercialized products such as insulating pipes/boards/blankets/translucent panels in industrial applications. As biomedical application aerogel technology is rapidly expanding due to the fact that it can provide a material that is health-acquiescent, it can be molded according to specific needs; it can have high yield, reproducibility and low toxicity, thus satisfying the biomedical requirements. Therefore, choosing the best biocompatible basic material can be subjected to specific design platforms to obtain an advanced material suitable for a specific biomedical application [21].
The best technology to provide very light weight, high specific surface area and coherent open porous matrix of the aerogel material is the supercritical fluid-based drying of gels, a technology for obtaining this innovative material [30]. As the supercritical drying process is the best technical option for aerogels, the design optimization of the technology is a subject of intense collaborative research and efforts [31,32].
Currently, this technology is based on the gentle drying technique that allows for the best preservation of the fragile solid network structure from which the material is generated [15]. Moreover, this robust workflow can be developed for producing aerogel-biomolecule-loaded materials to be used in future wound healing applications. The obtained aerogels have exquisite properties, like a narrow size distribution, high surface area, good porosity that can harbor drug encapsulation and can further sustain a controlled release of the drug. Additionally, this aerogel has a robust capacity to absorb wound exudate, transforming itself into a soft elastic hydrogel, prolonging the incorporated drug release for up to 72 h. The report shows that alginate-based aerogel capsules can both control drug release and manage wound exudate, in acute and chronic wounds. These alginate aerogels can be tailored as specific doses and drug-release kinetics in personalized medicine, to meet individual patients’ needs [15].
Other techniques that remove the pore’s moisture like ambient drying or freeze-drying can be used to obtain xerogels and cryogels. These technologies can lead to materials with comparable properties to aerogels. Therefore, xerogels and cryogels can have similarities with aerogels and this depends on the initial gel source, the actual solid content and the stability of the obtained 3D structure, due to the fact that mechanical properties are doubled by the morphology and the physicochemical properties [33]. When the applied technology is evaporative drying, solvent exchanges and sialylation steps are needed to circumvent the shrinkage of the fine porous structure. An outline of the main steps in obtaining xerogels, cryogels and aerogels is presented in Figure 1.
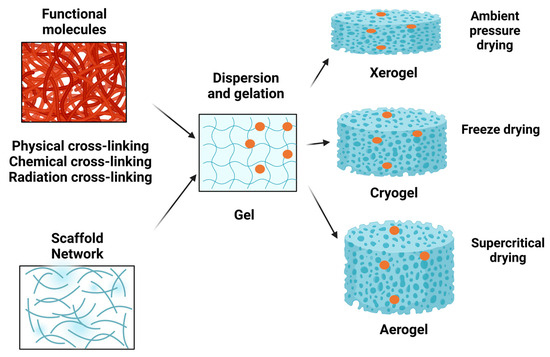
Figure 1.
Main procedure steps for obtaining xerogels, cryogels and aerogels. Using several methods (physical, chemical, radiation) of cross-linking, various functional molecules can be embedded in a network structure upon dispersion and gelation. Afterwards, the gel can be subjected to ambient pressure drying for obtaining a xerogel, or to freeze-drying for obtaining a cryogel or to supercritical drying for obtaining an aerogel.
For biomedical applications, biopolymers are gaining constant interest due to several properties. Hence, they can originate from renewable resources that have a good economic impact, being highly bio-compatible and bio-degradable [3]. In this setting, the search has been focusing on abundant natural polymers, e.g., cellulose, starch or pectin. All these biopolymers can be transformed into gels and moreover into aerogels that can then become scaffolding materials harboring tissue-regenerating cells, they can be turned into artificial cartilage, they can be tailored as blood vessels, they can heal wounds and so many other biomedical applications. Moreover, in the environmental domain, aerogels can adsorb noble metals in the recycling process or can purify water from pollutants adsorbing heavy metals, oil, organic compounds and other pollutants [34,35].
From an ecological/toxicological point of view, the bio-polymer-based aerogels offer important advantages because no toxic compounds are involved in their preparation. Hence, bio-polymer aerogels are “biologically-friendly” and can be subjected to further testing for biomedical applications. Recently, new bio-based aerogels, like nanocellulose, proving specific self-assembling capabilities, can be the new players in the biomedicine applications domain [36]. Nanocellulose aerogels in fact use an abundant source as cellulose, and so it can represent the third-generation of innovative aerogel materials characterized by high porosity and large specific surface area, but also proving good bio-compatibility properties of the cellulose. Nanocellulose aerogels can be used in industrial applications (e.g., thermal insulation, electromagnetic interference shielding, purification, energy storage) but also in biomedical applications [37].
Apart from the nanocellulose-based aerogels, silica-based aerogels have high chemical versatility but lower mechanical properties so that their application does not comply with domains that need strength and stiffness. However, silica aerogels’ chemistry can be improved by carefully choosing silane precursors, by hybridization with poly-organo-siloxanes, organic polymers or fibers. With these improvements, their mechanical properties, namely strength and flexibility, silica aerogels can enlarge their applicability [38]. Natural fibers can reinforce the dried silica aerogel composites [39]. Another recent tendency is to mechanically reinforce silica-based aerogels with carbon nanotubes, which are carbon aerogels of graphene [40]. Silica-based aerogels and aerogels based on cellulose, polyurethane and so on can be endowed with additional useful properties, hierarchical porosity, super-flexibility and shape memory [41].
In the biomedical domain, aerogels can be drug-delivery matrices, they can be tailored to match various physiological administration routes like oral, pulmonary/nasal inhalation, topical administration and so on. Within these administration routes, aerogels should sustain drug-release kinetics, be both robust to resist the host’s defense mechanisms and flexible enough to modulate itself in a new biological environment. Therefore, aerogels can improve drug bioavailability and can be tailored to have a controlled drug delivery [42]. For these biomedical applications, aerogels can be designed to have pores from few microns to few millimeters in diameter [43]. As further elaborated in the other sections of this paper, aerogel particles, with their high porosity, can be carriers for pulmonary delivery having 20–25 µm diameters and penetrating in the lung’s alveoli [44].
Biopolymer-based aerogels have an as important advantage as carriers because they can have an improved dissolution rate of hydrophobic or poorly hydrophilic drugs. This is important as drugs can have good specificity to a target but low dissolution rate that overcomes their efficacy. Aerogels can be tailored to have a high drug pay load, can sustain the stability of drugs and any other properties specific for oral, topical, pulmonary or nasal administration routes [4,17,45]. Consequently, designing innovative aerogels increases the therapeutic efficiency because, for example, an oral inhalation administration drug will have an improved lung deposition, an enhanced drug permeation and an overall positive impact on the healthcare system and economics [46].
Another expanding domain of biomedical application of aerogels is the wound healing domain. The healing process is complex and it involves an acute inflammatory reaction that generates the final regeneration of the injured tissue. But when the processes of healing are dysfunctional, e.g., in diabetes, the healing process is stalled and the wound becomes chronic. Therefore, wound dressings are essential for the physical barrier that protects the wound milieu from environmental contaminants. Furthermore, aerogels can be designed to incorporate bio-active molecules for an increased rate of wound-healing events. Aerogels need to regulate the release of the bio-active compounds on site, in a controlled manner and providing targeted therapeutic effects. Moreover, the aerogel should prevent infections and may form a wet medium within the lesion, equilibrating the wound exudate and accelerating the healing process. For example, besides bio-active molecules, aerogels can be designed to incorporate sensors. In order to evaluate the protease activity within the lesion, the cellulose-based aerogel can be designed to have an incorporated sensor that senses the protease activity, this sensor evaluating wound pathology [47]. The healthcare market of wound dressing is expanding, so aerogels incorporated in advanced wound dressings [48] can impact the healthcare system in acute and chronic wounds from diabetic foot ulcer to pressure ulcer patients [49].
