Abstract
In organic agriculture, synthetic pesticides and treatments are substituted by natural remedies with interesting success for product yield and environmental outcomes, but the safety of these bio-based products needs to be assessed in vertebrate and human models. Therefore, in this paper we assessed the safety profile of sweet chestnut (Castanea sativa) wood distillate (WD) on the different cellular components of tissues implied in transcutaneous absorption. We investigated the viability of different cell lines mimicking the skin (HaCaT keratinocytes), mucosa (A431), connective (normal human dermal fibroblasts, NHDF) and vascular (human umbilical vein endothelial cells, HUVEC) tissues after exposure to increasing concentrations (0.04–0.5%, v/v, corresponding to 1:2800–1:200 dilutions) of WD. A short exposure to increasing doses of WD was well tolerated up to the highest concentration. Instead, following a prolonged treatment, a concentration dependent cytotoxic effect was observed. Notably, a different behavior was found with the various cell lines, with higher sensitivity to cytotoxicity by the cells with higher proliferation rate and reduced doubling time (human keratinocytes). Moreover, to exclude an inflammatory effect at the not cytotoxic WD concentrations, the expression of the main inducible markers of inflammation, cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase-1 (mPGES-1), were assessed, and no improvement was found both after brief and prolonged exposure. In conclusion, our data exclude any inflammatory and cytotoxic effect at the lowest WD concentrations, namely 0.07% and 0.04%, mimicking some recommended dilutions of the product and the potential exposure doses for the operators in agriculture. Nevertheless, higher concentrations showed a safe profile for short time usage, but caution should be used by farmers following persistent product exposure.
1. Introduction
The sustainable application of natural products and bio-based compounds in the different productive and industrial supply-chain is an urgent requirement worldwide. Under this scenario, many natural compounds and derivatives are being used in place of synthetic chemical products, with the aim of allowing the same benefits but with lower toxicity for the environment and the human health. Pyrolytic bio-derivates, extracted from wood plants, bamboo, herbaceous plants, and algae are largely used in the agriculture practices [1]. The different features of wood distillates converge in a consolidated strengthening power in agricultural production [2]. In fact, in spite of the biomass from which they are extracted and the subsequent processing methods, an increase in agricultural production and plant resistance to biotic and abiotic stress, which adversely affect agricultural production or product quality, is reported. However, the different quality of these bio-derivates, the variability in the amount of pyrolysis derivates, together with the presence of wood tar, involve a potential risk for human health. To define the safety of new products, their activity should be assessed in human cells or tissues, mimicking the potential exposure of professionals and users.
An Italian example of these novel bio-based products is represented by BioDea-pyroligneous acid, also called wood distillate/wood vinegar, extracted from residual virgin sweet chestnut (Castanea sativa) biomass. Wood distillate (WD) used in agriculture improves crop production for its ability to reduce abiotic and biotic stress in plants, to induce antimicrobials and antiparasitic action, to strengthen roots and stems, avoiding evapotranspiration and improving the assimilation of microelements. Conveying nutritive and fundamental molecules for the resistance of the plant, through reduction of water clusters, WD is also characterized by indirect nutraceutical properties [3]. Furthermore, as recently reported by Vannini and coworkers, the foliar application of WD in combination with lecithin, an additive rich in phospholipids and used as biosurfactant, quickly stimulated a c.a. 50% increase in chlorophyll content and growth of lettuce (Lactuca sativa L.) [4]. Recently, a pre-biotic activity of WD has been reported also for soil biota [5]. Therefore, WD can be regarded as a valid candidate for the replacement of several chemical products routinely used worldwide in the agri-food industry [1,6]. WD derives from the waste wood from deforest management, it is extracted by pyrolysis, exploiting the physiological water contained in the lymph of the wood, following a temperature gradient. Pyrolysis is a thermochemical process that leads to the thermal degradation of materials in the absence or near absence of oxygen [7]. This slow pyrolysis destroys the organic bonds, and the plant biomass is reduced to a gaseous product (organic vapors) and a solid charcoal. The water vapor obtained by pyrolysis is condensed and collected using filters and cold traps [5,8]. The preliminary extract undergoes a further natural filtration before being left to decant for almost three mouths. This process allows to obtain an aqueous liquid-fraction rich in oxygenated compounds, biodegradable and stable: the pyroligneous acid or WD. The nature of the raw materials, heating rate and temperature strongly influence the chemical composition and the characteristics and properties of pyrolysis product [9,10,11].
In recent years, evidence have been reported about the profitable use of WD extracted from different wood types, mainly owing to its antioxidant activity [12] and its derivates [13] on different soil organisms [14]. The ecotoxicology profile of WD was recently assessed on aquatic biota, excluding any risk of heavy metal accumulation in an aquatic fern model [15]. At the same time, some cytotoxic effects of natural extracts have been demonstrated, as in the case of essential oils commonly used in traditional Chinese medicine [16,17]. To the best of our knowledge, no studies about WD have been published to evaluate the effects of this natural extract on human cells and tissues, taking into consideration both safety issues and the possible improvement of human health [18,19,20].
To this purpose, we assessed the effect of increasing concentrations of sweet chestnut WD on human cell models, representative of epidermis, mucosal membranes, and other cells on which WD could interact following exposure or absorption. Indeed, most of the chemical products manipulated in the agri-food industry are assimilated transcutaneously or by inhalation, causing inflammation and hazard to health [21,22,23]. We thus investigated the behavior, after exposure (short and prolonged) to WD, of an immortalized keratinocyte cell model (HaCaT) that represents the major cell type of the epidermis [24], of an epidermoid carcinoma (A431), extensively used for studies on the effect of xenobiotics in human skin and mucosa [25,26,27] and of normal human dermal fibroblasts (NHDF), stromal cells involved in the control of skin homeostasis and a balanced wound healing process [28]. We also evaluated the effect of WD on human vascular endothelial cells (HUVEC), which is indicative of the effects related to absorption and blood distribution of WD.
