Abstract
The increasing demand for avocado consumption has led to a vast generation of waste products. Despite the high nutritional value of avocados, the waste generated from their processing poses a significant environmental challenge. Therefore, the development of a sustainable approach to avocado waste management is a major concern. Biorefinery presents a promising approach to the valorization of avocado waste components, including the seed, peel, and pulp residues. This paper explores the potential of avocado waste biorefinery as a sustainable solution to produce bio-based products. Several approaches, including extraction, hydrolysis, fermentation, and biodegradation, to obtain valuable products such as starch, oil, fiber, and bioactive compounds for food or feed goods have been proposed. The review also highlights the approaches towards addressing challenges of energy security and climate change by utilizing avocado waste as a source to produce biofuels such as biogas, biodiesel, and bioethanol. In conclusion, the development of avocado waste biorefinery presents a promising avenue for sustainable development. This process can efficiently convert the avocado waste components into valuable bio-based products and clean energy sources, contributing to the attainment of a circular economy and a more sustainable future.
1. Introduction
The avocado (Persea americana Mill.) is a member of the Lauraceae family, and due to its high nutritional value, it has become a very desirable fruit in the market [1,2]. Mexico is a world leader in the cultivation and exportation of avocados, with a total production of 2.4 million tons in 2022 [3]. The size and shape of an avocado vary according to the variety; it is generally green in the immaturity stages, becoming brownish when ripe [4]. Avocado is composed of three main parts: the pulp, which constitutes the largest portion with 65–73% of the fruit; a large and rounded seed that represents around 16–20%; and finally, the peel with 11–15% (w/w) [5,6]. Avocado is consumed primarily as a fresh product; nevertheless, currently, there is an increasing global demand for the production and processing of this fruit, which promotes the increment of avocado waste generation. The by-products generated during its processing, such as peel, seeds, and pulp, are discarded into the environment, causing ecological problems including greenhouse gas emissions, soil and water pollution, and attracting pests and rodents [5,7]. Avocado waste represents environmental challenges that require urgent attention.
Avocado waste generation is directly related to the harvested amounts and, hence, can be substantial in countries such as Mexico. The global avocado wastage is estimated at 40% of its total production, attributable to fruit discarded or wasted as by-products [8]. It is important to consider that inappropriate storage conditions can lead to losses of 80% of the total production, which is linked to microbiological and mechanical damage in middle-income countries [9]. In addition to this, the percentage of avocado waste depends on several factors, including the size of the avocado industry, the extent of processing, disposal practices, and the variety of the fruit. For example, the percentage of avocado by-products (peel and seeds) was higher in the ‘Fuerte’ than in the ‘Hass’ variety (31 and 24%, respectively) [10]. However, there are also differences between the data reported in the same cultivar, for example, 24 and 27% were reported for the Hass cultivar [6,10]. Otherwise, depending on the final product, the amount of waste can also vary. For example, during the production of cold-pressed avocado oil from seeds, a large amount of waste is generated, since for every 1000 kg of avocado processed, approximately 80 kg of the final product are obtained [11]. Whereas, in the case of the oil production from the pulp and guacamole, the amount of waste represents between 21 and 76% (w/w) of the whole fruit [12].
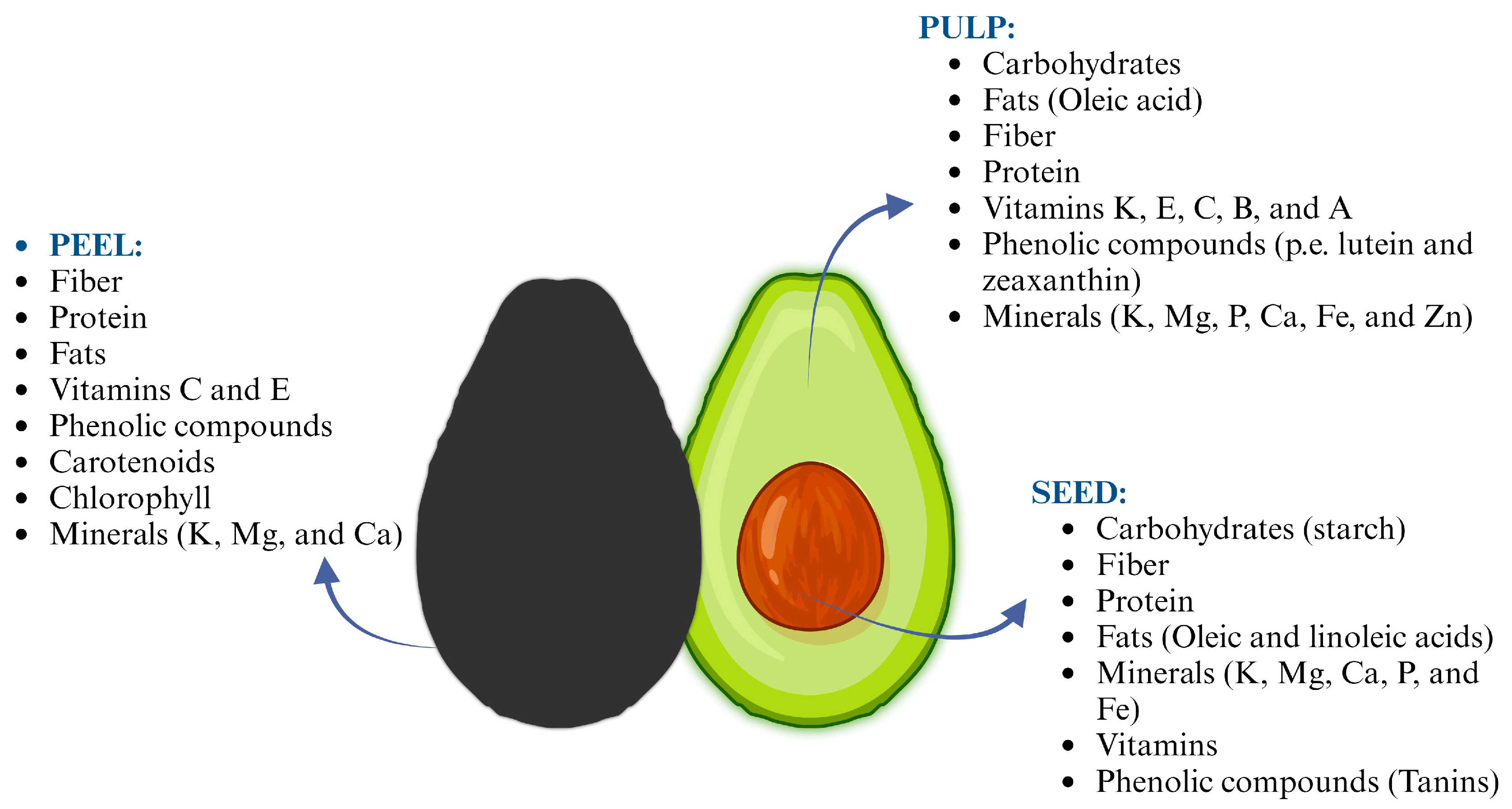
Avocado waste contains valuable nutrients and compounds, such as oil, carbohydrates, acetogenins, and dietary fiber (Figure 1), that can be recovered and used in the food, cosmetic, or pharmaceutical industries [4]. The green valorization of avocado waste is an essential aspect of sustainable development in the face of increasing environmental challenges, providing an opportunity to convert waste into valuable products while reducing environmental impacts [7,13]. This approach contributes to sustainable development, as it incorporates the principles of circular economy, promoting the efficient use of resources and reducing waste. The green valorization of avocado waste involves a process of extracting valuable compounds and materials from the avocado waste, promoting its reuse, and reintegration the avocado waste into the value [7,14,15,16]. This approach helps mitigate the negative environmental impact associated with the disposal of avocado waste while creating economic opportunities by providing alternative sources of raw materials for value-added products. Some sustainable solutions for avocado waste management include composting, anaerobic digestion, and the production of value-added products [7,14,16,17].
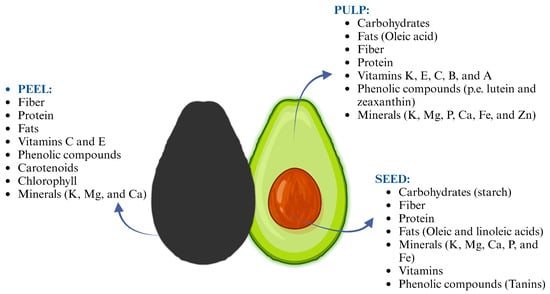
Figure 1.
General composition of peel, pulp, and seed of avocado fruit.
The sustainable valorization of avocado waste represents an opportunity for developed countries to increase their agricultural productivity and improve the livelihoods of farmers by creating additional income streams. Furthermore, this approach supports the sustainable development goals (SDGs), including reducing waste generation and promoting the efficient use of resources [14]. The health and environmental benefits of the green valorization of avocado waste can contribute to building resilient and sustainable agroecosystems, particularly in developing countries. This review aims to investigate the potential of converting avocado by-products, such as peels, seeds, and pulp, into valuable products through biotechnological and environmentally friendly processes, in line with the SDGs. Opportunities in the global market, trends, and challenges in the implementation of biorefinery for avocado waste are explored to contribute to the development of a circular economy model. This final section seeks to promote the concept of avocado waste biorefinery as a viable and environmentally friendly solution for dealing with avocado processing byproducts and to support sustainable practices and resource optimization in the avocado industry.
2. Composition and Properties of Avocado Residues
Avocado waste, particularly the seeds, has been the subject of various studies regarding its chemical composition and potential health benefits. The composition of avocado waste varies depending on the part of the fruit (Figure 1), as well as the processing methods used and fruit variety. Moreover, researchers around the world have determined that the physicochemical composition of the waste is affected by factors such as climate, geographic region, rainfall pattern, genetics, and level of maturity [18,19]. Carbohydrates are the main compounds in avocado seeds followed by fiber, protein, and fats. Whereas, the peel is mainly composed of fiber, protein, and fats, and the pulp is mainly composed of fats (Table 1). Avocado waste may have potential applications in various industries such as food, pharmaceuticals, cosmetics, and agriculture due to its bioactive properties and nutritional composition [5,20].

Table 1.
The chemical composition of the avocado seed, peel, and pulp is based on the variety.
The by-products of avocado fruit have received increasing attention in recent years because, in addition to the above-mentioned compounds, they are a potential source of bioactive compounds, including phenolic compounds, carotenoids, and sterols (Table 2). For example, the pulp and peel of the fruit also contain high levels of vitamins, particularly vitamin E, while the seeds contain higher levels of minerals and phenolic compounds [29,30]. Bioactive compounds have been shown to possess a wide range of health benefits, including antioxidant and anti-inflammatory properties, making them valuable ingredients in functional and nutraceutical foods. Carotenoids and phenolic compounds have been widely studied for their ability to promote health and prevent chronic diseases [31]. Overall, the exploration of the composition and properties of avocado waste is an area of active research with promising implications for sustainable waste management and potential value creation.

Table 2.
Bioactive compounds in avocado peel and seed extracts.
3. Alternatives for Avocado Waste Valorization
Several techniques for avocado waste valorization have been developed in recent years. One approach involves the extraction of antioxidants and bioactive compounds such as polyphenols, flavonoids, and carotenoids from the waste material using techniques such as microwave-assisted extraction and supercritical fluid extraction. These compounds have potential applications in the food and cosmetics industries due to their antioxidant and anti-inflammatory properties. Another way is the production of animal feed for poultry, pigs, and ruminants using avocado waste owing to its high protein and fiber content. Furthermore, bioplastics and biofuels are also alternatives for avocado waste valorization. All these techniques offer various opportunities for the valorization of avocado waste and contribute to sustainable waste management practices.
