Image Reconstruction with Reliability Assessment in Quantitative Photoacoustic Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Forward Model
2.2. Inverse Problem
2.3. Bayesian Approximation Error Modeling
2.4. Evaluating Credibility
3. Simulation Studies
3.1. Data Simulation
3.2. Inverse Problem
3.3. Prior Model
3.4. Approximation Error Statistics
3.5. Reliability of the Credibility Intervals
4. Results
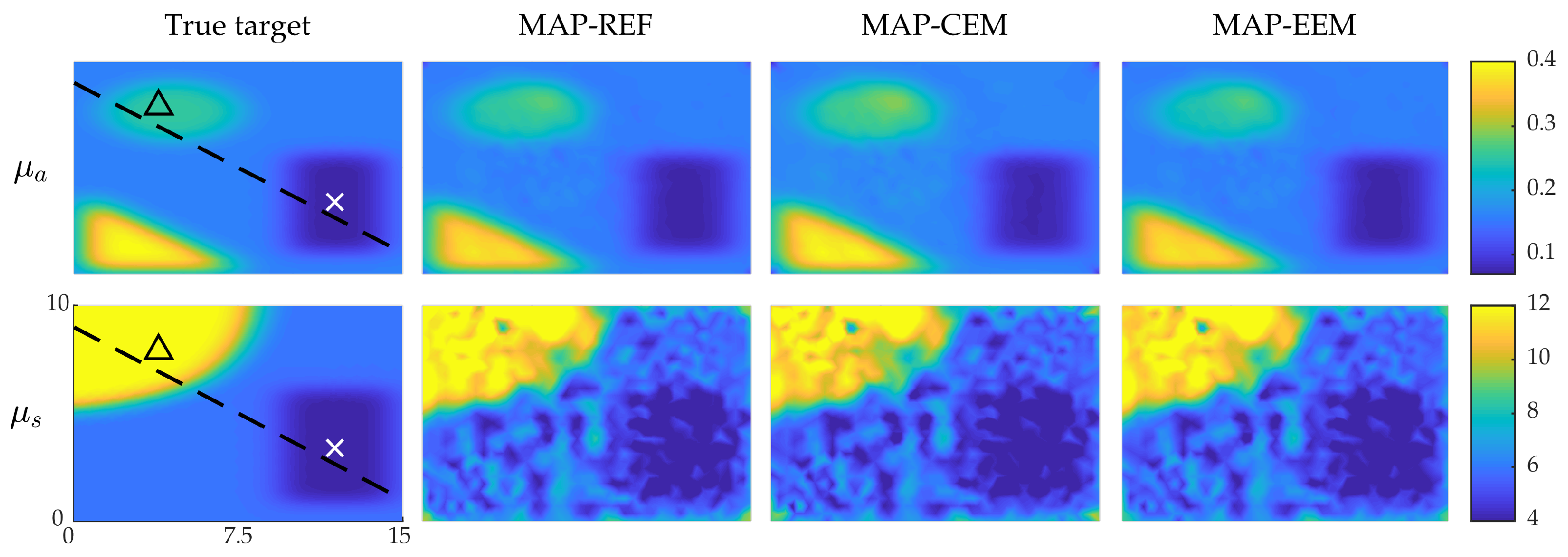
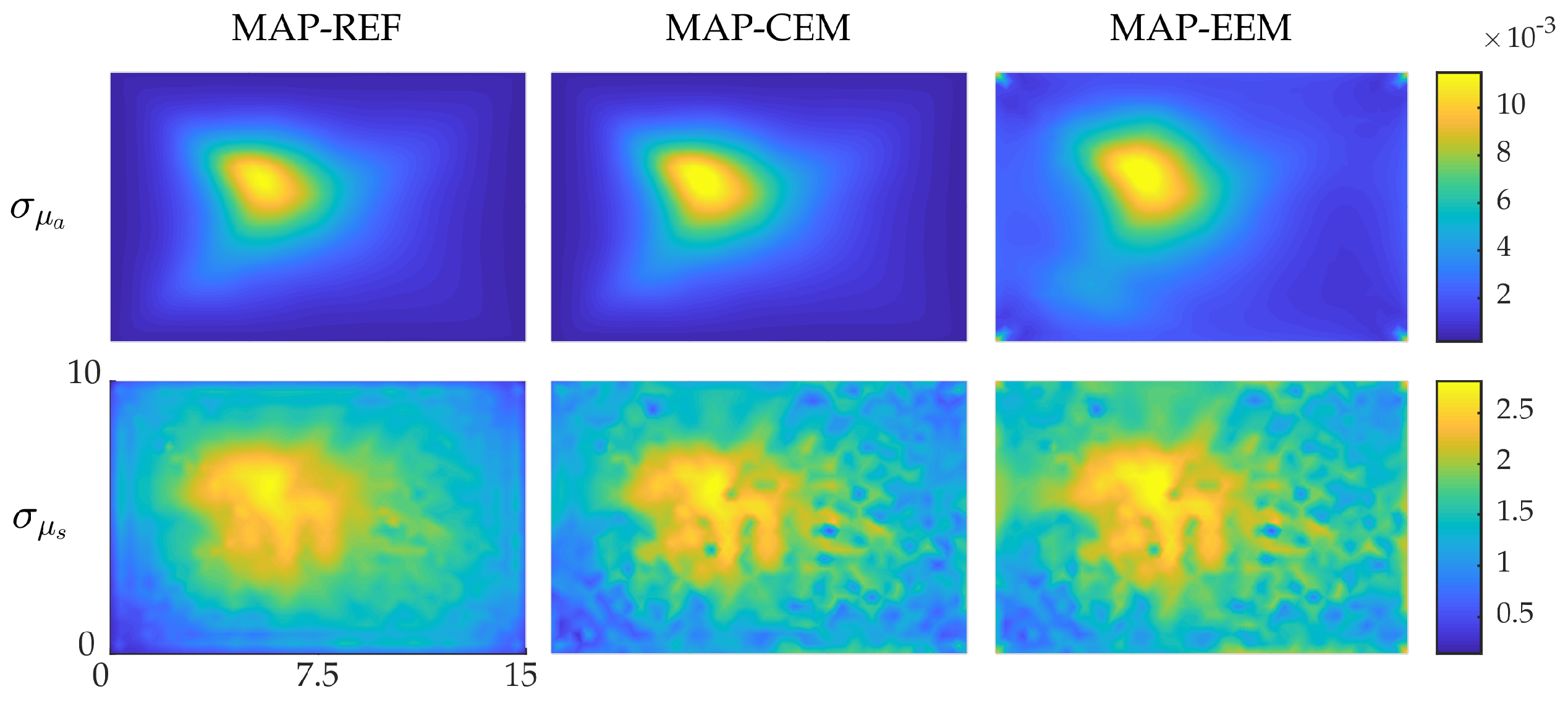
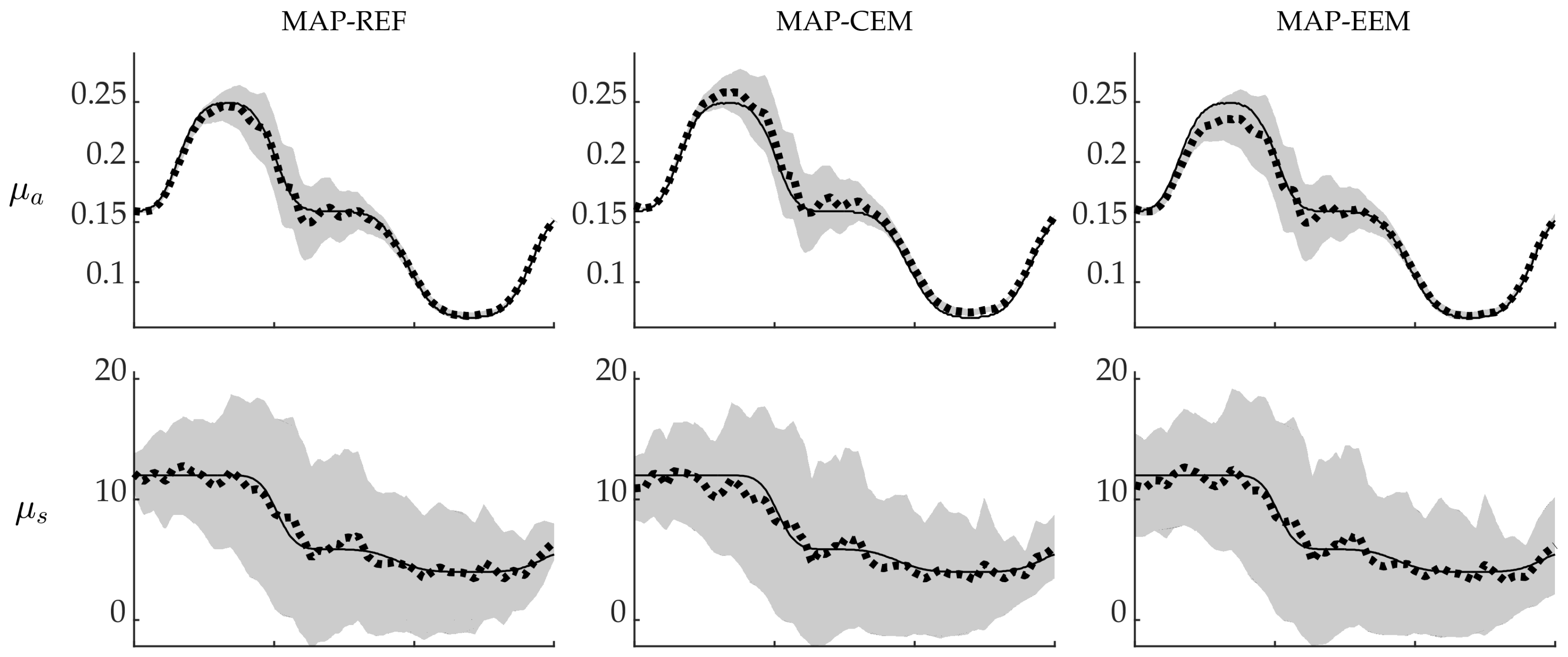
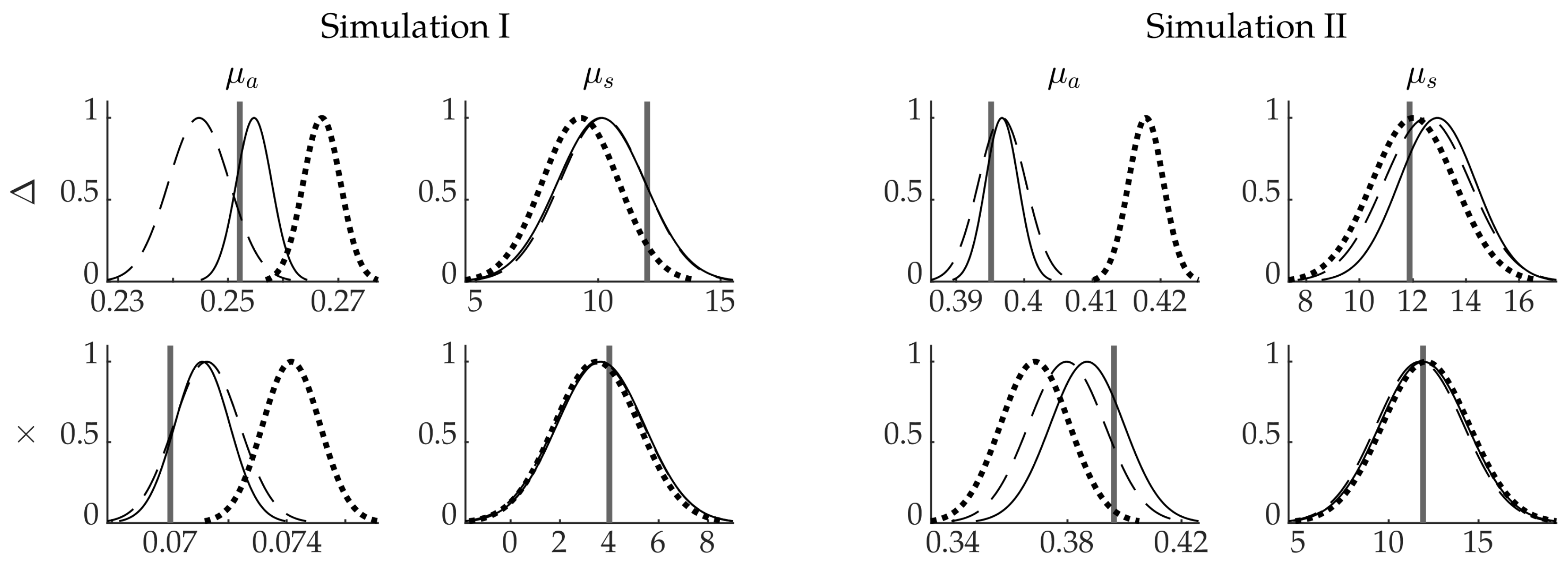
4.1. Simulation I: Smooth Inclusions
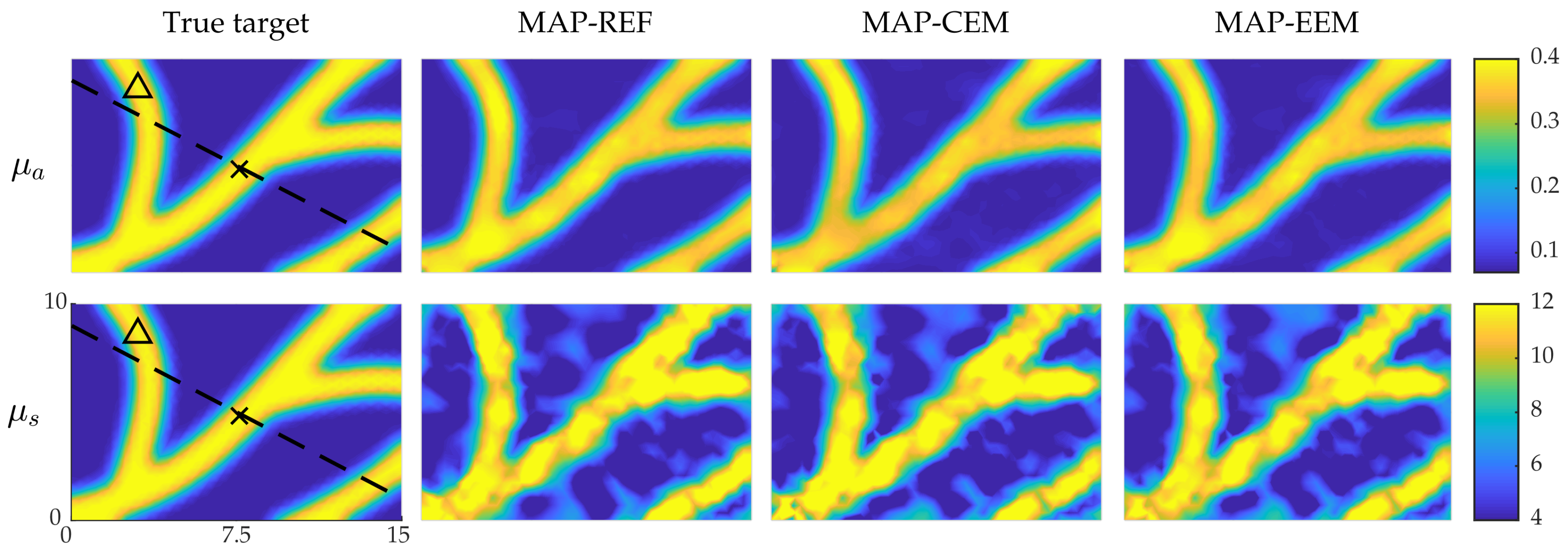
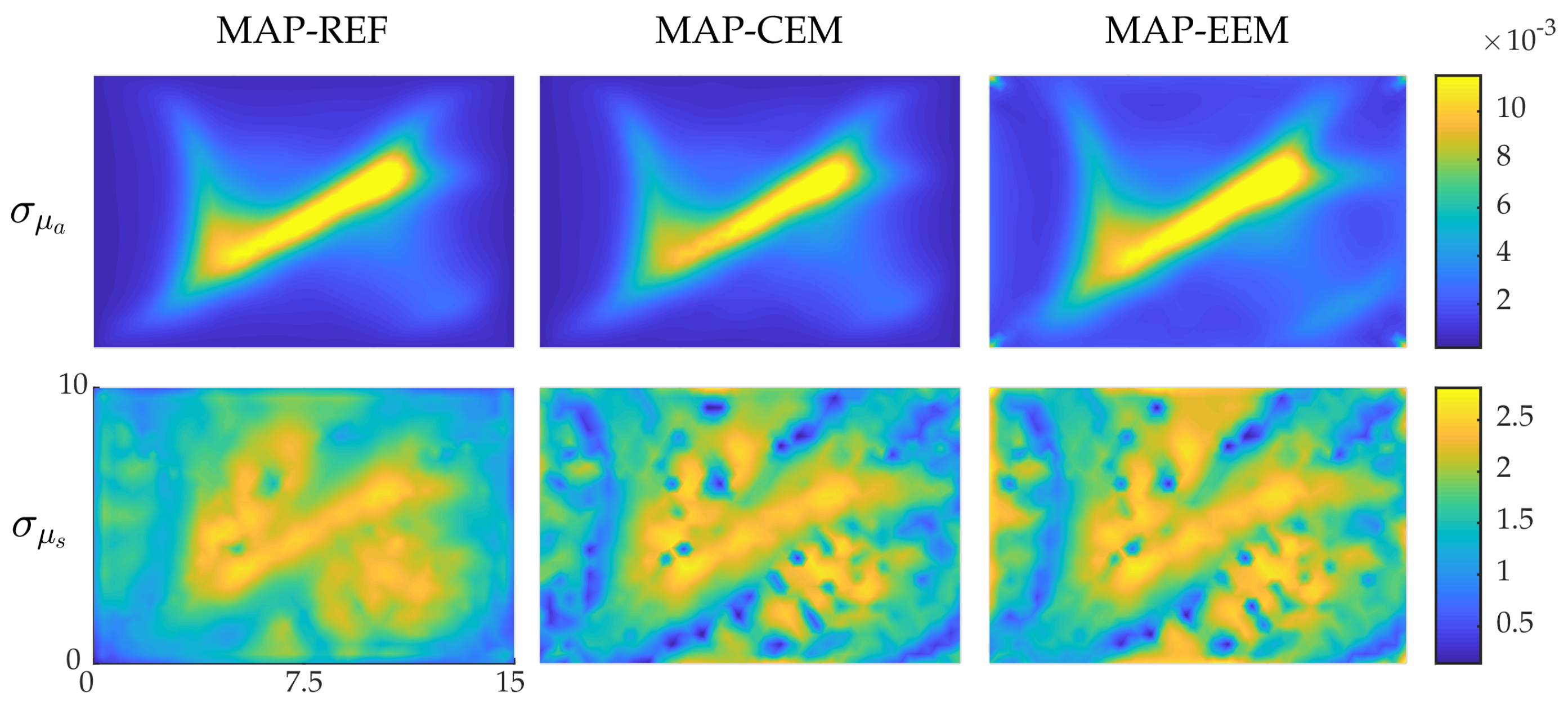
4.2. Simulation II: Blood-Vessel-Mimicking Inclusions
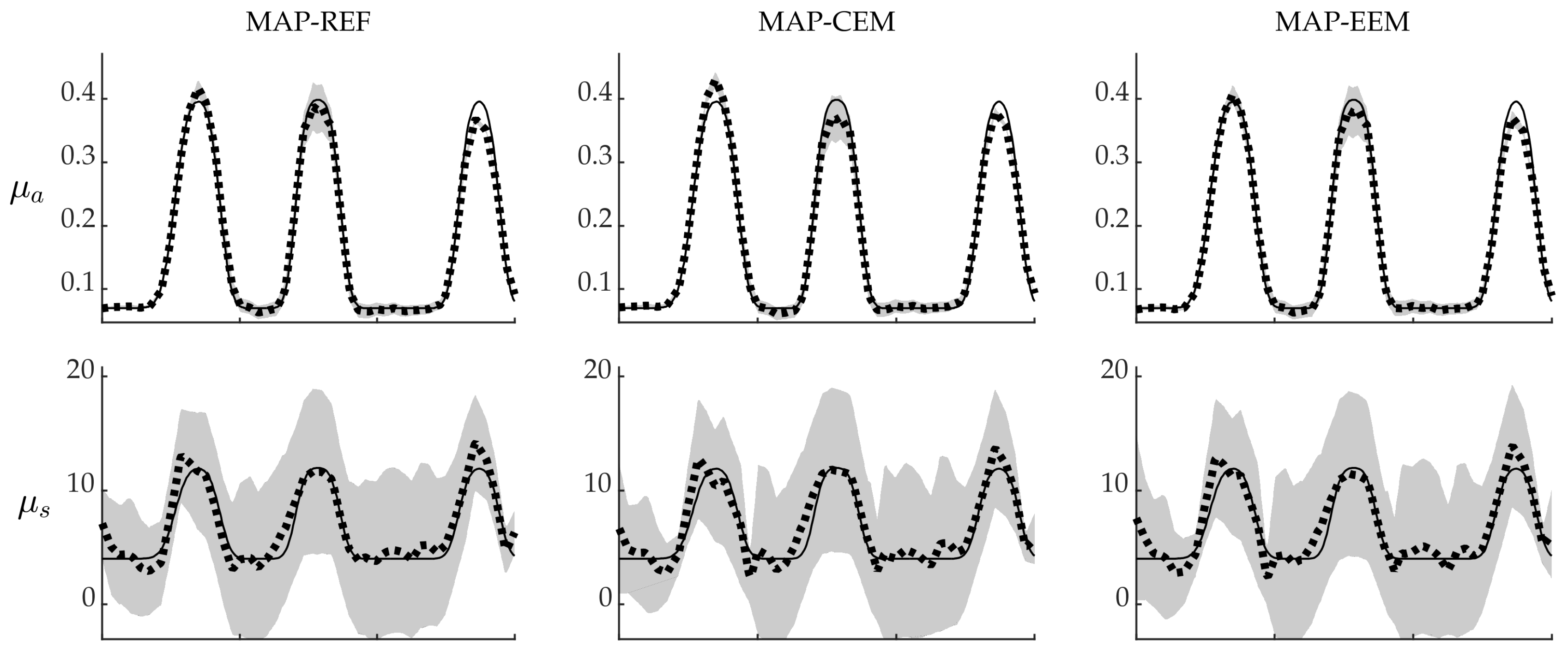
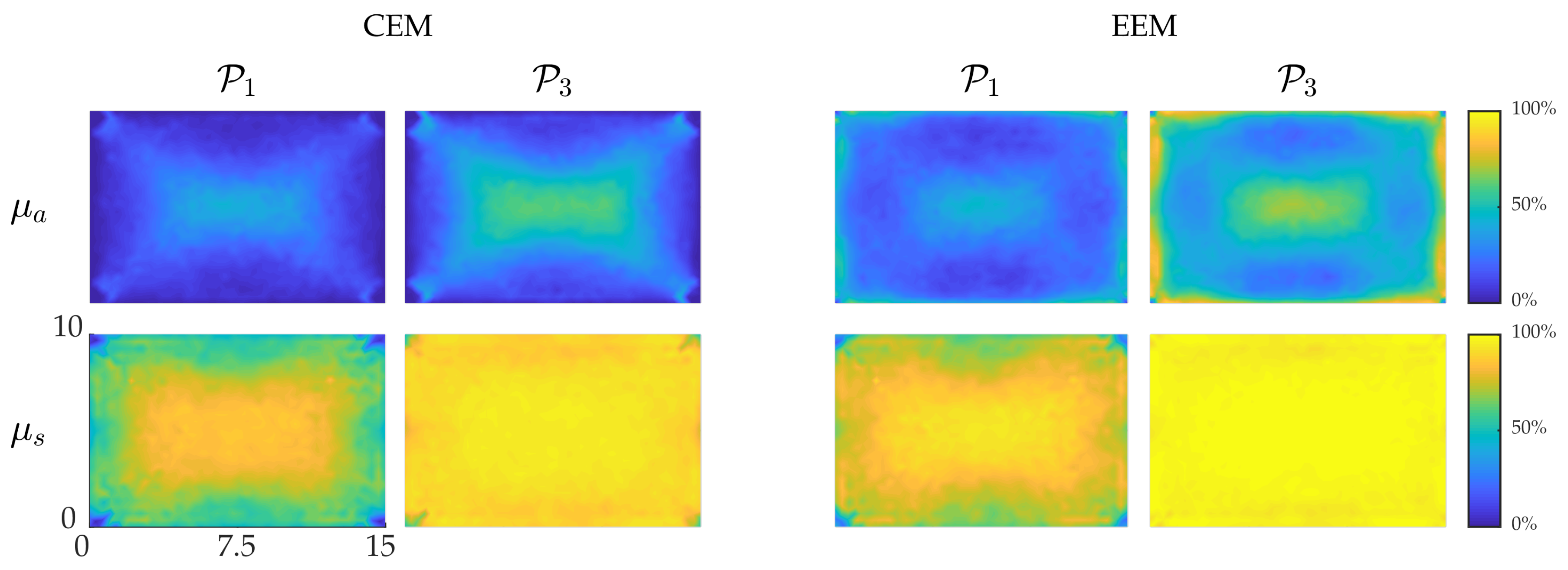
4.3. Reliability of the Credibility Intervals
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PAT | Photoacoustic tomography |
| QPAT | Quantitative photoacoustic tomography |
| MAP | Maximum a posteriori |
| RTE | Radiative transfer equation |
| DA | Diffusion approximation |
| FE | Finite element |
| FEM | Finite element method |
| BAE | Bayesian approximation error |
| CEM | Conventional error model |
| EEM | Enhanced error model |
| 2D | Two-dimensional |
| SD | Standard deviation |
| CDF | Cumulative distribution function |
References
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic tomography: Principles and advances. Electromagn. Waves 2014, 147, 1–22. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Wang, L.V. (Ed.) Photoacoustic Imaging and Spectroscopy; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.V. Photoacoustic tomography and sensing in biomedicine. Phys. Med. Biol. 2009, 54, R59–R97. [Google Scholar] [CrossRef]
- Wang, L.V. Prospects of photoacoustic tomography. Med. Phys. 2008, 35, 5758–5767. [Google Scholar] [CrossRef]
