Receive Beam-Steering and Clutter Reduction for Imaging the Speed-of-Sound Inside the Carotid Artery
Abstract
1. Introduction
- Blood motion: In conventional CUTE, Tx plane waves are sent forth to scan the full angular aperture that is possible within the limits of the transducer design, e.g., from −25° to 25°. For maintaining low clutter levels, synthetic Tx focusing is used, based on the coherent plane wave compounding [38] of acquisitions with finely spaced (e.g., 0.5°) Tx angles. Therefore, conventional CUTE typically requires more than 100 acquisitions. During the time required for this number of acquisitions, arterial blood flow leads to the decorrelation of blood echoes, resulting in phase noise and SoS artefacts.
- Tissue clutter: Higher order echoes from the tissue surrounding the carotid are interpreted as originating from inside the carotid, cluttering the blood echoes. Clutter has a higher intensity level than the blood echoes, leading to wrong echo phase shifts and thus falsified SoS reconstruction.
- Using Rx instead of Tx beam-steering for echo phase tracking, in order to reduce acquisition time and thus avoid/reduce blood flow-related artefacts. This approach is inspired by the commonly accepted principle of the reciprocity of sender and receiver, suggesting the equivalence of Tx- and Rx-steering.
- Taking advantage of the benefit of blood motion to reduce clutter: Pairwise subtraction of consecutive repetitions of the signal acquisition, spaced by small (on the order of a ms) time intervals, cancels static tissue clutter and thereby highlights the decorrelating weak blood echoes.
2. Materials and Methods
2.1. Conventional Tx CUTE
2.2. Full-Rx CUTE
2.3. Fast-Rx Subtraction Technique
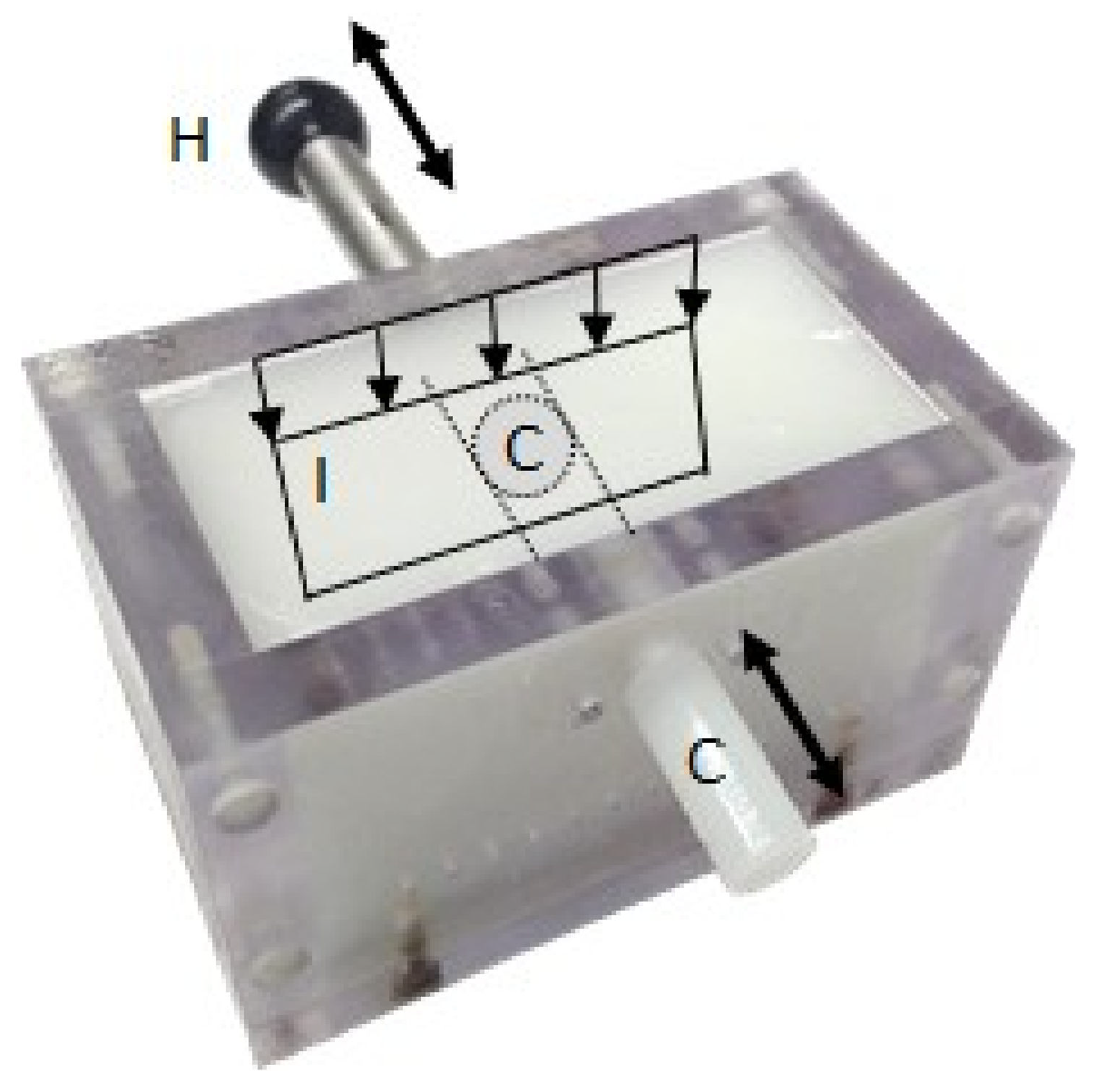
