Abstract
Direct Contact Membrane Distillation (DCMD) uses low heat sources to separate water from urea, which was then used as a plasticizer in regolith-based cement to make it more workable. The work investigated separating potable water and urea from artificial urine using DCMD and then characterizing the products. Water was successfully separated from the artificial urine solution as characterized by density, conductivity, pH, and substance concentrations. The concentrated urine solution was used in regolith-based cement cured under vacuum at temperatures that simulated temperatures that would be expected in construction on the Moon. Workability and other properties were improved by replacing water with concentrated urine solution in the mix.
Keywords:
artificial urine; membrane; flux; concentration; pH; density; ionic conductivity; lunar regolith; slump; compression strength 1. Introduction
To establish a human presence on the Moon, constructing structures will be essential. NASA plans to establish a base camp near the Moon’s south pole as part of the Artemis mission [1]. Transporting materials from Earth requires significant energy and complicates space mission logistics. This technical challenge can be addressed by using locally sourced materials. Concrete is a suitable building material, as it provides partial radiation shielding [2]. A cement binder can bond locally sourced fillers to create durable and strong concrete. Adequate workability of the concrete can be achieved by adding water to the binder–filler mixture to form a 3D structure. However, the strength of cement decreases with the addition of more water, and water is prone to evaporation in the Moon’s low-pressure environment, leading to shrinkage cracks. Moreover, transporting sufficient water from Earth is energy intensive. To mitigate these issues, small amounts of plasticizers can be added to the cement binder to enhance workability with less water. Plasticizers are typically produced from fossil fuels, such as coal or crude oil, and then would need to be transported from Earth as part of a space vehicle’s payload. The challenge lies in identifying a source of plasticizer that can be readily available at a lunar outpost.
Urea has been identified as an effective superplasticizer for regolith-based geopolymers [3] and is also known for its stabilizing properties [4]. In deep space, astronauts will produce urine, which is nearly always sterile [5] and contains valuable urea. However, urine is a highly dilute solution of urea, with 97% of it being water. In a deep space environment, it is essential to reuse this water. Therefore, this research study attempted to concentrate urine using Direct Contact Membrane Distillation (DCMD), measure the workability of regolith-based cement that replaces water with the concentrated solution, and cure the regolith-based cement samples under vacuum and appropriate lunar temperatures [6].
Traditional methods for extracting pure water from wastewater streams include standard distillation and vacuum distillation. Standard distillation is energy-intensive, while vacuum distillation, though it operates at lower temperatures, requires a complex vacuum system. Standard filtration methods necessitate frequent filter replacements, which involves resupply challenges.
In our lab, we have developed a novel yet simple method to separate pure water from wastewater, utilizing waste heat at temperatures as low as 50 °C. Such waste heat is likely available from various processes at a Moon outpost or on a spacefaring vessel. This method, DCMD, relies on the vapor pressure difference in water at different temperatures to separate water from solutes such as urea. DCMD is able to separate water from non-volatile compounds. The hydrophobic membrane allows water vapor to pass through while blocking hydrophilic solutes like urea, nitrates/nitrites, Ca2+, and K+. Once through the membrane, the pure water condenses in a cold water stream circulating above, making it available for reuse for washing and/or as irrigation water for the production of saline sensitive crops. This process, already utilized in desalination [7,8], relies on pressure differences from temperature differences in a closed system, making it feasible for low-gravity environments like the Moon’s surface or deep space. Hydrolyzed urine samples adjusted to a pH of 10.5 have been reported to be concentrated up to five times by DCMD [9]. Urine concentrated by DCMD has also been reported to be feasible to partially replace nutrients in hydroponic systems [10]. This study focuses on utilizing concentrated urine solutions as a substitute for DI water in concrete mixtures and examining their effect on the plasticization of lunar regolith. A secondary objective was investigating the beneficial side product of the process, purified water, for other uses.
2. Results and Discussion
2.1. Flux
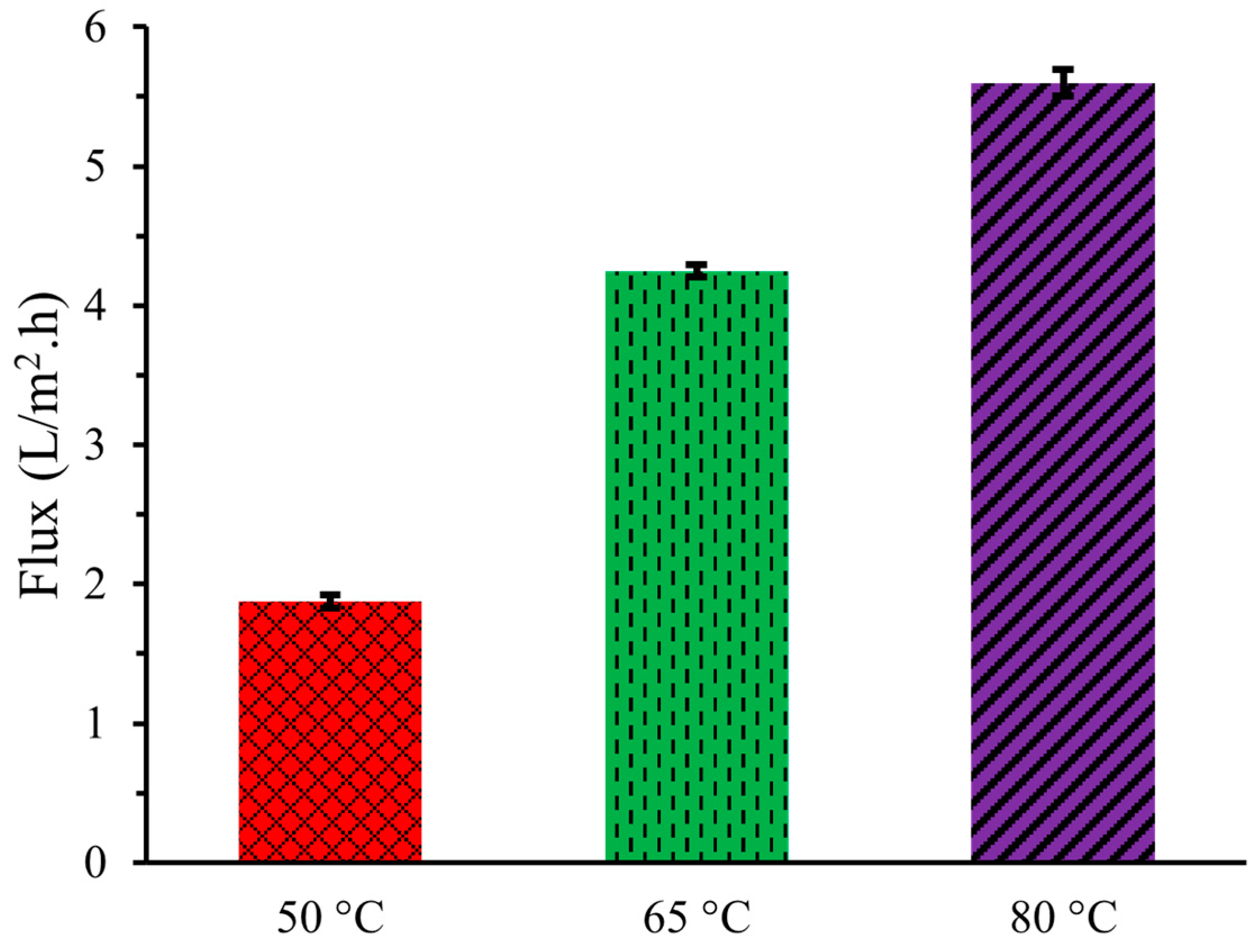
Figure 1 demonstrates that the flux, or the amount of pure water vapor passing through the PTFE membrane per unit area per unit time from the artificial urine solution increased with higher operating temperatures of DCMD. A greater temperature difference between the feed and permeating sides leads to a higher vapor pressure difference, resulting in increased flux. At an operating temperature of 50 °C, the flux of water vapor through the membrane was 1.88 ± 0.05 L/m2·h, which increased to 4.25 ± 0.05 L/m2·h and 5.06 ± 0.09 L/m2·h at 65 °C and 80 °C, respectively. These findings are consistent with the values reported by Lee et al. (2018) and Mohammad et al. (2015) [11,12]. This increase in flux was due to the higher feed temperatures enhancing the vapor pressure gradient across the membrane, thereby boosting water vapor transfer rates.
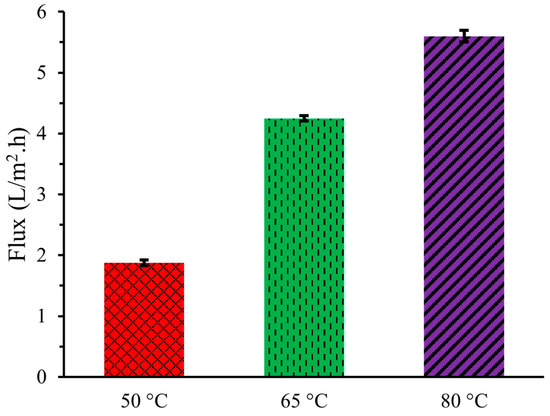
Figure 1.
Flux of artificial urine at three different temperatures during DCMD.
However, the membrane exhibited fouling effects with repeated use, defined as reduction in flux capacity. DCMD was run three times at each temperature, resulting in the measurement of water transfer and flux calculation being conducted three times for each temperature setting. After each run, the flux very slightly decreased due to fouling effects, with residual chemical substances accumulating on the bottom of the membrane, which increased with the number of runs. In the first trial, the flux, at an operating temperature of 50 °C, was recorded at 1.94 L/m2·h, which decreased to 1.91 L/m2·h and 1.78 L/m2·h in the second and third trials, respectively, indicating membrane fouling. Similarly, for the operating temperature of 65 °C, the flux in the first trial was measured at 4.38 L/m2·h, decreasing to 4.19 L/m2·h and 4.17 L/m2·h in the second and third trials. At 80 °C, the initial flux was 5.84 L/m2·h, which subsequently dropped to 5.57 L/m2·h and 5.39 L/m2·h in the second and third trials, respectively. Despite these variations, the average flux was used for analysis.
