Photoacoustic Ophthalmoscopy: Principle, Application, and Future Directions
Abstract
1. Introduction
2. Physical Principle of Photoacoustic Imaging
3. Requirement for Ocular Imaging
3.1. Safety Evaluation and Acquisition Speed
3.2. Photoacoustic Quantification
3.2.1. Photoacoustic Amplitudes
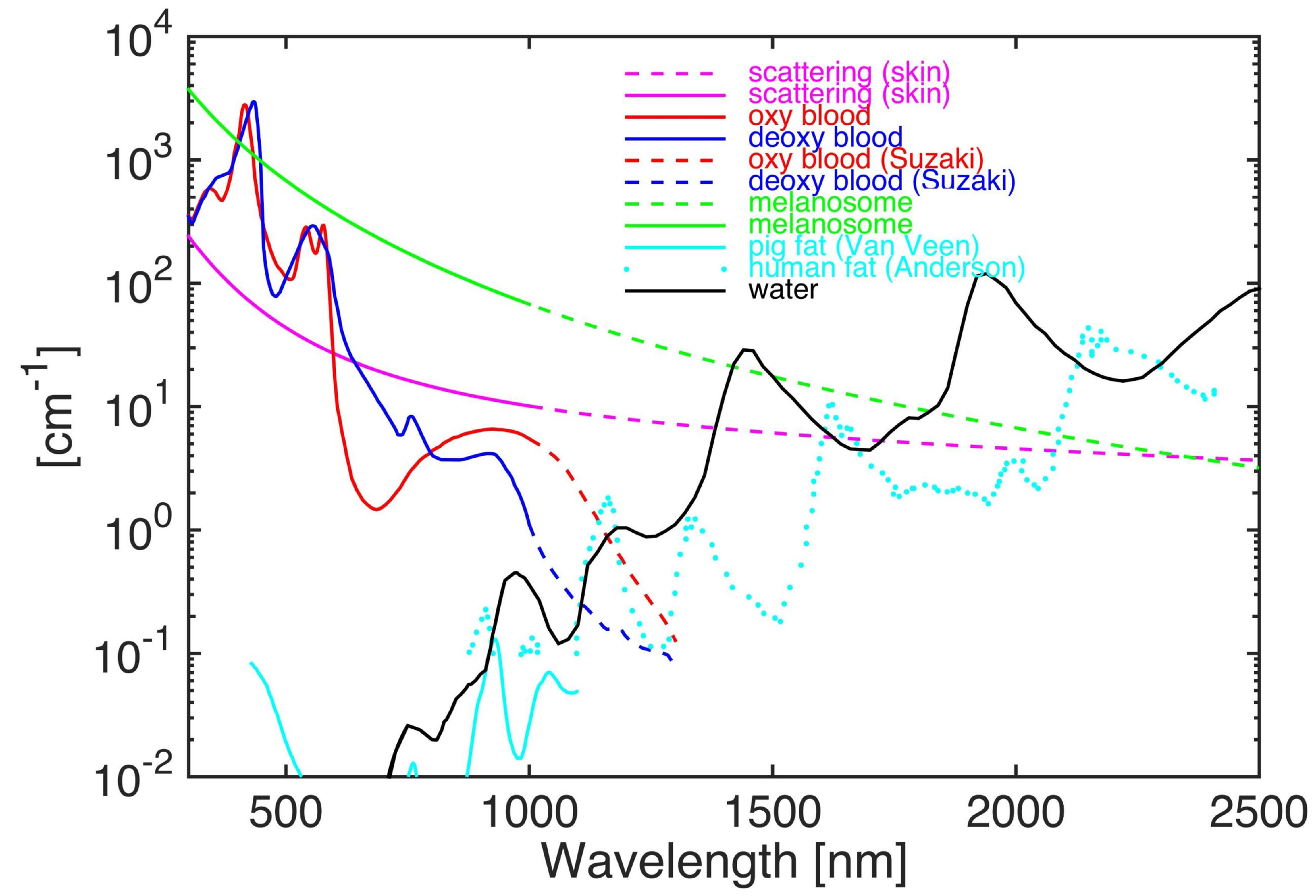
3.2.2. Photoacoustic Contrast
3.2.3. Oxygen Saturation (SO2)
3.3. Imaging Resolution of Photoacoustic Ophthalmoscopy
3.4. Scanning Mode of Photoacoustic Ophthalmoscopy
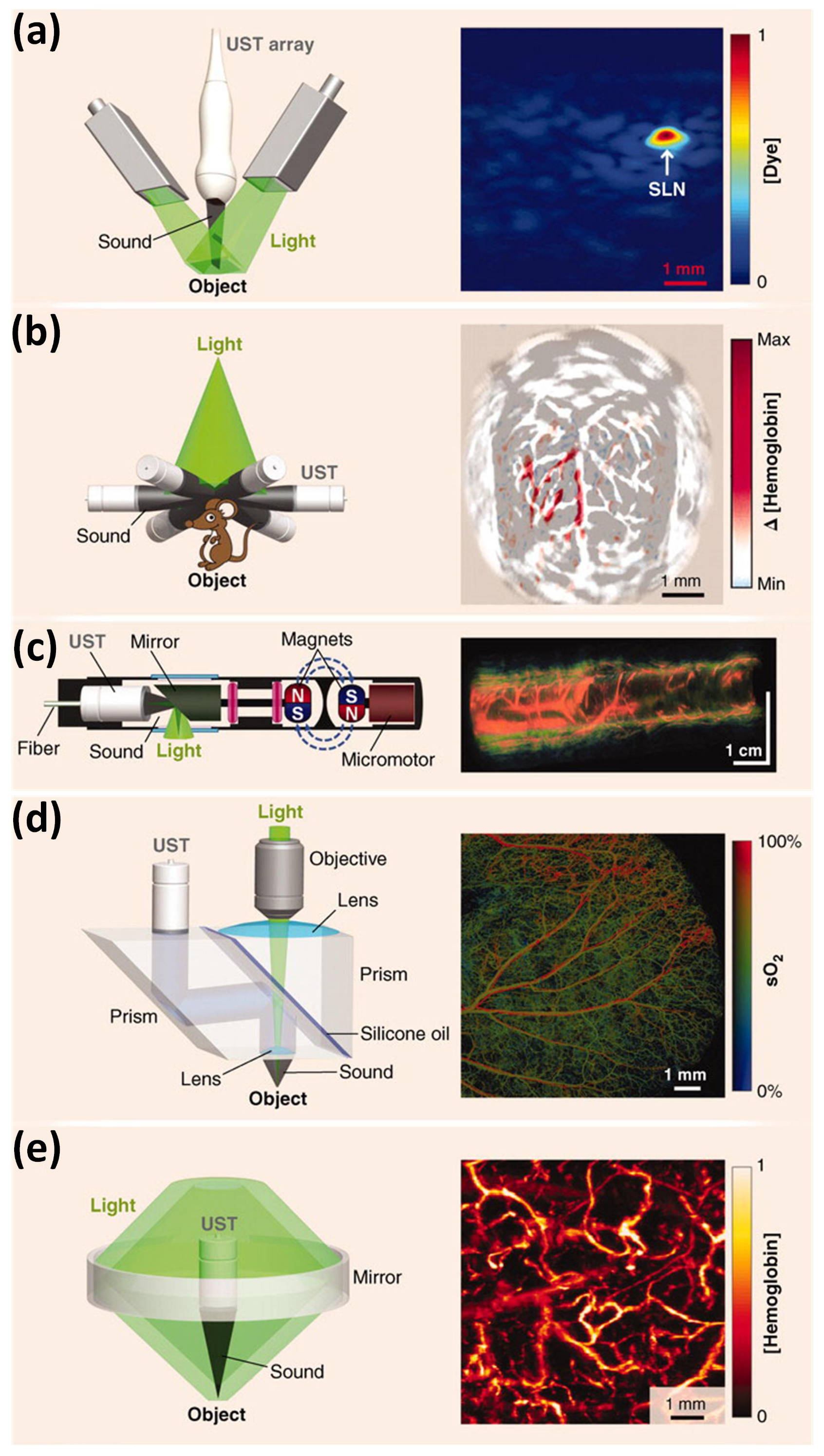
3.4.1. Mechanical Scanning
3.4.2. Optical Scanning
4. Contrast Agents
5. Specific Application of Photoacoustic Microscopy for Imaging of Retinal Diseases in Rodents
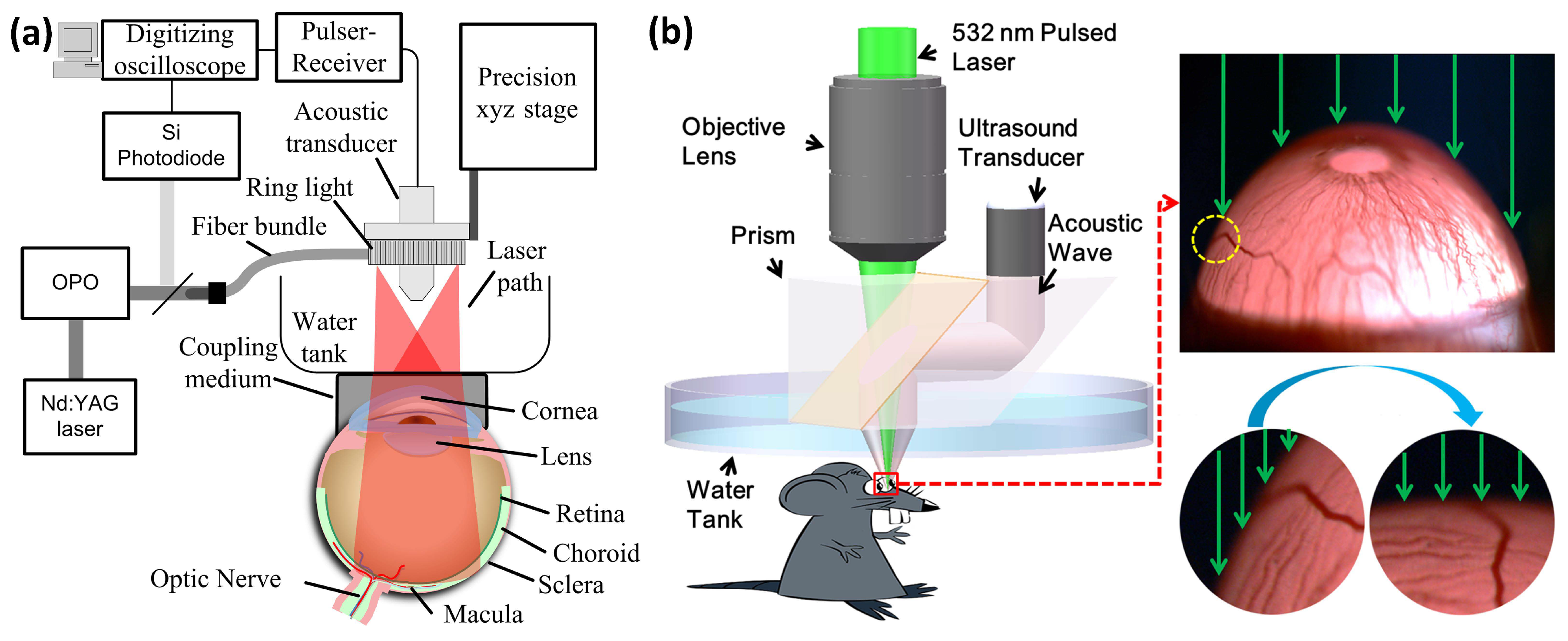
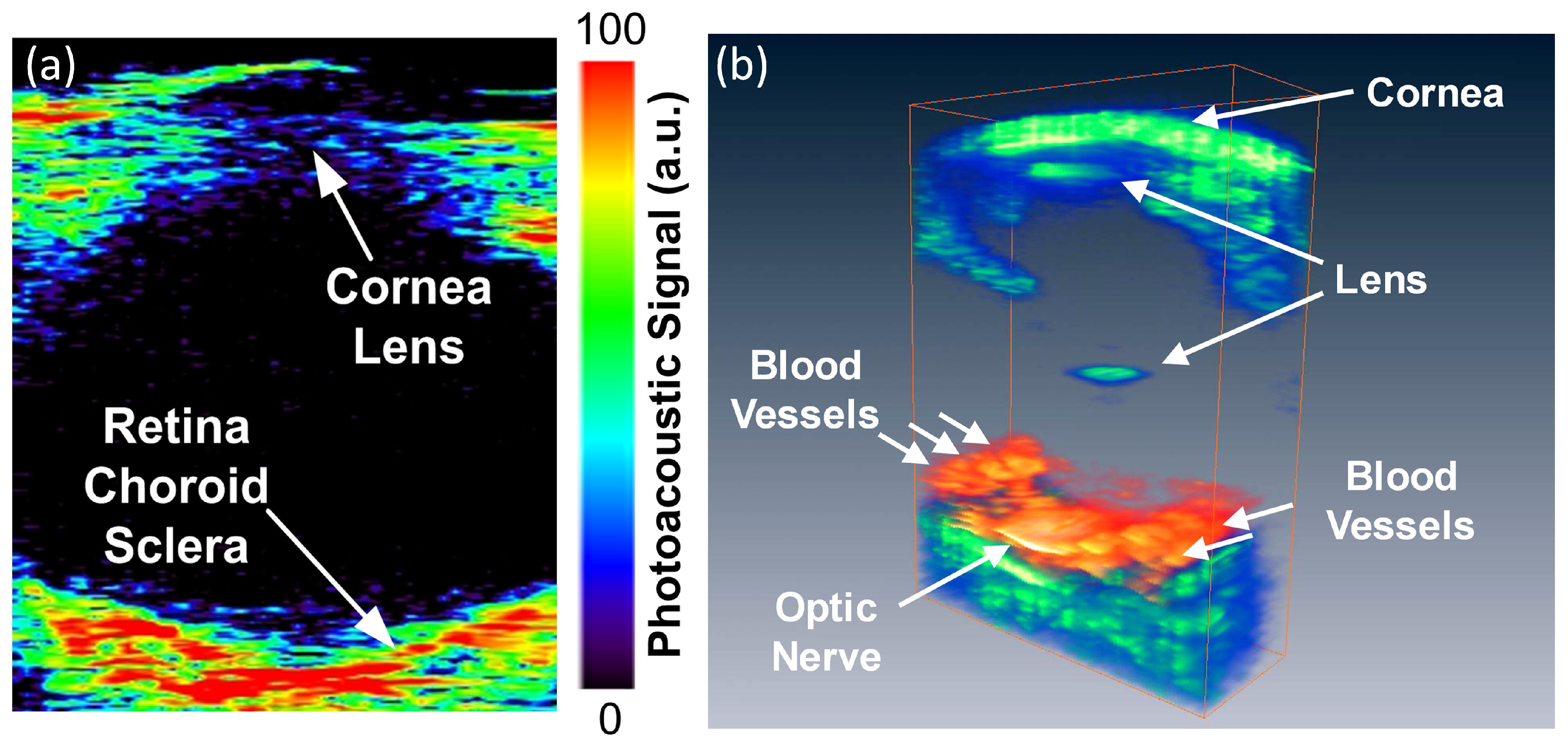
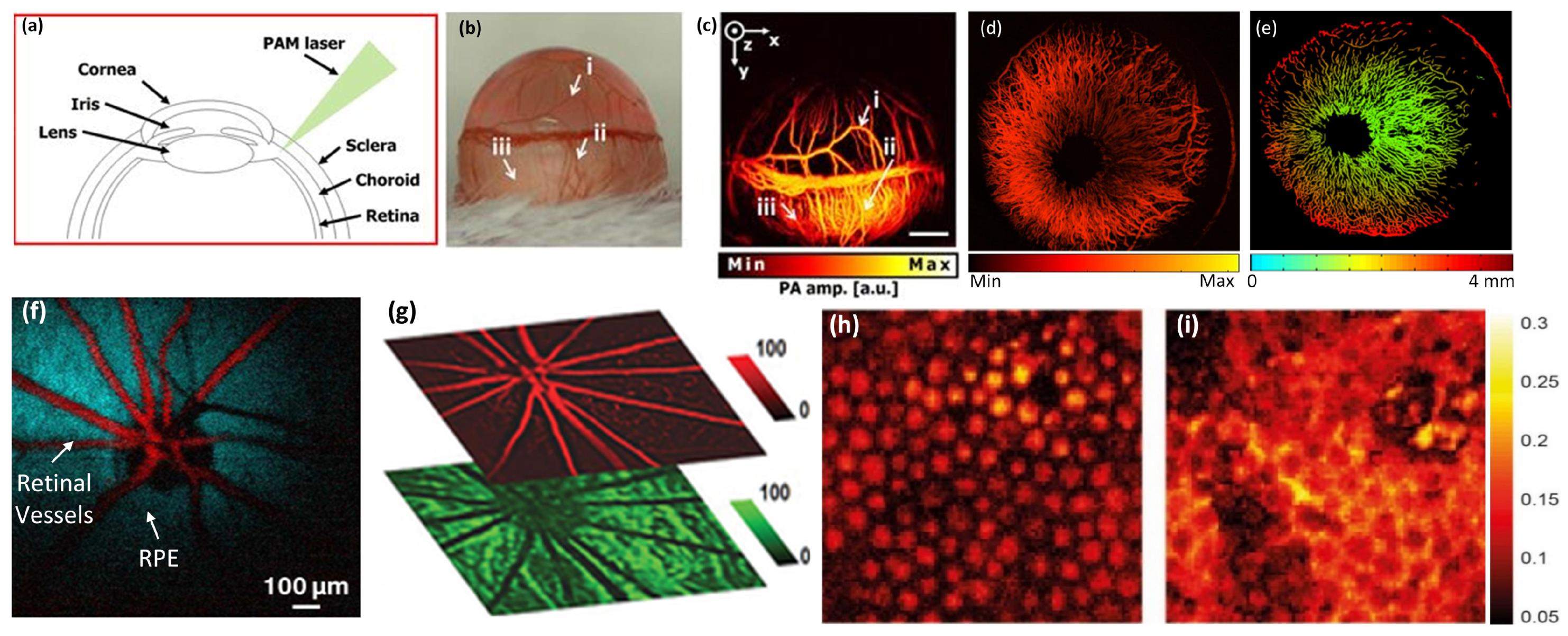
5.1. Anterior PA Imaging of the Eye
5.2. Retinal, Choroidal Vessels, Iris, Limbal Blood Vessels, and RPE
5.3. Corneal Neovascularization
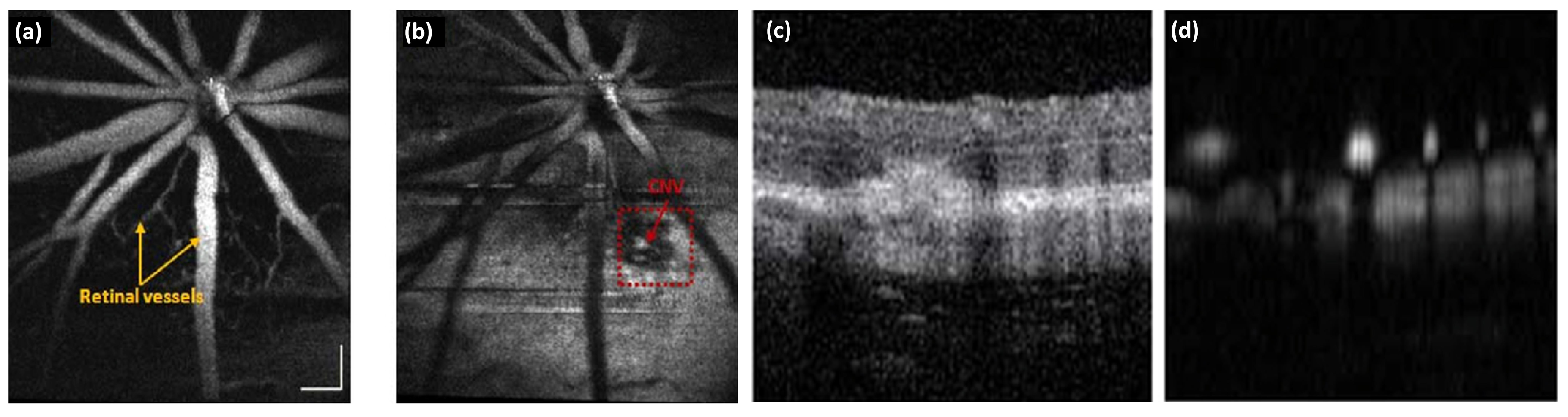
5.4. Choroidal Neovascularization
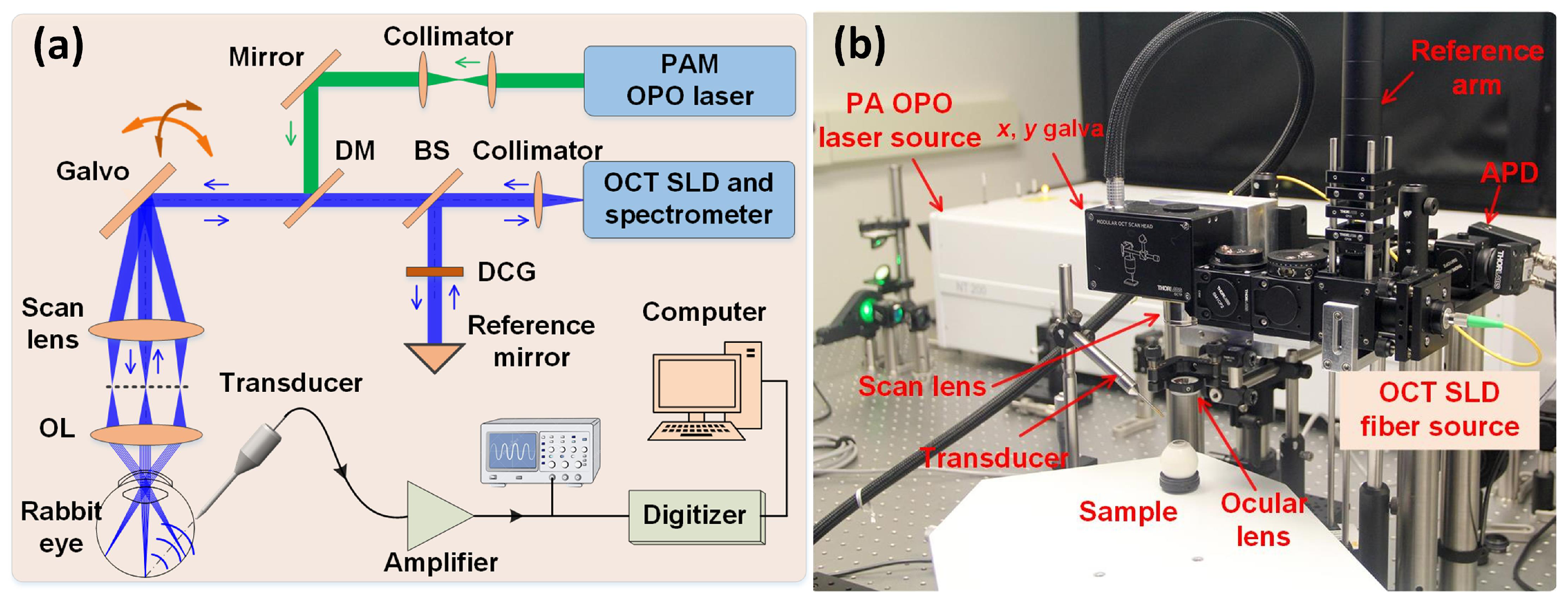
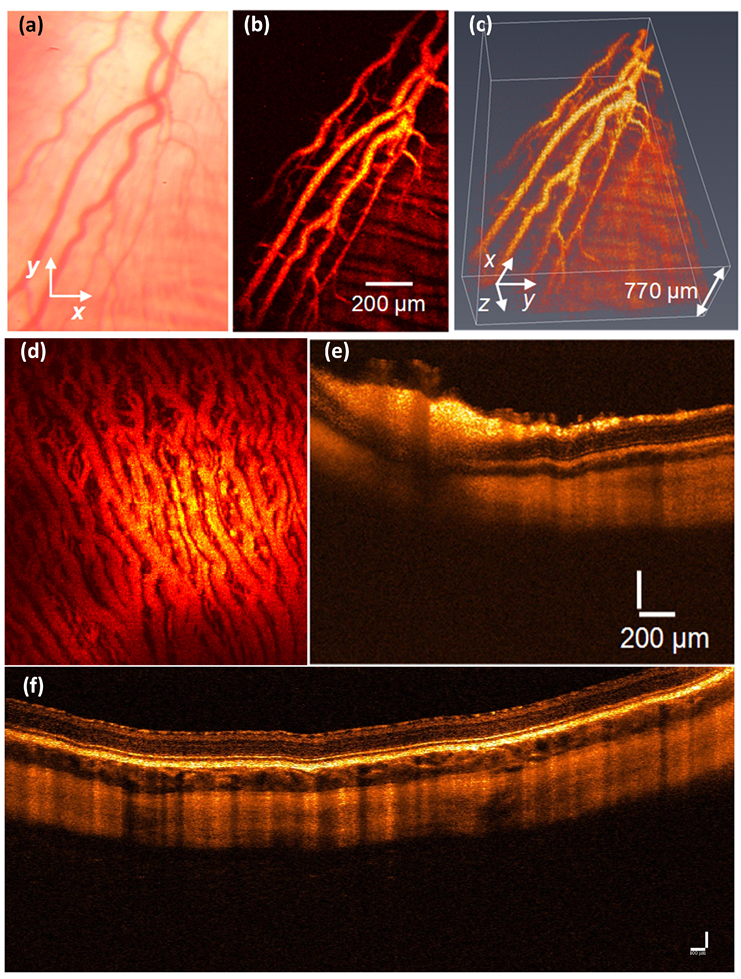
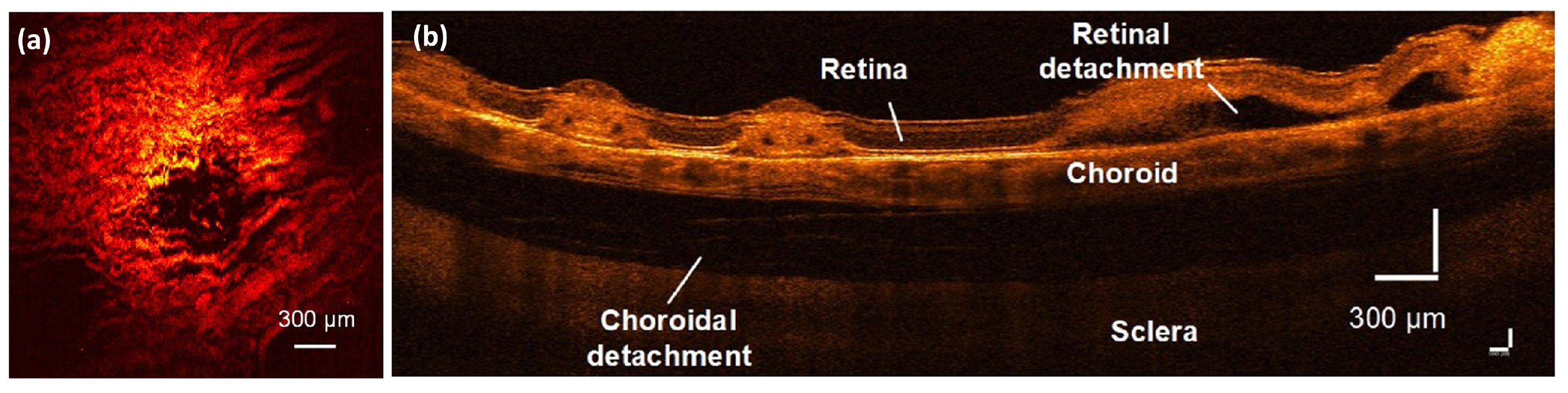
5.5. Retinal and Choroidal PAM Imaging in Larger Animal
5.6. Laser-Induced Burn in Choroidal Vessels
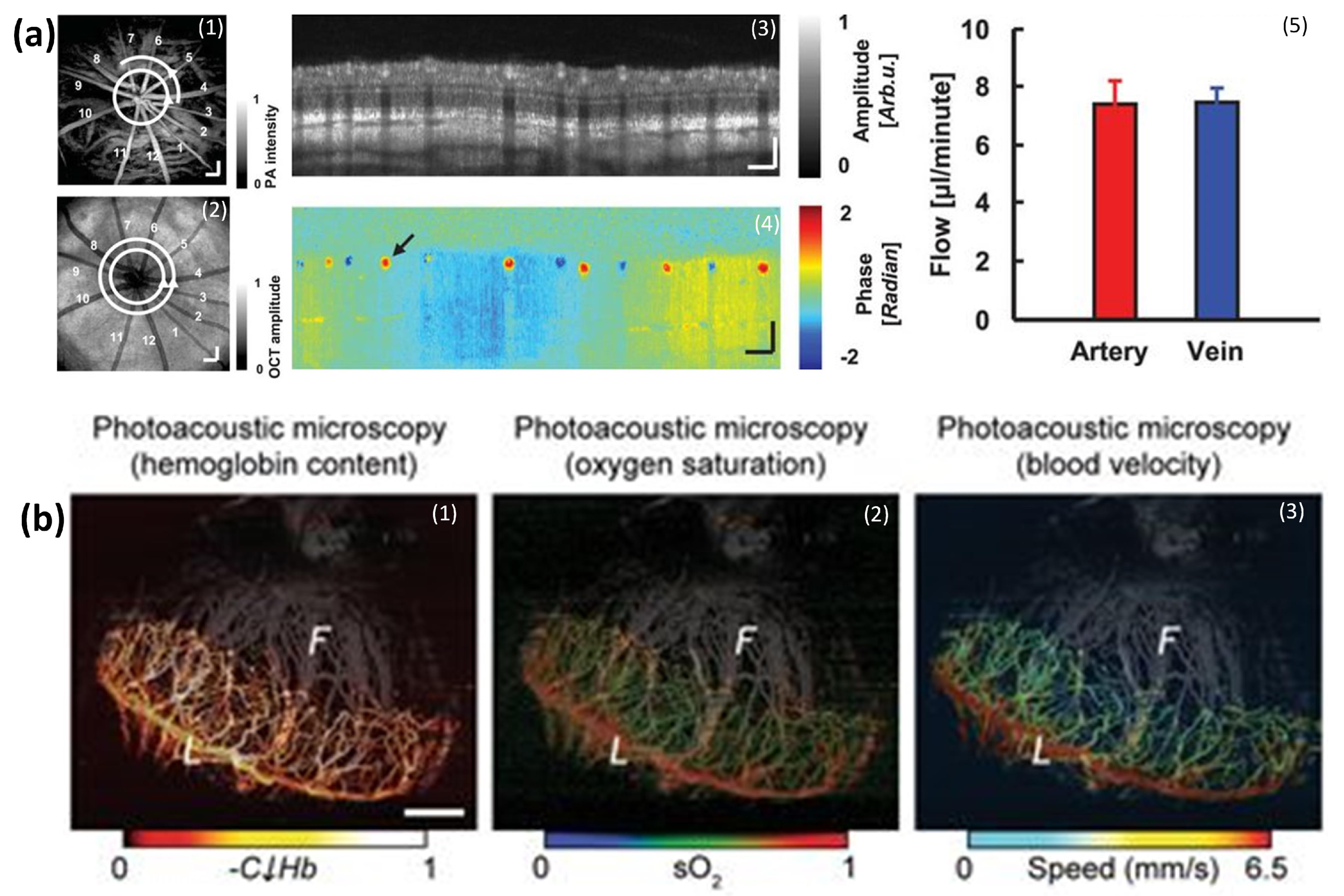
5.7. SO2 Measurements
5.8. Pre-Clinical
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Chan, T.; Friedman, D.S.; Bradley, C.; Massof, R. Estimates of incidence and prevalence of visual impairment, low vision, and blindness in the United States. JAMA Ophthalmol. 2018, 136, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; White, R.A.