Morphodynamic Features of Contrast-Enhanced Mammography and Their Correlation with Breast Cancer Histopathology
Abstract
1. Introduction
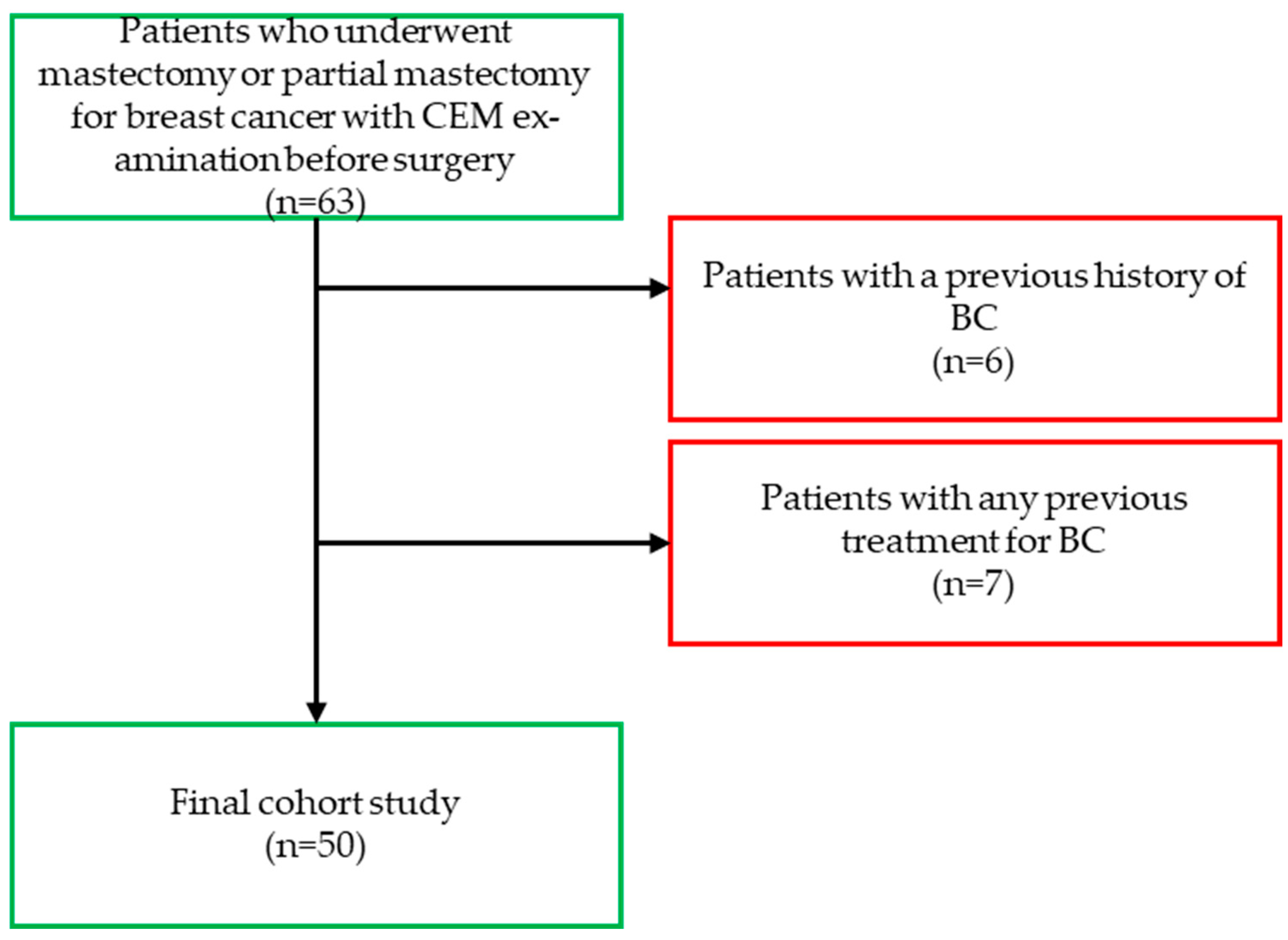
2. Materials and Methods
2.1. Study Design and Population
2.2. Image Acquisition
2.3. Image Preprocessing
2.4. Reader Study
2.5. Histopathological Evaluation
2.6. Statistical Analysis
3. Results
3.1. Study Population
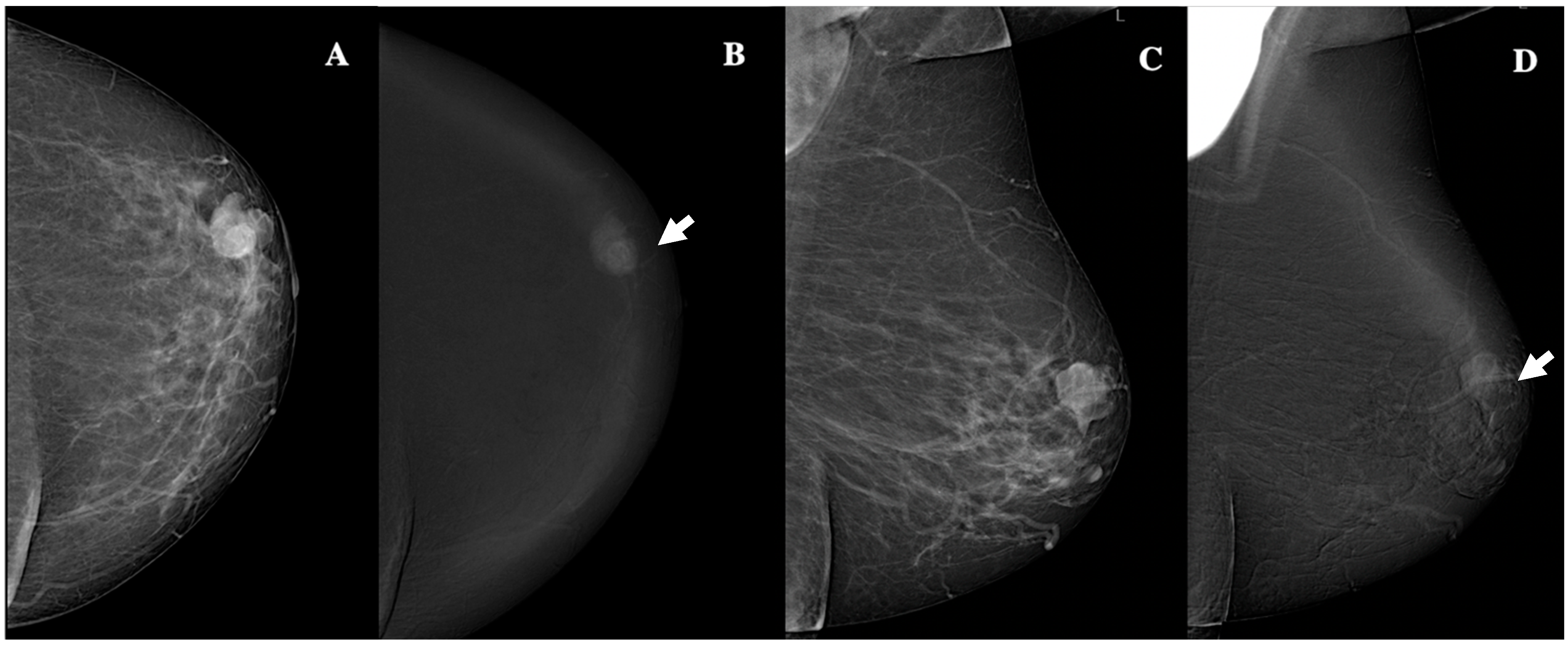
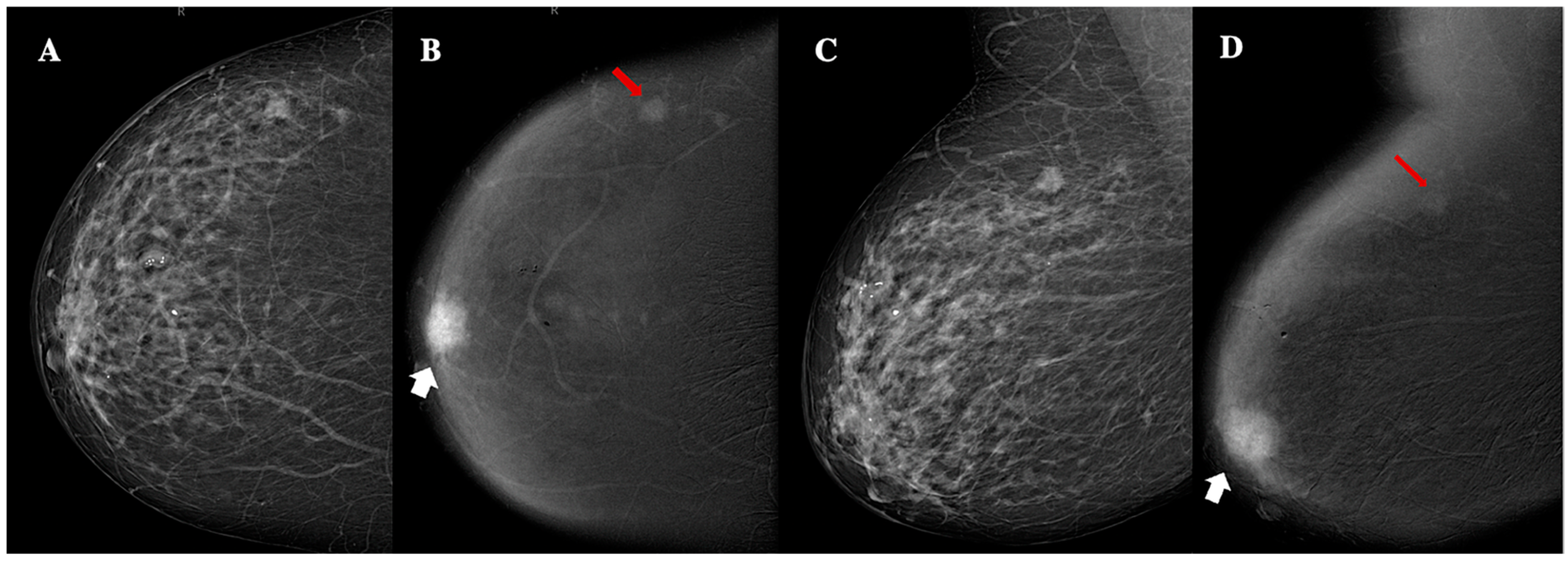
3.2. CEM Findings
3.3. Histopathological Findings
3.4. Relationship Between CEM Findings and Histopathological Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sibylle, L.; Philip, P.; Monica, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar]
- Jong, R.A.; Yaffe, M.J.; Skarpathiotakis, M.; Shumak, R.S.; Danjoux, N.M.; Gunesekara, A.; Plewes, D.B. Contrast-enhanced digital mammography: Initial clinical experience. Radiology 2003, 228, 842–850. [Google Scholar] [CrossRef]
- Ventura, C.; Baldassarre, S.; Cerimele, F.; Pepi, L.; Marconi, E.; Ercolani, P.; Floridi, C.; Argalia, G.; Goteri, G.; Giovagnoni, A. 2D shear wave elastography in evaluation of prognostic factors in breast cancer. Radiol. Medica 2022, 127, 1221–1227. [Google Scholar] [CrossRef]
- Lobbes, M.B.I.; Smidt, M.L.; Houwers, J.; Tjan-Heijnen, V.C.; Wildberger, J.E. Contrast-enhanced mammography: Techniques, current results, and potential indications. Clin. Radiol. 2013, 68, 935–944. [Google Scholar] [CrossRef]
