Abstract
The treatment of used vegetable oils (UVOs) with clays represents a pivotal step in their industrial recycling process as well as one of the most challenging topics for researchers. In particular, cheap, effective, and sustainable powders need to be explored in order to develop new processes which produce beneficial results in relation to economic and environmental aspects. In this context, five samples within commercial and waste vegetable oils were treated with two sodium- and calcium-based bentonites employing a low oil/bentonite ratio (0.15 wt%). The outcomes of the processes were monitored by FT-IR spectroscopy and compared with the data relative to the parent commercial edible oil. In particular, treatment of FT-IR data by multivariate statistical analysis allowed us to determine a chemical fingerprint characteristic of each sample. Important relationships between the overall chemical composition and the specific clay employed and the treatment time (2 or 4 h) were highlighted. Finally, N2 physisorption, TEM microscopy, and FT-IR analyses of the more efficient Na bentonite allowed us to characterize the material and thus to furnish all the information needed to set-up a general protocol for the partial regeneration of waste vegetable oil destined for further processing.
1. Introduction
The awareness of the importance of recycling wastes arising from urban areas has grown exponentially during the last years. In order to comply with the international targets of sustainability and CO2 emission, it is pivotal to apply circular economy models to the development of new processes and to the reconversion of existing ones [1,2]. In a circular economy, the employment of wastes as raw materials is fundamental [3,4,5] and, thus, many recent national and international legislative efforts have been directed to the endorsement of this practice [6].
In this context, waste oils, especially vegetal ones, are ideal candidates as raw materials for circular processes [7]. In fact, used vegetable oils (UVOs) are produced in large amounts worldwide, are widely diffuse, and easy to recycle. Additionally, from the processing of UVOs it is possible to obtain many chemicals with applications in several market segments [8]: from energy (burning), transport (biodiesel) [9,10], industry (lubrication), food (edible ingredients), cosmetics [11], constructions [12], to bioplastics.
Waste vegetable oils are constituted in large part by cooking oils from private kitchens and catering industries, and in minor percentage by animal fats and greases [13]. In the European Union, UVOs are considered urban wastes and they are registered in the European catalogue of wastes under the code 19 08 09 (edible oils and fats) [14]. From the chemical point of view, UVOs are made by a mixture of fatty acids (mainly linoleic, oleic, and linolenic) [15] with a variable degree of esterification and oxidation, contaminated by metals, food residues, and other particles [16,17]. Nowadays, UVOs are collected, stored and finally periodically delivered to a recycling plant, which in 90% of the cases produces biodiesel [18]. After collecting and before storage, the waste is usually treated in order to provide the recycling facility with a raw material of acceptable quality. This preliminary treatment is mainly directed to the adjustment of the total acidity, which must be more than 3–3.5 units to avoid undesired side reactions during the further esterification step (biodiesel production), and for the physical elimination of particles, including metal traces. Thus, filtration of synthetic and natural powders represents the procedure of choice for UVOs first treatment. Actually, clay minerals have been used for the clarification and bleaching [19] of oil samples for many years [20], resulting very effective in removing grease and impurities [21]. Clay minerals are cheap and available in many territories worldwide and their properties can be modified by proper activation protocols [22,23].
Despite the important advancement of the knowledge and related technology for the recycling of UVOs, filtration of porous materials still represents the best industrial procedure for recycling and purifying waste vegetable oils, restoring the colour, density, viscosity, and removing inorganic and solid contaminants. Recently, it was reported that waste oils treated with bentonites subjected to different pre-treatments show a different variation of their chemical composition, as different chemical groups are retained [24]. This specific affinity of selected clays for different chemical groups can be also exploited in other research fields, e.g., in the treatment of wastewaters [25,26].
In this context, many kinds of bentonites are available worldwide, which show different microstructural characteristics and effectiveness in purifying used vegetable oils, thus many experimental data are necessary in order to assess general trends aimed to develop new and more effective processes.
