Abstract
The inedible part (stones, husks, kernels, seeds) of the tree fruits that are currently processed in various regions of Greece constitutes a huge portion of the fruit processing solid waste that remains underexploited. In this review, the existing scientific background for the composition and content of fruit stone and seed in bioactive ingredients is highlighted for olives, stone fruits and citrus fruits that represent the economically most important tree crop products of the country. The content of bioactive compounds may vary considerably depending on the quality of the raw material and the treatment during processing. However, both the hydrophilic and the lipophilic fractions of the seeds contain significant amounts of the primary and the secondary plant metabolites. Among them, phytosterols and several types of polyphenols, but also squalene, tocopherols and some other terpenoids with a unique structure are of particular importance for the utilization and valorization of stones and seeds. Official and scholar records about the current management practices are also presented to highlight the dynamics of the Greek fruit sector. Prospects for the regionalization of fruit seed wastes, in line with EU-promoted Research and Innovation Strategies (RIS) for Smart Specialization are critically discussed.
1. The Fruit Sector in Greece
Fruits and vegetables constitute an important sector in the agricultural production of Greece holding very important share (about 39%) in the domestic agricultural economy, estimated at around 10 billion EUR [1,2]. Over the years 2009–2013, vegetables had the largest share of the total value of agricultural production (18%) followed by fruit production (16.6%) [2]. The major fruit production in Greece is derived from permanent tree crops (Cultivated areas of tree plantations and their production) and is comprised of olives (mainly oil producing), citrus (oranges, tangerines, lemons), stone fruits (peaches, apricots, cherries) and pome fruits (apples, pears) [3]. Greek table olives, oranges, peaches and apricots present high competitiveness in the global market [1,4]. Greece is among the top three EU producers of olives, peaches and citrus fruits [5] and the leading EU peach processor [6]. Noticeably, the production of clingstone peaches that are exclusively used for industrial processing is among the biggest in the world.
Although there is a long tradition in the production of table olives, olive oil, grape wine, and winery products in Greece with more than 80% of the corresponding crops intended for industrial processing, other types of processed fruits are still not fully developed. The part of the orange produce that is channeled to processing varies from year to year between 20–30% of the total production depending on crop quality and the quantities not absorbed in the fresh market. Typically, 15–20% of the total peach production is forwarded to industrial use and the rest is directed to the fresh fruit market [7]. This trend is partially due to the high revenues gained by exporting fresh Greek fruits (citrus, kiwifruits, table grapes, peaches/apricots), often much higher than those for processed products such as olive oil. The stone and citrus fruit processing industries in Greece are restricted to juices and canned fruits. Jams, fruits preserved in sugar syrup, dried, dehydrated, frozen, depitted fruits or ready-made salads are produced also in smaller quantities [6,7].
Fruit processing (from cleaning/sorting to packaging) generates huge amounts of solid waste that are either disposed in landfills or rivers, causing environmental hazards or at a lower extent they are recycled through livestock as feed resources. The industrial fruit solid waste includes the leaves, the spoiled fruits, the unused peels, pulp, or fibrous material along with the inedible part of the fruits (stones, husks, kernels, seeds). This latter part of the solid waste from fruit processing industries may reach up to 80% depending on the size of the pits and the type of processing. Valorization of the agro-industrial solid waste is challenging for the global food industry given the diversity in the type of fruit, heterogeneity among cultivars but also seasonality of production and various ways of end-use that require different ways of processing [1,8].
Figure 1 illustrates the regional distribution of the major types of fruit production in Greece that are, olives and stone fruits (peach, apricots, cherries) along with citrus ones. Three out of the 13 administrative regions of Greece, namely the regions of Central Macedonia (RCM), Peloponnese (RP) and Crete (RP), congregate > 50% of the orchards and other fruit tree plantations of the country. These regions, along with that of Central Greece (RCG) share the largest industrial specialization. RP and RC have the major revenues from virgin and crude pomace olive oil production, RI and RWG from citrus juice production while RCM from the canned fruit industry (involving cling peaches, apricots and very few quantities of pears, strawberries, and cherries). As the agro-industrial production in Greece is scattered throughout the countryside and the wastes are produced seasonally with significant differences in quantity and content, the challenges of implementing sustainable waste management are rather complex [9]. Most manufacturers are usually low to medium-sized enterprises that choose a disposal method in line with national laws and regulations. However, the overall cost including local alternatives, distance to various disposal facilities etc., also influences their decision. Waste minimization strategies are thus, expected to provide economically feasible solutions to the fruit processing industries contributing also to long-term energy sustainability at a national level [10].
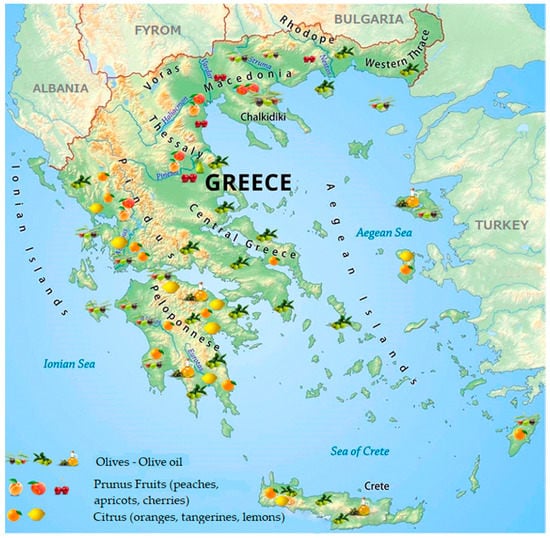
Figure 1.
Geographical distribution of the major fruit tree plantations in Greece.
In this review, emphasis will be given to show how the stone and seed wastes generated during the processing of tree fruits in various regions of Greece can be valorized and endorse the sustainability of the existing plants, in line with EU-promoted Research and Innovation Strategies (RIS) for Smart Specialization. The existing scientific background for the composition of tree fruit stones and seeds in bioactive ingredients and their valorization potential will be highlighted for olives, stone fruits, and oranges, the major types of tree fruits that are currently produced and processed in Greece.
2. Bioactive Ingredients in the Tree Fruit Stones and Seeds
2.1. Olive Fruit Stones and Seeds
The evergreen olive trees (Olea europaea L.) are the major tree plantations in Greece covering more than 70% of the tree crop area. They are cultivated in systematic orchards across the country even in less fertile, stony grounds where no other crop can thrive. Because the water demands are modest, olive production may often be the only sustainable source of income for communities in less favored areas. The fruit, which is classified as a drupe, is made up of the epicarp or skin (1–3% of the fruit weight), the mesocarp or flesh (70–80% of the total fruit mass), and a single woody shell of hardened endocarp, often called pit or stone (10–27% of the fruit weight), which encloses the seed (kernel) (2–4% of the fruit weight) [11]. Olive fruits are consumed only after processing which ends up to either table olives or olive oil. Both types of products are well-known natural sources of phenolic antioxidants that exert multiple biological functions [12,13,14] although the exact profile and content may vary a lot depending on the treatments during processing [15]. This aspect must be considered also when valorization of the generated by-products is envisioned.
Transformation of olives into canned table olives or olive oil follows in general the workflow presented in Figure 2. The diagram highlights the key production steps from each processing line that generates solid or semi-solid waste that is, whole olive stones and virgin pomace (containing crushed stone, seed, cellular fibrous, water), respectively. The former is the main solid by-product of the table olive industry, especially the pitted olives one (stuffed, sliced, paste etc.). When it comes to the olive pomace produced currently from two-phase or three-phase olive oil mills, the stone content generally varies from 28% in three-phase plants to 12% in the two-phase ones [16]. This actually refers only to the crushed stone (the wooden part) and not the released seeds which are fragmented by the current olive crushing systems and blended with the pulp [17]. A number of special industrial plants in Greece use the recovered stones as a fuel for their closed spaces, drying processes and greenhouse cultivations [18]. Even so, very few of the existing pomace processing plants in Greece apply de-stoning either prior to chemical treatment or after crude pomace oil extraction (see Figure 2). The remaining pitted material is then used for oil extraction or as a fertilizer, respectively. As the combustion of the exhausted dry pomace leads to the emission of toxic volatile compounds, the separation of stones and utilization of only this type of solid residue as fuel is often suggested [19]. Virgin olive oil production from de-stoned olives is another alternative that is under investigation for a number of reasons [20,21]. For example, it has been reported that de-stoning of olives prior to crushing and malaxation may stimulate lipoxygenase activity resulting in higher content of hexanal and a positive effect on the olive oil flavor [22]. On the other hand, malaxation of the paste with or without the stones is expected to affect the total phenol content of the olive oil as a result of the release of endogenous seed enzymes during the fruit crushing [17,23].
