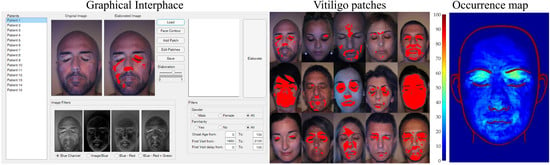
Semi-Automatic Tool for Vitiligo Detection and Analysis
Abstract
1. Introduction
2. Materials and Methods
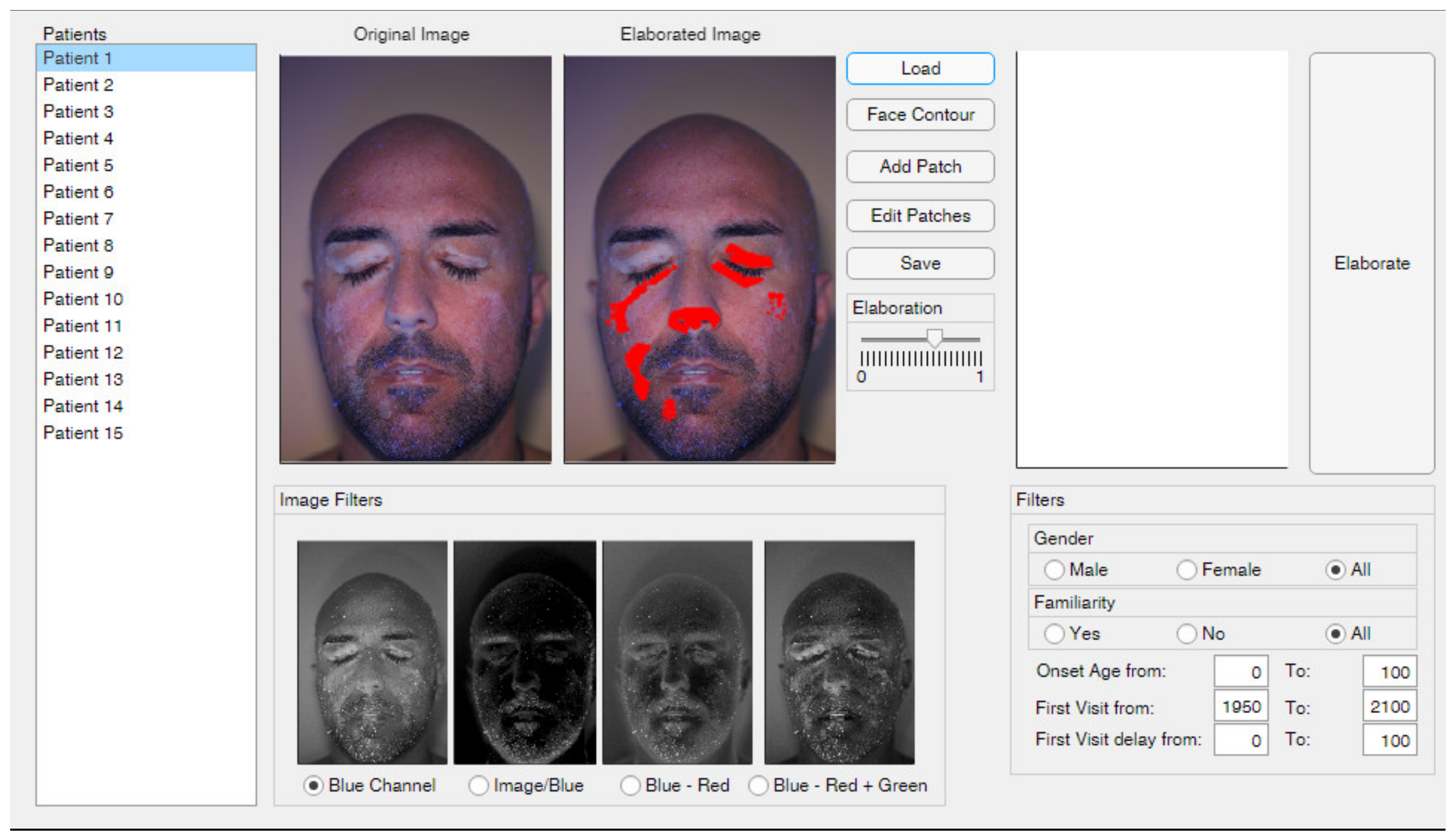
2.1. Graphical Interface
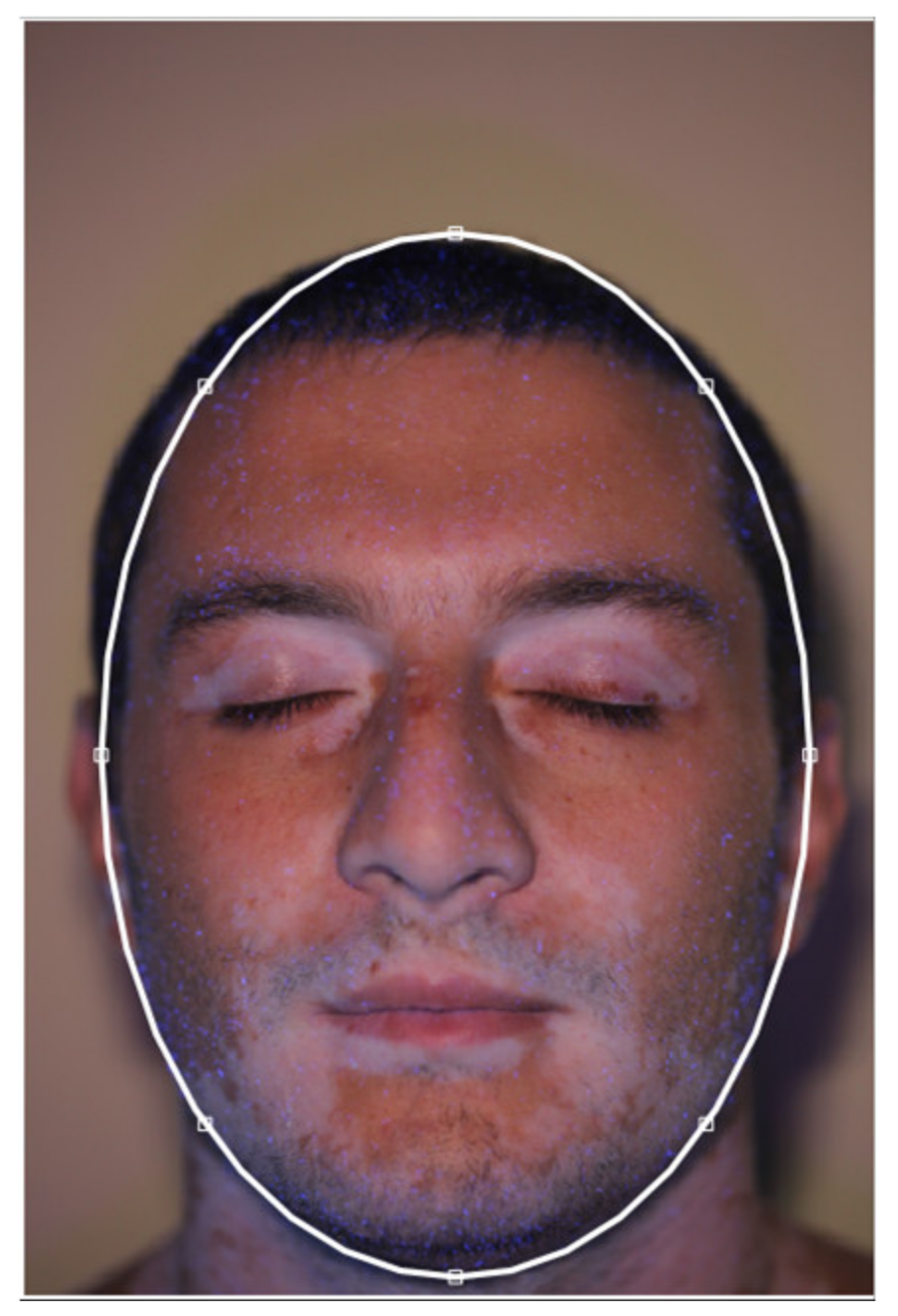
2.2. Face Contour
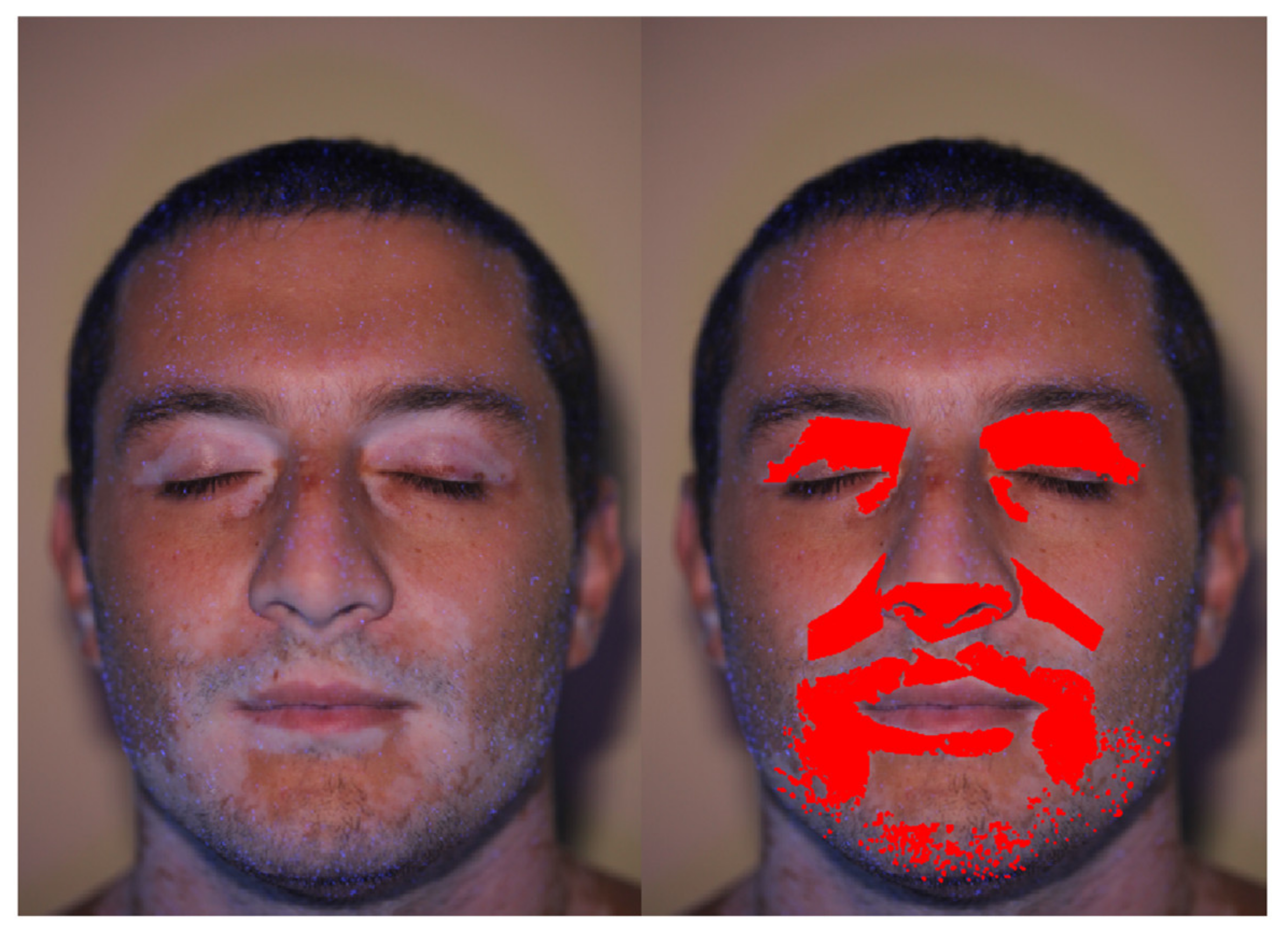
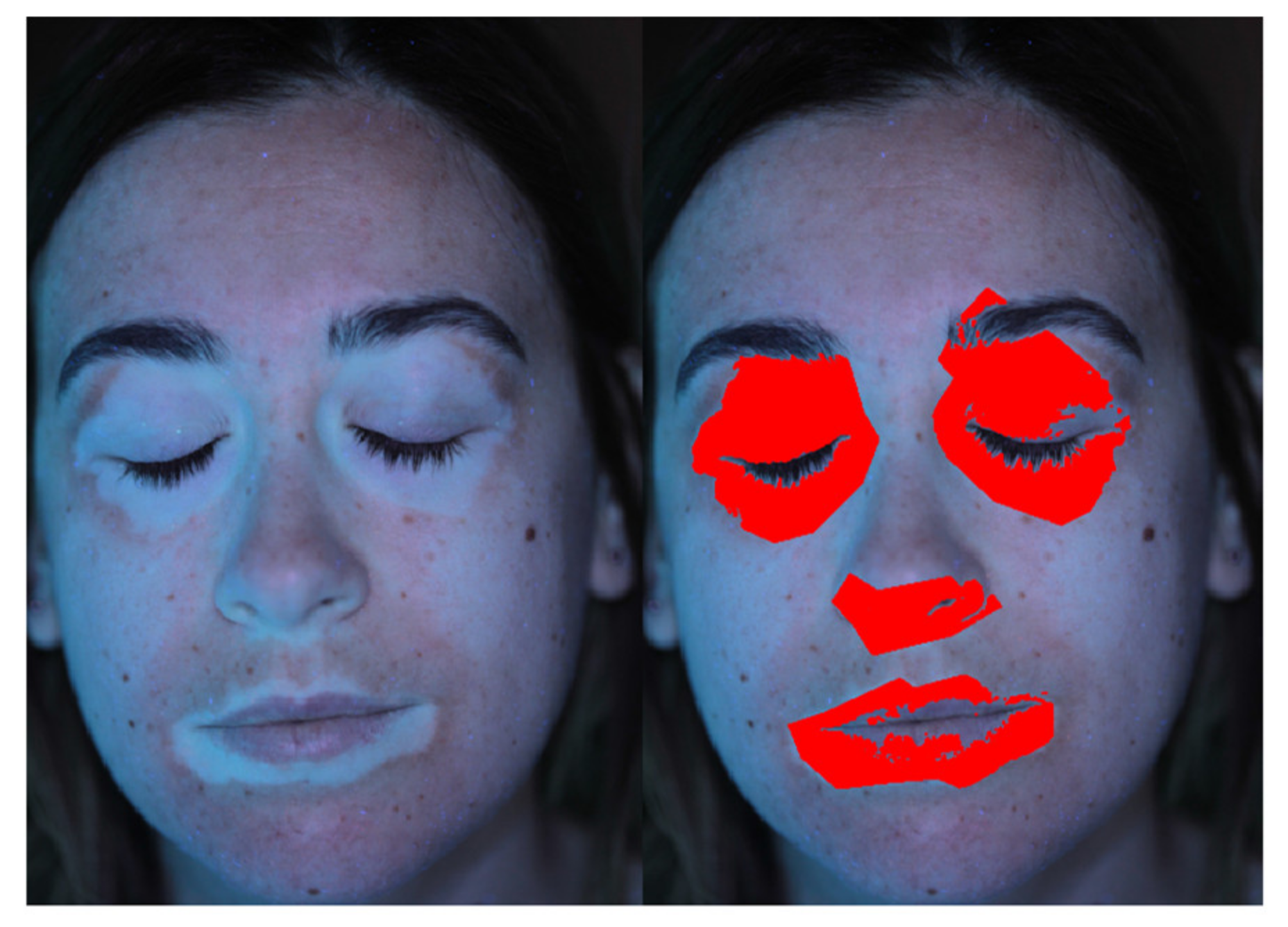
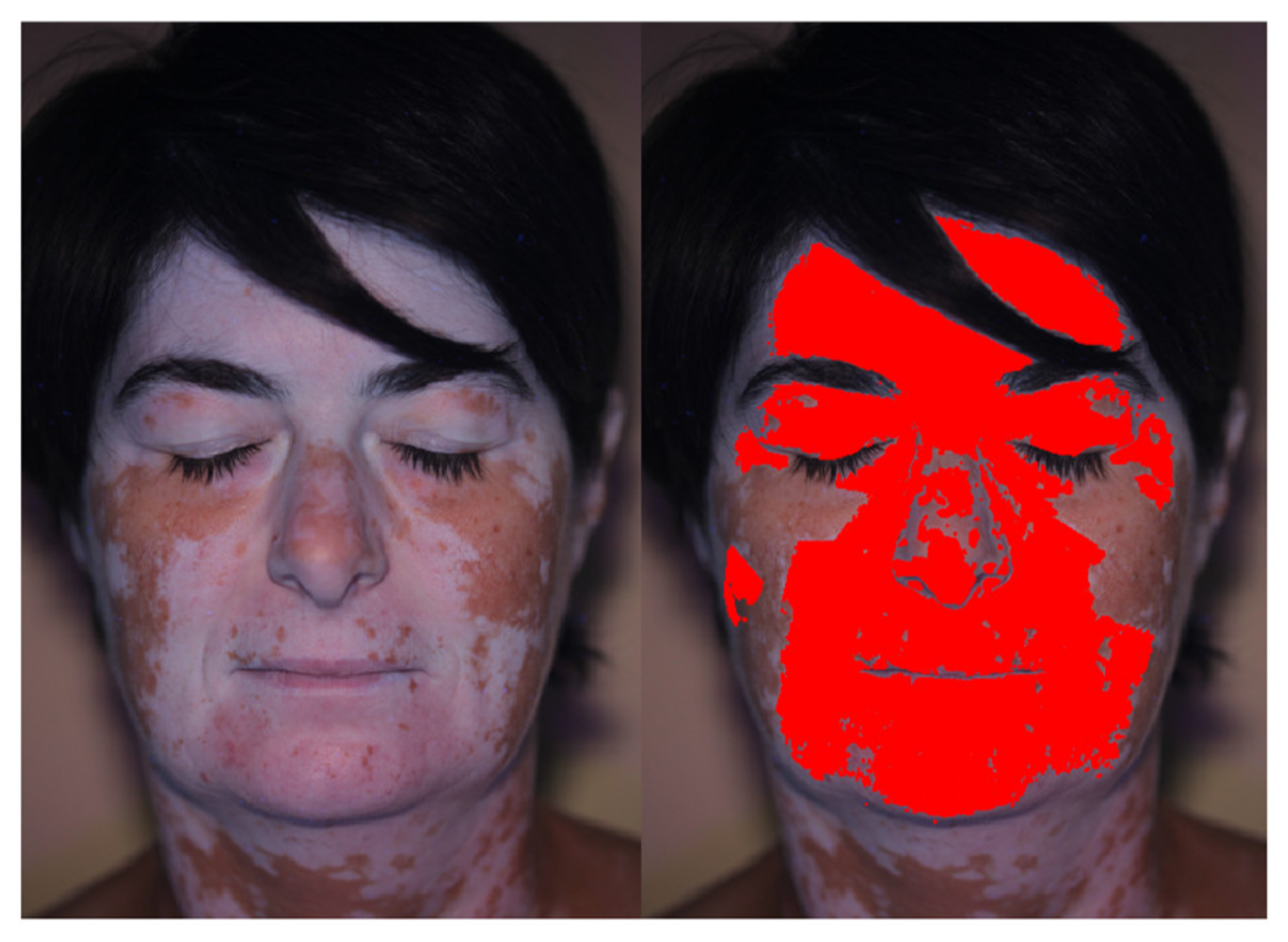
2.3. Add and Edit Patches
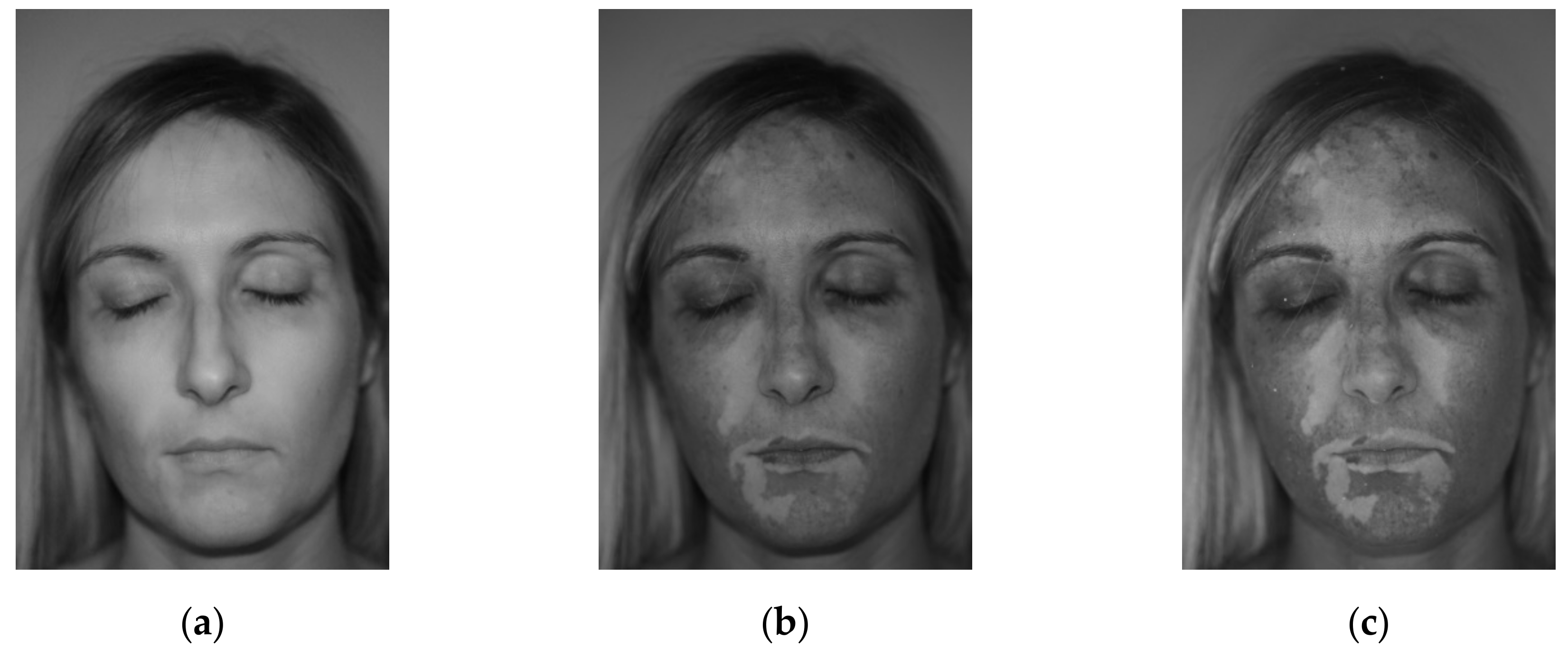
2.4. Image Filters
2.5. Elaboration
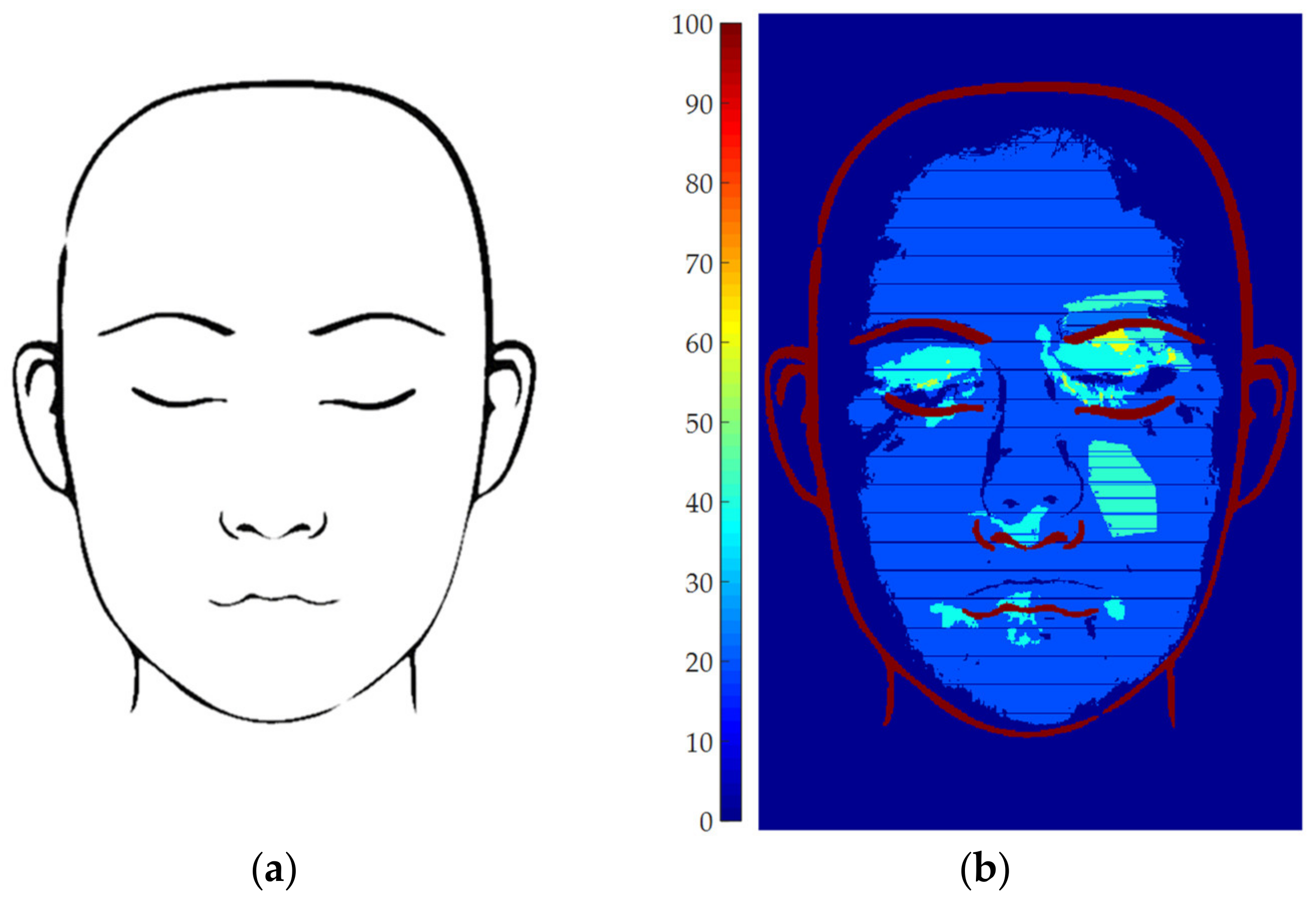
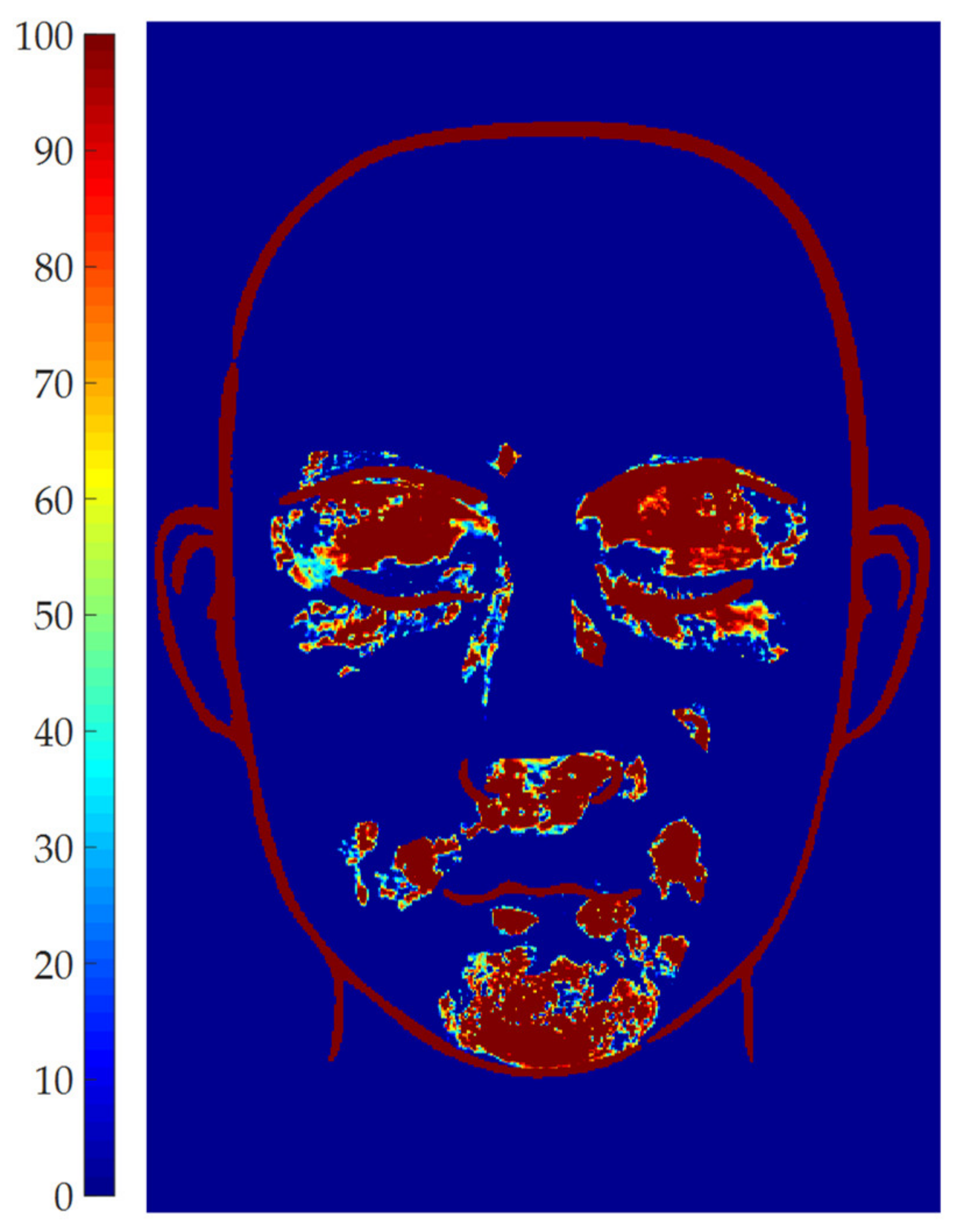
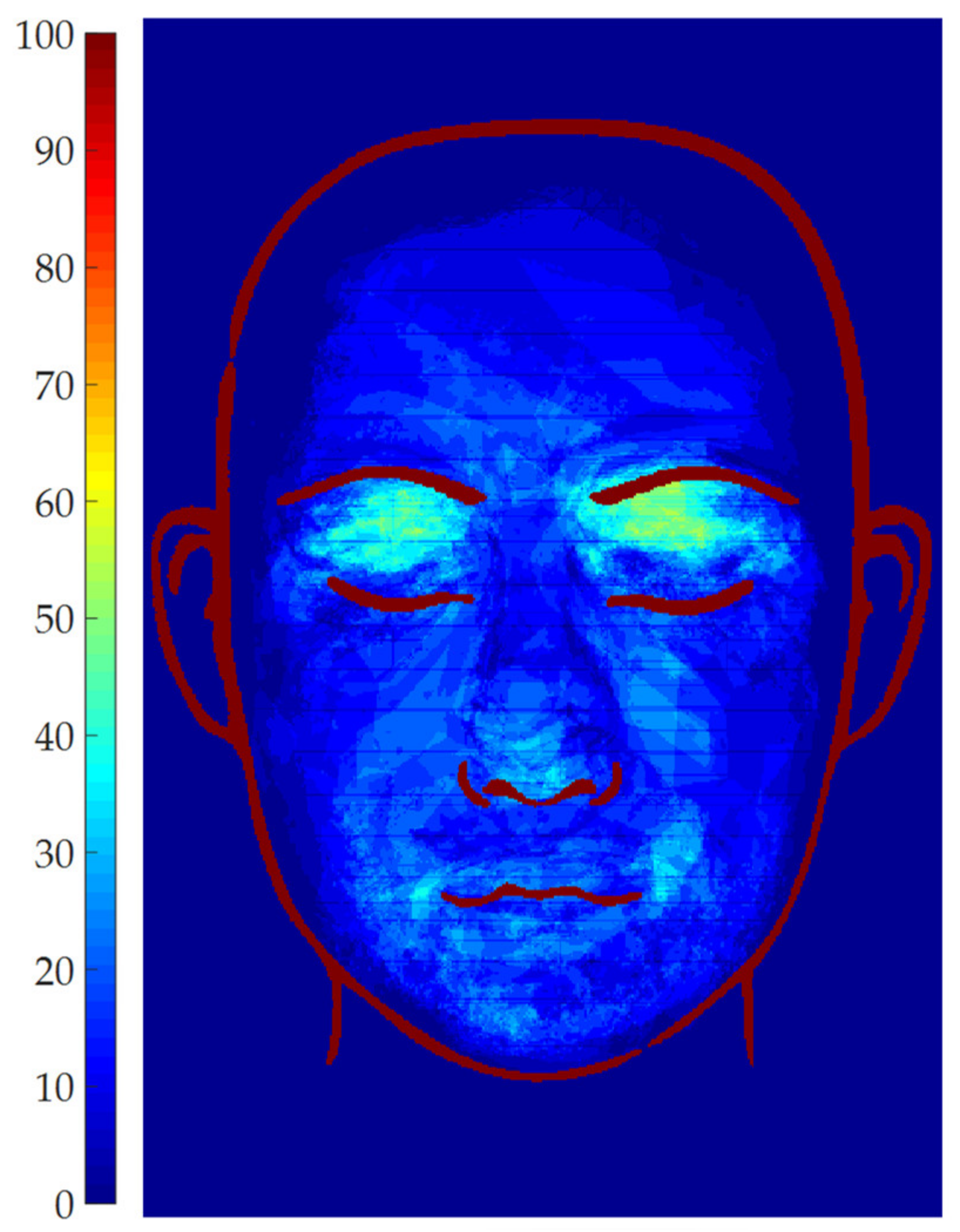
2.6. Results Representation