2. Materials and Methods
2.1. Wood Distillate
The WD tested in this study is extracted by Castanea sativa Mill. The chestnut WD is produced in Val di Chiana (Arezzo, Italy) by Bio-Esperia s.r.l. (RM Group Energy Solutions, Umbertide, Perugia, Italy) and distributed by BioDea©. It is obtained by a pyrolysis process, using a thermal gradient up to 75 °C and then left to settle for at least three months. This procedure confers to the distillate stable natural and biological characteristics. The amber-colored chestnut WD contains more than 300 synergistically active organic substances, mostly constituted by acetic acid (usually <10%, but up to 30%), phenols, polyphenols, and tannins (c.a.10–12%) and has an intrinsic acidity [3,29].
2.2. Cell Cultures
The experiments were performed on immortalized human keratinocytes (HaCaT), on a model of epidermoid carcinoma mimicking mucosa (A431), on normal human dermal fibroblasts (NHDF) and human umbilical vein endothelial cells (HUVEC). HaCaT (Voden medical, Meda, MB, Italy), A431 (ATCC, American Tissue Culture Center, Manassas, VA, USA) and NHDF (Lonza, Verviers, Belgium) were grown in Dulbecco’s modified Eagle’s medium (DMEM 4500 mg/L, Euroclone, Milan, Italy) supplemented with 10% of fetal bovine serum (FBS, Euroclone, Milan, Italy). HUVEC (Lonza, Basel, Switzerland) were grown in endothelial grow medium (EGM-2, Lonza, Basel, Switzerland) added with 10% of FBS (Hyclone, Celbio, Milan, Italy). Each medium was completed with 2 mM glutamine, 100 units/ml penicillin and 0.1 mg/ml streptomycin (Sigma Aldrich, St. Louis, MO, USA). Cells were cultured in 10 cm diameter Petri dishes up to a confluent state, in a humidified incubator with 5% CO2. Cells were expanded by splitting 1:6 twice a week for HaCaT and A431, while 1:3 twice a week for NHDF and HUVEC. HaCaT were used until the passage 31, A431 until passage 38, NHDF until passage 7, and HUVEC until passage 5.
Doubling time is defined as the average duration of cell growth and division as reflected by the cell cycle “clock” [30]. The doubling time of each cell line used in the study was calculated by means of Doubling Time software [31] that follows the equation: Td(h) = T × [log2/(logN − logN0)] (Td represents doubling time, T represents time in logarithmic proliferative phase (h), N represents cell numbers at the end of logarithmic proliferative phase, N0 represents cell numbers at the beginning of logarithmic proliferative phase. These data derived from the exponential growth phase, based on the number of cells seeded and periodically counted, and are reported as mean ± SD.
2.3. Cell Viability Assay
In order to evaluate the effect of WD on the survival of each cell line, the colorimetric quantitative assay of MTT (Thiazolyl Blue Tetrazolium Bromide) was performed. HaCaT and A431 cells were seeded at the density of 2.5 × 103 cells in 100 µL for each well of a 96-well multiplate, in medium with 10% FBS. NHDF and HUVEC were seeded in 96-multiwell plates in medium with 10% FBS, at the density of 3 × 103 cells in 100 µL for each well. After adhesion, cells were starved (medium with 0.1% FBS) for 6 h. Medium was then removed and the cells were treated with increasing concentrations of WD, namely 0.04–0.07–0.14–0.2–0.33–0.5 % (v/v) corresponding to those recommended for agricultural use. Stimulation was performed in medium with 1% FBS and the experiments followed two protocols: (1) short simulation of 15 min and 1 h followed by 18 h incubation in fresh medium to mimic professional accidental exposure, and (2) 24 and 48 h continuous incubation to mimic a chronic persistent exposure. At the end of stimulation, 1.2 mM MTT solution was added (Sigma-Aldrich St. Louis, MO, USA) in PBS (phosphate buffered saline, Euroclone, Milan, Italy), followed by 4 h incubation at 37 °C. MTT solution was removed and replaced with DMSO to solubilize the formazan crystals. The different intensity of the color was detected through a microplate reader (Infinite 200 Pro, Tecan Life Sciences, Männedorf, Switzerland) at 540 nm [32]. Data were reported as 540 nm relative absorbance/well and normalized on the control and presented as fold change.
Inhibitory concentration 50 (IC50) on cell viability represents the concentration at which the tested substance exerts half of its maximal inhibitory effect. IC50 of WD was calculated for each cell line using IC50 Calculator software Quest Graph™ IC50 Calculator (v.1) [33]. Data are reported as mean ± SD.
2.4. Expression of Inflammatory Markers
Protein expression was evaluated by Western blot analysis. Cells were seeded at the density of 3 × 105 cells/60 mm diameter dishes, in medium supplemented with 10% FBS. After cell adhesion, subconfluent cells were treated for 1 h with increasing concentrations of WD 0.04–0.07–0.14% (v/v). Then, cells were maintained in fresh medium with 1% FBS for 24 h. In experiments aimed to mimic WD prolonged exposure, cells were treated for 24 h with the lower concentrations of WD, namely 0.04 and 0.07%. A fixed dose of interleukin-1β (IL-1β, Sigma Aldrich St. Louis, MO, USA), 100 ng/mL, was used as a positive control of inflammatory induction [34]. At the end of incubation, cells were washed twice with cold PBS and then lysed in 60 µL of CelLytic (Sigma-Aldrich St. Louis, MO, USA) added with sodium orthovanadate (Na3VO4·2H2O) (1 mM) and a cocktail of protease inhibitors (Sigma Aldrich St. Louis, MO, USA). After protein extraction and quantification, performed by Bradford method, 50 μg of protein/sample were subjected to electrophoresis in 4–12% Bis-Tris Gels (Life Technologies, Carlsbad, CA, USA). Separated proteins were then blotted onto nitrocellulose membranes, incubated overnight with anti-COX-2 (160112, Ann Arbor, MI, USA) and mPGES-1 (160140, Ann Arbor, MI, USA) primary antibodies. The detection of the signal was realized by enhanced chemiluminescence system (Biorad, Hercules, CA, USA). The expression of β-actin was used to normalize the results. Quantification of bands was performed through Fiji ImageJ software. Data are analyzed as ratio of the arbitrary densitometry unit (A.D.U.) of target protein respect to the reference protein β-actin and are reported as fold change vs. the basal control condition [35].