3.1. Production of Biofuels
3.1.1. Biodiesel
Biodiesel is a substitute for “diesel” obtained from petroleum. It is produced from vegetable oils such as soybean, canola, rapeseed, cottonseed, and maize, among others, and can also be obtained from animal fats or algae [12,48]. Biodiesel is a carbon-neutral fuel; this means that the amount of carbon produced by the combustion of biodiesel in engines is the same amount of carbon fixed by the plant during its growth [49]. The most common method for biodiesel production is the transesterification of triacylglycerides with alcohol [49,50]. Despite the many advantages of biodiesel production from vegetable oils, this process has the disadvantage of using food as a feedstock source; therefore, the use of vegetable oil from non-food sources is preferable. Some studies have been carried out to characterize the composition of the extractable oil from avocado seeds and evaluate its potential for biodiesel production, as well as to revalue the agro-industrial waste from avocado processing, in addition to facing the challenge of biofuel production (Table 3). Sutrisno et al. [51] described the transesterification of oil obtained from avocado seeds via the Soxhlet method, employing n-hexane. Biodiesel was obtained by a reaction with MeOH (20% of the weight of the oil), catalyzed with NaOH (1% of the weight of the oil), and allowed to settle for two days. Mixtures of 10 or 20% (v/v) avocado seed biodiesel and petroleum-derived diesel (called B10 and B20, respectively) were prepared. The performance of the B10 and B20 biodiesel blends was compared with the performance of palm oil biodiesel and pure petroleum-derived diesel, but with a lower sulfur content in comparison to the last one [51]. Otherwise, the biodiesel production using avocado seed oil by transesterification reaction, using 20% (v/v) methanol (MeOH) and 0.85% (w/v) catalyst (potassium hydroxide, KOH), with heating, produced a yield of 78% (v/w) [52]. Transesterification can also be carried out in situ, avoiding the extraction stage. The use of this technique allowed a yield of 94.4% (v/w) to be reached using avocado seeds (10 g) at the established optimal conditions (0.05% KOH, w/w, and 1.5 g of MeOH at 65 °C, 50 min of reaction with 600 rpm agitation, and tetrahydrofuran as extraction and reaction solvent) [53]. Therefore, the transesterification of avocado oil for biodiesel production is a sustainable and environmentally friendly process that provides a high yield, holding promise for both the renewable energy sector and waste reduction efforts. Moreover, the validation of biodiesel production by avoiding the oil extraction process with a high yield is an Important step to achieving the use of this waste for the obtention of added-value products. The optimization of a process that reduces unit operations enables the obtention of environmentally friendly products that are competitive with conventional products owing to the reduction in the cost of production that these actions could have.
Otherwise, heterogeneous catalysis transesterification reactions for obtaining avocado seed biodiesel have also gained attention [54]. In line with this, eggshell, a source of calcium oxide, has been used as a catalyst for transesterification reactions in the conversion of avocado oil to biodiesel. Under optimal conditions, the process allowed a yield of 75% to be reached (v/w, Table 3) [54]. In the search for an integrative approach to avocado waste valorization, the production of biodiesel from oil avocado seeds and peel was obtained using the Soxhlet method using n-hexane, producing a yield of 90% (Table 3) [55]. In these studies, performance parameters such as cetane number, free fatty acid content, and saponification value, among others, were similar to those of petroleum diesel, so it is important to scale up production and analyze its economic viability. Hence, biodiesel production from avocado waste presents a promising avenue for sustainable energy generation. By utilizing this waste product, not only can we reduce waste and environmental pollution, but we can also have a renewable source of energy that has the potential to replace conventional fossil fuels. To scale up biodiesel production based on avocado waste, further research is needed to optimize the production methods and ensure the economic viability of avocado-based biodiesel. This should be carried out considering the required amount of avocado oil, the yield, and the possible use of this oil in markets with a higher economic value.
3.1.2. Bioethanol
Along the same line of agro-industrial waste utilization, attention has been focused on the conversion of biowaste to bioethanol. In this regard, agro-industrial lignocellulosic wastes, such as cocoa pods, peanut shells, bagasse, and avocado seeds (P. americana), are common feedstocks for bioethanol production [56,57]. This process implies several physical, chemical, or biological pre-treatments, which are applied to be able to break down the lignocellulosic polymers into simpler components, i.e., polymeric compounds and fermentable sugars can be transformed into ethanol [12,52]. The pretreatment consisted of mechanical (particle size reduction by milling), chemical (heat treatment in alkaline solution), or microwave (alkaline solution with microwave heating) processes. Milled avocado waste provided a higher yield (178 mL/kg) in comparison to that obtained by using cocoa pods and peanut shells (43 to 61 mL/kg), which could be attributable to the high sugar content in avocados (336.4 mg/g) in comparison to cocoa pods (111.4 mg/g) and peanuts (82.3 mg/g) [16]. Another key point in bioethanol production is the optimization of the fermentation process; the optimal conditions to produce ethanol from a hydrolysate obtained from avocado seeds were settled at 3 days of fermentation, pH 5.5, and 30 °C, leading to a yield of 6.365% [56]. Although information about bioethanol production from avocado wastes is limited, there are already proposals for the integral use of avocados in biorefineries, where different types of products such as oil, xylitol, and ethanol could be obtained [51]. Harnessing avocado wastes for bioethanol production is a valuable alternative for bioenergy production in avocado-producer countries such as Mexico, where each 1 kg of ethanol generates 8.25 kWh [16].

Table 3.
Processing conditions applied for avocado waste valorization.
Table 3.
Processing conditions applied for avocado waste valorization.
| Avocado Source | Obtained Product | Pre-Treatment | Processing Technology | Treatment Conditions | Yield | Reference |
|---|---|---|---|---|---|---|
| Seed | Biodiesel | Sun-drying and milling. | Transesterification of oil obtained by Soxhlet extraction | Transesterification: 20% methanol; 1% NaOH as catalyst; 2 days reaction time. | 20% (v/w) | [51] |
| Seed | Biodiesel | Sun-drying and milling. | Transesterification of oil obtained by Soxhlet extraction | Transesterification: 20% methanol; 0.85% KOH as catalyst; 8–10 min reaction time | 78% (v/w) | [52] |
| Seed | Biodiesel | Sun-drying, milling, and sieving. | In situ transesterification | In situ transesterification: THF as extraction–reaction solvent, 0.05% KOH as catalyst; 5% MeOH; 65 °C; 50 min reaction time. | 94.4% (v/w) | [53] |
| Seed | Biodiesel | Milling and drying in oven. | Oil extraction with n-hexane and transesterification | Extraction: 65 °C during 4 h. Transesterification: 6:1 ratio of MeOH:avocado oil; 5% CaO as catalyst; 60 min reaction time | 75% (v/w) | [54] |
| Seed and peel | Biodiesel | Seed and peel: drying and milling. oil: acid pre-treatment with H2SO4. | Transesterification of pre-treated oil obtained by Soxhlet extraction | Transesterification: 8:1 MeOH:pre-treated oil ratio; 0.7% NaOH as catalyst; 60 min reaction time. | 90% (v/w) | [55] |
| Seed | Bioethanol | Seed: washing, drying, and milling. Thermal pre-treatment. Microwave pre-treatment. Enzymatic hydrolysis. | Sugar fermentation | Thermal pre-treatment: suspension of milled seeds in an alkaline solution (1% NaOH) placed for 10 min in a 13 L autoclave. Microwave pre-treatment: suspension of milled seeds in an alkaline solution (1% NaOH) placed in a microwave oven at 300 W for 25 min. Enzymatic hydrolysis: pH 5; 50 °C; 48 h | 102 mL/L (thermal pre-treatment) 154 mL/L (Microwave pre-treatment) | [16] |
| Seed | Bioethanol | - | Sugar fermentation | pH 5.5; 30 °C; 3 days. | 6.36% (v/w) | [56] |
| Seed | Biogas | Seed: washing, drying, and milling. Thermal pre-treatment. Enzymatic hydrolysis. | Biomass digestion | Thermal pre-treatment: suspension of milled seeds in an alkaline solution (1% NaOH) placed for 10 min in a 13 L autoclave. Enzymatic hydrolysis: pH 5; 50 °C; 48 h Biomass digestion: anaerobic process; 37 °C. | 214.2 NmL/g | [16] |
| Peel | Biogas | Sun-drying and milling. | Biomass co-digestion | Co-digestion: 50% avocado peel and 50% poultry manure; 25 °C; pH 7; 4 days. | - | [58] |
| Pulp | Biogas | - | Biomass co-digestion | Co-digestion: avocado pulp with cow manure, 1:1 ratio; 30 days | - | [59] |
| Seed | Bioplastic | Starch extraction. | Manufacturing of bioplastic by casting method | Heating a solution of starch and chitosan in a 7:3 ratio. Addition of ethylene glycol (5, 10, 15, 20, and 25%), 70–90 °C. | - | [60] |
| Peel and Seed | Bioplastic (Biofilm) | Starch extraction. | Biofilm by the casting method | Biofilm with plasticizer: heating a solution of 2 g of starch and 0.5 g of glycerol in 70 mL of water. Thirty min with agitation. Drying over plates for 72 h at 30° C. Biofilm without plasticizer: Heating a solution of 2 g of starch in 70 mL of water. Thirty min with agitation. Drying over plates for 72 h at 30 °C. | 11.38% (starch yield extraction, w/w) | [61] |
| Seed | Bioplastic | Starch extraction. Extraction of cellulose from sugar palm fibers. | Manufactured bioplastic | A 7:3 ratio of starch to microcrystalline cellulose, using Schweizer’s reagent as solvent. Glycerol as a plasticizer. Stirring at 85 °C. | 16% (starch yield extraction, w/w) | [62] |
| Seed | Polylactic acid (PLA) | Seed hydrolysate. | Fermentation of avocado seed hydrolysates | Performed in 1 L fermenters; 37 °C; pH 7; 400 rpm; aeration rates (0.0–0.5 vvm); 0.037 gdcw/L as initial inoculum. | - | [63] |
| Commercial avocado oil | Polyhydroxyalkanoate (PHA) | - | Fermentation with avocado oil | Batch cultivation with C/N ratio of 14; avocado oil was added in different concentrations: 5, 10, 15, 20, and 25% (v/v). | 70.83% (v/v) | [64] |
| Seed | Snack | Freeze-drying. Oven-drying. | Extruding | Extrusion of brown rice, barley, and freeze-dried and oven-dried avocado seed through a 5 mm die opening at 130 °C (generated by friction). | - | [11] |
| Seed | Snack | Freeze-drying. | - | Formulation of a snack with different proportions of avocado seed powder (6, 12, and 18%). | - | [28] |
| Seed | Snack | Slicing, blanching, drying, and milling. | Extruding by hot air puffing | Extrusion at 110–115 °C of maize and avocado seed flour in several proportions (90:10, 80:20, 70:30, 0:100, and 100:0); screw speed of 100 rpm. Hot air puffing: 70 °C; 4 h. | - | [65] |
| Seed | Avocado Seed Power | Slicing and milling. Homogenized seeds were mixed with water and sonicated then vacuum filtered. | Spray drying | Spray-drying in different conditions of feed flow rate (20–25 mL/min) and inlet temperature (160–208 °C). | 24.46–35.47% | [66] |
| Seed | Extract | Milling, freeze-drying. | Extraction | Extraction: ethanol concentrations (26–93%), time (2.77–55.22 min), and temperature (26.36–93.64 °C) at different values. Cooled to 5 °C, centrifugated, and stored at −20 °C. | - | [67] |
| Peel (Hass variety) | Emulsion | Washing, freeze-drying, and milling. | Extraction and emulsion formulations. | Extraction: Soxhlet method, in a 1:36 ratio of ethanol to avocado. Three h of extraction. Emulsion formulation: oil-in-water and water-in-oil. | 23%(extraction) | [68] |
| Skins, pits, and leftover flesh | Vermicompost | Pre-composting of the organic residues. | Uses earthworms to break down organic materials. | Layers of shredded avocado waste and bedding material (paper, leaves, etc.) at room temperature. | - | [69] |
| Seed | Biochar | Avocado seeds cut. | Pyrolysis | Heating at temperatures between 400 and 550 °C. | - | [70] |
| Skins, pits, and leftover flesh | Liquid biofertilizer | Selection and isolation of active microorganisms. | Anaerobic digestion. | Fermentation for 90 h at 30 °C. | - | [71] |
THF: Tetrahydrofuran. All the studies contained in this table were carried out at a laboratory scale, which implies a technology readiness level of 4.
