An Overview of X-ray Photon Counting Spectral Imaging (x-CSI) with a Focus on Gold Nanoparticle Quantification in Oncology
Abstract
1. Introduction
- Physical enhancement mechanisms are those that improve radiotherapy response of cells due to physical interactions between incident radiation and the AuNPs themselves. These can include increased energy deposition (due to the higher Z-number of gold producing a higher probability of interaction with the incident radiation beam) and production of more ionising radiation types when irradiated (production of secondary X-ray fluorescence, Auger electrons, and photoelectrons [15]). These effects have some geometric dependence, but are largely thought to depend primarily on the amount of Au present in the treated cells.
- Chemical enhancement mechanisms are those that improve radiotherapy response of cells due to an increase in the number of reactive oxygen species (ROS) that are produced in the presence of AuNPs compared to in their absence [16]. These effects can be related to interactions between water molecules and the AuNP surface [17,18], and so generally are assumed to depend on the available AuNP surface area within the treated cells.
- Biological enhancement mechanisms are those that alter cell behaviour or metabolism in such a way as to predispose the cells to radiotherapy damage. These range from molecular changes such as inhibition of DNA repair [19], to cell cycle changes that cause accumulation of cells in radiosensitive phases [20]. The mechanisms behind some of these effects are not well understood, though they can reasonably be assumed to relate either to AuNP surface area or the levels of ROS produced by AuNP presence.
2. Requirements of X-ray Detectors for AuNP Quantification
2.1. Scintillators vs. Direct Converters
2.2. Photon Counting vs. Energy Integration
2.2.1. Energy Integration
2.2.2. Photon Counting
2.3. Using Spectral Information
3. Advantages of x-CSI for In Vivo AuNP Imaging
3.1. X-ray Imaging vs. Other Imaging Modalities
3.2. x-CSI vs. Traditional X-ray Imaging Techniques
4. x-CSI-Specific Design Considerations/Limitations and Their Mitigation
5. AuNP Quantification with x-CSI
5.1. Requirements
- Calculating the volume of the unit cell [89] that is taken up by a single gold atom;
- Determining the volume of AuNPs used in the study;
- Dividing the answer from 2 by the answer from 1 to calculate the number of Au atoms per AuNP;
- Multiplying the answer from 3 by the reported molarities of AuNPs (n.b. the molarity may need to be calculated based on other reported metrics first) to determine the effective number density of Au atoms;
- Multiplying the answer from 4 by the atomic mass of Au (197) to yield the Au atom density in g/L (which is equivalent to mg/mL).
| AuNP Diameter (nm) | Reported AuNP Concentration | Standardised Density (mg/mL) | Reference |
|---|---|---|---|
| 1.9 | 2.4 µM | 1.0 × 10−1 | [87] |
| 1.9 | 0.24 µM | 1.0 × 10−2 | |
| 13 | 10 nM | 1.3 × 10−1 | [90] |
| 30 | 2.4 mg/mL | 2.4 | [86] |
| ?? | 36 µg/mL | 3.6 × 10−2 | [91] |
| 14 | 7 × 109 NPs/mL | 1.9 × 10−4 | [92] |
| 50 | 7 × 109 NPs/mL | 8.8 × 10−3 | |
| 74 | 7 × 109 NPs/mL | 2.9 × 10−2 | |
| 1.9 | 12 µM/500 µg/mL | 5.0 × 10−1 | [93] |
| 2.7 | 0.5 mg/mL | 5.0 × 10−1 | [94] |
| 14 | 1.25 nM | 2.1 × 10−2 | [95] |
| 14 | 2.5 nM | 4.2 × 10−2 | |
| 14 | 5 nM | 8.3 × 10−2 | |
| 1.9 | 12 µM/500 µg/mL | 5.0 × 10−1 | [96] |
| 1.9 | 12 µM/500 µg/mL | 5.0 × 10−1 | [97] |
| 12 | 1 mM | 1.0 × 104 | [98] |
| 7 | 5.5 µmol/mL | 1.1 × 104 | [99] |
| 47 | 50 µM | 3.1 × 104 | [100] |
| 10.8 | 15 µM | 1.1 × 102 | [101] |
| 6.1 | 0.4 mM | 5.5 × 102 | [102] |
| 6.1 | 1 mM | 1.4 × 103 | |
| 4.7 | 500 µM | 3.1 × 102 | [103] |
| 14.8 | 1.5 µg/mL | 1.5 × 10−3 | [104] |
| 14.8 | 15 µg/mL | 1.5 × 10−2 | |
| 1.9 | 0.25 mM | 1.0 × 10 | [105] |
| 1.9 | 0.5 mM | 2.1 × 10 | |
| 1.9 | 1 mM | 4.2 × 10 | |
| 1.9 | 12 µM/500 µg/mL | 5.0 × 10−1 | [106] |
| 13 | 20 nM | 2.7 × 10−1 | [107] |
| 16 | 20 nM | 5.0 × 10−1 | [108] |
| 49 | 20 nM | 1.4 × 10 | |
| 30 | 15 nM | 2.5 | [109] |
| <2 | 50 µg/mL | 5.0 × 10−2 | [85] |
| 10.8 | 15 nM | 1.1 × 10−1 | [110] |
| 4.8 | 0.05 mM | 3.4 × 10 | [111] |
| 12.1 | 0.05 mM | 5.4 × 102 | |
| 27.3 | 0.05 mM | 6.2 × 103 | |
| 46.6 | 0.05 mM | 3.1 × 104 |
5.2. Achievements
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moeller, B.J.; Richardson, R.A.; Dewhirst, M.W. Hypoxia and radiotherapy: Opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007, 26, 241–248. [Google Scholar] [CrossRef]
