Abstract
There is approximately 30% of grey sedge (Lepironia articulata) residue remaining from weaving production that could add value to support zero waste management. Therefore, the aim of this research was to study the feasibility of using a residue of grey sedge or Krajood strips from weaving production to form a value-added product. To obtain preliminary data, Krajood strip residue was examined for its biological and physical properties. In addition, the biological and physical properties of Krajood strip residue in combination with loam soil (KSRL) were examined and compared with the properties of loam soil (LS) itself. The results showed that the total microbe and moisture content of the Krajood strip residue was significantly higher than that of the products made from Krajood strips (KS). The stress value of Krajood strips was higher than the stress values of other samples except for that of a bag made of paper. Identification of bacteria and mold by MALDI Biotyper and DNA sequencing compared with BLAST revealed the presence of the types of soil microbes that benefit plants. KSRL was enriched with larger amounts of the primary elements important for plant growth: nitrogen, phosphorus, and potassium, and the three second tier elements. The pH of KS, LS, and KSRL were 6.40 ± 0.14, 5.87 ± 0.04, and 5.26 ± 0.02, respectively. These results could support the use of this beneficial residue for bioresource sustainability.
1. Introduction
Grey Sedge (Lepironia articulata) or Krajood, a tall slender tightly rhizomatous macrophyte that forms large dense swards of foliage in the family Cyperaceae, is a weed that grows readily in muddy lakes or wetland found in Madagascar, India, Sri Lanka, southern China, Southeast Asia, and various islands of the western Pacific, as well as northern, eastern, and southern Australia [1]. In Thailand, it is particularly abundant in southern and eastern regions. The stems that are three years old can be used for weaving into a wide range of products. Data from community enterprises revealed that there was approximately 30 percent of Krajood strip residue after each production process. Usually, the residue is discarded and accumulates in the soil beside the production area or is burned off, causing air pollution. Open burning is a common source of air pollution in both rural and urban areas, and causes the release of dust, ash, soot, smoke, gases, and toxic substances such as carbon monoxide, nitrogen dioxide, and volatile organic compounds. These spread in the atmosphere, affecting health, causing annoyance, and obscuring visibility, which may cause accidents on the road. It also destroys the soil structure. Burning one ton of plant residue will cause the release of 2–14 kg of dust particles smaller than 2.5 microns [2].
Accumulation of organic matter content of sedge (Carex sprengelii) has been reported [3]. Major and trace elements of sedge species, which is in the same family Cyperaceae, can increase fodder value [4]. Application of organic residues, such as coconut shell hair, Muntingia calabura leaves, and Samanea saman leaves on some soil properties, could increase soil organic matter [5]. It was also mentioned that proper recycling of sunflower residue improved the biological, chemical, and physical properties of soil [6]. Grey sedge (L. articulata) can take up and retain nutrients [7]. Grey sedge can be exploited as a new source of natural colorants that have potential uses in the dye and food colorant industries, with six different phenolic compounds being detected from L. articulata [8].
To prevent the effects of such actions mentioned above, we focused on turning the residue of grey sedge to value-added products. However, there has been little research on using the grey sedge residue to create new products. Therefore, we aimed to study the feasibility of using grey sedge (Lepironia articulata) or Krajood strip residue from the weaving industry in a way to create a value-added product with zero waste, instead of burning it, which results in serious air pollution. To achieve this proposal, the preliminary research was the study of the physical and microbial properties of Krajood strip residue. The properties of loam soil (LS) and Krajood strip residue in combination with loam soil (KSRL) were then compared to obtain the data for plant growth enrichment. The results of this study can support the turning of the grey sedge residue to a value-added product, soil nutrients supplement material.
2. Materials and Methods
2.1. Materials
Krajood strip (Figure 1a), Krajood paper (Figure 1b), the residue from plain weave pattern (Figure 1e), and the residue from cross weave pattern (Figure 1f) were collected from the Krajood Ban Kum Pae Product Group Community Enterprise, Cha-uat District, Nakhon Si Thammarat Province, Thailand.

