Whole CNS 3D Cryo-Fluorescence Tomography Shows CSF Clearance along Nasal Lymphatics, Spinal Nerves, and Lumbar/Sacral Lymph Nodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Usage
2.2. Quantum Dot Infusion
2.3. Cryo-Fluorescence Tomography (CFT)
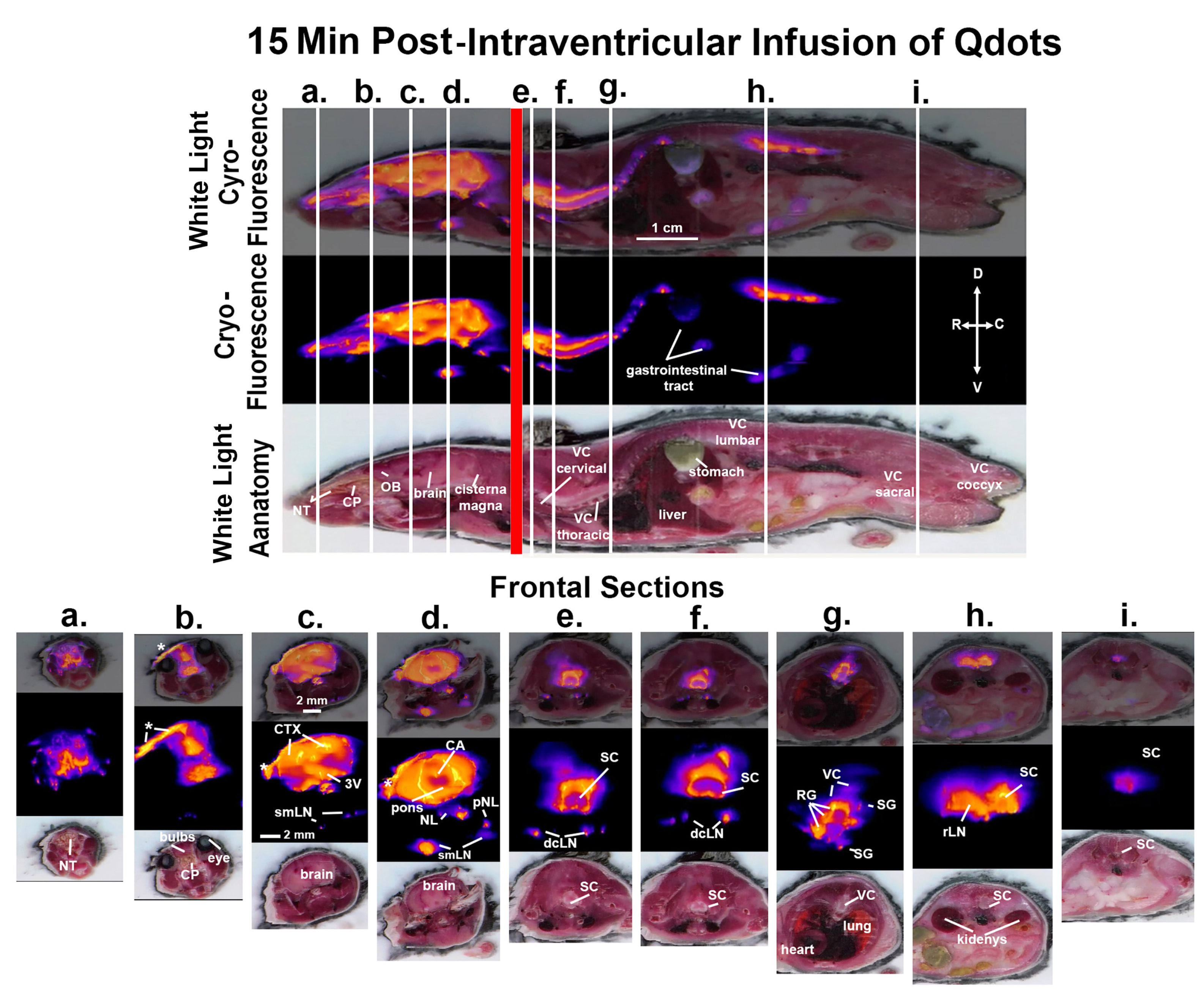
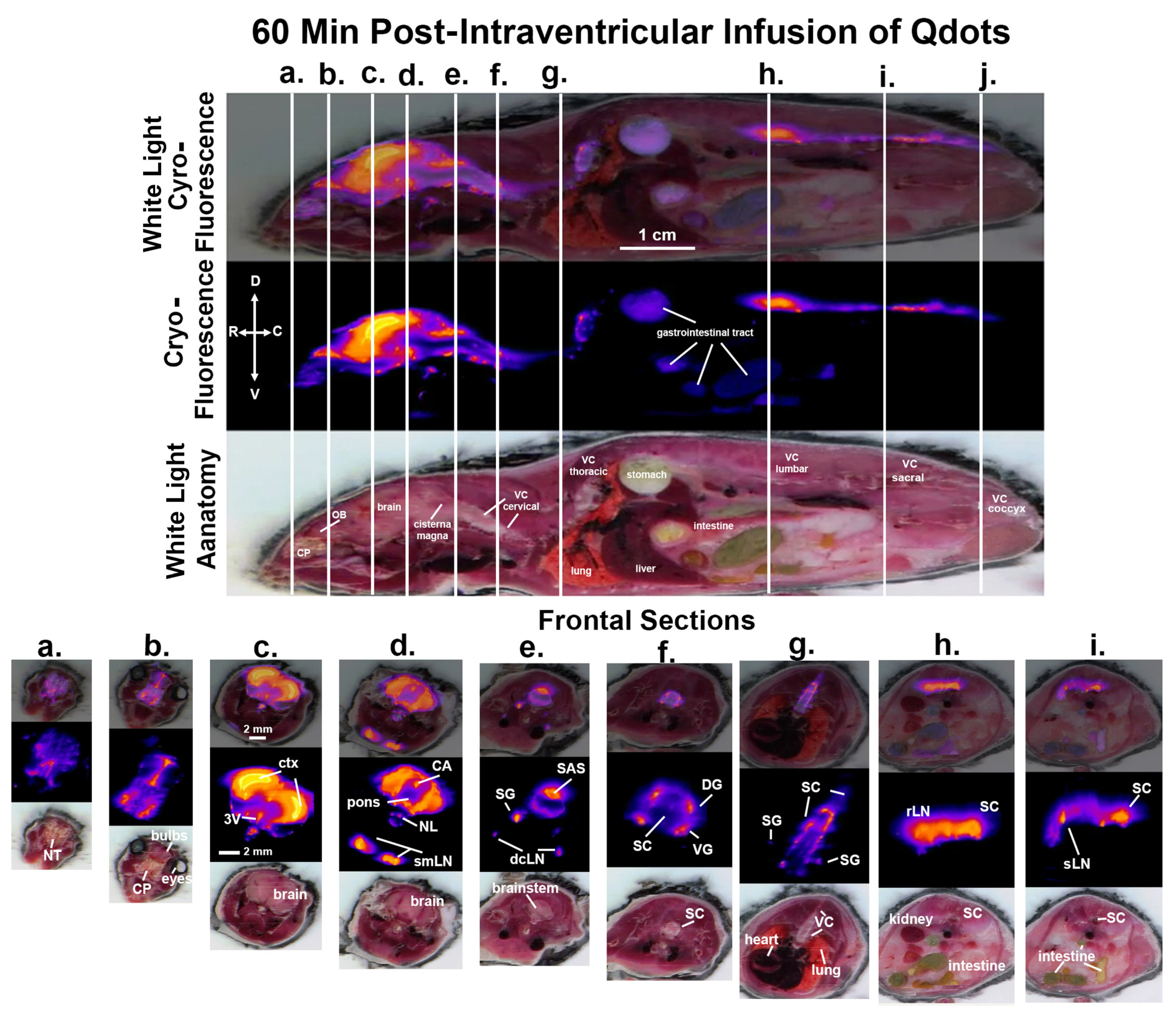
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benveniste, H.; Lee, H.; Volkow, N.D. The Glymphatic Pathway: Waste Removal from the CNS via Cerebrospinal Fluid Transport. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2017, 23, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Proulx, S.T. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell. Mol. Life Sci. 2021, 78, 2429–2457. [Google Scholar] [CrossRef] [PubMed]
- Cserr, H.F.; Harling-Berg, C.J.; Knopf, P.M. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992, 2, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.R.; Perry, V.H.; Weller, R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008, 34, 131–144. [Google Scholar] [CrossRef]
- Cai, X.; Qiao, J.; Kulkarni, P.; Harding, I.C.; Ebong, E.; Ferris, C.F. Imaging the effect of the circadian light-dark cycle on the glymphatic system in awake rats. Proc. Natl. Acad. Sci. USA 2020, 117, 668–676. [Google Scholar] [CrossRef]
- Tuura, R.O.; Volk, C.; Callaghan, F.; Jaramillo, V.; Huber, R. Sleep-related and diurnal effects on brain diffusivity and cerebrospinal fluid flow. NeuroImage 2021, 241, 118420. [Google Scholar] [CrossRef]
- Eide, P.K.; Vinje, V.; Pripp, A.H.; Mardal, K.A.; Ringstad, G. Sleep deprivation impairs molecular clearance from the human brain. Brain 2021, 144, 863–874. [Google Scholar] [CrossRef]
- Johnston, M.; Zakharov, A.; Papaiconomou, C.; Salmasi, G.; Armstrong, D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cereb. Fluid Res. 2004, 1, 2. [Google Scholar] [CrossRef]
- Kida, S.; Pantazis, A.; Weller, R.O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 1993, 19, 480–488. [Google Scholar] [CrossRef]
- Norwood, J.N.; Zhang, Q.; Card, D.; Craine, A.; Ryan, T.M.; Drew, P.J. Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. Elife 2019, 8, e44278. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.H.; Kim, S.H.; Ham, J.S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.H.; Hong, Y.K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Cifuentes, M.; Fernandez, L.P.; Perez, J.; Perez-Figares, J.M.; Rodriguez, E.M. Distribution of intraventricularly injected horseradish peroxidase in cerebrospinal fluid compartments of the rat spinal cord. Cell Tissue Res. 1992, 270, 485–494. [Google Scholar] [CrossRef]
