Abstract
The exponential growth of waste plastic accumulation has had an irreversible and lasting impact on the world. An imminent threat to marine and terrestrial ecosystems of massive proportions, plastic waste accumulation is a global problem that will not only have to be tackled by current generations but for many generations to follow. The scale of current recycling technologies and efforts to reduce consumption by for-profit and non-profit institutions, governments, and consumers will need to be rapidly increased to combat the negative impacts plastic waste has had on the planet since its conception. This is especially the case in areas with limited infrastructure to properly collect, manage, and dispose of plastic waste. Solutions to plastic waste accumulation crisis that are appropriate for the developing world are urgently needed. Conversion of plastic waste to liquid fuel by slow pyrolysis is a technology that is particularly suitable for developing countries due to its ability to convert polyolefin waste plastic into a useful product, thus preventing its eventual accumulation in the ecosystem. However, in developing countries, conversion techniques that do not rely on sophisticated technologies are needed. Since processing time and operating temperature are the simplest variables to control, an analytical study has been conducted to assess how the molecular composition of plastic derived fuel oil (PDFO) is impacted by these parameters. The results of gas chromatography-mass spectrometry (GC-MS) and thermogravimetric analysis (TGA) studies of PDFO from high- and low-density polyethylene plastic waste produced using appropriate technology techniques are presented alongside a comparison with traditional diesel fuel and kerosene. This approach is novel in that it differs from previously conducted research, which has studied the use of catalysts, additives, or single operating temperatures to assess the composition of PDFO. Therefore, this research contribution presents a simplistic and inexpensive approach for tuning PDFO composition in appropriate technology settings.
1. Introduction
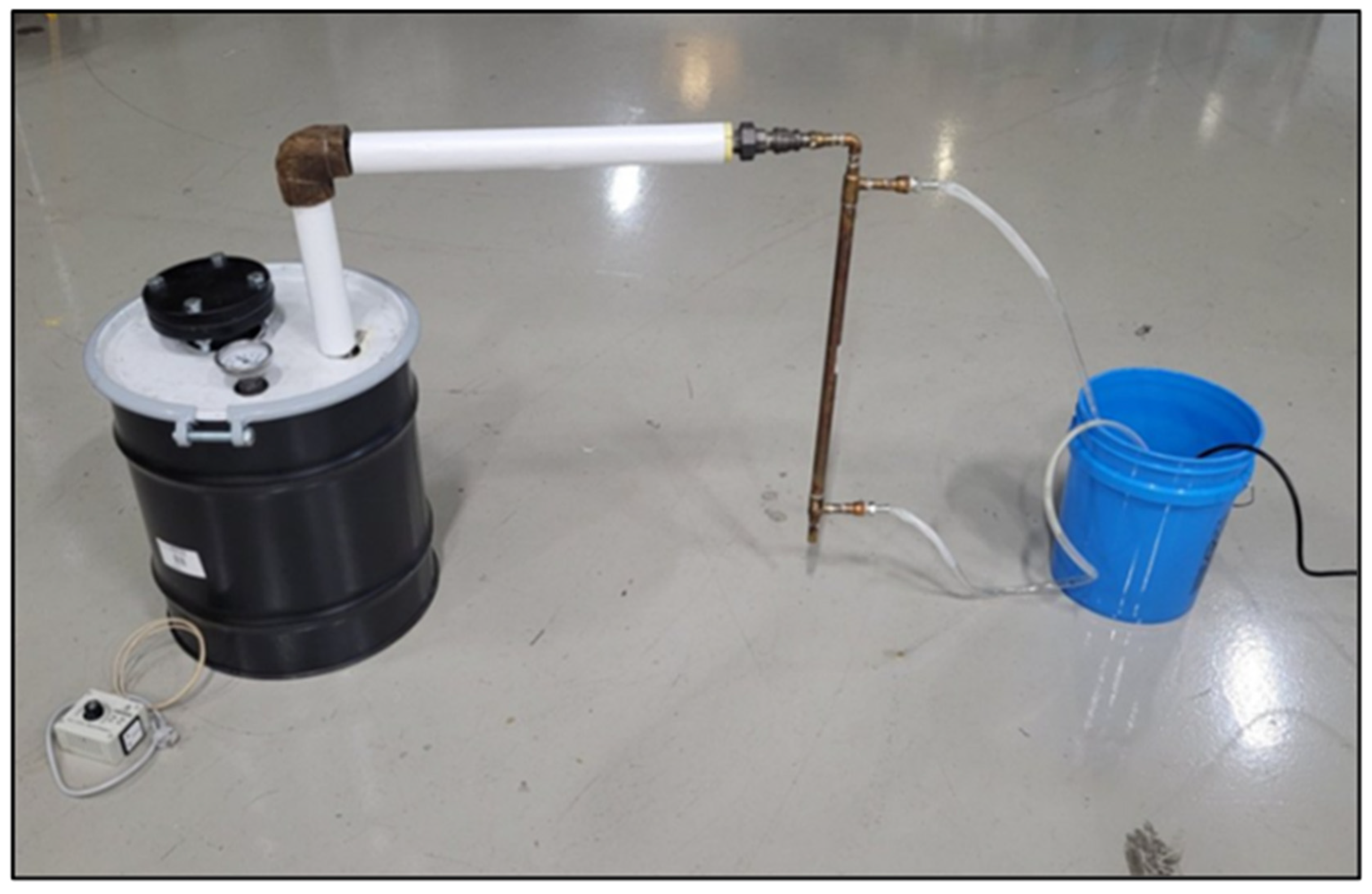
Recycling post-consumer use plastic is a significant challenge due to the heterogeneity of waste streams. This challenge is compounded in developing or infrastructure limited regions [1,2,3,4,5,6,7]. As a result, much of the post-consumer waste generated in the developing world ends up in the environment, where breakdown into microplastics and its migration up the food chain pose a potential threat to human health [8,9,10,11,12,13,14,15,16,17,18,19,20]. One solution for reducing the accumulation of mixed polyolefin plastic waste is to convert it into fuel oil via slow pyrolysis [21,22,23,24,25,26,27,28,29,30,31,32,33]. This process can be used to generate fuel oil with properties similar to either traditional diesel fuel or traditional kerosene, depending upon the pyrolysis temperature [23,24,32,33]. A low-tech process for reducing waste plastic accumulation in developing regions has been proposed and detailed by Joshi and Seay [34,35,36] and Joshi et al. [37,38]. Termed as Trash to Tank (3T), this process converts post-consumer waste plastic into plastic-derived fuel oil (PDFO) via slow pyrolysis using the principles of appropriate technology [39]. In order to conform to the principles of appropriate technology, Joshi and Seay [34,35,36] and Joshi et al. [37,38] have designed a simple, low cost and robust 3T Processor that safely carries out the pyrolysis reaction chemistry at desired temperatures. A photo of the 3T Processor is shown in Figure 1. The 3T processor has been conceived using an open-source design that can be easily built by individuals in developing regions. The processor is low-cost, easy to build and operate, and prioritizes simplicity over sophisticated controls. A schematic of the 3T processor is illustrated in Figure 2. The process consists of a 30-L insulated retort housed in a standard 20-gallon steel drum. The retort is heated using a 240-volt electric band heater and the vapor fuel oil product is condensed using ambient temperature water in a double-pipe heat exchanger. The feedstock used for the 3T processor is a chemical mixture of polyolefin plastics; namely, high-density polyethylene (HDPE) and low-density polyethylene (LDPE). The thermal decomposition of these polymers by slow pyrolysis yields a chemical mixture of hydrocarbon chains similar in carbon number to those found in traditional diesel and kerosene [34,40]. PDFO has beneficial applications in developing regions as an alternative to traditional diesel or kerosene. Particularly, it can be used in diesel generators, vehicles, kerosene cookstoves, and lamps, serving as a reliable source of energy for transportation, cooking and lighting [34].