2.5. Statistical Analysis
The experimental data are presented as mean ± SD of at least three independent experiments. Data normality was checked with the Shapiro–Wilk test and equality of variances with the Levene test. One-way ANOVA followed by the Bonferroni’s multiple comparisons test was used to evaluate differences among groups. A value of p < 0.05 was considered statistically significant. All the statistical analysis were performed using GraphPad Prism version 8.3.0 for Windows [36].
3. Results
3.1. Acidity of Wood Distillate Was Differentially Buffered by Culture Media
WD is characterized by a high acidity, with a pH of c.a. 3, per se not tolerated by human cells. For this reason, we firstly tested a range of WD dilutions in media to understand its buffering capacity. To define the proper concentrations to be evaluated in human cell viability, WD was diluted in the different media used to cultivate the cellular models under investigation (DMEM added with 1% FBS and EBM-2 with 1% FBS). The range of WD concentrations, from 0.04 to 0.5%, showed a different pH value, veered after the incubation (Figure 1). As expected, lower doses of WD (0.04 and 0.07%) showed a more alkaline pH, veered toward values closer to basal medium, while the higher concentrations of WD had lower pH values and a higher acidification of cell media (Figure 1b,c). Nevertheless, DMEM showed a prominent ability to buffer acidity of WD compared to EBM-2 (Figure 1c). Each concentration of WD, incubated with EBM-2, retains a pH grade closer to acidity than the same ones diluted in DMEM.
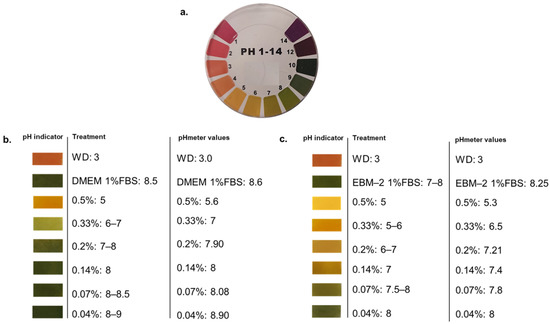
Figure 1.
Measurement of pH in cell culture media containing diluted WD. (a) The litmus test was applied using 50 µL of each solution on litmus paper, and a colour comparison was used to approximately evaluate pH level. A CyberScan pH 510 m (Thermo Scientific, Eutech instruments, Malaysia) was used to confirm the data. (b) pH of WD dilutions in DMEM containing 1% FBS, used to treat HaCaT, A431tumor cells and NHDF. (c) pH of WD dilutions in EBM-2 containing 1% FBS, used to treat HUVEC.
3.2. WD Differentially Affected Cell Viability
To evaluate the potential cytotoxic effect of WD on different cell lines, a viability assay was performed by exposing cultured cells to increasing concentrations of WD for different timelines. To mimic the potential exposure during manipulation in agriculture, brief exposure times of WD (15 min and 1 h) were tested on keratinocytes (HaCaT), mucosal model (A431) and fibroblasts (NHDF). Cell viability was evaluated after 18 h incubation with fresh medium, mimicking operator rinsing. WD was assayed at concentrations ranging from 0.04 and 0.5% (corresponding to 1:2800–1:200 dilutions). The brief exposure to WD (15 min and 1 h) did not alter cell viability in NHDF (Figure 2c), while the highest concentration (0.5%) impaired HaCaT and A431 viability of c.a. 25%, at both exposure times (Figure 2a,b, respectively). The higher incubation time (1 h) documented a stronger toxicity of higher concentrations respect to 15 min exposure.
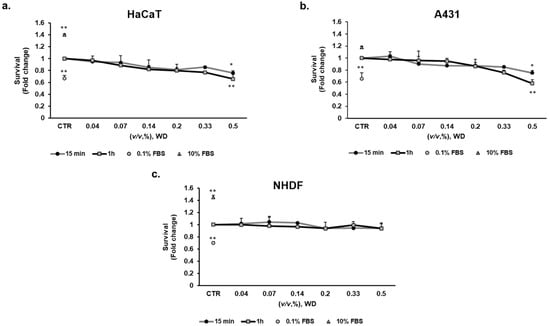
Figure 2.
Effect of WD on cell viability, evaluated by the MTT test: short exposure. Keratinocytes HaCaT (a), model of mucosal epithelial cells A431 (b) and fibroblasts NHDF (c) were exposed to increasing concentrations of WD (0.04–0.5%, v/v), under experimental condition of medium with 1% FBS for 15 min and 1 h. Viability was measured after 18 h of incubation in fresh medium by MTT test. Survival data were calculated as 540 nm relative absorbance/well. Data in the graphs are reported as fold change (means ± SD), giving 100% to the control condition (CTR: medium with 1% serum). (n = 3). * p < 0.05, ** p < 0.01 vs. untreated cells.
These results demonstrated the safety of diluted WD in agriculture with no relevant cytotoxic effect on the tissues involved in early phases of transcutaneous absorption for short time exposure. Higher concentrations instead affected cell viability. In fact, a significant cytotoxic effect was observed in keratinocytes and model of mucosal epithelial cells, treated with the highest concentration of WD.
Nevertheless, conventional prolonged exposure to WD have to be considered to evaluate the product toxicity, even if it is not foreseen in practice. For this reason, 24 and 48 h incubation with WD was performed to evaluate the effect of persistent cutaneous and mucosal contact. Concentrations of WD, ranging between 0.04 and 0.33% (corresponding to 1:2800–1:300 dilutions), were tested on keratinocytes (HaCaT), model of mucosal epithelial cells (A431), fibroblasts (NHDF) and endothelial cells (HUVEC) for 24 and 48 h. The highest concentrations of WD, 0.2 and 0.33%, caused a significant decrease of cell survival, already after 24 h of exposure and more evidently after 48 h, as reported for each cell model (Figure 3). The sensitivity to lower concentrations was different in the various cell lines. Keratinocytes showed a significant decrease of cell survival, of c.a. 25–30% already at 0.07% of WD (Figure 3a). This impairment of cell viability resulted enhanced up to approximately 35% with prolonged exposure to WD, as observed after 48 h of incubation (Figure 3b). A similar responsiveness was evident in A431 cells (Figure 3c,d). Indeed, at both times, an almost 30% of reduction in cell viability was observed already under treatment with 0.14% of WD. The cytotoxic effect became more evident with longer exposure (Figure 3d).
