Translation of Medical AR Research into Clinical Practice
Abstract
1. Introduction and Related Work
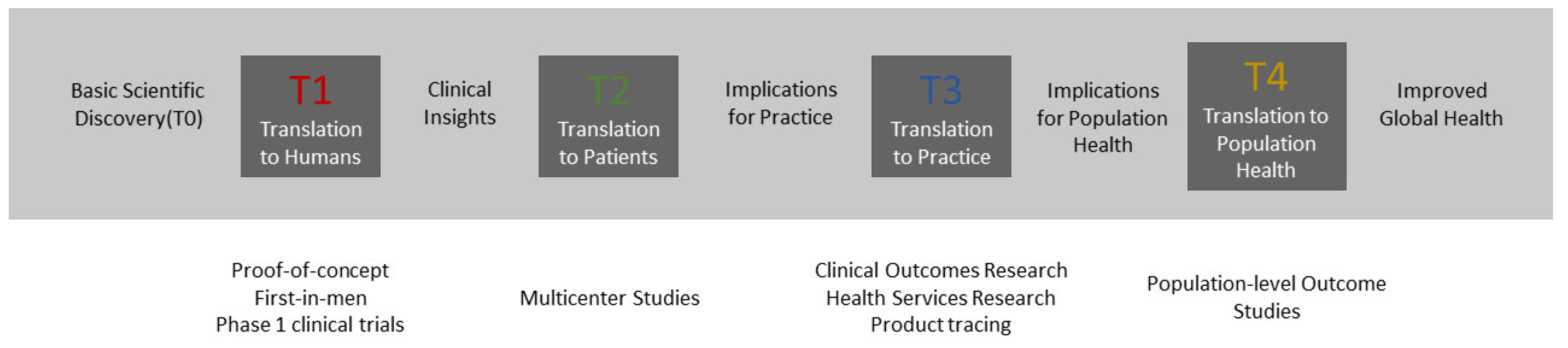
2. Incremental Research and Development towards Translation from Phase T0 to T2
2.1. Early Concept and Research Prototype (TRL1–TRL3)
2.2. Integrated Prototype Development (TRL4–TRL5)
2.3. Clinical Evaluation (TRL 6–TRL7)
2.4. Implementation into Practice (TRL8–TRL9)
3. Challenges and Opportunities
3.1. Technical Challenges
3.2. OR-X—A Translational Center for Surgical Excellence
3.3. Exploitation Strategies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eckert, M.; Volmerg, J.S.; Friedrich, C.M. Augmented Reality in Medicine: Systematic and Bibliographic Review. JMIR mHealth uHealth 2019, 7, e10967. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.; Seibold, M.; Bogo, F.; Farshad, M.; Pollefeys, M.; Fürnstahl, P.; Navab, N. Towards markerless surgical tool and hand pose estimation. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Jud, L.; Fotouhi, J.; Andronic, O.; Aichmair, A.; Osgood, G.; Navab, N.; Farshad, M. Applicability of Augmented Reality in Orthopedic Surgery—A Systematic review. BMC Musculoskelet. Disord. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Salah, Z.; Preim, B.; Elolf, E.; Franke, J.; Rose, G. Improved Navigated Spine Surgery Utilizing Augmented Reality Visualization. In Bildverarbeitung für die Medizin; Springer: Berlin/Heidelberg, Germany, 2011; pp. 319–323. [Google Scholar]
- Casari, F.A.; Navab, N.; Hruby, L.A.; Kriechling, P.; Nakamura, R.; Tori, R.; Nunes, F.D.L.D.S.; Queiroz, M.C.; Fürnstahl, P.; Farshad, M. Augmented Reality in Orthopedic Surgery Is Emerging from Proof of Concept Towards Clinical Studies: A Literature Review Explaining the Technology and Current State of the Art. Curr. Rev. Musculoskelet. Med. 2021, 14, 192–203. [Google Scholar] [CrossRef]
- Szilagyi, P.G. Translational Research and Pediatrics. Acad. Pediatr. 2009, 9, 71–80. [Google Scholar] [CrossRef]
- Bastogne, T. iQbD: A Technological Readiness Level-Indexed Quality-by-Design Paradigm for Medical Device Engineering. J. Med. Devices 2022, 16, 021008. [Google Scholar] [CrossRef]
- Carrillo, F.; Esfandiari, H.; Müller, S.; von Atzigen, M.; Massalimova, A.; Suter, D.; Laux, C.J.; Spirig, J.M.; Farshad, M.; Fürnstahl, P. Surgical Process Modeling for Open Spinal Surgeries. Front. Surg. 2021, 8, 776945. [Google Scholar] [CrossRef]
- Farshad, M.; Fürnstahl, P.; Spirig, J.M. First in man in-situ augmented reality pedicle screw navigation. N. Am. Spine Soc. J. (NASSJ) 2021, 6, 100065. [Google Scholar] [CrossRef]
- Farshad, M.; Spirig, J.M.; Suter, D.; Hoch, A.; Burkhard, M.D.; Liebmann, F.; Farshad-Amacker, N.; Fürnstahl, P. Operator independent reliability of direct augmented reality navigated pedicle screw placement and rod bending. N. Am. Spine Soc. J. (NASSJ) 2021, 100084. [Google Scholar] [CrossRef]