With the aim to extend the current portfolio of cheap and sustainable clays for UVOs processing, the regeneration of model UVO by treatment with Na- or Ca-based Algerian bentonites is herein reported. A spectroscopic characterization by meaning of FT-IR was conducted on both clays and oil samples, prior to and after treatment. The variation of some additional physical parameters, such as conductivity, salinity, pH, turbidity, and density in the oil samples, was also monitored and discussed. Microstructural features of Na clays, which showed better results, were further characterized by N2 physisorption and TEM microscopy.
2. Results and Discussion
2.1. FT-IR and Principal Component Analysis
Samples of UVO were partially regenerated by processing with Na (UVO Na filtered) or Ca (UVO Ca filtered) clays. The process was monitored by FT-IR after 2 and 4 h and the results compared with the spectra of the parent commercial Elio oil and the raw UVO (Figure 1).
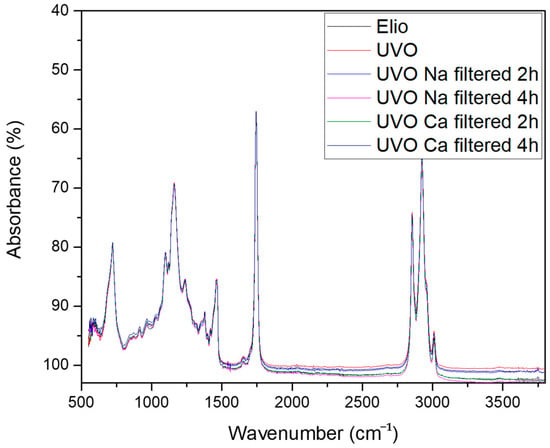
Figure 1.
FT-IR spectra of edible oil Elio, Used Vegetable Oil (UVO), and UVO filtered, respectively) on Na and Ca clays (2 and 4 h).
From a visual and qualitative analysis of the FT-IR profiles reported in Figure 1, it is possible to detect the typical major constituents of vegetable oils. According to Alexa and coworkers [27], it is possible to detect the symmetrical and asymmetrical vibrations ν(C-H) of the CH2 and CH3 aliphatic groups from the alkyl rest of the triglycerides between 3100 and 2800 cm−1, the absorption relative to the presence of saturated fatty acids at about 1750 cm−1, and the vibration bands related to the -CH2- and =CH2 groups between 1600 and 1400 cm−1. Finally, C=O vibrations (1150–1060 cm−1), C-C bond (from 1100 to 800 cm−1), C=O bond (970–800 cm−1), and carbohydrate chain vibrations (under 450 cm−1) can be appreciated.
As expected, from a visual inspection of the FT-IR spectra it is not possible to distinguish between edible, waste, and treated samples. This is due to the fact that the changes in the chemical composition of a vegetable oil prior and after frying are really minimal and the overall nature and distribution of the fatty acids profile becomes almost identical [28]. Even the regeneration of UVOs by treatment with clays did not change the overall chemical profile. In fact, it retains solids and small particles (as metal traces), and can affect the distribution of the volatile fraction [24], which is difficult to observe by qualitative FT-IR. Nevertheless, most of the information is hidden in the spectra raw data and it is possible to extract it by opportune multivariate analysis for building a chemical fingerprint characteristic of each type of oil. This technique has been already applied to GC-MASS [26], X-ray [29], NMR [30], FT-IR, and UV-VIS [31] raw data of vegetable oils.
In this context, Analysis of the Variance (ANOVA) and a Principal Component Analysis (PCA) was performed in order to determine the distances between each family of samples (Figure 2).
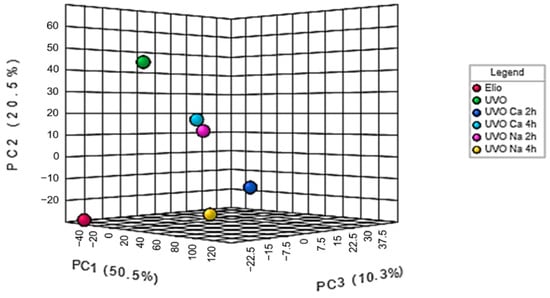
Figure 2.