Figure 1.
Samples used in this research: (a) Krajood strips (KS), (b) paper made from Krajood (PK), (c) shirt backing paper (SP), (d) a bag made of paper (BP), (e) residue of plain weave pattern (RPP), (f) residue of cross weave pattern (RCP), (g) loam soil, (h) Krajood strip residue in combination with loam soil (KSRL) at day 0, (i) Krajood strip residue in combination with loam soil (KSRL) at day 60.
2.2. Methods
2.2.1. Measurement of the Moisture Content (AOAC 1999)
Krajood strips, Krajood paper, woven Krajood products and other commercial paper products were randomly collected. Crucibles that had been dried in a hot air oven at 105 °C, for 3 h and cooled down in a desiccator were weighed. One gram of each sample was weighed into each crucible, which was then placed in a hot air oven at 105 °C, for 3 h, then cooled down in a desiccator. The crucible and its dried sample were then weighed, and moisture content was calculated as follows;
where
- W1 = weight of sample with crucible before drying (g)
- W2 = weight of sample with crucible after drying (g).
2.2.2. Tensile Strength Determination
The tensile strength of the Krajood strips was compared with paper made from Krajood, a bag made of paper, shirt backing paper, the residues of plain weave patterns and cross weave patterns. The tensile properties were measured by texture analyzer to test stress and mechanical properties with a 1 kN load cell load at 50 mm/min speed. The tensile strength was calculated using Equations (2)–(4) [9,10]:
where σ is tensile strength, F is the maximum load, and A is the cross-sectional area.
Young’s modulus was calculated using the linear graph slope of stress related to strain from the original point to the yielding point of all samples.
where
- ΔL is the range length for each sample,
- L0 is the original span length,
- Y is Young’s modulus for each sample,
- σ is stress, and ε is strain.
A precision digital micrometer was employed (Mitutoyo, Kanagawa, Japan) to the closest 0.001 mm at five random spots on each Krajood strip to determine strip thickness; the mean value was measured for each strip. The measurements of barrier and mechanical properties of each sample were shown based on the mean thickness values.
2.2.3. Microbial Examination and Identification
Microbial Observation and Identification from Krajood Strips and Papers Made from Krajood
Filamentous fungi were examined using 3MTM PetrifilmTM Rapid Yeast and Mold Count Plate. A quantity of 25 grams of the sample was transferred to 225 mL of 3MTM Buffered Peptone Water Broth. One ml of each ten-fold serial dilution of 0.1, 0.01, and 0.001 M of the sample was transferred to each 3MTM PetrifilmTM plate, then incubated at 25 °C for 72 h. The number of filamentous fungi colonies on the petrifilm was multiplied by the dilution factor and reported in colony-forming units per gram (CFU/g). Different filamentous fungi were streaked on PDA (Potato Dextrose Agar, HIMEDIA) to ensure a pure culture. Then, the slide culture technique was applied to identify filamentous fungi under a compound bright field microscope and compared with standards.
Microbial Observation and Identification from KSRL and LS
Total bacteria count from the loam soil (LS) and KSRL were detected using 3M™ Petrifilm™ Aerobic Count Plates. The samples were ten-fold serial diluted then transferred to each of the 3MTM Petrifilm plates, which were incubated at 35 °C for 48 h. The average number of microorganisms obtained from the colony counting was reported as colony-forming units per gram (CFU/g). Pure cultures were obtained by the cross-streak method on nutrient agar plate. The MALDI Biotyper® system was used to identify bacteria from the KSRL and LS.