- Liebmann, F.; Roner, S.; von Atzigen, M.; Scaramuzza, D.; Sutter, R.; Snedeker, J.; Farshad, M.; Fürnstahl, P. Pedicle screw navigation using surface digitization on the Microsoft HoloLens. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1157–1165. [Google Scholar] [CrossRef]
- Spirig, J.M.; Roner, S.; Liebmann, F.; Fürnstahl, P.; Farshad, M. Augmented reality-navigated pedicle screw placement: A cadaveric pilot study. Eur. Spine J. 2021, 30, 3731–3737. [Google Scholar] [CrossRef]
- Wanivenhaus, F.; Neuhaus, C.; Liebmann, F.; Roner, S.; Spirig, J.M.; Farshad, M. Augmented reality-assisted rod bending in spinal surgery. Spine J. 2019, 19, 1687–1689. [Google Scholar] [CrossRef]
- Lalys, F.; Jannin, P. Surgical process modelling: A review. Int. J. Comput. Aided Radiol. Surg. 2014, 9, 495–511. [Google Scholar] [CrossRef]
- Neumuth, T. Surgical Process Modelling. Innov. Surg. Sci. 2017, 2, 123–137. [Google Scholar]
- Dennler, C.; Bauer, D.E.; Scheibler, A.-G.; Spirig, J.; Götschi, T.; Fürnstahl, P.; Farshad, M. Augmented reality in the operating room: A clinical feasibility study. BMC Musculoskelet. Disord. 2021, 22, 451. [Google Scholar] [CrossRef]
- Dennler, C.; Jaberg, L.; Spirig, J.; Agten, C.; Götschi, T.; Fürnstahl, P.; Farshad, M. Augmented reality-based navigation increases precision of pedicle screw insertion. J. Orthop. Surg. Res. 2020, 15, 174. [Google Scholar] [CrossRef]
- Maier-Hein, L.; Vedula, S.S.; Speidel, S.; Navab, N.; Kikinis, R.; Park, A.; Eisenmann, M.; Feussner, H.; Forestier, G.; Giannarou, S.; et al. Surgical data science for next generation interventions. Nat. Biomed. Eng. 2017, 1, 691–696. [Google Scholar] [CrossRef]
- Maier-Hein, L.; Eisenmann, M.; Feldmann, C.; Feussner, H.; Forestier, G.; Giannarou, S.; Gibaud, B.; Hager, G.D.; Hashizume, M.; Katic, D.; et al. Surgical Data Science: A Consensus Perspective. arXiv 2018, arXiv:1806.03184. [Google Scholar]
- Liebmann, F.; Stütz, D.; Suter, D.; Jecklin, S.; Snedeker, J.G.; Farshad, M.; Fürnstahl, P.; Esfandiari, H. SpineDepth: A Multi-Modal Data Collection Approach for Automatic Labelling and Intraoperative Spinal Shape Reconstruction Based on RGB-D Data. J. Imaging 2021, 7, 164. [Google Scholar] [CrossRef]
- García-Vázquez, V.; Von Haxthausen, F.; Jäckle, S.; Schumann, C.; Kuhlemann, I.; Bouchagiar, J.; Höfer, A.-C.; Matysiak, F.; Hüttmann, G.; Goltz, J.P.; et al. Navigation and visualisation with HoloLens in endovascular aortic repair. Innov. Surg. Sci. 2018, 3, 167–177. [Google Scholar] [CrossRef]