- Diekmann, F.; Freyer, M.; Diekmann, S.; Stengel, A.; Fischer, T.; Bick, U. Evaluation of contrast-enhanced digital mammography. Eur. J. Radiol. 2011, 78, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Polat, D.S.; Evans, W.P.; Dogan, B.E. Contrast-enhanced digital mammography: Technique, clinical applications, and pitfalls. Am. J. Roentgenol. 2020, 215, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Covington, M.F.; Salmon, S.; Weaver, B.D.; Fajardo, L.L.; Rhodes, D.J. State-of-the-art for contrast-enhanced mammography. Br. Inst. Radiol. 2024, 97, 695–704. [Google Scholar] [CrossRef]
- Ainakulova, A.S.; Zholdybay, Z.Z.; Kaidarova, D.R.; Inozemtceva, N.I.; Gabdullina, M.O.; Zhakenova, Z.K.; Panina, A.S.; Toleshbayev, D.K.; Amankulov, J.M. Contrast-enhanced spectral mammography without and with a delayed image for diagnosing malignancy among mass lesions in dense breast. Wspolczesna Onkol. 2021, 25, 17–22. [Google Scholar] [CrossRef]
- Sorin, V.; Yagil, Y.; Yosepovich, A.; Shalmon, A.; Gotlieb, M.; Neiman, O.H.; Sklair-Levy, M. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. Am. J. Roentgenol. 2018, 211, W267–W274. [Google Scholar] [CrossRef]
- Bernardi, D.; Vatteroni, G.; Acquaviva, A.; Monti, S.; Bariani, M.; Petrillo, A. Contrast-enhanced mammography versus MRI in the evaluation of neoadjuvant therapy response in patients with breast cancer: A prospective study. Am. J. Roentgenol. 2022, 219, 884–894. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Lobbes, M.B.I.; Scaranelo, A.M.; Margolies, L.R.; Gilbert, F.J.; Lee, C.H. Contrast-enhanced mammography: State of the art. Radiology 2021, 299, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Pötsch, N.; Vatteroni, G.; Clauser, P.; Baltzer, P.A.T.; Helbich, T.H. Contrast-enhanced mammography versus contrast-enhanced breast MRI: A systematic review and meta-analysis. Radiology 2022, 305, 94–103. [Google Scholar] [CrossRef]