Molecular Identification
Total genomic DNA was extracted from the mycelium of isolates grown in Potato Dextrose Agar (HIMEDIA) incubated in an incubator at 28 °C for 4 days. Pure cultures were preliminarily identified by compound bright field microscope. Six selected strains were further identified using molecular methods. DNA extraction was carried out with an E.Z.N.A. plant DNA Kit from Omega, BIOTEK. Genomic DNA was used as a template to amplify the fungal ITS regions with the universal ITS primers and ITS1/ITS4 using the polymerase chain reaction (PCR Applied Biosystems) [11]. The PCR reaction procedure was set up as follows: step 1, 94 °C 4 min; Step 2, 94 °C 30 s, 55 °C 30 s, 72 °C 30 s, 30 cycles in step 2; step 3, 72 °C 5 min; and step 4, 4 °C held. The amplified DNA samples were submitted for sequencing and capillary electrophoresis was run through an ABI 3130 × l sequencer (Applied Biosystems) [12]. DNA sequencing was compared with Basic Local Alignment Search Tool (BLAST) programs in the GenBank to identify the sequence homology with closely related organisms. Similarity ≥99% was reported.
2.2.4. pH Measure of LS, KS and KSRL
A multi-parameter analyzer (Consort C1010) was used to measure the pH of the samples. The pH measurement was carried out on 10 grams of each sample that had been homogenized with 10 mL of distilled water.
2.2.5. Elemental Analysis
Sample Preparation and Analysis Method
Krajood strips, KSRL, and loam soil were dried in a hot air oven at 70 °C, and then the samples were ground in a small mill to pass through a 40-mesh sieve. Nitrogen content was determined by wet digestion followed by assay using Kjeldahl distillation and titration. The other elements (P, K, Ca, Mg, Na, S, Fe, Mn, Cu, Zn, Cd, and Pb) were detected according to the method used by Faithfull [13]. The samples were ground in a small mill to pass through a 40-mesh sieve before incineration in a muffle furnace (CABOLITE AAF1100) at 550 °C for 5 h. Each ash sample was dissolved in 10 mL of 1 N HCl and distilled water, and then assayed by ICP-OES (Perkin Elmer Optical Emission Spectrometer AVIO500) [14].
2.2.6. Statistical Analysis
All calculations were performed in three replications and the data were reported as the mean standard deviation (SD). Significant differences between means were evaluated using a one-way analysis of variance (ANOVA) test and Duncan’s multiple range test (SPSS Inc., Chicago, IL, USA). A value less than 0.05 was considered statistically significant.
3. Results
Krajood strip residue used in this research was from trimming off the excess in Krajood weaving products. Normally filamentous fungi can grow on products made from the parts of plants other than leather. Therefore, some of the finished products were further stained to prevent the attachment of filamentous fungi. In the preliminary part of this research, we examined two aspects of Krajood strip residue; the first part was the study of the physical and microbial properties of Krajood strip residue. Next, the microbial and physical properties of Krajood strip residue in a combination with loam soil (KSRL) were examined and compared with those from loam soil and Krajood strip residue.
Krajood strips naturally have a higher moisture content than paper made from Krajood and other products made from Krajood strips or other natural cellulose fibers (Table 1).

Table 1.
Percentage of moisture content of each sample.
To determine the strength of Krajood strips, other samples were also studied to compare how strong the Krajood strips were. Figure 2 shows the relationship between stress and strain of various materials. From the slopes of the graphs, which are linear showing Young’s modulus, values were as follows: Krajood strips (62.223 Mpa/mm2), the residue of cross weave pattern (23.089 Mpa/mm2), the residue of plain weave pattern (142.37 Mpa/mm2), bags made of paper (0.505 Mpa/mm2), shirt backing paper (12.298 Mpa/mm2), and paper made from Krajood (2.524 Mpa/mm2). The average tensile value of Krajood strips residue was 61.64 ± 0.51 N/mm2. The Young’s modulus values showed that Krajood strips had more strength but lower elasticity than paper made from Krajood, probably due to the higher fiber content of the paper made from Krajood.