- Yao, J.; Xia, J.; Maslov, K.I.; Nasiriavanaki, M.; Tsytsarev, V.; Demchenko, A.V.; Wang, L.V. Noninvasive photoacoustic computed tomography of mouse brain metabolism in vivo. Neuroimage 2013, 64, 257–266. [Google Scholar] [CrossRef]
- Cox, B.; Laufer, J.G.; Arridge, S.R.; Beard, P.C. Quantitative spectroscopic photoacoustic imaging: A review. J. Biomed. Opt. 2012, 17, 061202. [Google Scholar] [CrossRef]
- Kaipio, J.; Somersalo, E. Statistical and Computational Inverse Problems; Springer: New York, NY, USA, 2005. [Google Scholar]
- Pulkkinen, A.; Cox, B.T.; Arridge, S.R.; Kaipio, J.P.; Tarvainen, T. A Bayesian approach to spectral quantitative photoacoustic tomography. Inverse Probl. 2014, 30, 065012. [Google Scholar] [CrossRef]
- Bal, G.; Ren, K. On multi-spectral quantitative photoacoustic tomography in a diffusive regime. Inverse Probl. 2012, 28, 025010. [Google Scholar] [CrossRef]
- Cox, B.T.; Arridge, S.R.; Beard, P.C. Estimating chromophore distributions from multiwavelength photoacoustic images. J. Opt. Soc. Am. A 2009, 26, 443–455. [Google Scholar] [CrossRef]
- Laufer, J.; Cox, B.; Zhang, E.; Beard, P. Quantitative determination of chromophore concentrations form 2D photoacoustic images using a nonlinear model-based inversion scheme. Appl. Opt. 2010, 49, 1219–1233. [Google Scholar] [CrossRef]
- Razansky, D.; Baeten, J.; Ntziachristos, V. Sensitivity of molecular target detection by multispectral optoacoustic tomography (MSOT). Med. Phys. 2009, 36, 939–945. [Google Scholar] [CrossRef]
- Tarvainen, T.; Cox, B.T.; Kaipio, J.P.; Arridge, S.R. Reconstructing absorption and scattering distributions in quantitative photoacoustic tomography. Inverse Probl. 2012, 28, 084009. [Google Scholar] [CrossRef]
- Pulkkinen, A.; Kolehmainen, V.; Kaipio, J.P.; Cox, B.T.; Arridge, S.R.; Tarvainen, T. Approximate marginalization of unknown scattering in quantitative photoacoustic tomography. Inverse Probl. Imaging 2014, 8, 811–829. [Google Scholar]