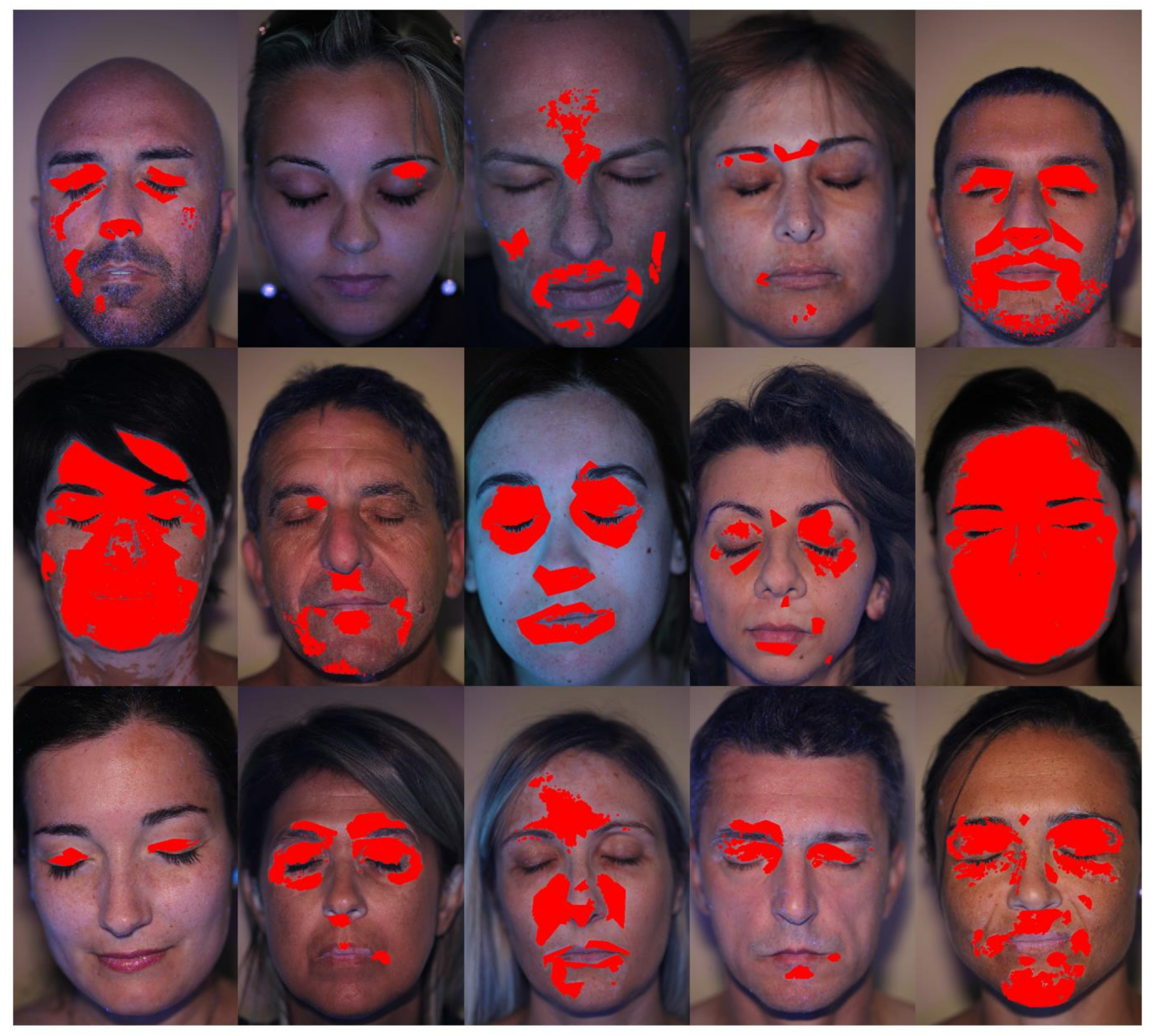
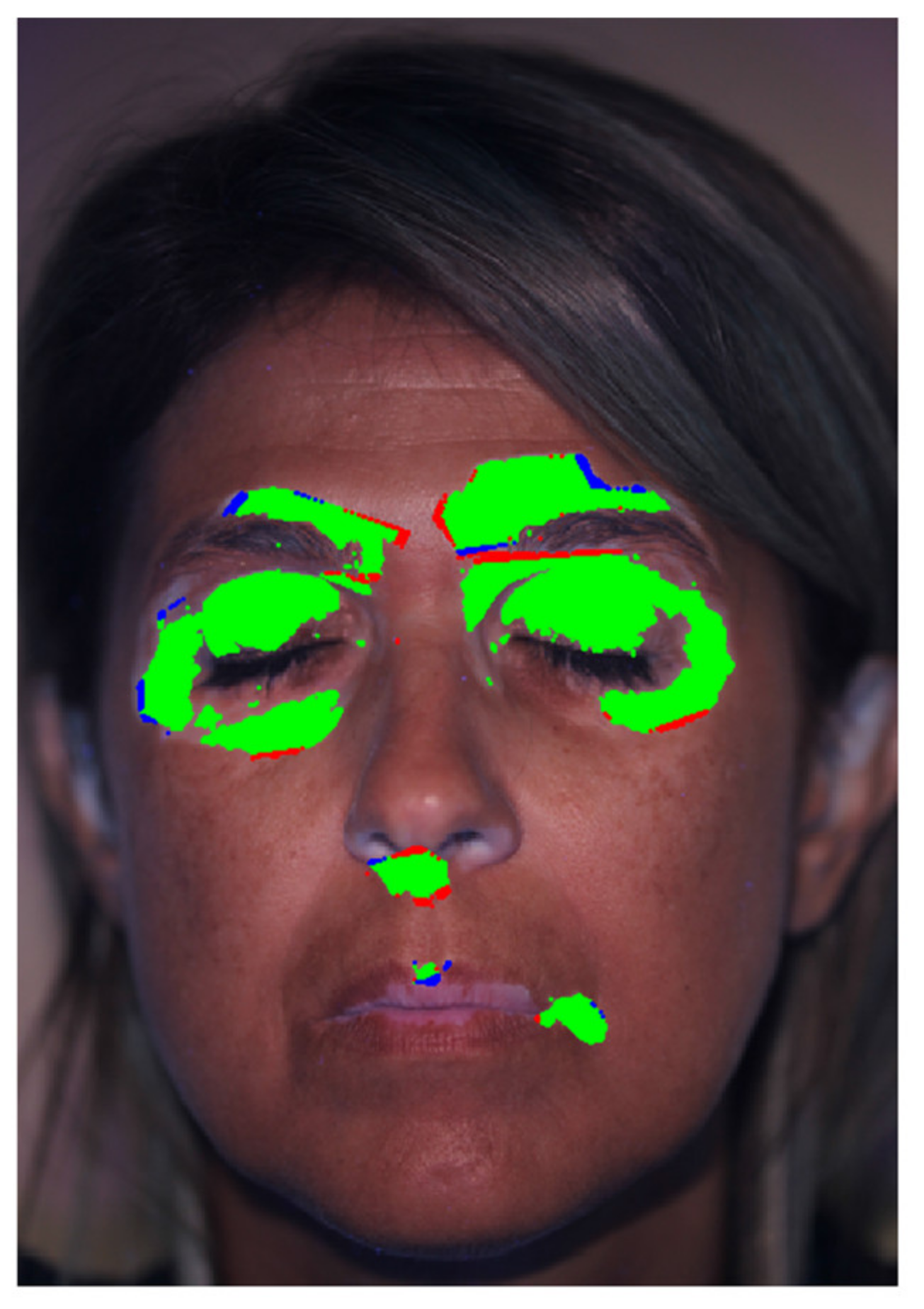
3. Results and Discussion
3.1. Parameters Setup
3.2. Patients set Elaboration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benzekri, L.; Hmamouchi, I.; Gauthier, Y. Possible patterns of epidermal melanocyte disappearance in nonsegmental vitiligo: A clinicopathological study. Br. J. Dermatol. 2014, 172, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Raam, L.; Kaleviste, E.; Šunina, M.; Vaher, H.; Saare, M.; Prans, E.; Pihlap, M.; Abram, K.; Karelson, M.; Peterson, P.; et al. Lymphoid Stress Surveillance Response Contributes to Vitiligo