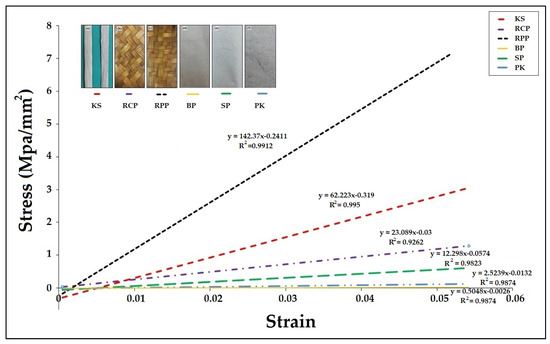
Figure 2.
Comparison of Young’s modulus for Krajood strips (KS), residue of cross weave pattern (RCP), residue of plain weave pattern (RPP), bag made of paper (BP), shirt backing paper (SP) and paper made from Krajood (PK).
The number of microbes found in the Krajood strips was significantly higher than that of paper made from Krajood (Table 2). The numbers of microbes detected (Table 2) coincided with the moisture content of the Krajood strips and paper made from Krajood (Table 1). The number of filamentous molds was not examined from the residue of the plain pattern and cross pattern pieces that were cut off from Krajood weaving products because they had already been stained to prevent the attachment of microorganisms in the products. In addition, a bag made of paper and a shirt backing paper were made from other materials that was not Krajood strip, so they were not detected for number of filamentous fungi.

Table 2.
Numbers of filamentous molds from Krajood strips and papers made from Krajood.
The type of fungi detected in Krajood strips was Aspergillus sp. (Figure 3), while Aspergillus sp. (Figure 4a), Curvularia sp. (Figure 4b), and Cladosporium sp. (Figure 4c) were observed in the paper made from Krajood.
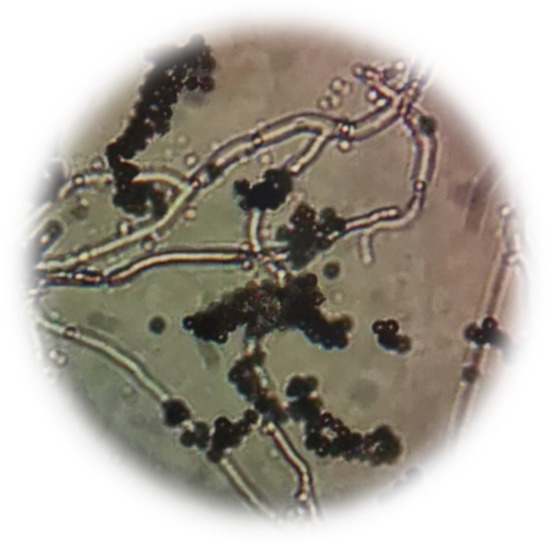
Figure 3.
Morphology of Aspergillus sp. detected from Krajood strips.
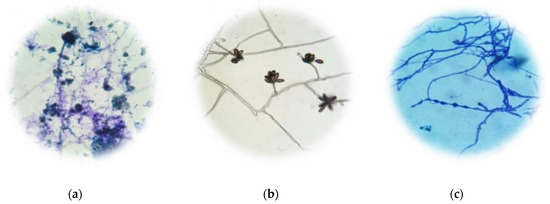
Figure 4.
Morphology of the types of molds detected in paper made from Krajood. Aspergillus sp. (a), Curvularia sp. (b), and Cladosporium sp. (c).
The Krajood strips are composed of natural fiber and can, therefore, be attacked to a greater extent by various bacteria, especially when they have accumulated in the soil, compared to the soil samples themselves, as shown in Table 3.

Table 3.
Numbers of total bacteria from loam soil (LS) and KSRL.
The numbers of bacteria found in those samples was consistent with the pH values examined from those samples (Table 4). It was apparent that samples with a neutral pH value were more prone to the growth of bacteria.

Table 4.
The pH of Krajood strips, and KSRL and loam soil.