- Lee, C.H.; Phillips, J.; Sung, J.S.; Giess, C.S.; Jochelson, M.S.; Margolies, L.R. ACR BI-RADS® Atlas-Mammography Contrast Enhanced Mammography (CEM); American College of Radiology: Reston, VA, USA, 2022. [Google Scholar]
- Nicosia, L.; Battaglia, O.; Venturini, M.; Pellegrino, S.; Priola, S.; Bernardi, D. Contrast-enhanced mammography BI-RADS: A case-based approach to radiology reporting. Insights Imaging 2024, 15, 37. [Google Scholar] [CrossRef]
- Magni, V.; Cozzi, A.; Muscogiuri, G.; Pellegrino, S.; Nicosia, L.; De Marco, P. Background parenchymal enhancement on contrast-enhanced mammography: Associations with breast density and patient characteristics. Radiol. Medica 2024, 129, 1303–1312. [Google Scholar] [CrossRef]
- Petrillo, A.; Fusco, R.; Petrosino, T.; Bianchi, F.; Ferrara, D.; Barone, C. A multicentric study of radiomics and artificial intelligence analysis on contrast-enhanced mammography to identify different histotypes of breast cancer. Radiol. Medica 2024, 129, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Bartolotta, T.V.; Militello, C.; Prinzi, F.; Ferraro, F.; Rundo, L.; Zarcaro, C.; Dimarco, M.; Orlando, A.A.M.; Matranga, D.; Vitabile, S. Artificial intelligence-based, semi-automated segmentation for the extraction of ultrasound-derived radiomics features in breast cancer: A prospective multicenter study. Radiol. Medica 2024, 129, 977–988. [Google Scholar] [CrossRef]