In the regenerative medicine domain, aerogel-based scaffolds can be tailored to mimic the extracellular matrix and specific bioactive molecules can be used to enhance regeneration and tissue integration [24,50]. Cellulose phosphate is a good cell scaffolding material because it enhances the growth of mesenchymal stem cells, it improves osteogenic differentiation, and does not display inflammatory responses [51]. An overview of aerogel applications in the biomedical field is presented in Figure 2 and Table 1.
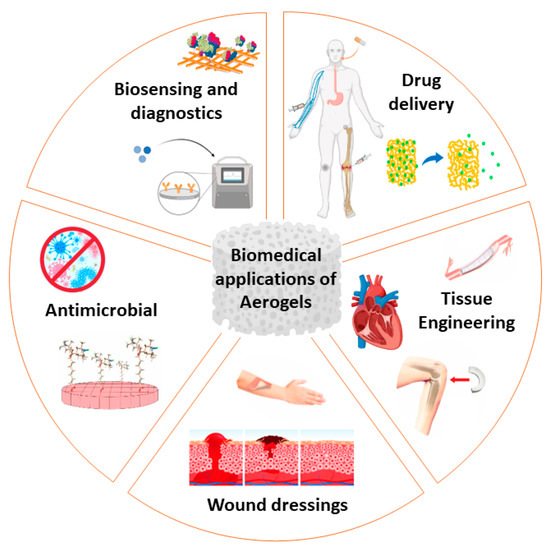
Figure 2.
Aerogels’ biomedical applications as tissue-engineering scaffolds, wound-dressing materials, biosensing and diagnostics, drug-delivery matrices and antimicrobial materials.

Table 1.
Aerogels in biomedical applications.
Overall, aerogels’ particularities are to be used in the biomedical domain, bringing new medical solutions on one hand and reducing healthcare expenses on the other.
3. Safety Regulation in Aerogels Testing
Aerogels based on biomaterials like silk fibroin [61], nanocellulose [62], chitosan [63] or alginate [64] are usually synthesized from natural sources subjected to cross-linking reactions [65]. These aerogels have very good biodegradability and biocompatibility and can be used in tissue engineering, drug delivery and biosensing. There are several characteristics of aerogels that make them biocompatible. Hence, they are characterized by high porosity and connectivity in open nanostructures that are advantageous to several physiological cellular processes like attachment, nutrition, oxygen availability, metabolism by-products and elimination [21]. As drug-delivery systems, aerogels can take advantage from several anorganic and organic structures, such as SiO2 [66], TiO2 [67], chitosan [68], pectin [69], carrageenan [70], alginate [71], starch [72] and cellulose [73].
In a published workflow for aerogels testing, the group of Keller et al. has shown the tiered testing strategy [11]; additional teams have applied the workflow in order to categorize and group nanomaterials [74,75,76].
The starting point is to establish, for the tested aerogel, the group of tests for similar materials [77]. Therefore, Keller et al. have included standard materials with conventional porous structures [11], materials that were further used by other groups to test silica aerogels [78]. The proposed flow starts with acellular screening (level 1), continues with in vitro toxicity (level 2) and ends with in vivo toxicity in level 3. Performing tests in all these levels, additional information is gained and the obtained results sieve the best compound intended for a biomedical application. Figure 3 depicts the proposed general workflow.
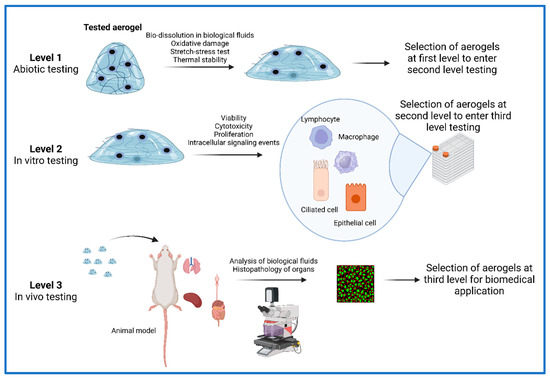
Figure 3.
Workflow for the testing procedures of an aerogel intended for biomedical application. At level 1, aerogels are tested for their bio-dissociation capacity in biological fluids, their oxidative abiotic capacity, their stretch potential if the intended use will comprise patch-type application and thermal stability if the intended use will be in thermal conditions that change. Upon first-level testing, the aerogels that meet the intended physical and chemical criteria will be tested at the second level in in vitro cellular cultures. Within level 2, the in vitro toxicity will comprise the testing of cellular viability, cytotoxicity, cellular proliferation and various other physiological important characteristics. Upon level 2 completion, aerogels will be selected by matching the best behavior in cellular conditions and will be subjected to in vivo testing in animal models. In level 3, various routes of testing will be performed and complex clinical and paraclinical parameters will be studied in the experimental animal. Histopathology of various organs and the analysis of relevant body fluids (plasma, bronchoalveolar fluid, etc.) will select the aerogel with the best in vivo behavior.
This testing strategy conforms to the Horizon 2020 GRACIOUS consortium focusing on the grouping of nanomaterials and developing the risk assessment process for practical application [79]. The specific tests conform with the tiered collection of methods developed earlier within the nanoGRAVUR framework [80] and with the nanomaterial’s life cycle and biological pathways developed by DF4nanoGrouping [81,82]. These mentioned projects that were developing guidelines for the nanomaterials domain were applied to materials in the nanometer range only, while recently Keller et al. applied them to the open-pore internal nanostructures [11].
3.1. Level 1
3.1.1. Testing the Oxidative Stress in Abiotic Conditions
The ferric reducing ability of the serum (FRAS) assay [83] is an analysis that detects the oxidative stress induction as part of the nanotoxicity of the materials. The FRAS assay actually detects the impairment of antioxidants in the human serum [84,85,86].
As identical classes of aerogels have the same chemical composition, they can differ due to different production processes in their porosity and grain size. Thus, porosity can influence the aerogel’s reactivity, and hence it can determine the biologically accessible surface area [11]. European_Chemical_Agency (ECHA) guidelines advise on grouping similar nanoforms within the same substance [87], and within this guideline, the reactivity tests suggest that all aerogels with the same chemical compositions (e.g., cellulose, alginate, silicate, polyurethane) have low surface reactivity.
The available surface of nanomaterials, in this case aerogels, impacts toxicity and the surface reactivity plays an important role in the interaction with the environment, biological systems, strongly driving health hazards [88].
The FRAS assay is simple, reproducible, widely used in the biomedical domain to measure the antioxidant capacity of a compound in many designs [89,90]. For example, FRAS tested in both human saliva and plasma could depict differences in oral hygiene [89]. Comparing various methods against FRAS, a recent study showed that this assay was the only test able to show a significant change in the total antioxidant capacity in hens subjected to drug-induced stress [90].