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; Resnikoff, S. Global prevalence of vision impairment and blindness: Magnitude and temporal trends, 1990–2010. Ophthalmology 2013, 120, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Coscas, F.; Glacet-Bernard, A.; Miere, A.; Caillaux, V.; Uzzan, J.; Lupidi, M.; Coscas, G.; Souied, E.H. Optical Coherence Tomography Angiography in Retinal Vein Occlusion: Evaluation of Superficial and Deep Capillary Plexa. Am. J. Ophthalmol. 2016, 161, 160–171.e2. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.S.; Oliver, S.C.; Coffee, R.E.; Hubschman, J.-P.; Schwartz, S.D. Ultra wide-field angiographic characteristics of branch retinal and hemicentral retinal vein occlusion. Ophthalmology 2010, 117, 780–784. [Google Scholar] [CrossRef] [PubMed]
- McAllister, I.L.; Yu, D.-Y.; Vijayasekaran, S.; Barry, C.; Constable, I. Induced chorioretinal venous anastomosis in experimental retinal branch vein occlusion. Br. J. Ophthalmol. 1992, 76, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, B.T.; Shu, X.; Liu, Q.; Liu, W.; Chen, S.; Beckmann, L.; Fawzi, A.A.; Zhang, H.F. Optical coherence tomography angiography of retinal vascular occlusions produced by imaging-guided laser photocoagulation. Biomed. Opt. Expess 2017, 8, 3571–3582. [Google Scholar] [CrossRef] [PubMed]
- Dysli, C.; Wolf, S.; Berezin, M.Y.; Sauer, L.; Hammer, M.; Zinkernagel, M.S. Fluorescence lifetime imaging ophthalmoscopy. Prog. Retin. Ey. Res. 2017, 60, 120–143. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wei, Q.; Liu, W.; Liu, T.; Yi, J.; Sheibani, N.; Fawzi, A.A.; Linsenmeier, R.A.; Jiao, S.; Zhang, H.F. A combined method to quantify the retinal metabolic rate of oxygen using photoacoustic ophthalmoscopy and optical coherence tomography. Sci. Rep. 2014, 4, 6525. [Google Scholar] [CrossRef]
