Abstract
The tannery industry is characterized by the consumption of a large quantity of water, around 30–40 m3 for processing 1000 kg of hide or skin. This amount becomes wastewater, containing about 300 kg of different chemicals, mainly refractory organic compounds, with high chemical oxygen demand (COD), total dissolved salts (TDS), chromium, and evolution of toxic gases, such as ammonia and sulfides, etc. The remaining tanning chemicals are released as effluent having high resistance against biological degradation, becoming a serious environmental issue. Usually, end-of-pipe treatment is not sufficient to meet the concerns of environmental issues. In terms of cleaner production options, the redesigning of the existing effluent treatment procedures with alternate or additional treatment techniques, which “supports resource recovery with no added chemicals”, is expected to give a sustainable solution for the management of toxic effluent. The Zero Liquid Discharge (ZLD) system serves to ensure zero water emission, as well as treatment facilities by recycling, recovery, and reuse of the treated wastewater using advanced cleanup technology. The international scenario shows the implementation of ZLD thanks to pressure from regulatory agencies. The ZLD system consists of a pre-treatment system with conventional physicochemical treatment, tertiary treatment, softening of the treated effluent, reverse osmosis (RO) treatment for desalination, and thermal evaporation of the saline reject from RO to separate the salts. By adopting this system, water consumption is reduced. Moreover, ZLD also becomes effective in disaster mitigation in areas where the tannery industry is a strong economic actor. With this review, we aim to give an outlook of the current framework.
1. Introduction
The leather industry is an important contributor to the global economy, as the annual global trade reached up to USD 414 billion in 2018, producing a broad range of leather products (footwear, clothing, gloves, handbags, purses, hats, and wristwatch straps) from the rawhide, where 95% of the raw materials are the by-products of meat and dairy industries [1,2]. Asian and European countries are the global leaders in exporting leather products with 48.5% of the worldwide export sale, as plenty of raw material is easily accessible in those nations [3]. In 2020, China was the largest producer of leather in the world (25% of the total production), followed by Brazil (10%), Russia, Italy, and India (7%) [4]. India has the highest livestock population (536.76 million) in the world, accounting for 11.54% of the total world livestock population and 56.7% of the world buffalo population [5]. India became one of the major players in the leather industry with the availability of livestock, water resources, trained laborers, and technology for processing leather. The export of this sector reached 2 billion ft2 in 2014, which is 10% of the world’s share, with 909 million pairs of leather footwear, 100 million pairs of shoe uppers, 16 million pieces of leather garments, 63 million pieces of leather goods, and 52 million pairs of gloves [6].
The tannery sector is a part of the leather processing industry where the raw leather is converted into finished material. It is considered the most contaminating sector due to the generation of toxic pollutants in every step of the process [7]. Global leather tanneries process 17 million tonnes of hides and skins per year, generate 600 million m3 of tannery wastewater (TWW), and discharge 350 million m3 of treated wastewater back into the environment [8]. In China, the tannery industry consumes over 1.4 hundred million m3 of water annually, while in Brazil, the annual consumption of water by this sector is identical to the water consumption of 5.5 million residents [9]. To convert rawhide into finished leather, the rawhide must undergo several mechanical and chemical processes. It is estimated that 30 L of effluent is generated for the processing of 1 kg of rawhide [10]. The tanning industry utilizes 30 to 40 m3 of water and about 300 kg of chemicals for processing 1 tonne of rawhide [11]. Only 20% of the raw materials are converted into usable leather products, whereas more than 60% of the raw materials are converted into solid and liquid waste [1].
The pollution of the liquid waste depends on the type of processed hides and skins and the used chemicals. The United Nations Industries Development Organization (UNIDO) estimated that 175 types of chemicals are involved in the tannery process [7]. Discharge of this effluent into freshwater without treatment or with just partial treatment has caused serious environmental impacts. Due to the increase in environmental concerns regarding these pollutants, countries have raised and implemented discharge standards with restrictions. In the tannery sector, the circular economy concept has been introduced to reduce waste generation and reuse resources, thereby preventing environmental pollution [12], in line with Sustainable Development Goal 12—responsible consumption and production.
In this review, we provide an overview of the (i) cleaner technological approaches in each sector of the tannery process, (ii) types of ZLD systems developed and how they are integrated with the cleaner technological approaches in the tannery sector, and (iii) case studies and the types of ZLD systems that are in operation to recover, recycle, and reuse the water, thereby reducing the freshwater demand, as well as avoiding the impacts of these pollutants on the environment. We also give a panorama of the ZLD application at an industrial scale all around the world.
2. Methodology
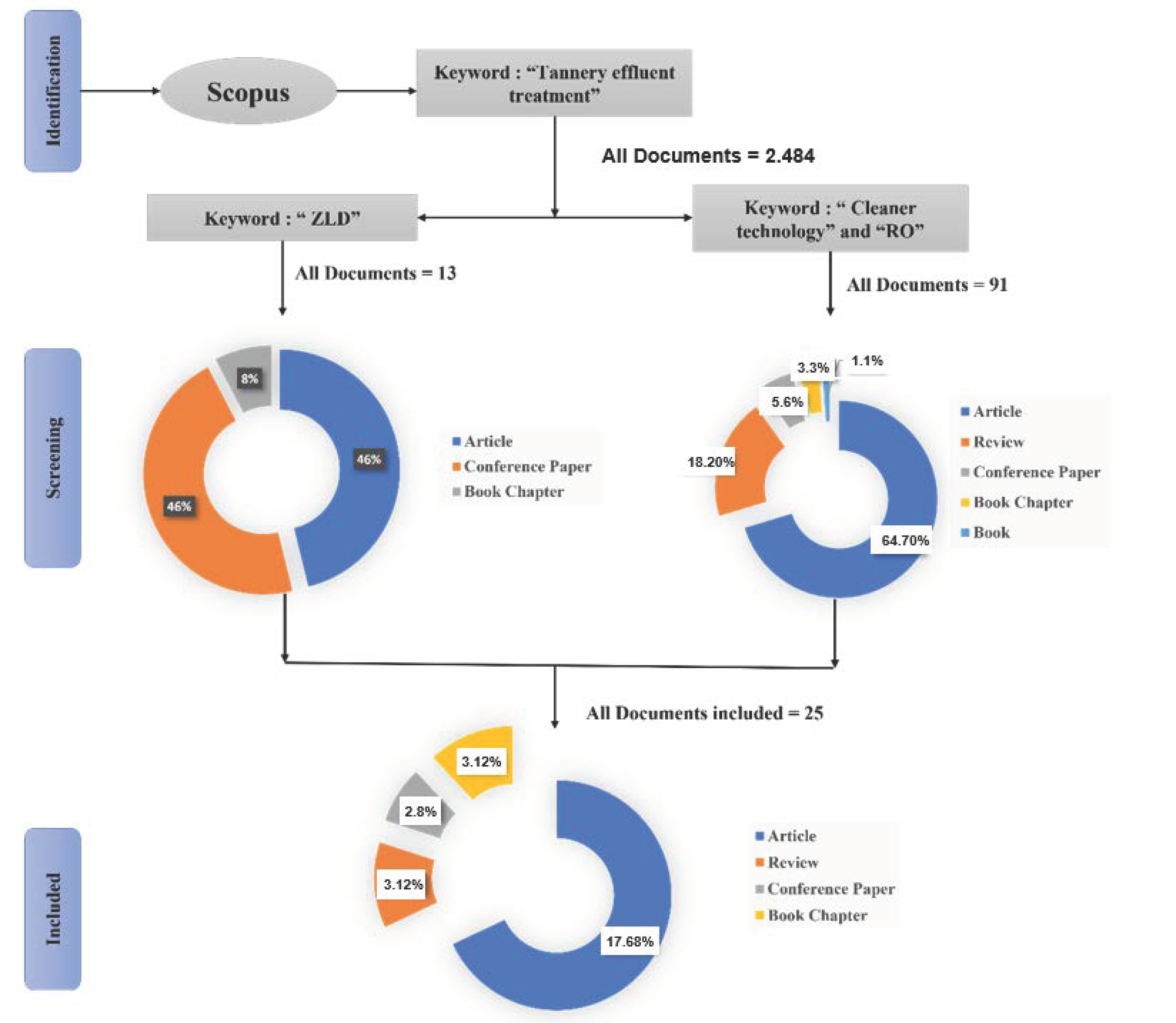
The literature review was performed using the scientific database Scopus. Using the algorithms offered in the Scopus database, a retrieval was performed for research publications from the year 2000 to 2022 using the words “tannery”, “cleaner”, “RO”, and “ZLD” in the keywords, title, or abstracts, as shown in Figure 1. Original research papers from the past ten years with the topic related to ZLD in the tannery sector were taken for the complete review.
Figure 1.
Prisma flow diagram for ZLD system in the tannery sector.
