Recycling of Pretreated Polyolefin-Based Ocean-Bound Plastic Waste by Incorporating Clay and Rubber
Abstract
1. Introduction
2. Results and Discussion
2.1. General Observations of Polymer Blends
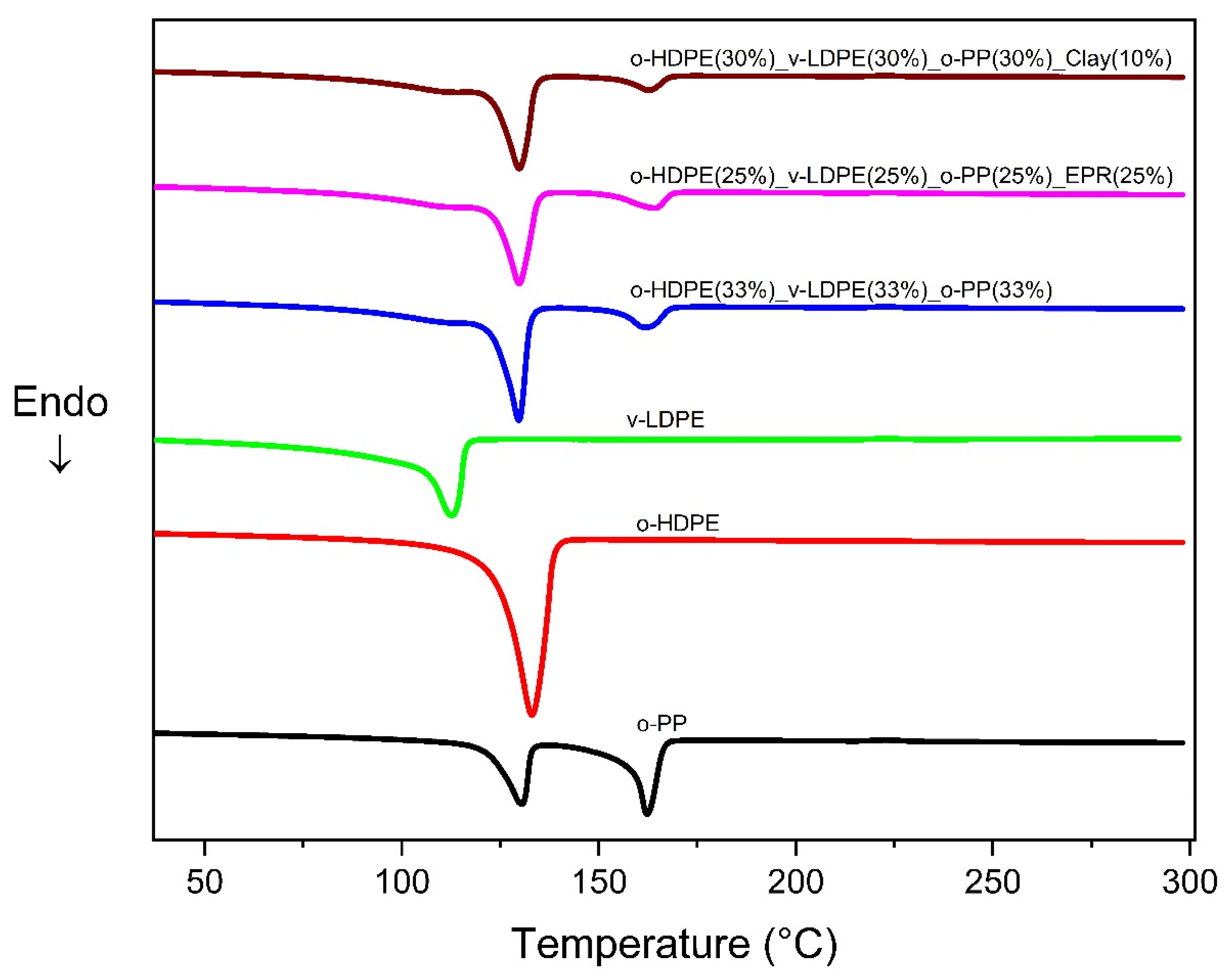
2.2. Differential Scanning Calorimetry (DSC)
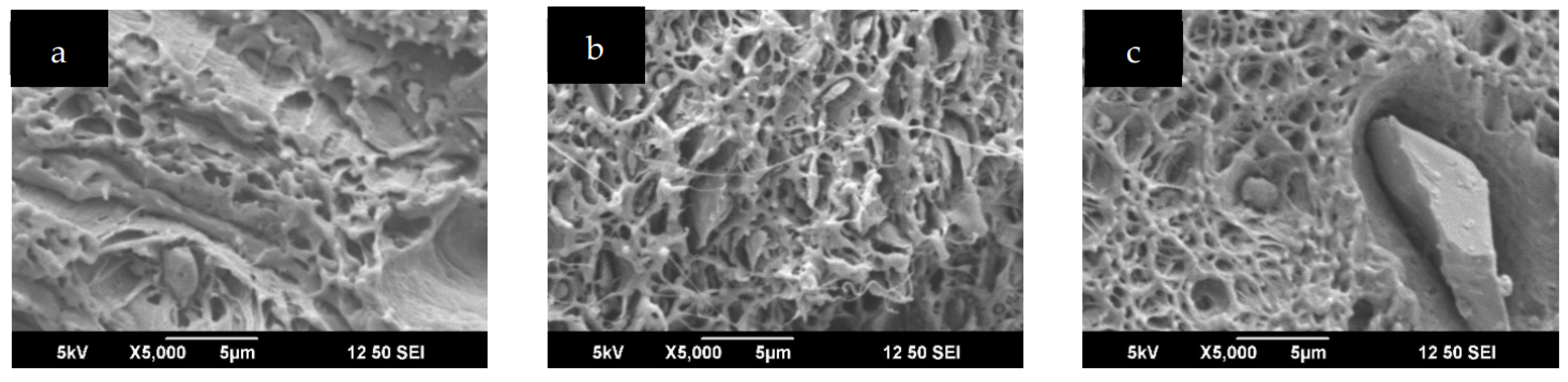
2.3. Scanning Electron Microscopy (SEM)
2.4. Rheology
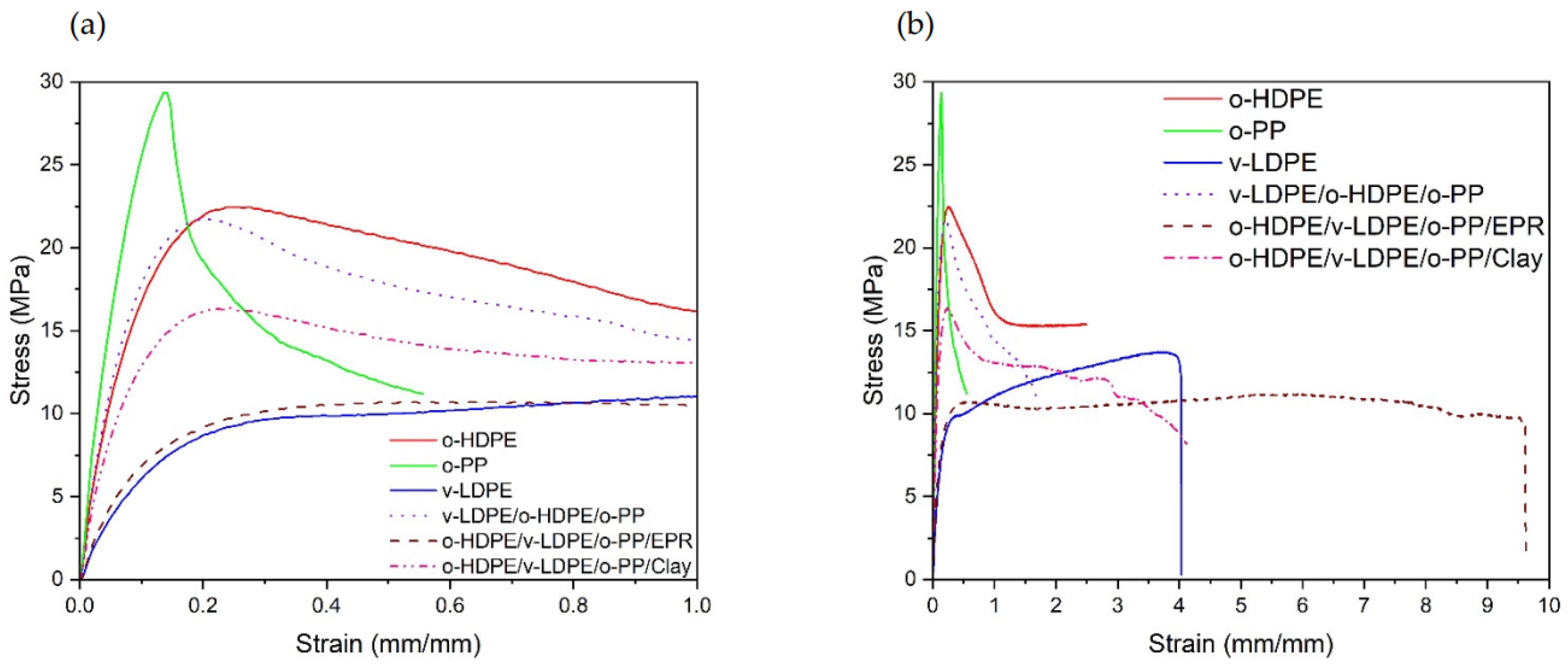
2.5. Tensile Testing
3. Materials and Methods
3.1. Compounding of Materials
3.2. Extruding Printer Filament
3.3. Printing Tensile Speciments (i.e., Dogbones)
3.4. Characterization of Polymer Blends
3.4.1. Differential Scanning Calorimetry
3.4.2. Scanning Electron Microscopy
3.4.3. Rheology
3.4.4. Tensile Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.S.; Sreedevi, A.V.; Kumar, A.B. First report of microplastic ingestion by the alien fish Pirapitinga (Piaractus brachypomus) in the Ramsar site Vembanad Lake, south India. Mar. Pollut. Bull. 2020, 160, 111637. [Google Scholar] [CrossRef]
- Athey, S.N.; Albotra, S.D.; Gordon, C.A.; Monteleone, B.; Seaton, P.; Andrady, A.L.; Taylor, A.R.; Brander, S.M. Trophic transfer of microplastics in an estuarine food chain and the effects of a sorbed legacy pollutant. Limnol. Oceanogr. Lett. 2020, 5, 154–162. [Google Scholar] [CrossRef]
- Jung, M.R.; Balazs, G.H.; Work, T.M.; Jones, T.T.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Brignac, K.C.; Hyrenbach, K.D.; Jensen, B.A.; et al. Polymer Identification of Plastic Debris Ingested by Pelagic-Phase Sea Turtles in the Central Pacific. Environ. Sci. Technol. 2018, 52, 11535–11544. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Pang, Y.X.; Jia, D.M.; Hu, H.J.; Hourston, D.J.; Song, M. Effects of a compatibilizing agent on the morphology, interface and mechanical behaviour of polypropylene/poly(ethylene terephthalate) blends. Polymer 2000, 41, 357–365. [Google Scholar] [CrossRef]
- Xanthos, M.; Young, M.W.; Biesenberger, J.A. Polypropylene/polyethylene terephthalate blends compatibilized through functionalization. Polym. Eng. Sci. 1990, 30, 355–365. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Mistretta, M.C.; La Mantia, F.P. Processing-morphology-property relationships of polyamide 6/polyethylene blend-clay nanocomposites. Express Polym. Lett. 2013, 7, 873–884. [Google Scholar] [CrossRef]