2.2. Concentration
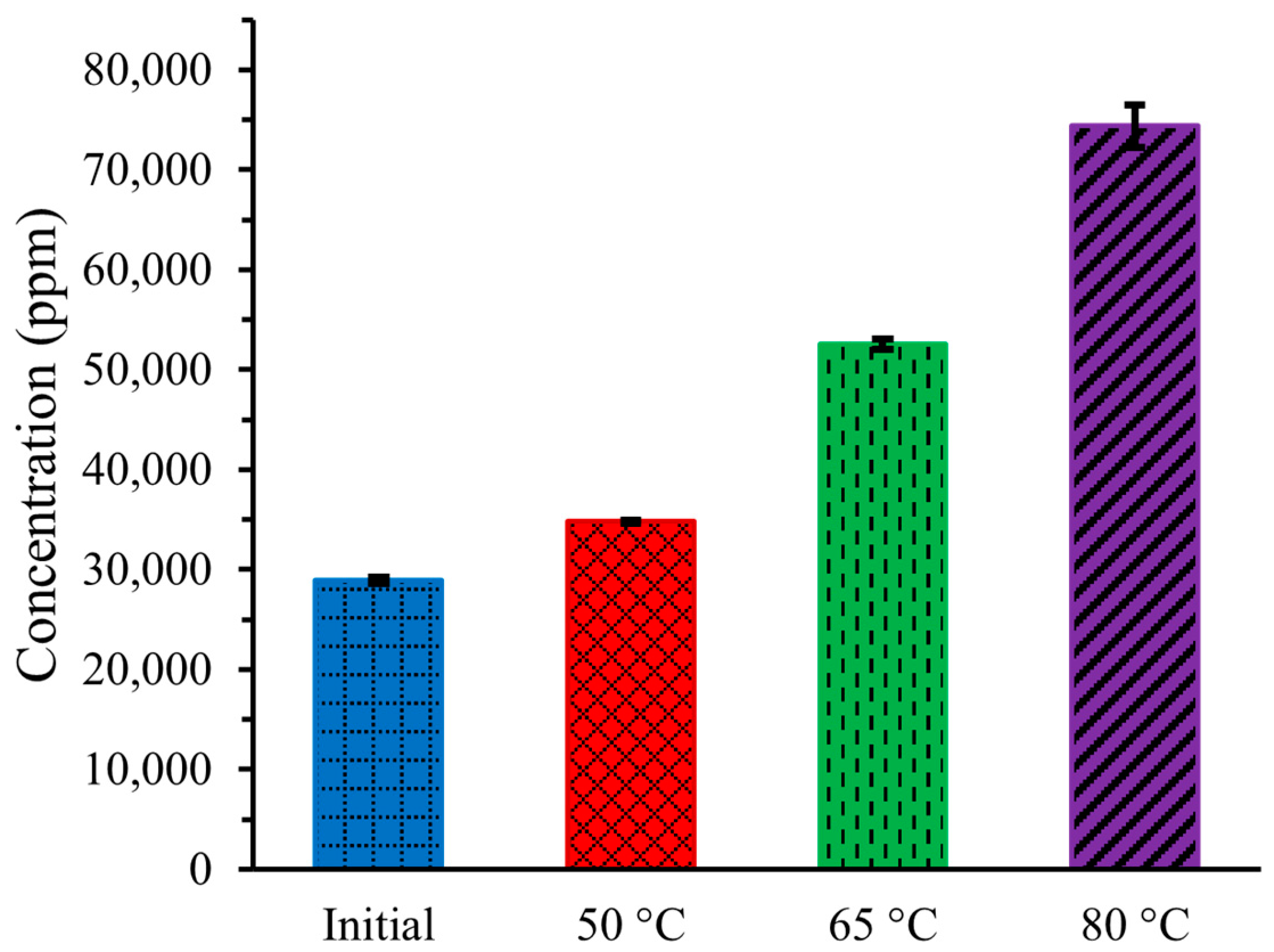
Figure 2 illustrates the concentration of substances in artificial urine both initially and after an 8 h operation of DCMD at three different temperatures. The concentration of chemical substances increases with the operating temperature of DCMD. The initial concentration of substances, including all chemical compounds, was 28,926 ± 317 ppm. After 8 h of DCMD operation, the concentration increased to 34,768 ± 168 ppm, 52,559 ± 534 ppm, and 74,349 ± 2108 ppm at temperatures of 50 °C, 65 °C, and 80 °C, respectively.
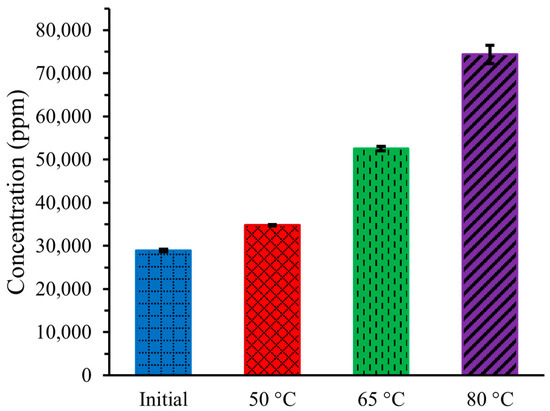
Figure 2.
Concentration of artificial urine at three different temperatures during DCMD.
The increased concentration at higher temperatures occurred because more pure water was transferred from the hot urine stream to the cold DI water stream due to the higher vapor pressure gradient, resulting in a more concentrated remaining urine stream.
2.3. Density
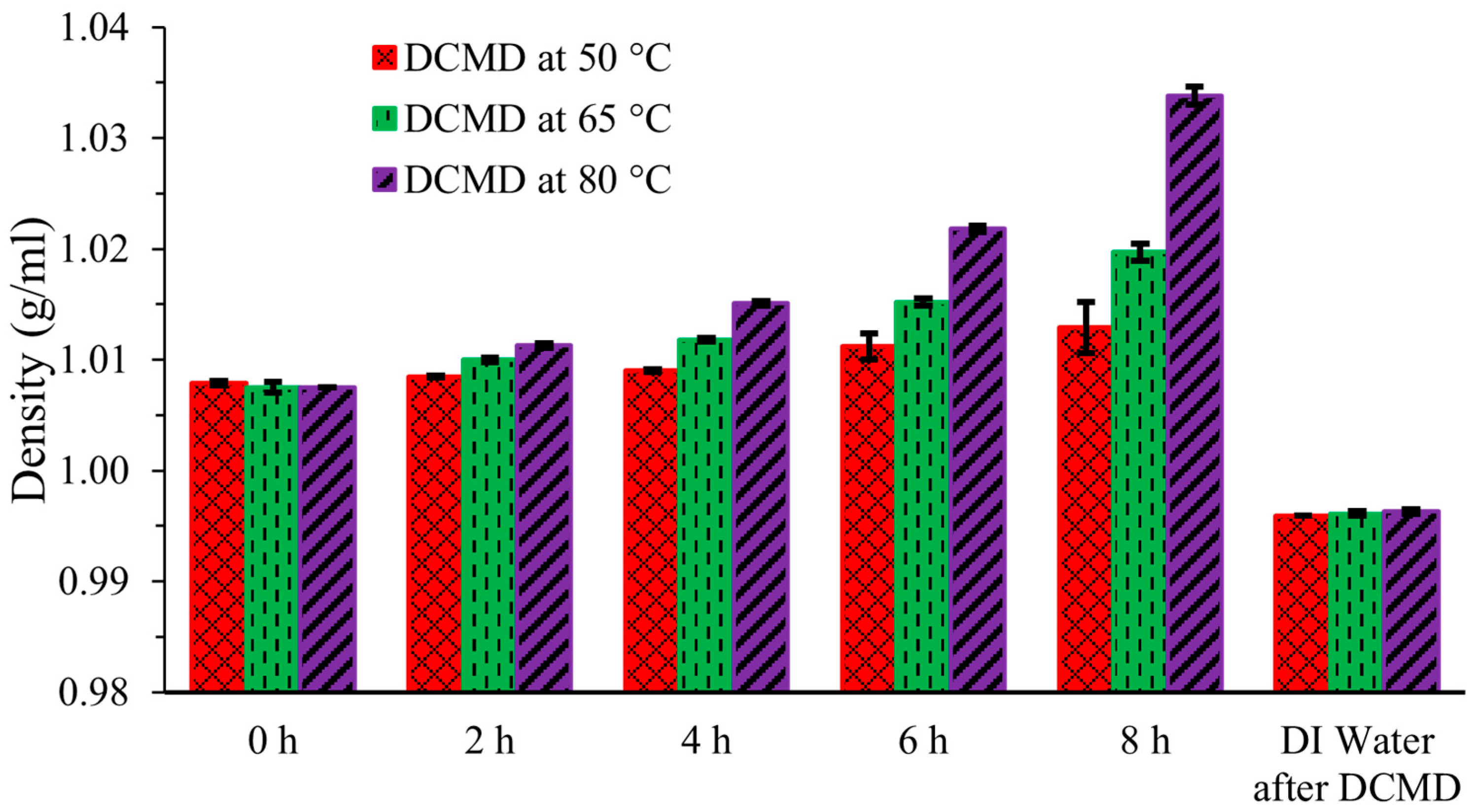
Figure 3 displays the density of the liquid streams. Densities of the artificial urine feed streams increased with elapsed time and with increased temperature as water molecules in vapor form, which are more volatile and smaller, passed through the membrane. The heavy, large, and less volatile components like urea remained in the solutions. The initial density at 0 h was 1.0075 ± 0.0005 g/mL, and it increased as water evaporated, particularly at the higher operating temperatures of 65 °C and 80 °C compared to 50 °C. After 8 h, the density of the simulated urine increased from its initial value to 1.0129 ± 0.0023 g/mL, 1.0197 ± 0.0008 g/mL, and 1.0338 ± 0.0027 g/mL at 50 °C, 65 °C, and 80 °C, respectively. In contrast, the density of DI water in the cold permeate stream was recorded as 0.9959 ± 0.0000 g/mL, 0.9961 ± 0.0004 g/mL, and 0.9963 ± 0.001 g/mL for the 50 °C, 65 °C, and 80 °C, respectively. These values are nearly identical across all temperatures, as the cold permeate stream was essentially only water. Minor variations can be attributed to human and instrument error, which are negligible.
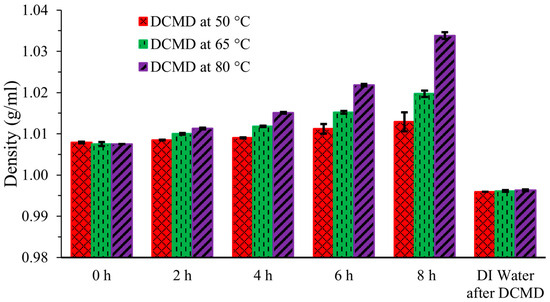
Figure 3.
Density of artificial urine at three different temperatures during DCMD.