- Elliott, S.P.; Malaeb, B.S. Long-term urinary adverse effects of pelvic radiotherapy. World J. Urol. 2010, 29, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, E.; Centurion, B.S.; Ferreira, L.H.C.; De Souza, A.P.; Damante, J.H.; Rubira-Bullen, I.R. Oral adverse effects of head and neck radiotherapy: Literature review and suggestion of a clinical oral care guideline for irradiated patients. J. Appl. Oral Sci. 2011, 19, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Nimalasena, S.; Gothard, L.; Anbalagan, S.; Allen, S.; Sinnett, V.; Mohammed, K.; Kothari, G.; Musallam, A.; Lucy, C.; Yu, S.; et al. Intratumoral Hydrogen Peroxide with Radiation Therapy in Locally Advanced Breast Cancer: Results from a Phase 1 Clinical Trial. Int. J. Radiat. Oncol. 2020, 108, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-J.; Chu, S.-W.; Liao, E.-C.; Fan, C.-H.; Chan, H.-L.; Wei, K.-C.; Yeh, C.-K. Normalization of Tumor Vasculature by Oxygen Microbubbles with Ultrasound. Theranostics 2019, 9, 7370–7383. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, P.; Wu, T.; Pan, W.; Li, N.; Tang, B. Organelle-localized radiosensitizers. Chem. Commun. 2020, 56, 10621–10630. [Google Scholar] [CrossRef]
- Johnke, R.M.; Sattler, J.A.; Allison, R.R. Radioprotective agents for radiation therapy: Future trends. Future Oncol. 2014, 10, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, H.; Jiang, R.; Wang, Z. The clinical effect study on malignant tumors with chronoradiotherapy. Biol. Rhythm. Res. 2014, 46, 249–255. [Google Scholar] [CrossRef]
- Greco, C.; Pares, O.; Pimentel, N.; Louro, V.; Santiago, I.; Vieira, S.; Stroom, J.; Mateus, D.; Soares, A.; Marques, J.; et al. Safety and Efficacy of Virtual Prostatectomy with Single-Dose Radiotherapy in Patients with Intermediate-Risk Prostate Cancer. JAMA Oncol. 2021, 7, 700. [Google Scholar] [CrossRef]
- Nichelatti, E.; Ronsivalle, C.; Picardi, L.; Montereali, R.M. Optimization of the theoretical dose distribution in the ‘Spread out Bragg Peak’ (SOBP) region in proton therapy by means of semi-analytical techniques. In Proceedings of the Nuovo Cimento della Societa Italiana di Fisica C, Online, 14–18 September 2020. [Google Scholar] [CrossRef]
- Pricker, S.P. Medical uses of gold compounds: Past, present and future. Gold Bull. 1996, 29, 53–60. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active targeting of gold nanoparticles as cancer therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bao, C.; Liang, S.; Fu, H.; Wang, K.; Deng, M.; Liao, Q.; Cui, D. RGD-conjugated silica-coated gold nanorods on the surface of carbon nanotubes for targeted photoacoustic imaging of gastric cancer. Nanoscale Res. Lett. 2014, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, K.T.; McMahon, S.J.; Taggart, L.E.; Prise, K.M. Radiosensitization by gold nanoparticles: Effective at megavoltage energies and potential role of oxidative stress. Transl. Cancer Res. 2013, 2, 269–279. [Google Scholar]
- Howard, D.; Sebastian, S.; Le, Q.V.-C.; Thierry, B.; Kempson, I. Chemical Mechanisms of Nanoparticle Radiosensitization and Radioprotection: A Review of Structure-Function Relationships Influencing Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 579. [Google Scholar] [CrossRef]
- Cheng, N.N.; Starkewolf, Z.; Davidson, R.A.; Sharmah, A.; Lee, C.; Lien, J.; Guo, T. Chemical Enhancement by Nanomaterials under X-ray Irradiation. J. Am. Chem. Soc. 2012, 134, 1950–1953. [Google Scholar] [CrossRef]
- Gilles, M.; Brun, E.; Sicard-Roselli, C. Quantification of hydroxyl radicals and solvated electrons produced by irradiated gold nanoparticles suggests a crucial role of interfacial water. J. Colloid Interface Sci. 2018, 525, 31–38. [Google Scholar] [CrossRef]
- Turnbull, T.; Douglass, M.; Williamson, N.H.; Howard, D.; Bhardwaj, R.; Lawrence, M.; Paterson, D.J.; Bezak, E.; Thierry, B.; Kempson, I.M. Cross-Correlative Single-Cell Analysis Reveals Biological Mechanisms of Nanoparticle Radiosensitization. ACS Nano 2019, 13, 5077–5090. [Google Scholar] [CrossRef]
- Xu, W.; Teng, L.; Pang, B.; Li, P.; Chuanqing, Z.; Huang, P.; Zhang, C.; Qiushi, R.; Wenbin, H.; Fu, S. The radiosensitization of melanoma cells by gold nanorods irradiated with MV X-ray. Nano Biomed. Eng. 2012, 4, 6–11. [Google Scholar] [CrossRef][Green Version]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Li, F.; Jin, X.; Li, J.; Chen, W.; Li, Q. Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells. Theranostics 2018, 8, 1824–1849. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Bromma, K.; Cicon, L.; Beckham, W.; Chithrani, D.B. Gold nanoparticle mediated radiation response among key cell components of the tumour microenvironment for the advancement of cancer nanotechnology. Sci. Rep. 2020, 10, 12096. [Google Scholar] [CrossRef]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef]
- Jiang, X.; Du, B.; Tang, S.; Hsieh, J.; Zheng, J. Photoacoustic Imaging of Nanoparticle Transport in the Kidneys at High Temporal Resolution. Angew. Chem. Int. Ed. 2019, 58, 5994–6000. [Google Scholar] [CrossRef]
