Abstract
The primary production of sulfide concentrates includes smelting to copper matte or blister copper, conversion of matte to blister copper, and refining to copper. Smelting, converting, and fire-refining can use a limited amount of secondary materials. Molten copper can effectively dissolve many metals, from valuable noble metals to harmful impurities such as bismuth. However, some of the impurity metals in copper are valuable in other applications. In this paper, we outline the main material flows in copper smelting and electrorefining and describe how minor metals can be recovered from secondary raw materials using copper as a carrier material. We will use a system integrated approach to define the factors that affect the recovery of different metals and copper quality. Metals typical in copper production are used as examples, like noble metals, As, Bi, Se, and Te, including metals in the EU critical raw materials list like PGM and Sb.
1. Introduction
The refinery production of copper in 2015 was estimated to be 22.9 million tonnes [1]. In 2014, the refinery production was 22.2 Mt, of which approximately 14.2 Mt was primary electrorefined and fire-refined and 4.1 Mt electrowon, and secondary production was 3.9 Mt [2]. Based on U.S. Geological Survey (USGS) dataseries [3] during 1990–2011, the increase of primary copper production was 0.39 Mt/year whereas the increase of secondary copper production was less than 0.07 Mt/year. The global consumption of refined copper was 22.9 Mt in 2014 [2]. In the 2010s, the global consumption has exceeded the global production [2]. The recycling input ratio has decreased from 35% to about 30% during the last ten years [1].
Copper uses can be classified, for example, into electrical, electronics and communications, construction, transportation, industrial machinery and equipment, and consumer and general products [1]. Electrical and electronic products include wire and equipment for the power and telecommunication utilities, business electronics, and lighting and wiring devices. Building construction includes electrical wire, plumbing and heating, air conditioning and commercial refrigeration, builders’ hardware, and architectural uses. Transportation equipment includes road, rail, marine, air, and space vehicles. Industrial machinery and equipment includes in-plant equipment, industrial valves and fittings, nonelectrical instruments, vehicles, and heat exchangers. Consumer and general products includes different appliances, cord sets, ordnance and ammunition, consumer electronics, fasteners and closures, coinage, utensils and cutlery, and miscellaneous products [4].
Copper scrap is generated at all stages in the life span of a copper product, including metal production, product manufacturing, and post-consumer disposal [5]. Scrap produced in metal production, like copper anode scrap, is called home scrap. Scrap from product manufacturing is new scrap and scrap collected as obsolete products is old scrap [6]. The purest copper scrap is re-melted and recast without refining to non-electrical applications. Less pure copper scrap is smelted in a primary or secondary smelter and refined. Alloy scrap is usually recycled directly to make a new alloy, as there is no advantage to smelt and refine it to pure copper [6]. Copper scrap grades are specified, for example, in Technical Specification CEN/TS 13388:2013 “Copper and copper alloys. Compendium of compositions and products” by the European Committee for Standardization (CEN). The CEN/TS 13388 describes 13 different copper scrap grades with maximum impurity levels and sources. The CEN/TS 13388 also describes 11 different brass scrap grades and six alloy grades originating from heat exchangers. The Institute of Scrap Recycling Industries, Inc. (ISRI) publishes Scrap Specifications Circular that describes over 40 different copper and copper alloy scrap grades by code words [7]. The ISRI scrap grades are defined using material and its source, and most of the descriptions include minimum copper levels and sometimes maximum impurity levels.
A deciding factor in the use of secondary sources is the required quality of the produced metal. Copper can be recycled repeatedly without the loss of important properties due to extremely efficient electrolytic refining. The previous process stages such as converting and fire-refining also play an important role in the production of high-quality copper. The traditional smelting, converting, and electrorefining route for primary copper production and some material flows of impurities and valuable by-products will be discussed.
2. Primary Copper Production
The EU Commission Implementing Decision 2016/1032 establishing BAT for the non-ferrous metals industries defines primary production as the production of metals using ores and concentrates and secondary production as the production of metals using residues and/or scraps, including remelting and alloying processes. Traditional copper primary production uses chalcopyrite-pyrite concentrates as the raw material, but copper scrap and other secondary materials are recycled into different furnaces. The main copper mineral chalcopyrite can contain about 15 associated elements, like noble metals, As, Sb, Bi, etc., whereas the secondary materials may include more than 40 elements as alloys and compounds for product functionality reasons [8,9]. To maximize the resource efficiency of secondary materials, it is necessary to consider a product-centric or mineral-centric approach instead of focusing on a single major metal as in the material-centric approach [9]. Product- and mineral-centric approaches take into account the flows of minor valuable and harmful elements in the process.