- Mamonov, A.V.; Ren, K. Quantitative photoacoustic imaging in radiative transport regime. Commun. Math. Sci. 2014, 12, 201–234. [Google Scholar] [CrossRef]
- Bal, G.; Ren, K. Multi-source quantitative photoacoustic tomography in a diffusive regime. Inverse Probl. 2011, 27, 075003. [Google Scholar] [CrossRef]
- Banerjee, B.; Bagchi, S.; Vasu, R.M.; Roy, D. Quantitative photoacoustic tomography from boundary pressure measurements: Noniterative recovery of optical absorption coefficient from the reconstructed absorbed energy map. J. Opt. Soc. Am. A 2008, 25, 2347–2356. [Google Scholar] [CrossRef]
- Saratoon, T.; Tarvainen, T.; Cox, B.T.; Arridge, S.R. A gradient-based method for quantitative photoacoustic tomography using the radiative transfer equation. Inverse Probl. 2013, 29, 075006. [Google Scholar] [CrossRef]
- Cezaro, A.D.; Cezaro, F.T.D.; Suarez, J.S. Regularization approaches for quantitative Photoacoustic tomography using the radiative transfer equation. J. Math. Anal. Appl. 2015, 429, 415–438. [Google Scholar] [CrossRef]
- Alberti, G.S.; Ammari, H. Disjoint sparsity for signal separation and applications to hybrid inverse problems in medical imaging. Appl. Comput. Harmon Anal. 2017, 42, 319–349. [Google Scholar] [CrossRef]
- Nykänen, O.; Pulkkinen, A.; Tarvainen, T. Quantitative photoacoustic tomography augmented with surface light measurements. Biomed. Opt. Express 2017, 8, 4380–4395. [Google Scholar] [CrossRef]
- Zemp, R.J. Quantitative photoacoustic tomography with multiple optical sources. Appl. Opt. 2010, 49, 3566–3572. [Google Scholar] [CrossRef]
- Yin, L.; Wang, Q.; Zhang, Q.; Jiang, H. Tomographic imaging of absolute optical absorption coefficient in turbid media using combined photoacoustic and diffusing light measurements. Opt. Lett. 2007, 32, 2556–2558. [Google Scholar] [CrossRef]