3.1.3. Biogas
Biogas is a flammable gas composed mainly of methane (CH4, 50–60%, v/v), and CO2 (30–45%, v/v), H2S, and NH3 can be found in small quantities. It is produced by the anaerobic digestion of organic matter carried out by microorganisms, which is why its composition is so variable. CH4 is the relevant gas in this mixture, as it is the actual gas of concern due to its use as an energy source [7]. Biogas obtention by using agro-wastes, including avocado pulp, for its high oil content has been widely studied. Almeida and Cadena [72] reported that biogas production from avocados was lower compared to sources with high protein (soybean), sugar (blackberries), or fiber content (sugarcane bagasse). This is probably because the high content of long-chain fatty acids in avocados could inhibit the microorganisms in the methanogenesis phase. Furthermore, a significant concentration of lipids inhibited methane production due to the formation of long-chain fatty acids [72,73]. Therefore, the use of avocado pulp may not be an appropriate approach for biogas production. In line with this, avocado seeds have been used for biogas production. A thermal–chemical (steam + NaOH) pretreatment, followed by the application of cellulases, allowed the authors to obtain 214.2 NmL/g of biogas, which is a higher value compared to the biomass without enzymatic treatment (161.5 NmL/g) [16]. Hence, by using the proper pretreatment, the avocado seeds can have a higher potential for CH4 production in comparison to other agro-waste such as bananas, potato peels (90%, v/w) [74], cocoa pods (65%, v/w), and peanut shells (40%, v/w) [16].
Other studies have explored the strategy of carrying out biogas production through the co-digestion process, which has shown that under certain conditions, the biogas production yield can increase compared to using a single substrate due to a synergistic effect [7,53,58]. In the study by Kenasa and Kena [58], a mixture of avocado peels and manure from different animals was assessed. The higher accumulative biogas production (453.5 mL) was achieved using 50% (w/w) avocado peel and 50% (w/w) poultry manure after 4 days of incubation at 25 °C, 0.5% (w/w) salt, and a pH of 7. All the feedstock containing avocado peel waste showed fast and higher biogas generation in comparison with the treatment without agro-waste. On the other hand, Tura et al. [7], evaluated the production of biogas through a combination of different fruit and vegetable wastes (including avocado) with cow manure. They showed that through this strategy, it is possible to obtain 105.5 mL of biogas/kg of biomass. In a similar study, the synergistic effect between cow manure and avocado pulp was described, and this combination was able to achieve an increase of 113% (v/w) concerning the production of cow manure separately [59]. Therefore, the production of biogas from avocado waste presents a sustainable approach to waste management and energy generation because 1 m3 CH4 is equivalent to 11 kWh [16]. The pretreatment with enzymes followed by the co-digestion process is a valuable alternative to convert avocado waste into bioenergy, further reducing residues and contributing to a circular economy. With continued research and development, biogas production from avocado waste has the potential to play an important role in the transition toward a more sustainable energy system.
3.2. Production of Biodegradable Plastics
Bioplastics are polymers made or obtained from renewable natural resources or certain microorganisms. They are an alternative to traditional plastics, acquired through the polymerization or condensation of molecules from petroleum refineries [75]. Bioplastics represent an opportunity to address climate change as they help to reduce the emission of greenhouse gases (mainly CO2) [75,76,77]. To obtain bioplastics, it is usual to process food products, such as maize, potato, and tapioca, among others, mainly to obtain polysaccharides [78,79,80]. As this may affect the food supply, the use of other sources to produce bioplastics, such as agro-industrial waste, is currently being explored [77]. The use of avocado seeds has gained interest in recent years to produce bioplastics since this process requires a large volume of raw materials. In addition to this, avocado seeds have a high content of starch (Table 2) [60]. A method for processing avocado wastes to obtain functional films by using avocado peels and seeds (35 and 65%, w/w) has been proven. This process includes acid hydrolysis with acetic acid, followed by plasticization with polyglicerine-3 and subsequent blending with pectin. The resulting film was suitable for food contact applications owing to its mechanical qualities, optical clarity, outstanding oxygen barrier capabilities, high antioxidant activity, biodegradability, and component migration control [81]. The addition of plasticizers helps to improve the mechanical properties of avocado films. In this sense, the mechanical properties of films made of starch from avocado seeds and chitosan were improved by the addition of ethylene glycol. Of particular interest is the linear increment in tensile strength and elongation at break as the plasticizer concentration increases from 5 to 35% (w/w) [60]. Moreover, glycerol was used to improve the mechanical properties (elongation increased four times) and water vapor permeability of the bioplastic obtained by extracting starch from avocado seeds, in contrast to the bioplastic without the addition of glycerol [61]. On the other hand, microcrystalline cellulose from sugar palm fiber was added to the bioplastic obtained from avocado seed starch as reinforcement in different proportions. Using this strategy, it was found that the best properties of the bioplastic were obtained with a 7:3 ratio of starch to microcrystalline cellulose. The bioplastic developed a tensile strength of 20.87 mPa and an elongation at break of 6.22% [62]. The bioplastics from avocado waste have great potential for use in foods with low moisture content, owing to their tendency to absorb water, promoting the loss of quality properties [61]. Furthermore, by mixing the starch from avocado seeds with other hydrocolloids and plasticizers, bioplastics with different properties for a wide range of applications can be developed, covering the requirements of each perishable food.
The production of bioplastics such as polylactic acids (PLAs) and polyhydroxyalkanoates (PHAs) using avocado waste as feedstock has also been described (Table 3). Modified Escherichia coli was used to synthesize lactate from avocado seed hydrolysate, which was used for the subsequent production of PLAs [63]. In other cases, avocado oil was used as the carbon source for PHA production by Cupriavidus necator H16. In this study, different proportions of avocado oil were used for PHA production, concluding that the best yield was reached by adding 20% (v/v) oil in the culture medium with nitrogen limitation [64]. Furthermore, different ratios of arabinose/avocado oil to obtain PHAs have been assessed; this is of particular importance since 0.1% (w/v) of arabinose has proven to encourage the wild-type synthase in C. necator H16 to express itself and polymerize longer-side chain monomers such as 3-hydroxyoctanoate (3HO) and 3-hydroxydecanoate (3HD) when compared to co-polymers made entirely of 3-hydroxybutyrate. This structural modification has a direct effect on the properties of the polymer; in this case, the melting temperature of the co-polymers is dramatically lowered by the inclusion of even trace amounts of 3HO and 3HD [82]. Hence, the culture medium composition should be properly optimized and aimed at producing the bioplastic with the required conditions for the target application. The production of biodegradable plastics from avocado waste has gained significant attention in recent years as a sustainable solution to the ever-increasing problem of plastic waste. Biodegradable polymers offer a potential alternative to traditional plastics that take hundreds of years to decompose and can cause severe damage to the environment.
3.3. Production of Animal Feed
Another way to promote the valorization of avocado waste is by feed production. Avocado seeds have been found to contain metabolic energy and can be used as a feed ingredient. However, the use of avocado waste in feed production must be limited due to the presence of antinutritional substances or tannins [83]. Despite this limitation, studies have shown that incorporating avocado waste into animal feed can have positive effects on body weight gain, feed efficiency, and other production performance parameters [84,85]. Evan et al. [84] performed an exploratory study carried out on lambs, which showed that the inclusion of 10% avocado meal in the diet after 84 days had a positive effect on weight increment in comparison with the control without affecting the growth behavior of the goats, and no significant effect on carcass traits was observed [86]. However, diets containing 14.8% avocado pulp and seed as a supplement for dairy goats showed a decrease in milk production and milk fat content, and this was linked with avocado lipid oxidation, which also caused off-flavor [84]. Regarding pig feeding with avocado wastes, a formulation containing sorghum meal (53.7%), soybean meal (12.9%), avocado paste (30.0%) (made of grounded whole avocados), CaHPO4H·2H2O 1.0%, CaCO3 (1.2%), NaCl (0.2%), vitamins, and trace elements (1.0%) was assessed (all percentages in w/w). Avocado consumption significantly changed the amount and make-up of intramuscular fat, lowering the lipid content in the muscle longissimus thoracis and raising the level of unsaturation. During cool storage, the muscles from treated pigs exhibited considerably decreased lipid and protein oxidation rates. Additionally protected from oxidation was the color of the treated pigs’ muscles [87]. In the case of broiler poultry, the feed consisted of grounded corn seeds (23.5%, w/w) and pomegranate-avocado pulp and seed wastes (76.5%, w/w). This supplementation had a positive impact on meat quality, particularly in the improvement of the fatty acid composition, such as ω-3 fatty acids, a reduced ratio of ω6/ω3 and cholesterol levels, and enhanced antioxidant activity. Additionally, compared to the commercial corn silage used for broiler breeding, this mixture was 50% less expensive [88]. Utilizing agricultural waste, particularly avocado residues, in the optimized concentrations for feed formulations could be an intriguing strategy for cutting expenses while promoting sustainability and environmental welfare. However, it is important to mention that avocado wastes, particularly peels and seeds, contain persin (1.81 ± 0.35 mg persin/g avocado seed) [15]. This compound is an acetogenin formed from the production of long-chain fatty acids and has a similar structure to linoleic acid. Persin can induce non-infectious mastitis and agalactia owing to the extensive coagulation necrosis of the secretory acinar epithelium and interstitial edema, congestion, and hemorrhage. It has been demonstrated that at a dose between 60 and 100 mg/kg, persin induces the above-mentioned effects on mammary glands in lactating mice. However, doses above 100 mg/kg induced necrosis of myocardial fibers and hydrothorax in the animals [89]. Hence, it is important to have information about the concentration of this compound in the feed or reduce the constant consumption of products that do not report the persin concentration.
3.4. Production of Natural Fertilizers
Avocado industry waste generates opportunities to develop products with greater value-adding from these wastes to promote a sustainable green economy [90]. The composition of this waste is organic matter with a high concentration of nutrients, essentially phosphorus and nitrogen, which bring to this waste an important fertilizing capacity [91]. Several types of biofertilizers can be produced from avocado industry waste, and we will explain some of these in the next subsections.
3.4.1. Compost
Composting involves the decomposition of organic matter through the action of microorganisms, resulting in a nutrient-rich fertilizer that can be used to improve soil quality and plant growth. In addition, this process provides a mature product that is free of pathogenic microorganisms and stable for use in agriculture [92,93]. To study the degree of organic matter decomposition during composting, the most frequently used parameters to assess humification include the carbon/nitrogen (C/N) ratio, the humic acid/fulvic acid ratio, and the humification ratio. The C/N ratio is an indicator of the availability of nitrogen during composting. As composting progresses, the C/N ratio decreases due to the decomposition of carbon-rich organic matter and the release of nitrogen. A lower C/N ratio is generally considered to indicate a more mature compost [7,11]. Perez-Murcia et al. [91] studied different composting methods combining avocado leaves with another agri-food sludge. They found that the N contents were the highest at the beginning of the process, with a lesser increase with time. However, they found that this product showed low values of the germination index, less than 60%, which indicates potential phytotoxicity possibly associated with the greater presence of polyphenolic compounds. The humification ratio is a measure of the degree of humification of organic matter during composting. Humification is the process by which organic matter is transformed into stable, humic substances. A higher humification ratio indicates a more advanced stage of humification and a more mature compost [91,94]. The humic acid/fulvic acid ratio is a measure of the relative proportions of different types of organic matter in compost. Humic acids are complex organic molecules that are more resistant to decomposition, while fulvic acids are smaller and more easily degraded. A higher humic acid/fulvic acid ratio is generally associated with a more mature compost [94]. The composting of avocado waste is one of the most critical areas for sustainable avocado waste management, helping farmers reduce their dependence on chemical fertilizers.
3.4.2. Vermicompost
Avocado waste can be processed through vermicomposting to produce a nutrient-rich fertilizer. Vermicomposting is a process that uses earthworms to break down organic materials into nutrient-rich compost that can be used as fertilizer for plants [95]. Avocado waste, including skins, pits, and leftover flesh, contains valuable nutrients, such as nitrogen, potassium, and phosphorus, that are essential for plant growth. By vermicomposting avocado waste, the earthworms can help break down the waste and convert it into a nutrient-rich compost that can be used to nourish plants [69,96]. To vermicompost avocado waste, layers of shredded avocado waste and bedding materials such as shredded newspaper, cardboard, or leaves are formed. Then, the earthworms are added to the bin and left to do their work. Over time, the earthworms break down the avocado waste and produce rich compost that can be used to fertilize plants. It is important to note that not all types of earthworms are suitable for vermicomposting. Red worms, also known as red wigglers, are the most commonly used species for vermicomposting because they thrive in organic waste and produce high-quality compost [69].