3.1.2. Testing the Dissolution Characteristics in Biological Fluids in Acellular Conditions
Testing aerogels for their dissolution capacity in biological fluids is important because, in their future application, aerogels will be subjected to the unbiased contact to specific biological fluids; therefore, their characteristics should be thoroughly analyzed in these conditions. Keller et al. tested the dissolution of aerogels in lysosomal fluid (pH 4.5) and gastric simulant (pH 1.6) and it was reported that almost all tested aerogels had a higher dissolution in lysosomal fluid than the gastric simulant [11]. The results show that bio-dissolution is determined by the molecular composition; hence, all the tested polyurethane (PU) aerogels ranked the lowest dissolution capacity, while the tested silica or alginate-based aerogels ranked the highest [11]. Similarly, in their dissolution testing, both the grain size that is related to the outer surface and the interior structure (total specific surface) can influence bio-dissolution. The authors point out that, after aerogel production, the details regarding the entire interior surface accessibility are not perfectly known, but the bio-dissolution correlates with it. Therefore, the relative ranking of the two tested silica aerogels as well as the two tested alginate aerogels correlate to their bio-dissolution rankings, indicating that the interior surface is critical for dissolution [11]. Earlier studies have shown that the release of a drug like ketoprofen from starch, alginate and pectin aerogels was sensitive to an acid pH, namely pH 4 [2,91]. Aerogels made from starch, pectin and alginate were loaded with the anti-inflammatory drug ketoprofen and the release behavior of the drug was evaluated in the 1.2–6.8 pH range, matching gastric and intestinal pH conditions. Drug release from starch aerogel was governed by dissolution mechanisms. The alginate aerogel exhibited accelerated drug release stimulated by the gastric pH. The authors point out that the release profiles of drugs from aerogels are governed by the type of the aerogel matrix. Therefore, in the drug-release biomedical applications, the nanostructured materials of aerogels can be good drug carriers [2].
Beforehand, when testing aerogels, the interior surface of the compounds is decisive for their dissolution. When analyzing aerogels based on alginate, pectin, chitosan and cellulose, although there is a degree of bio-dissolution, these characteristics will not become a health hazard. Nevertheless, after dissolution, the remaining solids can lose the internal nanostructure even if just low quantities are dissolved. From the hazard screening perspective, if the aerogels collapse in biological fluids, they will lose their nano-specific properties. Nevertheless, when an aerogel is carrying a compound, this carrier should be stable in biological fluids as its stability increases the release kinetics of drugs and the various matrix possibilities can be fine-tuned for both the drug loading and release [4].
3.1.3. Stretch-Stress Tests
Recent papers have shown the several analyses that can be used to test the stretch capacity of aerogels, mainly when they are intended to be used in patch-like physiological conditions. As aerogels can be generally described as elastic or fragile materials, several types of tests have been proposed, described in detail by Woignier et al. These diverse tests analyze parameters that influence the overall mechanical behavior of aerogels, e.g., pore volume, pore size, internal connectivity and various chemical bounds. Out of all the parameters, the pore size dictates the stretch-stress properties of an aerogel [92]. Thus, Woignier et al. describes several techniques for the dynamic characterization of materials using ultrasounds, Brillouin scattering and dynamic mechanical analysis in order to measure the elastic properties as well as the attenuation and internal friction testing. The presented experimental results show the values of the elastic and fracture properties in concordance with the material porosity. In the compression testing, aerogels are shown to behave like plastic materials. The presented data of different techniques characterize the overall mechanical behavior of aerogels, giving information on pore volume and size, internal connectivity and various internal bounds content. From all of these presented results, it can be concluded that pore size characteristics dominate the mechanics of aerogels [92].
3.2. Level 2—In Vitro Cellular Models for Testing Aerogels
Choosing the best cellular model depends on the intended use of the aerogel with/without a drug load. For example, if the route is foreseen as the respiratory system, either the intended or accidental inhalation of the NR8383 alveolar macrophage assay can be used [85,93]. The authors tested 18 inorganic nanomaterials, two nanoplatelets and two nanosized organic pigments on the NR8383 alveolar macrophage assay. After putting the cells in contact with this extended array of nanomaterials, several markers were evaluated: Lactate dehydrogenase (LDH), glucuronidase (Glu) and tumor necrosis factor alpha (TNF-α). The results have shown that the NR8383 alveolar macrophage assay can distinguish between active and passive nanomaterials and can hence predict whether an in vivo short-term inhalation evaluation is necessary for human health hazard assessment [93].
LDH release, Glu and H2O2 are basic assays in the cellular testing level and are all cytotoxic tailored tests that can be completed with more subtle tests like inflammation assays, for example the induction of TNF-α. Therefore, although aerogels do not induce a cytotoxic effect in in vitro cellular models in terms of LDH, Glu and H2O2, they can activate TNF-α. All these tests have at least two important characteristics, one is the concentration of the aerogels that is in contact with the cells and the second one is the time of action. Analysis should be conducted by evaluating both dose and time of action, as even low doses can display, with a longer period of action, toxicity in in vitro models [94]. For example, evaluating nanoparticles generated by dental composites dust in order to establish the toxicity in NR8383 alveolar macrophages, it was shown that H2O2 and TNF-α markers were dependent on the dose of the nanoparticles but also on the time of exposure [94].
Aerogel particles can have various dimensions, but only the ones that have small external dimensions can be ingested by phagocytes in an experimental cellular model. For example, if macrophages are tested with large aerogel particles, this can lead to frustrated phagocytosis [95,96]. This type of phagocytosis appears when phagocytic cells are exposed to large surfaces that cells are trying to engulf. Immune cells will try to increase their area of contact, resulting in lamella-shaped cells that will partially cover the target. This three-dimensional process may activate immune cells, generating an inflammatory response [95]. Besides frustrated phagocytosis, aerogel particles can induce cellular surface reaction, induce the release of soluble molecules and in the end induce inflammation processes like cytokines secretion [97]. Thus, when A549 epithelial cells and rat alveolar macrophages were tested on large surfaces, this frustrated phagocytosis appeared to be followed by inflammatory processes. Moreover, the authors show that a predictive measure of pathogenicity is cell proliferation, a process that depends on the dose and duration of the exposure to the compounds [97].
As priorly stated, macrophage testing is useful for in vitro aerogels evaluation as they can be categorized as “active” or “passive” materials [93]. If two out of four parameters, namely LDH, GLU, TNF-α and H2O2 tests, were significantly increased at a certain threshold, then the particle is categorized as “active”. Interestingly, Keller et al. showed that, for aerogels, the most hydrophobic compound that they have tested induced a decrease in the H2O2 indicator that was due to the adsorption of the H2O2 indicator reagent in the system [11]. This observation proves that choosing as particular test is as important as properly designing the cellular in vitro test. Other studies have also mentioned for other high-surface-area nanomaterials, such as MWCNTs that have hydrophobic chemistry, these difficulties in choosing the right in vitro toxicity test [98,99,100]. For example, 23 engineered nanomaterials intended for application in the biomedical domain were evaluated in ten representative cell lines with three classical in vitro toxicity assays. Out of the 23 nanomaterials, 6 induced oxidative cell stress and 1 reduced cellular metabolic activity. None of the tested nanomaterials affected cell viability. The results show that surface chemistry, surface coating and chemical composition may induce cellular toxicity [100].
In vitro models using RAW 264.7 macrophages identified alginate as a good, non-toxic transporter for anti-inflammatory drugs. Likewise, alginate–lignin and alginate–starch aerogels were proven to be non-toxic for fibroblasts or for various other cells [48,54]. For bone regeneration, alginate–starch aerogels are important materials because they form hydroxyapatite crystals in a simulated body fluid due to the presence of calcium in their matrix. These aerogels do not display a cytotoxic effect, so cells colonize and grow on their surface. These results prove that alginate–starch aerogels can be used in bone regeneration [54]. Also in regenerative medicine, polyurea-nano-encapsulated aerogels have high mechanical strengths. These aerogels were proven to have good biocompatibility when platelets, plasma and vascular endothelial cells were tested in the in vitro model. The aerogel did not induce platelet activation or hinder their aggregation; no inflammatory markers were induced in plasma. The aerogel did not impose toxicity on the vascular endothelial cell culture, so this aerogel has good propensity as an implantable material [101].