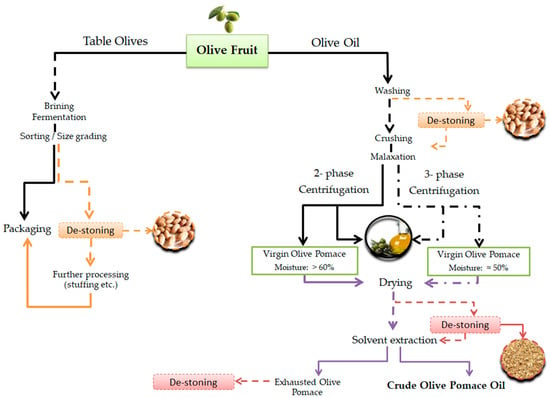
Figure 2.
General processing lines for table olive, olive oil and olive pomace oil production with emphasis on the stages that generate olive stone and seed wastes [9,24].
Currently, scientific research has focused more on the compositional characteristics of the wooden part of olive stones with regard to its heating or composting potential [25,26] rather than on the biochemical profile of the olive stones as a whole or of the olive seeds alone. The whole olive stone seems to be a good source of bioactive compounds [11,27,28,29,30,31]. Phenols constitute the most important family of bioactive constituents in the olive seeds, as it is the case with other parts of the olive drupe. The increasing interest in these compounds is due to their antioxidant properties and health enhancing effects, which have been attributed mainly to the presence of secoiridoids [14]. Nüzhenide is the predominant phenolic constituent of the polar seed extracts (see Figure 3) [30,31]. This secoiridoid metabolite has been detected only in the olive seed at levels ranging from 3.05 to 3.85 mg/g dry weight, depending on the cultivar [31]; its degradation during the malaxation process is considered to contribute to the accumulation of hydroxytyrosol in the olive paste [14]. Salidroside, an ester of tyrosol with oleic acid together with verbascoside, tyrosol, hydroxytyrosol, oleuropein and oleacein (3,4 DHPEA-EDA) are also present in the olive seeds, at much lower amounts [30,32]. Olive seed polar extracts have been shown to exert high radical scavenging activity but low total phenolic contents, as estimated colorimetrically. This can be explained considering that nüzhenide and related compounds possess a tyrosol unit in their structure, which shows a poor response toward the assay reagent [33].
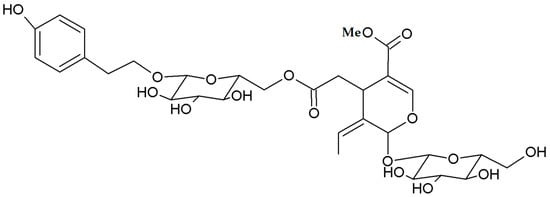
Figure 3.
Chemical structure of nüzhenide, the major phenolic constituent of the polar olive seed extracts.
Olive seeds contain also considerable amounts of oil reaching about 10% w/w, depending on the cultivar and the extraction method [34]. The oil extracted from the seeds is richer in total polyunsaturated fatty acids than that extracted from the pulp or the whole olive drupe. This is mainly because of its almost two-fold higher content in linoleic acid (C18:2) but also in some minor fatty acids such as C20:1 [35,36]. Compared with the oils from pulp or the whole olive fruit the seed oil seems to be almost two-fold richer in total phytosterols (4.13 mg/g vs. 1.87 mg/g in whole olive fruit oil) [37]. The sterol fraction of seed oil from seven Italian olive cultivars was quite rich in β-sitosterol, (>85% of its weight), but also in other minor components (campesterol, Δ7-stigmastenol, Δ7-avenasterol) [37]. The total content of phytosterols in the seed oil of olives seems to be of similar size to those found in the oil from other types of fruit seed wastes such as pomegranate, gooseberry or apricot [38,39] but also in commercial oilseed products (e.g., sunflower oil) that are good sources of phytosterols [40]. Recently, virgin olive seed oil from two Tunisian cultivars has been reported to contain also squalene [36]. This triterpenoid hydrocarbon that dominates the nonsaponifiable fraction of olive oil is a well-known singlet oxygen quencher with many applications in cosmetics [13,41,42]. Depending on the cultivar and the fruit maturity index, the squalene content of olive seed oil varied from 0.54 to 1.25 mg/g, being thus, much lower than that found in the oil from pulp or the whole olive fruit, but not negligible [36]. The reported values are comparable to those found in other agro-industrial by-products and wastes such as rice bran oil (0.04–1.52 mg/g) and red wine lees (0.54–1.54 mg/g) [9,42]. Regarding the total tocopherol content of olive seeds, it has been reported that it can range from 16.7 for Konservolia, to 38.8 mg/kg for Koroneiki cultivars(expressed as Vitamin E) [34]. However, Gonzalez-Hidalgo et al. [11] reported that α-tocopherol content in olive seeds of Manzanillia cultivar, recovered after table olive processing, may reach up to 100 mg/kg. These findings suggest that the levels of vitamin E in the olive seeds may vary a lot according to genetic factors but also the conditions of processing and the analytical method (e.g., sample pretreatment). The content of olive seed oils in carotenoids and chlorophylls has been also reported to be higher than that of the whole olive fruit although in general, the levels are rather low (3.16–4.08 mg/kg and 2.91–6.11 mg/kg) [36].
Table Olive and Olive Oil Sectors in Greece
The table olive and olive oil industrial sectors in Greece have developed substantially the last decades following an increase in their products demand due to better consumer awareness about their health benefits. Nowadays, table olives are cultivated by 64,000 producers and treated and processed by more than 100 organizations in the northern (Chalkidiki, Kavala, Thasos), centered (Phthiotida, Evia) and southern areas of Greece (Lakonia, Messenia). The annual output from table olive production has risen significantly during the last years reaching around 250,000 tons in 2016. It is estimated that more than 200,000 tons are exported to around 80 countries accounting for about 9.2% of total agricultural product exports of Greece and 8.0% of global table olive production [43,44]. Significant revenues are thus, produced for RCM, RCG and RP, respectively; the Regions of Eastern Macedonia and Thrace (REMT), Thessaly (RT) (Magnesia, Larissa), Epirus (RE) and Western Greece (RWG) (Acarnania, Arta) as well as the Region of North Aegean Islands (RNAI) seem also to gain income from table olive production and processing. 80% of the annual produce concerns three cultivars—“Chalkidiki”, “Konservolia” and “Kalamon”. The Interprofessional Table Olives Organization (DOEPEL) coordinates the groups involved in the production sector with individual farmers and co-operatives, but there are also members involved in processing, with organized units for the treatment, processing, standardization, packaging and marketing of table olives [44].
When it comes to olive oil, the respective sector is more fragmented and less well organized. The farms, dispersed primarily in the southern coastal areas of Greece, which are hot and dry, are of small size. This implies that potential profits are lower and depend on subsidies and own labor [45]. RP, RC and RCG share the major olive oil production (33, 33 and 14%, respectively) [7]. RWG along with RNAI and the region of Ionian Islands (RII) are also important producers. Olive oil is of relatively low importance to RCM but important for the rural economy of the Prefecture of Chalkidiki, as it constitutes a supplementary source of income for numerous families. Olive oil production in Greece ranges between 300,000–400,000 tons (almost 10–14% of global olive oil production most of which belongs to the highest quality category, that is, extra virgin olive oil [43,45]. However, its promotion as a high quality branded olive oil (e.g., PDO, PGI, organic cultivation, cultivar name) is still very limited in Greece. This is mainly due to (a) the fragmented nature of Greek olive oil mills and (b) the small size of bottling and labeling companies [45]. The majority of the olive oil mills are low to medium-size enterprises or cooperatives that lack effective knowledge or innovation management practices (e.g., vertically integrated production); their operation is often limited to the distribution of production subsidies to the farm owners instead of following a clear business strategy which would maximize their output [43,45]. Therefore, although there are around 2200 olive mills all over the country (37% in RP, 23% in RC, 17% in RCG) only a few large processors located in RP and RC, have the largest share in Greek olive oil trade [43,45].