3. Tanning Process and Related Environmental Issues
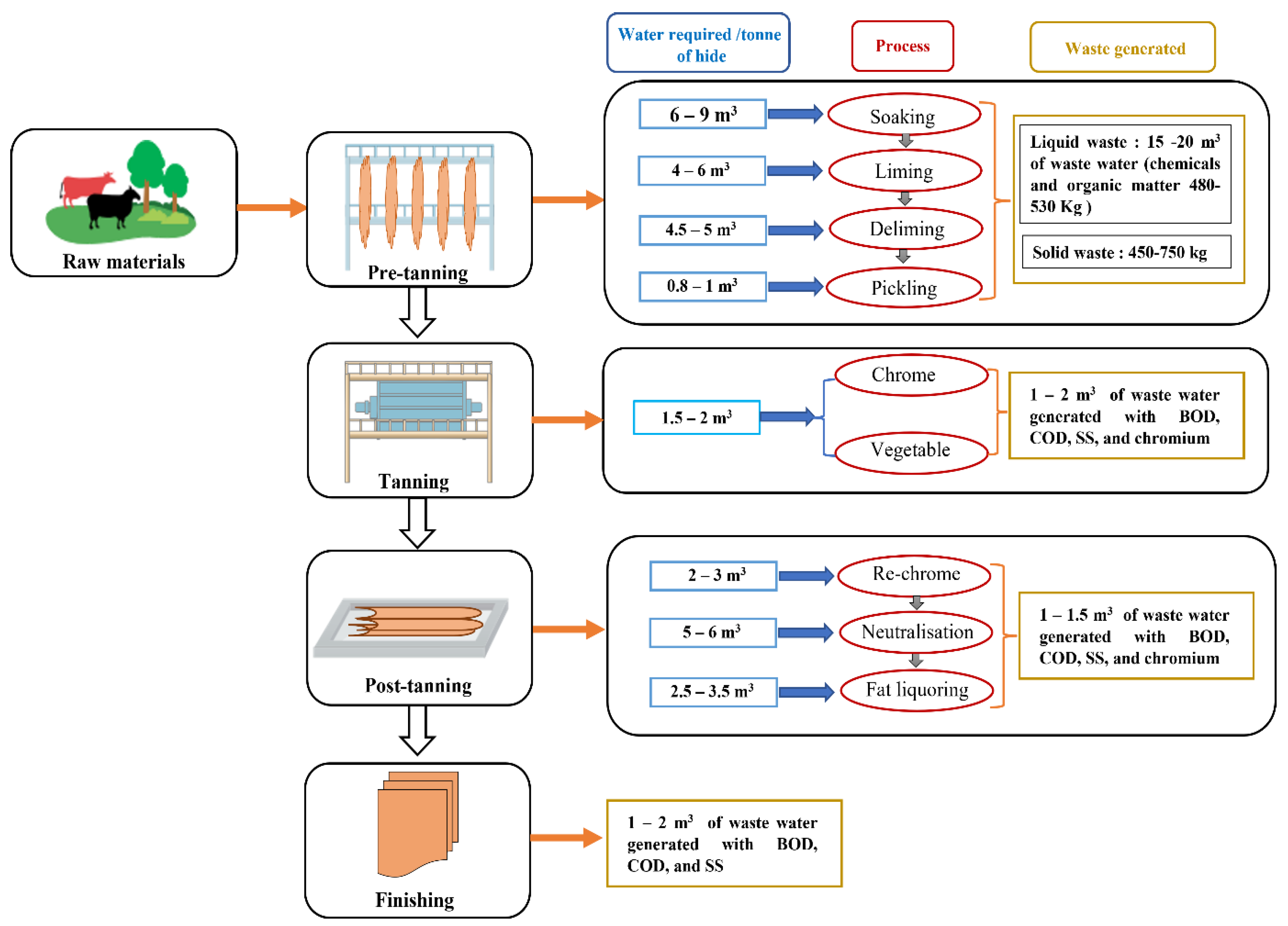
Leather manufacturing has four basic stages for processing the raw leather into finished leather, namely, the beam house stage, tanning stage, post-tanning, and finishing stage, each of them based on 10 to 15 operational steps generating solid and liquid waste, as shown in Figure 2 [13,14]. Raw leather is composed of three layers, namely, the dermis, epidermis, and subcutaneous layer, with the dermis layer consisting of 30–35% of the protein collagen, and the remainder being water and fats [15]. The tanning process involves the reaction between the collagen and chemicals to convert putrescible hide into non-putrescible hide [16]. To increase the hydrothermal stability of the leather, a basification process is employed, where hides soak in tanning liquor to fix the tanning material to the leather [17].
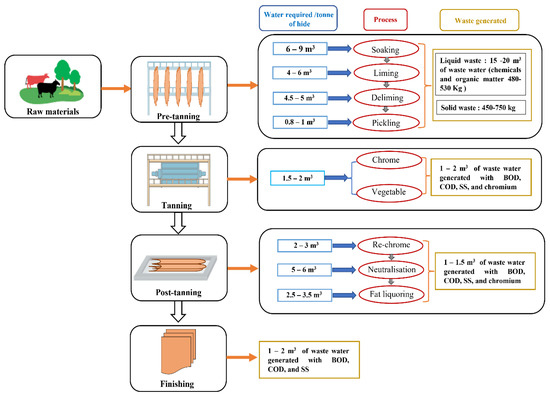
Figure 2.
Stages of the leather manufacturing sector with wastewater generated in each of them.
There are three major types of tanning: chrome tanning, vegetable tanning, and a combination of vegetable and chrome tanning [18]. The chrome tanning process involves the use of chromium ions (chromium sulfate) to create cross-linkage among free carboxyl groups present in collagen to make the hide resistant to bacterial degradation and high temperatures. The vegetable tanning process involves polyphenolic compounds, derived from catechol, to create a hydrogen bond between the tanning phenolic group to the collagen of the leather itself, creating stout stole leather [19]. Globally, 90% of the tanneries use chrome(III) sulfate as a tanning agent, and 80% of the total pollutants are generated in this step [13]. TWW is a dark brown liquid that contains high chemical oxygen demand (COD), biological chemical demand (BOD), total dissolved solids (TDS), phenolics, and hazardous chemicals such as chromium(III), resins, detergents, biocides, oils, and synthetic tannins, with a strong odor of ammonia [20].
TWW is highly toxic and has serious impacts on the soil by acidification, reducing soil fertility, leaching chromium into the deeper layer of soil, and altering the soil microbial communities. In surface water, TWW causes foaming, blocks sunlight penetration, and affects aquatic life by depleting dissolved oxygen in the water, triggering eutrophication, and increasing salinity in water [21,22]. TWW also causes several serious problems in living organisms, such as hematotoxicity in the common fish Tilapia mossambica and Labeo rohita, altered oxidative enzymes in Poecilia reticulata, embryonic toxicity to zebrafish, disrupted physiological and cytological processes in plants, hormonal imbalance, compromised reproductive fitness in rats, acute embryotoxicity in sea urchins, and genotoxic effects in the snail Pila globose [23].
Hexavalent chromium Cr(VI) generated in tanneries is recognized as highly toxic as it directly interacts with intercellular proteins and nucleic acids, causing mutagenic, carcinogenic, and teratogenic effects in animals and humans [24]. Pentachlorophenol (PCP), a polychlorinated aromatic compound used as a preservative for leather, is an important biocide from a toxicological perspective [25]. Cr(VI) and PCP exposure can cause chronic fatigue, infertility, neuralgic pain in the legs, hypothyroidism, dermatitis, ulcers, and lung cancer in humans [26,27]. Considering their effects on living organisms, the United States Environment Protection Agency (USEPA) has designated them as “priority pollutants” [28]. TWW contains chromium (120–200 mg/L), COD (2500–8000 mg/L), BOD (1200–3000 mg/L), chloride (6000–9500 mg/L), sulfates (1600–2500 mg/L), suspended solids (2000–5000 mg/L), and total dissolved solids (TDS) (9000–18000 mg/L) [29]. TDS is a major environmental concern as it makes the receiving water matrix (rivers and lakes) unfit for irrigation and livestock. It is estimated that every year, 3 million tonnes of common salt are discharged into water recipients worldwide [30]. Such toxic effects on the ecosystem have raised serious environmental concerns to develop an adequate cleaner treatment technology with discharge standards to combat environmental threats.
4. Clean Technology Approach in Tanneries
Circular economy practices are an integral part of sustainable development to meet the present needs without compromising the needs of the future. The “6R” principle (reduce, reuse, recycle, recover, redesign, and re-manufacture), which is an evolution of the “3R” principle (reduce, reuse, and recycle), is currently being followed by industries to reach the Sustainable Development Goals by 2030 [31]. In tanneries, waste minimization can be achieved through adopting cleaner technologies, as these processes aim to replace or avoid the usage of harmful chemicals and eliminate the generation of hazardous wastes. Studies have been performed to investigate the cleaner production approach in the leather processing industry, as shown in Table 1. Five clean technology approaches can be considered in leather tanning processing:
- High chrome exhaustion: this approach reduces the discharge of chrome by 91% by working without float in the tanning process and replacing formic and sulfuric acid with sulphonic aromatic acid in the process. This approach is 42% more economical than the traditional tanning one [32,33].
- Use of enzymes in the dehairing bath: enzymes are added in the soaking phase to improve the water uptake and to degrade the proteins and fats present in the skin [34]. This approach reduces the processing time.