3.4.3. Biochar
Biochar is a form of charcoal that is produced by heating organic material, such as wood or agricultural waste, in a low-oxygen environment. Avocado waste can be converted into biochar through pyrolysis. Biochar is a carbon-rich material that can be used as a soil amendment to improve soil structure, increase water-holding capacity, and enhance nutrient retention [97]. When avocado seeds are used to produce biochar, the process involves heating the waste in a kiln or oven at temperatures between 400 and 800 °C. This results in the production of a stable, carbon-rich material with a higher heating value [98]. Biochar made from avocado waste has been found to have a high content of carbon and other nutrients, such as nitrogen and phosphorus, which can help improve soil fertility and support plant growth [70]. The porous structure of biochar also can retain water and nutrients, which can help reduce nutrient runoff and soil erosion. Studies have shown that biochar from avocado seeds impregnated with Mg-(hydr)oxide (post-pyrolysis) demonstrated the benefits of metal impregnation regarding enhancing phosphate adsorption and its potential use for water treatment due to its performance for aqueous phosphate removal [99].
3.4.4. Liquid Biofertilizers
A suspension that has agriculturally useful microorganisms, such as phosphobacteria, Rhizobium, and Azospirillum, is a liquid biofertilizer. The advantage of liquid biofertilizers over conventional carrier-based biofertilizers is a higher competition potential with the native population, longer shelf life, and stability in storage up to 45 °C. Avocado waste can also be used to produce liquid biofertilizers through a process known as anaerobic digestion. During this process, microorganisms break down organic matter in the absence of oxygen, producing a nutrient-rich liquid fertilizer that can be applied directly to plants or used as a soil amendment [100]. Otherwise, the biofertilizer could be made by a mixture of different organic materials, as proposed in US patent 11,512,029 B21, where manure, organic waste such as avocado, and microorganisms that metabolize nutrients are used to increase nutritional value while contributing to providing humus, limiting leaching and soil erosion, balancing microorganisms, and reducing environmental contamination [71].
The use of avocado waste as fertilizers offers several advantages, including (i) waste reduction: composting is an effective way to reduce the volume of avocado waste generated by the industry. By diverting avocado waste from landfills, composting helps to reduce greenhouse gas emissions and environmental pollution. (ii) Soil enrichment and composting procedures: nutrient-rich organic material can be used to improve soil fertility, enhance water retention, and promote plant growth. (iii) Cost savings: composting can save farmers’ and growers’ money by reducing the need for synthetic fertilizers and improving soil health, leading to healthier crops and higher yields. Composting seems to be the easier and cheaper alternative for avocado waste management. However, it can be a challenge due to some limitations, including (i) time and space requirements: composting requires time and space to allow the waste to decompose and transform into a usable product; (ii) high moisture content makes it difficult to compost it without the addition of dry organic materials such as straw or wood chips; (iii) improper composting process can result in a product with pathogen microorganisms such as Escherichia coli and Salmonella; and (iv) variability in composition, due to the composition of avocado waste can vary depending on factors such as the variety of avocado, the stage of ripeness, and the method of processing, which can affect the quality and consistency of the compost [70,99,101].
3.4.5. Avocado Waste as a Substratum for the Cultivation of Mushrooms and Microorganisms
Edible mushrooms have been a part of human culture since time immemorial and are very popular due to their sensory qualities and appealing culinary qualities. Mushrooms are in high demand these days because of their numerous nutritional and health benefits. They have a high protein, vitamin, and mineral content and are low in calories, carbohydrates, fat, and sodium, and owing to this, they are considered superfoods [102,103]. Mushroom cultivation substrates vary depending on the species of mushroom being grown, as different species require different types and compositions of the substrate. Some common substrates include sawdust, straw, compost, and various agricultural and forestry byproducts [104]. These substrates can be combined to achieve the desired mixture of nutrients, moisture, and texture required for optimal mushroom growth [105]. Under the concept of circular economy, agro-industrial by-products such as avocado waste can be used as substrates for the obtention of added-value products. Of particular interest is mushroom production due to its global market size, which was estimated at USD 50.3 billion in 2021 and is expected to grow at a compound annual growth rate of 9.7% from 2022 to 2030. The increasing vegan population across the globe eating a high-protein diet is expected to be a major driver for the market during the forecast period [106]. Avocado waste can be used as a substrate for mushroom cultivation. Studies have shown that avocado peels can be a great substrate for oyster mushrooms such as Pleurotus ostreatus [17,105]. However, the yield produced by this substrate can be lower than using orange and pineapple peels, but it is important to mention that the mushroom cultivated on a substrate of the avocado peel showed higher radical scavenging activities. This was attributable to avocado waste having a high content of phenolic compounds (Table 2), which are absorbed by the mushrooms during their cultivation [17]. Otherwise, a culture medium made from avocado seed hydrolysates has been developed for the growth and support of lactic acid bacteria. This medium supplemented with the proper nutrients and additives (peptone, meat extract, yeast, Tween 80, dipotassium phosphate, sodium acetate, ammonium citrate, magnesium sulfate, and manganese sulfate) was able to increase the biomass growth and lactic acid production of Lactobacillus sp. In addition, a preliminary economic estimate indicated that this alternative medium could be at least 17% cheaper than the conventional MRS medium for lactic acid production. However, the strain used in this study showed low yields and produced low lactic acid concentrations compared to other processes based on starchy biomass materials [107]. However, it is important to carry out the optimization of the concentration of the avocado wastes used in the formation of substrates and culture media due to the presence of persin in peels and seeds. Persin is a toxin contained in the idioblastic oil cells of avocado fruits and leaves and is believed to act as a natural insecticide and fungicide; hence, it could limit the growth and development of cultivated organisms [15]. In addition to this, like any organic material, avocado waste can be susceptible to contamination by competing microorganisms, molds, or pathogens. Proper sterilization or pasteurization techniques should be employed to reduce contamination risks as in any other substrate [17]. Furthermore, it is important to consider that the rate at which avocado waste decomposes may differ from other substrates, affecting the overall duration and efficiency of mushroom cultivation. Finally, the substrate texture could be different than that of traditional substrates such as straw or sawdust, which can impact mycelium colonization and fruiting [17,105]. Nevertheless, using fruit waste substrates such as avocado peels for mushroom or microorganism cultivation under the proper optimized conditions is a good way to convert agricultural waste into a value-added product and reduce the amount of waste sent to landfills.
3.5. Production of Food Ingredients and Supplements
As previously mentioned, the amount of waste produced by avocado processing has become an opportunity due to the ample possibilities of using and reintegrating it into the production chain and revaluing it [11,108]. Avocado wastes are rich in compounds of interest, such as starch, antioxidants, lipids, and a wide range of other phytochemicals that can be of potential use in the food industry [19]. The avocado seed has been studied by several research groups that have found many phenolic compounds with antioxidant activity, in fact with a greater amount than that contained in the pulp or peel [109,110]. As described above, the application of seed extracts in various foods has gained much attention. For example, Permal et al. [11] developed a ready-to-eat extruded snack from freeze-drying seeds. The persin and amygdalin contents were 2.6 × 10−6 and 0.68 mg/g, respectively, which were non-toxic to humans. In agreement with this, the detected levels of amygdalin and persin in cereal snacks added with different concentrations of avocado seed powder (6, 12, and 18%) were negligible, while the developed product showed a parallel increment of the dietary fiber content. Their results are consistent with those observed in other research groups, where avocado seed powder has been found to contribute to a substantial increase in the content of phenolic compounds. In addition, with 6% avocado seed powder, the content of polyphenols increased approximately fivefold [28]. Another study in which a snack was obtained from avocado seed flour and maize showed that a proportion of 20% avocado seed flour is enough to obtain a product with antioxidant activity [65]. The reduction in the concentration of these compounds in the functional products has been linked to the sublimation process during the freeze-drying processing of avocado waste [11,28].
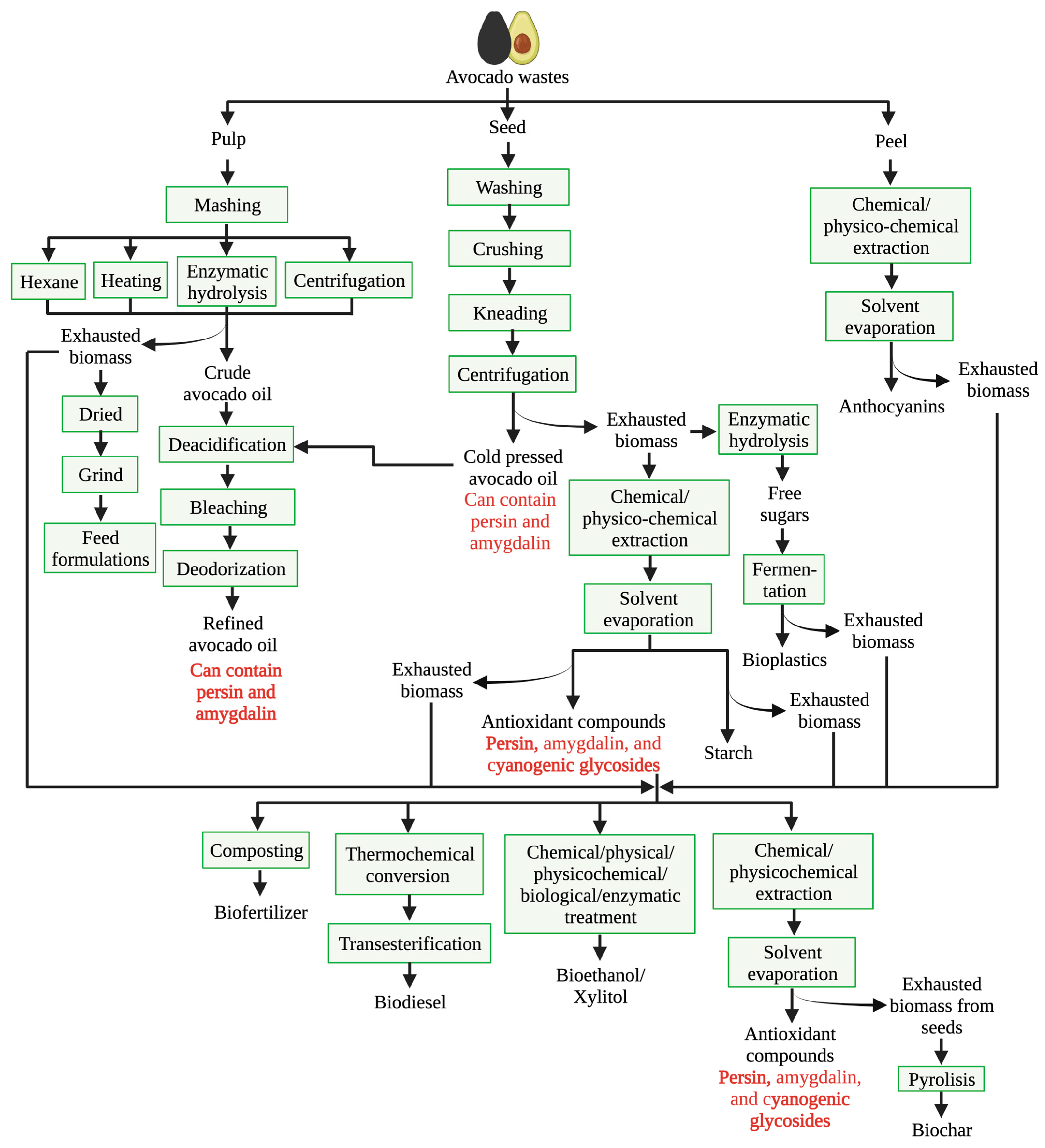
Other research groups have evaluated avocado seed extracts processed in different ways for their potential application in the food industry. Avocado seed extracts have anti-inflammatory activity and potential use as a food ingredient [111]. However, as with any plant extract, its bioactive compounds are susceptible to decomposition by exposition to environmental conditions. For this, the implementation of techniques such as spray-drying represents a reliable alternative to protect them by incorporating natural polymers [66]. In line with this, the oil/water emulsion of avocado seed extracts and egg albumin had an antioxidant effect on hamburger meat since it was found that this meat showed no signs of oxidation for 8 days [67]. Most of the studies have been based on avocado seed extracts; however, the use of peel extracts has also received interest from other research groups, as shown in a study by Ferreira et al. [68] in which the antimicrobial activity of an avocado peel extract in mayonnaise was investigated. They found that the ethanolic extract could inhibit bacterial growth with better performance than that shown by ascorbic acid. In addition to the presence of oils and phenolic compounds, avocado seed is also a great source of starch. The starch from this source has a gelatinization temperature range of 56–74 °C, a solubility of 19–20%, and a swelling power of 28–30 g water/g starch, meaning that it has potential as an ingredient in food systems [66,68]. Therefore, avocado wastes have a great potential to obtain high-added-value products; however, this process can involve several steps, which are common in the processing of pulp, peels, and seeds. This could be harnessed to obtain more than one product from these wastes (Figure 2).