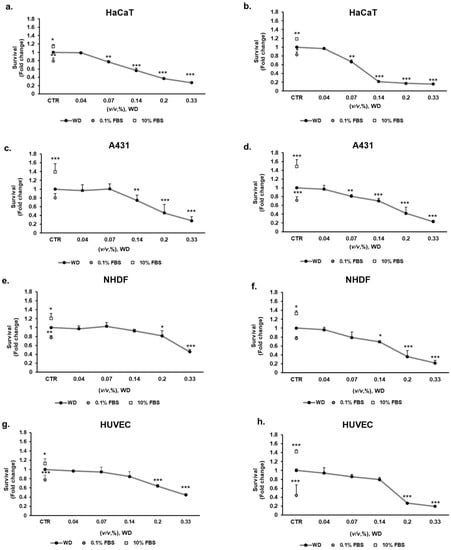
Figure 3.
Effect of WD on cell viability, evaluated by the MTT test: prolonged exposure. Keratinocytes HaCaT (a,b), model of mucosal epithelial cells A431 (c,d), fibroblasts NHDF (e,f), and endothelial cells HUVEC (g,h), were exposed to increasing concentrations of WD (0.04–0.33%, v/v), under experimental condition of medium with 1% FBS. Viability was measured after 24 h (a,c,e,g) and 48 h (b,d,f,h) of incubation by MTT test. Survival data were calculated as 540 nm relative absorbance/well. Data in the graphs are reported as fold change (means ± SD), giving 100% to the control condition (CTR: medium with 1 % serum). (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. untreated cells.
Compared to epidermal and mucosal cells, fibroblasts (NHDF), and endothelial cells (HUVEC) demonstrated a lower sensitivity to WD (Figure 3e–h). At 24 h (Figure 3e,g) and, more severely, at 48 h (Figure 3f,h), 0.2–0.33% of WD induced a significant decrease of NHDF and HUVEC viability. Likewise, at higher dilutions (0.07–0.04%) of WD, cell survival remained partially constant at both timelines (Figure 3e–h). An approximately 20% of reduction in cell survival was observed for both cell lines, under 24 h of treatment with 0.14% of WD (Figure 3e,g). The impairment of cell viability become closer to 30% with longer exposure, such as 48 h (Figure 3f,h).
Overall, these data document a different sensitivity of the cells to WD dosage, with the highest levels of the extract showing cytotoxicity, while the lower concentrations were safe and not toxic.
3.3. The Longer Doubling Time Improved Cell Resistance to WD
Behind the different sensitivity to WD, we investigated the proliferation rate of different cells, as a key of this difference. For each cell line, the doubling time was calculated based on experimental observations during the high proliferative phase (Table 1). Keratinocytes (HaCaT) doubled faster than other cell models, in approximately 15 h, similar to tumor cells (20 h for A431 cells). A longer doubling time, around 30 h, was observed in endothelial cells (HUVEC) and fibroblasts (NHDF), coherently with their slower proliferation rate. Interestingly, a correlation between doubling time and viability IC50 arose, comparing the behaviours and the data about the cell lines. A shorter doubling time increases cell sensitivity to WD, reducing its IC50, as evident in Table 1. This correlation is already known as a mechanism behind the adaptation of cell to many treatments, due to an acquired resistance [37,38]. Coherently with this ratio, HaCaT, featured by the highest proliferation rate, showed a severe sensitivity to WD that exerts half of its maximum inhibitory effect already at 0.12%, after 24 h, and up to 0.08% with prolonged exposure. Similarly, tumor cells displayed a marked doubling ability, slightly longer than HaCaT. Moreover, the proliferative rate is comparable between these two cell types, the IC50 of WD on A431 is obviously higher (0.19 and 0.17% at 24 and 48 h, respectively). It is known that tumor cells tolerate better the acidification of local environment, a frequent feature related to processes of tumor proliferation and progression or inflammation [32,39,40]. According to the slower and quite close doubling time, on HUVEC and NHDF, the IC50 of WD resulted in a range of high doses of WD, 0.25–0.31% at 24 h and 0.18% after 48 h of treatment.

Table 1.
Relation between IC50 of WD on cell viability and the doubling time of each cell type. IC50 of WD, at 24 and 48 h, on keratinocytes (HaCaT), model of mucosal epithelial cells (A431), fibroblasts (NHDF) and endothelial cells (HUVEC) was calculated by Quest Graph™ IC50 Calculator. These values were put in relation with the doubling time of each cell line, assessed during the exponential growth phase by Doubling Time software. Pearson’s correlation coefficient (r) of 0.9864 (p = 0.0136) defined the relation between IC50 of WD and doubling time after 24 h, and r value of 0.8515 (p = 0.1485) defined that after 48 h.
The correlation between IC50 of WD and doubling time was assessed by Pearson’s correlation coefficient. High correlation value (r = 0.9864, p = 0.0136) was found for IC50 of WD after 24 h, significatively related to doubling time. The close correlation slightly decreased after 48 h of exposure to WD (r = 0.8515, p = 0.1485). This relation between IC50 of WD and doubling time demonstrated that the high growth rate avoids acquiring resistance and adaption to WD exposure.
3.4. The Exposure of Cells to WD Did Not Induce an Inflammatory Phenotype
In order to further characterize the safety of WD, the inflammatory potential of not cytotoxic conditions of exposure to WD was assessed. The protein expression of the major enzymes involved in the synthesis of inflammatory prostanoids, cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase-1 (mPGES-1), was investigated in keratinocytes (HaCaT), model of mucosal epithelial cells (A431), and fibroblasts (NHDF), as cutaneous cellular models. COX-2 and mPGES-1 expression was evaluated in each cell model under basal condition and following a brief incubation with increasing concentrations of WD, ranging from 0.04 and 0.14% (1 h). Protein expression in cells treated with WD was compared to those in cells under treatment with IL-1β (used at 100 ng/mL), a known inflammatory inducer [41]. According to Western blot analysis (Figure 4), no induction of COX-2 expression was monitored in HaCaT cells briefly exposed to 0.04 and 0.07% concentrations of WD, but a slightly increase of COX-2 expression was observed following treatment with higher dose [0.14%] of WD (Figure 4a,b). No change in mPGES-1 expression was found (Figure 4a,c). A decrease of COX-2 expression and mPGES-1 was shown by A431, treated with lower doses [0.04–0.07%] of WD (Figure 4d–f). In NHDF no increase in COX-2 and mPGES-1 was detected (Figure 4d–f). An interesting decrease of COX-2 expression, in relation to the increasing concentration of WD, was observed in NHDF (Figure 4d,e). The significant decrease of COX-2 production in cutaneous fibroblasts, reported after treatment with 0.14 % of WD, taken together the obvious reduction of inflammatory markers in A431, could support the anti-inflammatory properties of the polyphenols presents in the distillate.