Figure 1.
3T processor designed using the principles of appropriate technology.
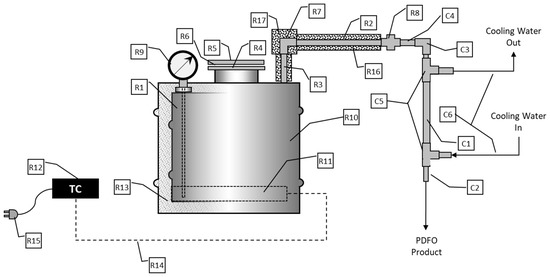
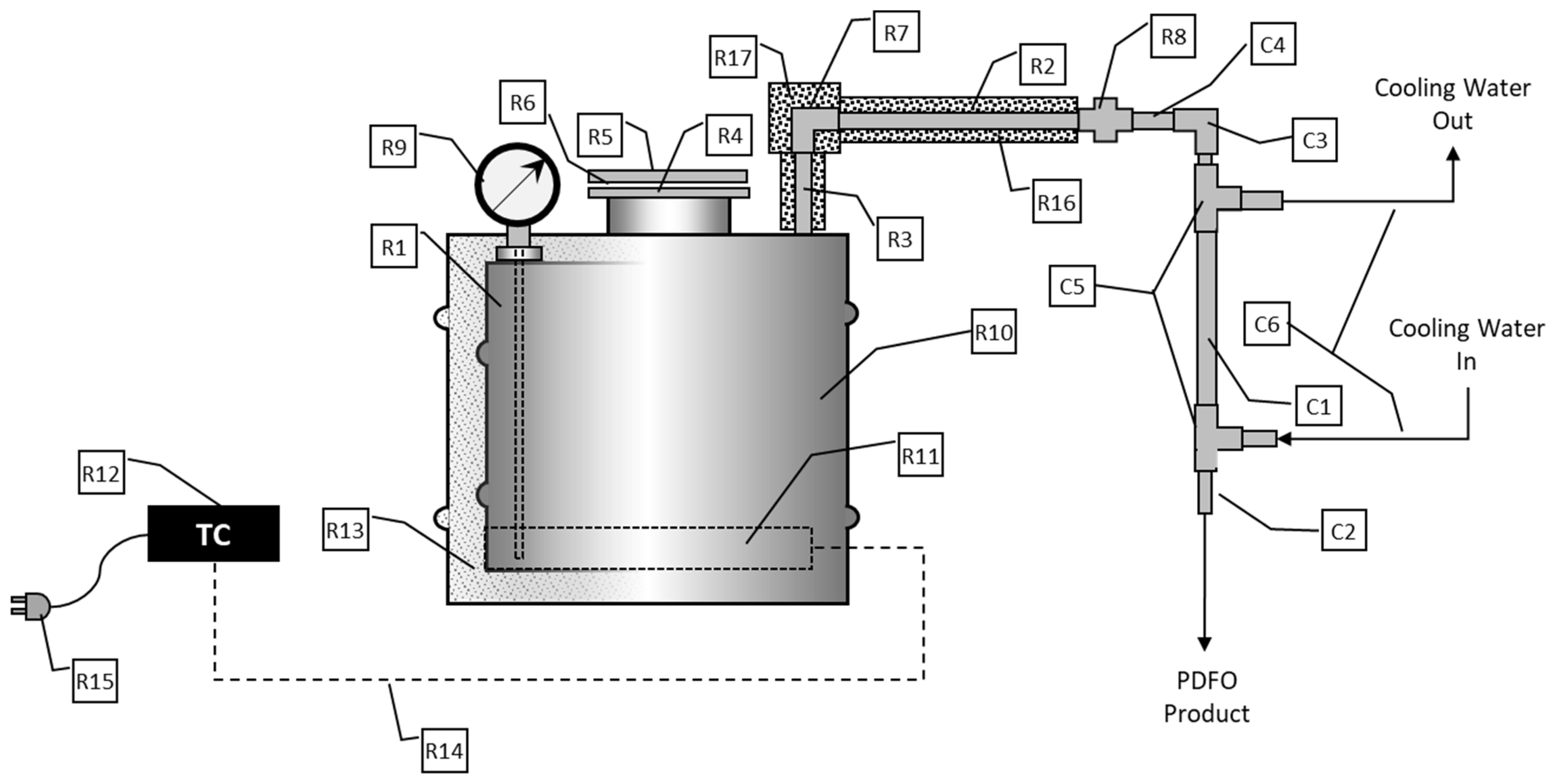
Figure 2.
Schematic of 3T processor. R1: Fabricated carbon steel retort; R2: 3/4′′ CS Pipe-24′′ long; R3: 3/4′′ CS Pipe-6′′ Long; R4: 3′′ Screw on Flange; R5: 3′′ Blind Flange; R6: Flange Gasket; R7: 3/4′′ CS Pipe Elbow; R8: 3/4′′ CS Pipe Union; R9: Analog Bi Metal Thermometer; R10: 20 Gallon CS Open Head Drum; R11: Heating Element, 240 V, 1900W; R12: Electronic heater controller; R13: High temperature thermal insulation; R14: NEMA Plug w/Wire Leads; R15: High Temperature Wire; R16: 3/4′′ Pipe Insulation (3 Feet); R17: 3/4′′ Elbow Insulation; C1: 3/4′′ Copper Tubing-24′′ Long; C2: 1/2′′ Copper Tubing-30′′ Long; C3: 1/2′′ Copper Elbow; C4: 1/2′′ Solder to 3/4′′ Male NPT Adapter; C5: Copper Right Angle Tee Reducer; C6: 5/8′′ Clear Tubing (10 feet).
Due to the similarity of PDFO to diesel and kerosene, the objective and novelty of this research contribution is to analyze the molecular composition of PDFO produced from HDPE and LDPE as a function of temperature and processing time. Since pyrolysis temperature and processing time are the two variables that can be easily controlled, they are useful for adjusting the slow pyrolysis reaction chemistry in appropriate technology settings. By using gas chromatography-mass spectrometry (GC-MS) and thermogravimetric analysis (TGA), the composition and stability of liquid PDFO can be assessed and compared with traditional diesel and kerosene. This approach differs from previous GC-MS and TGA-based literature analysis of PDFO where researchers have primarily focused on: the pyrolysis of plastic with catalysts [40,41,42,43,44,45,46]; coprocessing of plastic with oil producing biomass [47,48,49], lubrication oils [50,51], coal [52], and semicoke [53]; analyzed a single operating temperature [54]; or analyzed the composition of the plastic instead of PDFO [55,56]. Since the co-pyrolysis of plastic with additives, such as catalysts, lubrication oils, coal, and semicoke can increase costs and complexity, this research contribution focuses on the utilization of an inexpensive, simplistic, and yet novel approach for adjusting readily available tuning parameters—temperature and time—to study PDFO composition in appropriate technology settings.