In the wound healing domain, the vancomycin-loaded chitosan aerogel was tested. The chitosan aerogels had enhanced water sorption capacity and air permeability. Analyzing the vancomycin release, it was shown that this aerogel had a fast drug release. The aerogel was suitable for the fibroblasts’ regenerative growth and the antibiotic was effective upon cutaneous Staphylococus aureus possible infection. This study brought additional arguments in favor of aerogels to be used in wound healing [48].
Functionalizing silica–gelatin aerogel with methotrexate, the cytotoxicity on tumor cell lines (SCC VII and HL-60) was tested. In vitro experiments have shown that methrotrexate is released from the aerogels inducing an anti-tumoral effect. It is interesting that, as the aerogel is based on silica–gelatin aerogel, the antitumoral effect of the released drug is dependent on the collagenase activity of the tumoral cell; hence, a controlled release performed by the actual targeted cell [102].
3.3. Level 3—In Vivo Testing of Aerogel
After passing the first two levels of testing, the third level should be carefully planned in order to avoid using unnecessary animal models [103,104]. Therefore, an aerogel with low abiotic reactivity, no cytotoxicity in LDH, GLU and H2O2 tests can go forward in in vivo design. For example, an intratracheal instillation of a particulate aerogel revealed in the bronchoalveolar fluid a dominant population of alveolar macrophages and lack of polymorphonuclear granulocytes [11]. The biopsy and organ weight remained unchanged, with the exception of mediastinal lymph nodes that increased on day 21 of instillation. In the bronchoalveolar lavage fluid cells that are in majority alveolar macrophages, a slightly decreased viability accompanied by the appearance of granular eosinophilic material was detected. In in vivo animal models, when the instillation of particle aerogels is performed, all tested enzyme activities should be found decreased at control levels at least on day 21 post-instillation. In the case of foreign material presence in the lungs or granulomatous broncho-interstitial inflammation, various cell infiltrations should be assessed from day 3 to at least day 21. The dose-dependent effect should be evaluated in the animal models as low doses can have no effect on the target organ while just slightly more concentrated particles can induce a significant effect. Therefore, on day 21, foreign material can be present in macrophages, while alveolar histiocytosis, cell infiltration and granulomatous broncho-interstitial inflammation can be detectable based on just a slightly increased concentration from the lowest one.
Histopathology of the target organs is mandatory, but as other organs can be affected, careful evaluation should also be conducted, for example, on spleen (a highly immune-sensitive organ to overall toxicity).
Inhaled aerogel particles can have a very low toxicity in the lungs, eliciting a mild transient inflammation. The mechanism of early TNF-α induction was reported in studies using J774 murine macrophages tested with polyurethane particles [105], or crystalline silica particles [106]. Nevertheless, as ALP (alkaline phosphatase), the marker of epithelial lung cells, is not found to be increased, and BALF (bronchoalveolar lavage fluid) enzymes parameters normalize, the third level of evaluation can indicate a good toleration and a low toxicity for the lung, as the target organ chosen in this example [105].
Recent in vivo studies on aerogels focused on their future drug delivery in biomedical applications [16]. For example, when testing in animal models aerogels carrying an anti-tumoral drug, Taxol, it was shown that the intestinal absorption and blood levels increased over 10-fold in comparison to the classical formulation of Taxol. The active compound of Taxol, paclitaxel, was investigated in terms of bio-distribution in animal models of MCF7 subcutaneous xenografts and its superiority was proven in comparison to the classical formulation [16].
An aerogel based on silica, starch and alginate can be administered in animal models via gavage or introduced subcutaneously/intramuscularly to test whether the aerogels elicit local inflammation [23]. In implant experiments, a recent study tested the short and long-term biocompatibility of polyurea crosslinked silica aerogel implants. The authors obtained good tolerability even over twenty months. A normal thin fibrous capsule was found around the aerogel implant and no toxicity in the tissues surrounding the implants nor in the distant organs was found. These studies show that silica-based aerogels are good implantable biomaterials [23].
4. Health Risk Outlines
Aerogels are innovative nanostructured materials, with high porosity and which can therefore be used as materials with special and diverse properties. Aerogels display a light weight, have a high specific surface area and tunable surface chemistry. Aerogels are the subject of emerging applications because they can be designed as next-generation aerogels for current biomedical applications within pharmaceutics, regenerative medicine, wound healing, plastic surgery and many more. Environmental applications, like sound and thermal insulation, air and water cleaning, can also benefit from aerogel-based products. There is still a fragmentation between the speed of research development in the aerogels domain and their actual applications, so there is an increased need to accomplish the coupling academia–industry–national agencies. For example, although aerogels are important new candidates for tissue engineering, regenerative medicine and overall biomedical applications, we still do not have such a product on the market.
In terms of human health risk, we can consider the scenario wherein an aerogel analyzed in levels 2 and 3 gives moderate/slight or even reversible toxicity, but it does not give an accurate answer to the question of whether breathable aerogel dusts pose a health risk on humans. Occupational and epidemiological issues deserve more attention, especially when aerogels are used as insulator materials, and properly designing levels 1–3 testing can identify these issues [106].
Up to the present time, although the toxicity of aerogels is generally assessed, the actual health risk assessment of these new types of materials and other regulatory aspects are still not fully addressed. When an aerogel-based compound is registered, they do not code as a nanomaterial, but they have nanostructures in their composition; therefore, a hazard assessment should be addressed. When producing aerogels, one cannot neglect that, even if the intrinsic toxicity of the aerogel is low in general, inhalable or ingestible fragments can appear during production and manipulation. For example, in the industrial insulation production of silica and polyurethanes-based aerogel materials, a critical route of exposure to the nanoparticles is the possible inhalation of dust followed by pulmonary deposition. Another human activity that should be evaluated in terms of risk assessment of aerogels is during the installation and removal of aerogel-based insulation materials in houses [106]. Within the production and manipulation activities of the aerogels’ global regulation, it is highly necessary to prevent any risks to human health. In a recently published paper on the safety regulations for the manipulation of silica-based aerogels, the risks imposed to human health were highlighted [107].
From this perspective, this paper aspires to prove that the aerogels domain still needs specific regulatory guidelines and that studies that evaluate the potential on human health risks are urgently needed [108,109,110].
An outline of aerogels’ broad spread in terms of biomedical applications is summarized in Table 1. Although aerogels have good promise and high potential, there are still no mature aerogel-based products in the market for biomedical applications so far.
5. Conclusions
Aerogels are gaining increased applicability in various domains [111,112,113] and their toxicity testing should have specific pathways as they are nanoscale materials but do not actually fulfill the REACH definition of a nanomaterial. Aerogels’ nanostructures can raise concerns and they should all be addressed; therefore, a three-tier characterization is proposed to evaluate the biophysical, in vitro and in vivo toxicity. This evaluation may outline particular categories of aerogels. Groups of aerogels with similar chemical composition (e.g., chitosan, silicate, alginate, cellulose) prove similar effects in level 1 and 2 of testing, but at the third level of evaluation they may display particularities in accordance with the in vivo selected model. The properties recommended by the ECHA guidance for nanomaterials provide a tiered structure to assess the content and release of nanostructures in advanced materials. For complex advanced materials, the form of release should be assessed, and all the components, as an advanced material, can have more than one chemical component, as established by the NanoRelease stepwise decision framework within ISO TR 22293 [114,115,116]. Other classes of advanced materials in non-biological systems [117,118] can benefit from this testing workflow such as aerogels used in water purification systems [119], in electronics and in many other applications [120,121].