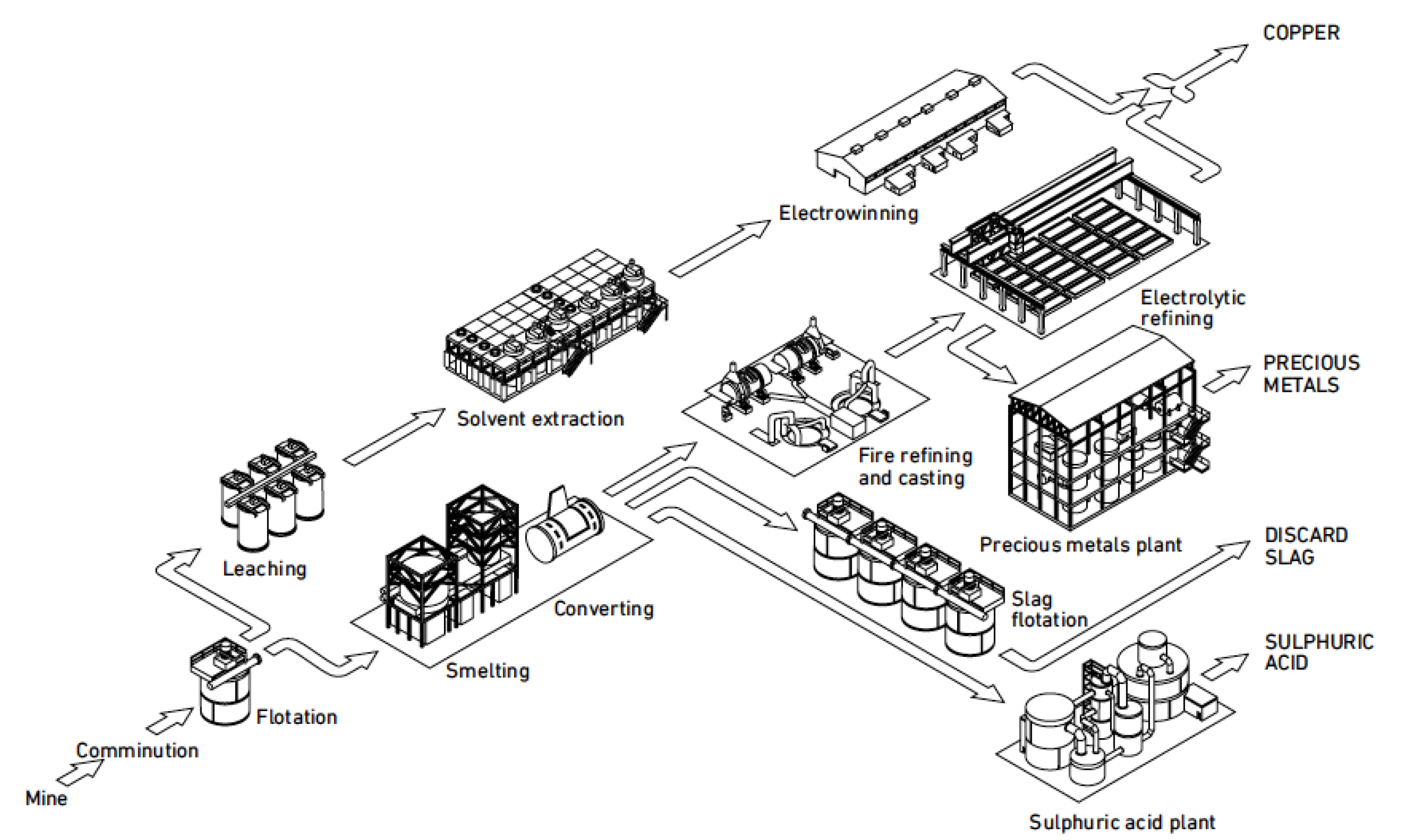
Copper can be extracted using various combinations of pyro- and hydrometallurgical methods. The main primary processing route is the smelting of sulfide concentrate to Cu2S-FeS matte followed by the converting of matte to blister copper that is fire-refined and electrorefined, and this process route produced 14.2 Mt in 2014 [2]. The hydrometallurgical leaching, solvent extraction, and electrowinning route produced 4.1 Mt in 2014 [2], but this process route will not be discussed in this paper as it is not used for secondary materials. The primary pyrometallurgical and hydrometallurgical processing routes are outlined in Figure 1. Secondary copper can be added at three locations in the primary smelting and electrorefining coppermaking process. The most common unit is the converter, but scrap is also added in the smelting and anode furnaces.