2. Materials and Methods
2.1. Collecting PDFO Samples
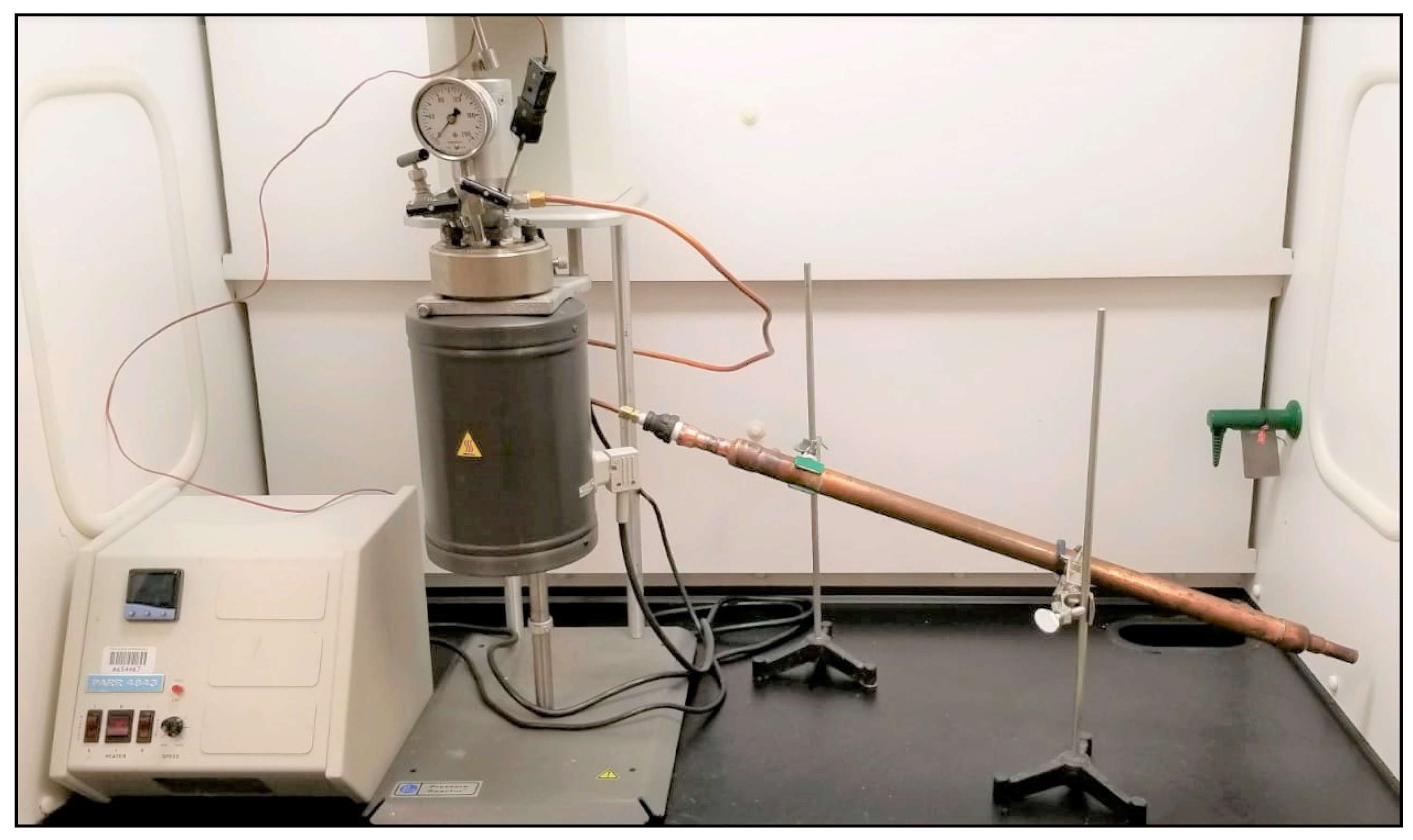
To understand the effect of temperature on the composition and stability of PDFO, the 3T processor was modeled using a bench-scale autoclave Parr pressure vessel reactor with a Parr 4843 controller, as shown in Figure 3. This setup was used to generate PDFO from HDPE and LDPE. These plastics were sourced from household waste, including milk jugs (HDPE) and zipper storage bags (LDPE). The plastics were cleaned and cut to small pieces for insertion in the reactor. The temperature of the slow-pyrolysis experiments was varied between 370–400 °C in increments of 10 °C for each plastic type. This range was chosen to reflect optimum PDFO production as a function of temperature, i.e., in general, temperatures below 370 °C produced minimal amount of PDFO, whereas above 400 °C produced wax for plastics such as HDPE. The process was operated at ambient pressure. The PDFO generated through slow pyrolysis was condensed in a double pipe heat exchanger cooled with ambient temperature water. A yield of 1 L of oil is collected for every 1 kg of plastic processed, which is consistent with previous results.
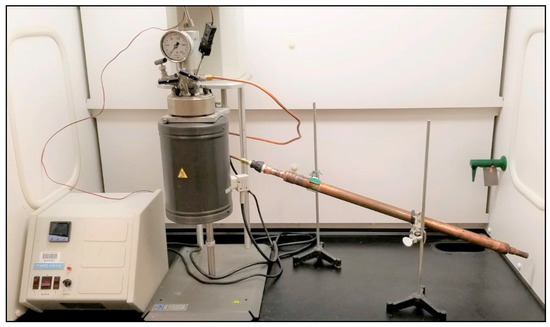
Figure 3.
Parr reactor and Parr 4843 controller setup for slow-pyrolysis experiments.
To understand the effect of sample retention time on the composition and stability of PDFO, the Parr bench-scale reactor and controller setup was used to generate PDFO from waste HDPE and LDPE at temperatures of only 370 °C and 400 °C. These temperatures were chosen to bookend the impact of temperature on the time-focused experimental runs. Hence, for observing the impact of time, fuel samples were collected at 30-min increments for 2 h after observing the first drop of fuel, or after approximately 4.5 h of starting the experiment for HDPE and 3.5 h for LDPE. The mass balance yields of the experimental runs for both HDPE and LDPE were determined to be on average 81.83% at 370 °C and 73.89% at 400 °C. The loss in mass can be attributed to non-condensable gases produced during thermal decomposition of the polymer chains as well as lower molecular weight hydrocarbon chains that could not be effectively condensed using cooling water. Ambient temperature cooling water in a double pipe heat exchanger was used for condensing during the experimental runs in order to approximate the conditions expected in a developing world setting.
2.2. Analyzing PDFO Samples
GC-MS studies were completed using an Agilent Technologies 7890 A gas chromatograph interfaced with an Agilent Technologies 5975 C mass spectrometer and triple-axis detector. Sample preparation involved taking 500 μL of each sample and diluting to 2.5 mL using pentanes. Injection volume for each sample was 5 μL. Analytes were separated using a capillary column (Agilent Technologies HP-5MS, 30 m 0.25 mm; i.d. 0.25 mm, Santa Clara, CA, USA) and ultra-high purity (>99.999%) helium gas as a mobile phase. The initial oven temperature was set at 60 °C, ramped to 200 °C at a rate of 10 °C/min, then ramped at 5 °C/min to 280 °C. The mass source, quadrupole, and injector were held at a constant temperature of 230 °C, 150 °C, and 300 °C, respectively. The most abundant 20–25 signals in each chromatogram were identified by comparing the fragmentation patterns from the mass spectrum to the National Institute of Standards and Technology (NIST) library. The area under the curve or each chromatogram signal was used to compare the relative abundances of each hydrocarbon present based upon the total number of carbons in each molecule (C8 through C30). To analyze the thermal stability of PDFO, TGA studies were completed in triplicate on each sample (5–8 mg) using a TA Instruments Q500 TGA with platinum pans. A heating rate of 10 °C/min from 30 to 600 °C under a constant dry nitrogen flow (40 mL/min) was utilized.