There are future paths that should be followed within the aerogels domain. First of all, as the latest definition of aerogels is over 25 years old, a new updated definition is currently being released. The updated definition of aerogels will put a better framework on the pathways that should be followed in the race to implement these materials in the biomedical domain and in various other domains. As aerogels for the biomedical domain can be roughly assimilated with the characteristics of extracellular matrix, the need to improve the strategies for preparing aerogel-based biomaterials with elasticity and strength that mimic tissue characteristics is imperious. Aerogels in the bone regeneration and wound healing domain are rather up-front in the research and testing stages, but other future domains could benefit from aerogels’ properties. Thus, another future domain that should be tackled is the neuro-regeneration domain, wherein guided regeneration is sought. Aerogels, with their variability in terms of functional matrices, could also be entering the guided neuro-regeneration domain in the future. Last but not least, another domain to be addressed in the future is in autoimmune diseases, wherein implantable aerogels could deliver specific drugs that could alleviate autoimmune symptomatology.
In the aerogel domain’s technological developments, their purpose-intended design for specific applications can only be implemented if the multi-sectorial approach is functional. Therefore, experts from extremely varied domains, such as chemical process engineering and physics, biological sciences, material sciences, drug delivery, regenerative medicine, pharmaceutics or toxicology, should have their efforts conjoined to put in use and apply this new type of material.
Author Contributions
Conceptualization, M.N. and A.C.B.; data curation, F.G., A.P., C.A.G.-G. and R.S.-V.; writing—original draft preparation, M.N., F.G., A.P., R.S.-V., C.A.G.-G. and A.C.B.; writing—review and editing, M.N. and A.C.B.; funding acquisition, M.N. and A.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This publication is based upon work funded by the European Cooperation in Science and Technology Project CA18125 “Advanced Engineering and Research of aeroGels for Environment and Life Sciences” (AERoGELS) https://www.cost.eu/actions/CA18125, accessed on 23 August 2023). This work was also supported by institutional project 31PFE/2022, MICINN (PID2020-120010RB-I00), Xunta de Galicia (ED431C 2020/17), Agencia Estatal de Investigación (AEI) and FEDER funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data availability can be found from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Selmer, I.; Kleemann, C.; Kulozik, U.; Heinrich, S.; Smirnova, I. Development of egg white protein aerogels as new matrix material for microencapsulation in food. J. Supercrit. Fluids 2015, 106, 42–49. [Google Scholar] [CrossRef]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym 2015, 117, 797–806. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel-based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- European Commission Recommendation on the Definition of Nanomaterial. Definition of Nanomaterial—European Observatory for Nanomaterials. 2011. Available online: europa.eu (accessed on 26 August 2023).
- Mech, A.; Wohlleben, W.; Ghanem, A.; Hodoroaba, V.D.; Weigel, S.; Babick, F.; Brüngel, R.; Friedrich, C.M.; Rasmussen, K.; Rauscher, H. Nano or Not Nano? A Structured Approach for Identifying Nanomaterials According to the European Commission’s Definition. Small 2020, 16, e2002228. [Google Scholar] [CrossRef] [PubMed]
- Wigger, H.; Wohlleben, W.; Nowack, B. Redefining environmental nanomaterial lows: Consequences of the regulatory nanomaterial definition on the results of environmental exposure models. Environ. Sci. Nano 2018, 5, 1372–1385. [Google Scholar] [CrossRef]
- Rauscher, H.; Kestens, V.; Rasmussen, K.; Linsinger, T.; Stefaniak, E.; European Commission; Joint Research Centre. Guidance on the Implementation of the Commission Recommendation 2022/C 229/01 on the Definition of Nanomaterial; Publications Office of the European Union: Luxembourg, 2023; ISSN 1831-9424. Available online: https://data.europa.eu/doi/10.2760/143118 (accessed on 23 August 2023).
- Steinhauser, K.G.; Sayre, P.G. Reliability of methods and data for regulatory assessment of nanomaterial risks. NanoImpact 2017, 7 (Suppl. C), 66–74. [Google Scholar] [CrossRef]
- Donaldson, K.; Poland, C.A. Nanotoxicity: Challenging the myth of nano-specific toxicity. Curr. Opin. Biotechnol. 2013, 24, 724–734. [Google Scholar] [CrossRef]
- Keller, J.G.; Wiemann, M.; Groters, S.; Werle, K.; Vennemann, A.; Landsiedela, R.; Wohlleben, W. Aerogels are not regulated as nanomaterials, but can be assessed by tiered testing and grouping strategies for nanomaterials. Nanoscale Adv. 2021, 3, 3881–3893. [Google Scholar] [CrossRef]
- Oberdorster, G.; Kuhlbusch, T.A.J. In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact 2018, 10 (Suppl. C), 38–60. [Google Scholar] [CrossRef]
- Charalabidis, A.; Sfouni, M.; Bergstrom, C.; Macheras, P. The Biopharmaceutics Classification System (BCS) and the Biopharmaceutics Drug Disposition Classification System (BDDCS): Beyond guidelines. Int. J. Pharm. 2019, 566, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract—Influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524–546. [Google Scholar] [CrossRef]
- Sellitto, M.R.; Amante, C.; Aquino, R.P.; Russo, P.; Rodríguez-Dorado, R.; Neagu, M.; García-González, C.A.; Adami, R.; Del Gaudio, P. Hollow Particles Obtained by Prilling and Supercritical Drying as a Potential Conformable Dressing for Chronic Wounds. Gels 2023, 9, 492–505. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Sunargulov, T. Changes in the respiratory organs in experimental dust inhalation with silica aerogel. Arkhiv Patol. 1966, 28, 15–20. [Google Scholar]
- Krishnakumari, M.K. Comparative acute oral toxicity of some mineral pesticides to albino rats. In Proceedings of the Symposium on Pesticides, Mysore, India; 1964; pp. 335–338. Available online: https://ir.cftri.res.in/3545/ (accessed on 23 August 2023).