- Ren, K.; Gao, H.; Zhao, H. A hybrid reconstruction method for quantitative PAT. SIAM J. Imaging Sci. 2013, 6, 32–55. [Google Scholar] [CrossRef]
- Pulkkinen, A.; Cox, B.T.; Arridge, S.R.; Goh, H.; Kaipio, J.P.; Tarvainen, T. Direct estimation of optical parameters from photoacoustic time series in quantitative photoacoustic tomography. IEEE Trans. Med. Imaging 2016, 35, 2497–2508. [Google Scholar] [CrossRef]
- Ding, T.; Ren, K.; Vallélian, S. A one-step reconstruction algorithm for quantitative photoacoustic imaging. Inverse Probl. 2015, 31, 095005. [Google Scholar] [CrossRef]
- Shao, P.; Harrison, T.; Zemp, R.J. Iterative algorithm for multiple illumination photoacoustic tomography (MIPAT) using ultrasound channel data. Biomed. Opt. Express 2012, 3, 3240–3248. [Google Scholar] [CrossRef]
- Song, N.; Deumié, C.; Silva, A.D. Considering sources and detectors distributions for quantitative photoacoustic tomography. Biomed. Opt. Express 2014, 5, 3960–3974. [Google Scholar] [CrossRef]
- Haltmeier, M.; Neumann, L.; Rabanser, S. Single-stage reconstruction algorithm for quantitative photoacoustic tomography. Inverse Probl. 2015, 31, 065005. [Google Scholar] [CrossRef]
- Gao, H.; Feng, J.; Song, L. Limited-view multi-source quantitative photoacoustic tomography. Inverse Probl. 2015, 31, 065004. [Google Scholar] [CrossRef]
- Hochuli, R. Monte Carlo Methods in Quantitative Photoacoustic Tomography. Ph.D. Thesis, University College London, London, UK, 2016. [Google Scholar]
- Tick, J.; Pulkkinen, A.; Tarvainen, T. Image reconstruction with uncertainty quantification in photoacoustic tomography. J. Acoust. Soc. Am. 2016, 139, 1951. [Google Scholar] [CrossRef]
- Tick, J.; Pulkkinen, A.; Lucka, F.; Ellwood, R.; Cox, B.T.; Kaipio, J.P.; Arridge, S.R.; Tarvainen, T. Three dimensional photoacoustic tomography in Bayesian framework. J. Acoust. Soc. Am. 2018, 144, 2061–2071. [Google Scholar] [CrossRef]