Brevibacterium linens is a Gram-positive rod-shaped bacterium that was isolated from soil samples using MALDI Biotyper, while Bacillus cereus was detected in the Krajood soil. The DNA sequencing of six isolates was searched for on the NCBI’s GenBank nucleotide database using the program BLAST to compare the similarities of DNA sequences. The results showed 100% maximum identity with Aspergillus niger strain TAA114, Penicillium janthinellum strain SC56A03, and Trichoderma virens strain F7 in the Krajood soil, while another three isolates from soil samples showed 99.55%, 99.73%, and 99.59% maximum identity of Virgibacillus halophilus strain 5B73C, Humicola fuscoatra, and Curvularia aeria strain FMR 11667, respectively.
The elements detected from each sample, loam soil (LS), KSRL, and Krajood strips residue, are shown in Table 5. These are elements that plants normally take in. Nitrogen, potassium sulfur, copper, zinc, boron, and sodium were detected at significantly higher levels in most Krajood strips compared with KSRL and loam soil, while phosphorus, calcium, magnesium, iron, manganese, aluminum, cobalt, chromium, and nickel were detected at significantly higher levels in the KSRL. Lead was found at a significantly higher concentration in the loam soil. The results revealed that KSRL was enriched with larger amounts of the primary elements nitrogen, phosphorus, and potassium normally needed for plant growth. In addition, the further three important elements for plant growth, calcium, magnesium, and sulfur, were also found at enriched levels in KSRL, while the loam soil did not contain enough of the nutrients (Table 5).

Table 5.
The concentration (ppm) of major and minor elements detected in the samples.
4. Discussion
The results of the testing of the physical properties of grey sedge residues implied that the different densities of the samples affected the Young’s modulus, as was also mentioned by Mizeral et al. [15] and Aydin and Ozveren [16]. In addition, the strips were strong and stiff enough to be used in a composite with other materials to up-value them, and an example of this was their use in papercrete, a new eco-friendly construction material [17]. Natural fibers from plants are generally suitable for reinforcing polymers, due to their strength, toughness, and low density, which is lower than that of glass fibers, and is, therefore, suitable for lightweight composite production. In addition, the properties of natural fibers from plants, such as tensile strength, percentage of elongation, and elastic modulus, can be improved for better properties. Therefore, using natural fibers from plants as reinforcing materials in plastics, instead of synthetic fibers, has become a growing trend [18,19,20,21,22,23]. Grey sedge can be an interesting source of reinforcing material for laboratory research, but for continuing mass production, the amount of raw material which is grey sedge must be sufficient for the production process.
Hence, we focused on the feasibility of turning grey sedge residue to other value-added products for zero waste management. Therefore, we started to study the physical and microbial properties of its residue, and the results of our work revealed that natural fiber from plants like grey sedge at the moisture content of 11.40 ± 0.17% still detected a large number of microorganisms. Therefore, if it accumulates properly in soil, it improves the quality of the soil and is suitable for cultivation, since microorganisms identified from grey sedge residue are those which affect the number and types of other microorganisms in the soil that will affect the quality of the soil for cultivation.
Important microorganisms isolated and identified from loam soil are halotolerant or halophilic ones. It is implied that loam soil in this area is saline soil so it might not be suitable for cultivation. Grey sedge residue is composed of natural fiber and, therefore, natural fermentation of grey sedge that has been accumulated in soil occurs and enhances the growth of some important filamentous molds that are different to those detected from loam soil itself. The results demonstrate that most of the main microorganisms isolated and identified from loam soil are halotolerant or halophilic ones and that the pH of soil was acidic. However, various filamentous molds that produce secondary metabolites that benefit plants were isolated and identified from the KSRL with its neutral pH condition (Table 5). These filamentous molds also enhance a friendly environment to plants, since they play roles as biocontrol agents against plant pathogens. Furthermore, some of them help to positively alter environmental conditions by degrading toxic pollutants in the soil. Soil microbes play a predominant role in the agro-ecosystem. They can affect soil nutrient cycling, organic matter formation and decomposition, and soil structure, thus influencing ecosystem stability. The application of biocontrol agents can perturb the composition and function of soil microbial communities. This affects the soil quality for sustainable plant production and the promotion of plant health.