- Hennen, S.N.; Xing, W.; Shui, Y.-B.; Zhou, Y.; Kalishman, J.; Andrews-Kaminsky, L.B.; Kass, M.A.; Beebe, D.C.; Maslov, K.I.; Wang, L.V. Photoacoustic tomography imaging and estimation of oxygen saturation of hemoglobin in ocular tissue of rabbits. Exp. Eye. Res. 2015, 138, 153–158. [Google Scholar] [CrossRef]
- Ning, B.; Kennedy, M.J.; Dixon, A.J.; Sun, N.; Cao, R.; Soetikno, B.T.; Chen, R.; Zhou, Q.; Shung, K.K.; Hossack, J.A. Simultaneous photoacoustic microscopy of microvascular anatomy, oxygen saturation, and blood flow. Opt. Lett. 2015, 40, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Maslov, K.I.; Zhang, Y.; Xia, Y.; Wang, L.V. Label-free oxygen-metabolic photoacoustic microscopy in vivo. J. Biomed. Opt. 2011, 16, 076003. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, S.; Xing, D. In vivo detection of hemoglobin oxygen saturation and carboxyhemoglobin saturation with multiwavelength photoacoustic microscopy. Opt. Lett. 2012, 37, 3414–3416. [Google Scholar] [CrossRef] [PubMed]
- Taruttis, A.; Ntziachristos, V. Advances in real-time multispectral optoacoustic imaging and its applications. Nat. Photon. 2015, 9, 219. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Kim, H.; Manivasagan, P.; Jung, M.J.; Kim, S.W.; Oh, J.; Kang, H.W. Doxorubicin-fucoidan-gold nanoparticles composite for dualchemo-photothermal treatment on eye tumors. Oncotarget 2017, 8, 113719–113733. [Google Scholar]
- Nguyen, V.P.; Park, S.; Oh, J.; Wook Kang, H. Biocompatible astaxanthin as novel contrast agent for biomedical imaging. J. Biophotonics 2016. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Oh, J.; Park, S.; Wook Kang, H. Feasibility of photoacoustic evaluations on dual-thermal treatment of ex vivo bladder tumors. J. Biophotonics 2016. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Kim, J.; Ha, K.-L.; Oh, J.; Kang, H.W. Feasibility study on photoacoustic guidance for high-intensity focused ultrasound-induced hemostasis. J. Biomed. Opt. 2014, 19, 105010. [Google Scholar] [CrossRef] [PubMed]
- de la Zerda, A.; Paulus, Y.M.; Teed, R.; Bodapati, S.; Dollberg, Y.; Khuri-Yakub, B.T.; Blumenkranz, M.S.; Moshfeghi, D.M.; Gambhir, S.S. Photoacoustic ocular imaging. Opt. Lett. 2010, 35, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. photon. 2009, 3, 503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Puliafito, C.A.; Jiao, S. Photoacoustic ophthalmoscopy for in vivo retinal imaging: Current status and prospects. Ophthalmic Surg. Lasers Imaging Retin. 2011, 42, S106–S115. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhang, W.; Nguyen, V.P.; Wang, X.; Paulus, Y.M. Novel Photoacoustic Microscopy and Optical Coherence Tomography Dual-modality Chorioretinal Imaging in Living Rabbit Eyes. J. Vis. Exp. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; O’Neill, B. Imaging high-intensity focused ultrasound-induced tissue denaturation by multispectral photoacoustic method: An ex vivo study. Appl. Opt. 2013, 52, 1764–1770. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.F. Photoacoustic imaging of the eye: A mini review. Photoacoustics 2016, 4, 112–123. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Q.; Paulus, Y.M. New Frontiers in Retinal Imaging. Int. J. Ophthalmic Res. 2016, 2, 148–158. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Kim, S.W.; Kim, H.; Kim, H.; Seok, K.H.; Jung, M.J.; Ahn, Y.-c.; Kang, H.W. Biocompatible astaxanthin as a novel marine-oriented agent for dual chemo-photothermal therapy. PLoS ONE 2017, 12, e0174687. [Google Scholar] [CrossRef]