- Ebadi, H.; Yousefi, A.A.; Ouroumiehei, A.B.D.A. Reactive extrusion and barrier properties of PP/PET films. Iran. Polym. J. 2007, 16, 10. [Google Scholar]
- Calderón, B.A.; Thompson, C.W.; Barinelli, V.L.; McCaughey, M.S.; Sobkowicz, M.J. Effect of exchange reactions and free radical grafting on the high-speed reactive extrusion of poly(butylene succinate) and poly(propylene carbonate) blends. Polym. Eng. Sci. 2019, 59, 1986–1998. [Google Scholar] [CrossRef]
- Gug, J.I.; Sobkowicz, M.J. Improvement of the mechanical behavior of bioplastic poly(lactic acid)/polyamide blends by reactive compatibilization. J. Appl. Polym. Sci. 2016, 133, 133. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Shaw, W.J.D. Polymer Alloy Material and Process for Production Thereof. U.S. Patent No. 5,367,048, 22 November 1994. [Google Scholar]
- Utracki, L.A. Compatibilization of polymer blends. Can. J. Chem. Eng. 2002, 80, 1008–1016. [Google Scholar] [CrossRef]
- Rivas, B.L.; Pereira, E.D. Blends of acrylonitrile-butadiene-styrene/waste poly(ethylene terephthalate) compatibilized by styrene maleic anhydride. J. Appl. Polym. Sci. 2001, 80, 2593–2599. [Google Scholar] [CrossRef]
- La Mantia, F.P. The Role of Additives in the Recycling of Polymers. Macromol. Symp. 1998, 135, 157–165. [Google Scholar] [CrossRef]
- Huitric, J.; Ville, J.; Médéric, P.; Moan, M.; Aubry, T. Rheological, morphological and structural properties of PE/PA/nanoclay ternary blends: Effect of clay weight fraction. J. Rheol. 2009, 53, 1101–1119. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Y.; Wang, S.; Gui, Z.; Chen, Z.; Fan, W. Preparation of poly(propylene)/clay layered nanocomposites by melt intercalation from pristine montmorillonite (MMT). Polym. Adv. Technol. 2003, 14, 733–737. [Google Scholar] [CrossRef]
- Ke, Z.; Yongping, B. Improve the gas barrier property of PET film with montmorillonite by in situ interlayer polymerization. Mater. Lett. 2005, 59, 3348–3351. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Z.K.; Yin, J.; Wang, X.Y.; Qi, Z.E. Preparation and properties of hybrids of organo-soluble polyimide and montmorillonite with various chemical surface modification methods. Polymer 1999, 40, 4407–4414. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Giannelis, E.P. Synthesis and barrier properties of poly(ε-caprolactone)-layered silicate nanocomposites. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1047–1057. [Google Scholar] [CrossRef]