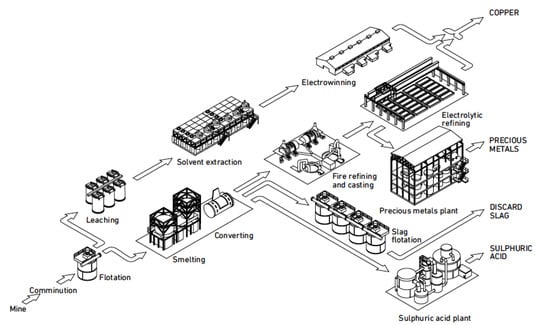
Figure 1.
The process routes of primary copper production [10].
System integration in engineering is a process to bring together the component sub-systems into one system and ensure that the subsystems function together as a system. The purpose of a primary smelter and refinery is to produce copper, so the operation of any sub-system should not compromise this task. The main process stages for copper production include feed preparation, smelting, converting, fire-refining, anode casting, and electrorefining, also together six process stages or subsystems. The treatment of offgases from smelting includes dust collection and recycling, and acid production. Sometimes, the offgas treatment can include impurity removal from dust before dusts are recycled back to the smelting furnace. The offgases from converting and fire-refining often contain so little SO2 that acid production cannot be achieved. The offgas treatment thus includes two to three subsystems. The treatment of slag includes cooling, crushing, grinding, and flotation. Most of the slags produced in different smelters have such a high copper concentration that they have to be processed to a new concentrate. Valuable metals not associated with copper compounds are usually lost in the discarded slag. The slag treatment thus includes three to four subsystems. The border between pyrometallurgical and hydrometallurgical stages is anode casting. Subsystems associated with electrorefining are electrolyte purification and anode slime treatment. The treatment of the electrorefining electrolyte includes Cu removal, Sb-Bi-As removal, and Ni removal, which make together three subsystems. The treatment of anode slime includes processes like decopperizing, selenium recovery, Doré smelting, tellurium recovery, and two to three electrowinning stages for noble metals recovery, so the anode slime treatment includes six to eight stages. A primary copper smelter and refinery thus has six subsystems for copper production and about 15 subsystems for internal recycling, waste treatment, and recovery of valuable products.
The primary smelter and refinery plant uses concentrate as a raw material, produces copper cathodes, sulphuric acid, and precious metals, and discards slag and offgases. Up to the production of blister copper in the converter, the main target is to remove iron and sulfur. After blister copper production, the purpose is to remove impurities. The primary raw material for smelters is copper sulphide concentrate with 20–30% Cu and the current average is ~25% Cu, which is significantly lower than in the beginning of 2000s [11]. The smelting subsystem produces matte with 45–75% Cu, the most common being 56–60% Cu [11]. The smelter furnace feed also includes recycled slag and smelter dusts and can include secondary materials. The converter subsystem takes in matte and secondary materials and produces blister copper (98–99% Cu). The fire-refining and casting of blister copper to anodes (>99% Cu) removes dissolved gases and some metallic impurities. The aim of final electrolytic refining is to produce cathodes with a maximum of 65 ppm metallic impurities. The main process stages to recover valuable elements and remove excess impurities are anode slime treatment and electrolyte purification.
The increasing copper concentration along the main process flow indicates that impure secondary materials should be fed in the beginning of the process and only pure materials should be used at later stages. Otherwise, impure secondary materials could compromise the copper quality. The use of secondary raw materials is determined by their impurity content and the capability of a unit process to remove those impurities. Slag and dust formation, heat balance, and the composition of the offgas that is sent to the acid plant must also be considered. As the phenomena in all process stages are complex, only approximate distributions of elements from input streams to output streams can be made.