Pathogenesis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Khurrum, H.; Alghamdi, K.M. Prepubertal and postpubertal vitiligo: A multivariate comparative study in 375 patients. An. Bras. Dermatol. 2017, 92, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Solak, B.; Dikicier, B.S.; Erdem, T.; Cosansu, N.C. Effects of age of onset on disease characteristics in non-segmental vitiligo. Int. J. Dermatol. 2017, 56, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, L.; Colucci, R.; Cammi, A.; Dragoni, F.; Moretti, S. Adult Onset Vitiligo: Multivariate Analysis Suggests the Need for a Thyroid Screening. BioMed Res. Int. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ren, Y.; Di, D.; Zhu, Q.; Luo, G. Segmental vitiligo treated by fire needle therapy: A case series. Eur. J. Dermatol. 2017, 28, 118–119. [Google Scholar]
- Jha, A.K.; Prasad, S.; Sinha, R. Bimatoprost ophthalmic solution in facial vitiligo. J. Cosmet. Dermatol. 2017, 17, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Lee, R.W. 365-nm narrowband Wood’s lamp for vitiligo and hypopigmentation disorders. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Klatte, J.L.; van der Beek, N.; Kemperman, P.M. 100 years of Wood’s lamp revised. Eur. Acad. Dermatol. Venereol. 2015, 29, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Gawkrodger, D.; Ormerod, A.; Shaw, L.; Mauri-Sole, I.; Whitton, M.; Watts, M.; Anstey, A.; Ingham, J.; Young, K. Guideline