- Precipitation of chrome: to recover and reuse chrome in spent liquor by raising the pH to minimize the solubility of chromium in the liquor [35].
- Recycling the dehairing bath: instead of discharging it to the treatment plant after a single use, it can be reused after a simple filtration [36].
- Recycling the chrome tanning bath (can reduce chrome use by 20%): reusing the contents in the tanning bath after a simple filtration process [37].

Table 1.
Cleaner production approaches in the leather manufacturing processing.
Table 1.
Cleaner production approaches in the leather manufacturing processing.
| Process | Waste Generated | Category of Approach | Cleaner Technology | Reference |
|---|---|---|---|---|
| Mechanical shaking | Salts | Reuse of collected salts | [38] | |
| Fleshing | 100 kg of solid waste/ton of processed hide | Recycling of dehairing bath | Recycling the solid waste to poultry feed or soap industry | [39] |
| Soaking and washing | Wastewater with brine solution (contaminated with fats, soap, and dirt) | Addition of sodium carbonate and sodium hydroxide to increase the pH for better results | [39,40] | |
| Liming and unhairing | Wastewater contaminated with lime, hair, and sulfides | Using enzymes in the dehairing bath | Enzyme-assisted unhairing to reduce COD and sulfide content in the wastewater and recycling of spent liquor | [40,41] |
| Deliming | Wastewater contaminated with lime, hair, and sulfides | Using organic acid and carbon dioxide-based deliming and reusing the deliming liquor | [40,41,42] | |
| Pickling | No wastewater is released | Decreasing the pH to 2.4 to increase the tanning conditions and using a salt-free organic acid pickling system | [40,43] | |
| Tanning | Wastewater with chromium | High chrome exhaustion and precipitation of chrome | Adding soda-ash to increase pH from 2.4 to 4.1 to improve chromium utilization | [38] |
| Re-tanning | Wastewater contaminated with dyes and oils | Recycling of chrome tanning bath | Non-spraying dying methods are preferred with the use of liquid and low-dust dyes | [38] |
Globally, the tanning industry generates 550 million m3 of TWW yearly and is responsible for 40% of global chromium pollution [37,44,45]. Studies have indicated that chromium can be replaced with alternative tanning agents, such as zirconium, aluminum oxide, and titanium compounds, even if it is not economically acceptable for the tannery sector [46]. Titanium can be extracted from the waste obtained from the metal industry to replace chromium as a promising approach toward cleaner production [47]. A blended tanning approach is also an eco-friendly approach as it does not have any negative effects on the quality of the finished leather [48]. A blending of chemicals, such as titanyl sulfate with citrate, aluminum sulfate with polyhedral oligomeric silsesquioxane-methacrylic acid, aluminum with titanium, and chromium with zirconium, produces high-quality leather compared to that produced by chromium [38]. Unfortunately, these studies are still at the preliminary stage and are viewed as theoretical approaches in the industrial community. Therefore, strategies have shifted towards focusing on maximizing chrome uptake in the tannery process and reducing the chrome concentration in the TWW [49]. The advanced tanning process has enhanced the chrome uptake from 70% to 90%, thus reducing the chrome concentration in the wastewater [50]. Even though chrome concentration in the TWW is reduced, TDS in the effluent remains problematic and it can be controlled to some extent by adopting cleaner technology approaches such as (a) alternative preservation techniques to eliminate the use of salt [17,51], (b) shaking and brushing the salted hides to decrease the salt in TDS by 15% [52], and (c) recycling tanning floats to reduce the TDS in the effluent [40]. It must be noted that a significant part of the salinity in the effluent cannot be removed through such cleaner technologies.
5. Need for Water Recycling in Tanneries
In the tannery industry, water consumption has raised concerns about the availability of freshwater, as the World Bank has estimated that the water demand could increase by over 650% in the next three decades [53]. Water consumption is a major issue in tanneries, and great strides have been made to reduce and recycle water. Water usage can be reduced by 55–58% by using the cleaner technology approach, and recycling the wastewater derived from the pre- and post-tanning process can reduce water consumption by 67% [40]. Minimizing water usage in the leather process can also decrease the treatment cost of the effluents [40]. Treatment of TWW is a multistage process (primary, secondary, and tertiary treatments), where the effluent is treated to reduce the pollution load. In this way, the water can be reused or discharged into the environment after the treatment itself [9]. However, the pollutants, especially the metals, are not completely removed from the wastewater but transferred to disposable sludge. The treated water can be recycled back into the tanneries for non-potable purposes. For the safe disposal of treated wastewater into the environment, it must meet the discharge standards set by the pollution control board [54].
In developing countries, such as India, Bangladesh, and Pakistan, tanneries are often set up by small-scale industries and they do not have adequate financial and technical resources for an effluent treatment plant [28]. For these reasons, tannery associations have been established to run common effluent treatment plants (CETPs), where the effluent outlet from each associated company is sent. So, the wastewater is effectively and efficiently treated to meet the discharge standards or be recycled back to the companies themselves for reuse [55]. There is a need to redesign, upgrade, and integrate the new technological approach into the conventional treatment methods so that more water can be recycled back into the industry.
In recent years, membrane technologies, such as microfiltration (MF), ultrafiltration (UF), and nanofiltration (NF), have been adopted in the CETPs to treat the water by removing the salinity [56]. Global adoption of membrane technology has contributed to reducing the cost of operation, and the maintenance of their equipment has become simple because of technological improvements [57]. The efficiency of the MF and UF depends on the recovery of chromium in the residual water because of the porous nature of the membrane leading to internal fouling [58]. Membrane technologies are the most studied process in recycling water and chrome to save water and chemicals in the tannery sector.
Reverse osmosis (RO) is known to produce high-quality permeate water that can be reused in the production cycle, thus reducing the consumption of groundwater [11]. RO is a process more suitable to recover chromium from wastewater than adsorption, chemical precipitation, solvent extraction, and ion exchange. The reason is that separation can be achieved without the addition of chemicals and thermal energy, thus making the process ideally suitable for a cleaner technology approach [59]. Studies have shown that the TWW treatment consisting of a combination of physicochemical process, filtration, UF, and RO can produce high-quality permeate with 98% rejection of salts with improved quality of recovered water and COD [60]. The disposal of the brine solution containing salts from the RO operation is the main drawback [61].
6. Zero Liquid Discharge system
The brine solution discharged from a CETP has always high salinity (TDS = 15–25 g/L) and poses a high risk to health and the environment, as it cannot be used for irrigation or discharged into water recipients [62]. Due to the environmental impacts caused by these pollutants, many countries have laid down strict treatment policies and adopted ZLD for the treatment of TWW [9]. The ZLD system uses a closed water cycle technique so that no water is discharged from the tannery. This eliminates the risk of water contamination by brine discharge and maximizes water usage [58,63]. ZLD can be achieved through the following methods: thermal evaporation, reverse osmosis, electrodialysis, forward osmosis, and membrane distillation. Compared to other technologies, membrane technology is eco-friendly, and it is known to achieve a higher degree of separation without the use of chemicals and thermal energy and has shown to be a promising technology to achieve ZLD in the tannery industry [64]. The ZLD system allows the treatment facility to reclaim and reuse the treated wastewater by employing advanced wastewater treatment technology [65]. Indeed, ZLD offers sustainable wastewater management globally, and the investment in ZLD technology is at least USD 100–200 million in developed countries [66]. Europe and the USA have the best-known standards for TWW treatment, followed by China, Brazil, and Southeast Asian countries [9]. In India, the environmental authorities and the tannery industry have adopted the ZLD system, as this treatment method eliminates almost the whole problem of TDS by removing all dissolved solids on-site. Therefore, this system is a way to reclaim and reuse all the water. The growth of the ZLD system is expected to increase 12% annually, reaching a USD 2.7 billion market value by 2030 [67].