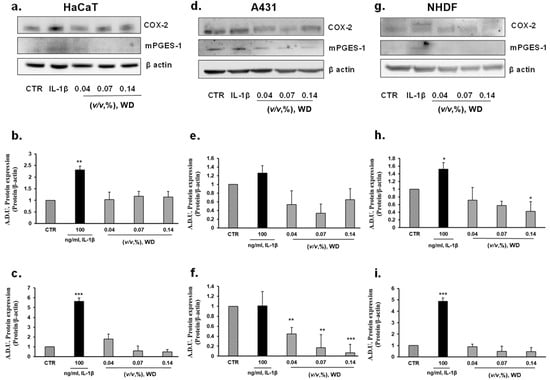
Figure 4.
Effect of WD on the expression of inflammatory markers COX-2 and mPGES-1. Keratinocytes HaCaT (a–c), model of mucosal epithelial cells (d–f) and fibroblasts NHDF (g–i) were treated with increasing doses of WD, 0.04–0.14%, for 1 h and after incubated for 24 h with fresh medium added with 1%FBS. Results were compared with untreated cells and cells under a fixed dose of interleukin-1β (IL-1β, 100 ng/mL), for 1 h. The treatments were performed under experimental condition of medium with 1% FBS. Signals were evaluated through Western blot and normalized on β-actin expression. Data were reported as arbitrary densitometry units (A.D.U.) ± SD of each signal/β-actin vs. basal control. (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. untreated cells (CTR).
Taken together the presented data support the safe profile of WD use in agriculture practices, especially for recommended uses of the product with short exposure times for the operators.
To investigate the potential inflammatory effect of prolonged exposure to WD, keratinocytes (HaCaT), model of mucosal epithelial cells(A431), fibroblasts (NHDF), and endothelial cells (HUVEC) were treated with no cytotoxic doses of WD (0.04 and 0.07%) for 24 h. No induction of COX-2 expression was observed in all the cell lines considered (Figure 5a–d). As reported by Western blot analysis, in HaCaT (Figure 5a), NHDF (Figure 5c) and HUVEC (Figure 5d), the expression of COX-2 resulted to be significantly enhanced following treatment with IL-1β, while it was absent or similar to control condition in cells exposed to WD. A slight, but not significant, decrease of COX-2 expression was shown in HaCaT (Figure 5a), A431 (Figure 5b) and NHDF (Figure 5c) under the exposure to 0.07% of WD. Regarding tumor cell A431, COX-2 was already expressed in the control condition (as previously reported by [42,43,44]), and is induced by IL-1β with no significant change following WD exposure (Figure 5b). Overall, these data document the safety of low WD doses, with no induction of inflammatory features. The induction of inflammation is a common effect of many chemicals used in the agri-food industry, and chronic exposure could affect the well-being of workers [21,22,23]. Therefore, the absence of inflammatory reactions found in our study, strengthens the safe profile of diluted WD.
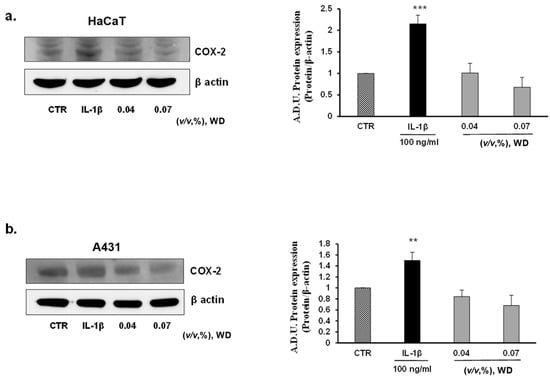
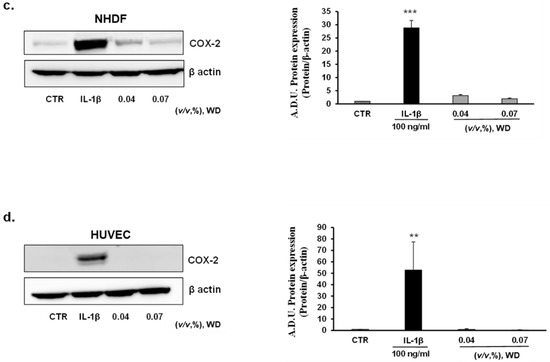
Figure 5.
Effect of prolonged exposure to WD on the expression of the inflammatory marker COX-2. Keratinocytes HaCaT (a), model of mucosal epithelial cells A431 (b), fibroblasts NHDF (c) and endothelial cells HUVEC (d) were treated with no cytotoxic doses of WD, 0.04–0.07%. Results were compared with untreated cells and cells under a fixed dose of Interleukin-1β (IL-1β, 100 ng/mL), for 24 h. The treatments were performed under experimental condition of medium with 1% FBS. Signals were evaluated through Western blot and normalized on β-actin. Data were reported as arbitrary densitometry units (A.D.U.) ± SD of each signal/ β-actin vs. basal control. (n = 3). ** p < 0.01, *** p < 0.01 vs. untreated cells (CTR).
4. Discussion
This experimental work aims to assess the effects of WD on the user’s health, following the exposure related to the agriculture practice. To date, no studies were published about the effect of this bio-derivate from sweet chestnuts on human tissues and health, therefore the presented data provide a preliminary in vitro safety analysis.