for the diagnosis and management of vitiligo. Br. J. Dermatol. 2008, 159, 1051–1076. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Jung, Y.S.; Jung, H.M.; Park, J.H.; Hann, S.-K. Classification of facial vitiligo: A cluster analysis of 473 patients. Pigment. Cell Melanoma Res. 2018, 31, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.P.D.S.; Hertz, A.; Luzio, P.; Paludo, P.; Azulay-Abulafia, L. Clinical and epidemiological characteristics of childhood vitiligo: A study of 701 patients from Brazil. Int. J. Dermatol. 2019, 59, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, K.; Kumar, A.; Taïeb, A.; Ezzedine, K. Assessment methods for the evaluation of vitiligo. J. Eur. Acad. Dermatol. Venereol. 2012, 26. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Okamura, K.; Araki, Y.; Suzuki, M.; Tanaka, T.; Abe, Y.; Nakano, S.; Yoshizawa, J.; Hozumi, Y.; Inoie, M.; et al. A novel three dimensional imaging method for the measurement of area in vitiligo and chemical leukoderma. J. Dermatol. Sci. 2016, 84, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.; Hussein, S.H.; Nugroho, H.; Fadzil, M.H.A. Objective assessment of vitiligo with a computerised digital imaging analysis system. Australas. J. Dermatol. 