3D PCA plot of edible, waste, and treated oil samples.
From the 3D PCA plot reported in Figure 2, it is possible to notice that all the samples turn out to be different, indicating the possibility of classifying the considered oils on the basis of the FT-IR profile. Edible Elio oil can be located in the down-left corner of the plot, far away from waste UVO (up-left), indicating that the frying process has affected the chemical composition of the vegetable oil. Regarding the treated samples, UVO treated for 2 h with Na clay or for 4 h with Ca clay seem to have a similar fingerprint, while between the samples processed for 4 h with Na clay and for 2 h with Ca clays, the first one appears as more similar to the original edible oil (Figure 2, yellow spot).
Additional FT-IR spectra were registered after 24 h of treatment as reported in the Supplementary Material (Figure S1). In that case, no relevant changes were observed with respect to the spectra recorded after 4 h, confirming the completion of the treatment in short times. This indicates that after 4 h of processing, even small amounts of Na-bentonite (0.15 wt%) can be effective in modifying the chemical composition of the waste vegetable oil.
Taking into consideration the PCA outcomes, Na clay was selected as reference material for a more detailed study (Section 3.1).
As the FT-IR analyses revealed a different fingerprint related to samples treated with different bentonites or even in different conditions, a detailed analysis of some physical properties of the oil prior and after treatment was performed. In particular, the pH, conductivity, TDS, turbidity, and density were measured for untreated and treated samples (Table 1).

Table 1.
Physicochemical parameters for samples of edible oil (EO), UVO, Na, and Ca treated oils.
The aim of the physicochemical analyses was to determine which conditions were more suitable for recycling waste vegetable oil samples for further industrial applications.
From the data reported in Table 1 it is possible to compare the performances of Na- and Ca-based bentonites. The first relevant aspect regards the variation of the pH, which ranges between about 5 and 5.5 points for edible and waste oils and increases between 1 and 1.5 units after treatment with clays. The lower values of pH observed after 4 h with respect to the ones registered after 2 h indicates that the system has reached an equilibrium in some point between in this window of time (2–4 h). Considering the basic nature of the clays herein used (10.8 for Na- and 9.88 for Ca-based ones), the protocol herein reported can be successful employed for acidic waste oils which cannot be delivered to a pilot plant if the pH value results lower than 3 units.
The increment of polar compounds and chemical groups in the treated oil is also reflected by the increment of the conductivity after 2 h, which seems stabilized after 4 h and shows even lower values that the ones observed in the edible and waste samples (0.8 for Na and 0.9 for Ca, respectively, against 0.1 of EO and UVO).
The increasing of TDS (both after 2 and 4 h) reveals the formation of dissolved matters in the analyzed samples. However, the measurements of the salinity suggest the absence of dissolved salts, indicating the non-ionic nature of the TDS. Regarding the turbidity, it increases to 69 NTU after frying, in agreement with the low transparency of the waste oil observed by a visual inspection. Probably, leaching from food and oxidation of the oil fatty acids produce and waxes and esters with high molecular weight. On the other side, the turbidity value decreased in the treated oils and after 4 h of treatment it results closer to the initial value relative to the oil before frying.
Regarding the density, which is affected by temperature, it decreases from 0.938 to 0.905, according to the observation of Sahasrabudhe and coworkers [31].
The analysis of the physical parameters reported in Table 1 confirm the preliminary indications obtained by the multivariate analysis based on the FT-IR data. In fact, considering the low amount of bentonite employed in each experiment (clay/oil ratio 0.15 wt%), the variation of the chemical composition, and thus of the physical parameters, obtained after 2 h and consolidated after 4 h, is excellent for a partially recycled vegetable oil.