6.1. Thermal-Based ZLD Systems
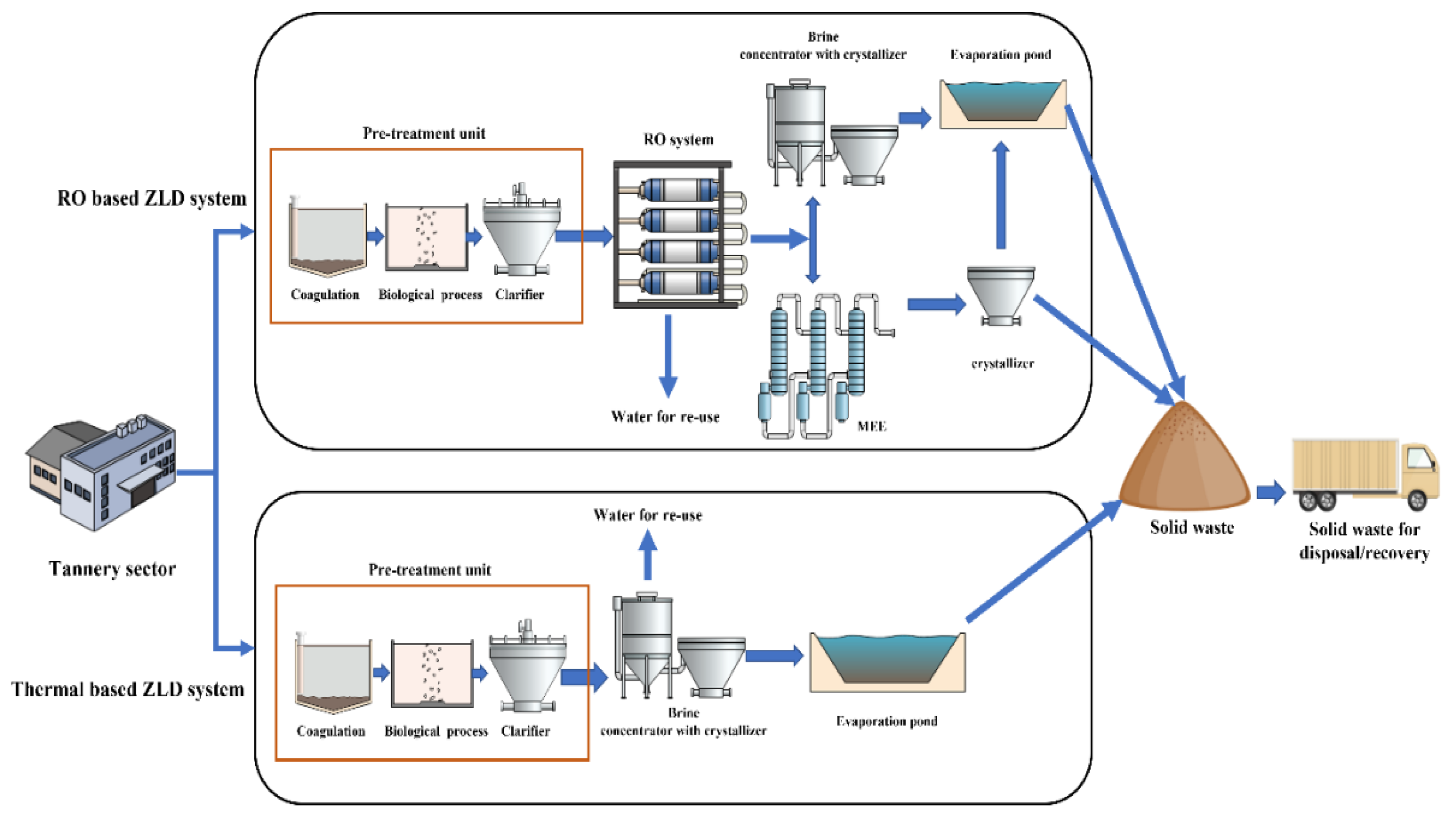
In the early stage of ZLD development, the systems were based on stand-alone thermal processes, where the wastewater generated from the conventional treatment plant was typically evaporated in a brine concentrator followed by a brine crystallizer or evaporation pond, as shown in Figure 3. Thermal desalination technologies such as mechanical vapor compression (MVC), multi-effect distillation (MED), and multistage flash (MSF) have been extensively used in seawater desalination plants [68]. MVC is employed in the ZLD systems of tanneries, where the feed water is mixed with brine slurry through the tubes of a heat exchanger, and superheated steam is used to evaporate the brine slurry by heat exchange [69]. Brine concentrators are capable of achieving 98% water recovery with solid concentrations up to 250 g/L [67]. Then, the concentrated brine is fed into a crystallizer for further water recovery by pumping through a submerged heat exchanger [70]. The treated water is collected and recirculated to the tannery sector for reuse. At the end of the ZLD system, salts are produced as by-products. They can be either disposed of through landfills or recovered as valuable salt products [71].
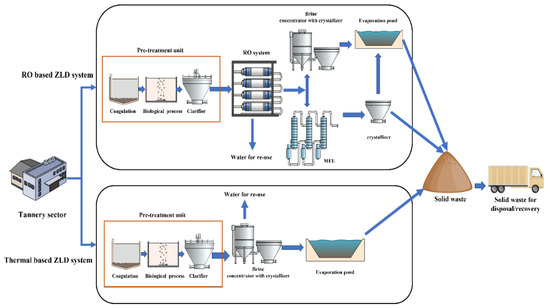
Figure 3.
Schematic illustration of thermal- and RO-based ZLD systems.
6.1.1. Drawbacks of Thermal-Based ZLD Systems
Even though the thermal-based ZLD system produces distillates having TDS <10 mg/L recyclable or reusable in the tannery sector, the consumption of large amounts of energy in the MVC brine concentrator (20–25 kWh/m3 of treated wastewater) raises concerns over energy conservation [67]. The energy consumption of the crystallizer unit after MVC is 52–66 kWh/m3 of wastewater [66]. Moreover, scaling and corrosion phenomena in the MVC system are problems that must be addressed and solved, or at least limited [72].
6.1.2. Approaches for Overcoming These Drawbacks
Steam-driven crystallizers are considered economically feasible for small-scale tanneries [73]. Adding anti-scalants such as calcium sulfate, calcium carbonate, strontium sulfate, or barium sulfate into the recirculating brine can prevent the scaling problem by keeping salt in the suspension [68]. Moreover, to counteract the corrosion problem, MVC equipment is manufactured using titanium and stainless-steel materials, thus increasing the capital cost [74,75].
Solar evaporation ponds exploit solar energy for concentrating the brine solution. This technique is applicable for treating a small volume of brine solution, and it represents a valid alternative to brine crystallizers in small-scale tanneries to limit the processing costs [76]. In some cases, improved solar evaporation ponds are used, where the brine solution is sprayed on the constructed land slope to enhance the brine-to-salt conversion process [77].
At last, incorporating an RO system in the ZLD can increase water recovery and reduce the volume of concentrated brine from entering the brine concentrator and crystallizer.
6.2. RO-Based ZLD Systems
When combined with a conventional thermal-based ZLD system, RO technology can decrease the volume of slurry entering the brine concentrator, thereby reducing energy consumption in MVC. RO eliminates the irreversible losses associated with evaporation and condensation in thermal processes [78]. This membrane section is located before the MVC brine concentrator to preconcentrate the feedwater so that it can reduce the load on the concentrator, as shown in Figure 3. RO uses around 2 kWh/m3 of wastewater, which is much lower than the consumption of the combination MVC concentrator + crystallizer [79].
6.2.1. Limitations of RO-Based ZLD Systems
The application of RO in the ZLD system is constrained by two limitations:
- (a)
- RO has a salinity limit and it is economical only when the salinity range is lower than 70 g/L [80].
- (b)
- Membrane fouling and scaling reduce the permeability and lifespan of RO membranes [58].
6.2.2. Systems for Overcoming These Limitations
Before feeding the wastewater to the RO section, suitable pre-treatment is necessary, such as chemical softening, ion exchange, and pH adjustment. Low pressure-driven membrane filters, such as MF, UF, and NF, can be used to pretreat the RO feed water [81]. Pilot-scale studies have shown that similar configurations are successful in fulfilling the requirements needed for the RO feed water [82].
7. Critical Assessment of ZLD Economics
The ZLD system is employed to ensure and enable the treatment facility to recycle, recover, and reuse treated wastewater. Installation of the ZLD system requires substantial capital costs and poses a great challenge technically and financially for tanneries. When ZLD systems for tanneries were installed for the first time, operation and maintenance costs were 7–10 times higher than the conventional effluent treatment plant [83,84]. Accumulation of solid waste from the system is one of the drawbacks of the ZLD system as it offers no re-use potential, being a mixture of salts. Despite benefits including yielding freshwater, the cost is still prohibitively high [85], even if the reduction in RO reject can drastically reduce the operational cost [86].
The energy required by a RO system is greatly utilized for pumping. The power consumption of the RO section is about 50% of the total treatment costs. The other costs are 37% for fixed costs, which include insurance and capital investment amortization, 7% for maintenance, 5% for membrane replacement, and 1% for labor and consumable chemicals [87]. Membrane pretreatment (MF, UF, and NF) of wastewater to feed to RO can increase the life of the RO membrane, reducing the cost of membrane replacement [88]. This operation has become mandatory for the sustainable approach in the ZLD system. Bench-scale and pilot-scale studies are being conducted to reduce the cost of implementing ZLD technology [82].
When ZLD is applied in tanneries, the method of action for dealing with the RO reject water must be specified. Solar evaporation pond is a traditional method that has been employed, but the land required for the pond system is high and the average evaporation rate is estimated to be 2.54–10.16 mm of water/day [89]. It is an effective method, especially in countries with warm weather conditions, as it can increase the evaporation rate. A sprinkler system in the pond can accelerate the evaporation rate. The evaporation ponds must be sealed with liners to avoid groundwater contamination and soil salinity [90]. The drawback is that water is not recovered, and the system is not effective during rains. Installing a multiple-effect evaporator can overcome this drawback. This technique allows the recovery of the evaporated water as condensate, with its recirculation into the industry for reuse [91].
The ZLD process has considerable capital and operating costs, but the system recovers 75% of the water and recycles it back for reuse, thus reducing the demand for freshwater [92]. Because of the impacts of the saline water on the environment, evaporation of this saline reject water to dryness is the only possible approach to disposal. This process of converting the salt liquor into solid waste forces additional charges on the ZLD system. The generated solid waste is composed of a mixture of salts such as chlorides, sulfates, carbonates, and nitrates, which raises the disposal problem for the tanneries, as the evaporated salts cannot be used for any other purposes [93]. The salt disposal would bring in additional costs that would make the whole ZLD system much more cumbersome. The capital investment and the operation cost challenge the sustainability of the whole system.