- Wagner, H.J.; Pilgrim, C.; Brandl, J. Penetration and removal of horseradish peroxidase injected into the cerebrospinal fluid: Role of cerebral perivascular spaces, endothelium and microglia. Acta Neuropathol. 1974, 27, 299–315. [Google Scholar] [CrossRef]
- Lam, M.A.; Hemley, S.J.; Najafi, E.; Vella, N.G.; Bilston, L.E.; Stoodley, M.A. The ultrastructure of spinal cord perivascular spaces: Implications for the circulation of cerebrospinal fluid. Sci. Rep. 2017, 7, 12924. [Google Scholar] [CrossRef]
- Ma, Q.; Decker, Y.; Muller, A.; Ineichen, B.V.; Proulx, S.T. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J. Exp. Med. 2019, 216, 2492–2502. [Google Scholar] [CrossRef]
- Bozanovic-Sosic, R.; Mollanji, R.; Johnston, M.G. Spinal and cranial contributions to total cerebrospinal fluid transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R909–R916. [Google Scholar] [CrossRef]
- Leaston, J.; Kulkarni, P.; Gharagouzloo, C.; Qiao, J.; Bens, N.; Ferris, C.F. Do We Swallow the Waste From Our Brain? Front. Neurosci. 2021, 15, 763780. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Gakuba, C.; Gaberel, T.; Goursaud, S.; Bourges, J.; Di Palma, C.; Quenault, A.; de Lizarrondo, S.M.; Vivien, D.; Gauberti, M. General Anesthesia Inhibits the Activity of the “Glymphatic System”. Theranostics 2018, 8, 710–722. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Zhang, S.L.; Sehgal, A. Regulation of the Blood-Brain Barrier by Circadian Rhythms and Sleep. Trends Neurosci. 2019, 42, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Tekieh, T.; Robinson, P.A.; Postnova, S. Cortical waste clearance in normal and restricted sleep with potential runaway tau buildup in Alzheimer’s disease. Sci. Rep. 2022, 12, 13740. [Google Scholar] [CrossRef] [PubMed]
- Wafford, K.A. Aberrant waste disposal in neurodegeneration: Why improved sleep could be the solution. Cereb. Circ.-Cogn. Behav. 2021, 2, 100025. [Google Scholar] [CrossRef] [PubMed]