2.2. Characterization of Na-Based Clay
The analysis of the chemical fingerprints of the partially regenerated oils, as well the variation of the physical parameters (especially turbidity) reported in Table 1 and discussed in the previous paragraph, highlighted the best performance of the Na-clay. As the bentonites herein tested are not commercially available, a characterization of the Na-clay used would facilitate the understanding of which microstructural characteristics are requested in order to reach the recycling performances reported. With this aim, N2-physisorption analysis and a TEM study were conducted. Bentonite pertains to the family of montmorillonite and thus it is characterized by the presence of Mg and Al as dominant components. The typical montmorillonite structure is organized in a series of layers made of a sandwich of one octahedral alumina sheet between two tetrahedral silica sheets (TOT) [26]. A random number of the tetrahedral Si atoms it is usually replaced by Al or Mg and/or by elements with a lower oxidation number. This arrangement produces a surface negatively charged which is balanced by the presence of inorganic cations as Na+ and Ca2+ located in the interlayer spaces [32]. Usually, the nature of these cations as well as the resulting pore size determine the behaviour of the specific bentonite and its application [33,34].
The bentonite herein employed was not modified or activated in any way, so a chemical-physical characterization was conducted in order to gain some knowledge about its structural features.
Samples of Na clay were subjected to N2 physisorption analysis. The considered samples exhibit a type IV isotherm and H2-isotherm loop, typical characteristic of mesoporous materials. Na clay shows a surface area of about 20 m2/g and a pore size distribution centred about at 4 nm (Figure 3).
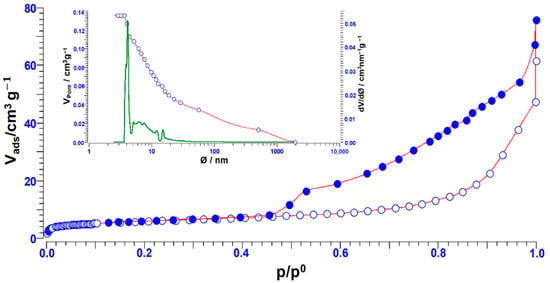
Figure 3.
N2 adsorption/desorption isotherm of Na clays.
TEM pictures (Figure 4) were used to elucidate the microstructure characteristics of the Na clays, which as expected were found to be unstructured. It is possible to observe that the clay layers are aligned in a parallel manner with the expansion of the interlayer space. The elementary analysis with EDAX data, reported in Table 2, highlights the presence of Na alkaline metal in the type of clay.
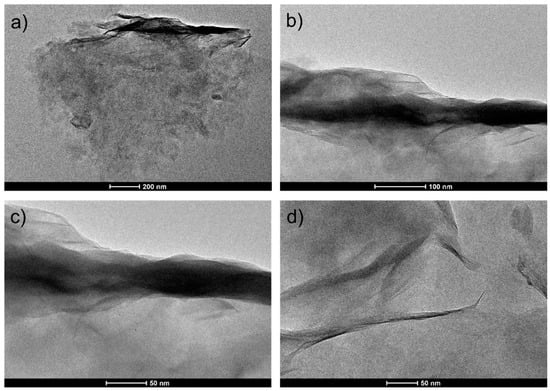
Figure 4.
Representative transmission electron microscopy images at different magnifications (a–d) of Na clays.

Table 2.
EDAX data for Na clays.
Finally, FT-IR spectroscopy was used to characterize the main chemical groups of Na clays and to determine any variation induced during the oil treatment (Figure 5).