2.1. Smelting
The smelting of copper concentrates to an iron and copper sulphide matte is done by flash smelting or bath smelting. Flash smelting methods include Outotec and INCO flash smelters. Flash smelting relies on the oxidation and smelting of airborne particles in dry feed. The reacted particles fall into a settler where the separation of matte and slag takes place. Matte and slag are tapped and processed further, and the offgases pass from the furnace through the uptake shaft to dust collection and a sulphuric acid plant. Bath smelting is carried out in a number of furnaces such as the reverberatory, blast, electric, Ausmelt/Isasmelt, Noranda, Mitsubishi, El Teniente, Baiyin, and Vanyukov furnaces. In bath smelting, the oxidation and smelting processes take place in a molten bath, and various slag and matte separation and tapping methods are used in different furnaces. The differences between bath processes are the position of air/oxygen or fuel addition points. Bath smelters usually have a separate holding furnace or settler. Based on 2016 survey data, 50% of smelters use flash smelting and 50% use bath smelting technology [11].
Both flash smelters and bath smelters use the fuel value of the feed material to smelt the charge. In the smelting of primary concentrates, the energy comes from the oxidation of sulphur. In the smelting of secondary materials, the fuel value is usually in polymer materials like printed circuit boards (PCB) in waste electrical and electronic equipment (WEEE). The flash smelting process cannot easily treat recycled scrap, because the feed has to be very dry and its particle size has to be less than 100 μm [12]. Bath smelting processes can deal with various raw materials as processing to small size and efficient drying are not needed. The stirring from gas blowing also promotes the smelting of scrap [12].
In the smelting of copper concentrates, the aim is to produce a liquid Cu2S-FeS matte. This is achieved by using a high temperature and low oxygen partial pressure. In matte smelting, iron and sulfur are removed into slag and offgases. The common slag chemistry in smelting is based on fayalite (2FeO·SiO2). The amount of iron in the concentrate affects the amount of slag formed during smelting. The matte and the slag are immiscible, and the lighter slag floats above the matte. Some of the impurities in the feed materials such as As, Bi, Sb, and Pb have a high affinity to copper and the slag must have the capacity to absorb them. Valuable metals such as precious metals will largely dissolve in copper and they must be contained in copper until the electrorefining step. Generally, the distribution of a metal between matte and slag is described by the distribution coefficient, Equation (1):
where [Me]matte and [Me]slag refer to the metal concentration (usually in wt-% or ppm) in the respective phases. The distribution coefficients are not constants and depend, for example, on temperature, copper grade, and oxygen partial pressure [12,13]. Increasing the matte grade results in a higher copper loss in slag and more As and less Bi and Pb in matte, whereas Sb and Ni distributions are not affected by matte grade [14,15]. With a higher copper grade and lower temperature, the distribution of noble metals to matte increases [13].
The intermediate product from smelting is either sulfide matte for converting or blister copper for fire refining. The distribution of elements between matte and slag or matte and offgas greatly depends on the smelting method and product. For example, the matte from the flash smelting furnace contains 90–95% of Ag, Au, and PGM, 60–70% of Sb, 30–75% of Bi, 15–40% of As, 70–80% of Ni, 85% of Se, and 60–80% of Te contained in the feed [6]. In bath smelting furnaces, less of the harmful impurities like As, Bi, and Sb distribute into the matte. The Ni and Se behave in bath smelting as in flash smelting. Some of the valuable metals can also end up in slag and flue dusts. The higher the matte copper grade, the more copper and other valuable metals are absorbed into the slag. The copper smelting slags usually contain over 1% Cu [11]. The slag is processed by flotation or electric arc furnace settling to recover copper that is then used in the smelter feed. The dusts are usually recycled back to smelting without further treatment [6].
2.2. Converting
Converting is the oxidation of the sulphide matte to blister copper. The most common converting process is the batch process that uses a cylindrical rotary Peirce-Smith converter to oxidize FeS to slag followed by the oxidation of Cu2S to blister copper, as represented in Reactions (2)–(4).
FeS + 1.5 O2 = FeO + SO2
Cu2S + 1.5 O2 = Cu2O + SO2
Cu2S + 2 Cu2O = 6 Cu + SO2
As Reactions (2) to (4) are exothermic, scrap is added for cooling. The amount of scrap can be more than 35% of the charge [6]. This cold material contains anode scrap returned from electrorefining, rejected anodes, copper-rich slag, and other rather pure materials. The excess heat in the converter also facilitates the breakdown of organic material, present, for example, in printed circuit boards and cable insulation [16].