- Cotton, R.T.; Frankenfeld, J.C. Silica Aerogel for Protecting Stored Seed or Milled Cereal Products from Insects. J. Econ. Entomol. 1949, 42, 553–561. [Google Scholar] [CrossRef]
- Gao, T.; Ihara, T.; Grynning, S.; Jelle, B.P.; Gunnarshaug Lien, A. Perspective of aerogel glazings in energy efficient buildings. Build. Environ. 2016, 95, 405–413. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An opinion paper on aerogels for biomedical and environmental applications. Molecules 2019, 24, 1815–1830. [Google Scholar] [CrossRef]
- Ferreira-Gonçalves, T.; Constantin, C.; Neagu, M.; Pinto Reis, C.; Sabri, F.; Simón-Vázquez, R. Safety and efficacy assessment of aerogels for biomedical applications. Biomed. Pharmacother. 2021, 144, 112356–112371. [Google Scholar] [CrossRef]
- Sabri, F.; Boughter, J.D., Jr.; Gerth, D.; Skalli, O.; Phung, T.C.; Tamula, G.R.; Leventis, N. Histological evaluation of the biocompatibility of polyurea crosslinked silica aerogel implants in a rat model: A pilot study. PLoS ONE 2012, 7, e50686. [Google Scholar] [CrossRef]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Keil, C.; Hübner, C.; Richter, C.; Lier, S.; Barthel, L.; Meyer, V.; Subrahmanyam, R.; Gurikov, P.; Smirnova, I.; Haase, H. Ca-Zn-Ag alginate aerogels for wound healing applications: Swelling behavior in simulated human body fluids and effect on macrophages. Polymers 2020, 12, 2741–2758. [Google Scholar] [CrossRef] [PubMed]
- Alnaief, M.; Obaidat, R.M.; Alsmadi, M.T.M. Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery. Polymers 2020, 12, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.X.; Zhang, Y.; Hu, T.; Li, W.X.; Li, Z.L.; Hu, H.J.; Zhu, S.R.; Chen, W.Z.; Zhou, C.S.; Jiang, G.B. Long-term antibacterial composite via alginate aerogel sustained release of antibiotics and Cu used for bone tissue bacteria infection. Int. J. Biol. Macromol. 2021, 167, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Liebner, F.; Pircher, N.; Schimper, C.; Haimer, E.; Rosenau, T. Aerogels: Cellulose-Based. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 2015. [Google Scholar]
- Durães, L.; Maleki, H.; Vareda, J.P.; Lamy-Mendes, A.; Portugal, A. Exploring the Versatile Surface Chemistry of Silica Aerogels for Multipurpose Application. MRS Adv. 2017, 2, 3511–3519. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogels in chemical engineering: Strategies toward tailor-made aerogels. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 307–334. [Google Scholar] [CrossRef]
- Sahin, I.; Uzunlar, E.; Erkey, C. Investigation of kinetics of supercritical drying of alginate alcogel particles. J. Supercrit. Fluids 2019, 146, 78–88. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, L.A.; García-González, A.C. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach. Molecules 2019, 24, 871–888. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569–2005603. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Markevicius, G.; Ladj, R.; Niemeyer, P.; Budtova, T.; Rigacci, A. Ambient-dried thermal superinsulating monolithic silica-based aerogels with short cellulosic fibers. J. Mater. Sci. 2017, 52, 2210–2221. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Silva, R.F.; Durães, L. Advances in carbon nanostructure–silica aerogel composites: A review. J. Mater. Chem. A 2018, 6, 1340–1369. [Google Scholar] [CrossRef]
- Ganesan, K.; Barowski, A.; Ratke, L.; Milow, B. Influence of hierarchical porous structures on the mechanical properties of cellulose aerogels. J. Sol-Gel Sci. Technol. 2019, 89, 156–165. [Google Scholar] [CrossRef]
- García-González, C.A.; López-Iglesias, C.; Concheiro, A.; Alvarez-Lorenzo, C. Chapter 16, Biomedical Applications of Polysaccharide and Protein Based Aerogels. In Biobased Aerogels: Polysaccharide and Protein-Based Materials; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 295–323. [Google Scholar]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144–2181. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Casielles, A.M.; Altay, A.; Bettini, R.; Alvarez-Lorenzo, C.; García-González, C.A. From the printer to the lungs: Inkjet-printed aerogel particles for pulmonary delivery. Chem. Eng. J. 2019, 357, 559–566. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Tucker, G.; DeSilva, B.; Dressman, J.; Ito, M.; Kumamoto, T.; Mager, D.; Mahler, H.-C.; Maitland-van der Zee, A.H.; Pauletti, G.M.; Sasaki, H.; et al. Current Challenges and Potential Opportunities for the Pharmaceutical Sciences to Make Global Impact: An FIP Perspective. J. Pharm. Sci. 2016, 105, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Liebner, F.; Condon, B.D. Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity. Sensors 2018, 18, 2334–2350. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M. Wound Healing Biomaterials–Volume 2: Functional Biomaterials; Elsevier Science: Cambridge, UK, 2016. [Google Scholar]
- García-González, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of Materials for Regenerative Medicine Using Supercritical Fluid Technology. Bioconjug. Chem. 2015, 26, 1159–1171. [Google Scholar] [CrossRef]
- Lu, T.H.; Li, Q.; Chen, W.S.; Yu, H.P. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Yin, W.; Rubenstein, D. Biomedical applications of aerogels. In Aerogels Handbook; Advances in sol–gel derived materials and, technologies; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 683–694. [Google Scholar]
- Power, M.; Hosticka, B.; Black, E.; Daitch, C.; Norris, P. Aerogels as biosensors: Viral particle detection by bacteria immobilized on large pore aerogel. J. Non-Cryst. Solids 2001, 285, 303–308. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Nypelö, T.; Salas, C.; Arboleda, J.; Hoeger, I.C.; Rojas, O.J. Cellulose nanofibrils. J. Renew. Mater. 2013, 1, 195–211. [Google Scholar] [CrossRef]
- Cumana, S.; Ardao, I.; Zeng, A.-P.; Smirnova, I. Glucose-6-phosphate dehydrogenase encapsulated in silica-based hydrogels for operation in a microreactor. Eng. Life Sci. 2014, 14, 170–179. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Smirnova, I.; García-González, C.A.; Gurikov, P. Pharmaceutical applications of aerogels. In Springer Handbook of Aerogels; Aegerter, M.A., Leventis, N., Koebel, M.M., Steiner III, S.A., Eds.; Springer: New York, NY, USA, 2023; pp. 1489–1504. [Google Scholar]
- Gaudio, P.D.; Auriemma, G.; Mencherini, T.; Porta, G.D.; Reverchon, E.; Aquino, R.P. Design of alginate-based aerogel for nonsteroidal anti-inflammatory drugs controlled delivery systems using prilling and supercritical-assisted drying. J. Pharm. Sci. 2013, 102, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liebner, F.; Dunareanu, R.; Opietnik, M.; Haimer, E.; Wendland, M.; Werner, C.; Maitz, M.; Seib, P.; Neouze, M.-A.; Potthast, A.; et al. Shaped hemocompatible aerogels from cellulose phosphates: Preparation and properties. Holzforschung 2012, 66, 317–321. [Google Scholar] [CrossRef]
- Tseng, P.; Napier, B.; Zhao, S.; Mitropoulos, A.N.; Applegate, M.B.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Directed assembly of bio-inspired hierarchical materials with controlled nanofibrillar architectures. Nat. Nanotechnol. 2017, 12, 474–480. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, Flexible, and Superhydrophobic Functionalized Cellulose Aerogel for Selective and Versatile Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Takeshita, S.; Sadeghpour, A.; Malfait, W.J.; Konishi, A.; Otake, K.; Yoda, S. Formation of Nanofibrous Structure in Biopolymer Aerogel during Supercritical CO2 Processing: The Case of Chitosan Aerogel. Biomacromolecules 2019, 20, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of Aerogels, Cryogels and Xerogels of Alginate: Effect of Molecular Weight, Gelation Conditions and Drying Method on Particles’ Micromeritics. Molecules 2019, 24, 1049–1061. [Google Scholar] [CrossRef]
- Feng, J.; Su, B.-L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications (Review Article). Chem. Soc. Rev 2021, 50, 3842–3888. [Google Scholar] [CrossRef]
- Mohammadian, M.; Jafarzadeh Kashi, T.S.; Erfan, M.; Soorbaghi, F.P. Synthesis and characterization of silica aerogel as a promising drug carrier system. J. Drug Deliv. Sci. Technol. 2018, 44, 205–212. [Google Scholar] [CrossRef]
- Wu, K.C.W.; Yamauchi, Y.; Hong, C.Y.; Yang, Y.H.; Liang, Y.H.; Funatsu, T.; Tsunoda, M. Biocompatible, surface functionalized mesoporous titania nanoparticles for intracellular imaging and anticancer drug delivery. Chem. Commun. 2011, 47, 5232–5234. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744–115758. [Google Scholar] [CrossRef]
- Veronovski, A.; Tkalec, G.; Knez, Z.; Novak, Z. Characterisation of biodegradable pectin aerogels and their potential use as drug carriers. Carbohydr. Polym. 2014, 113, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, D.A.S.; Paninho, A.I.; Cordeiro, T.; Nunes, A.V.M.; Fonseca, I.M.; Pereira, C.; Matias, A.; Ventura, M.G. Properties of κ-carrageenan aerogels prepared by using different dissolution media and its application as drug delivery systems. Mater. Chem. Phys. 2020, 253, 123290–123300. [Google Scholar] [CrossRef]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and hybrid alginate-hyaluronic acid aerogel microspheres as potential carrier for pulmonary drug delivery. J. Supercrit. Fluids 2019, 150, 49–55. [Google Scholar] [CrossRef]
- De Marco, I.; Iannone, R.; Miranda, S.; Riemma, S. An environmental study on starch aerogel for drug delivery applications: Effect of plant scale-up. Int. J. Life Cycle Assess. 2018, 23, 1228–1239. [Google Scholar] [CrossRef]
- Yue, J.; Xi, X.; Yu, C.; Liu, L.; Rui, Y.; Guoxin, S. Hierarchically structured cellulose aerogels with interconnected MXene networks and their enhanced microwave absorption properties. J. Mater. Chem. C 2018, 6, 8679–8687. [Google Scholar]
- Arts, H.; Hadi, M.; Keene, A.M.; Kreiling, R.; Lyon, D.; Monika Maier, M.; Michel, K.; Thomas Petry, T.; Sauer, U.G.; Warheit, D.; et al. A critical appraisal of existing concepts for the grouping of nanomaterials. Regul. Toxicol. Pharmacol. 2014, 70, 492–506. [Google Scholar] [CrossRef]
- Oomen, G.; Bleeker, E.A.; Bos, P.M.; van Broekhuizen, F.; Gottardo, S.; Groenewold, M.; Hristozov, D.; Hund-Rinke, K.; Irfan, M.A.; Marcomini, A.; et al. Grouping and read-across approaches for risk assessment of nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 13415–13434. [Google Scholar] [CrossRef]
- Oomen, G.; Steinhäuser, K.G.; Bleeker, E.A.J.; van Broekhuizen, F.; Sips, A.; Dekkers, S.; Wijnhoven, S.W.P.; Sayre, P.G. Risk assessment frameworks for nanomaterials: Scope, link to regulations, applicability, and outline for future directions in view of needed increase in efficiency. NanoImpact 2018, 9 (Suppl. C), 1–13. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Appendix for Nanoforms Applicable to the Guidance on Registration and Substance Identication, in ECHA-19-H-14-EN; ECHA: Helsinki, Finland, 2019; Available online: https://www.echa.europa.eu (accessed on 20 August 2023).