- Huang, J.C.; Zhu, Z.K.; Yin, J.; Qian, X.F.; Sun, Y.Y. Poly(etherimide)/montmorillonite nanocomposites prepared by melt intercalation: Morphology, solvent resistance properties and thermal properties. Polymer 2001, 42, 873–877. [Google Scholar] [CrossRef]
- Mofokeng, T.G.; Ray, S.S.; Ojijo, V. Structure–property relationship in PP/LDPE blend composites: The role of nanoclay localization. J. Appl. Polym. Sci. 2018, 135, 46193. [Google Scholar] [CrossRef]
- Vazquez, A.; López, M.; Kortaberria, G.; Martín, L.; Mondragon, I. Modification of montmorillonite with cationic surfactants. Thermal and chemical analysis including CEC determination. Appl. Clay Sci. 2008, 41, 24–36. [Google Scholar] [CrossRef]
- Taguet, A.; Cassagnau, P.; Lopez-Cuesta, J.M. Structuration, selective dispersion and compatibilizing effect of (nano)fillers in polymer blends. Prog. Polym. Sci. 2014, 39, 1526–1563. [Google Scholar] [CrossRef]
- Gelfer, M.Y.; Song, H.H.; Liu, L.; Hsiao, B.S.; Chu, B.; Rafailovich, M.; Si, M.; Zaitsev, V. Effects of organoclays on morphology and thermal and rheological properties of polystyrene and poly(methyl methacrylate) blends. J. Polym. Sci. Part B Polym. Phys. 2002, 41, 44–54. [Google Scholar] [CrossRef]
- Si, M.; Araki, T.; Ader, H.; Kilcoyne, A.L.D.; Fisher, R.; Sokolov, J.C.; Rafailovich, M.H. Compatibilizing bulk polymer blends by using organoclays. Macromolecules 2006, 39, 4793–4801. [Google Scholar] [CrossRef]
- Moghbelli, E.; Sue, H.J.; Jain, S. Stabilization and control of phase morphology of PA/SAN blends via incorporation of exfoliated clay. Polymer 2010, 51, 4231–4237. [Google Scholar] [CrossRef]
- Leszczyńska, A.; Njuguna, J.; Pielichowski, K.; Banerjee, J.R. Polymer/montmorillonite nanocomposites with improved thermal properties. Part I. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim. Acta 2007, 453, 75–96. [Google Scholar] [CrossRef]