2.4. Ionic Conductivity
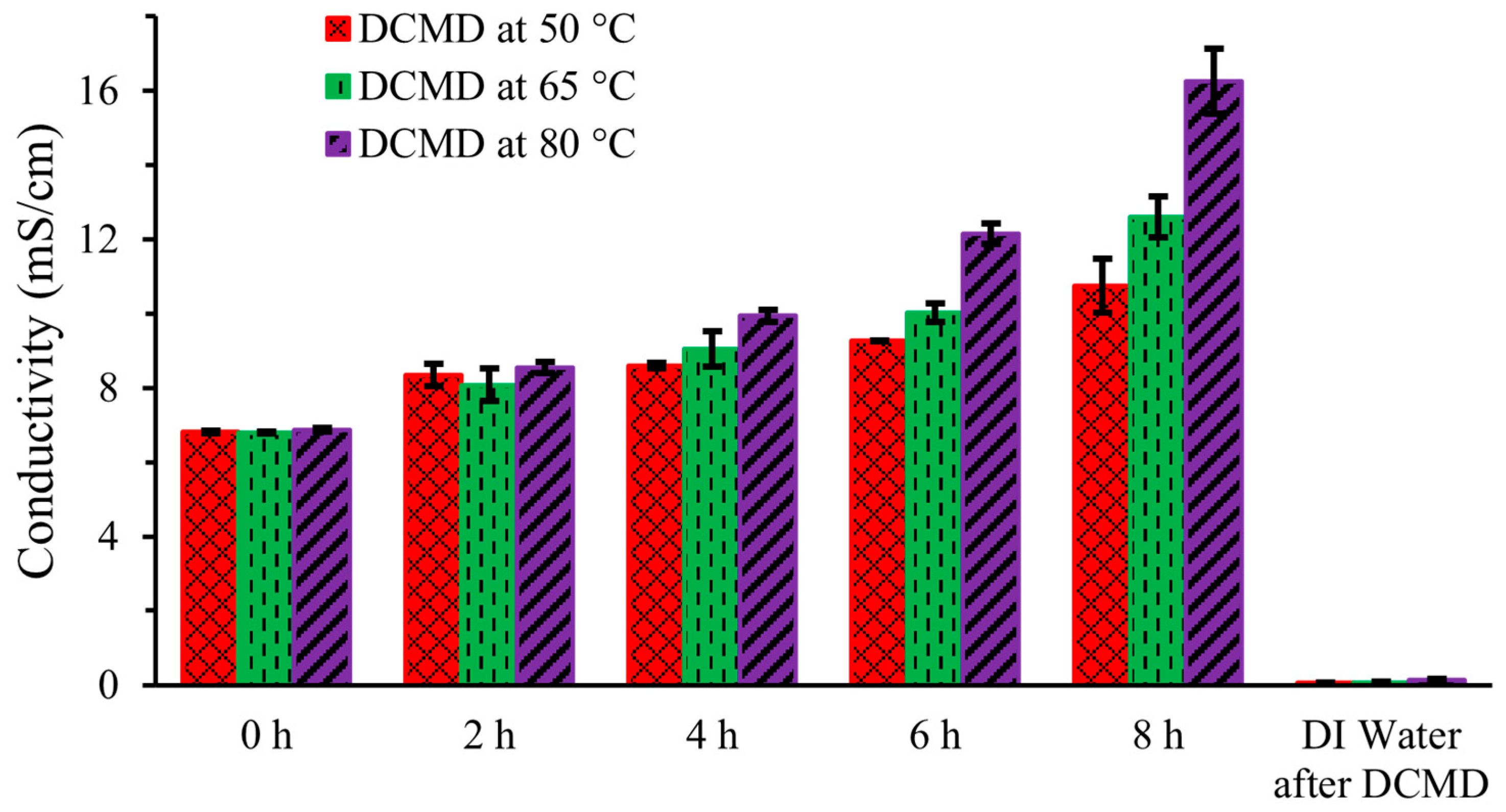
Figure 4 shows the ionic conductivity of artificial urine in the feed stream during DCMD and the conductivity of DI water in the permeate stream after 8 h of operation at three different temperatures (50 °C, 65 °C, and 80 °C). Measurements were taken at 0 h, 2 h, 4 h, 6 h, and 8 h, and they demonstrate a linear increase in ionic conductivity at each temperature. This increase is due to the movement of water through the hydrophobic membrane, leaving behind solutes in the feed solution, resulting in an increase in the ionic concentration and conductivity. The ionic conductivity increased from an initial value of 6.81 ± 0.05 mS/cm to 10.75 ± 0.72 mS/cm, 12.60 ± 0.55 mS/cm, and 16.26 ± 0.87 mS/cm after 8 h at 50 °C, 65 °C, and 80 °C, respectively. The higher increase in conductivity at elevated temperatures was attributed to the enhanced water removal rate. This findings is consistent with the previous published research [13]. The DI water’s conductivity was negligible at 0.07 ± 0.02 mS/cm for each operating temperature. This result is congruent with another research group’s findings [14], indicating that the PTFE membrane effectively blocked all ionic compounds at the end of DCMD.
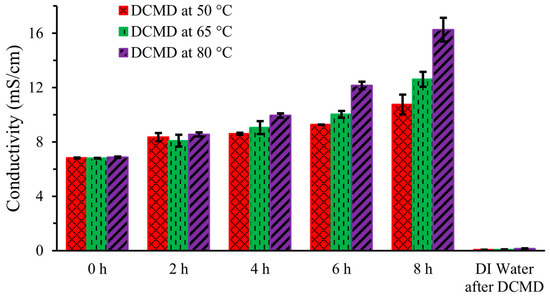
Figure 4.
Conductivity of artificial urine during DCMD.
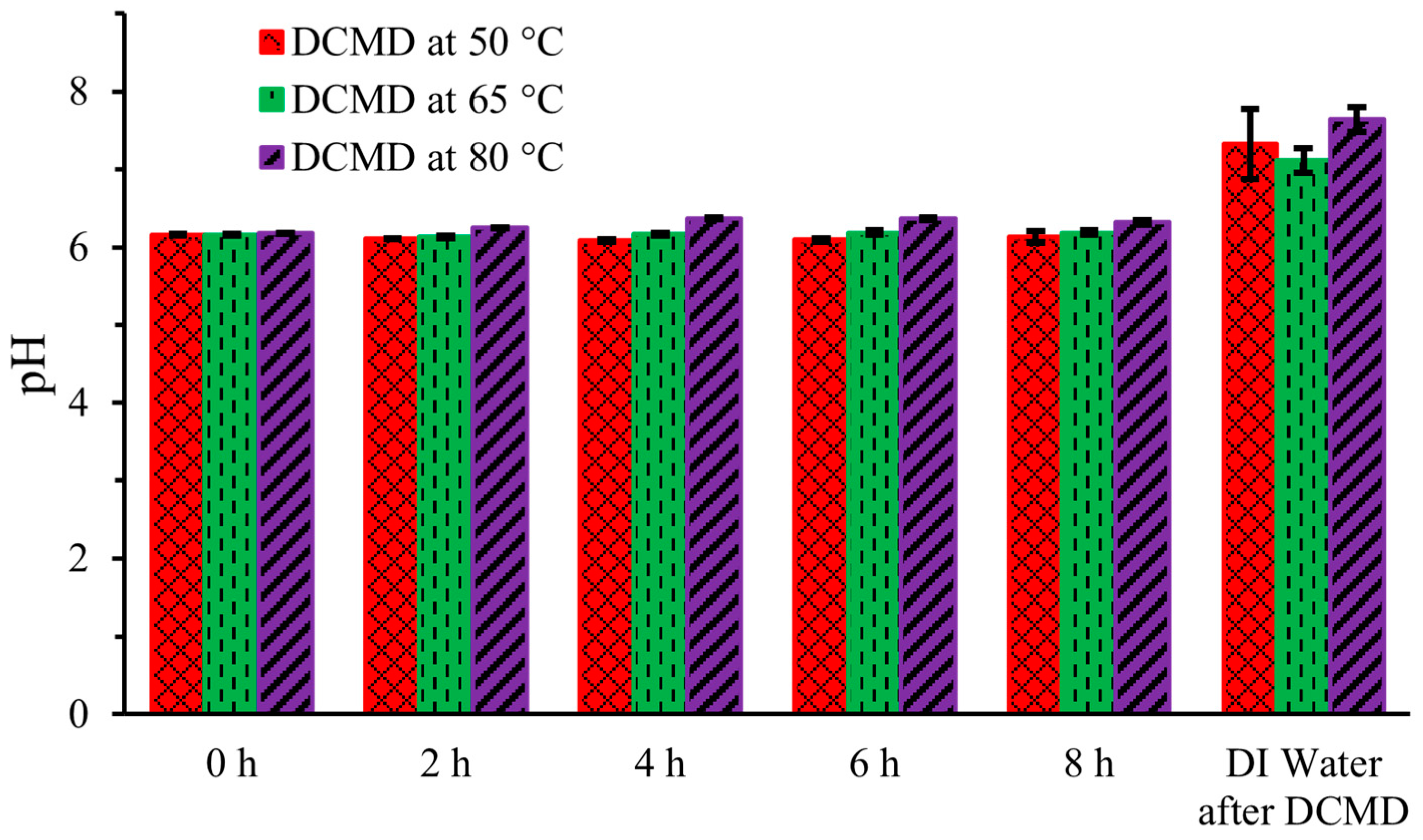
2.5. pH
The pH of human urine ranges from 4.5 to 8 [15] Figure 5 illustrates the pH levels of the artificial urine hot stream at five intervals—0 h (initial), 2 h, 4 h, 6 h, and 8 h (final)—during DCMD, as well as the pH of the DI water in the cold stream after the completion of DCMD at the three different temperatures. Initially, at 0 h, the pH of the artificial urine, measured immediately after preparation, was 6.2, and it remained unchanged throughout the DCMD process. The pH also did not vary with the operating temperature of DCMD, except for minor fluctuations attributable to the meter itself. The pH remained constant because only pure water was transferred from the hot urine stream through the membrane, with no addition or removal of protonic solvents from the hot feed stream.
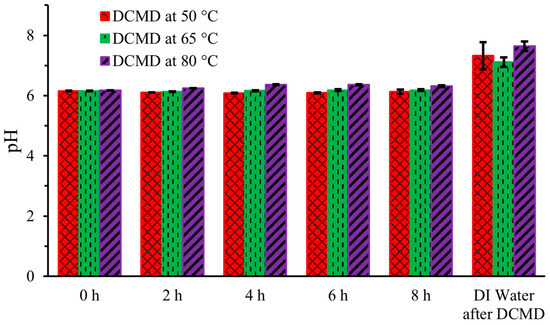
Figure 5.
pH of artificial urine during DCMD.