- Migliaro, G.; Bicchierai, G.; Valente, P.; Amato, F.; Vanzi, B.; Ventura, C. Contrast enhanced mammography (CEM) enhancing asymmetry: Single-center first case analysis. Diagnostics 2023, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gong, W.; Xie, Y.; Sheng, L. Correlation between the CEM imaging characteristics and different molecular subtypes of breast cancer. Radiology 2023, 72, 103595. [Google Scholar] [CrossRef] [PubMed]
- Fogante, M.; Tagliati, C.; De Lisa, M.; Berardi, R.; Giuseppetti, G.M.; Giovagnoni, A. Correlation between apparent diffusion coefficient of magnetic resonance imaging and tumor-infiltrating lymphocytes in breast cancer. Radiol. Medica 2019, 124, 581–587. [Google Scholar] [CrossRef]
- Prochowski Iamurri, A.; Ponziani, M.; Macchini, M.; Fogante, M.; Pistelli, M.; De Lisa, M.; Berardi, R.; Giuseppetti, G.M. Evaluation of multifocality and multicentricity with breast magnetic resonance imaging in each breast cancer subtype. Clin. Breast Cancer 2018, 18, e231–e235. [Google Scholar] [CrossRef] [PubMed]
- Soylu Boy, F.N.; Goksu, K.; Tasdelen, I. Association between lesion enhancement and breast cancer in contrast-enhanced spectral mammography. Acta Radiol. 2023, 64, 74–79. [Google Scholar] [CrossRef]
- Marzogi, A.; Baltzer, P.A.T.; Kapetas, P.; Milos, R.I.; Bernathova, M.; Helbich, T.H.; Clauser, P. Is the level of contrast enhancement on contrast-enhanced mammography (CEM) associated with the presence and biological aggressiveness of breast cancer? Diagnostics 2023, 13, 754. [Google Scholar] [CrossRef]
- Travieso-Aja, M.M.; Naranjo-Santana, P.; Fernández-Ruiz, C.; Rodríguez-Abreu, D.; García-Hernández, N. Factors affecting the precision of lesion sizing with contrast-enhanced spectral mammography. Clin. Radiol. 2018, 73, 296–303. [Google Scholar] [CrossRef]