A dilution range of WD, from 0.5 to 0.04%, was tested on each component of tissues implied in percutaneous absorption, separately. The cytotoxic effect of WD was evaluated on cell lines mimicking the skin (HaCaT), mucosa (A431), connective (NHDF) and vascular (HUVEC) tissues. To evaluate the potential cytotoxic or pro-inflammatory effect of WD on different cell lines, cultured cells were exposed to increasing concentrations of WD for different timelines, reproducing in vitro both accidental and prolonged exposure to the WD dilutions used in the field. The considerable content of acetic acid confers to WD a high acidity (pH of c.a. 3), a condition incompatible with human cell viability [3,32]. Therefore, the buffering capacity of media, used to cultivate the cellular models under investigation (DMEM added with 1% FBS and EBM-2 with 1% FBS) was assessed. DMEM showed a prominent ability to buffer acidity of WD compared to EBM-2. In fact, each concentration of WD, incubated with EBM-2, showed a pH grade closer to acidity than the same ones diluted in DMEM. Although WD is a bio-derivate with corroborating action and completely biodegradable, the different molecules and waste products of pyrolysis, tar, and other components, forming this complex matrix, imply a potential hazard for human tissues. In addition, it has been recently assessed that many essential oils and natural derivates, also used in natural medicine, exert a prooxidant and cytotoxic effect, especially in lung and skin tissues [16,17]. Therefore, following the identification of proper concentration of WD that mimic different agricultural applications, viability assays were performed on the cell lines chosen in this work. A brief exposure to WD was indicative of an accidental exposure of the operators to WD dilutions. For this reason, these tests involved only the cellular models affected by an early event of skin contact and transcutaneous absorption, keratinocytes (HaCaT), model of mucosal epithelial cells (A431), and fibroblasts (NHDF). The brief exposure to highest concentration (0.5%) impaired HaCaT and A431 viability of c.a. 25%, while the NHDF viability was less affected by WD. The longer incubation time (1 h) documented a stronger toxicity of higher concentrations respect to 15 min exposure. Coherently with these data, diluted WD (0.2% or below) showed a safe profile with no relevant cytotoxic effect on the tissues involved in early phases of transcutaneous absorption for short time exposure, which are closer to time of suggested usage.
Next, a prolonged exposure (24 and 48 h) was performed, excluding the highest concentration of WD (0.5%) from the following experiments, because of the strong cytotoxic effect shown already after 1 h of treatment. The viability assays involved also endothelial cells, to investigate the effects related to the final potential absorption and blood distribution of WD. A significant decrease of cell survival, of c.a. 25–30%, already at the lowest concentration and after 24 h of exposure, was observed in HaCaT keratinocytes and A431 that result the cells most sensitive to WD. On the contrary, fibroblasts and endothelial cells demonstrated a lower sensitivity to WD, compared to epidermal and mucosal cells. At 24 h and, more severely, at 48 h, the highest concentration of WD (0.2–0.33%) leaded to a significant decrease of fibroblast and endothelial cell viability.
To investigate the unexpected difference in cell sensitivity to WD, the proliferation rate was considered as a key factor. Doubling time, assessed as an index of cell proliferation, was correlated with IC50 for each cell line. Interestingly, the Pearson’s correlation coefficient values confirmed the close and significant correlation between intrinsic doubling time and IC50 of WD on viability, comparing the various cell lines assayed. As reported in Table 1, faster doubling time was correlated with lower IC50. A shorter doubling time increased cell sensitivity to WD, reducing its IC50. This correlation is already known as a mechanism behind the adaptation of cells to various treatments, due to an acquired resistance [37,38]. Emblematically, the highest proliferation rate of HaCaT resulted in a severe sensitivity to WD, which exerted half of its maximum inhibitory effect already at 0.12%, after 24 h, and up to 0.08% with prolonged exposure. Likewise, tumor cells are characterized by a high doubling ability, slightly longer than HaCaT. Moreover, beside the comparable proliferative rate between these two cell types, the IC50 of WD on A431 was obviously higher (0.19 and 0.17% at 24 and 48 h, respectively). In fact, it is known that tumor cells viability is less affected by the acidification of local environment, a frequent feature related to processes of tumor proliferation and progression or inflammation [32,39,40]. Coherently with the slower and quite similar doubling time of HUVEC and NHDF, the IC50 of WD was close to a range of high doses, 0.25–0.31% at 24 h and 0.18% after 48 h of treatment. This relation between IC50 of WD and doubling time demonstrated that the high growth rate avoids acquiring resistance and adaption to WD exposure. This mechanism provides a key concept to explain the different sensitivity to WD found in these cell models.
The inflammatory condition is a common side effect of many chemicals used in the agri-food industry, and chronic exposure could affect the well-being of workers [21,22,23]. The potential corrosive action of the components that form the complex matrix of WD and the waste product of pyrolysis that could remain in this bio-derivate, imply the need to investigate the induction of an inflammatory state in human tissues [1]. To deeply detail the potential pro-inflammatory reaction, not cytotoxic conditions of exposure to WD were assessed by Western blot analysis. To reproduce an in vitro model of inflammation due to an accidental exposure, the protein expression of the major enzymes involved in the synthesis of inflammatory prostanoids, cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase-1 (mPGES-1), was investigated in keratinocytes (HaCaT), model of mucosal epithelial cells (A431), and fibroblasts (NHDF), as cutaneous cellular models. Protein expression in cells treated with WD was compared to those in cells under treatment with IL-1β, a known cytokine inflammatory inducer [41]. The brief exposure (1 h) to increasing concentrations of WD, ranging from 0.04 and 0.14%, did not trigger the expression of COX-2 and mPGES-1 expression in each cell model. Similarly, HaCaT, A431, NHDF and HUVEC exposed to 0.04 and 0.07% of WD for 24 and 48 h did not show increase of COX-2 and mPGES-1 expression. The inflammatory markers, significantly increased under treatment with IL-1β, retained a lower level in cells exposed to WD, excluding any pro-inflammatory effect of Biodea product. The absence of inflammatory reactions found in our study, strengthens the safe profile of diluted WD. Furthermore, the slight decrease of COX-2 expression observed in keratinocytes mucosal epithelial cells and fibroblasts under the exposure to 0.07% of WD may represent a potential contribution of polyphenols, contained in WD, that confers an anti-inflammatory effect.