- Vercauteren, T.; Unberath, M.; Padoy, N.; Navab, N. CAI4CAI: The Rise of Contextual Artificial Intelligence in Computer-Assisted Interventions. Proc. IEEE 2020, 108, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Jecklin, S.; Jancik, C.; Farshad, M.; Fürnstahl, P.; Esfandiari, H. X23D—Intraoperative 3D Lumbar Spine Shape Reconstruction Based on Sparse Multi-View X-ray Data. J. Imaging 2022, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Feussner, H.; Wilhelm, D.; Navab, N.; Knoll, A.; Lüth, T. Surgineering: A new type of collaboration among surgeons and engineers. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 187–190. [Google Scholar] [CrossRef] [PubMed]

| TRL | Description |
|---|---|
| 1 | Basic principles observed and reported in a hypothesis. State-of-the-art reviewed and scientific proposal articulated. |
| 2 | Technology concept defined through clinical requirements, system components, and technical specifications. |
| 3 | Development and verification of a research prototype providing proof-of-concept of some key performance features. |
| 4 | Integrated prototype finalization and validation in a laboratory environment. Besides key performance, additional factors such as safety or adverse events are evaluated. |
| 5 | Validation of the prototype in relevant environment (ex vivo human/in vivo animal). Generation of pre-clinical data to justify in vivo studies. |
| 6 | Near-final device demonstrated safety in its first use on patients in a realistic, but highly controlled and standardized environment (“first-in-human” phase 1 study). |
| 7 | Device demonstrated safety and efficacy in a controlled operation environment through a randomized controlled trial (phase 2 study). |
| 8 | Clinical effectiveness, safety, and risks in using the device under real-world conditions are successfully investigated in large multi-center studies (phase 3 studies) |
| 9 | Regulatory approval for using the device in routine treatment obtained. Long term effectiveness and usage is monitored through post-market surveillance (phase 4 studies). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seibold, M.; Spirig, J.M.; Esfandiari, H.; Farshad, M.; Fürnstahl, P. Translation of Medical AR Research into Clinical Practice. J. Imaging 2023, 9, 44. https://doi.org/10.3390/jimaging9020044
Seibold M, Spirig JM, Esfandiari H, Farshad M, Fürnstahl P. Translation of Medical AR Research into Clinical Practice. Journal of Imaging. 2023; 9(2):44. https://doi.org/10.3390/jimaging9020044
Chicago/Turabian StyleSeibold, Matthias, José Miguel Spirig, Hooman Esfandiari, Mazda Farshad, and Philipp Fürnstahl. 2023. "Translation of Medical AR Research into Clinical Practice" Journal of Imaging 9, no. 2: 44. https://doi.org/10.3390/jimaging9020044
APA StyleSeibold, M., Spirig, J. M., Esfandiari, H., Farshad, M., & Fürnstahl, P. (2023). Translation of Medical AR Research into Clinical Practice. Journal of Imaging, 9(2), 44. https://doi.org/10.3390/jimaging9020044