- Keswani, R.K.; Tian, C.; Peryea, T.; Girish, G.; Wang, X.; Rosania, G.R. Repositioning Clofazimine as a Macrophage-Targeting Photoacoustic Contrast Agent. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Tian, C.; Qian, W.; Shao, X.; Xie, Z.; Cheng, X.; Liu, S.; Cheng, Q.; Liu, B.; Wang, X. Plasmonic nanoparticles with quantitatively controlled bioconjugation for photoacoustic imaging of live cancer cells. Adv. Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, X.; Liu, Q.; Paulus, Y.M. Photoacoustic Imaging in Ophthalmology. Int. J. Opthalmol. Eye Sci. 2015, 8, 126–132. [Google Scholar] [CrossRef]
- Jiao, S.; Jiang, M.; Hu, J.; Fawzi, A.; Zhou, Q.; Shung, K.K.; Puliafito, C.A.; Zhang, H.F. Photoacoustic ophthalmoscopy for in vivo retinal imaging. Opt. Epxress 2010, 18, 3967–3972. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H.; Kong, F.; Chen, Y.C.; Lloyd, H.O.; Kim, H.H.; Cannata, J.M.; Shung, K.K.; Coleman, D.J. High-Resolution Photoacoustic Imaging of Ocular Tissues. Ultrasound. Med. Biol. 2010, 36, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Hennen, S.N.; Xing, W.; Shui, Y.-B.; Zhou, Y.; Kalishman, J.; Andrews-Kaminsky, L.B.; Kass, M.A.; Beebe, D.C.; Maslov, K.I.; Wang, L.V. Photoacoustic tomography imaging and estimation of oxygen saturation of hemoglobin in ocular tissue of rabbits. Exp. Eye Res. 2015, 138, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rao, B.; Maslov, K.; Wang, L.V. Label-free photoacoustic ophthalmic angiography. Opt. Lett. 2010, 35, 1–3. [Google Scholar] [CrossRef]
- Generic tissue optical properties. Available online: https://omlc.org/news/feb15/generic_optics/index.html. (accessed on 5 December 2018).