Overall, these data document a different sensitivity of the cells to WD dosage and exposure time, with the highest levels of the extract showing cytotoxicity, while the lower amounts were safe and not toxic. These opposite features boost the great need to better characterize the effect of WD on human cells, to indicate the appropriate application of this important product, keeping a safe profile for workers and professionals. Indeed, the highest concentrations of WD, which are those employed in the field-scale crops, hydroponics, and fertigation (i.e., 1:200–1:500, respectively 0.5–0.2%, v/v of WD), maintain the reported cytotoxicity on cells representative of percutaneous absorption. Consequently, the use of this product in the agri-food industry at these concentrations requires suitable protective devices (i.e., gloves or eye protections), careful handling and thorough rinsing in case of skin contact to avoid any possible damage to workers. The lower concentrations (i.e., 1:700–1:1400, respectively 0.14–0.07% v/v of WD), usually applied in floriculture and seed treatments, not only guarantee the corroborating action of WD, but also the safeguard of the users.
5. Conclusions
The outcomes of this study delineate the safe profile of WD from sweet chestnut feedstock, better describing the range of careful utilization and the toxicity on human cell models of transcutaneous absorption. Several findings were reported about sweet chestnut WD properties and effects in agricultural practices, plant and soil enrichment, and nutraceutical advantages. On this scenario, our study aims to profile WD safety from a novel point of view: the agriculture workers and professionals exposed to WD during manipulation and application. The data collected on in vitro models of skin and endothelium showed no cytotoxicity of highly diluted product. On the other hand, the application of less diluted doses of WD needs a careful utilization, supported by specific protective devices, already indicated in the product label when used in agriculture [45]. In fact, it is suggested wearing protective gloves and clothes, to use protection for eyes and face, avoiding using the wood distillate in an environment not well ventilated [45]. On the whole, this study supports the applicability of WD in agriculture, but with a careful use by farmers in respect to the protection of themselves and the environment.
Author Contributions
A.F. and V.C. designed and performed experiments and wrote the paper. S.L. and L.M. provided critical review of manuscript. L.M. designed, conceived, and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
University of Siena funds in support of research (PSR) to L.M. and S.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Experimental data can be available upon request to corresponding author.
Acknowledgments
The authors thank Bio-Esperia s.r.l. (RM Group Energy Solutions) and BioDea for providing the WD product. Francesco Barbagli (Bio-Esperia srl and Biodea©) is acknowledged for his helpful suggestions.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Grewal, A.; Lokanadha, L.A.; Gunupuru, R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrol. 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood Vinegar as a Complex Growth Regulator Promotes the Growth, Yield, and Quality of Rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- BioDea. Datasheet—Distillato di Legno. 2020. Available online: https://www.biodea.bio/website/wp-content/uploads/2019/11/BioDea-scheda-informativa-Distillato-di-Legno-BIO.pdf (accessed on 1 January 2020).
- Vannini, A.; Moratelli, F.; Monaci, F.; Loppi, S. Effects of wood distillate and soy lecithin on the photosynthetic performance and growth of lettuce (Lactuca sativa L.). SN Appl. Sci. 2021, 3, 113. [Google Scholar] [CrossRef]
- Cardelli, R.; Becagli, M.; Marchini, F.; Saviozzi, A. Soil biochemical activities after the application of pyroligneous acid to soil. J. Compil. CSIRO Soil Res. 2020, 58, 461–467. [Google Scholar] [CrossRef]
- Mmojieje, J.; Hornung, A. The Potential Application of Pyroligneous Acid in the UK Agricultural Industry. J. Crop. Improv. 2015, 29, 2. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermoconversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Mansur, D.; Yoshikawa, T.; Norinaga, K.; Hayashi, J.; Tago, T.; Masuda, T. Production of ketones from pyroligneous acid of woody biomass pyrolysis over an iron-oxide catalyst. Fuel 2013, 103, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Zakaria, Z.A. Pyroligneous acid-the smoky acidic liquid from plant biomass. Appl. Microbiol. Biotechnol. 2015, 99, 611–622. [Google Scholar] [CrossRef]
- Hou, X.; Qiu, L.; Luo, S.; Kang, K.; Zhu, M.; Yao, Y. Chemical constituents and antimicrobial activity of wood vinegars at different pyrolysis temperature ranges obtained from Eucommia ulmoides Olivers branches. RSC Adv. 2018, 8, 40941–40949. [Google Scholar] [CrossRef] [Green Version]
- Misuri, F.; Marri, L. Antibacterial activity of wood distillate from residual virgin chestnut biomass. Eur. J. Wood Prod. 2020, 79, 237–239. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, C.H.; Liang, M.T.; Gao, Z.J.; Wu, Y.W.; Chuang, L.Y. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules 2016, 30, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, A.; Jain, K.; Darah, I. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata. Food Chem. 2008, 107, 1151–1160. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Effects of wood distillate (pyroligneous acid) on sensitive bioindicators (lichen and moss). Ecotoxicol. Environ. Saf. 2020, 204, 111117. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Uptake of Trace Elements in the Water Fern Azolla filiculoides after Short-Term Application of Chestnut Wood Distillate (Pyroligneous Acid). Plants 2020, 9, 1179. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Changhwan, A.; Jae-Hwan, L.; Mi-Jin, P.; Jae-Woo, K.; Jiyoon, Y.; Yeong-Min, Y.; Eui-Bae, J. Cytostatic effects of plant essential oils on human skin and lung cells. Exp. Ther. Med. 2020, 19, 2008–2018. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Morbidelli, L. Polyphenol-based nutraceuticals for the control of angiogenesis: Analysis of the critical issues for human use. Pharmacol. Res. 2016, 111, 384–393. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 21, 87. [Google Scholar] [CrossRef] [Green Version]