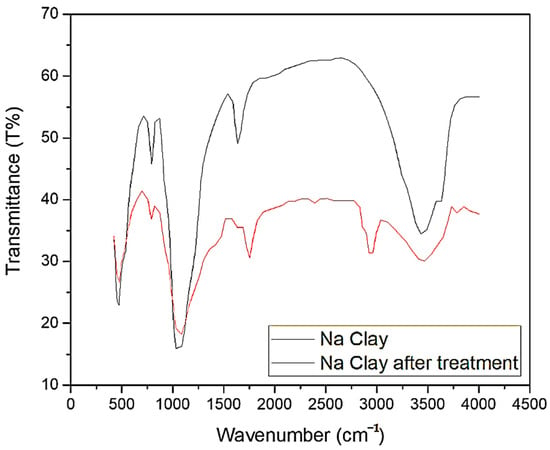
Figure 5.
FT-IR of Na clay prior and after the UVO treatment (4 h).
The signals corresponding to the main chemical groups present in bentonite samples are in agreement with the same analysis performed on similar samples of Sardinian bentonite [35]. In particular, the typical bending vibration peak at 917 cm−1 (Al-Al-OH), 519 cm−1 (Si-O-Al), and 419 cm−1 (Si-O) confirm the montmorillonite structure of the bentonite [36]. Additionally, stretching of the –OH group of intra-layers water can be observed respectively at 3436 cm−1 and 1636 cm−1. Additional FT-IR spectra of the Na clays after 24 h of treatment are reported in the Supplementary Material (Figure S1).
3. Materials and Methods
3.1. Raw Materials
Commercial edible frying oil Elio brand (80% soya and 20% sunflower) form Algerian market (ELIO) was analysed and after that used for 10 cycles of deep frying, generating the samples of UVO herein discussed.
Two types of clays (sodium, Na, and calcium, Ca) were brought from a natural cave in the Maghnia region in Western Algeria, by National Company of Non-Ferrous Mining Products (ENOF).
3.2. Physico Chemical Analyses
Conductivity meter Inolab type (from German manufacturer represented, in Algeria, by the commercial company HEXX) was employed for measuring the conductivity, salinity, and TDS (total dissolved solids). Electrical conductivity characterizes the ability of a material or a solution to allow electrical charges to move freely and therefore allow the passage of an electrical current. Salinity refers to the quantity of salts dissolved in a liquid, in particular water, which is a powerful solvent for many minerals. Salinity should not be confused with the hardness of the water, which is related to its dosage of calcium and magnesium. Total dissolved solids (TDS) represents the total concentration of substances dissolved in water. pH meter Inolab Level 1 brand (from Germany manufacturer represented, in Algeria, by the commercial company HEXX), with a digital display fitted with a combined glass electrode was used for determining the pH of the samples.
Turbidimeter HACH DR/800 Colorimeter brand (from Germany manufacturer represented, in Algeria, by the commercial company HEXX) used to measure the turbidity which refers to the content of a fluid in matters which disturb it.
Density was determined by the classical volumetric method as the ratio between the density (ρ) of the oil and the water (Equation (1)).
d = ρ(oil)/ρ(water) with ρ(oil) = m/v
Fourier Transform Infrared spectrometer Perkin Elmer type (Model SPECTRUM 100, from USA manufacturer represented, in Algeria, by the commercial company HTDS) was used to characterize oils and clays before and after processing, as well as the bentonite samples.
N2 sorption isotherms were collected on a Sorptomatic 1990 instrument (Fisons Instruments, Thermo Instrument Systems Inc., Waltham, MA, USA) and the data were analysed according to BET and BJH methods [37,38]. The following experimental procedure was used: 200 mg of sample were located in a quartz tube and degassed under high vacuum (1 × 10−3 bar) at 250 °C for 24 h and the residual volume was evaluated through helium before the measurements.
The transmission electron microscopy (TEM) was performed on a Fei Technai 200 kV instrument (Thermo Fisher Scientific, Waltham, MA, USA) operated at an accelerating voltage of 200 kV. EDAX (energy dispersive X-ray spectroscopy) spectrum was recorded for elementary analysis. The analysis of TEM data and images was carried out using Digital Micrograph (Gatan, Inc., Pleasanton, CA, USA) and TIA software (Thermo Fisher Scientific, Waltham, MA, USA).