- Akhter, F.; Soomro, S.A.; Inglezakis, V.J. Silica aerogels; a review of synthesis, applications and fabrication of hybrid composites. J. Porous Mater. 2021, 28, 1387–1400. [Google Scholar] [CrossRef]
- Stone, V.; Gottardo, S.; Bleeker, E.A.J.; Braakhuis, H.; Dekkers, S.; Fernandes, T.; Haase, A.; Hunt, N.; Hristozov, D.; Jantunen, P.; et al. A framework for grouping and read-across of nanomaterials—Supporting innovation and risk assessment. Nano Today 2020, 35, 100941–100956. [Google Scholar] [CrossRef]
- Wohlleben, W.; Hellack, B.; Nickel, C.; Herrchen, M.; Hund-Rinke, K.; Kettler, K.; Riebeling, C.; Haase, A.; Funk, B.; Kühnel, D.; et al. The nanoGRAVUR framework to group (nano) materials for their occupational, consumer, environmental risks based on a harmonized set of material properties, applied to 34 case studies. Nanoscale 2019, 11, 17637–17654. [Google Scholar] [CrossRef]
- Arts, J.H.E.; Hadi, M.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Saueret, U.G.; et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol. 2015, 71, S1–S27. [Google Scholar] [CrossRef]
- Arts, J.H.E.; Hadi, M.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Neubauer, N.; Petry, T.; et al. Case studies putting the decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regul. Toxicol. Pharmacol. 2016, 76, 234–261. [Google Scholar] [CrossRef]
- Achawi, S.; Feneon, B.; Pourchez, J.; Forest, V. Assessing biological oxidative damage induced by graphene-based materials: An asset for grouping approaches using the FRAS assay. Regul. Toxicol. Pharmacol. 2021, 127, 105067–105095. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-F.; Bello, D.; Schmidt, D.F.; Pal, A.K.; Stella, A.; Isaacs, J.A.; Rogers, E.J. Mapping the Biological Oxidative Damage of Engineered Nanomaterials. Small 2013, 9, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Hellac, B.; Wiemannd, M.; Giustia, A.; Werlee, K.; Haase, A.; Wohlleben, W. Nanomaterial categorization by surface reactivity: A case study comparing 35 materials with four different test methods. NanoImpact 2020, 19, 100234–100252. [Google Scholar] [CrossRef]
- Hellack, B.; Nickel, C.; Albrecht, C.; Kuhlbusch, T.A.J.; Boland, S.; Baeza-Squiban, A.; Wendel Wohlleben, W.; Schins, R.P.F. Analytical methods to assess the oxidative potential of nanoparticles: A review. Environ. Sci. Nano 2017, 4, 1920–1934. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Appendix R.6-1 for Nanoforms Applicable to the Guidance on QSARs and Grouping of Chemicals, in ECHA-19-H-15-EN; ECHA: Helsinki, Finland, 2019. [Google Scholar]
- Magro, C.; Paz-Garcia, J.; Ottosen, L.; Mateus, E.; Ribeiro, A. Sustainability of construction materials: Electrodialytic technology as a tool for mortars production. J. Hazard. Mater. 2018, 2018, 421–427. [Google Scholar] [CrossRef]
- Gawron-Skarbek, A.; Kontarska-Krauza, M.; Dynowska, B.; Guligowska, A.; Prymont-Przymińska, A.; Nowak, D.; Kostka, T. Salivary and plasma native and non-urate total antioxidant capacity versus oral health status in older non-smoking adults. Arch. Oral Biol. 2019, 107, 104515–104530. [Google Scholar] [CrossRef]
- Cecchini, S.; Fazio, F. Assessment of Total Antioxidant Capacity in Serum of Healthy and Stressed Hens. Animals 2020, 10, 2019. [Google Scholar] [CrossRef]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on risk assessment of the application of nanoscience and nano-technologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar] [PubMed]
- Woignier, T.; Primera, J.; Alaoui, A.; Despetis, F.; Calas-Etienne, S.; Faivre, A.; Duffours, L.; Levelut, C.; Etienne, P. Techniques for characterizing the mechanical properties of aerogels. J. Sol-Gel Sci. Technol. 2020, 93, 6–27. [Google Scholar] [CrossRef]
- Wiemann, M.; Vennemann, A.; Sauer, U.G.; Wiench, K.; Ma-Hock, L.; Landsiedel, R. An in vitro alveolar macrophage assay for predicting the short-term inhalation toxicity of nanomaterials. J. Nanobiotechnol. 2016, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Cokic, S.M.; Asbach, C.; Hoet, P.; Godderis, L.; Reichl, F.X.; Van Meerbeek, B.; Vennemann, A.; Wiemann, M. Interaction of rat alveolar macrophages with dental composite dust. Part. Fibre Toxicol. 2016, 13, 62–75. [Google Scholar] [CrossRef]
- Mularski, A.; Marie-Anaïs, F.; Mazzolini, J.; Niedergang, F. Observing Frustrated Phagocytosis and Phagosome Formation and Closure Using Total Internal Reflection Fluorescence Microscopy (TIRFM). Methods Mol. Biol. 2018, 1784, 165–175. [Google Scholar] [PubMed]
- Donaldson, K.; Seaton, A. A short history of the toxicology of inhaled particles. Part. Fibre Toxicol. 2012, 9, 13–25. [Google Scholar] [CrossRef]
- Cullen, R.T.; Miller, B.G.; Davis, J.M.; Brown, D.M.; Donaldson, K. Short-term inhalation and in vitro tests as predictors of fiber pathogenicity. Environ. Health Perspect. 1997, 105 (Suppl. S5), 1235–1240. [Google Scholar]
- Worle-Knirsch, J.M.; Pulskamp, K.; Krug, H.F. Oops they did it again! carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006, 6, 1261–1268. [Google Scholar] [CrossRef]
- Wohlleben, W.; Kolle, S.N.; Hasenkamp, L.-C.; Böser, A.; Vogel, S.; von Vacano, B.; van Ravenzwaay, B.; Landsiedel, R. Artifacts by marker enzyme adsorption on nanomaterials in cytotoxicity assays with tissue cultures. J. Phys. Conf. Ser. 2011, 304, 012061–012072. [Google Scholar] [CrossRef]
- Kroll, A.; Dierker, C.; Rommel, C.; Hahn, D.; Wohlleben, W.; Schulze-Isfort, C.; Göbbert, C.; Voetz, M.; Hardinghaus, F.; Schnekenburger, J. Cytotoxicity screening of 23 engineered nanomaterials using a test matrix of ten cell lines and three different assays. Part. Fibre Toxicol. 2011, 8, 9–28. [Google Scholar] [CrossRef]