- Joon, D.L.; Smith, D.; Tacey, M.; Schneider, M.; Harris, B.; Ong, W.L.; Foroudi, F.; Jenkins, T.; Wada, M.; Chao, M.; et al. A phantom study to contrast and compare polymer and gold fiducial markers in radiotherapy simulation imaging. Sci. Rep. 2021, 11, 8931. [Google Scholar] [CrossRef]
- Si-Mohamed, S.; Cormode, D.P.; Bar-Ness, D.; Sigovan, M.; Naha, P.C.; Langlois, J.-B.; Chalabreysse, L.; Coulon, P.; Blevis, I.; Roessl, E.; et al. Evaluation of spectral photon counting computed tomography K-edge imaging for determination of gold nanoparticle biodistribution in vivo. Nanoscale 2017, 9, 18246–18257. [Google Scholar] [CrossRef]
- Dong, Y.C.; Hajfathalian, M.; Maidment, P.S.N.; Hsu, J.C.; Naha, P.C.; Si-Mohamed, S.; Breuilly, M.; Kim, J.; Chhour, P.; Douek, P.; et al. Effect of Gold Nanoparticle Size on Their Properties as Contrast Agents for Computed Tomography. Sci. Rep. 2019, 9, 14912. [Google Scholar] [CrossRef]
- Pickford Scienti, O. On the Potential of Multi-Spectral X-ray and Photoacoustic Imaging to Facilitate Gold Nanoparticle Mediated Dose-Enhanced Radiotherapy; Institute of Cancer Research: London, UK, 2021. [Google Scholar]
- MARS for Clinicians. MARS Bioimaging Limited. 2020. Available online: https://www.marsbioimaging.com/clinicians/ (accessed on 24 February 2021).
- Siemens. NAEOTOM Alpha® with Quantum Technology. 2021. Available online: https://www.siemens-healthineers.com/computed-tomography/photon-counting-ct-scanner/naeotom-alpha (accessed on 28 November 2021).
- GE Healthcare. Karolinska Institutet & MedTechLabs Kickoff the World’s First Clinical Evaluation of GE Healthcare’s Photon Counting CT Technology with Deep Silicon Detectors. 2021. Available online: https://www.ge.com/news/press-releases/karolinska-institutet-medtechlabs-kickoff-the-worlds-first-clinical-evaluation-of-ge#_edn1 (accessed on 30 November 2021).
- Noid, G.; Tai, A.; Liu, Y.; Li, X. TH-CD-202-03: Enhancing Soft-Tissue CT Contrast for Radiation Therapy Using Mono-Energetic Decompositions of Dual Energy CT. Med. Phys. 2016, 43, 3876–3877. [Google Scholar] [CrossRef]
- Goo, H.W.; Goo, J.M. Dual-Energy CT: New Horizon in Medical Imaging. Korean J. Radiol. 2017, 18, 555. [Google Scholar] [CrossRef]
- Matsui, K.; Machida, H.; Mitsuhashi, T.; Omori, H.; Nakaoka, T.; Sakura, H.; Ueno, E. Analysis of coronary arterial calcification components with coronary CT angiography using single-source dual-energy CT with fast tube voltage switching. Int. J. Cardiovasc. Imaging 2014, 31, 639–647. [Google Scholar] [CrossRef]
- Petersilka, M.; Bruder, H.; Krauss, B.; Stierstorfer, K.; Flohr, T.G. Technical principles of dual source CT. Eur. J. Radiol. 2008, 68, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, L.; Duan, X.; Xi, Y.; Lewis, M.A.; Pearle, M.S.; Antonelli, J.A.; Goerne, H.; Kolitz, E.M.; Abbara, S.; Lenkinski, R.E.; et al. Dual-layer spectral detector CT: Non-inferiority assessment compared to dual-source dual-energy CT in discriminating uric acid from non-uric acid renal stones ex vivo. Abdom. Radiol. 2018, 43, 3075–3081. [Google Scholar] [CrossRef]
- Mori, I.; Machida, Y.; Osanai, M.; Iinuma, K. Photon starvation artifacts of X-ray CT: Their true cause and a solution. Radiol. Phys. Technol. 2013, 6, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Shirono, J.; Araki, H.; Budden, B.; Cai, L.; Kawata, G.; Miyazaki, H.; Qiang, Y.; Ye, Z.; Zhan, X.; et al. Modeling Photon Counting Detector Anode Street Impact on Detector Energy Response. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 5, 476–484. [Google Scholar] [CrossRef]
- Rajendran, K.; Voss, B.A.; Zhou, W.; Tao, S.; Delone, D.R.; Lane, J.I.; Weaver, J.M.; Carlson, M.L.; Fletcher, J.G.; McCollough, C.H.; et al. Dose Reduction for Sinus and Temporal Bone Imaging Using Photon-Counting Detector CT With an Additional Tin Filter. Investig. Radiol. 2020, 55, 91–100. [Google Scholar] [CrossRef]
- Pourmorteza, A.; Symons, R.; Reich, D.; Bagheri, M.; Cork, T.; Kappler, S.; Ulzheimer, S.; Bluemke, D. Photon-Counting CT of the Brain: In vivo Human Results and Image-Quality Assessment. Am. J. Neuroradiol. 2017, 38, 2257–2263. [Google Scholar] [CrossRef] [PubMed]
- Rebuffel, V.; Dinten, J.-M. Dual-energy X-ray imaging: Benefits and limits. Insight-Non-Destructive Test. Cond. Monit. 2007, 49, 589–594. [Google Scholar] [CrossRef]
- Mendonca, P.; Lamb, P.; Sahani, D.V. A Flexible Method for Multi-Material Decomposition of Dual-Energy CT Images. IEEE Trans. Med. Imaging 2014, 33, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Scienti, O.L.P.P.; Bamber, J.C.; Darambara, D.G. The effects of spectral X-ray photon counting detector parameters on detector performance: Thickness and pitch. IEEE Access 2020, 8, 196541–196552. [Google Scholar] [CrossRef]