- Liu, H.; Mead, J.L.; Stacer, R.G. Thermoplastic elastomers and rubber-toughened plastics from recycled rubber and plastics. Rubber Chem. Technol. 2002, 75, 49–63. [Google Scholar] [CrossRef]
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Börger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.K.; Pascoe, C.; Wyles, K.J. Global ecological, social and economic impacts of marine plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Phelan, A.A.; Ross, H.; Setianto, N.A.; Fielding, K.; Pradipta, L. Ocean plastic crisis—Mental models of plastic pollution from remote Indonesian coastal communities. PLoS ONE 2020, 15, e0236149. [Google Scholar] [CrossRef] [PubMed]
- Bidegain, G.; Paul-Pont, I. Commentary: Plastic waste associated with disease on coral reefs. Front. Mar. Sci. 2018, 5, 5. [Google Scholar] [CrossRef]
- Ryan, P.G. Land or sea? What bottles tell us about the origins of beach litter in Kenya. Waste Manag. 2020, 116, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, C.R.; Barlow, J.W.; Paul, D.R. Blends from reprocessed coextruded products. J. Appl. Polym. Sci. 1981, 26, 9–16. [Google Scholar] [CrossRef]
- Bertin, S.; Robin, J.J. Study and characterization of virgin and recycled LDPE/PP blends. Eur. Polym. J. 2002, 38, 2255–2264. [Google Scholar] [CrossRef]
- Larsen, Å.G.; Olafsen, K.; Alcock, B. Determining the PE fraction in recycled PP. Polym. Test. 2021, 96, 107058. [Google Scholar] [CrossRef]
- Strapasson, R.; Amico, S.C.; Pereira, M.F.R.; Sydenstricker, T.H.D. Tensile and impact behavior of polypropylene/low density polyethylene blends. Polym. Test. 2005, 24, 468–473. [Google Scholar] [CrossRef]
- Fonseca, C.A.; Harrison, I.R. An investigation of co-crystallization in LDPE/HDPE blends using DSC and TREF. Thermochim. Acta 1998, 313, 37–41. [Google Scholar] [CrossRef]
- Zhao, L.; Choi, P. A review of the miscibility of polyethylene blends. Mater. Manuf. Process. 2006, 21, 135–142. [Google Scholar] [CrossRef]
- Hossen Beg, M.D.; Bin Kormin, S.; Bijarimi, M.; Zaman, H.U. Effects of different starch types on the physico-mechanical and morphological properties of low density polyethylene composites. J. Polym. Eng. 2015, 35, 793–804. [Google Scholar] [CrossRef][Green Version]