- Ishimaru, A. Wave Propagation and Scattering in Random Media; Academic Press: New York, NY, USA, 1978; Volume 1. [Google Scholar]
- Arridge, S.R. Optical tomography in medical imaging. Inverse Probl. 1999, 15, R41–R93. [Google Scholar] [CrossRef]
- Kaipio, J.; Somersalo, E. Statistical inverse problems: Discretization, model reduction and inverse errors. J. Comput. Appl. Math. 2007, 198, 493–504. [Google Scholar] [CrossRef]
- Tarvainen, T.; Pulkkinen, A.; Cox, B.T.; Kaipio, J.P.; Arridge, S.R. Bayesian image reconstruction in quantitative photoacoustic tomography. IEEE Trans. Med. Imaging 2013, 32, 2287–2298. [Google Scholar] [CrossRef]
- Kolehmainen, V.; Schweiger, M.; Nissilä, I.; Tarvainen, T.; Arridge, S.R.; Kaipio, J.P. Approximation errors and model reduction in three-dimensional diffuse optical tomography. J. Opt. Soc. Am. A 2009, 26, 2257–2268. [Google Scholar] [CrossRef]
- Arridge, S.R.; Kaipio, J.P.; Kolehmainen, V.; Schweiger, M.; Somersalo, E.; Tarvainen, T.; Vauhkonen, M. Approximation errors and model reduction with an application in optical diffusion tomography. Inverse Probl. 2006, 22, 175–195. [Google Scholar] [CrossRef]
- Tarvainen, T.; Kolehmainen, V.; Pulkkinen, A.; Vauhkonen, M.; Schweiger, M.; R Arridge, S.; Kaipio, J. Approximation error approach for compensating modelling errors between the radiative transfer equation and the diffusion approximation in diffuse optical tomography. Inverse Probl. 2009, 26, 015005. [Google Scholar] [CrossRef]
- Tarvainen, T.; Kolehmainen, V.; Kaipio, J.P.; Arridge, S.R. Corrections to linear methods for diffuse optical tomography using approximation error modelling. Biomed. Opt. Express 2010, 1, 209–222. [Google Scholar] [CrossRef]
- Mozumber, M.; Tarvainen, T.; Kaipio, J.P.; Arridge, S.R.; Kolehmainen, V. Compensation of modeling errors die to unknown domain boundary in diffuse optical tomography. J. Opt. Soc. Am. A 2014, 31, 1847–1855. [Google Scholar] [CrossRef]