- Nicosia, L.; Bozzini, A.C.; Palma, S.; Mantini, G.; Marini, P.; Pruneri, G. Contrast-enhanced spectral mammography and tumor size assessment: A valuable tool for appropriate surgical management of breast lesions. Radiol. Medica 2022, 127, 1228–1234. [Google Scholar] [CrossRef]
- Cozzi, A.; Schiaffino, S.; Sardanelli, F. The emerging role of contrast-enhanced mammography. Quant. Imaging Med. Surg. 2019, 9, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, A.; Nicosia, L.; Pruneri, G.; Maisonneuve, P.; Meneghetti, L.; Renne, G.; Vingiani, A.; Cassano, E.; Mastropasqua, M.G. Clinical performance of contrast-enhanced spectral mammography in pre-surgical evaluation of breast malignant lesions in dense breasts: A single-center study. Breast Cancer Res. Treat. 2020, 184, 723–731. [Google Scholar] [CrossRef]
- Wessam, R.; Gomaa, M.; Fouad, M.A.; Omar, W.; Mohamed, M. Added value of contrast-enhanced mammography in assessment of breast asymmetries. Clin. Radiol. 2019, 92, 20180245. [Google Scholar] [CrossRef]
- Dominique, C.; Callonnec, F.; Berghian, A.; Defta, D.; Vera, P.; Modzelewski, R.; Decazes, P. Deep learning analysis of contrast-enhanced spectral mammography to determine histoprognostic factors of malignant breast tumours. Eur. Radiol. 2022, 32, 4834–4844. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, A.; Fusco, R.; Di Bernardo, E.; Petrosino, T.; Barretta, M.L.; Porto, A.; Granata, V.; Di Bonito, M.; Fanizzi, A.; Massafra, R.; et al. Prediction of breast cancer histological outcome by radiomics and artificial intelligence analysis in contrast-enhanced mammography. Cancers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Piccolo, C.L.; Sarli, M.; Pileri, M.; Costa, F.; Chiarenza, M.; Tagliati, C. Radiomics for predicting prognostic factors in breast cancer: Insights from contrast-enhanced mammography (CEM). J. Clin. Med. 2024, 13, 6486. [Google Scholar] [CrossRef]