- Tashiro, K.; Izuchi, M.; Kobayashi, M.; Stein, R.S. Cocrystallization and Phase Segregation of Polyethylene Blends between the D and H Species. 3. Blend Content Dependence of the Crystallization Behavior. Macromolecules 1994, 27, 1221–1227. [Google Scholar] [CrossRef]
- Datta, N.K.; Birley, A.W. ThermaL Analysis of Polyethylene Blends. Plast. Rubber Processing Appl. 1982, 2, 237–245. [Google Scholar]
- Lipatov, Y.S.; Moisya, Y.G.; Semenovich, G.M. Packing density of the chains in the boundary layers of polymers. Polym. Sci. U.S.S.R. 1977, 19, 146–151. [Google Scholar] [CrossRef]
- Ström, G.; Fredriksson, M.; Stenius, P. Contact angles, work of adhesion, and interfacial tensions at a dissolving Hydrocarbon surface. J. Colloid Interface Sci. 1987, 119, 352–361. [Google Scholar] [CrossRef]
- Krump, H.; Luyt, A.S.; Molefi, J.A. Changes in free surface energy as an indicator of polymer blend miscibility. Mater. Lett. 2005, 59, 517–519. [Google Scholar] [CrossRef]
- Kratofil, L.J.; Ptiček, A.; Hrnjak-Murgić, Z.; Jelenčić, J.; Mlinac-Mišak, M. Compatibilization effects in SAN/EPDM blends prepared by reactive extrusion. J. Elastomers Plast. 2007, 39, 371–382. [Google Scholar] [CrossRef]
- Ptiček Siročić, A.; Hrnjak-Murgić, Z.; Jelenčić, J. The surface energy as an indicator of miscibility of SAN/EDPM polymer blends. J. Adhes. Sci. Technol. 2013, 27, 2615–2628. [Google Scholar] [CrossRef]
- Taheri, M.; Morshedian, J.; Khonakdar, H.A. Effect of compatibilizer on interfacial tension of SAN/EPDM blend as measured via relaxation spectrums calculated from Palierne and Choi-Schowalter models. Polym. Bull. 2011, 66, 363–376. [Google Scholar] [CrossRef]
- Abhilash, S.S.; Luckose, R.; Lenin Singaravelu, D. Processing and characterization of HDPE and MDPE processed by rotational moulding. Mater. Today Proc. 2019, 27, 2029–2032. [Google Scholar] [CrossRef]
- ExxonMobil VistalonTM 404 Ethylene Propylene Copolymer Rubber Datasheet. Available online: http://www.lookpolymers.com/polymer_ExxonMobil-Vistalon-404-Ethylene-Propylene-Copolymer-Rubber.php (accessed on 2 March 2022).
- OR.190222 (HDPE) Technical Data Sheet|Oceanworks. Available online: https://app.oceanworks.co/products/rec1efHk9KpfO9POU/tds (accessed on 28 February 2022).