However, the pH of the DI water on the cold side ranged from 7 to 7.5, showing little variation across the three operating temperatures of DCMD. This consistency indicates both the potential potability and effectiveness of the membrane. For comparison, bottled drinking water typically has a pH between 6.9 and 7.5 [16].
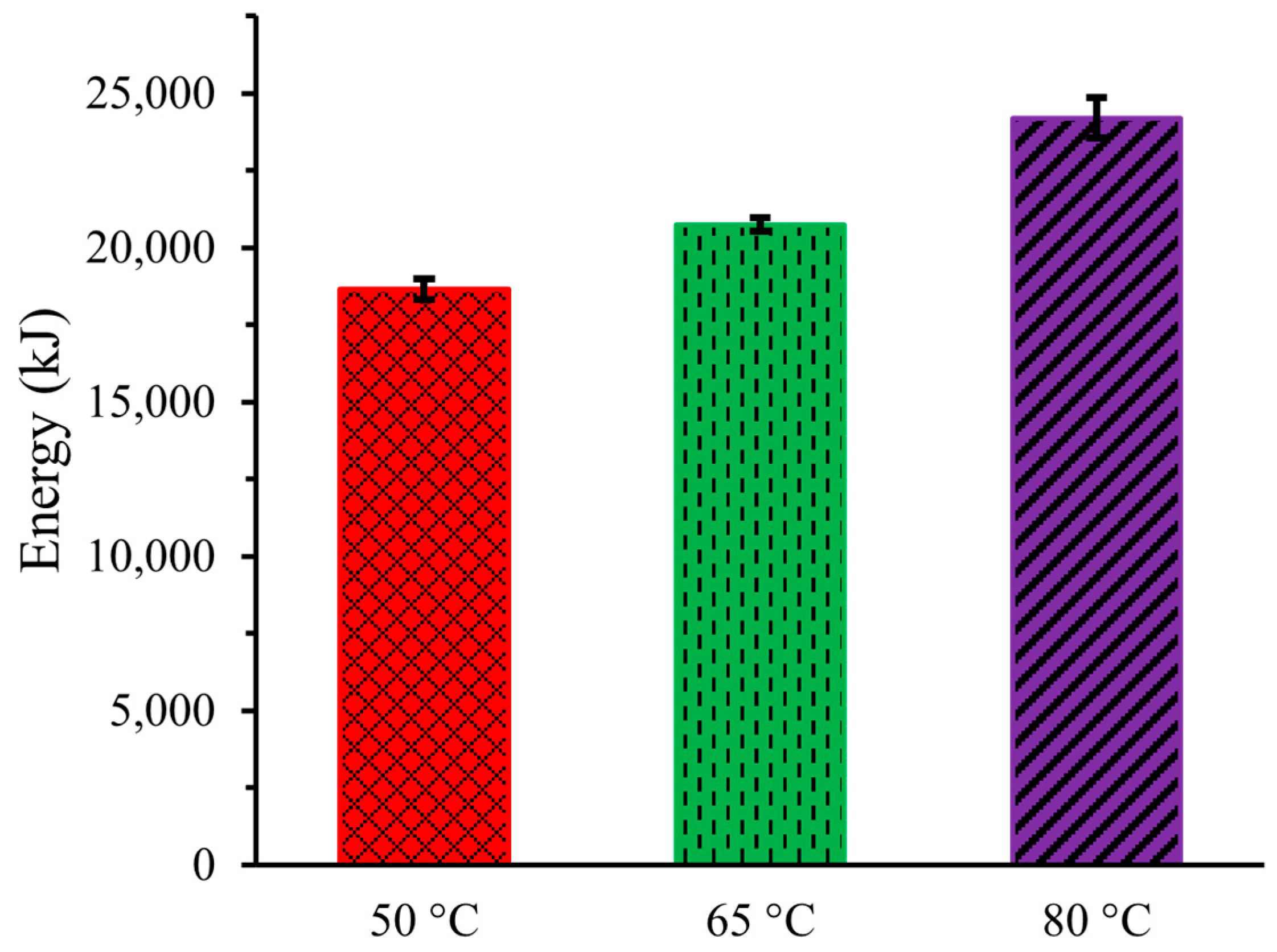
2.6. Energy
Figure 6 illustrates the energy needed in the DCMD system at the three operating temperatures for the 8 h experiments. The energy consumption at 50 °C was 18,648 ± 324 kJ (5.18 ± 0.09 kWh), which increased to 20,736 ± 216 kJ (5.76 ± 0.06 kWh) at 65 °C, and then to 24,192 ± 648 kJ (6.72 ± 0.18 kWh) at 80 °C. When expressed in specific energy consumption, these values correspond to 368.53 ± 6.27 kWh/m3, 409.53 ± 4.57 kWh/m3, and 477.54 ± 12.96 kWh/m3 for operating temperatures of 50 °C, 65 °C, and 80 °C, respectively. The higher energy consumption at elevated temperatures was attributed to the additional energy required to heat the urine solution and the more energy-intensive cooling system needed to condense the higher-temperature water vapor. The findings indicate that DCMD of artificial urine may be more energy efficient than similar research conducted by Zakrzewska-Trznadel et al. (1999) [17].
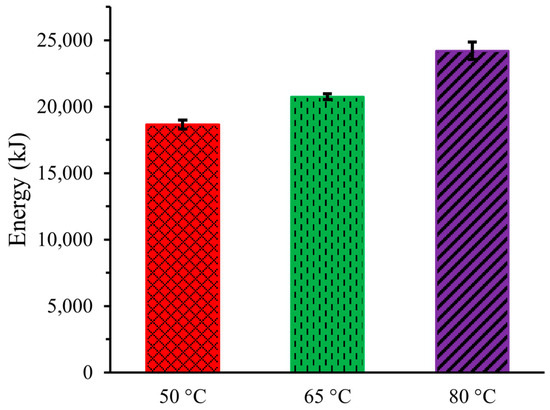
Figure 6.
Energy consumption for DCMD of Artificial Urine at Three Different Temperatures.
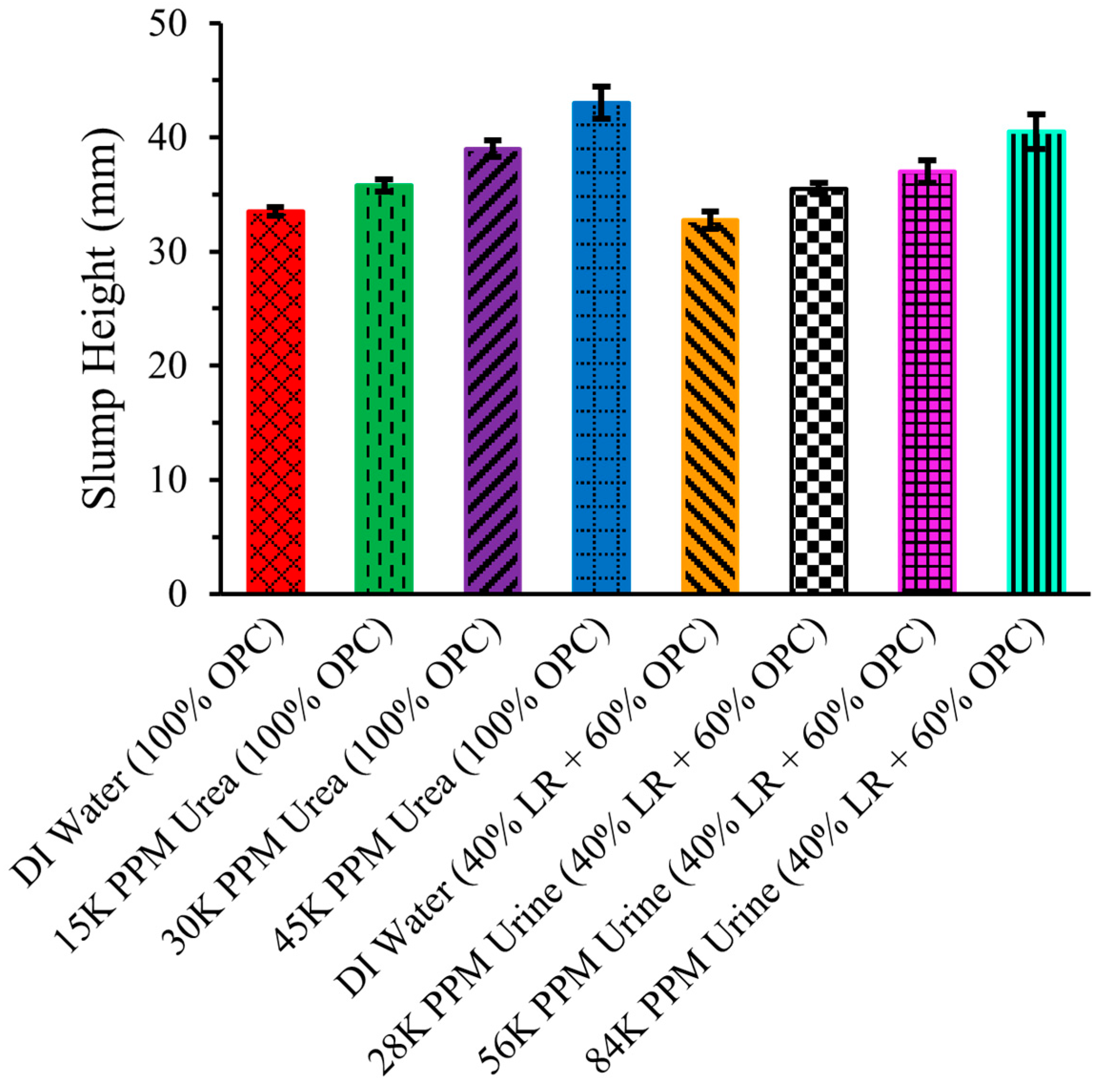
2.7. Slump Test
Figure 7 illustrates the slump height of eight distinct types of cement mixtures, including ordinary Portland cement (OPC) with DI water, three different concentrations of urea solution (15,000 ppm, 30,000 ppm, and 45,000 ppm), and 40% simulated lunar regolith LHS-1 (LR) and 60% OPC with DI water and with three different concentrations of urine solution (28,000 ppm, 56,000 ppm, and 84,000 ppm). In all eight cement mixtures, the liquid-to-solid ratio was maintained at 0.45, with the solid being either OPC alone or a combination of LR and OPC, and the liquid being DI water, urea solution, or urine solution.