3. Results and Discussion
3.1. Effect of Temperature
3.1.1. GC-MS Results
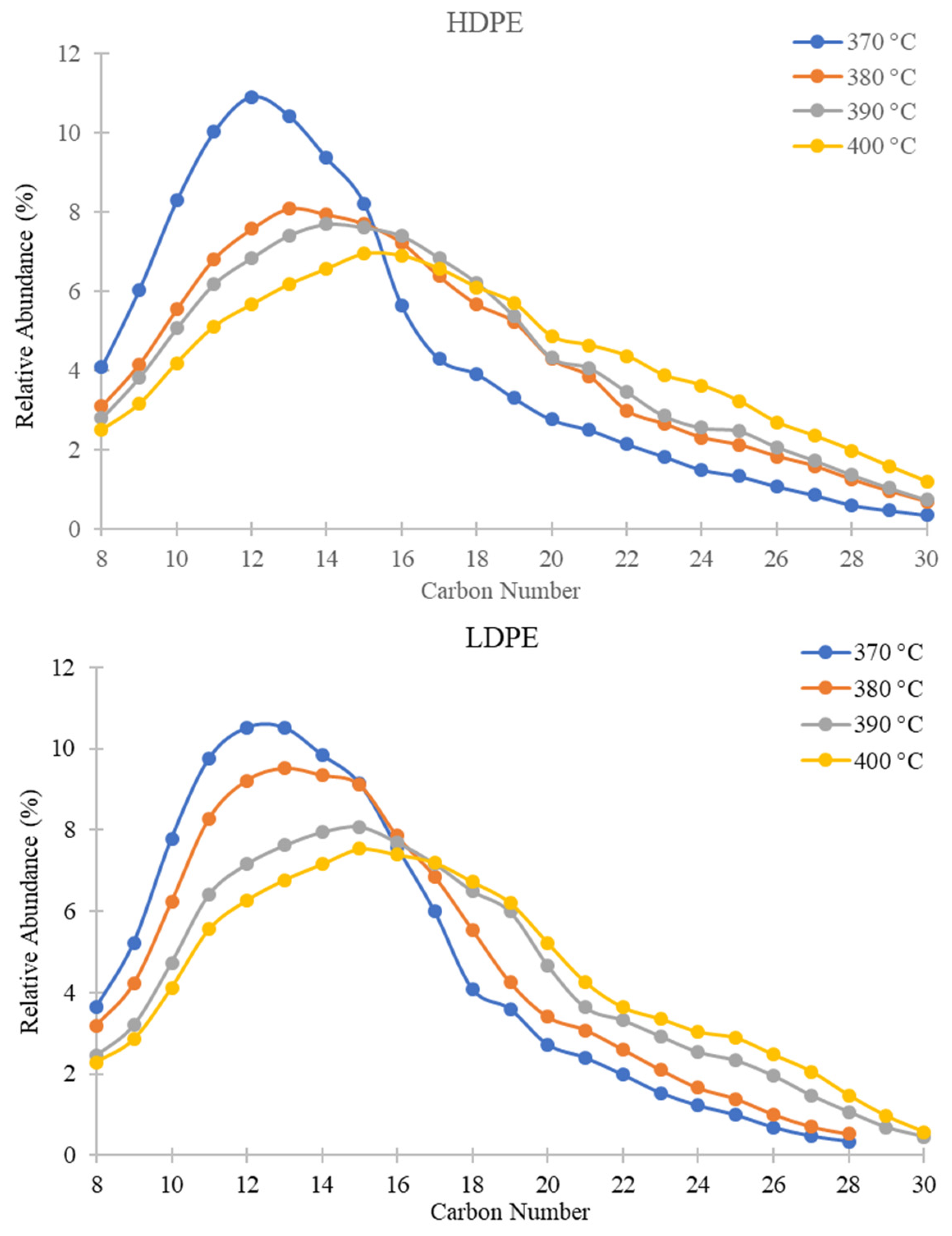
The effect of temperature variation on hydrocarbon distribution was investigated first by GC-MS. The results indicated that the hydrocarbons present in PDFO composition of all plastics analyzed were primarily aliphatic (alkanes and alkenes) hydrocarbons due to the depolymerization chemistry of the polyethylene, which undergo a random chain scission mechanism during pyrolysis [22,40,41,45,46,57,58,59]. For PE based plastics, hydrocarbons in the range of C8–C30 were observed. The identity of the most abundant signals were identified according to their fragmentation pattern from the mass spectrum and the relative abundances from each gas chromatograph were compared and averaged over a set of runs. The results of these analyses are shown in Figure 4. In comparison with petroleum-derived distillates, diesel fuel (No. 2 Fuel Oil) contains approximately 75–90% aliphatic alkanes and cycloalkanes, and 10–25% aromatics and olefins/alkenes [60,61]. Kerosene (No.1 Fuel Oil) is a light distillate primarily consisting of branched chain alkanes, cycloalkanes, and mixed aromatic cycloalkanes [62].
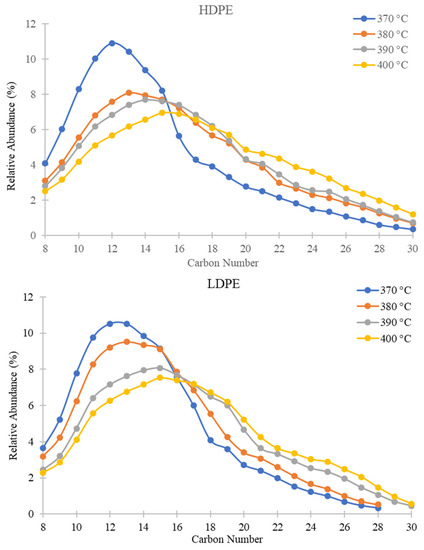
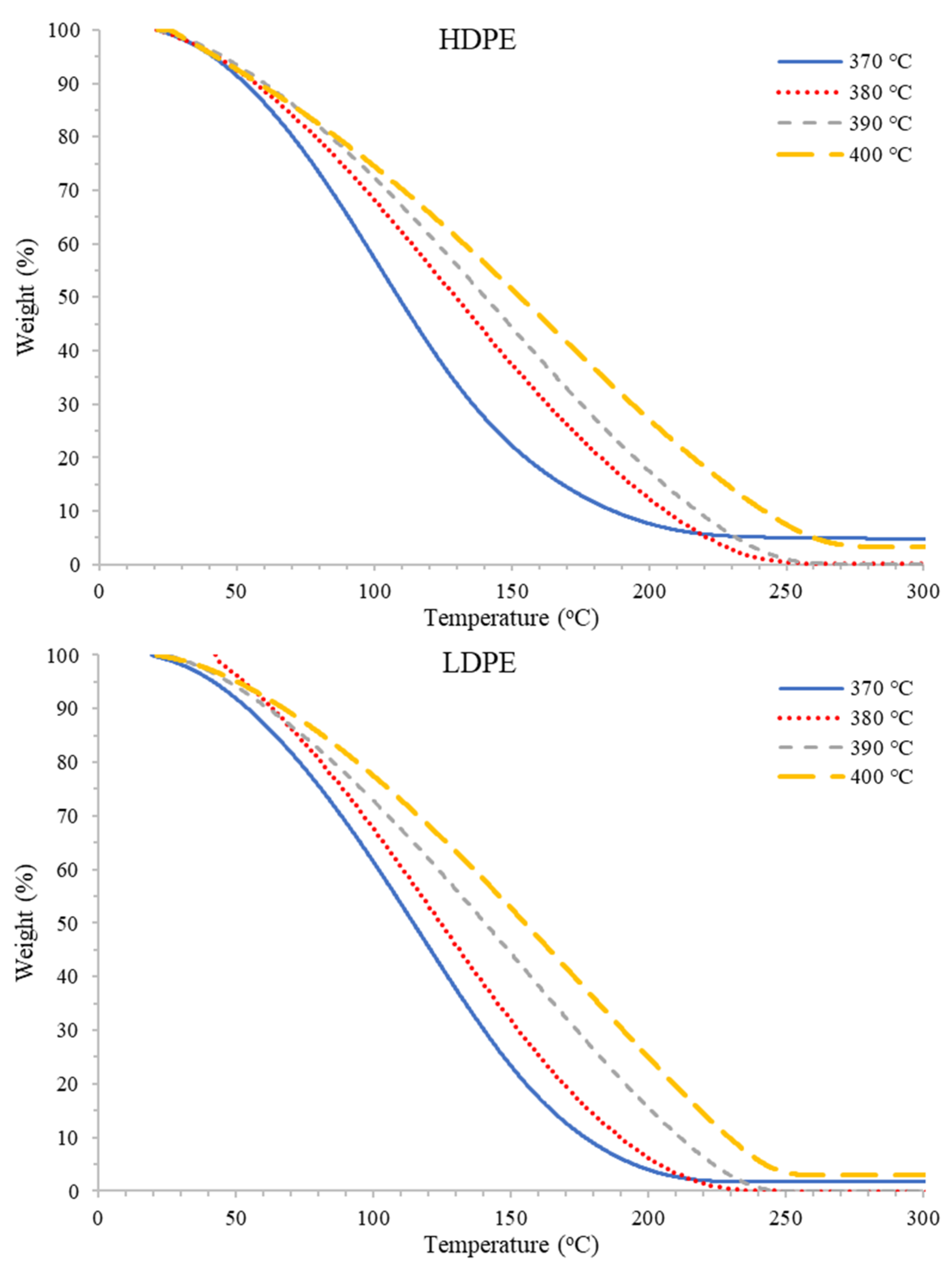
Figure 4.