- La Forgia, D.; Fanizzi, A.; Campobasso, F.; Bellotti, R.; Didonna, V.; Lorusso, V.; Moschetta, M.; Massafra, R.; Tamborra, P.; Tangaro, S.; et al. Radiomic analysis in contrast-enhanced spectral mammography for predicting breast cancer histological outcome. Diagnostics 2020, 10, 708. [Google Scholar] [CrossRef] [PubMed]
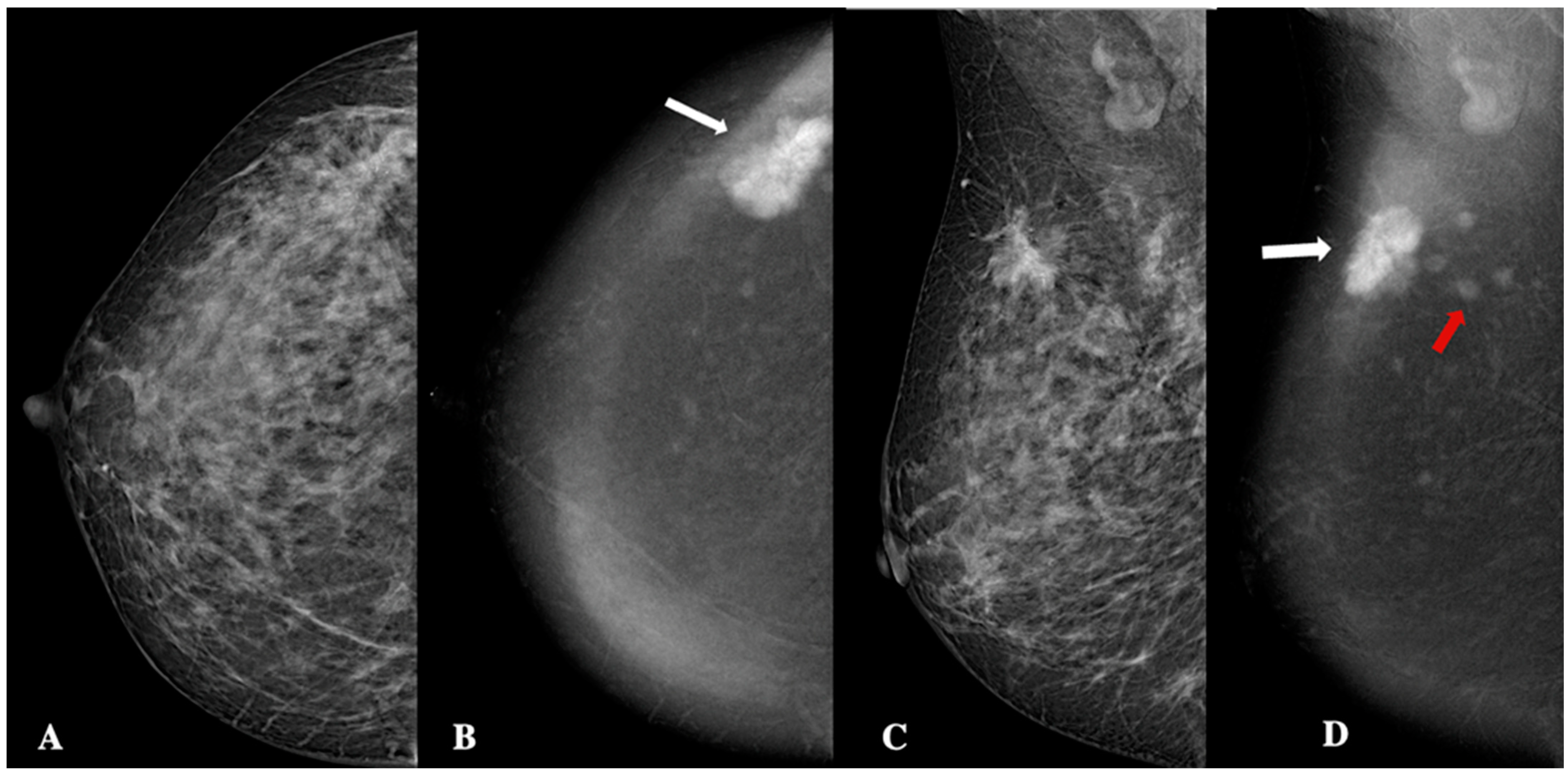
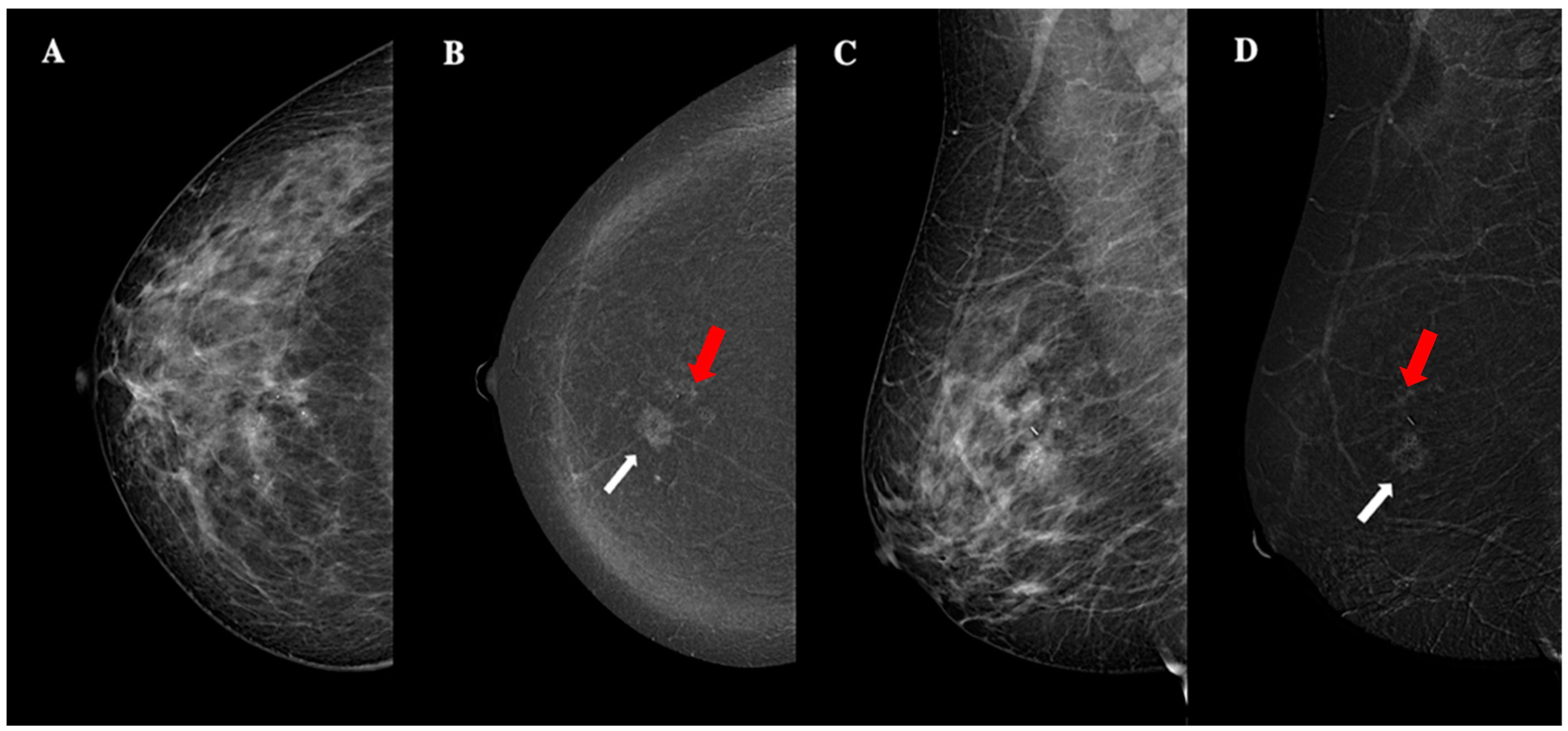
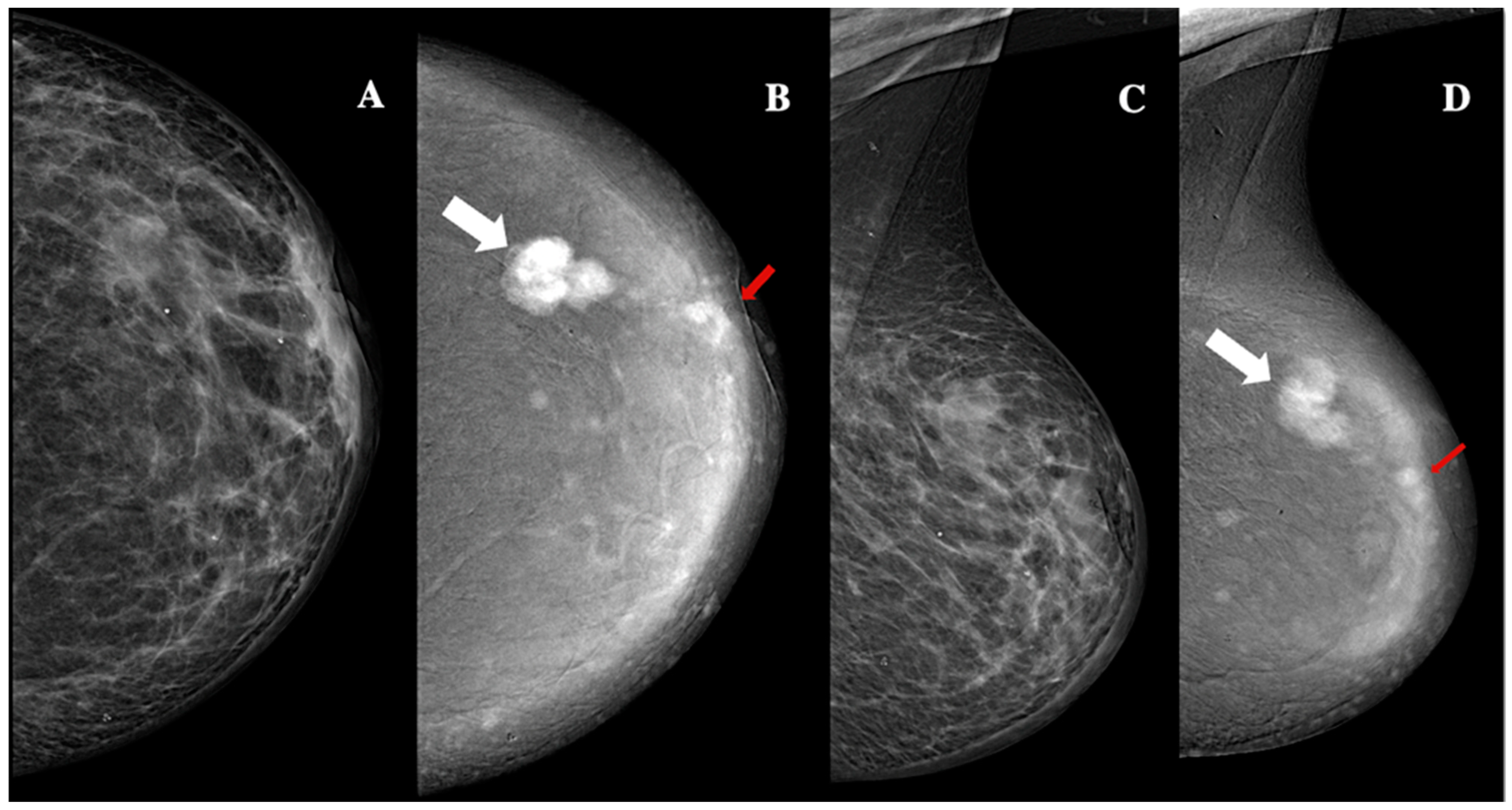
| Demographic characteristics | Age (year, mean ± SD) | 57.2 ± 13.7 |
| Body mass index (kg/m2, mean ± SD) | 23.7 ± 8.1 | |
| Clinical characteristics | Presence of palpable nodule (n, %) | 13 (26%) |
| Presence of nipple secrection (n, %) | 3 (6%) | |
| Presence of retraction (n, %) | 2 (4%) | |
| Presence of cutaneous retraction (n, %) | 3 (6%) | |
| Presence of palpable adenopathy (n, %) | 4 (8%) | |
| Family history of breast cancer (n, %) | 17 (34%) |
| Breast composition | A (almost entirely fatty) (n, %) | 4 (8%) |
| B (scattered fibroglandular areas) (n, %) | 30 (60%) | |
| C (heterogeneously dense) (n, %) | 14 (28%) | |
| D (extremely dense) (n, %) | 2 (4%) | |
| Microcalcifications | Present (n, %)/absent (n, %) | 11 (22%)/39 (78%) |
| Architectural distorsion | Present (n, %)/absent (n, %) | 34 (68%)/16 (32%) |
| Mean lesion dimension | Size (mm—mean ± SD) | 23.4 ± 10.9 |
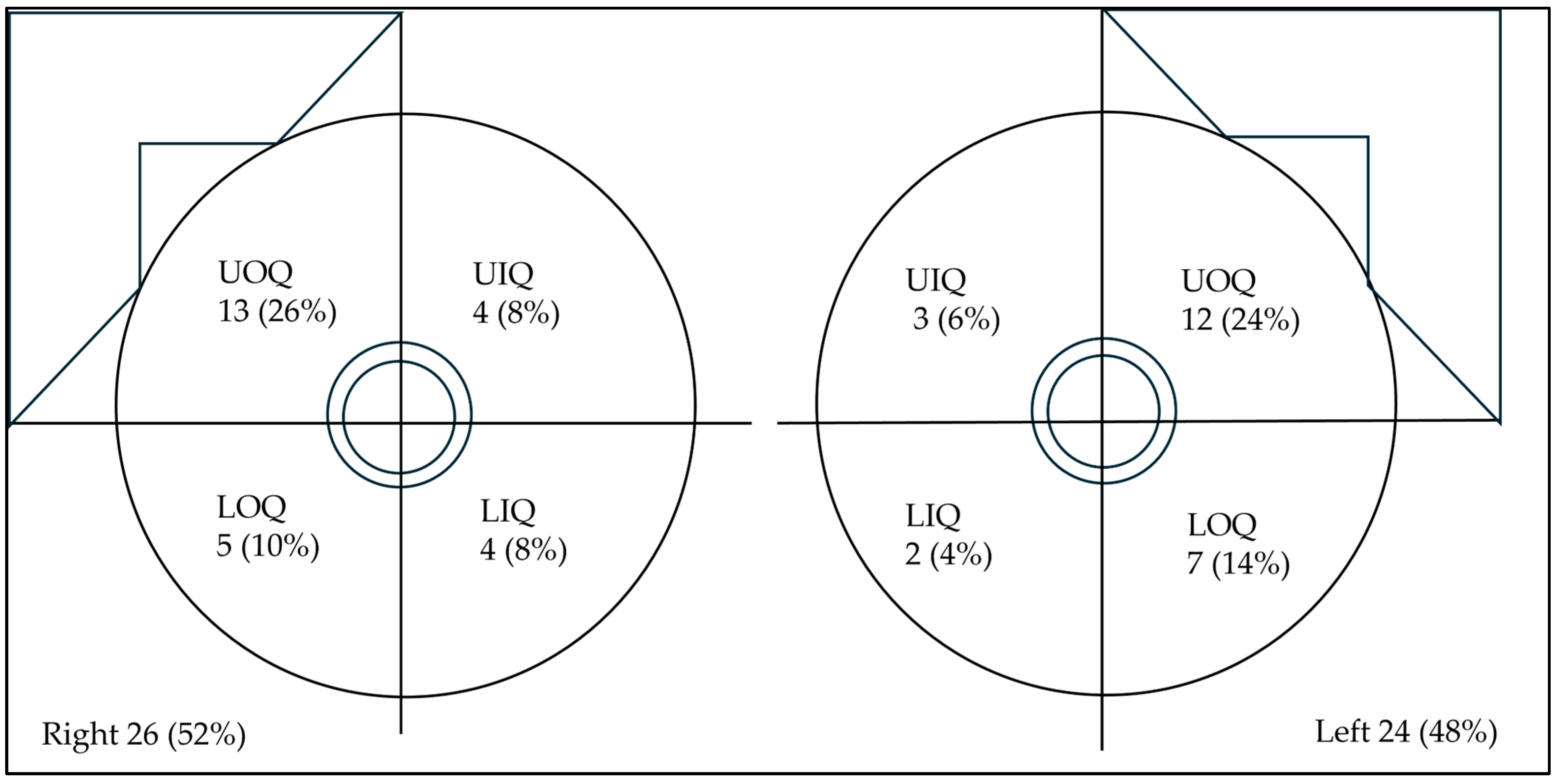
| Lesion side | Right (n,%)/left (n, %) | 26 (52%)/24 (48%) |