From the secondary processing unit plants, the olive pomace oil ones are very active members of the sector. Currently, 35 to 40 olive pomace-processing units operate in Greece. Most of them are dispersed in Crete (RC), Lesvos (RNAI), Patra (RWG), Lamia (RCG) and Messenia (RP). As they are often located close to inhabited areas they cause serious noise and odor problems, especially during their operation period (early November to late April). Most of the existing plants lack the storage and drying facilities required to receive and manage high moisture olive pomace, such as that from the two-phase olive oil mills (see Figure 2). The transportation and storage of this material is of high environmental risk as it retains a high organic content (sugars, acids, fat, trace elements, polyalcohols, pectins, polyphenols, etc.) that is not easily degraded by natural processes [10]. Additionally, because its oil content is lower than that of the conventional raw material, the whole pomace oil production becomes less profitable for processors. In Greece, crude pomace oil production involves heated extraction with solvents such as hexane. The solvent is recovered by distillation and the solvent-free, pomace oil is sold in pharmaceutical companies or refineries, mainly in Spain and Italy; only few olive pomace oil units possess their own refinery plants (one in RC) so that production of edible olive pomace oil in Greece is very low [43]. It is estimated that, each year 200,000 tons of virgin olive pomace are processed in Greece producing around 8500 tons of crude pomace oil and 100,000 tons of dry, exhausted pomace with total value of 5.5 and 5.0 million euros, each [43].
2.2. Prunus Fruit Stones and Seeds
Fruits from the deciduous trees of the genus Prunus, such as Prunus persica (peach), P. armeniaca (apricot) and P. avium or P. cerasus (sweet and sour cherry, respectively) are also classified as drupes; they are often called stone fruits because the shell of the endocarp that encloses their kernels or seeds is hard like a stone [46]. Peach (including nectarine), apricot and sour cherry are the main types of stone fruits that are currently processed in Greece for the production of canned fruits (halves, dices and slices), fruit cocktails, and compotes but also for juices and puree concentrates [47]. Figure 4 shows the general schemes for processing fresh stone fruits in Greece. Regardless of the type of fruit or end-product, the removal of stones after washing and peeling of the fruits is a common procedure [46].
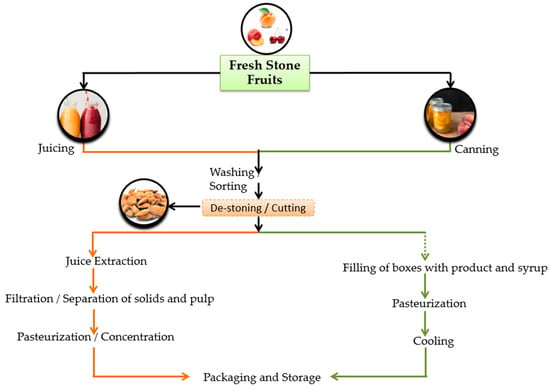
Figure 4.
The processing of fresh stone fruits in Greece.
The stones of apricot and cherries represent almost 30% of the fruit weight while those of peaches are comparatively smaller (ca. 7% of the fruit weight). Irrespectively of the Prunus stone/fruit weight value, the enclosed seeds represent 6–7% of the total fruit weight [48]. Stones constitute the largest part (70–80%) of the solid waste from Prunus fruit canneries and juice processors. The remainder contains fruits unsuitable for processing, fruit peels, screenings, etc. in the form of sludge. Due to their high energy and low ash content values, the peach and apricot stones are often separated within the processing plant and used as solid fuel to reduce the cost of the production heat supply [18,49]. Alternatively, peeled stones of peach and apricot are sold in confectionery plants to be used to produce persipan, a substitute of marzipan. In that case, chemical treatment to remove toxic cyanogenic glycosides such as prunasin and amygdalin, is a prerequisite [50]. Amygdalin, accumulating mainly in the shell of the seeds [51] is present at relatively high concentrations, reaching 3.89 mg/g in cherry, 6.81 mg/g in peach or even 14.37 mg/g in apricot seeds (see Figure 5) [52]. Generation of high levels of hydrogen cyanide through hydrolysis of these compounds in aqueous-alcoholic media is thus, an actual health risk [53]. The lethal dose of amygdalin which is estimated to 0.5–3.5 mg/kg body weight (bw), is likely to be exceeded by the consumption (grinding or chewing) of less than half of a large apricot seed [50].
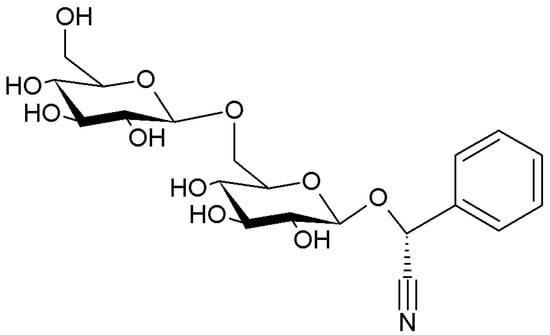
Figure 5.
Chemical structure of amygdalin, the major cyanogenic glycoside in Prunus seeds.
Pharmaceutical companies exploit Prunus stones to produce oil for cosmetics [39]. This type of use requires the crushing of stones and recovery of the seeds (kernels). The seeds of peach, apricot and cherries are very well appreciated for their oil content and bioactive compounds present therein. For example, it has been reported that supplementation of hypercholesteremic rats with apricot seed oil favors their liver antioxidant condition by causing a significant increase in the activity of antioxidant enzymes (glutathione peroxidase and catalase) [54]. Zhang et al. [55] proposed the same mechanism of activity to support the cardioprotective effects of apricot seed oil.
Lazos [56] was the first who studied extensively the composition and oil characteristics of some by-products from Greek Prunus cultivars and reported that peach seeds are superior to apricot and sour cherry ones in terms of oil yield (48% vs. 38% and 26%, respectively). Several studies that were carried out later with fruits from various cultivation areas and/or maturity stages showed that this statement cannot be generalized. Thus, peach and apricot seeds have comparable potential for oil extraction (37–56%) [57], often much higher than that of sour cherries (18–31%) [58]. The seed oils from these three types of Prunus fruits are considered rich sources of monounsaturated fatty acids, mainly oleic acid, moderate sources of linoleic acid and poor in saturated fatty acids. Oleic acid is the predominant fatty acid (43.9–78.5%) followed by linoleic (9.7–37%) and palmitic (4.9–7.3%) ones [48,57,58,59,60,61,62,63]. Currently, nine sterols have been detected in the seed oil of different apricot and sour cherry cultivars [58,64]. The total sterol content of apricot and sour cherry seed oils is most probably of similar size, ranging from 2.2 to 10.4 mg/g and consisting mainly (>75%) of β-sitosterol [56,58,64]. These levels can be considered relatively high as they fall within the range of total phytosterol values reported for most of the conventional plant sources such as the corn, soybean and sunflower oils [40,65]. The sour cherry seed oil contains low amounts of other potentially active microconstituents such as squalene (0.66–1.03 mg/g) and carotenoids (1.7–17.5 mg/kg) [58,66]. The Prunus seed oils present a great variation in the content and composition of vitamin E-active compounds [57]. γ-Tocopherol is the major constituent; its content in the seed oil of various sweet and bitter apricot cultivars was found to vary from 141.6 to 330.2 mg/kg [57] or from 424.8 to 732.7 mg/kg [67] while they were much higher in sour cherry seed oil (891–1333 mg/kg of oil) [58]. Noticeably, the content of these materials in γ-tocopherol is comparable to those of plant oils derived from nuts (e.g., peanuts) and oilseeds (corn, soya) [65].