- Visualsonic Inc. Vevo LAZR-Advanced multimodal imaging platform. Available online: https://www.visualsonics.com/sites/default/files/Vevo%20LAZR%20Brochure.pdf (accessed on 5 December 2018).
- Tian, C.; Zhang, W.; Nguyen, V.P.; Huang, Z.; Wang, X.; Paulus, Y.M. Integrated photoacoustic microscopy, optical coherence tomography, and fluorescence microscopy for multimodal chorioretinal imaging. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2018, San Francisco, CA, USA, 28 January–1 February 2018; SPIE: Bellingham, WA, USA, 2018; p. 104945U. [Google Scholar]
- Kang, J.; Kim, D.; Wang, J.; Han, Y.; Zuidema, J.M.; Hariri, A.; Park, J.H.; Jokerst, J.V.; Sailor, M.J. Enhanced Performance of a Molecular Photoacoustic Imaging Agent by Encapsulation in Mesoporous Silicon Nanoparticles. Adv. Mater. 2018, 1800512. [Google Scholar] [CrossRef]
- Hariri, A.; Wang, J.; Kim, Y.; Jhunjhunwala, A.; Chao, D.L.; Jokerst, J.V. In vivo photoacoustic imaging of chorioretinal oxygen gradients. J. Biomed. Opt. 2018, 23, 036005. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, W.; Mordovanakis, A.; Wang, X.; Paulus, Y.M. Noninvasive chorioretinal imaging in living rabbits using integrated photoacoustic microscopy and optical coherence tomography. Opt. Epxress 2017, 25, 15947–15955. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Oh, J.; Park, S.; Wook Kang, H. Feasibility of photoacoustic evaluations on dual-thermal treatment of ex vivo bladder tumors. J. Biophotonics 2017, 10, 577–588. [Google Scholar] [CrossRef]
- Song, K.H.; Stein, E.W.; Margenthaler, J.A.; Wang, L.V. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J. Biomed. Opt. 2008, 13, 054033. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-R.; Hovhannisyan, V.A.; Chao, Y.-C.; Chao, S.-L.; Chiang, S.-J.; Lin, S.-J.; Dong, C.-Y.; Chen, C.-C. Multiple release kinetics of targeted drug from gold nanorod embedded polyelectrolyte conjugates induced by near-infrared laser irradiation. J. Am. Chem. Soc. 2010, 132, 14163–14171. [Google Scholar] [CrossRef] [PubMed]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Delori, F.C.; Webb, R.H.; Sliney, D.H. Maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices. J. Opt. Soc. Am. A 2007, 24, 1250–1265. [Google Scholar] [CrossRef]
- ANSI Z136.1. Available online: https://www.lia.org/store/product/ansi-z1361-2014-safe-use-lasers-electronic-version (accessed on 5 December 2018).
- Robinson, D. The mechanics of human saccadic eye movement. J. Physiol. 1964, 174, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, T.; Wen, R.; Li, Y.; Puliafito, C.A.; Zhang, H.F.; Jiao, S. Optical coherence photoacoustic microscopy for in vivo multimodal retinal imaging. Opt. Lett. 2015, 40, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Song, H.B.; Kim, J.; Lee, B.J.; Managuli, R.; Kim, J.H.; Kim, J.H.; Kim, C. In vivo photoacoustic imaging of anterior ocular vasculature: A random sample consensus approach. Sci. Rep. 2017, 7, 4318. [Google Scholar] [CrossRef] [PubMed]
- Maslov, K.; Stoica, G.; Wang, L.V. In vivo dark-field reflection-mode photoacoustic microscopy. Opt. Lett. 2005, 30, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Wang, L.; Maslov, K.; Wang, L.V. Integrated optical- and acoustic-resolution photoacoustic microscopy based on an optical fiber bundle. Opt. Lett. 2013, 38, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, L.V. Photoacoustic tomography and sensing in biomedicine. Phys. Med. Biol. 2009, 54, R59. [Google Scholar] [CrossRef] [PubMed]
- Maslov, K.; Zhang, H.F.; Hu, S.; Wang, L.V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 2008, 33, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. Fast optical-resolution photoacoustic microscopy using a 2-axis water-proofing MEMS scanner. Sci. Rep. 2015, 5, 7932. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wei, Q.; Liu, T.; Kuai, D.; Zhang, H.F.; Burke, J.M.; Jiao, S. Integrating photoacoustic ophthalmoscopy with scanning laser ophthalmoscopy, optical coherence tomography, and fluorescein angiography for a multimodal retinal imaging platform. J. Biomed. Opt. 2012, 17, 061206. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, Q.; Gao, Q.; Song, W.; Zhang, H.F.; Yuan, X. In vivo blind-deconvolution photoacoustic ophthalmoscopy with total variation regularization. J. Biophotonics 2018, e201700360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Galanzha, E.I.; Shashkov, E.V.; Moon, H.-M.; Zharov, V.P. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat. Nanotechnol. 2009, 4, 688–694. [Google Scholar] [CrossRef]
- de la Zerda, A.; Bodapati, S.; Teed, R.; May, S.Y.; Tabakman, S.M.; Liu, Z.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Family of enhanced photoacoustic imaging agents for high-sensitivity and multiplexing studies in living mice. ACS Nano. 2012, 6, 4694–4701. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, L.; Nie, L.; Sun, X.; Cheng, L.; Wu, C.; Niu, G.; Chen, X.; Liu, Z. Visualization of protease activity in vivo using an activatable photo-acoustic imaging probe based on CuS nanoparticles. Theranostics 2014, 4, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.; Zhou, M.; Song, S.; Huang, Q.; Hazle, J.; Li, C. Copper sulfide nanoparticles as a new class of photoacoustic contrast agent for deep tissue imaging at 1064 nm. Acs. Nano. 2012, 6, 7489–7496. [Google Scholar] [CrossRef]
- Kim, J.B.; Park, K.; Ryu, J.; Lee, J.J.; Lee, M.W.; Cho, H.S.; Nam, H.S.; Park, O.K.; Song, J.W.; Kim, T.S. Intravascular optical imaging of high-risk plaques in vivo by targeting macrophage mannose receptors. Sci. Rep. 2016, 6, 22608. [Google Scholar] [CrossRef]
- Hu, J.; Ortgies, D.H.; Aguliar Torres, R.; Fernández, N.; Porto, L.; Martín Rodríguez, E.; García Solé, J.; Jaque, D.; Alfonso, F.; Rivero, F. Quantum Dots Emitting in the Third Biological Window as Bimodal Contrast Agents for Cardiovascular Imaging. Adv. Funct. Mater. 2017, 27, 1703276. [Google Scholar] [CrossRef]
- Hu, J.; Sanz-Rodríguez, F.; Rivero, F.; Rodríguez, E.M.; Torres, R.A.; Ortgies, D.H.; Solé, J.G.; Alfonso, F.; Jaque, D. Gold nanoshells: Contrast agents for cell imaging by cardiovascular optical coherence tomography. Nano Res. 2018, 11, 676–685. [Google Scholar] [CrossRef]