| Lesion location | UOQ (n,%)/UIQ (n,%) | 25 (50%)/7 (14%) |
| LOQ (n,%)/LIQ (n,%) | 12 (24%)/6 (12%) | |
| Lesion shape | Oval (n, %)/round (n, %)/irregular (n,%) | 31 (62%)/9 (18%)/10 (20%) |
| Lesion margins | NC (n, %)/C (n, %) | 7 (14%)/43 (86%) |
| Lesion internal enhancement | Homogeneous (n, %) | 8 (16%) |
| Heterogeneous (n, %) | 37 (74%) | |
| Rim enhancement (n, %) | 5 (10%) | |
| Lesion enhancement conspicuity | Low (n, %) | 25 (50%) |
| Moderate (n, %) | 10 (20%) | |
| High (n, %) | 15 (30%) | |
| Associated features | Nipple retraction (n, %) | 4 (8%) |
| Nipple invasion (n, %) | 2 (4%) | |
| Skin retraction (n, %) | 2 (4%) | |
| Skin thickening (n, %) | 3 (6%) | |
| Skin invasion (n, %) | 9 (18%) | |
| Axillary adenopathy (n, %) | 3 (6%) | |
| Background parenchymal enhancement | Minimal (n, %) | 26 (52%) |
| Mild (n, %) | 15 (30%) | |
| Moderate—marked (n, %) | 9 (18%) |
| Mean lesion dimension | Size (mm) (mean ± SD) | 23.7 ± 14.3 |
| Histological phenotype | Ductal (n, %) | 36 (72%) |
| Lobular (n, %) | 14 (28%) | |
| Grading (Elston–Ellis) | Grade 1 (n, %) | 7 (14%) |
| Grade 2 (n, %) | 25 (50%) | |
| Grade 3 (n, %) | 18 (36%) | |
| Immunophenotype | Luminal-A (n, %) | 18 (36%) |
| Luminal-B (n, %) | 26 (52%) | |
| HER2-enriched (n, %) | 2 (4%) | |
| Triple-negative (n, %) | 4 (8%) | |
| Lymphovascular invasion | Present (n, %)/absent (n, %) | 16 (32%)/34 (68%) |
| CEM Findings | Histopathological/Clinical Characteristics | Statistical Relationship |
|---|---|---|
| Tumor dimension | Tumor size | Positive correlation (ρ = 0.788, p < 0.001) |
| Grade 3 | Significant association (p = 0.017) | |