Apart from oil, the defatted sour cherry seed extract seems to exhibit interesting medicinal properties. For example, a strong cardioprotective effect was evidenced in some preclinical studies of recovery of myocardial ischemia-reperfusion injuries in rats. Their protective role has been attributed to the presence of simple phenolic compounds (caffeic, ferulic, p-coumaric acid), flavonoids (quercetin, apigenin, rhamnetin, scutellacein, and pinocembrin), stilbenes as well as oligomeric pro- and anthocyanidins [68,69,70,71].
Stone Fruit Canners and Juice Producers in Greece
Most of the tree plantations devoted to stone fruits (peaches, apricots, cherries) in Greece are found in plains (62%) while the remainder in mountainous (21%) and in semi-mountainous areas (17%) [7]. RCM (Imathia, Pella) holds about 95% of the total area under peach trees [6]; 65–70% of the total area under cherry trees but also important number of apricot tree cultivations (mainly Chalkidiki). The rest of the latter are mainly found in RP (Korinth, Argos/Peloponnese) but also in RT (Tirnavos/Thessaly) [6]. From 2012 to 2017, the production of peaches (including nectarines) in Greece ranged between 577,000 to 788,000 tons with almost half of it dedicated only to clingstone peaches [6,72], mainly for canning. Regarding apricots, the available data for the period 2009–2013 denote that 10,000–14,000 tons of the fruits were destined for canning and 15,000–17,000 tons for juice production. The numbers are only indicative as the annual output depends substantially on the weather conditions. Currently, there are 26 peach canners and 10 apricot ones in Greece [73]. It is worth noting that the Association of Greek Canners is one of the leading dealers in the world controlling more than 40% of the canned peach trade. On the other hand, 40 juice production units process around 30,000 tons of peaches and produce around 7000–8000 tons of peach juice [7]. Most of the plants are located around the territories of Imathia and Pella (RCM) while few others are close to Larissa (RT) and Argolida (RP). Greek cherries production (around 60,000 tons/year) is mainly destined for the fresh market, with only the sour cultivars (3000 tons/year) to be processed into jams, spoon sweets, and the ”vissinada”, a traditional sour cherry concentrate [47,72]. The usual processing period of sour cherries lasts from May to June, for apricots from mid-June to mid-July and for peaches from mid-July until mid-September [47].Taking into account the available reports and statistical facts it can be suggested that canneries and juice processors in Greece generate annually more than 20,000 tons of solid waste from peaches, apricots and cherries processing.
2.3. Citrus Seeds
The processing of sweet and sour orange (Citrus sinensis L. and C. aurantium L. respectively), mandarin (C. reticulata), lemon (C. limon (L.) Burm) and other citrus fruits to produce juice represents one of the most important food industries in the world. Because of the high worldwide demand, citrus cultivars have undergone numerous genetic modifications to satisfy industrial requirements and boost their economic impact. For example, seedless varieties have evolved to avoid pressure or fine filtering of crushed seed during juice processing and the concomitant release of bitter compounds [74]. Nevertheless, citrus seeds can be recovered as whole depending on the juice processing technology. Being located in the interior of segments that are inside the flavedo, the citrus seeds amount to 0.1–5.0% of the fruit mass. Thus, they may constitute an important part of the solid residuals that are generated during the process (along with ground peels and segment membranes). However, their levels vary greatly depending on the fruit type, cultivar, maturity, and the technology of the production process.
The last decades, the bitter constituents of citrus seeds have gained remarkable attention by several groups of medical scientists because of a series of biological functions that they can exert in vivo (anticancer, antiviral, anti-inflammatory etc.), presenting thus, a great potential for biomedical applications [75]. From a chemical point of view, they are classified as highly oxygenated triterpenes, known also as limonoids. In their aglycone form, limonoids account for the delayed bitterness in citrus juice. Their glycosylated forms, which are abundant in the juice and pulp of citrus fruits, are rather tasteless. Citrus seeds constitute the only natural source of limonoid aglycones. The total limonoid content and composition may vary a lot depending on the type of cultivar but also the method of analysis [76]. Bonaccorsi and co-workers [77,78] have reported that lemon and orange seeds contain as many limonoids as 375 and 114 mg/kg, respectively. These values were by far lower than those reported for lemon and orange seeds (18.93 and 22.33 mg/g dry seed, respectively) by other authors [79]. Noteworthy, a labor-intensive sample preparation protocol followed in the latter study probably resulted in higher extraction yields. Limonoid glycosides that are also present in citrus seeds are most often less abundant, almost half in content than their corresponding aglycones [79]. The average concentration of total limonoid glucosides and the corresponding aglycones in seeds from lemons, grapefruits, tangerines, and oranges has been reported to be 6.1 and 13.5 mg/g, respectively [80]. Limonin is consistently the most abundant constituent and one of the six limonoid aglycones (limonin, nomilin, obacunoic acid, ichangin, deoxylimonoic acid and nomilinic acid) that have been identified to be inherently bitter [75]. Limonin, along with some derivatives and analogs (limonin 17-β-d-glucopyranoside, limonin carboxymethoxime, and deoxylimonin) are considered strong antineoplastic agents [81]. On the other hand, two nomilin derivatives (deacetylnomilin and nomilin glucoside) have been reported to be the most effective inhibitors of estrogen receptor- positive breast cancer cells [75]. The furan group that is a common structural feature of all limonoids seems to be the site of several physiological activities. For example, changes in the A ring of the limonoid nucleus can lead to a loss of anticancer activity [81]. Currently, there is no evidence that biological effects involve antioxidant action. This could be because the structure of these compounds lacks conjugated unsaturation and electron delocalization potential that would enhance their in vivo activity against reactive oxygen species (see Figure 6).
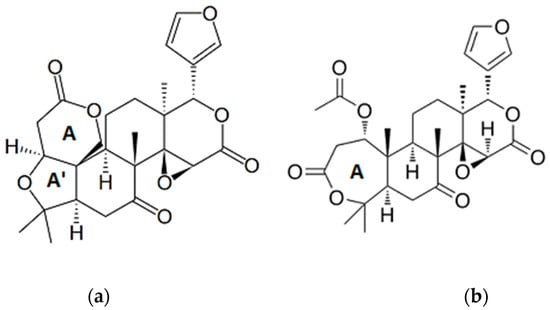
Figure 6.
Chemical structure of (a) limonin and (b) nomilin, the major bioactive limonoids found in Citrus seeds.
Phenolic compounds, also present in the citrus seeds are more probable to account for the antioxidant properties of their polar extracts. The composition and content level of individual phenolics and even synergistic phenomena are likely to affect the antioxidant potential of specific cultivars. The total phenolic content of mandarin seeds (C. reticulate Blanco cv. Chachiensis) was found to range from 2.3 to 3.0 mg GAE/g of dry sample [82]. The respective content in Tunisian mandarin and bitter orange seeds(C. reticulate Blanco and C. aurantium) harvested at three ripening stages (immature, semimature, and commercial mature) was found to vary from 0.68 to 2.11 mg GAE/g dry sample [83]. Moreover, lemon seeds were found richer than the corresponding fruit juice in total phenols (2.1–3.4 mg GAE/kg fresh weight). Interestingly, the total flavonoid content of the seeds was found to exceed more than 50-fold that of the juice [84]. Methanol extracts of citrus seeds are rich in flavones and glycosylated flavanones although the exact contents may fluctuate considerably according to a plethora of biotic and abiotic factors. For example, the seed content of glycosylated flavanones were found to range from 0.04 to 3.28 mg/g dry extract in different citrus cultivars [85]. However, total flavanones up to 47.4 mg/g dry extract have been reported in orange seeds recovered after industrial juice processing [78]. Hesperidin is the major flavanone found in sweet orange, naringin in bitter orange seeds and eriocitrin in lemon seeds (see Figure 7) [77,83,85]. Hesperidin is the major flavanone in mandarin only until the semi mature stage; at the stage of fruit maturity the phenolic fraction of seeds is dominated by the presence of gallic acid. Altogether, the research findings indicate that citrus seeds may constitute a valuable source of phenolic antioxidants provided that the raw material is selected according to compositional data and standard requirements.