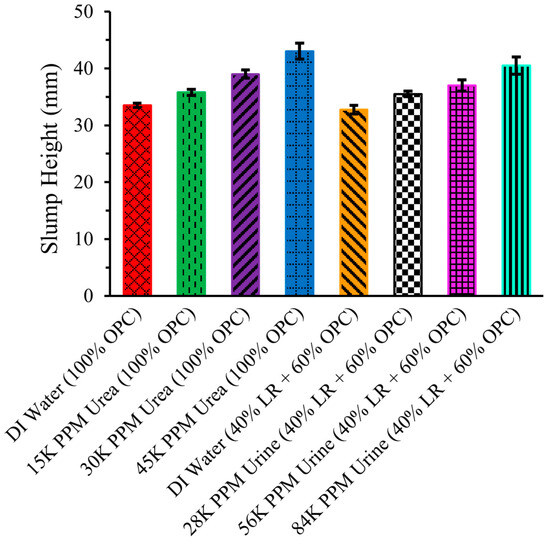
Figure 7.
Slump height of LR-based cement mixtures. Standard error bars are shown.
The slump height of the cement mixture of OPC with DI water, represented by the leftmost bar, was 33.5 ± 0.35 mm. By replacing DI water with a urea solution containing 15,000 ppm, urea increased the slump height to 35.75 ± 0.53 mm. This increase in workability is attributed to the addition of urea, which acts as a plasticizer, supporting the findings of Pilehvar et al. [3]. As the concentration of urea increased to 30,000 ppm and 45,000 ppm, the slump height continued to rise, reaching 39 ± 0.71 mm and 43 ± 1.41 mm, respectively. The presence of urea enhances workability by breaking down hydrogen bonds among the molecules comprising cement.
However, the slump height of the cement mixture of 40% LR and 60% OPC with DI water was slightly lower compared to pure (100%) OPC with DI water. LR has a larger particle size compared to OPC, a fact that may explain lower slump values. LR may also absorb more water than OPC [18]. Nevertheless, the slump height increased when DI water was replaced with urine solution, with a continuing upward trend as the concentration of the urine solution increased. The other non-urea components in artificial urine solutions may also decrease slump values. The ions involved may compete with urea in interacting with cement particles to cause a slightly lower workability [19].
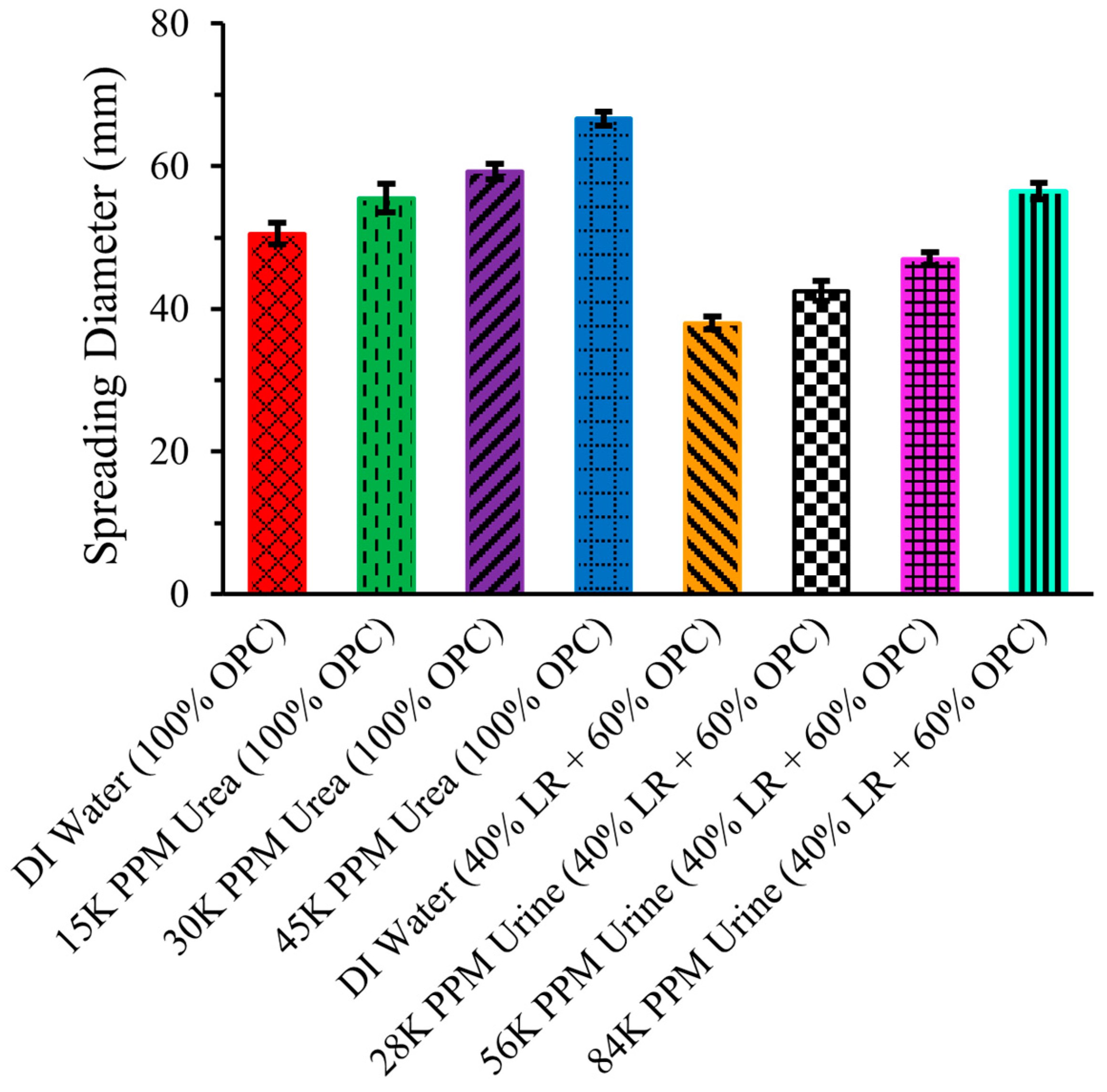
Figure 8 illustrates the slump spread of the same eight cement mixtures mentioned above.
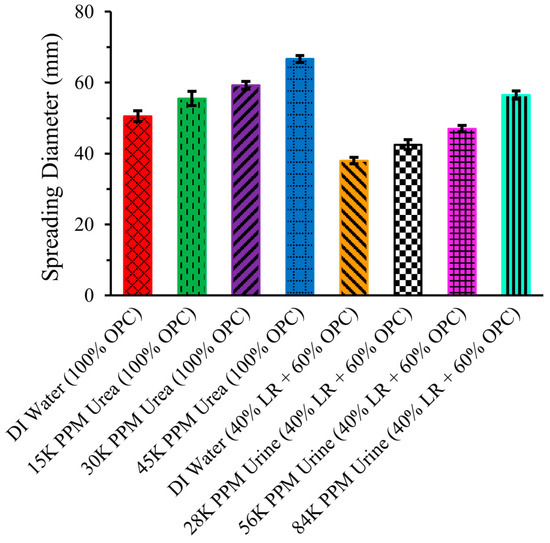
Figure 8.
Slump spreading of LR-based cement mixtures.
The spreading of the cement mixture followed the same trend as the slump height. For OPC with DI water, the spreading was 50.5 ± 1.52 mm, increasing with the addition of urea solutions. With urea concentrations of 15,000 ppm, 30,000 ppm, and 45,000 ppm, the spreading increased to 55.5 ± 2.02 mm, 59.25 ± 1.14 mm, and 66.63 ± 0.96 mm, respectively. However, the spreading of the cement mixture of 40% LR and 60% OPC with DI water was 38 ± 0.91 mm, which was lower compared to the 50.5 ± 1.52 mm spreading of the pure (100%) OPC with DI water mixture. This decrease in slump spreading with LR replacement of OPC probably occurred for the same reasons described in the previous section for the observed slump height changes. Nonetheless, the spreading of the 40% LR and 60% OPC mixture increased when DI water was replaced with urine solutions. With a 28,000 ppm urine solution, the spreading was 42.5 ± 1.38 mm, and it further increased to 47 ± 0.91 mm and 56.5 ± 1.19 mm with 56,000 ppm and 84,000 ppm urine solutions, respectively.
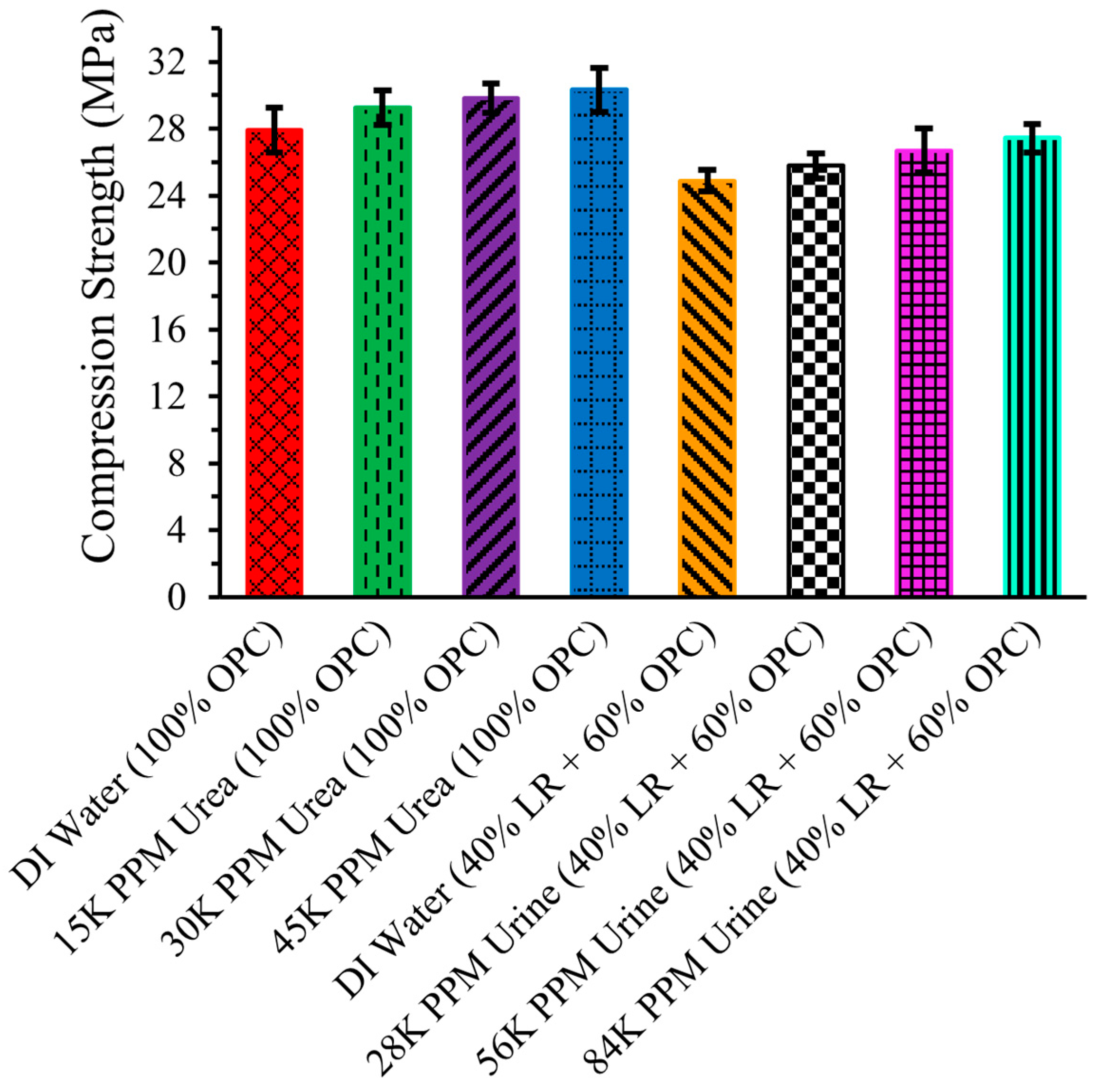
2.8. Compression Strength
Figure 9 illustrates the compression strength of LR-based cement cylinders. The compression strength of the cement cylinder made with OPC and DI water was 27.89 ± 1.34 MPa. The average strength increased when urea solutions were used instead of DI water. Specifically, the compression strength of the cement cylinder with 15,000 ppm urea solution was 29.25 ± 1.04 MPa, and it further increased to 29.81 ± 0.89 MPa and 30.31 ± 1.33 MPa with 30,000 ppm and 45,000 ppm urea solutions, respectively. The error in measurements means that little difference in compression strength can be ascertained for the urea solution data compared to that for DI water.