- OR.190252 (PP) Technical Data Sheet|Oceanworks. Available online: https://app.oceanworks.co/products/rec2Qj7VUBZackzFU/tds (accessed on 28 February 2022).
- ExxonMobil ExxonMobilTM LDPE LD 123.LN Low Density Polyethylene Resin. Available online: https://exxonmobilchemical.ulprospector.com/en-US/ds243948/ExxonMobilTMLDPELD123.LN.aspx?I=58933&U=0 (accessed on 25 February 2022).
- Certene® SGM-140—Muehlstein—Datasheet. Available online: https://omnexus.specialchem.com/product/t-muehlstein-certene-sgm-140 (accessed on 25 February 2022).
- ExxonMobil PaxonTM BA50-100 High Density Polyethylene Resin. Available online: https://exxonmobilchemical.ulprospector.com/en-US/ds244451/PaxonTMBA50-100.aspx?I=58933&U=1 (accessed on 25 February 2022).
- Martey, S.; Addison, B.; Wilson, N.; Tan, B.; Yu, J.; Dorgan, J.R.; Sobkowicz, M.J. Hybrid Chemomechanical Plastics Recycling: Solvent-free, High-Speed Reactive Extrusion of Low-Density Polyethylene. ChemSusChem 2021, 14, 4280–4290. [Google Scholar] [CrossRef] [PubMed]

| Base Polymers | Additives | Characteristics of Polymer Blends | ||||||
|---|---|---|---|---|---|---|---|---|
| v-LDPE | o-HDPE | o-PP | v-PS | Clay | SMB | EPR | ||
| 1 | 23 g | 23 g | 23 g | - | 30 g | - | - | Brittle, Poor dispersion of clay in the blend |
| 2 | 30 g | 30 g | 30 g | - | 10 g | - | - | Flexible; Poor dispersion of clay |
| 3 | 33 g | 33 g | 33 g | - | - | - | - | Flexible |
| 4 | 25 g | 25 g | 25 g | - | - | - | 25 g | Very flexible |
| 5 | 19 g | 19 g | 19 g | 19 g | - | 5 g | 19 g | Flexible |
| 6 | 25 g | 25 g | 25 g | 25 g | - | - | - | Quite brittle |
| 7 | 23 g | 23 g | 23 g | 23 g | 8 g | - | - | Flexible, Poor dispersion of clay in the blend with visible clay particles |
| 8 | 17 g | 17 g | 17 g | 17 g | 10 g | 5 g | 17 g | Flexible |
| Polymers | o-HDPE | v-LDPE | o-PP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔH (J/g) | %X | Tm (°C) | ΔH (J/g) | %X | Tm (°C) | ΔH (J/g) | %X | |
| v-LDPE | - | - | - | 112.7 | 137.3 | 46.9 | - | - | - |
| o-HDPE | 133.1 | 201.6 | 68.8 | - | - | - | |||
| o-PP | 130.4 | 41.8 | 20.2 | - | - | - | 162.2 | 50.9 | 24.6 |
| o-HDPE (33%) _v-LDPE (33%) _o-PP (33%) | 129.7 | 197.6 | 67.4 | - | - | - | 162.0 | 54.6 | 26.4 |
| o-HDPE (25%) _v-LDPE (25%) _o-PP (25%) _EPR (25%) | 129.8 * | 212.9 * | 72.7 * | - | - | - | 164.5 | 57.5 | 27.8 |