Relative abundances of C8–C30 hydrocarbons for PDFO as a function of temperature for HDPE waste (top) and LDPE waste (bottom) as determined from GC-MS analysis.
In Figure 4, the percent relative abundance of predominantly present hydrocarbons for HDPE and LDPE shifts from left to right as temperature increases. For instance, the peak of the curves shifts from C12 at 370 °C to C15 at 400 °C for both plastics. For the two inner temperatures of 380 °C and 390 °C, percent relative abundance shifts incrementally towards 400 °C. Table 1 summarizes the predominant hydrocarbons present in PDFO derived from each plastic at the two outer temperatures. As the temperature increases, the fraction of heavier hydrocarbons in the PDFO composition increases. This phenomenon may be attributed to the boiling point of heavier hydrocarbons, which vaporize at higher temperatures. For reference, the literature reported diesel and kerosene hydrocarbon ranges which are also reported in Table 1 [60]; however, these ranges vary widely in the literature; diesel hydrocarbon ranges have been reported from C7–C24 and C8–C17 for kerosene [62,63,64,65].

Table 1.
Summary of predominant hydrocarbons in PDFO as a function of temperature and plastic type.
3.1.2. TGA Results
Representative TGA thermograms for each PDFO thermal degradation as a function of temperature and waste plastic source are presented in Figure 5. As temperature increased for all plastics analyzed under this study, an increase in thermal stability, or a decrease in volatility, of the PDFO was observed. These results suggest that as the pyrolysis temperature increases, heavier and longer hydrocarbon chains are produced and distilled during the chain scission mechanism [62].
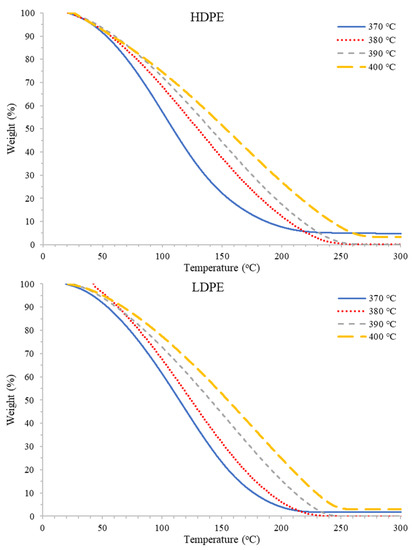
Figure 5.
Representative TGA thermograms for PDFO for HDPE waste (top) and LDPE waste (bottom) as a function of temperature.
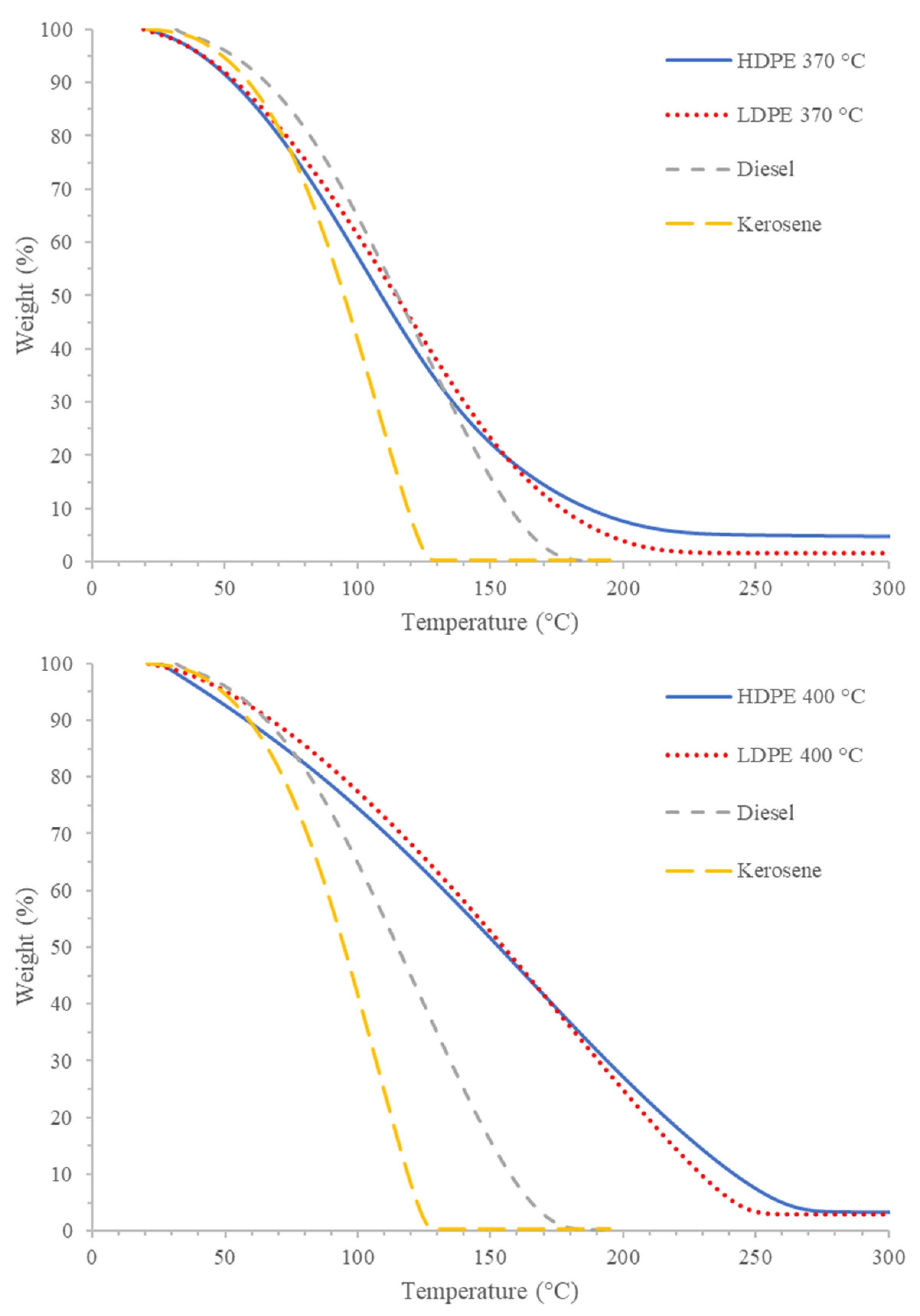
Figure 6 depicts the comparison of PDFO thermal degradation with that of diesel and kerosene. In general, the trend observed in terms of stability is kerosene < diesel < PDFO. However, at lower temperatures of 370 °C, the rate of weight loss for PDFO is more similar to diesel and kerosene than at higher temperatures of 400 °C. This further alludes to the increased presence of longer chain hydrocarbons present in PDFO at increased temperatures.