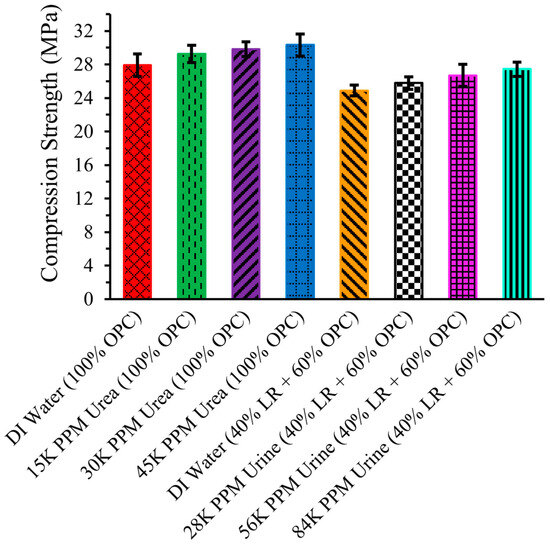
Figure 9.
Compression Strength of LR Based Cement Cylinder.
The compression strength of the cement cylinders made with 40% LR and 60% OPC using DI water was 24.89 ± 0.67 MPa, which was lower than that of the cylinder made with pure OPC and DI water. This reduction in strength can be attributed to the different chemical composition and particle size of LR compared to OPC.
Nonetheless, the compression strength appeared to increase when urine solution was used. Specifically, the strength was 25.77 ± 0.75 MPa with a 28,000 ppm urine solution. The strength further increased with higher concentrations of urine solution, reaching 26.69 ± 1.33 MPa with 56,000 ppm and 27.43 ± 0.85 MPa with 84,000 ppm. These results indicate that using waste urine in space could be viable for construction purposes on the Moon.
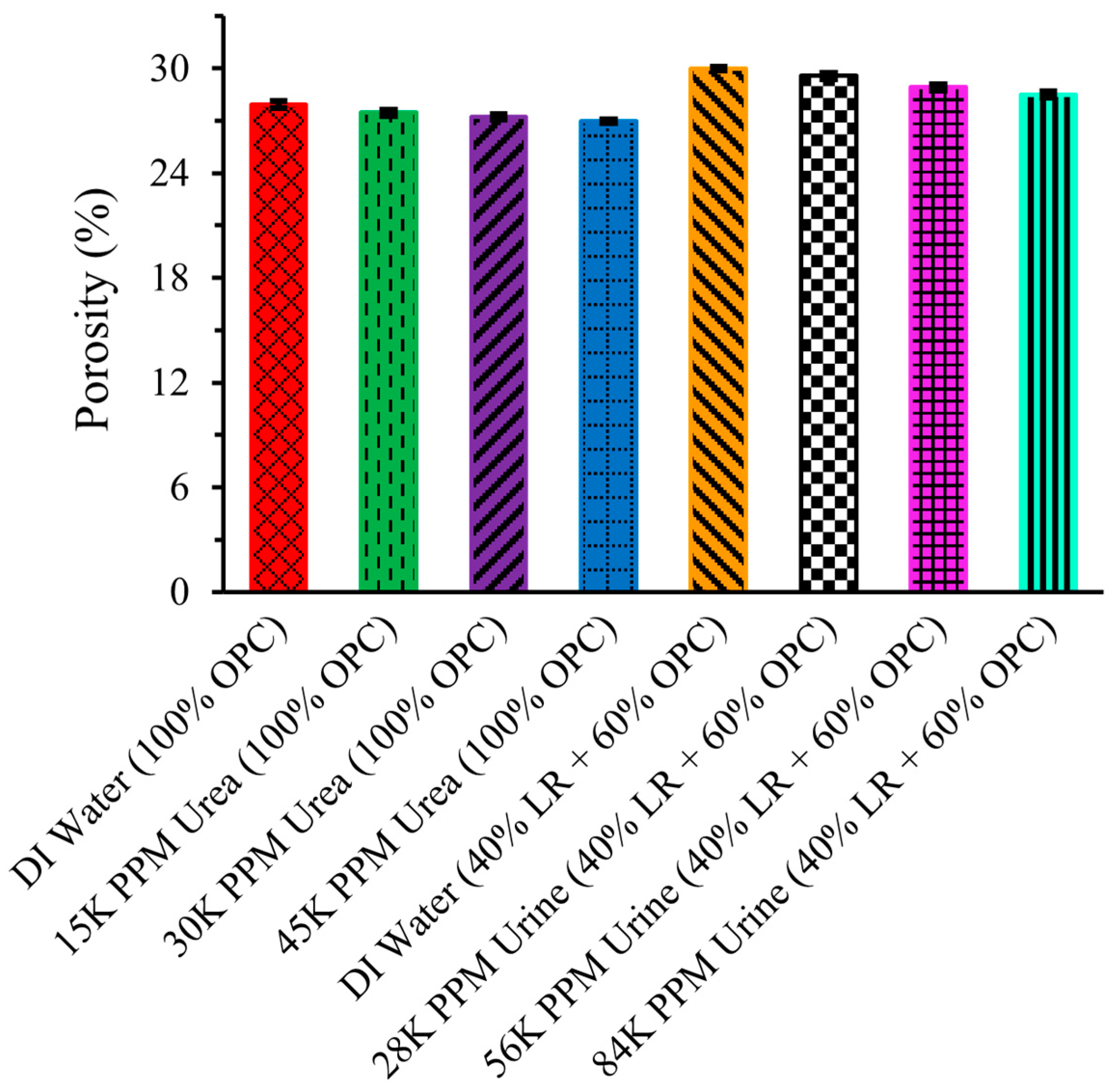
2.9. Porosity
Figure 10 illustrates the porosity of LR-based cement cylinders, highlighting the negative correlation between compression strength and porosity—higher compression strength indicates lower porosity, while higher porosity results in increased brittleness and reduced durability [20]. The leftmost bar shows that the porosity of the cement structure made with pure OPC and DI water was 27.92 ± 0.2 percent, which decreased to 27.46 ± 0.18 percent when urea solution was used instead of DI water. This trend aligns logically with the inverse relationship to compression strength, with porosity further decreasing to 27.22 ± 0.15 percent and 26.96 ± 0.09 percent for 30,000 ppm and 45,000 ppm urea solutions, respectively.
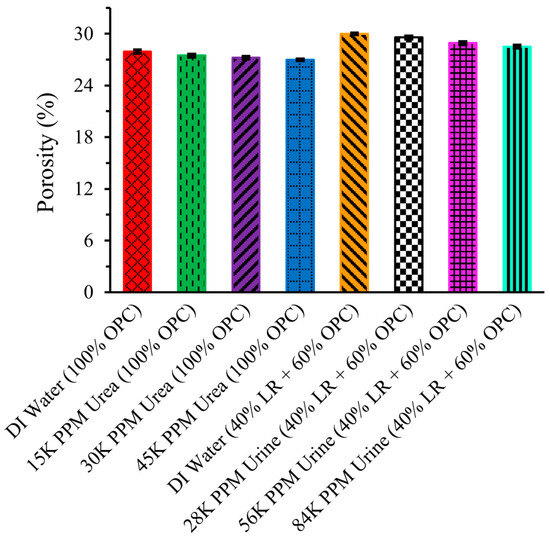
Figure 10.
Porosity of LR-based cement.
The porosity of the cement structure made with 40% LR and 60% OPC using DI water is significantly higher than that of the structure made with pure OPC and DI water. However, the porosity continuously decreases with the increasing concentration of urine solution, indicating that using urine solution instead of water is more beneficial for construction purposes on the Moon. Specifically, the porosity decreased to 29.56 ± 0.16 percent, 28.91 ± 0.16 percent, and 28.49 ± 0.17 percent for 28,000 ppm, 56,000 ppm, and 84,000 ppm urine solutions, respectively. This trend in decreasing porosity with increasing concentrations of urea or urine solution suggests that waste urine could be effectively utilized for lunar construction.
3. Materials and Methods
3.1. Raw Materials and Chemicals
The chemicals, amounts, and suppliers are listed in Table 1 for the preparation of artificial urine.

Table 1.
List of Chemical Compounds of Artificial Urine.
The simulated Lunar Regolith LHS-1 (LR) was purchased from Exolith (Orlando, FL, USA) and commercial grade ordinary Portland cement from Quikrete Portland cement (Bowman, SC, USA) type I/II (no. 1124) was purchased from Lowes, which complies with the American Society for Testing Materials (ASTM) C 150 specifications. The chemical compounds of stimulated Lunar Regolith and ordinary Portland cement are in Table 2.

Table 2.
Lunar Regolith (LR) and OPC composition in wt.% concentrations.