2.4. Phantom Description
2.5. Acquisition System
2.6. US Image Reconstruction and Phase Tracking
2.7. SoS Image Reconstruction
3. Results
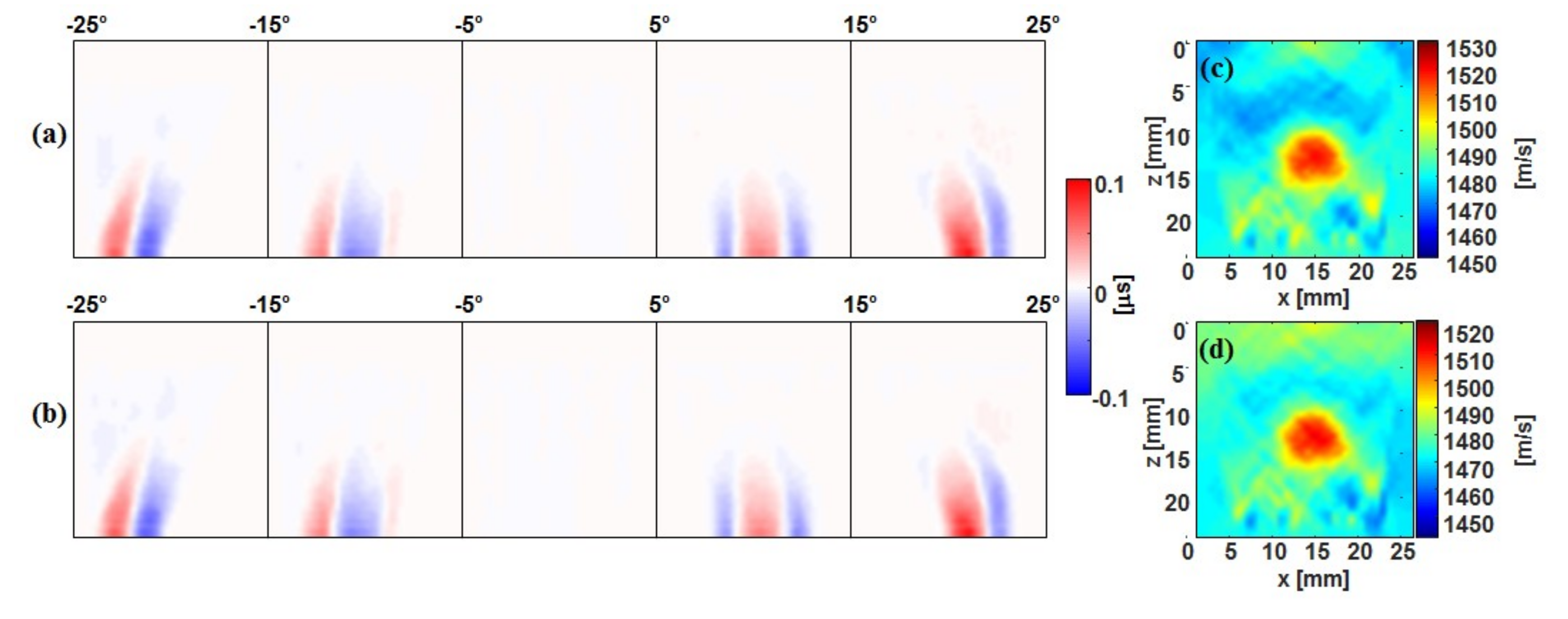
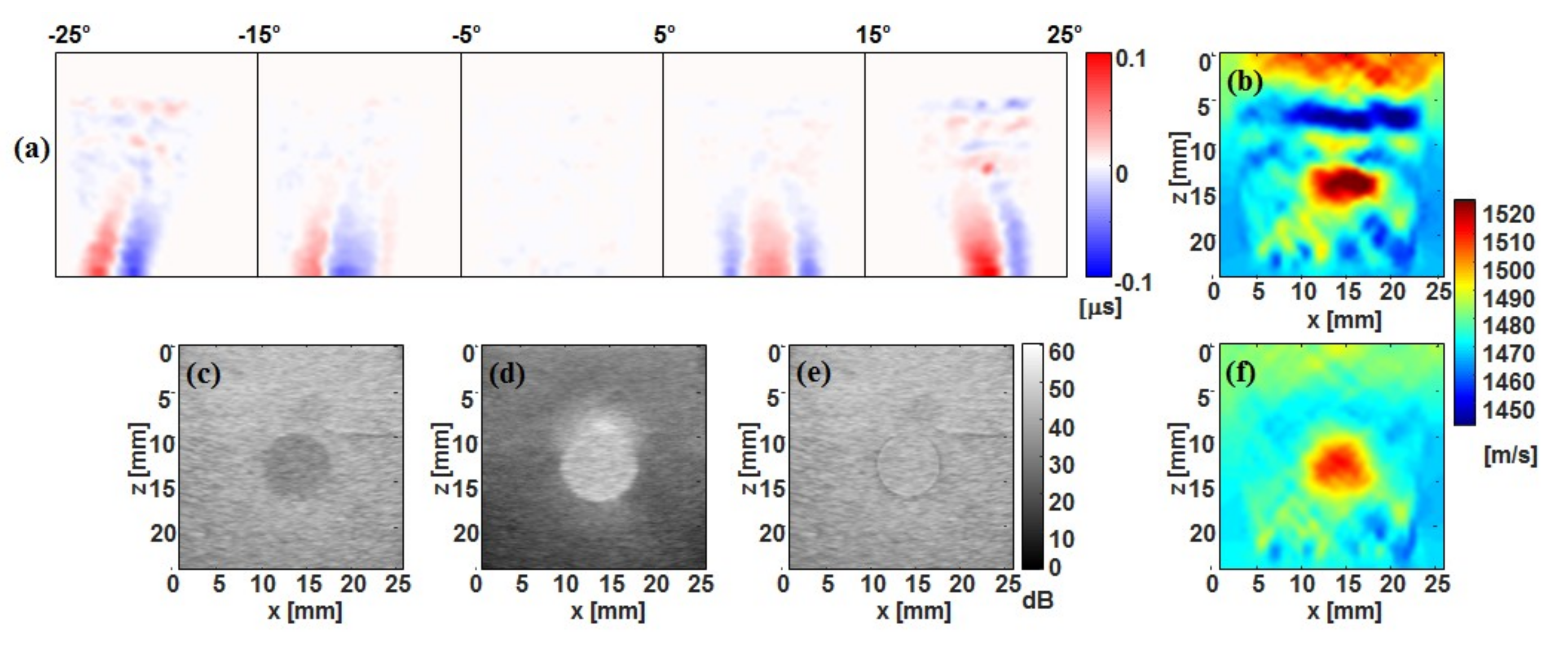
3.1. Tx Versus Full-Rx CUTE
3.2. Fast-Rx Subtraction Technique
4. Discussion and conclusion
- In the phantom, the simulated flow speed was identical across the lumen cross section. In an in vivo scenario, blood in the centre of the lumen moves faster than blood near the periphery. So, a simple subtraction would lead to nonuniform echo strength, converging to zero towards the vessel wall. The subtraction corresponds to a linear ramp filter in the frequency domain of the “slow” time axis (repetitions of acquisitions). To achieve a more uniform echo level, one may make use of wall filters, similar to what is used in Doppler flow imaging.