| Non-circumscribed margins | Luminal-B | Significant association (p = 0.001) |
| High lesion conspicuity | Luminal-B (n, %) | Significant association (p = 0.001) |
| Triple-negative (n, %) | Significant association (p = 0.001) | |
| Tumor size | Positive correlation (ρ = 0.517, p < 0.001) | |
| BPE | Age | Negative correlation (ρ = −0.286, p = 0.049) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, C.; Fogante, M.; Marconi, E.; Franca Simonetti, B.; Borgoforte Gradassi, S.; Carboni, N.; Lenti, E.; Argalia, G. Morphodynamic Features of Contrast-Enhanced Mammography and Their Correlation with Breast Cancer Histopathology. J. Imaging 2025, 11, 80. https://doi.org/10.3390/jimaging11030080
Ventura C, Fogante M, Marconi E, Franca Simonetti B, Borgoforte Gradassi S, Carboni N, Lenti E, Argalia G. Morphodynamic Features of Contrast-Enhanced Mammography and Their Correlation with Breast Cancer Histopathology. Journal of Imaging. 2025; 11(3):80. https://doi.org/10.3390/jimaging11030080
Chicago/Turabian StyleVentura, Claudio, Marco Fogante, Elisabetta Marconi, Barbara Franca Simonetti, Silvia Borgoforte Gradassi, Nicola Carboni, Enrico Lenti, and Giulio Argalia. 2025. "Morphodynamic Features of Contrast-Enhanced Mammography and Their Correlation with Breast Cancer Histopathology" Journal of Imaging 11, no. 3: 80. https://doi.org/10.3390/jimaging11030080
APA StyleVentura, C., Fogante, M., Marconi, E., Franca Simonetti, B., Borgoforte Gradassi, S., Carboni, N., Lenti, E., & Argalia, G. (2025). Morphodynamic Features of Contrast-Enhanced Mammography and Their Correlation with Breast Cancer Histopathology. Journal of Imaging, 11(3), 80. https://doi.org/10.3390/jimaging11030080