Therefore, supplementation of loam soil with grey sedge residue might well be an effective way to enrich soil with nutrients. However, since each plant requires a different proportion of soil nutrients, the data obtained from this research may, therefore, be applicable to some kinds of plants. In addition, there are many conditions that affect plant growth apart from the management of the grey sedge residue at field level. Further studies can be applied based on these data.
5. Conclusions
Grey sedge residue is a good source of macroelements and microelements. The soil with the composition of grey sedge residue induces the growth of microorganism that are beneficial to the increase of soil nutrients. Therefore, it can increase the soil quality for plant growth, and on the other hand this is another method to eliminates waste material to zero.
Author Contributions
Conceptualization, K.C. and N.S.; methodology, all authors; writing—original draft preparation, K.C., N.S. and N.P.; writing—review and editing, K.C. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We would like to thank Pittaya Kobkanjanasin, a consultant in the Green Industry under the Department of Industrial Promotion, Thailand for coordinating with the Krajood Ban Kum Pae Product Group Community Enterprise, Cha-uat District, Nakhon Si Thammarat Province, Thailand. We also thank Pojchanad Pathaburee from Office of Scientific Instruments and Testing, Prince of Songkhla University, Hat Yai District, Songkhla Province, Thailand, and Ruethaitip Khaopong from the Ward Medic Limited Partnership, for facilitating the use of molecular methods for microbial identification.
Conflicts of Interest
The authors declare that there are no conflict of interest associated with this study.
References
- Govaerts, R.H.A.; Simpson, D.A.; Bruhl, J.J.; Egorova, T.; Goetghebeur, P.; Wilson, K.L. World Checklist of Cyperaceae: Sedge; Royal Botanic Gardens, Kew: Richmond, UK, 2007; p. 765. [Google Scholar]
- Cheewaphongphan, P.; Garivait, S. Bottom up approach to estimate air pollution of rice residue open burning in Thailand. APJAS 2013, 49, 139–149. [Google Scholar] [CrossRef]
- Meehan, M.A.; DeKeyser, E.S.; Sedivec, K.K.; Norland, J.E. Nutrition composition of Sprengel’s sedge (Carex sprengelii). Can. J. Plant Sci. 2012, 92, 867–871. [Google Scholar] [CrossRef]
- Janyszek-Soltysiak, M.; Grzelak, M.; Gajewski, P.; Jagodzinski, A.M.; Gawel, E.; Wronska-Pilarek, D. Mineral contents in Aboveground Biomass of Sedges (Carex L., Cyperaceae). Energies 2021, 14, 8007. [Google Scholar] [CrossRef]
- Srisutham, M.; Nontasri, T.; Polthanee, A. Influence of adding various organic residues on some soil properties, growth and yield of Chilli Padi (Capsicum flutescens Linn.) grown under greenhouse conditions. Khon Kaen Agric. J. 2020, 48, 879–886. [Google Scholar] [CrossRef]
- Babu, S.; Rana, D.S.; Yadav, G.S.; Singh, R.; Yadav, S.K. A Review on Recycling of Sunflower Residue for Sustaining Soil Health (Review article). Int. J. Agron. 2014, 2014, 601049. [Google Scholar] [CrossRef]
- Wurochekke, A.A.; Harun, N.A.; Mohamed, R.M.S.R.; Kassim, A.H.B.M. Constructed Wetland of Lepironia Articulata for household greywater treatment. APCBEE Procedia 2014, 10, 103–109. [Google Scholar] [CrossRef]