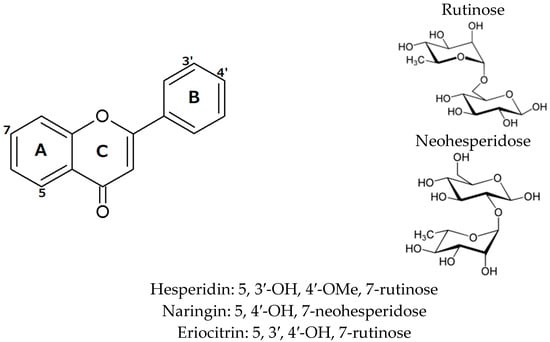
Figure 7.
Chemical structures of the major bioactive flavones found in orange and lemon seeds.
Oil extraction from the citrus seeds is another practice of commercial interest [79,86,87,88]. Citrus seeds may contain from 26 to 59% of oil [87,89]. As in the case of other specialty oils from fruit seeds and kernels, citrus seed oils are also considered to be rich in minor bioactive constituents. The orange, lemon, and mandarin (tangerine) seed oils proved to be a good source of essential fatty acids (C18:2 n-6 and C18:3 n-3) with lemon seed oil having more than 50% of linoleic plus linolenic fatty acids. In general, linoleic acid, followed by palmitic and oleic acids were the major fatty acids found in orange, lemon and mandarin seed oils [87]. However, it has been shown that oleic acid prevails in the oil of certain species, subspecies or hybrids e.g. C. paradisi (grapefruit), C. aurentium sbs pamara, and C. limon (interdonato) [89]. β-Sitosterol (1.20–1.22 mg/g) along with low amounts of campesterol and cholesterol have been found in the seed oils of several orange cultivars accounting for a total of 1.29–1.33 mg/g. As suggested by the researchers, these values are higher than those reported for other types of seed oils (e.g., pistachio) but intercomparisons are highly subjective to the conditions of analysis employed [88]. Currently, there are very few reports on the squalent content of citrus seed oils; Uenos et al. have detected only traces of squalene in the seeds from C. junos using supercritical CO2 and Soxhlet extraction [90].
As often indicated by their intensely yellow color, citrus seed oils contain several types of pigments. However, lipophilic carotenoids such as β-cryptoxanthin, β-carotene and lutein have been detected at rather low levels, ranging widely from 0.3 to 26.7 mg/kg [87,88,91]. The carotenoid contents of lemon seed oil produced by cold-pressing or solvent extraction were not significantly different [91]. Even so, lutein was the predominant constituent in orange, lemon and mandarin seed oils accounting for 59.4, 80.9 and 64.3% w/w of total carotenoids [87]. Moreover, lemon seeds that were recovered after juice processing were not found to contain detectable amounts of carotenes and xanthophylls, indicating that the conditions of industrial treatments and storage were most probably detrimental for the stability of this type of compounds [77]. Thus, the color of the citrus seed oil is more likely to be associated with the abundance of phenolic compounds such as polymethoxy flavones. The literature on this topic is rather scarce but indicates that orange, lemon, and mandarin seed oils may contain similar levels of total phenols. The reported values vary greatly; Cassia et al. [87] found approximately 1.0–1.2 mg GAE/g oil of orange, lemon and mandarin seeds while a few years later they reported somewhat higher values for orange seed oils (3.8–4.9 mg GAE/g oil) [88]. Recently, Guneser and Yilmaz reported that the total flavonoid content of cold-pressed and solvent-extracted lemon seed oil may reach up to 11.6 mg/g, as estimated by HPLC-DAD analysis [91]. The authors found that eriocitrin, hesperidin, naringin and neohesperidin as the most common flavonoids along with gallic and trans-ferulic acids as the most abundant phenolic acids dominated the phenolic fraction of both types of oils. The tocopherol contents of orange, lemon, and mandarin seed oils ranged widely from 74.71 to 748.11 mg/kg with α-tocopherol being the most abundant form. Lower levels of total tocopherols (19–195 mg/kg) have been evidenced in the case of several citrus cultivars from Turkey and Vietnam [89]. In the latter study, α- and γ-tocotrienols were also detected at levels ranging from 0.1 to 10.0 mg/kg oil. The reported values denote an interesting trend for accumulation of vitamin E-active compounds in citrus seeds which needs further investigation.
The citrus juice industry worldwide, retains a high level of innovation regarding the production of added-value by-products such as essential oil and aromatic constituents, carotenoids, dietary fiber concentrates etc. For example, citrus by-products are commonly used to fortify feedstuffs or as a source of clouding agents for citrus beverages [92]. In Greece, apart from essential oil and an aqueous solution called “anthonero”, dry pulp pellets of 10–12% moisture content are produced by drying of substandard fruits, peels, pulp, leaves etc. (80–85% moisture) in specially designed plants. The residual water is concentrated to a semi-solid sugary material (molasse) that is mixed with dry pulp and sold as animal feed or sold directly for biogas production. However, most of the juice plants dispose the solid waste to a landfill or mix it with wastewater and discharge to neighboring rivers (e.g., Evrotas, Lakonia) causing serious problems in their ecosystems [93]. Whatsoever, high transportation costs, lack of disposal sites, and the accumulation of waste with high organic load in open dumps renders these practices economically unfavorable. None of the existing plants possess a waste management plan that would allow the collection of sufficient quantities of seeds in one place most probably due to its high cost.
The Citrus Juice Sector in Greece
Fresh and concentrate juice producers that use exclusively fresh fruits as raw materials but also concentrate juice processors are involved in the Greek juice sector. Fresh oranges, tangerines, lemon, and grapefruit are purchased from Greek producers in quantities that may vary from year to year depending on the availability and prices. According to the Greek Ministry of Rural Development and Food, in 2014 there were 10 citrus juice processing units in Greece; seven located in RP (Argolida, Lakonia), one in RI (Arta), one in RC (Chania) and one in RNAI (Chios). During their operation period which lasts from October to late July, they processed around 135,000 tons of oranges to produce approximately 8289 tons of orange juice, 1500 tons of tangerines, 500 tons of lemon and 800 tons of grapefruit [94]. Sweet orange cultivars (Valencia, Salustiana) along with the blood-red or Sanguini ones (Taroco, Dolco) but also the so called “common Greek” (local varieties in Chios, Arta, Chania, South Peloponnese) that contain many seeds are mainly used for juice production. The widely grown and seedless Navel-type cultivars (Washington Navel, LineaLate, Navelate) are used only in blends with sweet cultivars due to their high content in bitter limonoids. When it comes to Greek mandarins, only 2% of the total annual production is processed and the rest is consumed as fresh. Due to the predominance of the seedless cultivar type “clementine” in the fresh fruit market, the use of the common Mediterranean cultivar for processing is more favored. The fruits of this cultivar are seeded and superior in taste. Among the citrus fruit producing regions, RI (Thesprotia, Arta) and RWG (Aitoloakarnania) along with RP (Argolida) have the largest share in clementine production which lasts from January to May. The common mandarin variety is mainly grown in Chios (RNAI), Kalimnos (RSAI) and Chania (RC) from December to February [95]. A very low percentage of the Greek lemon fruits (around 1.0–1.5%) is destined for processing and juice production given the high demands for fresh lemons (60,000–80,000 tons) that cannot be covered by domestic production (30,000–50,000 tons). Therefore, imported fruits are processed mainly for soft-drink production [95]. The main lemon variety in Greece is “Maglini”, a local variety of northern Peloponnese which produces strongly aromatic fruit, with a quite sour juice; the early cultivar Interdonato along with Verna and Eureka may also be used for processing. Lemon production is limited to the southern areas of the country belonging almost exclusively to RP and is more intensive from December to May [94,95].