3.3. Experimental Protocol
On a laboratory scale, about 100 g of UVO was mixed with 15 mg of Na or Ca clay and the mixture stirred for 2 h and 4 h. The ratio clay/waste oil was 0.15 wt% which is about ten times less than the usual amount employed for vegetable oil bleaching (1–3%) [39]. Regarding the processing timing, considering the low clay/oil ratio, short treatments (30 min or 1 h) were not considered. The resulting product was filtered on a funnel equipped with a small cotton portion. Then, the processed oil was analysed without any further treatment while the residual clay was dried before analysis.
4. Conclusions
Processing of vegetable oils (both edible and waste) by treatment with natural clays represents a pivotal step in several industrial processes. The effectiveness of the procedure combined with the wide availability of cheap and sustainable natural clays represents an incentive to the developing of optimized treatments. A preliminary screening between two types of Algerian clay revealed different performances of Na- or Ca-based bentonites, with the first one being more effective for the regeneration of waste vegetable oils arising from deep frying. A chemical fingerprint of commercial, waste, and recycled (under different conditions) samples was determined by statistical multivariate analysis of FT-IR data. Comparison of the different fingerprints revealed as even small variations in the procedure (e.g., 2 or 4 h) can have not negligible consequences on the chemical composition of the samples. The analysis of some physical parameters, such as pH, conductivity, TDS, salinity, turbidity, and density, confirmed the statistical results, revealing that 4 h of treatment with Na-clays represents the best compromise for obtaining a recycled oil matrix ready to be further processed. Thus, an improved protocol, which employs low clay/oil ratio (0.15 wt%) and reasonable processing times (2–4 h), was developed and described. Finally, a specific characterization of the Na-clay was conducted by meaning of N2 physisorption, SEM microscopy, and FT-IR spectroscopy.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/recycling6040068/s1, Figure S1: FT-IR spectra of waste cooking oil sample (WCO) and samples treated with Na-clays (TCO) after 4 and 24 h, Figure S2: FT-IR spectra of edible cooking oil (CO), waste cooking oil (WCO) and 24 h treated cooking oil with Na-clay (TCO), Figure S3: FT-IR spectra of Na-caly prior (SC) and after 24 h of waste cooking oil treatment (SC 24 h), Figure S4: Cooking oil (CO), Waste Cooking Oil (WCO), 2 h treated sample (TCO 2 h), and 4 h treated samples (TCO 4 h) with Na-clay.
Author Contributions
Conceptualization, data analysis, writing the original draft, and reviewing. A.M., S.T. and Z.T.; sampling, A.S., S.B., S.K.A.; Analysis, A.S., Z.T., S.G., N.S.; Funding acquisition, A.M. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was funded by the WORLD Project-RISE, a project that has received funding from the European Union’s Horizon 2020 research and innovation programme, under the Marie Skłodowska-Curie, Grant Agreement No. 873005. Moreover, The Algerian Directorate General of Scientific Research and Technological Development (DGRSDT) and the Algerian Ministry of Higher Education and Scientific Research (MESRS) are greatly thanked for their financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the support of the CeSAR UNISS, Centro Servizi di Ateneo per la Ricerca of the University of Sassari, for making available several instrumental techniques to carry out materials characterization.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Corona, B.; Shen, L.; Reike, D.; Rosales Carreón, J.; Worrell, E. Towards sustainable development through the circular economy—A review and critical assessment on current circularity metrics. Resour. Conserv. Recycl. 2019, 151, 104498. [Google Scholar] [CrossRef]