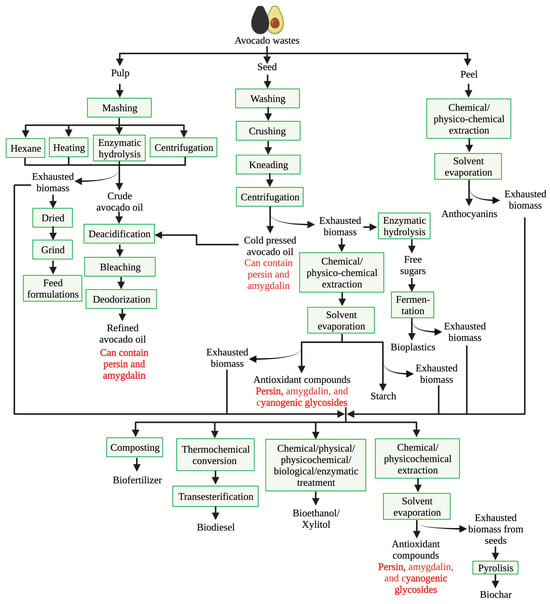
Figure 2.
Main process involved in the conventional avocado waste biorefinery. Compounds in red are substances that could affect human or animal health after constant consumption in high amounts.
4. Role of Avocado Waste Valorization in the Compliance of Sustainable Development Goals
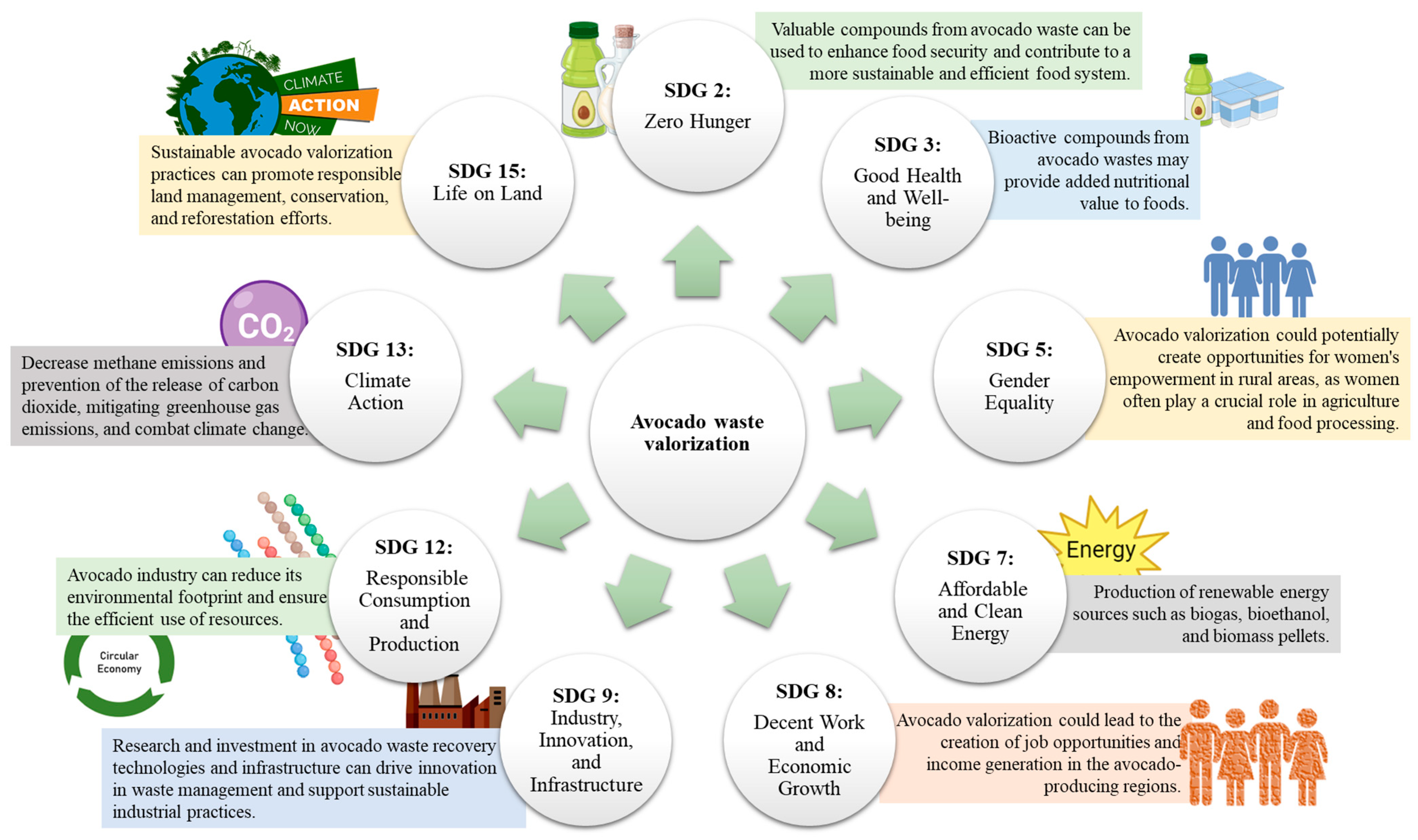
Avocado waste valorization has the potential to contribute to the compliance of several sustainable development goals (SDGs) as it provides a sustainable solution for waste management and can lead to the creation of value-added products [27,88,112]. This is an important topic as it pertains to the sustainable utilization of agricultural waste and the achievement of global sustainable development objectives. Avocado waste is an organic residue that can be utilized to produce bio-products, including biodegradable plastics, biogas, biofuels, and food ingredients [12,29,62]. This provides an opportunity to reduce waste generation, increase resource efficiency, and support the transition to a circular bioeconomy.
The valorization of avocado waste through various biorefinery processes has the potential to contribute significantly to the SDGs (Figure 3). The avocado fruit is a source of food and income for many communities. The valorization of avocado waste can lead to the production of value-added products, such as avocado oil, flour, and pulp, which can be used as food ingredients, reducing food waste and contributing to food security (SDG 2: Zero Hunger) [13,32]. Otherwise, the extraction of the high content of antioxidants and other bioactive compounds in avocado waste leads to the production of nutraceuticals, functional foods, and dietary supplements that promote good health and well-being (SDG 3: Good Health and Well-being) [13,68].
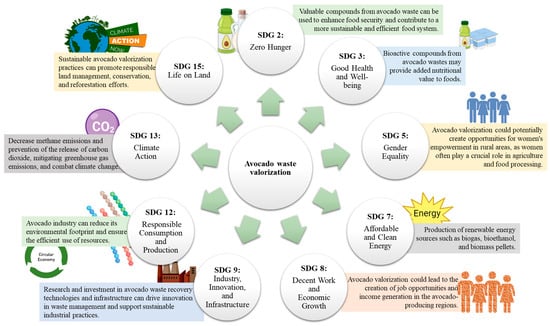
Figure 3.
Importance of avocado waste biorefinery in compliance with the sustainable development goals (SDG).
Moreover, the use of avocado waste as a feedstock for bioenergy production can contribute to the transition towards renewable energy sources such as biogas, biofuels, and other forms of energy, reduce reliance on fossil fuels, and promote sustainable energy (SDG 7: Affordable and Clean Energy) [12,113]. Avocado waste conversion into bioenergy can reduce greenhouse gas emissions and mitigate climate change. The use of bioenergy can also help to reduce deforestation and land-use change, which are major contributors to carbon emissions (SDG 13: Climate Action) [18,90]. The valorization of agro-waste can create employment opportunities and contribute to economic growth. In this sense, small- and medium-sized enterprises can be established to process the waste into value-added products, creating jobs and increasing income for local communities (SDG 8: Decent Work and Economic Growth). All these actions that result in the production of value-added products from the waste contribute to a circular economy, where waste is viewed as a resource rather than a liability (SDG 12: Responsible Consumption and Production)[12,113]. The production of value-added products from avocado residues reduces waste generation, promotes sustainable consumption and production, and contributes to the transition towards renewable energy sources, all while providing economic and social benefits. Several international companies harness avocado wastes for the development of added-value products that are well-positioned in the market (Table 4). Even in these successful cases, a collaborative effort between different stakeholders, including governments, industries, and academia, is required to promote research and the implementation of sustainable management practices to achieve the SDGs.

Table 4.
Commercial products based on avocado wastes or that incorporate avocado by-products in their formulation.
5. Trends and Challenges for Avocado Waste Valorization
The new bio-economy vision rests on unlocking the full potential of all types of sustainably sourced biomass, including residual biomasses such as avocado waste from the avocado processing industry, and it is based on reducing waste and maximizing resource efficiency [92]. The tendency in the avocado industry is to reduce waste by finding innovative uses for avocado waste. Returning to what was said above, avocado waste can be composted to produce a nutrient-rich soil amendment, which can be used to support plant growth and reduce the need for chemical fertilizers.
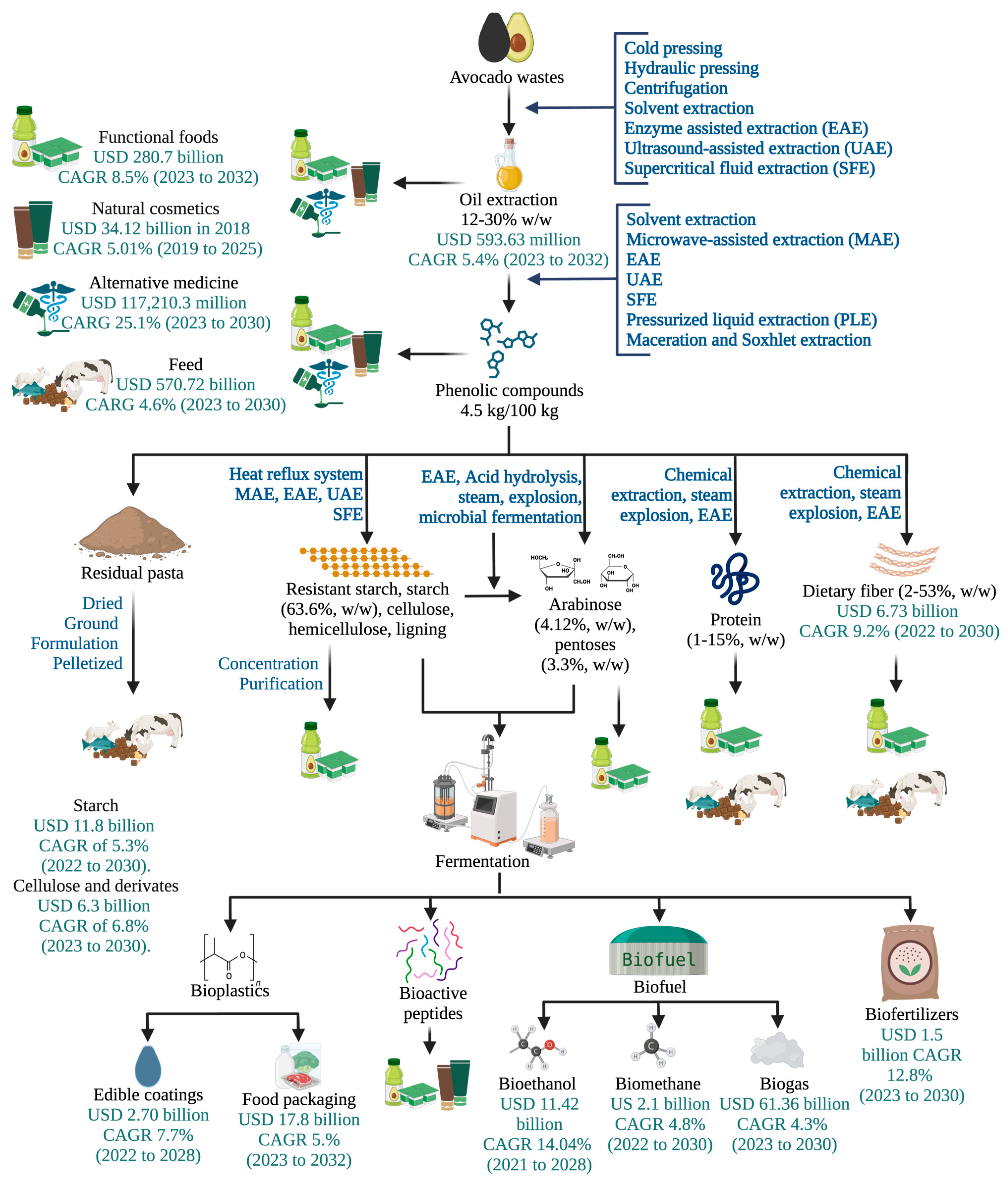
Additionally, avocado waste can be used as a feedstock for biogas production, which is a renewable energy source that can be used to generate electricity or fuel vehicles. Another trend in waste reduction consists of finding innovative uses for avocado waste. For example, avocado waste can be used to produce food ingredients such as dietary fiber, antioxidants, and colorants, which can be used in a variety of food products. The avocado industry has the potential to support sustainable development by reducing waste, improving resource efficiency, and promoting economic growth (Figure 4). By finding innovative uses for avocado waste, the industry can reduce its environmental footprint, create new business opportunities, and contribute to a more sustainable future [101,113]. In addition, there is a growing focus on the development of innovative processing technologies that can improve the efficiency of avocado waste valorization. These technologies include high-pressure processing, enzymatic hydrolysis, and microwave-assisted extraction, which can enable the extraction of the target compounds while maintaining or improving their properties [32,112].