- Mallidi, S.; Larson, T.; Tam, J.; Joshi, P.P.; Karpiouk, A.; Sokolov, K.; Emelianov, S. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano Lett. 2009, 9, 2825–2831. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Frey, W.; Kim, S.; Homan, K.; Kruizinga, P.; Sokolov, K.; Emelianov, S. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt. Express 2010, 18, 8867–8878. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, X.; Wang, X.; Ku, G.; Gill, K.L.; O'Neal, D.P.; Stoica, G.; Wang, L.V. Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain. Nano Lett. 2004, 4, 1689–1692. [Google Scholar] [CrossRef]
- Qian, W.; Murakami, M.; Ichikawa, Y.; Che, Y. Highly efficient and controllable PEGylation of gold nanoparticles prepared by femtosecond laser ablation in water. J. Phys. Chem. 2011, 115, 23293–23298. [Google Scholar] [CrossRef]
- Liba, O.; SoRelle, E.D.; Sen, D.; de La Zerda, A. Contrast-enhanced optical coherence tomography with picomolar sensitivity for functional in vivo imaging. Sci. Rep. 2016, 6, 23337. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Yamagata, M.; Okamoto, Y.; Akiyama, Y.; Takahashi, H.; Kawano, T.; Katayama, Y.; Niidome, Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Controll. Release 2006, 114, 343–347. [Google Scholar] [CrossRef]
- Li, Z.; Huang, P.; Zhang, X.; Lin, J.; Yang, S.; Liu, B.; Gao, F.; Xi, P.; Ren, Q.; Cui, D. RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy. Mol. Pharm. 2009, 7, 94–104. [Google Scholar]
- Eidelman, S.; Hayes, K.; Olive, K.E.; Aguilar-Benitez, M.; Amsler, C.; Asner, D.; Babu, K.; Barnett, R.; Beringer, J.; Burchat, P. Review of particle physics. Phys. Lett. B 2004, 592, 1–5. [Google Scholar] [CrossRef]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef]
- Troyan, S.L.; Kianzad, V.; Gibbs-Strauss, S.L.; Gioux, S.; Matsui, A.; Oketokoun, R.; Ngo, L.; Khamene, A.; Azar, F.; Frangioni, J.V. The FLARE™ Intraoperative Near-Infrared Fluorescence Imaging System: A First-in-Human Clinical Trial in Breast Cancer Sentinel Lymph Node Mapping. Ann. Surg. Oncol. 2009, 16, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bauer, A.Q.; Akers, W.J.; Sudlow, G.; Liang, K.; Shen, D.; Berezin, M.Y.; Culver, J.P.; Achilefu, S. Hands-free, wireless goggles for near-infrared fluorescence and real-time image-guided surgery. Surgery 2011, 149, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Jones, G.E.; Neligan, P.C.; Newman, M.I.; Phillips, B.T.; Sacks, J.M.; Zenn, M.R. Intraoperative laser angiography using the SPY system: Review of the literature and recommendations for use. Ann. Surg. Innov. Res. 2013, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Ivanova, A.; Boggess, J.F. Robotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: A feasibility study. Gynecol. Oncol. 2012, 124, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Thimsen, E.; Sadtler, B.; Berezin, M.Y. Shortwave-infrared (SWIR) emitters for biological imaging: A review of challenges and opportunities. Nanophotonics 2017, 6, 1043–1054. [Google Scholar] [CrossRef]
- Lapierre-Landry, M.; Gordon, A.Y.; Penn, J.S.; Skala, M.C. In vivo photothermal optical coherence tomography of endogenous and exogenous contrast agents in the eye. Sci. Rep. 2017, 7, 9228. [Google Scholar] [CrossRef] [PubMed]
- Lapierre-Landry, M.; Gordon, A.Y.; Skala, M. In vivo Photothermal Optical Coherence Tomography of Gold Nanorods in the Mouse Eye. In Proceedings of the Bio-Optics: Design and Application, San Diego, CA, USA, 2–5 April 2017; Optical Society of America: Washington, DC, USA, 2017; p. BoM3A. 2. [Google Scholar]
- Si, P.; Yuan, E.; Liba, O.; Winetraub, Y.; Yousefi, S.; SoRelle, E.; Yecies, D.; Dutta, R.; de la Zerda, A. Gold Nanoprisms as Optical Coherence Tomography Contrast Agents in the Second Near Infrared Window for Enhanced Angiography in Live Animals. ACS Nano. 2018, 322545. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, G.; Lin, R.; Gong, X.; Song, L.; Li, T.; Wang, W.; Zhang, K.; Qian, X.; Zhang, H. Three-dimensional Hessian matrix-based quantitative vascular imaging of rat iris with optical-resolution photoacoustic microscopy in vivo. J. Biomed. Opt. 2018, 23, 046006. [Google Scholar]
- Shu, X.; Li, H.; Dong, B.; Sun, C.; Zhang, H.F. Quantifying melanin concentration in retinal pigment epithelium using broadband photoacoustic microscopy. Biomed. Opt. Expess 2017, 8, 2851–2865. [Google Scholar] [CrossRef]
- Kelly-Goss, M.R.; Ning, B.; Bruce, A.C.; Tavakol, D.N.; Yi, D.; Hu, S.; Yates, P.A.; Peirce, S.M. Dynamic, heterogeneous endothelial Tie2 expression and capillary blood flow during microvascular remodeling. Sci. Rep. 2017, 7, 9049. [Google Scholar] [CrossRef]
- Dai, C.; Li, L.; Liu, W.; Wang, F.; Zhou, C. In vivo time-serial evaluation of laser-induced choroidal neovascularization in rats simultaneously using photoacoustic microscopy and optical coherence tomography. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2018, San Francisco, CA, USA, 28 January–1 February 2018; SPIE: Bellingham, WA, USA, 2018; p. 1049469. [Google Scholar]
- Liu, T.; Li, H.; Song, W.; Jiao, S.; Zhang, H.F. Fundus Camera Guided Photoacoustic Ophthalmoscopy. Curr. Eye Res. 2013, 38, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wei, Q.; Jiao, S.; Zhang, H.F. Integrated Photoacoustic Ophthalmoscopy and Spectral-domain Optical Coherence Tomography. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A. A schematic eye for the rabbit. Vis. Res. 1972, 12, 123–138. [Google Scholar] [CrossRef]
- A Compendium of Tissue Optical Properties. Available online: http://omlc.org/spectra/hemoglobin/ (accessed on 5 December 2018).
- Song, W.; Wei, Q.; Liu, T.; Kuai, D.; Zhang, H.F.; Burke, J.M.; Jiao, S. Integrating photoacoustic ophthalmoscopy with scanning laser ophthalmoscopy, optical coherence tomography, and fluorescein angiography for a multimodal retinal imaging platform. J. Biomed. Opt. 2012, 17, 061206. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Schultz, K.M.; Zhang, K.; Sasman, A.; Gao, F.; Kume, T.; Zhang, H.F. In vivo corneal neovascularization imaging by optical-resolution photoacoustic microscopy. Photoacoustics 2014, 2, 81–86. [Google Scholar] [CrossRef]
- Laser Biological Hazards-Eyes. Available online: https://ehs.oregonstate.edu/laser/training/laser-biological-hazards-eyes (accessed on 5 December 2018).