- Symons, R.; Krauss, B.; Sahbaee, P.; Cork, T.E.; Lakshmanan, M.N.; Bluemke, D.A.; Pourmorteza, A. Photon-counting CT for simultaneous imaging of multiple contrast agents in the abdomen: An in vivo study. Med. Phys. 2017, 44, 5120–5127. [Google Scholar] [CrossRef] [PubMed]
- Nasirudin, R.A.; Mei, K.; Panchev, P.; Fehringer, A.; Pfeiffer, F.; Rummeny, E.J.; Fiebich, M.; Noël, P.B. Reduction of metal artifact in single photon-counting computed tomography by spectral-driven iterative reconstruction technique. PLoS ONE 2016, 10, e0124831. [Google Scholar]
- Broeke, L.V.; Grillon, M.; Yeung, A.W.K.; Wu, W.; Tanaka, R.; Vardhanabhuti, V. Feasibility of photon-counting spectral CT in dental applications—a comparative qualitative analysis. BDJ Open 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Sigovan, M.; Si-Mohamed, S.; Bar-Ness, D.; Mitchell, J.; Langlois, J.-B.; Coulon, P.; Roessl, E.; Blevis, I.; Rokni, M.; Rioufol, G.; et al. Feasibility of improving vascular imaging in the presence of metallic stents using spectral photon counting CT and K-edge imaging. Sci. Rep. 2019, 9, 19850. [Google Scholar] [CrossRef]
- Si-Mohamed, S.; Tatard-Leitman, V.; Laugerette, A.; Sigovan, M.; Pfeiffer, D.; Rummeny, E.J.; Coulon, P.; Yagil, Y.; Douek, P.; Boussel, L.; et al. Spectral Photon-Counting Computed Tomography (SPCCT): In-vivo single-acquisition multi-phase liver imaging with a dual contrast agent protocol. Sci. Rep. 2019, 9, 8458. [Google Scholar] [CrossRef]
- Symons, R.; Pourmorteza, A.; Sandfort, V.; Ahlman, M.A.; Cropper, T.; Mallek, M.; Kappler, S.; Ulzheimer, S.; Mahesh, M.; Jones, E.C.; et al. Feasibility of Dose-reduced Chest CT with Photon-counting Detectors: Initial Results in Humans. Radiology 2017, 285, 980–989. [Google Scholar] [CrossRef]
- Roeder, R.K.; Curtis, T.E.; Nallathamby, P.D.; Irimata, L.E.; McGinnity, T.L.; Cole, L.E.; Vargo-Gogola, T.; Cowden Dahl, K.D. Nanoparticle imaging probes for molecular imaging with computed tomography and application to cancer imaging. In Medical Imaging 2017: Physics of Medical Imaging; SPIE: Bellingham, WA, USA, 2017; Volume 10132, p. 101320X. [Google Scholar] [CrossRef]
- Aamir, R.; Chernoglazov, A.I.; Bateman, C.; Butler, P.H.; Anderson, N.G.; Bell, S.T.; Panta, R.K.; Healy, J.L.; Mohr, J.L.; Rajendran, K.; et al. MARS spectral molecular imaging of lamb tissue: Data collection and image analysis. J. Instrum. 2014, 9, P02005. [Google Scholar] [CrossRef]
- Amma, M.R.; Butler, A.P.H.; Raja, A.; Bamford, B.; Butler, P.H.; Walker, P.; Matanaghi, A.; Adebileje, S.A.; Anderson, N.; Anjomrouz, M.; et al. Assessment of metal implant induced artefacts using photon counting spectral CT. In Proceedings of the Developments in X-ray Tomography XII, San Diego, CA, USA, 27 September 2019; Volume 11113, p. 46. [Google Scholar] [CrossRef]
- DenOtter, T.D.; Schubert, J. Hounsfield Unit. In StatPearls; StatPerals Publishing: Bethesda, MA, USA, 2021. [Google Scholar]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Ballabriga, R.; Alozy, J.; Blaj, G.; Campbell, M.; Fiederle, M.; Frojdh, E.; Heijne, E.H.M.; Llopart, X.; Pichotka, M.P.; Procz, S.; et al. The Medipix3RX: A high resolution, zero dead-time pixel detector readout chip allowing spectroscopic imaging. J. Instrum. 2013, 8, C02016. [Google Scholar] [CrossRef]
- Moghiseh, M.; Aamir, R.; Panta, R.K.; de Ruiter, N.J.A.; Chernoglazov, A.; Healy, J.; Butler, A.P.H.; Anderson, N.G. Discrimination of Multiple High-Z Materials by Multi- Energy Spectral CT—A Phantom Study. JSM Biomed. Imaging Data 2016, 3, 1007. [Google Scholar]
- Anderson, N.G.; Butler, A.P.; Scott, N.J.A.; Cook, N.J.; Butzer, J.S.; Schleich, N.; Firsching, M.; Grasset, R.; De Ruiter, N.; Campbell, M.; et al. Spectroscopic (multi-energy) CT distinguishes iodine and barium contrast material in MICE. Eur. Radiol. 2010, 20, 2126–2134. [Google Scholar] [CrossRef]
- Balegamire, J.; Vandamme, M.; Chereul, E.; Si-Mohamed, S.; Maache, S.A.; Almouazen, E.; Ettouati, L.; Fessi, H.; Boussel, L.; Douek, P.; et al. Iodinated polymer nanoparticles as contrast agent for spectral photon counting computed tomography. Biomater. Sci. 2020, 8, 5715–5728. [Google Scholar] [CrossRef]
- Pan, D.; Schmieder, A.H.; SenPan, A.; Yang, X.; Wickline, S.A.; Roessl, E.; Proksa, R.; Schirra, C.O.; Lanza, G.M. Molecular Imaging with Spectral CT Nanoprobes. In Design and Applications of Nanoparticles in Biomedical Imaging; Springer: Singapore, 2017; pp. 385–402. [Google Scholar]