- In an in vivo scenario, the background tissue is not perfectly stationary due to the pulsating blood flow, which will lead to residual “stationary” clutter inside the vessel lumen even after subtraction. One strategy to minimise motion is by choosing an optimum timing of the fast-Rx acquisitions in between successive heart beats. This can be achieved by synchronising the acquisition with a motion-detection algorithm or an electrocardiogram. A different and complementary strategy is motion tracking and motion compensation before subtraction or wall filtering.
- The low signal-to-noise ratio (SNR) of blood echoes can produce phase noise and thus artefacts in the SoS reconstruction. SNR can be improved by repeating the fast-Rx acquisition multiple times, similar to what is done in Doppler flow imaging, and averaging the phase correlation maps before determining the echo phase shift.
- In our present study, lumen motion occurred only in between repetitions, but not during a single acquisition sequence. In an in vivo situation, blood flow occurs during the few plane wave transmissions of each single fast-Rx acquisition sequence. Even though the number of Tx angles is short enough that echo decorrelation does not occur, the blood motion will add a phase shift bias. One strategy to reduce this influence is to invert the Tx angle sequence in successive fast-Rx acquisitions, so that the phase shift due to blood flow cancels out in the final average phase shift.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kato, K.; Sugimoto, H.; Kanazumi, N.; Nomoto, S.; Takeda, S.; Nakao, A. Intra-operative application of real-time tissue elastography for the diagnosis of liver tumours. Liver Int. 2008, 28, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.J.; Zhu, Y.; Spalding, C.S. In vivo real-time freehand palpation imaging. Ultrasound Med. Biol. 2003, 29, 427–435. [Google Scholar] [CrossRef]
- Shiina, T.; Nitta, N.; Ueno, E.I.; Bamber, J.C. Real time tissue elasticity imaging using the combined autocorrelation method. J. Med. Ultrason. 2002, 26, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Laufer, J.; Delpy, C.; Elwell, P.; Beard, P. Quantitative spatially resolved measurement of tissue chromophore concentrations using photoacoustic spectroscopy: Application to the measurement of blood oxygenation and haemoglobin concentration. Phys. Med. Biol. 2007, 52, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Held, K.G.; Jaeger, M.; Ricka, J.; Frenz, M.; Akarcay, H.G. Multiple irradiation sensing of the optical effective attenuation coefficient for spectral correction in handheld OA imaging. Photoacoustics 2016, 4, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.; Laufer, J.G.; Arridge, S.R.; Beard, P.C. Quantitative spectroscopic photoacoustic imaging: A review. J. Biomed. Opt. 2012, 17, 0612021–06120222. [Google Scholar] [CrossRef]
- Jansen, K.; van Soest, G.; van der Steen, A.F.W. Intravascular photoacoustic imaging: A new tool for vulnerable plaque identification. Ultrasound Med. Biol. 2014, 40, 1037–1048. [Google Scholar] [CrossRef]
- Wu, M.; Jansen, K.; van der Steen, A.F.W.; van Soest, G. Specific imaging of atherosclerotic plaque lipids with two-wavelength intravascular photoacoustics. Biomed. Opt. Exp. 2015, 6, 3276–3286. [Google Scholar] [CrossRef]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef]
- Niederhauser, J.J.; Jaeger, M.; Lemor, R.; Weber, P.; Frenz, M. Combined ultrasound and optoacoustic system for real-time high-contrast vascular imaging in vivo. IEEE Trans. Med. Imaging 2005, 24, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, K.; van den Berg, P.J.; Rabot, O.; Kohl, A.; Tisserand, S.; Brands, P.; Steenbergen, W. Handheld probe integrating laser diode and ultrasound transducer array for ultrasound/photoacoustic dual modality imaging. Opt. Express 2014, 22, 26365–26374. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.A.; Steenbergen, W.; Manohar, S. Handheld probe-based dual mode ultrasound/photoacoustics for biomedical imaging. In Frontiers in Biophotonics for Translational Medicine; Springer: Singapore, 2016; pp. 209–247. [Google Scholar]