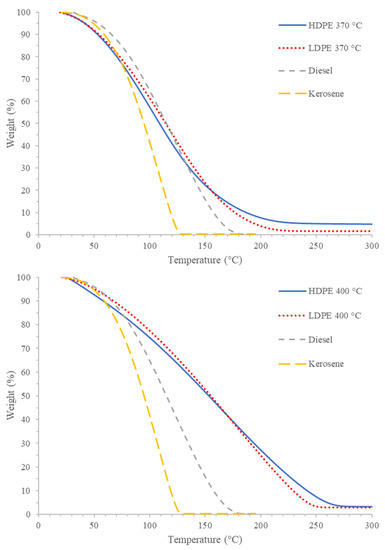
Figure 6.
Representative TGA thermograms of PDFO at 370 °C (top) and 400 °C (bottom) with diesel and kerosene.
The higher stability of PDFO also leads to increased fuel quality, or the reduction in the degradation of the fuel at ambient conditions due to polymerization, acidity, oxidation, emulsion, and microorganism infestation [66]. However, as with diesel combustion, the efficiency of PDFO combustion is a function of the engine technology [62]. Additionally, the CG-MS results confirmed that no sulfur-containing compounds were present in the product, meaning that no oxides of sulfur were emitted during combustion.
3.2. Effect of Time
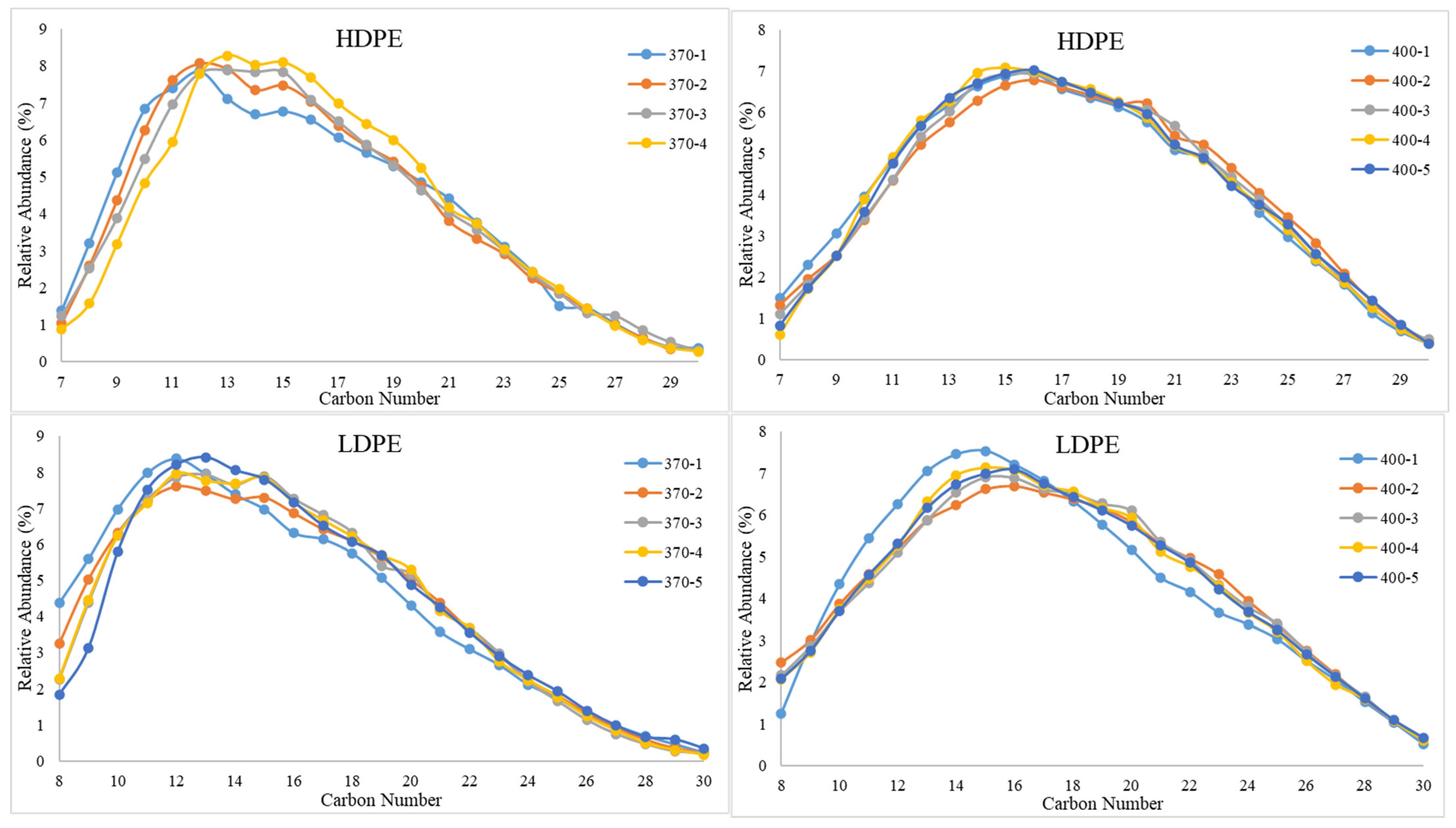
The GC-MS results for studying the impact of sample retention time on composition are presented in Figure 7. The results portray that sample retention time did not have a significant impact on composition. Except for HDPE at 400 °C, the peak hydrocarbon chain length increases by approximately 1–2 carbon numbers from the initial time increment to the final time increment. This implies that slightly heavier hydrocarbons are exiting the reactor at increased run times. However, a clear visible shift in composition is only noticed as temperature increases, that is the peak hydrocarbon chains for all sample run times shifting from approximately C12 to C16 and as temperature increases from 370 °C to 400 °C. As for the TGA results for the effect of sample retention time on the stability of PDFO, no significant changes in thermal stability were observed as sample retention time increased.
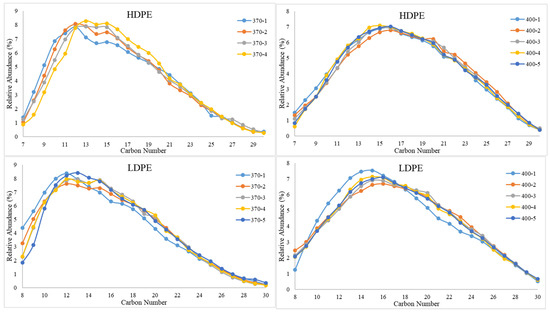
Figure 7.
Relative abundances of C8–C30 hydrocarbons for PDFO as a function of temperature and sample time for HDPE waste (top) and LDPE waste (bottom). Note, sample naming convention, “Temperature-Sample Number”; i.e., “370-1” indicates the first sample collected at 370 °C. Samples were collected every hour.