- Astiz, M.; de Alaniz, M.J.; Marra, C.A. The oxidative damage and inflammation caused by pesticides are reverted by lipoic acid in rat brain. Neurochem. Int. 2012, 61, 1231–1241. [Google Scholar] [CrossRef]
- Barbasz, A.; Kreczmer, B.; Skórka, M.; Czyżowska, A. Toxicity of pesticides toward human immune cells U-937 and HL-60. J. Environ. Sci. Health B 2020, 55, 719–725. [Google Scholar] [CrossRef]
- Shah, H.K.; Basu, T.S.; Banerjee, D. Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: An in vitro study. Chemosphere Elsevier 2020, 246, 125691. [Google Scholar] [CrossRef]
- Lee, J.H. Chapter 3—Keratinocyte Differentiation and Epigenetics. In Epigenetics and Dermatology; Qianjin, L., Christopher, C., Chang, B., Richardson, C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 37–52. [Google Scholar] [CrossRef]
- Smina, T.P.; Mohan, A.; Ayyappa, K.A.; Sethuraman, S.; Krishnan, U.M. Hesperetin exerts apoptotic effect on A431 skin carcinoma cells by regulating mitogen activated protein kinases and cyclins. Cell Mol. Biol. 2015, 30, 92–99. [Google Scholar] [CrossRef]
- Li, C.C.; Yu, F.S.; Fan, M.J.; Chen, Y.Y.; Lien, J.C.; Chou, Y.C.; Lu, H.F.; Tang, N.Y.; Peng, S.F.; Huang, W.W.; et al. Anticancer effects of cantharidin in A431 human skin cancer (Epidermoid carcinoma) cells in vitro and in vivo. Environ. Toxicol. 2017, 32, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Del Favero, G.; Woelflingseder, L.; Janker, L.; Neuditschko, B.; Seriani, S.; Gallina, P.; Sbaizero, O.; Gerner, C.; Marko, D. Deoxynivalenol induces structural alterations in epidermoid carcinoma cells A431 and impairs the response to biomechanical stimulation. Sci. Rep. 2018, 8, 11351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Ma, X.; Dong, J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J. Anal. Appl. Pyrolysis. 2010, 87, 24–28. [Google Scholar] [CrossRef]
- Bertuzzi, A.; Gandolfi, A.; Sinisgalli, C.; Starace, G.; Ubezio, P. Cell loss and the concept of potential doubling time. Cytometry 1997, 29, 34–40. [Google Scholar] [CrossRef]
- Roth, V. Doubling Time Computing. 2006. Available online: http://www.doubling-time.com/compute.php (accessed on 20 January 2020).
- Ciccone, V.; Filippelli, A.; Angeli, A.; Supuran, C.T.; Morbidelli, L. Pharmacological Inhibition of CA-IX Impairs Tumor Cell Proliferation, Migration and Invasiveness. Int. J. Mol. Sci. 2020, 21, 2983. [Google Scholar] [CrossRef] [Green Version]
- Quest Graph™ IC50 Calculator; AAT Bioquest, Inc.: Sunnyvale, CA, USA, 2021; Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 20 January 2020).
- Ciccone, V.; Monti, M.; Antonini, G.; Mattoli, L.; Burico, M.; Marini, F.; Maidecchi, A.; Morbidelli, L. Efficacy of AdipoDren® in Reducing Interleukin-1-Induced Lymphatic Endothelial Hyperpermeability. J. Vasc. Res. 2016, 53, 255–268. [Google Scholar] [CrossRef]
- Ciccone, V.; Zazzetta, M.; Morbidelli, L. Comparison of the Effect of Two Hyaluronic Acid Preparations on Fibroblast and Endothelial Cell Functions Related to Angiogenesis. Cells 2019, 8, 1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GraphPad Prism Version 8.3.0 for Windows; GraphPad Software: San Diego, CA, USA, 2019; Available online: www.graphpad.com (accessed on 15 October 2019).
- Wang, Q.; Cui, K.; Espin-Garcia, O.; Cheng, D.; Qiu, X.; Chen, Z.; Moore, M.; Bristow, R.G.; Xu, W.; Der, S.; et al. Resistance to bleomycin in cancer cell lines is characterized by prolonged doubling time, reduced DNA damage and evasion of G2/M arrest and apoptosis. PLoS ONE 2013, 8, e82363. [Google Scholar] [CrossRef] [Green Version]
- Eigenmann, M.J.; Frances, N.; Lavé, T.; Walz, A.C. PKPD modeling of acquired resistance to anti-cancer drug treatment. J. Pharmacokinet. Pharmacodyn. 2017, 44, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, A.; Rauschner, M.; Gießelmann, M.; Reime, S.; Haupt, V.; Thews, O. Extracellular Acidosis Modulates the Expression of Epithelial-Mesenchymal Transition (EMT) Markers and Adhesion of Epithelial and Tumor Cells. Neoplasia 2019, 21, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Terzuoli, E.; Ziche, M.; Morbidelli, L. H2S dependent and independent anti-inflammatory activity of zofenoprilat in cells of the vascular wall. Pharmacol. Res. 2016, 113 Pt A, 426–437. [Google Scholar] [CrossRef]
- Donnini, S.; Finetti, F.; Solito, R.; Terzuoli, E.; Sacchetti, A.; Morbidelli, L.; Patrignani, P.; Ziche, M. EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. FASEB J. 2007, 21, 2418–2430. [Google Scholar] [CrossRef] [Green Version]
- Bazzani, L.; Donnini, S.; Finetti, F.; Christofori, G.; Ziche, M. PGE2/EP3/SRC signaling induces EGFR nuclear translocation and growth through EGFR ligands release in lung adenocarcinoma cells. Oncotarget 2017, 8, 31270–31287. [Google Scholar] [CrossRef] [Green Version]
- Terzuoli, E.; Bellan, C.; Aversa, S.; Ciccone, V.; Morbidelli, L.; Giachetti, A.; Donnini, S.; Ziche, M. ALDH3A1 Overexpression in Melanoma and Lung Tumors Drives Cancer Stem Cell Expansion, Impairing Immune Surveillance through Enhanced PD-L1 Output. Cancers 2019, 11, 1963. [Google Scholar] [CrossRef] [Green Version]
- Safety Sheet of Wood Distillate; BioDea© and Esperia s.r.l. (RM Group Energy Solutions): Arezzo, Italy, 2020; Available online: https://www.biodea.bio/website/wp-content/uploads/2020/01/SCHEDA-DI-SICUREZZA-DISTILLATO-DI-LEGNO.pdf (accessed on 1 January 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).