- Boussiba, S.; Vonshak, A. Astaxanthin Accumulation in the Green Alga Haematococcus pluvialis1. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Lee, C.; Han, S.; Kim, S.; Jeon, M.; Jeon, M.Y.; Kim, C.; Kim, J. Combined photoacoustic and optical coherence tomography using a single near-infrared supercontinuum laser source. Appl. Opt. 2013, 52, 1824–1828. [Google Scholar] [CrossRef]
- Wang, T.; Nandy, S.; Salehi, H.S.; Kumavor, P.D.; Zhu, Q. A low-cost photoacoustic microscopy system with a laser diode excitation. Biomed. Opt. Expess 2014, 5, 3053–3058. [Google Scholar] [CrossRef]
- Kolkman, R.G.; Steenbergen, W.; van Leeuwen, T.G. In vivo photoacoustic imaging of blood vessels with a pulsed laser diode. Lasers Med. Sci. 2006, 21, 134–139. [Google Scholar] [CrossRef]
- Chen, S.-L.; Xie, Z.; Ling, T.; Guo, L.J.; Wei, X.; Wang, X. Miniaturized all-optical photoacoustic microscopy based on microelectromechanical systems mirror scanning. Opt. Lett. 2012, 37, 4263–4265. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, S.-L.; Ling, T.; Guo, L.J.; Carson, P.L.; Wang, X. Pure optical photoacoustic microscopy. Opt. Epxress 2011, 19, 9027–9034. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Lloyd, H.; Silverman, R.H.; Chitnis, P.V. A frequency-domain non-contact photoacoustic microscope based on an adaptive interferometer. J. Biophotonics 2018, e201700278. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, A.; Chitnis, P.V.; Silverman, R.H. All-optical photoacoustic microscopy (AOPAM) system for remote characterization of biological tissues. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2014, San Francisco, CA, USA, 1–6 February 2014; SPIE: Bellingham, WA, USA, 2014; p. 89432Y. [Google Scholar]
- Xu, G.; Wang, C.; Feng, T.; Oliver, D.E.; Wang, X. Non-contact photoacoustic tomography with a laser Doppler vibrometer. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2014, San Francisco, CA, USA, 1–6 February 2014; SPIE: Bellingham, WA, USA, 2014; p. 894332. [Google Scholar]
- Wu, N.; Ye, S.; Ren, Q.; Li, C. High-resolution dual-modality photoacoustic ocular imaging. Opt. Lett. 2014, 39, 2451–2454. [Google Scholar] [CrossRef] [PubMed]


| Specification | PAT | PAOM | Vevo LAZR | REFERENCE |
|---|---|---|---|---|
| Axial Resolution | ~15 µm | ~15–37 µm | 44–75 µm | [23,38,39,40,41] |
| Lateral Resolution | ~45 µm | 4.1 µm | [19,39,42,43] | |
| Acquisition Time | >10 min | ~2.7 s (2 × 2 mm2)–60 s (3 × 3 mm2) | 74 s (10 mm) | [27,42,44] |
| Imaging Depth | ~ 5 cm | ~1–3 mm | ~2 cm | [23] |
| Field of view (FOV) | 30 × 30 mm | <10 × 10 mm2 | ~14–23 mm wide | [27] |
| Application | Anterior of the eye: whole eye tissues | Anterior and posterior of the eyes: retinal vessels, choroidal vessels, and capillaries | Anterior of the eye, SO2 | [22,34,41] |
| Wavelength | 532, NIR window | 405–2100 nm | 680–970 nm | [20,38,42] |
| Transducer | Single element, Linear Arrays, and planar | Single element | Linear Arrays, and planar | [16,18,19,20,21,27,38,41] |
| Coupling media | Ultrasound gel | Ultrasound gel, water, BSS | Ultrasound gel | [23,25,39,42] |
| Image acquisition Mode | PAT and US | PAM | PAT, US, and Pulse Doppler | [23,38,39,41] |
| Lateral Resolution (µm) | Axial Resolution (µm) | Frequency (MHz) | Bandwidth (MHz) | REFERENCE |
|---|---|---|---|---|
| 5 | 15 | 75 | 100 | Hao et al. [24] |
| 3 | 50 | 50 | Jeon et al. [51] | |
| 4.1 | 37 | 27 | 16 | Tian et al. [25] |
| 3.6 | 27.7 | 50 | 50 | Kim et al. [56] |
| Contrast Agents | Characterization | Application |
|---|---|---|
| Organic agents | ||
| Indocyanine green | High penetration depth | PAM, Indocyanine green angiography (ICGA) |
| Evans blue | Soluble and easy clearance via recticuloendothelial system (RES) | Microvascular network imaging |
| Cy7 fluorophore [63] | Non-toxic, biocompatible, biodegradability, high stability ad long circulation time | OCT contrast agent, tumor imaging agents |
| Fluorescein sodium dye | Biocompatibility | FA |
| Inorganic agents | ||
| Gold nanorod [70,80,81] | High penetration depth; relative slow tissue clearance | PAM, OCT, photothermal OCT, biomarker for molecular imaging |
| Gold nanoprisms [82] | Work in second near infrared window; deeper image depth | Enhanced OCT angiography |
| Retinal Disease | Advantages | Drawback | References |
|---|---|---|---|
| Choroidal Neovascularization | High resolution Moderate depth resolution Strong optical absorption | Small animals: mice and rat | [86,94] |
| Corneal Neovascularization | High resolution Moderate depth resolution Strong optical absorption Angiography | Small animals: mice and rats | [51,85,92] |
| SO2 | Measure optical absorption of hemoglobin directly More accurate than oximetry and multi-wavelength fundus photography | Requirement of image registration, and post-image processing | [27,51,85] |
| Retinal oxygen metabolic rate (rMRO2) | Accurate and non-invasive quantification Derive from the measured SO2, blood flow and vessels diameter | Requirement of image registration | [9] |
| Blood flow | Accurate | Combination with OCT | [85] |
| Scanning Method | Acquisition Time | Imaging Size | Wavelength | Energy | Application | References |
|---|---|---|---|---|---|---|
| Mechanical scanning | 90 min | 12 × 8 mm2 | 740 nm | 0.5 mJ/cm2 | Eye tissues | de la Zerda et al. [22] |
| Mechanical scanning | 120 min | 2 × 2 mm2 | 570 nm and 578 nm | 40 nJ | Iris microvasculature | Hu et al. [36] |
| Mechanical scanning | 20 min | 3 × 3 mm2 | 532 nm | 80 nJ | Corneal neovascularization | Liu et al. [87] |
| Mechanical scanning | 6.5 min | 2 × 2 mm2 | 532 nm | 500 nJ | Iris microvasculature | Wu et al. [103] |
| Optical scanning | 2.7 s | 2 × 2 mm2 | 532 nm | 40 nJ | Retinal blood vessels, and RPE | Jiao et al. [33] |
| Optical scanning | 2.7 s | 2 × 2 mm2 | 570 nm, 578 nm, and 588 nm | 40 nJ | SO2, retinal and choroidal vessels | Song et al. [57] |
| Optical scanning | 65 s | 3 × 3 mm2 | 570 nm | 80 nJ | Retinal and choroidal blood vessels in rabbit | Chao et al. [25,42] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.P.; Paulus, Y.M. Photoacoustic Ophthalmoscopy: Principle, Application, and Future Directions. J. Imaging 2018, 4, 149. https://doi.org/10.3390/jimaging4120149
Nguyen VP, Paulus YM. Photoacoustic Ophthalmoscopy: Principle, Application, and Future Directions. Journal of Imaging. 2018; 4(12):149. https://doi.org/10.3390/jimaging4120149
Chicago/Turabian StyleNguyen, Van Phuc, and Yannis M. Paulus. 2018. "Photoacoustic Ophthalmoscopy: Principle, Application, and Future Directions" Journal of Imaging 4, no. 12: 149. https://doi.org/10.3390/jimaging4120149
APA StyleNguyen, V. P., & Paulus, Y. M. (2018). Photoacoustic Ophthalmoscopy: Principle, Application, and Future Directions. Journal of Imaging, 4(12), 149. https://doi.org/10.3390/jimaging4120149