- Laufer, J.; Johnson, P.; Zhang, E.; Treeby, B.; Cox, B.; Pedley, B.; Beard, P. In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy. J. Biomed. Opt. 2012, 17, 0560161–0560168. [Google Scholar] [CrossRef] [PubMed]
- Toi, M.; Asao, Y.; Matsumoto, Y.; Sekiguchi, H.; Yoshikawa, A.; Takada, M.; Kataoka, M.; Endo, T.; Kawaguchi-Sakita, N.; Kawashima, M.; et al. Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array. Sci. Rep. 2017, 7, 41970. [Google Scholar] [CrossRef] [PubMed]
- Sarvazyan, A.P.; Urban, M.W.; Greenleaf, J.F. Acoustic waves in medical imaging and diagnostics. Ultrasound Med. Biol. 2013, 39, 1133–1146. [Google Scholar] [CrossRef]
- Bamber, J.C.; Hill, C.R.; King, J.A. Acoustic properties of normal and cancerous human liver-II Dependence on tissue structure. Ultrasound Med. Biol. 1981, 7, 135–144. [Google Scholar] [CrossRef]
- Greenleaf, J.F.; Bahn, R.C. Clinical imaging with transmissive ultrasonic computerized tomography. IEEE Trans. Biomed. Eng. 1981, 28, 177–185. [Google Scholar] [CrossRef]
- Carson, P.; Meyer, C.; Scherzinger, A.; Oughton, T. Breast imaging in coronal planes with simultaneous pulse-echo and transmission ultrasound. Science 1981, 214, 1141–1143. [Google Scholar] [CrossRef]
- Scherzinger, A.; Belgam, R.; Carson, P.; Meyer, C.; Sutherland, J.; Bookstein, F.; Silver, T. Assessment of ultrasonic computed tomography in symptomatic breast patints by discriminant analysis. Ultrasound Med. Biol. 1989, 15, 21–28. [Google Scholar] [CrossRef]
- Duric, N.; Littrup, P.; Poulo, L.; Babkin, A.; Pevzner, R.; Holsapple, E.; Rama, O.; Glide, C. Detection of breast cancer with ultrasound tomography: First results with the computed ultrasound risk evaluation (CURE) prototype. Med. Phys. 2007, 34, 773–785. [Google Scholar] [CrossRef]
- Wiskin, J.; Borup, D.; Johnson, S.; Berggren, M. Non-linear inverse scattering: High resolution quantitative breast tissue tomography. J. Acoust. Soc. Am. 2012, 131, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Nebeker, J.; Nelson, T.R. Imaging of sound speed using reflection ultrasound tomography. J. Ultrasound Med. 2012, 31, 1389–1404. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, N.V.; Zapf, M.; Dapp, R.; Hopp, T.; Kaiser, W.A.; Gemmeke, H. First results of a clinical study with 3D ultrasound computer tomography. In Proceedings of the 2013 IEEE International Ultrasonics Symposium (IUS), Prague, Czech Republic, 21–25 July 2013. [Google Scholar]
- Zografos, G.; Liakou, P.; Koulocheri, D.; Liovarou, I.; Sofras, M.; Hadjiagapis, S.; Orme, M.; Marmarelis, V. Differentiation of BIRADS-4 small breast lesions via multimodal ultrasound tomography. Eur. Radiol. 2015, 25, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.E.; Ophir, J.; Wilson, L.S.; Chen, C.F. Pulse-echo ultrasound speed measurements: Progress and prospects. Ultrasound Med. Biol. 1991, 17, 633–646. [Google Scholar] [CrossRef]
- Shin, H.-C.; Prager, R.; Gomersall, H.; Kingsbury, N.; Treece, G.; Gee, A. Estimation of average speed of sound using deconvolution of medical ultrasound data. Ultrasound Med. Biol. 2010, 36, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.; Trahey, G.E. The direct estimation of sound speed using pulse-echo ultrasound. J. Acoust. Soc. Am. 1998, 104, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, M.; Held, G.; Peeters, S.; Preisser, S.; Grünig, M.; Frenz, M. Computed ultrasound tomography in echo mode for imaging speed of sound using pulse-echo sonography: Proof of principle. Ultrasound Med. Biol. 2015, 41, 235–250. [Google Scholar] [CrossRef]