- Massey, A.; Boag, M.K.; Magnier, A.; Bispo, D.P.; Khoo, T.K.; Pountney, D.L. Glymphatic System Dysfunction and Sleep Disturbance May Contribute to the Pathogenesis and Progression of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 12928. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.F. Rethinking the Conditions and Mechanism for Glymphatic Clearance. Front. Neurosci. 2021, 15, 624690. [Google Scholar] [CrossRef]
- Refinetti, R.; Menaker, M. The circadian rhythm of body temperature. Physiol. Behav. 1992, 51, 613–637. [Google Scholar] [CrossRef]
- Buhr, E.D.; Yoo, S.H.; Takahashi, J.S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 2010, 330, 379–385. [Google Scholar] [CrossRef]
- Morf, J.; Schibler, U. Body temperature cycles: Gatekeepers of circadian clocks. Cell Cycle 2013, 12, 539–540. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Normoyle, K.P.; Jackson, K.; Spitler, K.; Sharrock, M.F.; Miller, C.M.; Best, C.; Llano, D.; Du, R. Brain temperature and its fundamental properties: A review for clinical neuroscientists. Front. Neurosci. 2014, 8, 307. [Google Scholar] [CrossRef]
- Mrozek, S.; Vardon, F.; Geeraerts, T. Brain temperature: Physiology and pathophysiology after brain injury. Anesthesiol. Res. Pract. 2012, 2012, 989487. [Google Scholar] [CrossRef]
- Zhu, M.; Ackerman, J.J.; Yablonskiy, D.A. Body and brain temperature coupling: The critical role of cerebral blood flow. J. Comp. Physiol. B 2009, 179, 701–710. [Google Scholar] [CrossRef]
- Mathieu, E.; Gupta, N.; Macdonald, R.L.; Ai, J.; Yucel, Y.H. In vivo imaging of lymphatic drainage of cerebrospinal fluid in mouse. Fluids Barriers CNS 2013, 10, 35. [Google Scholar] [CrossRef]
- Moinuddin, S.M.; Tada, T. Study of cerebrospinal fluid flow dynamics in TGF-beta 1 induced chronic hydrocephalic mice. Neurol. Res. 2000, 22, 215–222. [Google Scholar] [CrossRef]
- Ma, Q.; Ries, M.; Decker, Y.; Müller, A.; Riner, C.; Bücker, A.; Fassbender, K.; Detmar, M.; Proulx, S.T. Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol. 2019, 137, 151–165. [Google Scholar] [CrossRef]
- Liu, S.; Lam, M.A.; Sial, A.; Hemley, S.J.; Bilston, L.E.; Stoodley, M.A. Fluid outflow in the rat spinal cord: The role of perivascular and paravascular pathways. Fluids Barriers CNS 2018, 15, 13. [Google Scholar] [CrossRef]
- Miura, M.; Kato, S.; von Ludinghausen, M. Lymphatic drainage of the cerebrospinal fluid from monkey spinal meninges with special reference to the distribution of the epidural lymphatics. Arch. Histol. Cytol. 1998, 61, 277–286. [Google Scholar] [CrossRef]