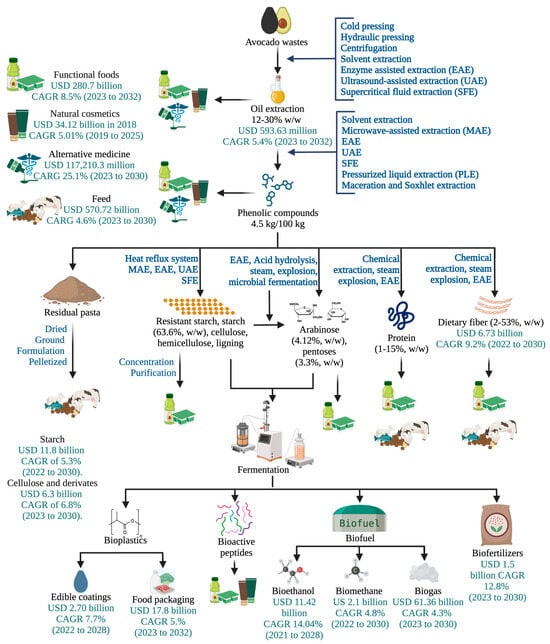
Figure 4.
Integral biorefinery of avocado waste incorporating emerging technologies and global market value of the products. CARG: compound annual growth rate. Flow lines indicate the use of the biomass derived from the previous process.
Some challenges associated with the green valorization of avocado waste, including (i) the logistics of collecting and transporting avocado waste, can be challenging, especially for small-scale producers. The waste is highly perishable and requires careful handling to prevent spoilage and maintain its quality. (ii) The scale-up of avocado waste valorization processes can be challenging due to the variability in the waste stream. The waste can vary in composition, quality, and quantity, making it difficult to develop standardized processes that can be easily scaled up. (iii) The production of value-added products from avocado waste can have environmental impacts, such as the consumption of energy and water resources and the generation of waste streams. It is important to ensure that the environmental impact of these processes is minimized and that sustainable practices are followed. (iv) The regulatory framework for avocado waste valorization is still evolving, and there is a lack of clarity regarding the approval processes for the use of avocado waste in food, cosmetics, and pharmaceutical products [114].
In addition to the mentioned points, all the proposals for waste valorization must be effective and economical strategies that consider the technical and economic feasibility of the process. At the same time, they must ensure environmental protection and safety. Even with all the studies carried out on waste valorization, more research and the development of new valorization strategies should be encouraged to create new market opportunities for avocado waste by-products. This will require the collaboration of all stakeholders in avocado production, including researchers, farmers, and policymakers, to promote sustainable practices.
6. Conclusions
The avocado waste biorefinery is a promising solution for achieving more sustainable development. The use of avocado waste as a feedstock to produce biofuels, bioplastics, and other value-added products not only helps to reduce waste but also offers an alternative to traditional fossil fuels and plastics. Additionally, the development of such a biorefinery creates new revenue streams and jobs, thus contributing to the local economy. This approach creates economic opportunities, particularly in developing countries, potentially reducing poverty while mitigating environmental pollution caused by the disposal of waste. As we continue to seek ways to reduce our environmental impact and move towards a more sustainable future, initiatives like this pave the way for innovative and practical solutions to promote the effective utilization and reutilization of waste resources that benefit both the environment and society. To achieve this, several challenges should be addressed, including logistics, scale-up, environmental impact, and regulation. The adopted strategies to solve these drawbacks must be effective and economically feasible. Addressing these challenges will require collaboration between industry, academia, and government to develop innovative solutions that promote the sustainable use of avocado waste.
Author Contributions
Conceptualization, M.I.-M.; writing—original draft preparation, T.S.-C., F.G.C., A.P., M.I.-M. and L.A.-G.; writing—review and editing, T.S.-C., F.G.C., A.P., M.I.-M., and L.A.-G.; visualization, M.I.-M. and L.A.-G.; supervision, M.I.-M. and L.A.-G.; project administration, L.A.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diniz do Nascimento, L.; Moraes, A.A.B.d.; Costa, K.S.d.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G.d. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Bhore, S.J.; Ochoa, D.S.; Al Houssari, A.; Zelaya, A.L.; Yang, R.; Chen, Z.; Siddiqui, S.D.; da Silva, S.S.; Schumann, M.; Zhang, Z.; et al. The Avocado (Persea americana Mill.): A Review and Sustainability Perspectives. Preprints 2021, 2021120523. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAOSTAT) Cultivos. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 9 May 2023).
- Bill, M.; Sivakumar, D.; Thompson, A.K.; Korsten, L. Avocado Fruit Quality Management during the Postharvest Supply Chain. Food Rev. Int. 2014, 30, 169–202. [Google Scholar] [CrossRef]
- Jimenez, P.; Garcia, P.; Quitral, V.; Vasquez, K.; Parra-Ruiz, C.; Reyes-Farias, M.; Garcia-Diaz, D.F.; Robert, P.; Encina, C.; Soto-Covasich, J. Pulp, Leaf, Peel and Seed of Avocado Fruit: A Review of Bioactive Compounds and Healthy Benefits. Food Rev. Int. 2021, 37, 619–655. [Google Scholar] [CrossRef]
- García-Vargas, M.C.; Contreras, M.; Gómez-cruz, I.; Romero-garcía, J.M.; Castro, E. Avocado-Derived Biomass: Chemical Composition and Antioxidant Potential. Proc. West Mark Ed Assoc. Conf. 2021, 70, 100. [Google Scholar]
- Mekonnen Tura, A.; Seifu Lemma, T. Production and Evaluation of Biogas from Mixed Fruits and Vegetable Wastes Collected from Arba Minch Market. Am. J. Appl. Chem. 2019, 7, 185. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G.A. Avocado Fruit and By-Products as Potential Sources of Bioactive Compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Sandoval-Contreras, T.; Calderón-Santoyo, M. Sodium Alginate Coatings Added with Meyerozyma caribbica: Postharvest Biocontrol of Colletotrichum gloeosporioides in Avocado (Persea americana Mill. cv. Hass). Postharvest Biol. Technol. 2020, 163, 111123. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Avocado By-Products as Inhibitors of Color Deterioration and Lipid and Protein Oxidation in Raw Porcine Patties Subjected to Chilled Storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef]
- Permal, R.; Leong Chang, W.; Seale, B.; Hamid, N.; Kam, R. Converting Industrial Organic Waste from the Cold-Pressed Avocado Oil Production Line into a Potential Food Preservative. Food Chem. 2020, 306, 125635. [Google Scholar] [CrossRef] [PubMed]
- García-Vargas, M.C.; Contreras, M.D.M.; Castro, E. Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts. Appl. Sci. 2020, 10, 8195. [Google Scholar] [CrossRef]
- Dias, P.G.I.; Sajiwanie, J.W.A.; Rathnayaka, R.M.U.S.K. Chemical Composition, Physicochemical and Technological Properties of Selected Fruit Peels as a Potential Food Source. Int. J. Fruit Sci. 2020, 20, S240–S251. [Google Scholar] [CrossRef]
- Ibáñez-Forés, V.; Bovea, M.D.; Segarra-Murria, J.; Jorro-Ripoll, J. Environmental Implications of Reprocessing Agricultural Waste into Animal Food: An Experience with Rice Straw and Citrus Pruning Waste. Waste Manag. Res. 2023, 41, 653–663. [Google Scholar] [CrossRef]
- Permal, R.; Chia, T.; Arena, G.; Fleming, C.; Chen, J.; Chen, T.; Chang, W.L.; Seale, B.; Hamid, N.; Kam, R. Converting Avocado Seeds into a Ready to Eat Snack and Analysing for Persin and Amygdalin. Food Chem. 2023, 399, 134011. [Google Scholar] [CrossRef]
- Vintila, T.; Ionel, I.; Rufis Fregue, T.T.; Wächter, A.R.; Julean, C.; Gabche, A.S. Residual Biomass from Food Processing Industry in Cameroon as Feedstock for Second-Generation Biofuels. Bioresources 2019, 14, 3731–3745. [Google Scholar] [CrossRef]
- Otieno, O.D.; Mulaa, F.J.; Obiero, G.; Midiwo, J. Utilization of Fruit Waste Substrates in Mushroom Production and Manipulation of Chemical Composition. Biocatal. Agric. Biotechnol. 2022, 39, 102250. [Google Scholar] [CrossRef]
- Nyakang’i, C.O.; Ebere, R.; Marete, E.; Arimi, J.M. Avocado Production in Kenya in Relation to the World, Avocado By-Products (Seeds and Peels) Functionality and Utilization in Food Products. Appl. Food Res. 2023, 3, 100275. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado By-Products: Nutritional and Functional Properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. Bioactive Characterization of Persea americana Mill. by-Products: A Rich Source of Inherent Antioxidants. Ind. Crops Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef]
- Bora, P.S.; Narain, N.; Rocha, R.V.M.; Queiroz Paulo, M. Characterization of the Oils from the Pulp and Seeds of Avocado (Cultivar: Fuerte) Fruits. Grasas Y Aceites. 2001, 52, 171–174. [Google Scholar] [CrossRef]
- Arukwe, U.; Amadi, B.A.; Duru, M.K.C.; Agomou, E.N.; Adindu, E.A.; Odika, P.C.; Lele, K.C.; Egejuru, L.; Anudike, J. Chemical Composition of Persea americana Leaf, Fruit and Seed. IJRRAS 2012, 11, 346–349. [Google Scholar]
- Vinha, A.F.; Moreira, J.; Barreira, S.V.P. Physicochemical Parameters, Phytochemical Composition and Antioxidant Activity of the Algarvian Avocado (Persea americana Mill.). J. Agric. Sci. 2013, 5, 100–109. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Navarro, R.; Díaz-Calderón, P.; Fuentealba, C.; Astudillo-Castro, C.; Toledo, L.; Enrione, J.; Galvez, L. Industrial Avocado Waste: Functional Compounds Preservation by Convective Drying Process. J. Food Eng. 2017, 198, 81–90. [Google Scholar] [CrossRef]
- Ejiofor, N.C.; Ezeagu, I.E.; Ayoola, M.B.; Umera, E.A. Determination of the Chemical Composition of Avocado (Persea americana) Seed. Adv. Food Technol. Nutr. Sci. Open J. 2018, 2, S51–S55. [Google Scholar] [CrossRef]
- Haque, S.K.M. Extraction and Characterization of Oil from Avocado Peels. J. Mex. Chem. Soc. 2021, 65, 347–356. [Google Scholar] [CrossRef]
- Siol, M.; Sadowska, A. Chemical Composition, Physicochemical and Bioactive Properties of Avocado (Persea americana) Seed and Its Potential Use in Functional Food Design. Agriculture 2023, 13, 316. [Google Scholar] [CrossRef]
- Amado, D.A.V.; Detoni, A.M.; De Carvalho, S.L.C.; Torquato, A.S.; Martin, C.A.; Tiuman, T.S.; Aguiar, C.M.; Cottica, S.M. Tocopherol and Fatty Acids Content and Proximal Composition of Four Avocado Cultivars (Persea americana Mill). Acta Aliment 2019, 48, 47–55. [Google Scholar] [CrossRef]