In copper converting the distribution of minor elements occurs between Cu2S and Cu phases. The Cu2S and Cu phases are almost pure and their activities close to unity. The distribution coefficients between metal and sulphide phases Lm/s(Me) are usually over 1 and they are mostly temperature-dependent [12]. This means that the minor elements tend to dissolve in blister copper. The distribution of noble metals in blister copper is high, enabling their recovery [17]. The distribution of elements in converting depends on the matte copper grade. With a low grade matte, more As, Sb, and Bi end up as dust in the offgas, whereas with a high grade matte, they dissolve in blister copper. The use of higher matte grades in smelters has led to increased levels of impurities in anode slimes due to the reduced amount of converter slag produced [6]. The As and Sb are enriched in molten copper. Bi is mostly volatilized, but also dissolves in copper [14]. Ni and Co are distributed into the slag. Valuable Ge, Ga, and In from WEEE are distributed into the slag [17].
2.3. Fire Refining and Anode Casting
Fire refining is used to control the sulphur, oxygen, and impurity levels in copper before the casting of anodes. Fire refining is done by first blowing air through molten copper to oxidise impurities and reduce the sulphur level. The slag is removed prior to the second blowing of hydrocarbons for the partial removal of dissolved oxygen from liquid copper. Anode furnaces are similar to the Peirce-Smith converter. The furnaces are charged with molten blister copper, anode scrap, and rejected anodes. The anode furnace is suited to melt and refine high-grade scrap with a copper content greater than 90% [16]. One way to recycle old scrap in primary production is to feed high-grade copper scrap, such as copper granules made from cables, directly into the anode furnace [16].
The distribution of impurity elements in fire refining can be divided into three groups using the equilibrium constant of Reaction (5):
Cu2O(l) + Me(l) = 2 Cu(l) + MeO(s,l)
The impurities with equilibrium constant K values of less than 10−2, like Au, Hg, Ag, Pt, Pd, Se, Tl, and Te, will not oxidize. These elements dissolve in molten copper and end up in the anode slime during electrorefining. Metals with K values higher than 103, like Fe, Zn, Na, Cr, Mn, Si, Ti, Al, Ba, Mg, Be, and Ca, will oxidize easily and they will not remain in anode copper. Elements with intermediate K values cause problems in the recovery or removal from copper, and they include Bi, Pb, Ga, Ni, Cd, Sb, As, Co, Ge, Sn, and In [18].
After the anode furnace treatment, the impure copper anodes contain an average of 99.2–99.3% Cu. The main impurities are Ni+Co (1370–2040 ppm), O (1460–1630 ppm), Pb (1000–1290 ppm), As (790–1480 ppm), Sb (240–350 ppm), and Bi (130–300 ppm), and valuable metals are Ag (710–810 ppm), Au (30–60 ppm), Se (340–410 ppm), and Te (90–110 ppm) [19]. The anodes contain different oxide, selenide, and telluride compounds. As, Sb, and Bi can result in floating slimes if the molar ratio of As/(Sb+Bi) in the anode is less than 2 [19]. A uniform anode composition is desired in order to achieve homogeneous dissolution during electrorefining.
2.4. Electrorefining
In the electrorefining, impure copper anodes are dissolved and copper is deposited as a pure cathode. The elements more noble than copper will report in anode slime and less noble elements dissolve into the electrolyte. Depending on the composition and mineralogy of the anode and composition of the electrolyte, several other reactions can happen, and these can affect the cathode quality. The aim of copper producers is to produce marketable cathode copper the required properties are described, for example, in technical specification CEN/TS 13388 “Copper and copper alloys. Compendium of compositions and products” describes copper cathode grade Cu-CATH-1 with a maximum of 65 ppm metallic impurities and grade Cu-CATH-2 with a minimum of 99.90% Cu and maximum of 0.03% metallic impurities (excluding Ag). Typically, Cu-CATH-1 is used to make electrolytic tough pitch copper that is suitable for most applications. The London Metal Exchange (LME) Grade A is identical to Cu-CATH-1 grade. New York Mercantile Exchange (NYMEX) uses ASTM B115-10 Cathode Grade 1 and Shanghai Futures Exchange (SHFE) uses GB/T 467-2010 specifications. These grades are almost the same as Cu-CATH-1, with minor differences in Bi, Se, Te, and Ni maximum levels.