- Antila, S.; Karaman, S.; Nurmi, H.; Airavaara, M.; Voutilainen, M.H.; Mathivet, T.; Chilov, D.; Li, Z.; Koppinen, T.; Park, J.H.; et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017, 214, 3645–3667. [Google Scholar] [CrossRef]
- Jacob, L.; Boisserand, L.S.B.; Geraldo, L.H.M.; de Brito Neto, J.; Mathivet, T.; Antila, S.; Barka, B.; Xu, Y.; Thomas, J.M.; Pestel, J.; et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat. Commun. 2019, 10, 4594. [Google Scholar] [CrossRef]
- Verma, A.; Hesterman, J.Y.; Chazen, J.L.; Holt, R.; Connolly, P.; Horky, L.; Vallabhajosula, S.; Mozley, P.D. Intrathecal (99m)Tc-DTPA imaging of molecular passage from lumbar cerebrospinal fluid to brain and periphery in humans. Alzheimer’s Dement. 2020, 12, e12030. [Google Scholar] [CrossRef]
- Bechter, K.; Schmitz, B. Cerebrospinal fluid outflow along lumbar nerves and possible relevance for pain research: Case report and review. Croat. Med. J. 2014, 55, 399–404. [Google Scholar] [CrossRef]
- Inoue, Y.; Masutani, Y.; Kiryu, S.; Haishi, T.; Yoshikawa, K.; Watanabe, M.; Shimada, M.; Ohtomo, K. Integrated lymphography using fluorescence imaging and magnetic resonance imaging in intact mice. Mol. Imaging 2011, 10, 317–326. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stokes, C.; White, E.F.; Toddes, S.; Bens, N.; Kulkarni, P.; Ferris, C.F. Whole CNS 3D Cryo-Fluorescence Tomography Shows CSF Clearance along Nasal Lymphatics, Spinal Nerves, and Lumbar/Sacral Lymph Nodes. J. Imaging 2023, 9, 45. https://doi.org/10.3390/jimaging9020045
Stokes C, White EF, Toddes S, Bens N, Kulkarni P, Ferris CF. Whole CNS 3D Cryo-Fluorescence Tomography Shows CSF Clearance along Nasal Lymphatics, Spinal Nerves, and Lumbar/Sacral Lymph Nodes. Journal of Imaging. 2023; 9(2):45. https://doi.org/10.3390/jimaging9020045
Chicago/Turabian StyleStokes, Christian, Eli F White, Steve Toddes, Nicole Bens, Praveen Kulkarni, and Craig F Ferris. 2023. "Whole CNS 3D Cryo-Fluorescence Tomography Shows CSF Clearance along Nasal Lymphatics, Spinal Nerves, and Lumbar/Sacral Lymph Nodes" Journal of Imaging 9, no. 2: 45. https://doi.org/10.3390/jimaging9020045
APA StyleStokes, C., White, E. F., Toddes, S., Bens, N., Kulkarni, P., & Ferris, C. F. (2023). Whole CNS 3D Cryo-Fluorescence Tomography Shows CSF Clearance along Nasal Lymphatics, Spinal Nerves, and Lumbar/Sacral Lymph Nodes. Journal of Imaging, 9(2), 45. https://doi.org/10.3390/jimaging9020045