- Jaeger, M.; Robinson, E.; Akarçay, H.; Frenz, M. Full correction for spatially distributed speed-of-sound in echo ultrasound based on measuring aberration delays via transmit beam steering. Phys. Med. Biol. 2015, 60, 4497–4515. [Google Scholar] [CrossRef]
- Jaeger, M.; Frenz, M. Quantitative imaging of speed of sound in echo ultrasonography. In Proceedings of the IEEE International Ultrasound Symposium 2015, Taipei, Taiwan, 23 October 2015. [Google Scholar]
- Lukanova, D.; Nikolov, N.; Genova, K.; Stankev, M.; Georgieva, E. The accuracy of noninvasive imaging techniques in diagnosis of carotid plaque morphology. Maced. J. Med. Sci. 2015, 3, 224–230. [Google Scholar] [CrossRef]
- Park, T. Evaluation of carotid plaque using ultrasound imaging. J. Cardiovasc. Ultrasound 2016, 24, 91–95. [Google Scholar] [CrossRef]
- Störk, S.; Feelders, R.A.; van den Beld, A.W.; Steyerberg, E.W.; Savelkoul, H.F.J.; Lamberts, S.W.J.; Grobbee, D.E.; Bots, M.L. Prediction of mortality risk in the elderly. Am. J. Med. 2006, 119, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Steinl, D.C.; Kaufman, B.A. Ultrasound imaging for risk assessment in atherosclerosis. Int. J. Mol. Sci. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Altedorneburg, G.; Droste, D.; Haas, N.; Kemény, V.; Nabavi, D.; Füzesi, L.; Ringelstein, E. Preoperative B-mode ultrasound plaque appearance compared with carotid endarterectomy specimen histology. Acta Neurol. Scand. 2000, 101, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.; Horvat, R.; Puchner, S.; Rinner, W.; Polterauer, P.; Lammer, J.; Minar, E.; Bucek, R.A. Plaque imaging of the internal carotid artery—Correlation of B-flow imaging with histopathology. AJNR 2007, 28, 122–126. [Google Scholar] [PubMed]
- Tanter, M.; Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Loupas, T.; Powers, J.T.; Gill, R.W. An axial velocity estimator for ultrasound blood flow imaging, based on a full evaluation of the Doppler equation by means of a two-dimensional autocorrelation approach. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995, 42, 672–688. [Google Scholar] [CrossRef]
| Tx Steering | Full Rx | Fast Rx | |
|---|---|---|---|
| Tx angles | Φ = −25°:2.5°:25° | Φ = 0° | Φ = 0° |
| Tx aperture | Φrad = 2.5°, 0.5° steps | Φrad = 5°, 0.5° steps | Φrad = 5°, 2.5° steps |
| Rx angles | Ψ = 0° | Ψ = −25°:2.5°:25° | Ψ = −25°:2.5°:25° |
| Rx aperture | Ψrad = 5° | Ψrad = 2.5° | Ψrad = 2.5° |
| CUTE angles | Φcute = −25°:10°:25° | Ψcute = −25°:10°:25° | Ψcute = −25°:10°:25° |
| Total acquisition time | 3.5 ms | 0.7 ms | 0.2 ms |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuriakose, M.; Muller, J.-W.; Stähli, P.; Frenz, M.; Jaeger, M. Receive Beam-Steering and Clutter Reduction for Imaging the Speed-of-Sound Inside the Carotid Artery. J. Imaging 2018, 4, 145. https://doi.org/10.3390/jimaging4120145
Kuriakose M, Muller J-W, Stähli P, Frenz M, Jaeger M. Receive Beam-Steering and Clutter Reduction for Imaging the Speed-of-Sound Inside the Carotid Artery. Journal of Imaging. 2018; 4(12):145. https://doi.org/10.3390/jimaging4120145
Chicago/Turabian StyleKuriakose, Maju, Jan-Willem Muller, Patrick Stähli, Martin Frenz, and Michael Jaeger. 2018. "Receive Beam-Steering and Clutter Reduction for Imaging the Speed-of-Sound Inside the Carotid Artery" Journal of Imaging 4, no. 12: 145. https://doi.org/10.3390/jimaging4120145
APA StyleKuriakose, M., Muller, J.-W., Stähli, P., Frenz, M., & Jaeger, M. (2018). Receive Beam-Steering and Clutter Reduction for Imaging the Speed-of-Sound Inside the Carotid Artery. Journal of Imaging, 4(12), 145. https://doi.org/10.3390/jimaging4120145