The composition of the anodes can affect both the operation of the electrorefining and the cathode quality. The dissolving Ni and As will affect electrolyte conductivity, viscosity, and density. These physical properties affect the energy consumption of electrorefining. Excess Ni and As in the electrolyte can also affect copper solubility and the copper deposition rate. Some of the anode impurities will form anode slimes that can adhere on the anode surface, fall to the electrolysis cell bottom, or form floating slimes. Adherent slimes can cause an increase in the cell voltage or even anode passivation. Unless the slimes fall rapidly to the bottom, they can move to the cathode surface, thus causing contamination.
The main cathode contamination mechanisms are electrolyte inclusions due to rough and uneven deposit growth, codeposition, and the inclusion of suspended or floating slimes [20]. Almost all lead, tin, and selenium from the anode is transferred into the bottom slime. Bismuth and antimony dissolve, but can also form floating slimes. Arsenic, nickel, and cobalt dissolve and remain in the solution. The most troublesome impurities, which are As, Sb, and Bi, dissolve into solution but they can form floating slimes when their concentrations are high enough due to accumulation in the solution. The amorphous floating slimes typically have compositions of Sb-As-O and Bi-As-O. To maintain cathode quality, both anode and electrolyte impurity levels have to be controlled.
2.5. Anode Slime Treatment and Electrolyte Purification
The anode slime from electrorefining is collected and processed to recover noble metals, Se and Te. Today, the average slime composition is 21.5% Cu, 12.7% Ag, 0.6% Au, 9.0% Se, 1.6% Te, 10.8% Pb, 2.5% Ni, 3.4% As, 1.5% Sb, and 1.5% Bi [19]. Lead is mostly as insoluble PbSO4. Silver and gold occur both in solid solution and as selenides and tellurides. Antimony, arsenic, and bismuth form a series of multiple oxides [6]. Typical anode slime treatment includes decopperizing to dissolve Cu, Ni, and Te for recovery, roasting to recover Se, and smelting the noble metals to Doré anodes followed by sequential electrolysis stages to recover Ag, Au, and PGM.
The purpose of electrolyte purification is to control the copper level and to remove excess impurities. The electrolyte purification consists of electrowinning to recover copper and crystallization to recover metal salts. The recovered and purified sulphuric acid and copper sulphate are used to make a new electrolyte. The As/Sb/Bi equilibrium that can cause the formation of floating slimes can also be used to control the levels of these metals, usually with the use of reducing compounds like SO2 and adsorptive materials [21].
3. Copper-Containing Secondary Materials
Secondary copper sources include primary production waste, industrial waste, and electronic waste. “Home scrap” is the scrap generated at the smelter or refinery and includes slag, dust, anode scrap, and slime etc. It is usually recycled at the plant. “New scrap” is generated at downstream metal fabricators, like in semifinished products (bar, tube, wire etc.). It can be recycled at the fabricator or in a primary production plant. Obsolete consumer products and WEEE belong to “old scrap”. In 2010, primary copper production was 16.2 Mt and secondary copper production from new scrap was 4.2 Mt and from old scrap was 5.2 Mt [22]. The amount of WEEE generated in 2010 was 33.8 Mt, containing about 3.5 Mt copper [22]. In 2014, the amount of WEEE was 41.8 Mt, of which only 15.5% was properly recycled [23]. The amount of WEEE is expected to increase to 50 Mt or 6.7 kg per capita in 2018 [23]. The increase in the WEEE amount is expected to require more secondary smelter capacity.
The technical specification CEN/TS 13388 describes 13 copper scrap grades with a minimum of 96–99.90% copper with the main impurities being iron, lead, and zinc, 11 brass scrap grades, and six heat exchanger tube scrap grades. The European standard EN 12861:1999 “Copper and copper alloys. Scrap” specifies the same scrap grades as CEN/TS 13388 and they are used for direct melting. This means that their impurity levels are low enough for the planned manufacturing purpose and electrolytic refining is not necessary. Copper alloys can contain antimony, arsenic and phosphorus to inhibit dezincification, lead, tellurium, selenium and sulfur for improved machinability, aluminium for resistance to impingement corrosion, and chromium to form a heat-treatable alloy. These alloying elements in copper scrap can become impurities when processing to new cathode copper.