- Othman, R.; Ramya, R.; Hassan, N.M.; Kamoona, S. GCTOF-MS and HPLC Identification of Phenolic Compounds with Different Fractional Extracts of Lepironia articulata. J. Pharm. Nutr. Sci. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Djafari Petroudy, S.R. Physical and mechanical properties of natural fibres. In Advanced High Strength Natural Composites in Construction, 2nd ed.; Gwen, J., Ed.; Matthew Deans: Cambridge, UK, 2017; pp. 59–83. [Google Scholar]
- Biswas, S.; Ahsan, Q.; Cenna, A.; Hasan, M.; Hassan, A. Physical and mechanical properties of jute, bamboo and coir natural fiber. Fibres Polym. 2013, 14, 1762–1767. [Google Scholar] [CrossRef]
- Manter, D.K.; Vivanco, J.M. Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. J. Microbiol. Methods 2007, 71, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Durney, B.C.; Crihfield, C.L.; Holland, L.A. Capillary electrophoresis applied to DNA: Determining and harnessing sequence and structure to advance bioanalyses (2009–2014). Anal. Bioanal. Chem. 2015, 407, 6923–6938. [Google Scholar] [CrossRef] [PubMed]
- Faithfull, N.T. Methods in Agricultural Chemical Analysis: A Practical Handbook; CABI Publishing: New York, NY, USA, 2002; p. 266. [Google Scholar]
- Momen, A.A.; Zachariadis, G.A.; Anthemidis, A.N.; Stratis, J.A. Use of fractional factorial design for optimization of digestion procedures followed by multi-element determination of essential and non-essential elements in nuts using ICP-OES technique. Talanta 2007, 71, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Mizera, C.; Herak, D.; Hrabe, P.; Kabutey, A. Effect of temperature and moisture content on tensile behaviour of false banana fibre. Int. Agrophysics 2017, 31, 377–382. [Google Scholar] [CrossRef][Green Version]
- Aydin, T.Y.; Ozveren, A. Effects of moisture content on elastic constants of fir wood. Eur. J. Wood Wood Prod. 2019, 77, 63–70. [Google Scholar] [CrossRef]
- Cardinale, T.; D’Amato, M.; Sulla, R.; Cardinale, N. Mechanical and physical characterization of papercrete as new eco-friendly construction. Mater. Appl. Sci. 2021, 11, 1011. [Google Scholar] [CrossRef]
- Rokbi, M.; Osmani, H.; Imad, A.; Benseddiq, N. Effect of chemical treatment on flexure properties of natural fiber-reinforced polyester composite. Procedia Eng. 2011, 10, 2092–2097. [Google Scholar] [CrossRef]
- Salaman, A.J. Tensile and impact properties of polystyrene matrix composites reinforced by Palm natural fibers and carbon fibers. Acad. Res. Int. 2012, 3, 114–116. [Google Scholar]
- Verma, D.; Gope, P.C.; Shandilya, A.; Gupta, A.; Maheshwari, M.K. Coir Fiber Reinforcement and Application in Polymer Composites: A Review. J. Mater. Environ. Sci. 2013, 4, 263–276. [Google Scholar]
- Rohit, K.; Dixit, S. A Review-Future Aspect of Natural Fiber Reinforced Composite. Polym. Renew. Resour. 2016, 7, 43–60. [Google Scholar] [CrossRef]
- Elanchezhiana, C.; Ramnathb, V.B.; Ramakrishnanc, G.; Rajendrakumard, M.; Naveenkumare, V.; Saravanakumar, M.K. Review on mechanical properties of natural fibre composites. Mater. Today Proc. 2018, 5, 1785–1790. [Google Scholar] [CrossRef]
- Azman, M.A.; Asyraf, M.R.M.; Khalina, A.; Petru, M.; Ruzaidi, C.M.; Sapuan, S.M.; Wan Nik, W.B.; Ishak, M.R.; Ilyas, R.A.; Suriani, M.J. Natural Fiber Reinforced Composite Material for Product Design: A Short Review. Polymers 2021, 13, 1917. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).