- Mozumber, M.; Tarvainen, T.; Arridge, S.R.; Kaipio, J.; Kolehmainen, V. Compensation of optode sensitivity and position errors in diffuse optical tomography using the approximation error approach. Biomed. Opt. Express 2013, 4, 2015–2031. [Google Scholar] [CrossRef]
- Heino, J.; Somersalo, E.; Kaipio, J.P. Compensation for geometric mismodelling by anisotropies in optical tomography. Opt. Express 2005, 13, 296–308. [Google Scholar] [CrossRef]
- Koponen, J.; Huttunen, T.; Tarvainen, T.; Kaipio, J.P. Bayesian approximation error approach in full-wave ultrasound tomography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 1627–1637. [Google Scholar] [CrossRef]
- Leino, A.; Pulkkinen, A.; Tarvainen, T. ValoMC: A Monte Carlo software and MATLAB toolbox for simulating light transport in biological tissues. OSA Contin. 2018. under review. [Google Scholar]
- Prahl, S.A.; Keijzer, M.; Jacques, S.L.; Welch, A.J. A Monte Carlo model of light propagation in tissue. SPIE Proc. Dosim. Laser Radiat. Med. Biol. 1989, IS5, 102–111. [Google Scholar]
- Gusev, V.E.; Karabutov, A.A. Laser Optoacoustics; American Institute of Physics: New York, NY, USA, 1993. [Google Scholar]
- Xu, M.; Wang, L.V. Universal back-projection algorithm for photoacoustic computed tomography. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 71, 016706. [Google Scholar] [CrossRef]
- Kunyansky, L.A. A series solution and a fast algorithm for the inversion of the spherical mean Radon transform. Inverse Probl. 2007, 23, S11–S20. [Google Scholar] [CrossRef]
- Agranovsky, M.; Kuchment, P. Uniqueness of reconstruction and an inversion procedure for thermoacoustic and photoacoustic tomography with variable sound speed. Inverse Probl. 2007, 23, 2089–2102. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.V. Time Reversal and Its Application to Tomography with Diffracting Sources. Phys. Rev. Lett. 2004, 92, 339021–339024. [Google Scholar] [CrossRef]