- Moghiseh, M.; Lowe, C.; Lewis, J.G.; Kumar, D.; Butler, A.; Anderson, N.; Raja, A.; Bombonati, A. Spectral Photon-Counting Molecular Imaging for Quantification of Monoclonal Antibody-Conjugated Gold Nanoparticles Targeted to Lymphoma and Breast Cancer: An In vitro Study. Contrast Media Mol. Imaging 2018, 2018, 2136840. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, J.R.; Farhadi, F.; Solomon, J.; Sahbaee, P.; Saboury, B.; Pritchard, W.F.; Jones, E.C.; Samei, E. Comparison of Low Dose Performance of Photon-Counting and Energy Integrating CT. Acad. Radiol. 2020, 28, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lane, J.; Carlson, M.; Bruesewitz, M.; Witte, R.; Koeller, K.; Eckel, L.; Carter, R.; McCollough, C.; Leng, S. Comparison of a Photon-Counting-Detector CT with an Energy-Integrating-Detector CT for Temporal Bone Imaging: A Cadaveric Study. Am. J. Neuroradiol. 2018, 39, 1733–1738. [Google Scholar] [CrossRef]
- Otfinowski, P.; Deptuch, G.; Maj, P. FRIC—a 50 μm pixel-pitch single photon counting ASIC with Pattern Recognition algorithm in 40 nm CMOS technology. J. Instrum. 2020, 15, C01016. [Google Scholar] [CrossRef]
- Ballabriga, R.; Alozy, J.; Bandi, F.N.; Campbell, M.; Egidos, N.; Fernandez-Tenllado, J.M.; Heijne, E.H.M.; Kremastiotis, I.; Llopart, X.; Madsen, B.J.; et al. Photon Counting Detectors for X-ray Imaging with Emphasis on CT. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 422–440. [Google Scholar] [CrossRef]
- Flohr, T.; Petersilka, M.; Henning, A.; Ulzheimer, S.; Ferda, J.; Schmidt, B. Photon-counting CT review. Phys. Med. 2020, 79, 126–136. [Google Scholar] [CrossRef]
- Ponchut, C. Correction of the charge sharing in photon-counting pixel detector data. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2008, 591, 311–313. [Google Scholar] [CrossRef]
- Krzyzanowska, A.; Deptuch, G.W.; Maj, P.; Grybos, P.; Szczygiel, R. Characterization of the Photon Counting CHASE Jr., Chip Built in a 40-nm CMOS Process with a Charge Sharing Correction Algorithm Using a Collimated X-ray Beam. IEEE Trans. Nucl. Sci. 2017, 64, 2561–2568. [Google Scholar] [CrossRef]
- Ballabriga, R.; Campbell, M.; Heijne, E.H.M.; Llopart, X.; Tlustos, L. The Medipix3 Prototype, a Pixel Readout Chip Working in Single Photon Counting Mode with Improved Spectrometric Performance. IEEE Trans. Nucl. Sci. 2007, 54, 1824–1829. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Sjolin, M. Digital count summing vs analog charge summing for photon counting detectors: A performance simulation study. Med. Phys. 2018, 45, 4085–4093. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.S. Coincidence Counters for Charge Sharing Compensation in Spectroscopic Photon Counting Detectors. IEEE Trans. Med. Imaging 2020, 39, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Ullberg, C.; Eriksson, C.; Urech, M.; Stewart, A. Photon counting dual energy X-ray imaging at CT count rates: Measurements and implications of in-pixel charge sharing correction. In Medical Imaging 2018: Physics of Medical Imaging; SPIE: Bellingham, WA, USA, 2018; Volume 10573, p. 1057319. [Google Scholar] [CrossRef]
- Scienti, O.L.P.P.; Bamber, J.C.; Darambara, D.G. CdTe Based Energy Resolving, X-ray Photon Counting Detector Performance Assessment: The Effects of Charge Sharing Correction Algorithm Choice. Sensors 2020, 20, 6093. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Santos, A.; Darambara, D.G. An investigation of performance characteristics of a pixellated room-temperature semiconductor detector for medical imaging. J. Phys. D Appl. Phys. 2009, 42, 175101. [Google Scholar] [CrossRef]
- Genocchi, B.; Scienti, O.P.; Darambara, D. Optimal configuration of a low-dose breast-specific gamma camera based on semiconductor CdZnTe pixelated detectors. J. Phys. Conf. Ser. 2017, 841, 12012. [Google Scholar] [CrossRef]
- Myronakis, M.E.; Zvelebil, M.; Darambara, D.G. Computational modelling of pixelated CdZnTe detectors for x- and γ-ray imaging applications. J. Instrum. 2012, 7, P03004. [Google Scholar] [CrossRef]
- Scienti, O.L.P.P.; Bamber, J.C.; Darambara, D. Inclusion of a Charge Sharing Correction Algorithm into an X-ray Photon Counting Spectral Detector Simulation Framework. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 5, 485–492. [Google Scholar] [CrossRef]
- Koenig, T.; Zuber, M.; Hamann, E.; Cecilia, A.; Ballabriga, R.; Campbell, M.; Ruat, M.; Tlustos, L.; Fauler, A.; Fiederle, M.; et al. How spectroscopic X-ray imaging benefits from inter-pixel communication. Phys. Med. Biol. 2014, 59, 6195–6213. [Google Scholar] [CrossRef]
- Trueb, P.; Zambon, P.; Broennimann, C. Assessment of the spectral performance of hybrid photon counting X-ray detectors. Med. Phys. 2017, 44, e207–e214. [Google Scholar] [CrossRef]
- A Crash Course on Handling DICOM Medical Imaging Data, POSTDICOM. 2021. Available online: https://www.postdicom.com/en/blog/handling-dicom-medical-imaging-data (accessed on 24 February 2021).