3. Future Perspectives
The production of stone and seed wastes from the all-year-round operation of tree fruit processors (olive oil mills or olive pomace oil processors, fruit canneries and juice producers) all over Greece is currently an under-exploited resource of valuable constituents. Considering that seeds represent the reproductive organ of the plant species under study, their composition is naturally rich in nutrients that promote growth/germination (e.g., lipids, phytosterols, amino acids) but also in secondary metabolites that regulate the plant defense against insects/animals and can turn from health beneficial to even fatal for humans. Table 1 summarizes the types of key bioactive ingredients per each type of tree fruit stone/seed included in this review.

Table 1.
Key bioactive ingredients found currently in hydrophilic and lipophilic fractions of the seeds of olives, prunus and citrus fruits.
Above all, the valorization of tree fruit stone and seed wastes as new added-value by-products would be an ecologically sustainable solution for its management provided that economically feasible schedules are implemented. Most often fixed rules about sustainable production such as the raw material and energy savings confine the needs for the development of new industrial processes and satisfaction of increasing market requirements. In the case of Greece, where the topography is particularly unfavorable of transportations (many mountainous and semi-mountainous areas along with a large number of peripheral islands and remote areas) regionalization of agro-industrial activities seems to be an one-way plan for future investments and enhancement of local economies. For example, the operational costs for industrial processing of the fruit stone/seed waste could be largely reduced if the residual material that is generated within one (or adjacent) region is shipped and collected into a single plant that is directly accessible by a large number of suppliers-fruit processors. The development and operational feasibility of such second-stage processing plants require clear business models that can target niche markets such as that of specialty oils, nutraceuticals with health claims, foods for specific groups of patients etc. For instance, the production of orange or peach seed oils with distinct organoleptic characteristics or seed extracts rich in valuable phytosterols and phenolic compounds requires innovative technological processes of high cost which however can be scaled down depending on the end-use of the added-value products.
The possibility to collect, dry, store, mix and then process different types of wasted by-products that are generated within the defined region is another management perspective that needs to be explored. Given the heterogeneity of the fruit seed wastes in terms of residual moisture, the drying facilities of each plant can take advantage of the local capacities in renewable energy sources, such as solar, wind and hydro plants. If end-product standardization and safety issues are well-described, such practices would help the new business to overwhelm the drying costs but also extend its seasonal operation. Considering the case of establishing a stone/seed processing plant in a region of Greece i.e., in the region of Central Macedonia, the selection of its location should satisfy several criteria; proximity to raw material suppliers/energy resources, consistency with regional cohesion policies that promote less competitive prefectures and EU rules towards a zero-waste economy. Thus, the plant should be very close to the major fruit canneries and juice producers of the country that operate intensively during the hot and dry period (from May to September) but also near to olive-oil mills and pomace processors that operate later in the season. The location should serve well also for the shipment of grape seeds from nearby wineries that operate mainly during the autumn months. Noticeably, grape seed by-products have gained a considerable market share during the last decade as sources of phenolic antioxidants and health promoting functions. Finally, the investors should consider also other important agro-industries of the RCM as potential suppliers; in that case, the valorization potential of solid wastes from the rice industry (rice hulls and bran) should be foreseen.
Acknowledgments
The authors sincerely thank the Editors for their kind invitation to contribute with an article and publish in the Special Issue “Food waste—strategies to reuse and prevention”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petropoulos, D.P. Analysis of the “Balassa” Index of fruit and vegetables in Greece (1981-2013). IORE J. Agro Crop Sci. 2016, 2, 1–10. [Google Scholar] [CrossRef]
- European Comission Agriculture and Rural Development. Agriculture in the European Union: Statistical and Economic Information; Agriculture and Rural Development European Commission: Brussel, Belgium, 2013. [Google Scholar]
- Food and Agriculture Organisation of the United Nations Statistics Division. Available online: http://www.fao.org/faostat/en/#data/QI (accessed on 5 February 2018).
- Klonaris, S.; Agiangkatzoglou, A. Competitiveness of Greek virgin olive oil in the main destination markets. Br. Food J. 2018, 120, 80–95. [Google Scholar] [CrossRef]
- Eurostat. Agriculture, Forestry and Fishery Statistics; Eurostat: Luxembourg, 2014; ISBN 9789279330056.
- United States Department of Agriculture. GAIN Report: Stone Fruit Annual EU-28; U.S. Mission to the European Union: Brussels, Belgium, 2017. [Google Scholar]
- Galanopoulos, K.; Mattas, K.; Baourakis, G. “Sixth Framework Program Priority 8.1 EU, Hellenic Ministry of Rural Development and Food. Market and Trade Policies for Mediterranean Agriculture: The Case of Fruit/Vegetable and Olive Oil”. Available online: http://www.minagric.gr/en/agro_pol/Works_en.htm (accessed on 17 December 2017).
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Naziri, E.; Nenadis, N.; Mantzouridou, F.T.; Tsimidou, M.Z. Valorization of the major agrifood industrial by-products and waste from Central Macedonia (Greece) for the recovery of compounds for food applications. Food Res. Int. 2014, 65, 350–358. [Google Scholar] [CrossRef]
- European Comission Environment DG. LIFE among the Olives: Good Practice in Improving Environmental Performance in the Olive oil Sector; Hervé, M., Ed.; European Comission Environment: Luxenburg, 2010; ISBN 978-92-79-14154-6. [Google Scholar]
- González-Hidalgo, I.; Bañón, S.; Ros, J.M. Evaluation of table olive by-product as a source of natural antioxidants. Int. J. Food Sci. Technol. 2012, 47, 674–681. [Google Scholar] [CrossRef]
- Tsimidou, M.Z. Virgin Olive Oil (VOO) and other olive tree products as sources of α-tocopherol. Updating and perspective. In Tocopherol: Sources, Uses and Health Benefits; Catala, A., Ed.; Nova Science Publisher: New York, NY, USA, 2012; pp. 1–21. [Google Scholar]
- Kalogeropoulos, N.; Tsimidou, M. Antioxidants in Greek virgin olive oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.G. Olives and Olive Oil Bioactive Constituents, 1st ed.; Boskou, D.G., Ed.; AOCS: Urbana, IL, USA, 2015; ISBN 9781630670429. [Google Scholar]
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenols in table olives. J. Agric. Food Chem. 2002, 50, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Pattara, C.; Cappelletti, G.M.; Cichelli, A. Recovery and use of olive stones: Commodity, environmental and economic assessment. Renew. Sustain. Energy Rev. 2010, 14, 1484–1489. [Google Scholar] [CrossRef]
- Amirante, P.; Clodoveo, M.L.; Tamborrino, A.; Leone, A.; Paice, A.G. Influence of the Crushing System: Phenol Content in Virgin Olive Oil Produced from Whole and De-Stoned Pastes; Elsevier Inc.: Amsterdam, The Netherlands, 2010; ISBN 9780123744203. [Google Scholar]
- Skoulou, V.; Zabaniotou, A. Investigation of agricultural and animal wastes in Greece and their allocation to potential application for energy production. Renew. Sustain. Energy Rev. 2007, 11, 1698–1719. [Google Scholar] [CrossRef]
- Regional Committee of Peloponesse. Available online: http://ppel.gov.gr/2016/12/ανακοίνωση-διαβούλευσης-στο-πλαίσιο/ (accessed on 5 Feburary 2018).