3.2. Methods
3.2.1. Preparation of Artificial Urine
Human urine contains over 3000 components, encompassing all ages, races, colors, genders, and regions. Among these components, more than 90 compounds are found with 100% occurrence, irrespective of gender or the time of day the urine is collected [10]. For practical and economical purposes, the formulation of artificial urine is simplified by including only 13 components (Table 1) with relatively higher concentrations [11]. In this study, artificial urine was prepared by dissolving the specified amounts of the 13 chemicals listed in Table 1 in 1000 mL of deionized (DI) water within a 2000 mL flask. The flask was placed on a hot plate with a magnetic stirrer set to 200 rpm at ambient temperature. Each compound was added sequentially, ensuring complete dissolution in the deionized water before adding the next. After all components were added, the mixture was stirred magnetically for 1 h to achieve a clear homogeneous solution. The solution was then sealed and stored at room temperature prior to the direct contact membrane distillation process.
3.2.2. Direct Contact Membrane Distillation (DCMD) of Artificial Urine
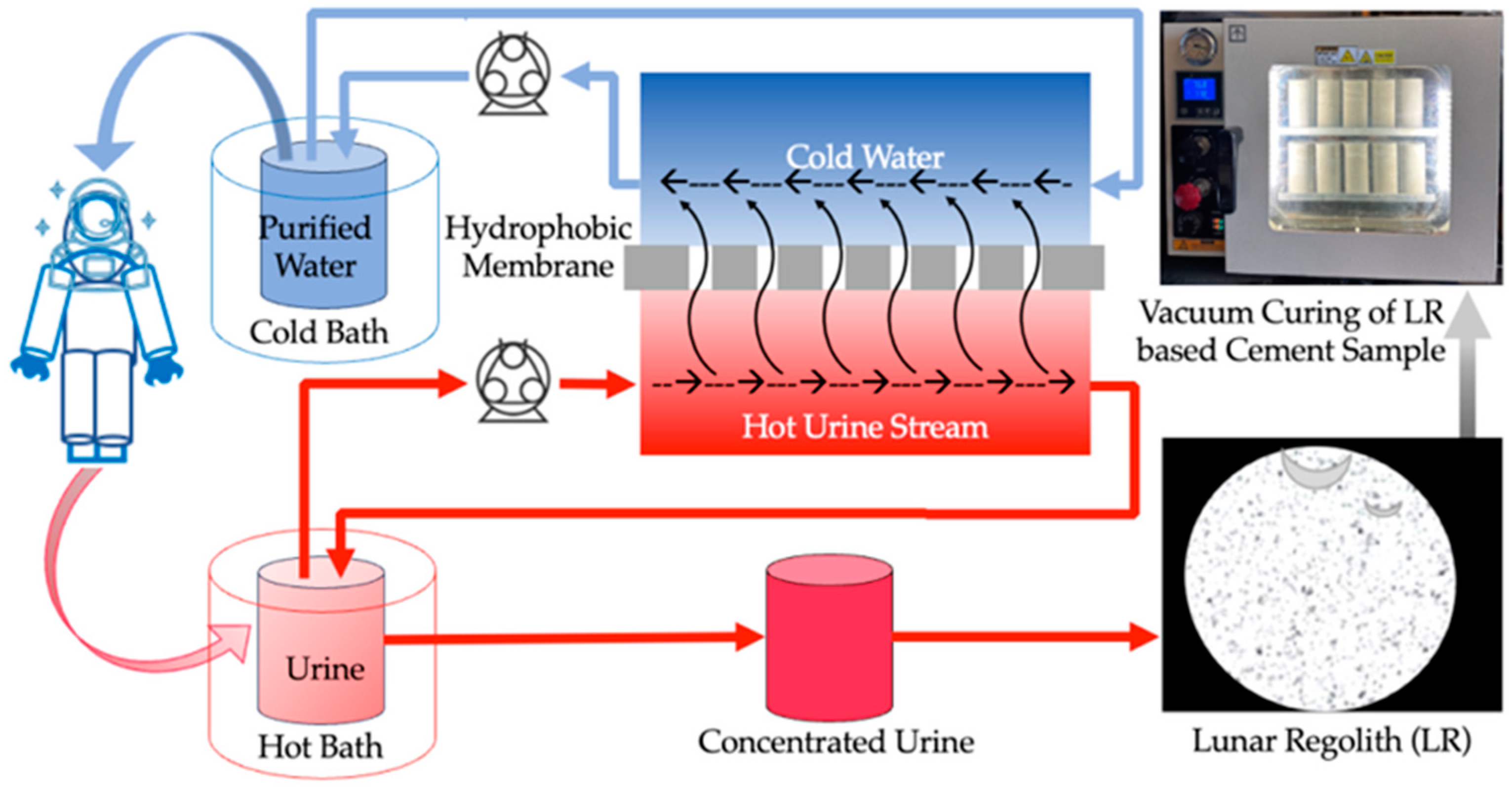
DCMD is an energy-efficient thermal separation process employing a hydrophobic, microporous membrane to separate a hot feed solution from cold water. The driving force behind DCMD is the temperature differential across the membrane, which establishes a vapor pressure gradient facilitating the transfer of water vapor from the hot feed side to the cold water side. The process involves direct contact of the hot feed urine solution with the cold DI water on the opposite side of the membrane. The hydrophobic nature of the membrane allows only water vapor to pass through its pores while retaining the liquid phase and dissolved larger or ionic substances. Operating at relatively low temperatures (50 °C, 65 °C, and 80 °C) and pressures compared to traditional desalination or wastewater treatment methods, DCMD offers simplicity and potentially lower implementation costs. In this study, 1 L of artificial urine was placed in a 1 L conical flask and submerged in a hot bath system (model 1130A, VWR Scientific, Philadelphia, PA, USA) maintained at three constant temperatures (50 °C, 65 °C, and 80 °C). Simultaneously, 200 mL of deionized (DI) water was placed in a 1 L glass bottle and chilled to approximately 0 °C using a chiller (model 9510, PolyScience, Niles, IL, USA). A PTFE membrane with a pore size of 0.45 µm was used for DCMD (145 × 97 mm flat sheet membrane, Sterlitech, Auburn, WA, USA). Peristaltic pumps (model 77200-50, Masterflex, Radnor, PA, USA) were used for fluid circulation. The experimental setup is shown in Supplementary Materials Figure S1. The process was conducted for 8 h, with 50 mL samples collected from the hot bath every 2 h to assess membrane effectiveness and observe changes. Analyses of membrane flux, density, ionic conductivity, pH, and energy consumption were conducted to ensure accurate evaluation of changes with DCMD treatment. The overall scheme is shown in Figure 11.
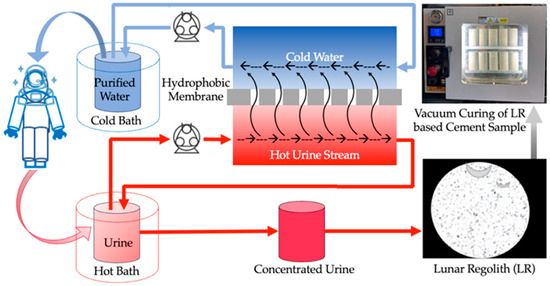
Figure 11.
Graphical representation of Direct Contact Membrane Distillation of artificial urine and vacuum curing of an LR-based cement sample.
Flux in DCMD is a critical parameter that determines the rate of vapor transfer through the membrane per unit area per unit time, thereby impacting the system’s efficiency and effectiveness. It reflects the performance of the membrane distillation process and is influenced by factors such as temperature difference, membrane properties, feed solution concentration, flow rates, temperature polarization, and membrane fouling. High flux values indicate superior performance, making it an essential metric for optimizing and evaluating DCMD systems. Flux is typically expressed in units such as liters per square meter per h (L/m2·h) or kg per square meter per h (kg/m2·h). The flux (J) in DCMD were calculated using the formula:
where m is the volume of the permeate collected (L), A is the effective membrane area (m2), and t is the time over which the permeate is collected (h).
In this study, DCMD was conducted at three different temperatures (50 °C, 65 °C, and 80 °C) for 8 h. After 8 h, the mass of DI water in the cold bath was determined by measuring the difference between the initial and final masses, and the concentration was calculated using the following formula:
where is the concentration of dissolved components in ppm, is the initial mass of dissolved components in mg, is the initial mass of water in kg, and is the transferred water in kg from the urine stream in the hot side of the bath to the DI water in the cold side of the bath. DCMD was run three times at each temperature, resulting in the measurement of water transfer and flux calculation being conducted three times per temperature.
The density of the urine solution in DCMD is pivotal in determining the operational effectiveness and efficiency of the process. Throughout the DCMD operation, alterations in the concentration of the urine solution occur due to water vaporization, resulting in corresponding changes in density. These density changes were systematically assessed using a 2 mL pycnometer at three distinct temperatures for the three process temperatures (50 °C, 65 °C, and 80 °C), with measurements taken at intervals of 0 h, 2 h, 4 h, 6 h, and 8 h, respectively.
Ionic conductivity, which gauges the flow of ions or the passage of current through ionic conduction in a solution, measures the presence of ions or ionic salts in both artificial urine and DI water. The ionic conductivity was measured for DI water and for the hot urine streams at intervals of 2 h, 4 h, 6 h, and 8 h during DCMD using a Starter 300C conductivity meter (Ohaus, Parsippany, NJ, USA).
The pH was measured for the DI water on the cold side after the completion of DCMD, as well as for the hot urine stream samples collected at intervals of 2 h, 4 h, 6 h, and 8 h during DCMD, using an Orion Star A111 pH meter (Thermo Scientific, Waltham, MA, USA).
Energy consumption analysis is essential for evaluating the efficacy and feasibility of the DCMD technique in water purification. While low-grade waste heat available on the Moon could reduce the need for additional energy, for experimental simplicity, we used the electric grid as the energy source. In DCMD, energy was required for three primary subsystems: heating the feed solution in the hot bath to convert water into vapor, pumping the fluid through the system, and cooling the slightly heated cool water back to the original cool side temperature. To accurately measure total energy consumption, an energy meter (model P4460.01, P3 International, New York, NY, USA) was connected to the source.
3.2.3. Preparation of Lunar-Regolith (LR)-Based Cement Sample
The standard method ASTM C192M was used to prepare samples, with concentrated synthetic urine used instead of DI water [12]. DI water was used as a control. A constant water, urine solution, or urea solution to cement ratio of 0.45 was used. For simplicity, a freshly made concentrated urine solution of the correct PPM was used. The cement paste to be batched was 40% simulated regolith LHS-1 and 60% ordinary Portland cement. Each batch was prepared using a KitchenAid 600TM mixer (Benton Harbor, MI, USA). The experimental mixture components are shown in Table 3.

Table 3.
Cement batch compounds.