- Ackerman, M.J. The Visible Human Project. Proceedings of the IEEE. 1998. Available online: https://www.nlm.nih.gov/research/visible/visible_human.html (accessed on 24 February 2021).
- Zhang, X.D.; Chen, J.; Luo, Z.; Wu, D.; Shen, X.; Song, S.-S.; Sun, Y.-M.; Liu, P.-X.; Zhao, J.; Huo, S.; et al. Enhanced Tumor Accumulation of Sub-2 nm Gold Nanoclusters for Cancer Radiation Therapy. Adv. Healthc. Mater. 2013, 3, 133–141. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Cai, Z.; Kwon, Y.L.; Lechtman, E.; Pignol, J.-P.; Reilly, R.M. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res. Treat. 2012, 137, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, K.T.; Coulter, J.; Jain, S.; Forker, J.; McMahon, S.; Schettino, G.; Prise, K.; Currell, F.; Hirst, D.G. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: Potential application for cancer therapy. Nanotechnology 2010, 21, 295101. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhattarai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C. How Can You Calculate How Many Atoms Are in a Nanoparticle? Available online: http://sustainable-nano.com/2016/07/28/how-many-atoms-are-in-a-nanoparticle/ (accessed on 24 February 2021).
- Chang, M.-Y.; Shiau, A.-L.; Chen, Y.-H.; Chang, C.-J.; Chen, H.H.-W.; Wu, C.-L. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008, 99, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, W.; Bao, Y.; Xu, H.; Qin, S.; Tu, Y. BSA capped Au nanoparticle as an efficient sensitizer for glioblastoma tumor radiation therapy. RSC Adv. 2015, 5, 40514–40520. [Google Scholar] [CrossRef]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; Van Prooijen, M.; Allen, C.; Bristow, R.; Hill, R.P.; Jaffray, D. Gold Nanoparticles as Radiation Sensitizers in Cancer Therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef]
- Coulter, J.A.; Jain, S.; Butterworth, K.T.; Taggart, L.; Dickson, G.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Trainor, C.; Hounsell, A.; et al. Cell type-dependent uptake, localization, and cytotoxicity of 1.9 nm gold nanoparticles. Int. J. Nanomed. 2012, 7, 2673–2685. [Google Scholar] [CrossRef]
- Cui, L.; Tse, K.; Zahedi, P.; Harding, S.M.; Zafarana, G.; Jaffray, D.A.; Bristow, R.G.; Allen, C. Hypoxia and Cellular Localization Influence the Radiosensitizing Effect of Gold Nanoparticles (AuNPs) in Breast Cancer Cells. Radiat. Res. 2014, 182, 475–488. [Google Scholar] [CrossRef]
- Geng, F.; Song, K.; Xing, J.Z.; Yuan, C.; Yan, S.; Yang, Q.; Chen, J.; Kong, B. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology 2011, 22, 285101. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Coulter, J.A.; Butterworth, K.; Hounsell, A.R.; McMahon, S.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.; Currell, F.; et al. Gold nanoparticle cellular uptake, toxicity and radiosensitisation in hypoxic conditions. Radiother. Oncol. 2014, 110, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Coulter, J.; Hounsell, A.R.; Butterworth, K.; McMahon, S.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.; Currell, F.; et al. Cell-Specific Radiosensitization by Gold Nanoparticles at Megavoltage Radiation Energies. Int. J. Radiat. Oncol. 2011, 79, 531–539. [Google Scholar] [CrossRef]
- Joh, D.Y.; Sun, L.; Stangl, M.; Al Zaki, A.; Murty, S.; Santoiemma, P.P.; Davis, J.J.; Baumann, B.; Alonso-Basanta, M.; Bhang, D.; et al. Selective Targeting of Brain Tumors with Gold Nanoparticle-Induced Radiosensitization. PLoS ONE 2013, 8, e62425. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Pujari, G.; Semwal, M.K.; Sarma, A.; Avasthi, D.K. In vitro studies on radiosensitization effect of glucose capped gold nanoparticles in photon and ion irradiation of HeLa cells. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2013, 301, 7–11. [Google Scholar] [CrossRef]