8. Application of ZLD in the Tannery Industry
The implementation of ZLD systems for the recovery of water depends on the graphical location and the regulatory agencies in the country where it is applied [87].
8.1. India
The increasing population and pollution load on freshwater sources have increased the freshwater demand in India. This has led to sustainable approaches in TWW treatment plants.
In 2015, the water conservation policy by the Indian government obliged high polluting industries to implement ZLD technologies [66]. The Indian ZLD market was valued to be USD 39 million, and it has grown continuously at a yearly rate of 7% since 2012 [94].
The Central Pollution Control Board has defined that the ZLD system must incorporate two-stage RO treatment and thermal evaporators after conventional wastewater treatment for separating salts from the saline liquor [95]. Solar evaporation ponds can be used to treat the RO reject water with proper liner-based ponds at small ZLD plants (wastewater production < 500 m3/d) and mechanical evaporators at larger ZLD plants [96].
In India, most of the ZLD systems have started installing mechanical evaporators due to the high land requirement for solar evaporation. This provides an advantage of recovering the water in the form of condensate [30].
TDS concentration of raw effluent generated by tanneries varies from 7 to 17 g/L, while it varies from 25 to 40 g/L in the RO reject [97,98]. The recovered permeate recovered from the RO system (70–80%) is combined with condensate derived from the thermal evaporator and recirculated into the process for reuse [99]. The RO rejects account for about 20–30% of the total wastewater generated [71].
In India, around 44% of the tanneries, or 934, are located in Tamil Nadu, with 60% of the national production [100]. The tanning sector is dominated by small-scale units with limited technical expertise and financial resources. In 2005, the Tamil Nadu Pollution Control Board directed tanners to install RO or similar systems to control TDS and expected tanners to reach the minimum national discharge standards [101]. Due to this, tanneries have installed 13 CETPs integrated with RO and evaporators to meet these discharge standards [102]. While assessing the performance of RO membrane technology employed in CETPs in and around Vellore in India, it is noted that the volume of effluent treated is around 415 thousand m3/year. The performance of the membrane technology and characteristics of the final brine solution are reported in Table 2 [30].

Table 2.
Performance of RO technology in CETPs in Tamil Nadu, India.
The RO reject is treated through multiple effect evaporators for further water recovery in the form of condensate. The final form of sludge contains 54% chlorides, 35% sodium, 0.9% calcium, 0.3% magnesium, 1.5% sulfates, 1.3% silica, and 11% moisture that cannot be reused and is disposed in sanitary landfills.
Capital investment, operating costs, and many technical issues related to membrane technologies remain unsolved in adopting the ZLD technologies in India.
8.2. United States
The USA operates the most ZLD systems in the world. The initial development of ZLD took place in 1970, when high saline effluent discharged into the Colorado River and increased the salinity of the river water, forcing the regulatory authority to implement ZLD [103]. Around 60 of 82 ZLD plants in the country were associated with the power industry sector to recycle water for reuse [66]. Understanding the importance of the ZLD system, the United States Environmental Protection Agency revised the guidelines regarding water discharge and promoted ZLD technologies [104]. In compliance with the new guidelines, incentives were provided for ZLD installations. It is estimated that the ZLD market will increase, reaching USD 244 million by 2025 [67].
In the USA, new technologies integrated with electrodialysis reversal (EDR) are being explored to reduce the salt load by 89%. It is estimated that its cost would be 5–8 USD/m3 of wastewater, reducing the challenges faced in brine management [105].
8.3. China
A new action plan was announced by China to tackle water pollution. Recovering the water from wastewater has made ZLD mandatory in the industry for the preservation of water resources and ecosystems [106]. The 14th 5-Year Plan included ZLD systems for environmental protection and the enhancement of water recovery and reuse [107]. Several ZLD systems have already been installed, with a treatment capacity of 110–2300 m3/h [67]. China has implemented the first forward osmosis-based ZLD system to improve the recovery of water and reduce energy consumption by eliminating the need for high-pressure hydraulics [108].
A preliminary study has been conducted to exploit the exhausted heat from flue gas generated from the flue gas desulfurization plant for the ZLD system. It has proven to be cost-effective [109].
8.4. Other Countries
In 2015 in the European Union, the amount of reused water was in the order of over 1 billion m3/year, and it is estimated that it will increase to 6 billion m3/year by 2025 [67].
Full-scale plants with membrane technology (MF, UF, and NF) for the pre-treatment of RO feed water have been implemented in the Middle East. The operation of the first industrial plant for wastewater treatment and recycling commissioned in Oman has a capacity of 7500 m3/day [67,110].
9. Discussion
Tanneries generate highly polluting wastewater, and to prevent the discharge of these pollutants into the environment and recover the water efficiently, a ZLD system needs to be configured properly. Before employing a ZLD system, proper research is necessary to study the characteristics of TWW so the installed scheme will be an effective and fool-proof ZLD scheme. If this target is not achieved, the cost of operation and maintenance will increase, affecting the sustainability of the company.
A novel technological approach must consider the aims of the considered technology, in terms of:
- Reduction in freshwater consumption;
- Increase in water recovery;
- Reduction in the cost of operation; and
- Reduction/disposal of generated solid waste/brine liquor.
Some of the novel approaches are listed below.
- In India, novel marine disposal of saline streams is explored as the desalination plant is integrated into a leather complex. It draws 30,000 m3/day and discharges 20,000 m3/day of the saline stream back into the Bay of Bengal under the overall control of environmental protection authorities with a special bio-control and dispersion system to safeguard the aquatic life [111].
- The volume of RO rejection can be reduced using an alternative technology known as the high-pressure RO system, where 30–40% of the water can be recovered from the rejected water of the RO plant. This reduces the volume of rejected water by 10%, thus reducing the cost of thermal evaporation by 35–40%. The overall operation cost can be reduced by 10–15% [112,113].
- RO-based ZLD systems can be improved by upgrading the system with a new technology named high-efficiency RO, with a recovery rate higher than 90% and a low tendency for scaling and fouling. It overcomes the restriction of salinity limit in RO and treats the brine concentrate efficiently and economically as RO [114].
- Membrane distillation incorporated in ZLD systems is a developing technology that has the potential to concentrate saline wastewater by using waste heat. This system is more economical than the brine concentrator as it can recover 60–90% of water and it works by the waste heat. Therefore, MD-ZLD has the potential to replace brine concentrators. However, at the moment, it has not yet been applied to large-scale treatment plants [115].
- Implementation of forward osmosis in ZLD is an alternative approach to the RO system. It is an energy-efficient separation technique to recover the water from the brine solution. Permeation of water molecules occurs under the influence of osmotic pressure without the requirement of hydraulic pressure. Less energy consumption and reversible membrane fouling are the advantages of this technology. It offers a cleaner alternative way to achieve higher water flux, thereby reducing the generation of brine solution [116,117].
- Electrodialysis can be used to preconcentrate the brine before the crystallizer as the ion exchange membrane eliminates the ions in the brine solution. As a result, a salt-depleted brine solution is obtained at the end of the process. Scaling and fouling can be easily removed by reversing the polarity of the electrodialysis. Pilot-scale studies have shown that this technology is capable of concentrating feed water to relatively high conductivity (above 120 mS/cm) compared to RO capability (80–90 mS/cm) [118].
- Solar evaporation technologies are suitable in arid regions and require low maintenance and low operating costs. Wind aid-intensified evaporation technology uses wind energy to evaporate the brine and can be 10-fold over the natural evaporation at the rate of 0.55–1.7 m3/h with 500 m2 of a wetted surface for 300–1000 m3/d [119].
- The use of chlorine-resistant membranes, such as sulfonated polysulfone composite membranes, can reduce membrane degradation, thus restricting the need for de-chlorination of the RO feed [120].
With this study, we illustrate that ZLD is an important wastewater management strategy that is being implemented globally, even if high operating costs and energy consumption remain limiting factors to ZLD technologies.
The impacts of pollution on the environment have resulted in freshwater scarcity, global climatic change, and the contamination of groundwater. Altogether, this has forced regulatory authorities to push industries to adopt ZLD. Advances in technology and exploring novel approaches to overcome all the limitations of ZLD technologies can make this approach more feasible and sustainable in the near future.