- King-Loeza, Y.; Ciprián-Macías, D.A.; Cardador-Martínez, A.; Martín-del-Campo, S.T.; Castañeda-Saucedo, M.C.; Ramírez-Anaya, J.d.P. Functional Composition of Avocado (Persea americana Mill. Var Hass) Pulp, Extra Virgin Oil, and Residues Is Affected by Fruit Commercial Classification. J. Agric. Food Res. 2023, 12, 100573. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.G.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estevez, M. Avocado (Persea americana Mill.) Phenolics, in Vitro Antioxidant and Antimicrobial Activities, and Inhibition of Lipid and Protein Oxidation in Porcine Patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Pintado, M.E.; Aguilar, C.N. Process Optimization of Microwave-Assisted Extraction of Bioactive Molecules from Avocado Seeds. Ind. Crops Prod. 2020, 154, 112623. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G.A. Phenolic Compound Profiles and Antioxidant Capacity of Persea americana Mill. Peels and Seeds of Two Varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Rosero, J.C.; Cruz, S.; Osorio, C.; Hurtado, N. Analysis of Phenolic Composition of Byproducts (Seeds and Peels) of Avocado (Persea americana Mill.) Cultivated in Colombia. Molecules 2019, 24, 3209. [Google Scholar] [CrossRef] [PubMed]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado Seed: A Comparative Study of Antioxidant Content and Capacity in Protecting Oil Models from Oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef] [PubMed]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; De Alencar, S.M. Exploration of Avocado By-Products as Natural Sources of Bioactive Compounds. PLoS ONE 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive Identification of Bioactive Compounds of Avocado Peel by Liquid Chromatography Coupled to Ultra-High-Definition Accurate-Mass Q-TOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; Martín-Belloso, O. Lipid Digestibility and Polyphenols Bioaccessibility of Oil-in-Water Emulsions Containing Avocado Peel and Seed Extracts as Affected by the Presence of Low Methoxyl Pectin. Foods 2021, 10, 2193. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Escalona-Buendía, H.B.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Pedroza-Islas, R.; Ponce-Alquicira, E. Optimization of the Antioxidant and Antimicrobial Response of the Combined Effect of Nisin and Avocado Byproducts. LWT Food Sci. Technol. 2016, 65, 46–52. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as Valuable Tools for the Determination of Phenolic and Other Polar Compounds in the Edible Part and by-Products of Avocado. LWT Food Sci. Technol. 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant Capacities, Procyanidins and Pigments in Avocados of Different Strains and Cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Del Pino-García, R.; Curiel, J.A.; Lozano-Sánchez, J.; Segura-Carretero, A. Functional Ingredient from Avocado Peel: Microwave-Assisted Extraction, Characterization and Potential Applications for the Food Industry. Food Chem. 2021, 352. [Google Scholar] [CrossRef] [PubMed]
- Soldera-Silva, A.; Seyfried, M.; Campestrini, L.H.; Zawadzki-Baggio, S.F.; Minho, A.P.; Molento, M.B.; Maurer, J.B.B. Assessment of Anthelmintic Activity and Bio-Guided Chemical Analysis of Persea americana Seed Extracts. Vet. Parasitol. 2018, 251, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Alkaltham, M.S.; Uslu, N.; Özcan, M.M.; Salamatullah, A.M.; Mohamed Ahmed, I.A.; Hayat, K. Effect of Drying Process on Oil, Phenolic Composition and Antioxidant Activity of Avocado (cv. Hass) Fruits Harvested at Two Different Maturity Stages. LWT Food Sci. Technol. 2021, 148, 111716. [Google Scholar] [CrossRef]
- de Oliveira, C.S.; Andrade, J.K.S.; Rajan, M.; Narain, N. Influence of the Phytochemical Profile on the Peel, Seed and Pulp of Margarida, Breda and Geada Varieties of Avocado (Persea americana Mill) Associated with Their Antioxidant Potential. Food Sci. Technol. 2022, 42, e25822. [Google Scholar] [CrossRef]
- Flores, M.; Ortiz-Viedma, J.; Curaqueo, A.; Rodriguez, A.; Dovale-Rosabal, G.; Magaña, F.; Vega, C.; Toro, M.; López, L.; Ferreyra, R.; et al. Preliminary Studies of Chemical and Physical Properties of Two Varieties of Avocado Seeds Grown in Chile. J. Food Qual. 2019, 2019, 3563750. [Google Scholar] [CrossRef]
- Wang, M.; Yu, P.; Chittiboyina, A.G.; Chen, D.; Zhao, J.; Avula, B.; Wang, Y.; Khan, I.A. Assessment of Avocado (Persea americana Mill) Oils. Molecules 2020, 25, 1453. [Google Scholar] [CrossRef]
- Kumar, J.A.; Sathish, S.; Prabu, D.; Renita, A.A.; Saravanan, A.; Deivayanai, V.C.; Anish, M.; Jayaprabakar, J.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Agricultural Waste Biomass for Sustainable Bioenergy Production: Feedstock, Characterization and Pre-Treatment Methodologies. Chemosphere 2023, 331, 138680. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Shukla, S.K.; Wang, R. Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts 2023, 13, 740. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. Bioenergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef]
- Sutrisno; Anggono, W.; Suprianto, F.D.; Santosa, C.D.; Suryajaya, M.; Gotama, G.J. Experimental Investigation of Avocado Seed Oil Utilization in Diesel Engine Performance. E3S Web Conf. 2019, 130, 01030. [Google Scholar] [CrossRef]
- Dagde, K.K. Extraction of Vegetable Oil from Avocado Seeds for Production of Biodiesel. J. Appl. Sci. Environ. Manag. 2019, 23, 215. [Google Scholar] [CrossRef]
- Deepalakshmi, S.; Sivalingam, A.; Thirumarimurugan, M.; Yasvanthrajan, N.; Sivakumar, P. In-situ Transesterification and Process Optimization of Biodiesel from Waste Avocado Seed. J. Chem. Pharm. Sci. 2014, 2014, 115–118. [Google Scholar]
- Valensya, D.; Rozalia, I.; Zuhra; Syamsuddin, Y. Utilization of Avocado Seed Waste as Raw Material for Producing Biodiesel with CaO Catalyst from Eggshell. IOP Conf. Ser. Mater. Sci. Eng. 2020, 845, 2020. [Google Scholar] [CrossRef]
- Chimezie, E.C.; Wang, Z.; Yu, Y.; Nonso, U.C.; Duan, P.G.; Kapusta, K. Yield Optimization and Fuel Properties Evaluation of the Biodiesel Derived from Avocado Pear Waste. Ind. Crops Prod. 2023, 191, 115884. [Google Scholar] [CrossRef]
- Woldu, A.R.; Ashagrie, Y.N.; Tsigie, Y.A. Bioethanol Production from Avocado Seed Wastes Using Saccharomyces cerevisiae. Am. J. Environ. Energy Power Res. 2015, 3, 1–9. [Google Scholar]
- Chen, S.J.; Chen, X.; Zhu, M.J. Xylose Recovery and Bioethanol Production from Sugarcane Bagasse Pretreated by Mild Two-Stage Ultrasonic Assisted Dilute Acid. Bioresour. Technol. 2022, 345, 126463. [Google Scholar] [CrossRef]
- Kenasa, G.; Kena, E. Optimization of Biogas Production from Avocado Fruit Peel Wastes Co-Digestion with Animal Manure Collected from Juice Vending House in Gimbi Town, Ethiopia. Ferment. Technol. 2019, 8, 1–6. [Google Scholar]
- Langat, K.; Njogu, P.; Kamau, J. Biogas Energy Potential from Co-Digestion of Avocado Pulp with Cow Manure in Kaitui Location, Kericho County, Kenya. Int. Res. J. Innov. Eng. Technol. 2018, 2, 28–34. [Google Scholar]
- Ginting, M.H.S.; Hasibuan, R.; Lubis, M.; Alanjani, F.; Winoto, F.A.; Siregar, R.C. Utilization of Avocado Seeds as Bioplastic Films Filler Chitosan and Ethylene Glycol Plasticizer. Asian J. Chem. 2018, 30, 1569–1573. [Google Scholar] [CrossRef]
- Jiménez, R.; Sandoval-Flores, G.; Alvarado-Reyna, S.; Alemán-Castillo, S.E.; Santiago-Adame, R.; Velázquez, G. Extraction of Starch from Hass Avocado Seeds for the Preparation of Biofilms. Food Sci. Technol. 2022, 42, e56820. [Google Scholar] [CrossRef]
- Sartika, M.; Lubis, M.; Harahap, M.B.; Afrida, E.; Ginting, M.H.S. Production of Bioplastic from Avocado Seed Starch as Matrix and Microcrystalline Cellulose from Sugar Palm Fibers with Schweizer’s Reagent as Solvent. Asian J. Chem. 2018, 30, 1051–1056. [Google Scholar] [CrossRef]
- Sierra-Ibarra, E.; Leal-Reyes, L.J.; Huerta-Beristain, G.; Hernández-Orihuela, A.L.; Gosset, G.; Martínez-Antonio, A.; Martinez, A. Limited Oxygen Conditions as an Approach to Scale-up and Improve d and l-Lactic Acid Production in Mineral Media and Avocado Seed Hydrolysates with Metabolically Engineered Escherichia coli. Bioprocess Biosyst. Eng. 2021, 44, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sánchez, A.; López-Cuellar, M.D.R.; Pérez-Guevara, F.; Figueroa López, U.; Martín-Bufájer, J.M.; Vergara-Porras, B. Synthesis of Poly-(R-Hydroxyalkanoates) by Cupriavidus Necator ATCC 17699 Using Mexican Avocado (Persea americana) Oil as a Carbon Source. Int. J. Polym. Sci. 2017, 2017, 6942950. [Google Scholar] [CrossRef]
- Arueya, G.L.; Oluwatobi, A.; Arueya, G.L. Avocado (Persia americana) Seed Processing into a Third Generation (3 g) Functional Food Snack: Nutritional, Antioxidative Stress and Safety Potentials. Afr. J. Food Sci. Technol. 2021, 12, 1–15. [Google Scholar]
- Alissa, K.; Hung, Y.C.; Hou, C.Y.; Lim, G.C.W.; Ciou, J.Y. Developing New Health Material: The Utilization of Spray Drying Technology on Avocado (Persea americana Mill.) Seed Powder. Foods 2020, 9, 139. [Google Scholar] [CrossRef]
- Gómez, F.S.; Peirósánchez, S.; Iradi, M.G.G.; Azman, N.A.M.; Almajano, M.P. Avocado Seeds: Extraction Optimization and Possible Use as Antioxidant in Food. Antioxidants 2014, 3, 439–454. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. From By-Product to Functional Ingredient: Incorporation of Avocado Peel Extract as an Antioxidant and Antibacterial Agent. Innov. Food Sci. Emerg. Technol. 2022, 80, 103116. [Google Scholar] [CrossRef]
- Bolaños Villarreal, A.P.; García Tumipamba, D.E.; Cuarán Sarzosa, F.V. Vermicomposting: Production of Humus and Biol. In Communication, Smart Technologies and Innovation for Society Proceedings of CITIS 2021; Rocha, A., López-López, P.C., Salgado-Guerrero, J.P., Eds.; Springer: Shoreham-by-Sea, UK, 2022. [Google Scholar]
- Demissie, H.; Gedebo, A.; Agegnehu, G. Agronomic Potential of Avocado-Seed Biochar in Comparison with Other Locally Available Biochar Types: A First-Hand Report from Ethiopia. Appl. Environ. Soil. Sci. 2023, 2023, 7531228. [Google Scholar] [CrossRef]
- Thanh Chi, L.; Ha, N. Multipurpose Fertilizer Composition and Method of Manufacturing the Same. U.S. Patent 11,512,029 B1, 2022. [Google Scholar]
- Streitwieser, D.A.; Cadena, I.A. Preliminary Study of Biomethane Production of Organic Waste Based on Their Content of Sugar, Starch, Lipid, Protein and Fibre. Chem. Eng. Trans 2018, 65, 661–666. [Google Scholar] [CrossRef]
- Sun, M.; Shi, Z.; Zhang, C.; Zhang, Y.; Zhang, S.; Luo, G. Novel Long-Chain Fatty Acid (LCFA)-Degrading Bacteria and Pathways in Anaerobic Digestion Promoted by Hydrochar as Revealed by Genome-Centric Metatranscriptomics Analysis. Appl. Environ. Microbiol. 2022, 88, e01042-22. [Google Scholar] [CrossRef]