- Rodriguez-Anton, J.M.; Rubio-Andrada, L.; Celemín-Pedroche, M.S.; Alonso-Almeida, M.D.M. Analysis of the relations between circular economy and sustainable development goals. Int. J. Sustain. Dev. World Ecol. 2019, 26, 708–720. [Google Scholar] [CrossRef]
- Zeller, V.; Towaa, E.; Degrez, M.; Achten, W.M.J. Urban waste flows and their potential for a circular economy model at city-region level. Waste Manag. 2019, 83, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.K.; Li, J.; Koh, L.; Ogunseitan, O.A. Circular economy and electronic waste. Nat. Electron. 2019, 2, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Pires, A.; Martinho, G. Waste hierarchy index for circular economy in waste management. Waste Manag. 2019, 95, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, J.; Martel Martín, S.; Baldino, S.; Prandi, C.; Mannu, A. European Union Legislation Overview about Used Vegetable Oils Recycling: The Spanish and Italian Case Studies. Processes 2020, 8, 798. [Google Scholar] [CrossRef]
- Mannu, A.; Garroni, S.; Ibanez Porras, J.; Mele, A. Available Technologies and Materials for Waste Cooking Oil Recycling. Processes 2020, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Mannu, A.; Ferro, M.; Di Pietro, M.E.; Mele, A. Innovative applications of waste cooking oil as raw material. Sci. Prog. 2019, 102, 153–160. [Google Scholar] [CrossRef] [PubMed]
- No, S.Y. Inedible vegetable oils and their derivatives for alternative diesel fuels in CI engines: A review. Renew. Sust. Energy Rev. 2011, 15, 131–149. [Google Scholar] [CrossRef]
- Panadare, D.C.; Rathod, V.K. Applications of Waste Cooking Oil Other Than Biodiesel. Iran. J. Chem. Eng. 2015, 12, 55. [Google Scholar]
- Biermann, U.; Meier, M.A.R.; Metzger, J.O.; Schafer, H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854. [Google Scholar] [CrossRef] [PubMed]
- Asli, H.; Ahmadinia, E.; Zargar, M.; Karim, M.R. Investigation on physical properties of waste cooking oil. Constr. Build. Mater. 2012, 37, 398. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Implementation of the Waste Framework Directive. Available online: http://ec.europa.eu/environment/waste/framework/list.htm (accessed on 22 July 2021).
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR determination of free fatty acids in vegetable oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef] [Green Version]
- Andrikopoulos, N.K.; Boskou, G.; Dedoussis, G.V.Z.; Chiou, A.; Tzamtzis, V.A.; Papathanasiou, A. Quality assessment of frying oils and fats from 63 restaurants in Athens, Greece. Food Serv. Technol. 2003, 3, 49–59. [Google Scholar] [CrossRef]
- Ziaiifar, A.M.; Achir, N.; Courtois, F.; Trezzani, I.; Trystram, G. Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep-fat frying process. Int. J. Food Sci. Technol. 2008, 43, 1410–1423. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Hymore, F.K. Effects of some additives on the performance of acid-activated clays in the bleaching of palm oil. Appl. Clay Sci. 1996, 10, 379–385. [Google Scholar] [CrossRef]
- Komadel, P. Acid activated clays: Materials in continuous demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Murray, H.H. Overview—Clay mineral applications. Appl. Clay Sci. 1991, 5, 379–395. [Google Scholar] [CrossRef]
- Luna, F.M.T.; Cecilia, J.A.; Saboya, R.M.A.; Barrera, D.; Sapag, K.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants. Materials 2018, 1, 1764. [Google Scholar] [CrossRef] [Green Version]