Author Contributions
Conceptualization, F.C., S.S. and G.P.G.; writing—original draft preparation, R.R. and S.S.; writing—review and editing, F.C., S.S. and G.P.G.; supervision, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sivaram, N.M.; Barik, D. Toxic waste from leather industries. In Energy from Toxic Organic Waste for Heat and Power Generation; Woodhead Publishing: Cambridge, UK, 2018; pp. 55–67. ISBN 9780081025284. [Google Scholar]
- Saran, S.; Chib, S.; Saxena, R.K. Biotechnology of leather: An alternative to conventional leather processing. A Handb. High Value Ferment. Prod. Hum. Welf. 2019, 2, 23–47. [Google Scholar]
- Saranya, D.; Shanthakumar, S. Opportunities for phycoremediation approach in tannery effluent: A treatment perspective. Environ. Prog. Sustain. Energy 2019, 38, e13078. [Google Scholar] [CrossRef]
- Omoloso, O.; Mortimer, K.; Wise, W.R.; Jraisat, L. Sustainability research in the leather industry: A critical review of progress and opportunities for future research. J. Clean. Prod. 2021, 285, 125441. [Google Scholar] [CrossRef]
- Singh, A. Livestock Production Statistics of India—2019. Available online: https://www.vetextension.com/livestock-animal-production-statistics-of-india-2019/ (accessed on 22 May 2022).
- Nisa, S. A Study of Export Potential of Indian Leather Industry and Strategies for Growth. SSRN’s Elibrary 2007. [Google Scholar] [CrossRef]
- Suman, H.; Sangal, V.K.; Vashishtha, M. Treatment of tannery industry effluent by electrochemical methods: A review. Mater. Today Proc. 2021, 47, 1438–1444. [Google Scholar] [CrossRef]
- Rajamani, S. Sustainable Environmental Technologies Including Water Recovery for Reuse from Tannery and Industrial Wastewater–Indian and Asian Scenario. Ann. Univ. Oradea Fascicle Text. Oradea Rom. 2017, 173–179. [Google Scholar]
- Zhao, C.; Chen, W. A review for tannery wastewater treatment: Some thoughts under stricter discharge requirements. Environ. Sci. Pollut. Res. 2019, 26, 26102–26111. [Google Scholar] [CrossRef]
- Doble, M.; Kruthiventi, A.K. Industrial Examples. In Green Chemistry and Engineering; Academic Press: Burlington, ON, Canada, 2007; pp. 245–296. ISBN 978-0-12-372532-5. [Google Scholar]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef]
- Moktadir, M.A.; Ahmadi, H.B.; Sultana, R.; Zohra, F.T.; Liou, J.J.H.; Rezaei, J. Circular economy practices in the leather industry: A practical step towards sustainable development. J. Clean. Prod. 2020, 251, 119737. [Google Scholar] [CrossRef]
- Famielec, S. Chromium concentrate recovery from solid tannery waste in a thermal process. Materials 2020, 13, 1533. [Google Scholar] [CrossRef]
- Sundar, V.J.; Ramesh, R.; Rao, P.S.; Saravanan, P.; Sridharnath, B.; Muralidharan, C. Water management in leather industry. J. Sci. Ind. Res. 2001, 60, 443–450. [Google Scholar]
- Sivakumar, V.; Swaminathan, G.; Rao, P.G.; Ramasami, T. Influence of ultrasound on diffusion through skin/leather matrix. Chem. Eng. Process. Process Intensif. 2008, 47, 2076–2083. [Google Scholar] [CrossRef]
- Thazeem, B.; Umesh, M.; Mani, V.M.; Beryl, G.P.; Preethi, K. Biotransformation of bovine tannery fleshing into utilizable product with multifunctionalities. Biocatal. Biotransformation 2021, 39, 81–99. [Google Scholar] [CrossRef]
- Saravanabhavan, S.; Thanikaivelan, P.; Rao, J.R.; Nair, B.U.; Ramasami, T. Reversing the conventional leather processing sequence for cleaner leather production. Environ. Sci. Technol. 2006, 40, 1069–1075. [Google Scholar] [CrossRef]
- Tariq, S.R.; Shah, M.H.; Shaheen, N. Comparative statistical analysis of chrome and vegetable tanning effluents and their effects on related soil. J. Hazard. Mater. 2009, 169, 285–290. [Google Scholar] [CrossRef]
- Covington, A.D. Modern tanning chemistry. Chem. Soc. Rev. 1997, 26, 111. [Google Scholar] [CrossRef]
- Hansen, É.; Monteiro de Aquim, P.; Hansen, A.W.; Cardoso, J.K.; Ziulkoski, A.L.; Gutterres, M. Impact of post-tanning chemicals on the pollution load of tannery wastewater. J. Environ. Manag. 2020, 269, 110787. [Google Scholar] [CrossRef]
- Saxena, G.; Chandra, R.; Bharagava, R.N. Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer: Cham, Switzerland, 2017; Volume 240, pp. 31–69. ISBN 978-3-319-42300-5. [Google Scholar]
- Bhattacharya, P.; Swarnakar, S.; Mukhopadhyay, A.; Ghosh, S. Exposure of composite tannery effluent on snail, Pila globosa: A comparative assessment of toxic impacts of the untreated and membrane treated effluents. Ecotoxicol. Environ. Saf. 2016, 126, 45–55. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Bharagava, R.N. Environmental Hazards and Toxicity Profile of Organic and Inorganic Pollutants of Tannery Wastewater and Bioremediation Approaches. In Bioremediation of Industrial Waste for Environmental Safety; Saxena, G., Bharagava, R.N., Eds.; Springer: Singapore, 2020; pp. 381–398. ISBN 978-981-13-1891-7. [Google Scholar]
- Costa, M.; Klein, C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef]
- Bauchinger, M.; Schmid, E.; Dresp, J.; Kolin-Gerresheim, J.; Hauf, R.; Suhr, E. Chromosome changes in lymphocytes after occupational exposure to toluene. Mutat. Res. Toxicol. 1982, 102, 439–445. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, I.S.; Dureja, P. Enrichment, isolation and characterization of pentachlorophenol degrading bacterium Acinetobacter sp. ISTPCP-3 from effluent discharge site. Biodegradation 2009, 20, 643–650. [Google Scholar] [CrossRef]
- Shekhar Thakur, I.; Verma, P.K.; Upadhaya, K.C. Involvement of plasmid in degradation of pentachlorophenol by Pseudomonas sp. from a chemostat. Biochem. Biophys. Res. Commun. 2001, 286, 109–113. [Google Scholar] [CrossRef]
- Verma, T.; Tiwari, S.; Tripathi, M.; Ramteke, P.W. Treatment and Recycling of Wastewater from Tannery. In Advances in Biological Treatment of Industrial Waste Water and Their Recycling for a Sustainable Future; Singh, R.L., Singh, R.P., Eds.; Springer: Singapore, 2019; pp. 51–90. ISBN 978-981-13-1468-1. [Google Scholar]
- Kavitha, P.R.; Ganapathy, G.P. Tannery process and its environmental impacts a case study: Vellore District, Tamil Nadu, India. J. Chem. Pharm. Sci. 2015, 8, 759–764. [Google Scholar]
- Buljan, J.; Emmanuel, K.V.; Viswanathan, M.; Bosnić, M.; Král, I. Assessment of Performance of Zero Liquid Discharge (ZLD) Operations in Some Tannery Clusters Vellore Districts, Tamil Nadu, India; United Nations Industrial Development Organization: Vienna, Austria, 2017. [Google Scholar]
- Karuppiah, K.; Sankaranarayanan, B.; Ali, S.M.; Jabbour, C.J.C.; Bhalaji, R.K.A. Inhibitors to circular economy practices in the leather industry using an integrated approach: Implications for sustainable development goals in emerging economies. Sustain. Prod. Consum. 2021, 27, 1554–1568. [Google Scholar] [CrossRef]
- Morera, J.M.; Bacardit, A.; Ollé, L.; Bartolí, E.; Borràs, M.D. Minimization of the environmental impact of chrome tanning: A new process with high chrome exhaustion. Chemosphere 2007, 69, 1728–1733. [Google Scholar] [CrossRef]
- Bacardit, A.; Morera, J.M.; Ollé, L.; Bartolí, E.; Dolors Borràs, M. High chrome exhaustion in a non-float tanning process using a sulphonic aromatic acid. Chemosphere 2008, 73, 820–824. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Thomas, L.; Goswami, M.; Giri, B.S.; Pandey, A. Industrial Enzymes. In Industrial Biorefineries and White Biotechnology; Pandey, A., Höfer, R., Taherzadeh, M., Nampoothiri, K.M., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 473–497. ISBN 9780444634535. [Google Scholar]
- Kanagaraj, J.; Chandra Babu, N.K.; Mandal, A.B. Recovery and reuse of chromium from chrome tanning waste water aiming towards zero discharge of pollution. J. Clean. Prod. 2008, 16, 1807–1813. [Google Scholar] [CrossRef]
- Collivignarelli, C.; Barducci, G. Waste recovery from the tanning industry. Waste Manag. Res. 1984, 2, 265–278. [Google Scholar] [CrossRef]
- Blackman, A. Adoption of Clean Leather-Tanning Technologies in Mexico. Discussion Papers 10881, Resources for the Future; Washington, DC, USA, 2005; DP 05-38; Available online: https://ageconsearch.umn.edu/record/10881 (accessed on 22 May 2022). [CrossRef]
- Al-Jabari, M.; Sawalha, H.; Pugazhendhi, A.; Rene, E.R. Cleaner production and resource recovery opportunities in leather tanneries: Technological applications and perspectives. Bioresour. Technol. Rep. 2021, 16, 100815. [Google Scholar] [CrossRef]
- Varaidzo S Dandira, I.M. Design of a Cleaner Production Framework to Enhance Productivity: Case Study of Leather Company. Int. J. Sci. Res. 2013, 2, 18–37. [Google Scholar]
- Raghava Rao, J.; Chandrababu, N.K.; Muralidharan, C.; Nair, B.U.; Rao, P.G.; Ramasami, T. Recouping the wastewater: A way forward for cleaner leather processing. J. Clean. Prod. 2003, 11, 591–599. [Google Scholar] [CrossRef]
- Galiana-Aleixandre, M.V.; Mendoza-Roca, J.A.; Bes-Piá, A. Reducing sulfates concentration in the tannery effluent by applying pollution prevention techniques and nanofiltration. J. Clean. Prod. 2011, 19, 91–98. [Google Scholar] [CrossRef]