- Vlyssides, A.G.; Loizides, M.; Karlis, P.K. Integrated strategic approach for reusing olive oil extraction by-products. J. Clean. Prod. 2004, 12, 603–611. [Google Scholar] [CrossRef]
- Boskou, D.G. Phenolic Compounds in Olives and Olive Oil. In Olive oil Minor Constituents and Health; Boskou, D.G., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 11–44. ISBN 9781420059939. [Google Scholar]
- Angerosa, F.; Basti, C.; Vito, R.; Lanza, B. Effect of fruit stone removal on the production of virgin olive oil volatile compounds. Food Chem. 1999, 67, 295–299. [Google Scholar] [CrossRef]
- Luaces, P.; Romero, C.; Gutierrez, F.; Sanz, C.; Perez, A.G. Contribution of olive seed to the phenolic profile and related quality parameters of virgin olive oil. J. Sci. Food Agric. 2007, 87, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, E.; Mantzouridou, F.T. Current status and future challenges of table olive processing wastewater valorization. Biochem. Eng. J. 2016, 112, 103–113. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Barampouti, E.M.P.; Mai, S.T. Physical characteristics of olive stone wooden residues: Possible bulking material for composting process. Biodegradation 2008, 19, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Vlyssides, A.G.; Loizidou, M.; Zorpas, A.A. Characteristics of solid residues from olive oil processing as bulking material for co-composting with industrial wastewaters. J. Environ. Sci. Health Part A 1999, 34, 737–748. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; De Dios Alché, J.; Rodríguez-García, M.I. Characterization of olive seed storage proteins. Acta Physiol. Plant. 2007, 29, 439–444. [Google Scholar] [CrossRef]
- Esteve, C.; Marina, M.L.; García, M.C. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015, 167, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Prenzler, P.D.; Lavee, S.; Antolovich, M.; Robards, K. Quantitative changes in phenolic content during physiological development of the olive (Olea europaea) cultivar Hardy’s Mammoth. J. Agric. Food Chem. 2003, 51, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Macchioni, A.; Montedoro, G.; Agrarie, I.; Costanzo, V.S.; Chimica, D.; Elce, V.; Sample, M.; et al. Phenolic compounds of olive fruit: One- and Two-Dimensional Nuclear Magnetic Resonance characterization of nuzhenide and its distribution in the constitutive parts of fruit. J. Agric. Food Chem. 1999, 12–18. [Google Scholar] [CrossRef]
- Maestro-Duran, R.; Leon-Cabello, R.; Ruiz-Gutierrez, V.; Fiestas, P.; Vazquez-Roncero, A. Glucosidos fenolicos amargos de las semillas del olivo (Olea europaea). Grasas Aceites 1994, 45, 332. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.V.; Boas, L.V. Phenolic compounds and antioxidant activity of Olea europaea L. Fruits and leaves. Food Sci. Technol. Int. 2006, 12, 385–396. [Google Scholar] [CrossRef]
- Moghaddam, G.; Heyden, Y.V.; Rabiei, Z.; Sadeghi, N.; Oveisi, M.R.; Jannat, B.; Araghi, V.; Hassani, S.; Behzad, M.; Hajimahmoodi, M. Characterization of different olive pulp and kernel oils. J. Food Compos. Anal. 2012, 28, 54–60. [Google Scholar] [CrossRef]
- Ranalli, A.; Pollastri, L.; Contento, S.; Di Loreto, G.; Iannucci, E.; Lucera, L.; Russi, F. Acylglycerol and fatty acid components of pulp, seed, and whole olive fruit oils. Their use to characterize fruit variety by chemometrics. J. Agric. Food Chem. 2002, 50, 3775–3779. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, A.; Flamini, G.; Ben Selma, Z.; Le Dréau, Y.; Artaud, J.; Abdelhedi, R.; Bouaziz, M. Olive oil quality is strongly affected by cultivar, maturity index and fruit part: Chemometrical analysis of volatiles, fatty acids, squalene and quality parameters from whole fruit, pulp and seed oils of two Tunisian olive cultivars. Eur. J. Lipid Sci. Technol. 2015, 117, 976–987. [Google Scholar] [CrossRef]
- Ranalli, A.; Pollastri, L.; Contento, S.; Loreto, G.D.; Lannucci, E.; Lucera, L.; Russi, F. Sterol and alcohol components of seed, pulp and whole olive fruit oils. Their use to characterise olive fruit variety by multivariates. J. Sci. Food Agric. 2002, 82, 854–859. [Google Scholar] [CrossRef]
- da Silva, A.C.; Jorge, N. Bioactive compounds of the lipid fractions of agro-industrial waste. Food Res. Int. 2014, 66, 493–500. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M. Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind. Crops Prod. 2016, 83, 329–338. [Google Scholar] [CrossRef]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- Tsimidou, M.Z. Squalene and tocopherols in olive oil. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 561–567. ISBN 9780123744203. [Google Scholar]
- Naziri, E.; Tsimidou, M.Z. Squalene, a desirable hydrocabon of virgin olive oil. In Olive Oil at the Core of the Mediterranean Diet; Greek Lipid Forum: Athens, Greece, 2015; pp. 44–55. [Google Scholar]
- E.C. BIC of Attica. Olive Oil and Olive Pomace Oil Sector Analysis & Brief analysis of Table Olives Sector; E.C. BIC of Attica: Athens, Greece, 2012. [Google Scholar]
- Table Olives. Available online: http://doepel.gr/en_US/ (accessed on 2 Feburary 2018).
- Mylonas, P. Olive Oil: Establishing the Greek Brand; National Bank of Greece: Athens, Greece, 2015. [Google Scholar]
- Siddiq, M. Stone fruits production, postharvest storage, processing and nutrition. In Agricultural and Food Biotechnology of Olea Europaea and Stone Fruits; Muzzalupo, I., Micali, S., Eds.; Bentham Science Publishers Ltd.: Sharjah, UAE, 2015; pp. 309–383. ISBN 978-1-68108-002-4. [Google Scholar]
- Valta, K.; Damala, P.; Panaretou, V.; Orli, E.; Moustakas, K.; Loizidou, M. Review and assessment of waste and wastewater treatment from fruits and vegetables processing industries in Greece. Waste Biomass Valoriz. 2016, 1–20. [Google Scholar] [CrossRef]
- Kamel, B.S.; Kakuda, Y. Characterization of the seed oil and meal from apricot, cherry, nectarine, peach and plum. J. Am. Oil Chem. Soc. 1992, 69, 492–494. [Google Scholar] [CrossRef]
- Arvelakis, S.; Gehrmann, H.; Beckmann, M.; Koukios, E.G. Preliminary results on the ash behavior of peach stones during fluidized bed gasification: Evaluation of fractionation and leaching as pre-treatments. Biomass Bioenergy 2005, 28, 331–338. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels. EFSA J. 2016, 14, 1–47. [Google Scholar] [CrossRef]
- García, M.C.; González-García, E.; Vásquez-Villanueva, R.; Marina, M.L. Apricot and other seed stones: Amygdalin content and the potential to obtain antioxidant, angiotensin I converting enzyme inhibitor and hypocholesterolemic peptides. Food Funct. 2016, 7, 4693–4701. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially- available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Transition of phenolics and cyanogenic glycosides from apricot and cherry fruit kernels into liqueur. Food Chem. 2016, 203, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, T.; Durmaz, G.; Ateş, B.; Erdoǧan, A. Protective effect of dietary apricot kernel oil supplementation on cholesterol levels and antioxidant status of liver in hypercholesteremic rats. J. Food Agric. Environ. 2009, 7, 61–65. [Google Scholar]
- Zhang, J.; Gu, H.-D.; Zhang, L.; Tian, Z.J.; Zhang, Z.Q.; Shi, X.C.; Ma, W.H. Protective effects of apricot kernel oil on myocardium against ischemia-reperfusion injury in rats. Food Chem. Toxicol. 2011, 49, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Lazos, E.S. Composition and oil characteristics of apricot, peach and cherry kernel. Grasas y Aceites 1991, 42, 127–131. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.M. Fatty acids and tocopherol contents of some prunus spp. kernel oils. J. Food Lipids 2009, 16, 187–199. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Soliven, A.; Segliņa, D. Composition of bioactive compounds in kernel oils recovered from sour cherry (Prunus cerasus L.) by-products: Impact of the cultivar on potential applications. Ind. Crops Prod. 2016, 82, 44–50. [Google Scholar] [CrossRef]