2014, 56, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, H.; Fadzil, M.H.A.; Shamsudin, N.; Hussein, S.H. Computerised image analysis of vitiligo lesion: Evaluation using manually defined lesion areas. Ski. Res. Technol. 2012, 19, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Low, M.; Raina, P. Automating Vitiligo Skin Lesion Segmentation Using Convolutional Neural Networks. Electr. Eng. Syst. Sci. 2019, 4. [Google Scholar]
- Bae, Y.; Nelson, J.S.; Jung, B. Multimodal facial color imaging modality for objective analysis of skin lesions. J. Biomed. Opt. 2009, 13, 064007. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, P.; Fiaschi, M.; Menchini, G. Semi-Automatic Tool for Vitiligo Detection and Analysis. J. Imaging 2020, 6, 14. https://doi.org/10.3390/jimaging6030014
Neri P, Fiaschi M, Menchini G. Semi-Automatic Tool for Vitiligo Detection and Analysis. Journal of Imaging. 2020; 6(3):14. https://doi.org/10.3390/jimaging6030014
Chicago/Turabian StyleNeri, Paolo, Michela Fiaschi, and Giovanni Menchini. 2020. "Semi-Automatic Tool for Vitiligo Detection and Analysis" Journal of Imaging 6, no. 3: 14. https://doi.org/10.3390/jimaging6030014
APA StyleNeri, P., Fiaschi, M., & Menchini, G. (2020). Semi-Automatic Tool for Vitiligo Detection and Analysis. Journal of Imaging, 6(3), 14. https://doi.org/10.3390/jimaging6030014