- Deng, W.; Chen, D.; Huang, M.; Hu, J.; Chen, L. Carbon dioxide deliming in leather production: A literature review. J. Clean. Prod. 2015, 87, 26–38. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Z.; Zhou, R.; Deng, W.; Wang, M.; Ma, S. Ecological utilization of leather tannery waste with circular economy model. J. Clean. Prod. 2011, 19, 221–228. [Google Scholar] [CrossRef]
- Bień, J.; Celary, P.; Wystalska, K. The problems in achieving sustainable development in the tannery industry in regard to sewage sludge management. J. Ecol. Eng. 2017, 18, 13–20. [Google Scholar] [CrossRef]
- Hutton, M.; Shafahi, M. Water pollution caused by leather industry: A review. In Proceedings of the ASME 2019 13th International Conference on Energy Sustainability, ES 2019, Collocated with the ASME 2019 Heat Transfer Summer Conference, American Society of Mechanical Engineers. Bellevue, WA, USA, 14–17 July 2019; Volume 59094, p. V001T10A002. [Google Scholar]
- Sreeram, K.J.; Ramasami, T. Sustaining tanning process through conservation, recovery and better utilization of chromium. Resour. Conserv. Recycl. 2003, 38, 185–212. [Google Scholar] [CrossRef]
- Mutlu, M.M.; Crudu, M.; Maier, S.S.; Deselnicu, D.C.; Albu, L.; Gulumser, G.; Bitlisli, B.O.; Basaran, B.; Tosun, C.C.; Adiguzel Zengin, A.C. Eko-leather: Properties of chromium-free leathers produced with titanium tanning materials obtained from the wastes of the metal industry. Ekoloji 2014, 23, 83–90. [Google Scholar] [CrossRef]
- China, C.R.; Maguta, M.M.; Nyandoro, S.S.; Hilonga, A.; Kanth, S.V.; Njau, K.N. Alternative tanning technologies and their suitability in curbing environmental pollution from the leather industry: A comprehensive review. Chemosphere 2020, 254, 126804. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J.; Jia, X.; Peng, B. A salt-free and chromium discharge minimizing tanning technology: The novel cleaner integrated chrome tanning process. J. Clean. Prod. 2016, 112, 1055–1063. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Y.; Chen, H.; Huang, H.; Liu, B. Mechanism of High Chrome Uptake of Tanning Pickled Pelt by Carboxyl-Terminated Hyper-Branched Polymer Combination Chrome Tanning. ChemistrySelect 2019, 4, 670–680. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Chandra Babu, N.K.; Sadulla, S.; Suseela Rajkumar, G.; Visalakshi, V.; Chandra Kumar, N. Cleaner techniques for the preservation of raw goat skins. J. Clean. Prod. 2001, 9, 261–268. [Google Scholar] [CrossRef]
- Thanikaivelan, P.; Rao, J.R.; Nair, B.U.; Ramasami, T. Recent trends in leather making: Processes, problems, and pathways. Crit. Rev. Environ. Sci. Technol. 2005, 35, 37–79. [Google Scholar] [CrossRef]
- Molden, D.; Amarasinghe, U.; Hussain, I. Water for Rural Development—Background Paper on Water for Rural Development; International Water Management Institute: Anand, India, 2001; Volume 32, ISBN 9290904593. [Google Scholar]
- Ahamed, M.I.N.; Kashif, P.M. Safety Disposal of Tannery Effluent Sludge: Challenges to Researchers- a Review. Int. J. Pharm. Sci. Res. 2014, 5, 733–736. [Google Scholar]
- Ramteke, P.W.; Awasthi, S.; Srinath, T.; Joseph, B. Efficiency assessment of Common Effluent Treatment Plant (CETP) treating tannery effluents. Environ. Monit. Assess. 2010, 169, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Roca, J.A.; Galiana-Aleixandre, M.V.; Lora-García, J.; Bes-Piá, A. Purification of tannery effluents by ultrafiltration in view of permeate reuse. Sep. Purif. Technol. 2010, 70, 296–301. [Google Scholar] [CrossRef]
- Zakmout, A.; Sadi, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Tannery effluent treatment by nanofiltration, reverse osmosis and chitosan modified membranes. Membranes 2020, 10, 378. [Google Scholar] [CrossRef]
- Suthanthararajan, R.; Ravindranath, E.; Chitra, K.; Umamaheswari, B.; Ramesh, T.; Rajamani, S. Membrane application for recovery and reuse of water from treated tannery wastewater. Desalination 2004, 164, 151–156. [Google Scholar] [CrossRef]
- Kiril Mert, B.; Kestioglu, K. Application of nanofiltration and reverse osmosis for tanning wastewater. Int. J. Environ. Res. 2014, 8, 789–798. [Google Scholar]
- Fababuj-Roger, M.; Mendoza-Roca, J.A.; Galiana-Aleixandre, M.V.; Bes-Piá, A.; Cuartas-Uribe, B.; Iborra-Clar, A. Reuse of tannery wastewaters by combination of ultrafiltration and reverse osmosis after a conventional physical-chemical treatment. Desalination 2007, 204, 219–226. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Sinha, S.; Singh, S.; Mallick, S. Comparative growth response of two varieties of Vigna radiata L. (var. PDM 54 and var. NM 1) grown on different tannery sludge applications: Efects of treated wastewater and ground water used for irrigation. Environ. Geochem. Health 2008, 30, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Byers, B. Zero discharge: A systematic approach to water reuse. Chem. Eng. 1995, 102, 96. [Google Scholar]
- Yang, F.; Huang, Z.; Huang, J.; Wu, C.; Zhou, R.; Jin, Y. Tanning wastewater treatment by ultrafiltration: Process efficiency and fouling behavior. Membranes 2021, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Lin, X.; Kong, X.; Duan, Q.; Wang, P.; Mei, X.; Ma, J. Making waves: Zero liquid discharge for sustainable industrial effluent management. Water 2021, 13, 2852. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef]
- Qiblawey, H.M.; Banat, F. Solar thermal desalination technologies. Desalination 2008, 220, 633–644. [Google Scholar] [CrossRef]
- Bostjancic, J.; Ludlum, R. Getting to Zero Discharge: How to Recycle That Last Bit of Really Bad Wastewater A Brief History of Evaporation. In Proceedings of the International Water Conference, Engineers Society of Western Pennsylvania, West Chester, PA, USA, 21–24 April 1996; Volume 57, pp. 290–295. [Google Scholar]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Boopathy, R.; Karthikeyan, S.; Mandal, A.B.; Sekaran, G. Characterisation and recovery of sodium chloride from salt-laden solid waste generated from leather industry. Clean Technol. Environ. Policy 2013, 15, 117–124. [Google Scholar] [CrossRef]
- Alasfour, F.N.; Abdulrahim, H.K. The effect of stage temperature drop on MVC thermal performance. Desalination 2011, 265, 213–221. [Google Scholar] [CrossRef]
- Griffin, S.J.; Schooley, K.E.; Solomon, R.L. The Advantage of Mixed Salt Crystallizers in Zero Liquid Discharge (ZLD) Wastewater Treatment Systems. GE Water Process Technol. 2021, 12, 998. [Google Scholar]
- Faes, W.; Van Bael, J.; Lecompte, S.; Verbeken, K. Optimization of heat exchanger design taking corrosion into account. Therm. Sci. Eng. Prog. 2022, 30, 101277. [Google Scholar] [CrossRef]
- Wang, C.P.; Wang, H.Z.; Ruan, G.L.; Wang, S.H.; Xiao, Y.X.; Jiang, L.D. Applications and prospects of titanium and its alloys in seawater desalination industry. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 688, p. 33036. [Google Scholar]
- Srithar, K.; Mani, A. Studies on solar flat plate collector evaporation systems for tannery effluent (soak liquor). J. Zhejiang Univ. Sci. 2006, 7, 1870–1877. [Google Scholar] [CrossRef]
- Srithar, K.; Mani, A. Open fibre reinforced plastic (FRP) flat plate collector (FPC) and spray network systems for augmenting the evaporation rate of tannery effluent (soak liquor). Sol. Energy 2007, 81, 1492–1500. [Google Scholar] [CrossRef]
- Al-Karaghouli, A.; Kazmerski, L.L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.L.; Arias Chavez, L.H.; Ben-Sasson, M.; Romero-Vargas Castrillón, S.; Yip, N.Y.; Elimelech, M. Desalination and reuse of high-salinity shale gas produced water: Drivers, technologies, and future directions. Environ. Sci. Technol. 2013, 47, 9569–9583. [Google Scholar] [CrossRef]
- Prihasto, N.; Liu, Q.F.; Kim, S.H. Pre-treatment strategies for seawater desalination by reverse osmosis system. Desalination 2009, 249, 308–316. [Google Scholar] [CrossRef]
- Loganathan, K.; Chelme-Ayala, P.; Gamal El-Din, M. Pilot-scale study on the treatment of basal aquifer water using ultrafiltration, reverse osmosis and evaporation/crystallization to achieve zero-liquid discharge. J. Environ. Manag. 2016, 165, 213–223. [Google Scholar] [CrossRef]
- Pérez-González, A.; Urtiaga, A.M.; Ibáñez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef]
- Almulla, A.; Eid, M.; Côté, P.; Coburn, J. Developments in high recovery brackish water desalination plants as part of the solution yp water quantity problems. Desalination 2003, 153, 237–243. [Google Scholar] [CrossRef]
- Li, W.; Krantz, W.B.; Cornelissen, E.R.; Post, J.W.; Verliefde, A.R.D.; Tang, C.Y. A novel hybrid process of reverse electrodialysis and reverse osmosis for low energy seawater desalination and brine management. Appl. Energy 2013, 104, 592–602. [Google Scholar] [CrossRef]
- Robertson, A.; Nghiem, L.D. Treatment of High TDS Liquid Waste: Is Zero Liquid Discharge Feasible? J. Water Sustain. 2011, 1, 1–11. [Google Scholar]
- Younos, T. The Economics of Desalination. J. Contemp. Water Res. Educ. 2009, 132, 39–45. [Google Scholar] [CrossRef]
- Mickley, M.C. Membrane Concentrate Disposal: Practices and Regulation; US Department of the Interior, Bureau of Reclamation, Technical Service: Denver, CO, USA, 2006; Volume 123, ISBN 1856173895.