| o-HDPE (30%) _v-LDPE (30%) _o-PP (30%) _clay (10%) | 129.9 | 173.0 | 59.1 | - | - | - | 162.9 | 37.8 | 18.2 |
| Polymers | Ultimate Tensile Strength (MPa) | Elongation at Peak Load (%) |
|---|---|---|
| v-LDPE | 13.3 ± 0.6 | 324.6 ± 66.1 |
| o-HDPE | 22.3 ± 0.8 | 25.3 ± 1.1 |
| o-PP | 31.6 ± 2.0 | 19.1 ± 4.4 |
| v-LDPE (33.3%)_o-HDPE (33.3%)_o-PP (33.3%) | 21.4 ± 0.9 | 21.7 ± 1.4 |
| v-LDPE (30%)_o-PP (30%)_o-HDPE (30%)_clay (10%) * | 16.4 | 24.2 |
| v-LDPE (25%)_o-PP (25%)_o-HDPE (25%)_EPR (25%) * | 11.2 | 540.5 |
| ExxonMobil (BA50-100) a | ExxonMobil (LD123.LN) | Certene TM SGM-140 | Oceanworks (OR.190222) | Oceanworks (OR.190252) | |
|---|---|---|---|---|---|
| Density (g/cm3) | 0.949 | 0.923 | 1.05 | 0.94–0.96 | 0.91–0.93 |
| Melt Index (g/10 min) | <0.10 (190 °C/2.16 kg) | 2.4 (190 °C/2.16 kg) | 14 (200 °C/5 kg) | 0.75 (190 °C/2.16 kg) | 3.55 (190 °C/2.16 kg) |
| Vicat softening Temperature (°C) | 120.0 | 92.0 | 92.8 | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martey, S.; Hendren, K.; Farfaras, N.; Kelly, J.C.; Newsome, M.; Ciesielska-Wrobel, I.; Sobkowicz, M.J.; Chen, W.-T. Recycling of Pretreated Polyolefin-Based Ocean-Bound Plastic Waste by Incorporating Clay and Rubber. Recycling 2022, 7, 25. https://doi.org/10.3390/recycling7020025
Martey S, Hendren K, Farfaras N, Kelly JC, Newsome M, Ciesielska-Wrobel I, Sobkowicz MJ, Chen W-T. Recycling of Pretreated Polyolefin-Based Ocean-Bound Plastic Waste by Incorporating Clay and Rubber. Recycling. 2022; 7(2):25. https://doi.org/10.3390/recycling7020025
Chicago/Turabian StyleMartey, Shawn, Keith Hendren, Nicholas Farfaras, Jesse C. Kelly, Matthew Newsome, Izabela Ciesielska-Wrobel, Margaret J. Sobkowicz, and Wan-Ting Chen. 2022. "Recycling of Pretreated Polyolefin-Based Ocean-Bound Plastic Waste by Incorporating Clay and Rubber" Recycling 7, no. 2: 25. https://doi.org/10.3390/recycling7020025
APA StyleMartey, S., Hendren, K., Farfaras, N., Kelly, J. C., Newsome, M., Ciesielska-Wrobel, I., Sobkowicz, M. J., & Chen, W.-T. (2022). Recycling of Pretreated Polyolefin-Based Ocean-Bound Plastic Waste by Incorporating Clay and Rubber. Recycling, 7(2), 25. https://doi.org/10.3390/recycling7020025