- Chamli, D.; Bootello, M.A.; Bouali, I.; Jouhri, S.; Boukhchina, S.; Martínez-Force, E. Chemical characterization and thermal properties of kernel oils from Tunisian peach and nectarine varieties of Prunus persica. Grasas y Aceites 2017, 68, 1–9. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ünver, A.; Arslan, D. A research on evaluation of some fruit kernels and/or seeds as a raw material of vegetable oil industry. Qual. Assur. Saf. Crop. Foods 2014, 7, 187–191. [Google Scholar] [CrossRef]
- Femenia, A.; Rosselló, C.; Mulet, A.; Cañellas, J. Chemical composition of bitter and sweet apricot kernels. J. Agric. Food Chem. 1995, 43, 356–361. [Google Scholar] [CrossRef]
- Górnaś, P.; Ramos, M.J.; Montano, M.C.; Rudzińska, M.; Radziejewska-Kubzdela, E.; Grygier, A. Fruit pits recovered from 14 genotypes of apricot (Prunus armeniaca L.) as potential biodiesel feedstock. Eur. J. Lipid Sci. Technol. 2017, 1700147, 1–10. [Google Scholar] [CrossRef]
- Yilmaz, C.; Gökmen, V. Compositional characteristics of sour cherry kernel and its oil as influenced by different extraction and roasting conditions. Ind. Crops Prod. 2013, 49, 130–135. [Google Scholar] [CrossRef]
- Rudzińska, M.; Górnaś, P.; Raczyk, M.; Soliven, A. Sterols and squalene in apricot (Prunus armeniaca L.) kernel oils: The variety as a key factor. Nat. Prod. Res. 2017, 31, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Belitz, H.D., Grosch, W., Schieberle, P., Eds.; Springer: Berlin, Germany, 2009; Volume 107, ISBN 9783540699330. [Google Scholar]
- Radenkovs, V.; Feldmane, D. Profile of lipophilic antioxidants in the by-products recovered from six cultivars of sour cherry (Prunus cerasus L.). Nat. Prod. Res. 2017, 31, 2549–2553. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Radziejewska-Kubzdela, E.; Mišina, I.; Biegańska-Marecik, R.; Grygier, A.; Rudzińska, M. Tocopherols, tocotrienols and carotenoids in kernel oils recovered from 15 apricot (Prunus armeniaca L.) genotypes. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 693–699. [Google Scholar] [CrossRef]
- Bak, I.; Lekli, I.; Juhasz, B.; Varga, E.; Varga, B.; Gesztelyi, R.; Szendrei, L.; Tosaki, A. Isolation and analysis of bioactive constituents of sour cherry (Prunus cerasus) seed kernel: An emerging functional food. J. Med. Food 2010, 13, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.E.; Gallyas, E.; Bak, I.; Rakotovao, A.; Boucher, F.; De Leiris, J.; Nagy, N.; Varga, E.; Tosaki, A. Heme oxygenase-1-related carbon monoxide and flavonoids in ischemic/reperfused rat retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Lekli, I.; Juhasz, B.; Nagy, N.; Varga, E.; Varadi, J.; Gesztelyi, R.; Szabo, G.; Szendrei, L.; Bacskay, I.; et al. Cardioprotective mechanisms of Prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1329–H1336. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Priksz, D.; Lampé, N.; Bombicz, M.; Kurucz, A.; Szabó, A.; Pósa, A.; Szabó, R.; Kemény-Beke, Á.; Remenyik, J.; et al. Protective effect of Prunus cerasus (sour cherry) seed extract on the recovery of ischemia/reperfusion-induced retinal damage in Zucker diabetic fatty rat. Molecules 2017, 22, 1782. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Greece: Stone Fruit Annual; United States Department of Agriculture: Washington, DC, USA, 2014.
- Greek Ministry of Rural Development and Food Apricots. Available online: http://www.minagric.gr/index.php/el/for-farmer-2/crop-production/oporokipeytika/1773-berikoka (accessed on 7 Febuary 2018).
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Manners, G.D. Citrus Limonoids: Analysis, bioactivity, and biomedical prospects. J. Agric. Food Chem. 2007, 55, 8285–8294. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Brodbelt, J.S.; Bhat, N.G.; Patil, B.S. Methods for the separation of limonoids from citrus. ACS Symp. Ser. 2006, 936, 34–51. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.; Torre, G.; Saro, M.; Dugo, P.; Mondello, L. Underestimated sources of flavonoids, limonoids and dietary fiber: Availability in lemon’s by-products. J. Funct. Foods 2014, 9, 18–26. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.; Inferrera, V.; Dugo, P.; Mondello, L. Underestimated sources of flavonoids, limonoids and dietary fiber: Availability in orange’s by-products. J. Funct. Foods 2015, 12, 150–157. [Google Scholar] [CrossRef]
- Minamisawa, M.; Yoshida, S.; Uzawa, A. The functional evaluation of waste yuzu (Citrus junos) seeds. Food Funct. 2014, 5, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Miyake, M.; Inaba, N.; Ayano, S.; Ifuku, Y.; Hasegawa, S. Limonoid glucosides of Satsuma mandarin (Citrus unshiu Marcov.) and its processing products. In Citrus Limonoids: Functional Chemicals in Agriculture and Foods; Berhow, M., Hasegawa, S., Manners, G., Eds.; American Chemical Society: Washington, DC, USA, 2000; pp. 107–119. [Google Scholar]
- Miller, E.G.; Taylor, S.E.; Berry, C.W.; Zimmerman, J.A. Citrus limonoids: Increasing importance as anticancer agents. In Citrus Limonoids: Functional Chemicals in Agriculture and Foods; Berhow, M., Hasegawa, S., Manners, G., Eds.; American Chemical Society: Washington, DC, USA, 2000; pp. 132–144. [Google Scholar]
- Wang, H.; Chen, G.; Guo, X.; Abbasi, A.M.; Liu, R.H. Influence of the stage of ripeness on the phytochemical profiles, antioxidant and antiproliferative activities in different parts of Citrus reticulata Blanco cv. Chachiensis. LWT Food Sci. Technol. 2016, 69, 67–75. [Google Scholar] [CrossRef]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crops Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Bocco, A.; Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidant activity and phenolic composition of Citrus peel and seed extracts. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Habib, M.A.; Hammam, M.A.; Sakr, A.A.; Ashoush, Y.A. Chemical evaluation of egyptian citrus seeds as potential sources of vegetable oils. J. Am. Oil Chem. Soc. 1986, 63, 1192–1196. [Google Scholar] [CrossRef]
- Cassia, R.M.; Mieko, K.; Neuza, J. Phytochemicals and antioxidant activity of Citrus seed oils. Food Sci. Technol. Res. 2012, 18, 399–404. [Google Scholar] [CrossRef]
- Jorge, N.; da Silva, A.C.; Aranha, C.P.M. Antioxidant activity of oils extracted from orange (Citrus sinensis) seeds. An. Acad. Bras. Cienc. 2016, 88, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Matthaus, B.; Özcan, M.M. Chemical evaluation of citrus seeds, an agro-industrial waste, as a new potential source of vegetable oils. Grasas y Aceites 2012, 63, 313–320. [Google Scholar] [CrossRef]
- Ueno, H.; Tanaka, M.; Machmudah, S.; Sasaki, M.; Goto, M. Supercritical carbon dioxide extraction of valuable compounds from Citrus junos seed. Food Bioprocess Technol. 2008, 1, 357–363. [Google Scholar] [CrossRef]
- Guneser, B.A.; Yilmaz, E. Bioactives, aromatics and sensory properties of cold-pressed and hexane-extracted lemon (Citrus limon L.) seed oils. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 723–731. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Varzakas, T.H. Fruit/fruit juice waste management: Treatment methods and potential uses of treated waste. In Waste Management for the Food Industries; Arvanitoyannis, I.S., Ed.; Elsevier Academic Press: Amsterdam, the Netherland, 2008; pp. 569–628. [Google Scholar]
- Karaouzas, I. Agro-industrial wastewater pollution in Greek river ecosystems. In The Rivers of Greece: Evolution, Current Status and Perspectives; Skoulikidis, N., Dimitriou, E., Karaouzas, I., Eds.; Springer-Verlag: Berlin, Germany, 2018; pp. 169–204. [Google Scholar]
- U.S. Department of Agriculture. GAIN Report: Greece Citrus Annual 2014; U.S. Department of Agriculture: Rome, Italy, 2014.
- Greek Ministry of Rural Development and Food Citrus. Available online: http://www.minagric.gr/index.php/el/for-farmer-2/crop-production/oporokipeytika/505-esperidoeidi/1713-stoixeia-esper-greece (accessed on 9 Febuary 2018).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).