3.3. Discussion
The results obtained from the GC-MS analyses indicated a significant shift from shorter hydrocarbon chain lengths to longer hydrocarbon chain lengths as the temperature increased. Shorter hydrocarbon chain lengths indicate a fuel oil product more similar to kerosene while longer chain hydrocarbon lengths would indicate a product more similar to diesel fuel. This indicates that operating temperatures nearer to 370 °C can be used to generate PDFO similar in composition to cooking fuel or lamp oil, while temperatures nearer to 400 °C can be used to generate PDFO similar in composition to a diesel fuel alternative. In a developing world setting where appropriate technology-based solutions are preferred, temperature is an effective control variable. Of course, adding reflux capabilities will result in a narrower range of hydrocarbon chain lengths; however, this is difficult to achieve using appropriate technology given the constraints of costs and simplicity.
The results from the TGA studies further confirmed that PDFO produced from this method resulted in a wider boiling point range than traditional fuels. Although the impacts of this wider boiling point range on the operation of diesel engines will require further study, the use of PDFO as an alternative to kerosene in cookstoves or lamps would be unlikely to result in adverse effects.
Additionally, the results from the analysis of PDFO produced at varying processing times indicated that the fuel composition remained relatively constant over the entire operating time. This is a significant finding for appropriate technology applications as it indicates that the operator need not be concerned with reaction time durations and can be assured of a consistent PDFO composition output, in case the slow pyrolysis process needed to be stopped before the end of a run. In a developing world setting, this implies that the early termination of a run will not be problematic on the quality of PDFO production due to electricity shortages or intermittent supply of electricity when operating the 3T Processor (depicted in Figure 1 and Figure 2).
4. Conclusions
This research contribution considered the impact of temperature and sample collection time as two variables affecting the composition and stability of PDFO derived from waste HDPE and LDPE using slow pyrolysis. The results indicated that temperature had a significant contribution on PDFO composition and can be used as an effective control variable in appropriate technology-based applications. The effect of sample retention time on composition was determined to be minimal, which means that early batch stoppage will not affect product consistency.
Slow pyrolysis provides an effective and simple method for eliminating plastic waste accumulation. By temperature tuning of the slow-pyrolysis reaction, conversion of waste plastic into products with properties similar to traditional kerosene or diesel fuel is possible. This discovery is especially beneficial for promoting the use of slow-pyrolysis-type appropriate technologies in the developing world to improve management of plastic waste and to generate valuable fuel products that can be directly used by the local communities.
Author Contributions
Conceptualization, J.R.S. and C.J.J.; methodology, C.J.J. and K.M.M.; software, K.M.M.; validation, C.J.J., K.M.M. and J.R.S.; formal analysis, C.J.J. and K.M.M.; investigation, C.J.J., K.M.M. and J.R.S.; resources, J.R.S. and K.M.M.; data curation, C.J.J. and K.M.M.; writing—original draft preparation, C.J.J.; writing—review and editing, J.R.S. and K.M.M.; supervision, J.R.S.; project administration, J.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw GC/MS data available from corresponding author.
Acknowledgments
The contribution of the University Appropriate Technology Research Team; Murray State University, Department of Chemistry and the Polymer and Materials Science Laboratory; and the individuals, Emily Garner, Reem Turkmani, Matthew Gilbert, Hemisha Joshi, and Ankit Jangid are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalanatarifard, A.; Yang, G.S. Identification of the Municipal Solid Waste Characteristics and Potential of Plastic Recovery at Bakri Landfill, Muar, Malaysia. J. Sust. Dev. 2012, 5, 11. [Google Scholar] [CrossRef]
- Minghua, Z.; Xiumin, F.; Rovettac, A.; Qichang, H.; LiuBingkai, F.V.; Giustic, A.; Yi, L. Municipal solid waste management in Pudong New Area, China. Waste Manag. 2009, 29, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.R.A.; Mokhtarani, N.; Mokhtarani, B. Municipal solid waste management in Rasht City, Iran. Waste Manag. 2009, 29, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Mrayyan, B.; Hamdi, M.R. Management approaches to integrated solid waste in industrialized zones in Jordan: A case of Zarqa City. Waste Manag. 2006, 26, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Seng, B.; Kaneko, H.; Hirayama, K.; Katayama-Hirayama, K. Municipal solid waste management in Phnom Penh, capital city of Cambodia. Waste Manag. Res. 2010, 29, 491–500. [Google Scholar] [CrossRef]
- Sujauddin, M.; Huda, M.S.; Hoque, R.A.T.M. Household solid waste characteristics and management in Chittagong, Bangladesh. Waste Manag. 2008, 28, 1688–1695. [Google Scholar] [CrossRef]
- Troschinetz, A.M.; Mihelcic, J.R. Sustainable recycling of municipal solid waste in developing countries. Waste Manag. 2009, 29, 915–923. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos.Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Barnes, D.K.; Walters, A.; Goncalves, L. Macroplastics at sea around Antarctica. Mar. Environ. Res. 2011, 70, 250–252. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Jayasiri, H.B.; Purushothaman, C.S.; Vennila, A. Quantitative analysis of plastic debris on recreational beaches in Mumbai, India. Mar. Poll. Bull. 2013, 77, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, J. Plastic Not-So-Fantastic: How the Versatile Material Harms the Environment and Human Health; Scientific American: New York, NY, USA, 2019; Available online: https://www.scientificamerican.com/article/plastic-not-so-fantastic/ (accessed on 15 May 2021).
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Parker, L. In a First, Microplastics Found in Human Poop; National Geographic Magazine: Washington, DC, USA, 2018; Available online: www.nationalgeographic.com (accessed on 15 May 2021).
- Parker, L. Plastics. Planet or Plastic? Natl. Geogr. 2018, 40–91. [Google Scholar]
- Rochman, C.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Swee Teh, S.; Thompson, R.C. Policy: Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef]
- Wieczorek, A.M.; Morrison, L.; Croot, P.L.; Allcock, A.L.; MacLoughlin, E.; Savard, O.; Brownlow, H.; Doyle, T.K. Frequency of microplastics in mesopelagic fishes from the Northwest Atlantic. Front. Mar. Sci. 2018, 5, 39. [Google Scholar] [CrossRef]
- Wilcox, C.; Sebille, E.V.; Hardesty, B.D. Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proc. Natl. Acad. Sci. USA 2015, 112, 11899–11904. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and Recovery Routes of Plastic Solid Waste (PSW): A Review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- DeNeve, D.; Joshi, C.; Higgins, J.; Seay, J. Optimization of an Appropriate Technology Based Process for Converting Waste Plastic in to Liquid Fuel via Thermal Decomposition. J. Sust. Dev. 2017, 10, 116. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K. Recovery of Hydrocarbon Liquid from Waste High Density Polyethylene by Thermal Pyrolysis. Braz. J. Chem. Eng. 2011, 28, 659–667. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Deak, G.; Jover, B. Thermal Degradation of Municipal Plastic Waste for Production of Fuel-Like Hydrocarbons. Polym. Degrad. Stab. 2004, 86, 357–366. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.K.; Mishra, D.K. Thermolysis of Waste Plastics to Liquid Fuel. A Suitable Method for Plastic Waste Management and Manufacture of Value Added Products—A World Prospective. Renew. Sustain. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Patil, L.; Varma, A.K.; Singh, G.; Mondal, P. Thermocatalytic Degradation of High Density Polyethylene into Liquid Product. J. Polym. Environ. 2017, 26, 1920–1929. [Google Scholar] [CrossRef]