- Richardson, L.L. Use of bleaching, clays, in processing edible oils. J. Am. Oil Chem. Soc. 1978, 55, 777–780. [Google Scholar] [CrossRef]
- Mannu, A.; Vlahopoulou, G.; Urgeghe, P.; Ferro, M.; Del Caro, A.; Taras, A.; Garroni, S.; Rourke, J.P.; Cabizza, R.; Petretto, G.L. Variation of the Chemical Composition of Waste Cooking Oils upon Bentonite Filtration. Resources 2019, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Taleb, Z.; Ramdani, A.; Berenguer, R.; Ramdani, N.; Adjir, M.; Taleb, S.; Morallón, E.; Nemmich, S.; Tilmatine, A. Combined ozonation process and adsorption onto bentonite natural adsorbent for the o-cresol elimination. Int. J. Environ. Anal. Chem. 2021, 1–18. [Google Scholar] [CrossRef]
- Feddal, I.; Taleb, Z.; Ramdani, A.; Herbache, H.; Taleb, S. Discoloration of contaminated water by an industrial dye: Methylene Blue, by two Algerian bentonites, thermally activated. Alger. J. Environ. Sci. Technol. 2019, 5, 1141. [Google Scholar]
- Alexa, E.; Dragomirescu, A.; Pop, G.; Jianu, C.; Dragoş, D. The use of FT-IR spectroscopy in the identification of vegetable oils adulteration. J. Food Agric. Environ. 2009, 7, 20–24. [Google Scholar]
- Mannu, A.; Ferro, M.; Colombo Dugoni, G.; Panzeri, W.; Petretto, G.L.; Urgeghe, P.; Mele, A. Improving the recycling technology of waste cooking oils: Chemical fingerprint as tool for non-biodiesel application. Waste Manag. 2019, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Mannu, A.; Panzeri, W.; Theeuwen, C.H.J.; Mele, A. An integrated approach to optimizing cellulose mercerization. Polymers 2020, 12, 1559. [Google Scholar] [CrossRef]
- Mannu, A.; Karabagias, I.K.; Di Pietro, M.E.; Baldino, S.; Karabagias, V.K.; Badeka, A.V. 13C NMR-based chemical fingerprint for the varietal and geographical discrimination of wines. Foods 2020, 9, 1040. [Google Scholar] [CrossRef]
- Sahasrabudhe, S.N.; Rodriguez-Martinez, V.; O’Meara, M.; Farkas, B.E. Density, viscosity, and surface tension of five vegetable oils at elevated temperatures: Measurement and modeling. Int. J. Food Prop. 2017, 20, 1965–1981. [Google Scholar] [CrossRef] [Green Version]
- Varma, R.S. Clay and clay-supported reagents in inorganic synthesis. Tetrahedron 2002, 58, 1235–1255. [Google Scholar] [CrossRef]
- Makhoukhi, B.; Didi, M.A.; Villemin, D.; Azzouz, A. Acid activation of Bentonite for use as a vegetable oil bleaching agent. Grasas Y Aceites 2009, 60, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.R.; Jenkins, D.B.; Ungermann, C.B. Bleaching with alternative layered minerals: A comparison with acid-activated montmorillonite for bleaching soybean oil. J. Am. Oil Chem. Soc. 1989, 66, 334–341. [Google Scholar] [CrossRef]
- Mannu, A.; Vlahopoulou, G.; Sireus, V.; Petretto, G.L.; Mulas, G.; Garroni, S. Bentonite as a Refining Agent in Waste Cooking Oils Recycling: Flash Point, Density and Color Evaluation. Nat. Prod. Commun. 2018, 13, 613–616. [Google Scholar] [CrossRef] [Green Version]
- Jalil, M.E.R.; Vieira, R.S.; Azevedo, D.; Baschini, M.; Sapag, K. Improvement in the adsorption of thiabendazole by using aluminum pillared clays. Appl. Clay Sci. 2013, 71, 55–63. [Google Scholar] [CrossRef]
- Song, K.; Sandi, G. Characterization of Montmorillonite Surfaces After Modification by Organosilane. Clays Clay Miner. 2001, 49, 119. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. The Solubility of Oil-Soluble Dyes in Aqueous Solutions of Stable Protecting Colloids as Examples of True Reversible Equilibrium. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lomić, G.A.; Kiš, E.E.; Dimić, E.B.; Romanić, R. Investigation of activated Al pillared clay efficiency in vegetable oil purification. Acta Period. Technol. 2014, 35, 31–36. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).