- Yin, W.; Venkitachalam, S.M.; Jarrett, E.; Staggs, S.; Leventis, N.; Lu, H.; Rubenstein, D.A. Biocompatibility of surfactant-templated polyurea–nanoencapsulated macroporous silica aerogels with plasma platelets and endothelial cells. J. Biomed. Mater. Res. Part A 2010, 92, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Király, G.; Veres, P.; Lázár, I.; Fábián, I.; Bánfalvi, G.; Juhász, I.; Kalmár, J. Controlled release of methotrexate from functionalized silica-gelatin aerogel microparticles applied against tumor cell growth. Int. J. Pharm. 2019, 558, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Burden, N.; Aschberger, K.; Chaudhry, Q.; Clift, M.J.D.; Doak, S.H.; Fowler, P.; Johnston, H.J.; Landsiedel, R.; Rowland, J.; Stone, V. The 3Rs as a framework to support a 21st century approach for nanosafety assessment. Nano Today 2017, 12, 10–13. [Google Scholar] [CrossRef]
- Nan, A.P.; Petit, A.; Yahia, L.; Huk, O.L.; Tabrizian, M. Cytotoxic reaction and TNF-a response of macrophages to polyurethane particles. J. Biomater. Sci. Polym. Ed. 2002, 13, 257–272. [Google Scholar] [CrossRef]
- Wiemann, M.; Vennemann, A.; Stintz, M.; Marín, R.R.M.; Babick, F.; Lindner, G.-G.; Schuster, T.B.; Brinkmann, U.; Krueger, N. Effects of ultrasonic dispersion energy on the preparation of amorphous SiO2 nanomaterials for in vitro toxicity testing. Nanomaterials 2019, 9, 11–43. [Google Scholar] [CrossRef]
- Moitra, S.; Tabrizi, A.F.; Machichi, K.I.; Kamravaei, S.; Miandashti, N.; Henderson, L.; Mukherjee, M.; Khadour, F.; Naseem, M.T.; Lacy, P.; et al. Non-Malignant Respiratory Illnesses in Association with Occupational Exposure to Asbestos and Other Insulating Materials: Findings from the Alberta Insulator Cohort. Int. J. Environ. Res. Public Health 2020, 17, 7085–7097. [Google Scholar] [CrossRef]
- Boccia, A.C.; Pulvirenti, A.; García-González, C.A.; Grisi, F.; Neagu, M. Compendium of Safety Regulatory for Safe Applications of Aerogels. Gels 2023, 9, 842. [Google Scholar] [CrossRef]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- Human Health and Environmental Exposure Assessment and Risk Characterization of Nanomaterials—Best Practice for REACH Registrants. In Proceedings of the Third GAARN Meeting, Helsinki, Finland, 30 September 2013; European Chemicals Agency: Helsinki, Finland, 2013. Available online: https://echa.europa.eu/documents/10162/788325/best_practices_human_health_environment_nano_3rd_en.pdf/a368f6df-fe40-4673-a041-83b63cc9f50b?t=1395828950292 (accessed on 8 November 2023).
- Kaur, A.; Singh, H. Nanomaterials in Environment: Sources, Risk Assessment, and Safety Aspect. In Advanced Functional Nanoparticles “Boon or Bane” for Environment Remediation Applications, 1st ed.; Environmental Contamination Remediation and, Management; Kumar, R., Kumar, R., Chaudhary, S., Eds.; Springer: Cham, Switzerland, 2023; pp. 75–93. [Google Scholar]
- DAMADEI. Design and Advanced Materials as a Driver of European Innovation. 2013. Available online: http://www.damadei.eu/ (accessed on 26 August 2023).
- MatSEEC. Knowledge and Technology Transfer in Materials Science and Engineering in Europe. 2015. Available online: www.esf.org/matseec (accessed on 26 August 2023).
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef]
- Patterson, G.; Hsieh, Y.-L. Tunable dialdehyde/dicarboxylate nanocelluloses by stoichiometrically optimized sequential periodate–chlorite oxidation for tough and wet shape recoverable aerogels. Nanoscale Adv. 2020, 2, 5623–5634. [Google Scholar] [CrossRef]
- ISO/TR 22293:2021; Evaluation of Methods for Assessing the Release of Nanomaterials from Commercial, Nanomaterial-Containing Polymer Composites. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://statnano.com/standard/iso/1406/ISOTR-22293-2021#ixzz89KDQnciS (accessed on 10 November 2023).
- Knight, D.J.; Deluyker, H.; Chaudhry, Q.; Vidal, J.M.; de Boer, A. A call for action on the development and implementation of new methodologies for safety assessment of chemical-based products in the EU—A short communication. Regul. Toxicol. Pharmacol. 2021, 119, 104837–104844. [Google Scholar] [CrossRef]
- Wohlleben, W.; Punckt, C.; Aghassi-Hagmann, J.; Siebers, F.; Menzel, F.; Esken, D.; Drexel, C.-P.; Zoz, H.; Benz, H.U.; Weier, A.; et al. Nanoenabled Products: Categories, Manufacture, and Applications: Protocols and Industrial Innovations. In Metrology and Standardization for Nanotechnology: Protocols and Industrial Innovations; Mansfield, E., Kaiser, D.L., Fujita, D., Van de Voorde, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 411–464. [Google Scholar]
- Westerhoff, P.; Alvarez, P.; Li, Q.; Gardea-Torresdey, J.; Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. Nano 2016, 3, 1241–1253. [Google Scholar] [CrossRef]
- Westerhoff, P.; Atkinson, A.; Fortner, J.; Wong, M.S.; Zimmerman, J.; Gardea-Torresdey, J.; Ranville, J.; Herckes, P. Low risk posed by engineered and incidental nanoparticles in drinking water. Nat. Nanotechnol. 2018, 13, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shen, Q.; Kawabata, Y.; Segawa, J.; Cao, X.; Guan, K.; Istirokhatun, T.; Yoshioka, T.; Matsuyama, H. Graphene quantum dots (GQDs)-assembled membranes with intrinsic functionalized nanochannels for high-performance nanofiltration. Chem. Eng. J. 2020, 420, 127602–127615. [Google Scholar] [CrossRef]
- Camboni, M.; Floyd, P.; Hanlon, J.; Pérez García, R. A State of Play Study of the Market for So Called “Next Generation” Nanomaterials; ECHA-2019-R-14-EN; Catalogue number ED-02-19-746-EN-N; European Union Observatory for Nanomaterials Publisher: Helsinki, Finland, 2019; ISBN 978-92-9020-726-9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).