- Khoshgard, K.; Hashemi, B.; Arbabi, A.; Rasaee, M.J.; Soleimani, M. Radiosensitization effect of folate-conjugated gold nanoparticles on HeLa cancer cells under orthovoltage superficial radiotherapy techniques. Phys. Med. Biol. 2014, 59, 2249–2263. [Google Scholar] [CrossRef]
- Kong, T.; Zeng, J.; Wang, X.; Yang, X.; Yang, J.; McQuarrie, S.; McEwan, A.; Roa, W.; Chen, J.; Xing, J.Z. Enhancement of Radiation Cytotoxicity in Breast-Cancer Cells by Localized Attachment of Gold Nanoparticles. Small 2008, 4, 1537–1543. [Google Scholar] [CrossRef]
- Liu, C.-J.; Wang, C.-H.; Chen, S.-T.; Chen, H.-H.; Leng, W.-H.; Chien, C.-C.; Wang, C.-L.; Kempson, I.; Hwu, Y.; Lai, T.-C.; et al. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Phys. Med. Biol. 2010, 55, 931–945. [Google Scholar] [CrossRef]
- Liu, C.-J.; Wang, C.-H.; Chien, C.-C.; Yang, T.-Y.; Chen, S.-T.; Leng, W.-H.; Lee, C.-F.; Lee, K.-H.; Hwu, Y.; Lee, Y.-C.; et al. Enhanced X-ray irradiation-induced cancer cell damage by gold nanoparticles treated by a new synthesis method of polyethylene glycol modification. Nanotechnology 2008, 19, 295104. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Jin, X.; He, P.; Zheng, X.; Dai, Z.; Ye, F.; Zhao, T.; Chen, W.; Li, Q. The dependence of radiation enhancement effect on the concentration of gold nanoparticles exposed to low- and high-LET radiations. Phys. Med. 2015, 31, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.N.; Bishara, N.; Ackerly, T.; He, C.F.; Jackson, P.; Wong, C.; Davidson, R.; Geso, M. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Taggart, L.E.; McMahon, S.J.; Currell, F.J.; Prise, K.M.; Butterworth, K.T. The role of mitochondrial function in gold nanoparticle mediated radiosensitisation. Cancer Nanotechnol. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Wang, Y.; Liu, Z.; Fu, L.; Hu, L. Enhancement of radiation effect and increase of apoptosis in lung cancer cells by thio-glucose-bound gold nanoparticles at megavoltage radiation energies. J. Nanoparticle Res. 2013, 15, 1642. [Google Scholar] [CrossRef]
- Wang, C.-H.; Zhang, S.-Y.; Fang, Q.; Shen, Z.-J.; Fan, Z.-J.; Jin, X.-F.; Zeng, Y.; Liu, Z.-Y.; Xie, H.-Z. Renal Dysfunction and hsCRP Predict Long-term Outcomes of Percutaneous Coronary Intervention in Acute Myocardial Infarction. Am. J. Med. Sci. 2015, 349, 413–420. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, J.Z.; Chen, J.; Ko, L.; Amanie, J.; Gulavita, S.; Pervez, N.; Yee, D.; Moore, R.; Roa, W. Enhanced radiation sensitivity in prostate cancer by gold-nanoparticles. Clin. Investig. Med. 2008, 31, E160–E167. [Google Scholar] [CrossRef]
- Roa, W.; Zhang, X.; Guo, L.; Shaw, A.; Hu, X.; Xiong, Y.; Gulavita, S.; Patel, S.; Sun, X.; Chen, J.; et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology 2009, 20, 375101. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.M.; Liu, P.X.; Liang, X.J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045–3059. [Google Scholar] [CrossRef]
- Jayarathna, S.; Ahmed, F.; O’Ryan, L.; Moktan, H.; Cui, Y.; Cho, S.H. Characterization of a Pixelated Cadmium Telluride Detector System Using a Polychromatic X-ray Source and Gold Nanoparticle-Loaded Phantoms for Benchtop X-ray Fluorescence Imaging. IEEE Access 2021, 9, 49912–49919. [Google Scholar] [CrossRef]