- Pinto, F.; Costa, P.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of plastic wastes. 1. Effect of plastic waste composition on product yield. J. Anal. Appl. Pyrolysis 1999, 51, 39–55. [Google Scholar] [CrossRef]
- Santaweesuk, C.; Janyalertadun, A. The Production of Fuel Oil by Conventional Slow Pyrolysis Using Plastic Waste from a Municipal Landfill. Int. J. Environ. Sci. Dev. 2017, 8, 168–173. [Google Scholar] [CrossRef]
- Sarker, M. Converting waste plastic to hydrocarbon fuel materials. Energy Eng. 2011, 108, 35–43. [Google Scholar] [CrossRef]
- Sarker, M.; Molla, M.; Rashid, M.M.; Rahman, M.S. Production of Valuable Heavy Hydrocarbon Fuel Oil by Thermal Degradation Process of Post-Consumer Municipal Polystyrene (PS) Waste Plastic in Steel Reactor. Energy Power 2012, 2, 89–95. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B. Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel 2016, 174, 164–171. [Google Scholar] [CrossRef]
- Wong, S.I.; Ngadia, N.; Abdullahb, T.A.T.; Inuwac, I.M. Current State and Future Prospects of Plastic Waste as Source of Fuel: A Review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Joshi, C.A.; Seay, J.R. An appropriate technology based solution to convert waste plastic into fuel oil in underdeveloped re-gions. J. Sustain. Dev. 2016, 9, 133–143. [Google Scholar] [CrossRef]
- Joshi, C.; Seay, J. Building momentum for sustainable behaviors in developing regions using Locally Managed Decentralized Circular Economy. Chin. J. Chem. Eng. 2019, 27, 1566–1571. [Google Scholar] [CrossRef]
- Joshi, C.A.; Seay, J.R. Total generation and combustion emissions of plastic derived fuels: A trash to tank approach. Environ. Prog. Sustain. Energy 2019, 39. [Google Scholar] [CrossRef]
- Joshi, C.; Seay, J.; Banadda, N. A Perspective on a Locally Managed Decentralized Circular Economy for Waste Plastic in Developing Countries. Environ. Prog. Sustain. Energy 2019, 38, 3–11. [Google Scholar] [CrossRef]
- Joshi, C.; Browning, S.; Seay, J. Combating plastic waste via Trash to Tank. Nat. Rev. Earth Environ. 2020, 1, 142. [Google Scholar] [CrossRef]
- Schumacher, E.F. Small Is Beautiful: Economics as If People Mattered; Harper & Row: New York, NY, USA, 1973. [Google Scholar]
- Budsaereechai, S.; Hunt, A.J.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv. 2019, 9, 5844–5857. [Google Scholar] [CrossRef]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, Ε.V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef]
- Chandrasekaran, S.R.; Kunwar, B.; Moser, B.R.; Rajagopalan, N.; Sharma, B.K. Catalytic thermal cracking of postconsumer waste plastics to fuels. 1. Kinetics and optimization. Energy Fuels 2015, 29, 6068–6077. [Google Scholar] [CrossRef]
- Kunwar, B.; Chandrasekaran, S.R.; Moser, B.R.; Deluhery, J.; Kim, P.; Rajagopalan, N.; Sharma, B.K. Catalytic thermal cracking of postconsumer waste plastics to fuels. 2. Pilot-scale thermochemical conversion. Energy Fuels 2017, 31, 2705–2715. [Google Scholar] [CrossRef]
- Liu, S.; Kots, P.A.; Vance, B.C.; Danielson, A.; Vlachos, D.G. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 2021, 7, eabf8283. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Ismail, I.M.I.; Nizami, A.S. Plastic waste to liquid oil through catalytic pyrolysis using natural and synthetic zeolite catalysts. Waste Manag. 2017, 69, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic pyrolysis of plastic waste: Moving toward pyrolysis based biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Aboulkas, A.; Nadifiyine, M. Investigation on pyrolysis of Moroccan oil shale/plastic mixtures by thermogravimetric analysis. Fuel Process. Technol. 2008, 89, 1000–1006. [Google Scholar] [CrossRef]
- Rahman, S. Pyrolysis of Waste Plastic Fish Bags to Useable Fuel Oil; Harris Centre, Memorial University: St. John’s, NL, Canada, 2018. [Google Scholar]
- Zhou, L.; Wang, Y.; Huang, Q.; Cai, J. Thermogravimetric characteristics and kinetic of plastic and biomass blends co-pyrolysis. Fuel Process. Technol. 2006, 87, 963–969. [Google Scholar] [CrossRef]
- Phetyim, N.; Pivsa-Art, S. Prototype co-Pyrolysis of used lubricant oil and mixed plastic waste to produce a diesel-like fuel. Energies 2018, 11, 2973. [Google Scholar] [CrossRef]
- Breyer, S.; Mekhitarian, L.; Rimez, B.; Haut, B. Production of an alternative fuel by the co-pyrolysis of landfill recovered plastic wastes and used lubrication oils. Waste Manag. 2017, 60, 363–374. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Zhou, L.; Huang, Q. Thermogravimetric analysis and kinetics of coal/plastic blends during co-pyrolysis in nitrogen atmosphere. Fuel Process. Technol. 2008, 89, 21–27. [Google Scholar] [CrossRef]
- Xing, X.; Wang, S.; Zhang, Q. Thermogravimetric analysis and kinetics of mixed combustion of waste plastics and semicoke. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Boadu, E.K.; Dapaah, S. Plastic waste to fuel via pyrolysis: A key way to solving the severe plastic waste problem in Ghana. Therm. Sci. Eng. Prog. 2019, 11, 417–424. [Google Scholar] [CrossRef]
- Akoueson, F.; Chbib, C.; Monchy, S.; Paul-Pont, I.; Doyen, P.; Dehaut, A.; Duflos, G. Identification and quantification of plastic additives using Pyrolysis-GC/MS: A review. Sci. Total Environ. 2021, 773, 145073. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Himber, C.; Boricaud, B.; Kazour, M.; Amara, R.; Cassone, A.-L.; Laurentie, M.; Paul-Pont, I.; Soudant, P.; Dehaut, A.; et al. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal. Bioanal. Chem. 2018, 410, 6663–6676. [Google Scholar] [CrossRef] [PubMed]
- Agboola, O.; Sadiku, R.; Mokrani, T.; Amer, I.; Imoru, O. Polyolefins and the environment. In Fibres; Woodhead Publishing: Cambridge, UK, 2017; pp. 89–133. [Google Scholar]
- CROW. Depolymerization, Polymer Properties Database. Crow Polymer Science. 2021. Available online: http://polymerdatabase.com/home.html (accessed on 17 June 2021).
- Zeus®. Technical Whitepaper: Thermal Degradation of Plastics; Zeus Industrial Products, Inc.: Orangeburg, SC, USA, 2005. [Google Scholar]
- U.S. Department of Health and Human Services. Toxicological Profile for Fuel Oil, Chapter 3, Chemical and Physical Information; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1995; pp. 105–109. [Google Scholar]
- Rentar Fuel Catalyst. What Are Hydrocarbons and What Hydrocarbons Are in Diesel? 19 April 2018. Available online: https://rentar.com/hydrocarbons-hydrocarbons-diesel/#:%7E:text=%E2%80%9CPetroleum-derived (accessed on 15 May 2021).
- Gad, S.C. Kerosene. Encycl. Toxicol. 2005, 2, 664–665. [Google Scholar]
- International Energy Association—Advanced Motor Fuels (IEA-AMF). Diesel and Gasoline. Available online: https://www.iea-amf.org/content/fuel_information/diesel_gasoline (accessed on 15 May 2021).
- ALS Global. Petroleum Fractions by Carbon Range. 2021. Available online: www.alsglobal.com (accessed on 15 May 2021).
- Gad, S.C. Diesel Fuel. Encycl. Toxicol. 2014, 3, 115–118. [Google Scholar]
- CorrosionpediaTM. Fuel Stability. Janatta Interactive. January 2018. Available online: https://www.corrosionpedia.com/definition/1644/fuel-stability (accessed on 15 May 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).