- Burgholzer, P.; Matt, G.J.; Haltmeier, M.; Paltauf, G. Exact and approximative imaging methods for photoacoustic tomography using an arbitrary detection surface. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2007, 75, 046706. [Google Scholar] [CrossRef]
- Treeby, B.E.; Zhang, E.Z.; Cox, B.T. Photoacoustic tomography in absorbing acoustic media using time reversal. Inverse Probl. 2010, 26, 115003. [Google Scholar] [CrossRef]
- Zhang, J.; Anastasio, M.A.; Rivière, P.I.; Wang, L.V. Effects of Different Imaging Models on Least-Squares Image Reconstruction Accuracy in Photoacoustic Tomography. IEEE Trans. Med. Imaging 2009, 28, 1781–1790. [Google Scholar] [CrossRef]
- Deán-Ben, X.L.; Buehler, A.; Ntziachristos, V.; Razansky, D. Accurate model-based reconstruction algorithm for three-dimensional optoacoustic tomography. IEEE Trans. Med. Imaging 2012, 31, 1922–1928. [Google Scholar] [CrossRef]
- Wang, K.; Su, R.; Oraevsky, A.A.; Anastasio, M.A. Investigation of iterative image reconstruction in three-dimensional optoacoustic tomography. Phys. Med. Biol. 2012, 57, 5399–5423. [Google Scholar] [CrossRef]
- Arridge, S.R.; Betcke, M.M.; Cox, B.T.; Lucka, F.; Treeby, B.E. On the adjoint operator in photoacoustic tomography. Inverse Probl. 2016, 32, 115012. [Google Scholar] [CrossRef]
- Cheong, W.F.; Prahl, S.A.; Welch, A.J. A Review of the Optical Properties of Biological Tissues. IEEE J. Quantum Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Tuchin, V.V. Optical properties of skin, subcutaneous, and muscle tissues: A review. J. Innov. Opt. Health Sci. 2011, 4, 9–38. [Google Scholar] [CrossRef]
- Rasmussen, C.E.; Williams, C.K.I. Gaussian Processes for Machine Learning; MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Pulkkinen, A.; Cox, B.T.; Arridge, S.R.; Kaipio, J.P.; Tarvainen, T. Quantitative photoacoustic tomography using illuminations from a single direction. J. Biomed. Opt. 2015, 20, 036015. [Google Scholar] [CrossRef]