- Manohar, N.; Reynoso, F.J.; Diagaradjane, P.; Krishnan, S.; Cho, S.H. Quantitative imaging of gold nanoparticle distribution in a tumor-bearing mouse using benchtop X-ray fluorescence computed tomography. Sci. Rep. 2016, 6, 22079. [Google Scholar] [CrossRef]
- Nie, X.; Chen, Y.; Meng, L.-J. Towards Zero Compton Background in X-ray Fluorescence Computed Tomography (XFCT). J. Nucl. Med. 2019, 60, 1352. [Google Scholar]
- Lechtman, E.; Chattopadhyay, N.; Cai, Z.; Mashouf, S.; Reilly, R.; Pignol, J.P. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys. Med. Biol. 2011, 56, 4631–4647. [Google Scholar] [CrossRef] [PubMed]
- Behrouzkia, Z.; Zohdiaghdam, R.; Khalkhali, H.R.; Mousavi, F. Evaluation of Gold Nanoparticle Size Effect on Dose Enhancement Factor in Megavoltage Beam Radiotherapy Using MAGICA Polymer Gel Dosimeter. J. Biomed. Phys. Eng. 2019, 9, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, A.; Horvat, M.; Smolčić, V.Š. Dealing with the positive publication bias: Why you should really publish your negative results. Biochem. Med. 2017, 27, 447–452. [Google Scholar] [CrossRef]
- Kempson, I. Mechanisms of nanoparticle radiosensitization. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1656. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Cooper, L.N.; Andreev, O.A.; Reshetnyak, Y.K.; Antosh, M.P. Gold Nanoparticles for Radiation Enhancement in vivo. Jacobs J. Radiat. Oncol. 2016, 3, 26–45. [Google Scholar]
- Mackey, M.A.; El-Sayed, M.A. Chemosensitization of Cancer Cells via Gold Nanoparticle-Induced Cell Cycle Regulation. Photochem. Photobiol. 2014, 90, 306–312. [Google Scholar] [CrossRef]
- Li, Q.; Huang, C.; Liu, L.; Hu, R.; Qu, J. Effect of Surface Coating of Gold Nanoparticles on Cytotoxicity and Cell Cycle Progression. Nanomaterials 2018, 8, 1063. [Google Scholar] [CrossRef]
- Hanžić, N.; Horvat, A.; Bibić, J.; Unfried, K.; Jurkin, T.; Dražić, G.; Marijanović, I.; Slade, N.; Gotić, M. Syntheses of gold nanoparticles and their impact on the cell cycle in breast cancer cells subjected to megavoltage X-ray irradiation. Mater. Sci. Eng. C 2018, 91, 486–495. [Google Scholar] [CrossRef]

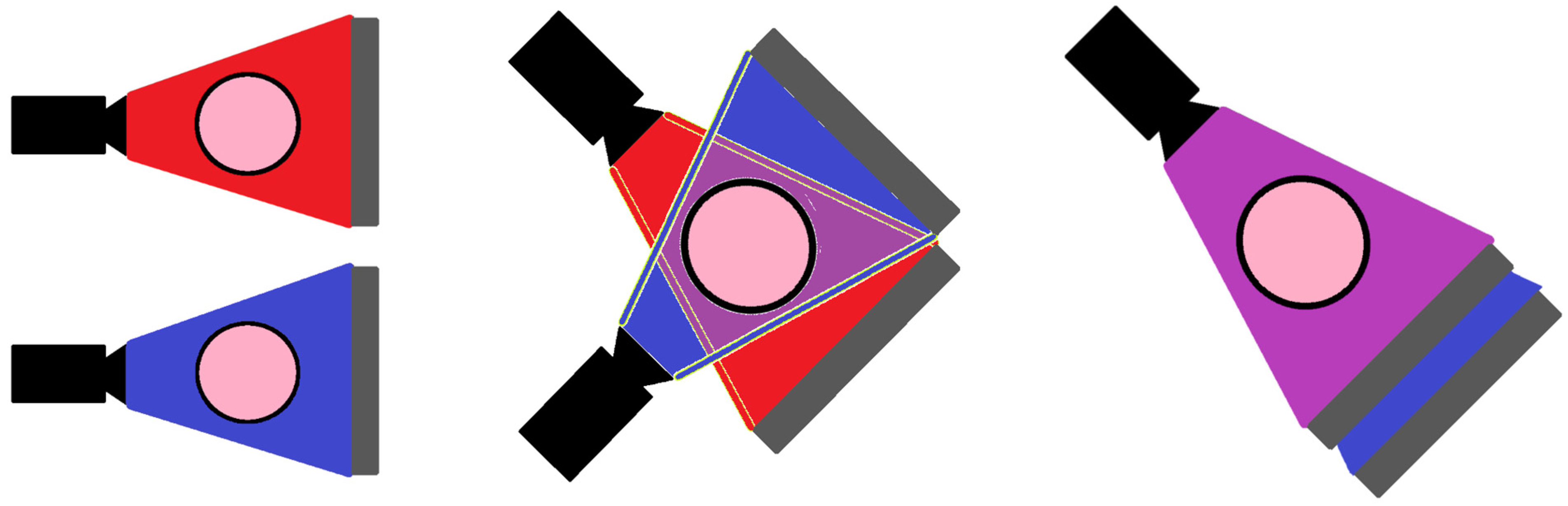
| Optimised Detector | MARS Detector | Phillips Healthcare Detector | |
|---|---|---|---|
| Pixel pitch (µm) | 200–250 | 110 | 500 |
| Sensor thickness (mm) | ~1.5 | 2 | 2 |
| CSCA NS | 3 × 3 or Hybrid | Hybrid | 1 × 1 or 2 × 2 (chequered) |
| CSCA NL | Dynamic | Dynamic | Static |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickford Scienti, O.L.P.; Darambara, D.G. An Overview of X-ray Photon Counting Spectral Imaging (x-CSI) with a Focus on Gold Nanoparticle Quantification in Oncology. J. Imaging 2022, 8, 4. https://doi.org/10.3390/jimaging8010004
Pickford Scienti OLP, Darambara DG. An Overview of X-ray Photon Counting Spectral Imaging (x-CSI) with a Focus on Gold Nanoparticle Quantification in Oncology. Journal of Imaging. 2022; 8(1):4. https://doi.org/10.3390/jimaging8010004
Chicago/Turabian StylePickford Scienti, Oliver L. P., and Dimitra G. Darambara. 2022. "An Overview of X-ray Photon Counting Spectral Imaging (x-CSI) with a Focus on Gold Nanoparticle Quantification in Oncology" Journal of Imaging 8, no. 1: 4. https://doi.org/10.3390/jimaging8010004
APA StylePickford Scienti, O. L. P., & Darambara, D. G. (2022). An Overview of X-ray Photon Counting Spectral Imaging (x-CSI) with a Focus on Gold Nanoparticle Quantification in Oncology. Journal of Imaging, 8(1), 4. https://doi.org/10.3390/jimaging8010004