- Pancharatnam, S. Transient Behavior of a Solar Pond and Prediction of Evaporation Rates. Ind. Eng. Chem. Process Des. Dev. 1972, 11, 287–292. [Google Scholar] [CrossRef]
- Ahmed, M.; Shayya, W.H.; Hoey, D.; Mahendran, A.; Morris, R.; Al-Handaly, J. Use of evaporation ponds for brine disposal in desalination plants. Desalination 2000, 130, 155–168. [Google Scholar] [CrossRef]
- Rajkumar, R.; Sathish, S.; Senthilkumar, P. Studies on enhancing the efficiency of ZLD plant for tannery effluent by implementing Low-Cost ambient air evaporator system. Rasayan J. Chem. 2018, 11, 13–17. [Google Scholar] [CrossRef]
- Martínez, J.; León, E.; Baena-Moreno, F.M.; Rodríguez-Galán, M.; Arroyo-Torralvo, F.; Vilches, L.F. Techno-economic analysis of a membrane-hybrid process as a novel low-energy alternative for zero liquid discharge systems. Energy Convers. Manag. 2020, 211, 112783. [Google Scholar] [CrossRef]
- Buljan, J.; Emmanuel, K.V.; Viswanathan, M.; Bosnić, M.; Král, I. Analysis of flow and energy aspects of Zero Liquid Discharge (ZLD) technology in treatment of tannery effluents in Tamil Nadu, India. In Proceedings of the 34th IULTCS Congress: Science and Technology for Sustainability of Leather, Chennai, India, 6 February 2017; pp. 244–259. [Google Scholar]
- Patel, T.; Bagrecha, D. Zero Liquid Discharge. Zero Waste 2019, 2, 13–23. [Google Scholar] [CrossRef]
- CPCB Guidelines on Techno—Economic Feasibility of Implementation of Zero Liquid Discharge (Zld) For Water Polluting Industries. Available online: https://ueppcb.uk.gov.in/downloads/view/28 (accessed on 22 May 2022).
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Afonso, M.D.; Jaber, J.O.; Mohsen, M.S. Brackish groundwater treatment by reverse osmosis in Jordan. Desalination 2004, 164, 157–171. [Google Scholar] [CrossRef]
- Ahmed, M.; Shayya, W.H.; Hoey, D.; Al-Handaly, J. Brine disposal from reverse osmosis desalination plants in Oman and the United Arab Emirates. Desalination 2001, 133, 135–147. [Google Scholar] [CrossRef]
- Vigneswaran, V.S.; Ganesh Kumar, P.; Jeyachandran, J.; Britto Sahayaraj, S.; Kumaresan, G. Usage of solar greenhouse evaporator to enhance dehydration and potable water extraction from tannery effluent. Process Saf. Environ. Prot. 2021, 147, 912–923. [Google Scholar] [CrossRef]
- Namasivayam, C. Reutilization of Industrial Solid Wastes for Industrial Effluent Treatments. Freshw. Manag. 2005, 2, 124. [Google Scholar]
- Govindasamy, P.; Madhavan, S.D.; Revathi, S.; Shanmugam, P. Performance evaluation of common effluent treatment plant for tanneries at Pallavaram CETP. J. Environ. Sci. Eng. 2006, 48, 213–220. [Google Scholar]
- TNPCB Details of Common Effluent Treatment Plants pertaining to the Cluster of Tannery Industries in Tamil Nadu. Available online: https://tnpcb.gov.in/cetp.php (accessed on 22 May 2022).
- Mickley, M. Survey of High-Recovery and Zero Liquid Discharge Technologies for Water Utilities; WateReuse Foundation: Alexandria, VA, USA, 2008. Available online: https://www.waterboards.ca.gov/water_issues/programs/grants_loans/water_recycling/research/02_006a_01.pdf (accessed on 22 May 2022).
- United States Environmental Protection Agency Effluent Limitations Guidelines and Standards for the Steam Electric Power Generating Point Source Category; Final Rule, 40 CFR Part 423. Fed. Regist. 2015, 80, 67838–67903.
- Kimberly-clark Economic zero liquid discharge at US chemical facility. Filtr. Sep. 2018, 55, 12. [CrossRef]
- Rajamani, S. Novel industrial wastewater treatment integrated with recovery of water and salt under a zero liquid discharge concept. Rev. Environ. Health 2016, 31, 63–66. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.J. Minimal Liquid Discharge (MLD) and Zero Liquid Discharge (ZLD) strategies for wastewater management and resource recovery-Analysis, challenges and prospects. J. Environ. Chem. Eng. 2020, 8, 104418. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Fu, J.; Hu, N.; Yang, Z.; Wang, L. Experimental study on zero liquid discharge (ZLD) of FGD wastewater from a coal-fired power plant by flue gas exhausted heat. J. Water Process Eng. 2018, 26, 100–107. [Google Scholar] [CrossRef]
- Nair, M.; Kumar, D. Water desalination and challenges: The Middle East perspective: A review. Desalin. Water Treat. 2013, 51, 2030–2040. [Google Scholar] [CrossRef]
- Rajamani, S.G. Innovative environmental technologies including water recovery for reuse from tannery and industrial wastewater—Indian and Asian scenario. In Proceedings of the International Conference on Advanced Materials and Systems, The National Research & Development Institute for Textiles and Leather-INCDTP, Bucharest, Romania, 20–22 October 2016; pp. 513–518. [Google Scholar]
- Günther, R.; Perschall, B.; Reese, D.; Hapke, J. Engineering for high pressure reverse osmosis. J. Memb. Sci. 1996, 121, 95–107. [Google Scholar] [CrossRef]
- Davenport, D.M.; Deshmukh, A.; Werber, J.R.; Elimelech, M. High-Pressure Reverse Osmosis for Energy-Efficient Hypersaline Brine Desalination: Current Status, Design Considerations, and Research Needs. Environ. Sci. Technol. Lett. 2018, 5, 467–475. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Treatment technologies for reverse osmosis concentrate volume minimization: A review. Sep. Purif. Technol. 2014, 122, 472–489. [Google Scholar] [CrossRef]
- Schwantes, R.; Chavan, K.; Winter, D.; Felsmann, C.; Pfafferott, J. Techno-economic comparison of membrane distillation and MVC in a zero liquid discharge application. Desalination 2018, 428, 50–68. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Memb. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Zhou, X.; Gingerich, D.B.; Mauter, M.S. Water Treatment Capacity of Forward-Osmosis Systems Utilizing Power-Plant Waste Heat. Ind. Eng. Chem. Res. 2015, 54, 6378–6389. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, H.; Lu, F.; Su, Y.; Li, W.; An, J.; Wang, Y.; Xu, T. Electrodialytic concentration of landfill leachate effluent: Lab- and pilot-scale test, and assessment. Sep. Purif. Technol. 2021, 276, 119311. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H.; Riaza, A.; Bernaola, F.J. Comparative study of brine management technologies for desalination plants. Desalination 2014, 336, 32–49. [Google Scholar] [CrossRef]
- Park, H.B.; Freeman, B.D.; Zhang, Z.B.; Sankir, M.; McGrath, J.E. Highly chlorine-tolerant polymers for desalination. Angew. Chemie—Int. Ed. 2008, 47, 6019–6024. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).