| Mesh | |||
|---|---|---|---|
| Data simulation | 104,472 | 52,654 | |
| Coarse mesh, basis for optical parameters | 2052 | 1085 | |
| Fine mesh | 32,832 | 16,649 | |
| Prior samples for evaluating the credibility | 1932 | 1024 |
| Reconstructions | 1.25 | 0.2 (1) | 6 (1) | 0.2 (1) | 6 (1) |
| Approximation error statistics | 1.25 | 0.2 | 6 | 0.067 | 2 |
| Reliability of the credibility intervals | 1.25 | 0.2 | 6 | 0.067 | 2 |
| MAP-REF | MAP-CEM | MAP-EEM | ||||
|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | |
| Simulation I | 3.6 | 11.0 | 5.1 | 12.5 | 3.7 | 11.1 |
| Simulation II | 6.7 | 15.2 | 9.5 | 16.5 | 7.6 | 15.7 |
| MAP-REF | MAP-CEM | MAP-EEM | ||||
|---|---|---|---|---|---|---|
| Time (s) | Iteration | Time (s) | Iteration | Time (s) | Iteration | |
| Simulation I | 18,081 | 9 | 445 | 11 | 296 | 10 |
| Simulation II | 22,480 | 10 | 507 | 12 | 337 | 11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hänninen, N.; Pulkkinen, A.; Tarvainen, T. Image Reconstruction with Reliability Assessment in Quantitative Photoacoustic Tomography. J. Imaging 2018, 4, 148. https://doi.org/10.3390/jimaging4120148
Hänninen N, Pulkkinen A, Tarvainen T. Image Reconstruction with Reliability Assessment in Quantitative Photoacoustic Tomography. Journal of Imaging. 2018; 4(12):148. https://doi.org/10.3390/jimaging4120148
Chicago/Turabian StyleHänninen, Niko, Aki Pulkkinen, and Tanja Tarvainen. 2018. "Image Reconstruction with Reliability Assessment in Quantitative Photoacoustic Tomography" Journal of Imaging 4, no. 12: 148. https://doi.org/10.3390/jimaging4120148
APA StyleHänninen, N., Pulkkinen, A., & Tarvainen, T. (2018). Image Reconstruction with Reliability Assessment in Quantitative Photoacoustic Tomography. Journal of Imaging, 4(12), 148. https://doi.org/10.3390/jimaging4120148