The slump cone test is a widely used, quick, and straightforward method for measuring the consistency or workability of fresh cement. It ensures that the cement mix is suitable for specific construction applications and meets desired specifications. After putting mixed cement in a cone, the cone is placed smaller side up on a flat surface. The cone is lifted off and the distance the mixture collapses down (the slump) is measured. Variations in slump can indicate changes in moisture content, aggregate grading, or mix proportions, helping to detect potential issues before the cement cures into a hardened cement paste. In this study, a mini slump cone test, a modified standard ASTM C143 [21], was used to simulate the conventional slump cone test [22]. After thorough mixing, the cement mixture was filled into a mini-slump cone (5.70 cm height, 1.88 cm top internal diameter, and 3.68 cm bottom internal diameter) in three layers, each approximately one-third of the cone’s height, and compacted by 13 strokes of rodding with the tamping rod uniformly over the cross-section on a grid plate. The cone was then carefully lifted upward, slowly without twisting to allow the cement to spread on the grid plate. The spreading diameters, both lateral and longitudinal, and the slump height of the samples (shown in Supplementary Materials Figure S2) were recorded to measure the workability of the cement mixture.
Following the slump testing, the LR-based cement mixture was poured into plastic cylindrical molds with dimensions of 5.08 cm diameter and 10.16 cm height, filling each mold in three layers that were approximately one-third of the cylinder’s height. Each layer was compacted using 13 strokes of rodding with a tamping rod to ensure uniformity. Subsequently, the cement samples were allowed to cure for 24 h at room temperature. After this initial curing period, the samples were removed from the molds and transferred to a vacuum oven (model AccuTemp-09s, Across International, Sparks, NV, USA), where they underwent a 28-day vacuum curing process at a temperature of 25 °C and a pressure of 3.95 kPa (29.7 mm Hg), replicating lunar conditions.
In the compression test, cylindrical specimens of the concrete mixture, cured under a vacuum for 28 days, were subjected to increasing compressive loads until failure occurred, following ASTM C39 standards [23], using a Universal Testing Machine (model CM-450-SF, East Palestine, OH, USA). The test applied a load rate of 241.3 kPa/s with computer-aided control until the specimen failed. Upon failure, the machine stopped applying force and recorded the maximum load sustained by the specimen, along with the corresponding deformation.
The water absorption method was used to measure the porosity of LR-based concrete samples [24]. After performing tensile tests, fractured pieces of each sample, weighing 5–10 g, were weighed and then submerged in deionized (DI) water for 24 h to ensure complete saturation. The saturated pieces were removed, lightly patted dry with a paper towel, and reweighed. Subsequently, the samples were placed in a drying oven at 105 °C for 24 h to remove moisture from the pores. After drying, the samples were cooled for 1 h and weighed again. The percentage of porosity was calculated using the equation:
where is the sample weight after saturation and Wd is the sample weight after drying.
4. Conclusions
DCMD is a straightforward, high-purity, cost-effective, and energy-efficient method that has successfully separated pure water from artificial urine. Numerical analyses of density, conductivity, and pH in the DI water underscore the efficacy of the PTFE membrane in water purification via DCMD. Flux measurements corroborate the practicality of DCMD, while energy assessments suggest its superior efficiency compared to other contemporary technologies. Furthermore, slump tests conducted on LR cement samples mixed with artificial urine demonstrate improved workability, attributed to urea acting as a plasticizer. Compression and porosity tests indicate that adding concentrated artificial urine to LR-based cement may improve these properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/recycling9050089/s1, Table S1: slump data; Table S2: compression strength data; Tables S3 and S4: porosity data; Table S5: flux and concentration data; Table S6: pH data; Table S7: conductivity data; Table S8: density data; Table S9: energy consumption data; Figure S1: experimental setup for DCMD; and Figure S2: slump test and compression test.
Author Contributions
Conceptualization, J.G.L. and M.T.; methodology, J.G.L. and M.T.; software, J.G.L. and M.T.; validation, M.T. and J.G.L.; formal analysis, J.G.L. and M.T.; investigation, S.T.G. and S.A.; resources, S.T.G. and S.A.; data curation, J.G.L. and M.T.; writing—original draft preparation, M.T.; writing—review and editing, J.G.L.; visualization, J.G.L. and M.T.; supervision, J.G.L. and S.A.; project administration, J.G.L.; funding acquisition, J.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NASA grant [Grant number: Cooperative Agreement Number: 80NSSC22M0030 (CFDA # 43.008)] and the Board of Regents of the State of Louisiana [Contract Number: LEQSF-EPS(2023)-RAP-47] with the help of LSU management.
Data Availability Statement
Data from this study are available from the corresponding author upon request.
Acknowledgments
The authors want to thank Audrey Shank and Emma Agan of Louisiana Tech University; Anne Meier, Gioia Massa, Ray Pitts, Trent Smith, and Ray Wheeler of Kennedy Space Center, NASA; NASA and the Louisiana Board of Regents (BoR); and the Louisiana NASA EPSCoR Team at LSU. Funding was provided under Cooperative Agreement Number 80NSSC22M0030.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Steigerwald, W. NASA’s Artemis Base Camp on the Moon Will Need Light, Water, Elevation. Available online: https://www.nasa.gov/humans-in-space/nasas-artemis-base-camp-on-the-moon-will-need-light-water-elevation/ (accessed on 25 July 2024).
- Özen, S.; Şengül, C.; Taşdemir, M.A.; Erenoğlu, T.; Çolak, Ü.; Reyhancan, İ.A. Properties of Heavyweight Concrete for Structural and Radiation Shielding Purposes. Arab. J. Sci. Eng. 2016, 41, 1573–1584. [Google Scholar] [CrossRef]
- Pilehvar, S.; Arnhof, M.; Pamies, R.; Valentini, L.; Kjøniksen, A.-L. Utilization of urea as an accessible superplasticizer on the moon for lunar geopolymer mixtures. J. Clean. Prod. 2020, 247, 119177. [Google Scholar] [CrossRef]
- Duan, H.; Jiang, Z.; Zhu, S.; Yao, P.; Sun, Q. New composite grouting materials: Modified urea–formaldehyde resin with cement. Int. J. Min. Sci. Technol. 2012, 22, 195–200. [Google Scholar] [CrossRef]
- Simha, P.; Zabaniotou, A.; Ganesapillai, M. Continuous urea–nitrogen recycling from human urine: A step towards creating a human excreta based bio–economy. J. Clean. Prod. 2018, 172, 4152–4161. [Google Scholar] [CrossRef]
- Horvath, T.; Hayne, P.O.; Paige, D.A. Thermal and illumination environments of lunar pits and caves; models and observations from the Diviner Lunar Radiometer Experiment. Geophys. Res. Lett. 2022, 49, e2022GL099710. [Google Scholar] [CrossRef]
- Hegde, C.; Ribeiro, R. Preparation and Characterization of Hydrophobic Membranes and Their Seawater Desalination Performance Study by Direct Contact Membrane Distillation. Nat. Environ. Pollut. Technol. 2022, 21, 1599–1608. [Google Scholar] [CrossRef]
- Sirkar, K.K.; Song, L. Pilot-Scale Studies for Direct Contact Membrane Distillation-Based Desalination Process. In Desalination and Water Purification Research and Development Program Report; U.S. Department of the Interior Bureau of Reclamation: Washington, DC, USA, 2009. [Google Scholar]
- Khumalo, N.; Nthunya, L.; Derese, S.; Motsa, M.; Verliefde, A.; Kuvarega, A.; Mamba, B.B.; Mhlanga, S.; Dlamini, D.S. Water recovery from hydrolysed human urine samples via direct contact membrane distillation using PVDF/PTFE membrane. Sep. Purif. Technol. 2019, 211, 610–617. [Google Scholar] [CrossRef]
- Tarikuzzaman, M.; Iqbal, M.A.; Lynam, J.G. Direct Contact Membrane Distillation of Artificial Urine for Sugar Beet Production in a Hydroponic System. J. Ecol. Eng. 2024, 25, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-G.; Kim, W.-S.; Choi, J.-S.; Ghaffour, N.; Kim, Y.-D. Dynamic solar-powered multi-stage direct contact membrane distillation system: Concept design, modeling and simulation. Desalination 2018, 435, 278–292. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Sagar, V.; Mekalip Lauren, M.; Lynam Joan, G. Membrane distillation of synthetic urine for use in space structural habitat systems. Green Process. Synth. 2024, 13, 1599–1604. [Google Scholar] [CrossRef]
- Fei, W.; Junfeng, L.; Da, L.; Zheng, L.; Jie, Z.; Ping, D.; Guochang, L.; Yujie, F. High-Efficiency Water Recovery from Urine by Vacuum Membrane Distillation for Space Applications: Water Quality Improvement and Operation Stability. Membranes 2022, 12, 629. [Google Scholar] [CrossRef] [PubMed]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A New Artificial Urine Protocol to Better Imitate Human Urine. Scientific Reports 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kulthanan, K.; Nuchkull, P.; Varothai, S. The pH of water from various sources: An overview for recommendation for patients with atopic dermatitis. Asia Pac. Allergy 2013, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska-Trznadel, G.; Harasimowicz, M.; Chmielewski, A.G. Concentration of radioactive components in liquid low-level radioactive waste by membrane distillation. J. Membr. Sci. 1999, 163, 257–264. [Google Scholar] [CrossRef]
- Jones, B.M.; Aleksandrov, A.; Dyar, M.D.; Hibbitts, C.A.; Orlando, T.M. Investigation of water interactions with Apollo lunar regolith grains. J. Geophys. Res. Planets 2020, 125, e2019JE006147. [Google Scholar] [CrossRef]
- Ma, B.; Yi, P.; Hongbo, T.; Zhenghang, L.; Xiufeng, D. Effect of Polyacrylic Acid on Rheology of Cement Paste Plasticized by Polycarboxylate Superplasticizer. Materials 2018, 11, 1081. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Su, L.; Wang, Y.; Zhang, L. Analysis of the Effect of Porosity in Concrete under Compression Based on DIP Technology. J. Mater. Civ. Eng. 2022, 34, 04021376. [Google Scholar] [CrossRef]
- ASTM C143/C143M-12; Standard Test Method for Slump of Hydraulic-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Akond, A.U.R.; Lynam, J.G. Deep eutectic solvent extracted lignin from waste biomass: Effects as a plasticizer in cement paste. Case Stud. Constr. Mater. 2020, 13, e00460. [Google Scholar] [CrossRef]
- ASTM C39/C39M-21; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Andreola, F.; Leonelli, C.; Romagnoli, M.; Miselli, P. Techniques Used to Determine Porosity. Am. Ceram. Soc. Bull. 2000, 79, 49–52. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).