- Spence, A.; Blanco Madrigal, E.; Patil, R.; Bajón Fernández, Y. Evaluation of Anaerobic Digestibility of Energy Crops and Agricultural By-Products. Bioresour. Technol. Rep. 2019, 5, 243–250. [Google Scholar] [CrossRef]
- Coppola, G.; Gaudio, M.T.; Lopresto, C.G.; Calabro, V.; Curcio, S.; Chakraborty, S. Bioplastic from Renewable Biomass: A Facile Solution for a Greener Environment. Earth Syst. Environ. 2021, 5, 231–251. [Google Scholar] [CrossRef]
- Klai, N.; Yadav, B.; El Hachimi, O.; Pandey, A.; Sellamuthu, B.; Tyagi, R.D. Agro-Industrial Waste Valorization for Biopolymer Production and Life-Cycle Assessment toward Circular Bioeconomy. In Biomass, Biofuels, Biochemicals; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 515–555. ISBN 9780128218785. [Google Scholar]
- Jiménez-Rosado, M.; Maigret, J.E.; Perez-Puyana, V.; Romero, A.; Lourdin, D. Revaluation of a Soy Protein By-Product in Eco-Friendly Bioplastics by Extrusion. J. Polym. Environ. 2022, 30, 1587–1599. [Google Scholar] [CrossRef]
- Sultan, N.F.K.; Johari, W.L.W. The Development of Banana Peel/Corn Starch Bioplastic Film: A Preliminary Study. Bioremediation Sci. Technol. Res. 2017, 5, 12–17. [Google Scholar] [CrossRef]
- de Azêvedo, L.C.; Rovani, S.; Santos, J.J.; Dias, D.B.; Nascimento, S.S.; Oliveira, F.F.; Silva, L.G.A.; Fungaro, D.A. Study of Renewable Silica Powder Influence in the Preparation of Bioplastics from Corn and Potato Starch. J. Polym. Environ. 2021, 29, 707–720. [Google Scholar] [CrossRef]
- Asrofi, M.; Sapuan, S.M.; Ilyas, R.A.; Ramesh, M. Characteristic of Composite Bioplastics from Tapioca Starch and Sugarcane Bagasse Fiber: Effect of Time Duration of Ultrasonication (Bath-Type). Mater. Today Proc. 2020, 46, 1626–1630. [Google Scholar] [CrossRef]
- Merino, D.; Bertolacci, L.; Paul, U.C.; Simonutti, R.; Athanassiou, A. Avocado Peels and Seeds: Processing Strategies for the Development of Highly Antioxidant Bioplastic Films. ACS Appl. Mater Interfaces 2021, 13, 38688–38699. [Google Scholar] [CrossRef]
- Flores-Sánchez, A.; Rathinasabapathy, A.; del Rocío López-Cuellar, M.; Vergara-Porras, B.; Pérez-Guevara, F. Biosynthesis of Polyhydroxyalkanoates from Vegetable Oil under the Co-Expression of FadE and PhaJ Genes in Cupriavidus Necator. Int. J. Biol. Macromol. 2020, 164, 1600–1607. [Google Scholar] [CrossRef]
- Pazla, R.; Jamarun, N.; Elihasridas, A.; Yanti, G.; Ikhlas, Z. The Impact of Replacement of Concentrates with Fermented Tithonia (Tithonia diversifolia) and Avocado Waste (Persea americana Miller) in Fermented Sugarcane Shoots (Saccharum officinarum) Based Rations on Consumption, Digestibility, and Production Perform. Arch. Anesthesiol. Crit. Care 2018, 4, 527–534. [Google Scholar] [CrossRef]
- de Evan, T.; Carro, M.D.; Yepes, J.E.F.; Haro, A.; Arbesú, L.; Romero-Huelva, M.; Molina-Alcaide, E. Effects of Feeding Multinutrient Blocks Including Avocado Pulp and Peels to Dairy Goats on Feed Intake and Milk Yield and Composition. Animals 2020, 10, 194. [Google Scholar] [CrossRef]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Sadeghi Mahoonak, A.R.; Rafipour, F. Development of Animal/ Plant-Based Protein Hydrolysate and Its Application in Food, Feed and Nutraceutical Industries: State of the Art. J. Clean Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- Job, B.; Id, L.C.; Fernando, G.; Roberto, V.; Jorge, B. Effect of Supplementation with Avocado Meal on Lamb Diets on Growth and Carcass Performance. Abanico Vet. 2021, 11, 1–12. [Google Scholar]
- Hernández-López, S.H.; Rodríguez-Carpena, J.G.; Lemus-Flores, C.; Grageola-Nuñez, F.; Estévez, M. Avocado Waste for Finishing Pigs: Impact on Muscle Composition and Oxidative Stability during Chilled Storage. Meat Sci. 2016, 116, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Leontopoulos, S.; Skenderidis, P.; Petrotos, K.; Giavasis, I. Corn Silage Supplemented with Pomegranate (Punica granatum) and Avocado (Persea americana) Pulp and Seed Wastes for Improvement of Meat Characteristics in Poultry Production. Molecules 2021, 26, 5901. [Google Scholar] [CrossRef] [PubMed]
- Oelrichs, P.B.; Ng, J.C.; Seawright, A.A.; Ward, A.; Schaffeler, L.; Macleod, J.K. Isolation and Identification of a Compound from Avocado (Persea americana) Leaves Which Causes Necrosis of the Acinar Epithelium of the Lactating Mammary Gland and the Myocardium. Nat. Toxins 1995, 3, 3344–3349. [Google Scholar] [CrossRef]
- Tesfaye, T.; Ayele, M.; Gibril, M.; Ferede, E.; Limeneh, D.Y.; Kong, F. Beneficiation of Avocado Processing Industry By-Product: A Review on Future Prospect. Curr. Res. Green Sustain. Chem. 2022, 5, 100253. [Google Scholar] [CrossRef]
- Pérez-Murcia, M.D.; Martínez-Sabater, E.; Domene, M.A.; González-Céspedes, A.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Barber, X.; López-Lluch, D.B.; Moral, R. Role of Proteins and Soluble Peptides as Limiting Components during the Co-Composting of Agro-Industrial Wastes. J. Env. Manag. 2021, 300. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Shahzad, K. Food Waste Recycling for Compost Production and Its Economic and Environmental Assessment as Circular Economy Indicators of Solid Waste Management. J. Clean Prod. 2021, 317, 128467. [Google Scholar] [CrossRef]
- Rothé, M.; Darnaudery, M.; Thuriès, L. Organic Fertilizers, Green Manures and Mixtures of the Two Revealed Their Potential as Substitutes for Inorganic Fertilizers Used in Pineapple Cropping. Sci. Hortic. 2019, 257, 108691. [Google Scholar] [CrossRef]
- Torres-Climent, A.; Gomis, P.; Martín-Mata, J.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Paredes, C.; Moral, R. Chemical, Thermal and Spectroscopic Methods to Assess Biodegradation of Winery-Distillery Wastes during Composting. PLoS ONE 2015, 10, e0138925. [Google Scholar] [CrossRef]
- Mohanapriya, T.; Sindhu, M.; Annapoorani, C.A. Comparative Analysis of Composting and Vermicomposting from Peel of Musa acuminata. Int. J. Zool. Investig. 2021, 7, 792–800. [Google Scholar] [CrossRef]
- González-Fernández, J.J.; Galea, Z.; Álvarez, J.M.; Hormaza, J.I.; López, R. Evaluation of Composition and Performance of Composts Derived from Guacamole Production Residues. J. Environ. Manage. 2015, 147, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Granados, P.; Mireles, S.; Pereira, E.; Cheng, C.L.; Kang, J.J. Effects of Biochar Production Methods and Biomass Types on Lead Removal from Aqueous Solution. Appl. Sci. 2022, 12, 5040. [Google Scholar] [CrossRef]
- Durak, H.; Aysu, T. Effect of Pyrolysis Temperature and Catalyst on Production of Bio-Oil and Bio-Char from Avocado Seeds. Res. Chem. Intermed. 2015, 41, 8067–8097. [Google Scholar] [CrossRef]
- Kang, J.; Parsons, J.; Gunukula, S.; Tran, D.T. Iron and Magnesium Impregnation of Avocado Seed Biochar for Aqueous Phosphate Removal. Clean Technol. 2022, 4, 690–702. [Google Scholar] [CrossRef]
- Riaz, U.; Mehdi, S.M.; Iqbal, S.; Khalid, H.I.; Qadir, A.A.; Anum, W.; Ahmad, M.; Murtaza, G. Bio-Fertilizers: Eco-Friendly Approach for Plant and Soil Environment. In Bioremediation and Biotechnology. Sustainable Approaches to Pollution Degradation; Hakeem, K.R., Bhat, R.A., Qadri, H., Eds.; Springer: Cham, Switzerland, 2020; p. 334. [Google Scholar]
- Rodríguez-Martínez, B.; Romaní, A.; Eibes, G.; Garrote, G.; Gullón, B.; del Río, P.G. Potential and Prospects for Utilization of Avocado By-Products in Integrated Biorefineries. Bioresour. Technol. 2022, 364, 128034. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Maouni, A.; Pinto da Silva, L.; Saidi, R.; Legssyer, M.; Lamrani, Z.; Esteves da Silva, J.C.G. Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke. Molecules 2023, 28, 1123. [Google Scholar] [CrossRef] [PubMed]
- Alkan, S.; Uysal, A.; Kasik, G.; Vlaisavljevic, S.; Berežni, S.; Zengin, G. Chemical Characterization, Antioxidant, Enzyme Inhibition and Antimutagenic Properties of Eight Mushroom Species: A Comparative Study. J. Fungi 2020, 6, 166. [Google Scholar] [CrossRef]
- Majib, N.M.; Sam, S.T.; Yaacob, N.D.; Rohaizad, N.M.; Tan, W.K. Characterization of Fungal Foams from Edible Mushrooms Using Different Agricultural Wastes as Substrates for Packaging Material. Polymers 2023, 15, 873. [Google Scholar] [CrossRef]
- Doroški, A.; Klaus, A.; Režek Jambrak, A.; Djekic, I. Food Waste Originated Material as an Alternative Substrate Used for the Cultivation of Oyster Mushroom (Pleurotus ostreatus): A Review. Sustainability 2022, 14, 12509. [Google Scholar] [CrossRef]
- Grand View Research Mushroom Market Size, Share & Trends Analysis Report by Product (Button, Shiitake, Oyster), by Form, by Distribution Channel, by Application (Food, Pharmaceuticals, Cosmetics), by Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/mushroom-market (accessed on 16 April 2023).
- Espinel-Ríos, S.; Palmerín-Carreño, D.M.; Hernández-Orihuela, A.L.; Martínez-Antonio, A. A Plackett-Burman Design for Substituting Mrs Medium Components with Avocado Seed Hydrolysate for Growth and Lactic Acid Production by Lactobacillus sp. Rev. Mex. De Ing. Quim. 2019, 18, 131–141. [Google Scholar] [CrossRef]
- Bangar, S.P.; Dunno, K.; Dhull, S.B.; Kumar Siroha, A.; Changan, S.; Maqsood, S.; Rusu, A.V. Avocado Seed Discoveries: Chemical Composition, Biological Properties, and Industrial Food Applications. Food Chem. X 2022, 16, 100507. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Chin, R.; Tan, S.T.; Tan, S.S. Phytochemicals and Antioxidant Activity of Ultrasound-Assisted Avocado Seed Extract. Malays. J. Anal. Sci. 2022, 26, 439–446. [Google Scholar]
- da Silva, G.G.; Pimenta, L.P.S.; Melo, J.O.F.; Mendonça, H. de O.P.; Augusti, R.; Takahashi, J.A. Phytochemicals of Avocado Residues as Potential. Molecules 2022, 27, 1892. [Google Scholar] [CrossRef]
- Dabas, D.; Ziegler, G.R.; Lambert, J.D. Anti-Inflammatory Properties of a Colored Avocado Seed Extract. Adv. Food Technol. Nutr. Sci. Open J. 2019, 5, 8–12. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Calderón-Santoyo, M.; Ascanio, G.; Ragazzo-Calderón, F.Z.; Parra-Saldívar, R.; Ragazzo-Sánchez, J.A. Harnessing Emerging Technologies to Obtain Biopolymer from Agro-Waste: Application into the Food Industry. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Colombo, R.; Papetti, A. Avocado (Persea americana Mill.) by-Products and Their Impact: From Bioactive Compounds to Biomass Energy and Sorbent Material for Removing Contaminants. A Review. Int. J. Food Sci. Technol. 2019, 54, 943–951. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).