The metal composition of different electronic scraps varies widely depending on age, origin, and product manufacturing techniques. In WEEE, including all types of collected products, copper makes up about 7% of the weight, the printed circuit boards 3.1%, and other non-ferrous metals (excluding Al) 1% [24]. These fractions could be recycled in copper production. The base metals in printed circuit boards include 11–40% Cu, 0.3–3% Pb, 0.3–2.7% Sn, 0.3–3.2% Zn, and 0.05–0.9% Ni; noble metals of 50–5700 ppm Ag, 10–1300 ppm Au, and 3–50 ppm PGM; and major impurities of up to 3500 ppm Sb, 690 ppm Bi, and 27 ppm As [24]. The major economic driver for the recycling of electronic waste is the recovery of precious metals, but copper has almost the same economic value as the noble metals [23]. WEEE has been processed in primary copper smelters, e.g., by Noranda in Canada as additions to Peirce-Smith converters and Boliden in Sweden who smelt WEEE containing scrap to black copper, and then add this to Peirce-Smith converters.
4. Distribution of Elements
The main primary raw material for copper smelting is CuFeS2-FeS2 concentrate that usually includes, besides Cu and Fe, Ag, Au, PGMs, As, Sb, Bi, Mo, Ni, Pb, Se, and Te. Considering the minor elements in copper concentrates, many of them are harmful to the environment or human health, cause operating difficulties or may affect product quality. In customs smelting, they are known as penalty elements and include, for example, As, Bi, Cd, Cl, Co, F, Hg, Ni, Pb, Sb, and Zn [25]. Copper scrap can introduce Mo, Ni, Mn, Sn, and Zn into the material flow. WEEE scrap contains precious metals Ag, Au, Pd, and Pt; base metals including Cu, Al, Ni, Sn, Zn, and Fe; and metals that need attention like Hg, Be, In, Pb, Cd, As, Sb, and Bi [26]. Scrap recycling shall not disturb electrorefining by excess impurities. Table 1 shows a compilation of matte and metal compositions in various stages of the primary smelting and electrorefining process. The composition ranges for concentrates in Table 1 is wide, because it includes several concentrate types. The concentrates from primary sulfides (chalcopyrite and bornite) contain 20% to 30% copper, whereas the secondary sulfide concentrates (chalcocite and covellite) can have copper concentrations of up to 40% [27]. Typically, the concentrations of the main elements are 22–32% Cu, 23–30% Fe, and 27–35% S [6]. For Cu-CATH-1 grade, all impurity metals do not have individual limits, and instead, the following combined limits apply:

Table 1.
Examples of phase compositions in primary smelting and electrorefining of copper (wt-%).
- (As + Cd + Cr + Mn + P + Sb) max. 15 ppm.
- (Bi + Se + Te) max. 3 ppm, (Se + Te) max. 3.0 ppm.
- (Co + Fe + Ni + Si + Sn + Zn) max. 20 ppm.
Based on the 2016 survey results, many electrorefineries can reach critical impurity levels in the cathode copper that are only 10% of the accepted maximum in Cu-CATH-1 [28].
The main secondary source of noble metals is WEEE scrap, and some silver can be in copper alloy scrap. In pyrometallurgical process steps, all the noble metals will dissolve into matte, black copper, blister copper, and anode copper. During electrolysis, the noble metals end up in anode slime from where they can be recovered by pyrometallurgical and electrolytic methods.
The secondary sources of base metals are metal scrap and WEEE scrap. Aluminium is rejected into slag, but can increase slag viscosity and copper losses. Most of the nickel and 50% of cobalt distribute into copper phases. Excess Ni in the anode will form compounds that produce slime in electrorefining. In electrorefining, nickel dissolves and is removed in the electrolyte purification circuit. Some of the nickel ends in anode slime. Pb, Zn, and Sn are rejected in slag or offgas, especially in bath smelting, and in converting, less than 10% of these end up in blister copper. Pb can also be removed in the anode furnace by using silica flux. Pb, Zn, and Sn end up in anode slime during electrorefining.
The main impurities that affect the electrolysis process are As, Sb, Bi, and Pb [28]. Their sources are primary raw materials and WEEE scrap. These metals have a complex effect on the anode metallurgy, but usually the target is to limit their concentrations and at the same time have a high ratio of As compared to the others. The aim in anode composition control is to ensure smooth dissolution, prevent excess slime, and prevent passivation. During smelting, Bi and some of the As and Sb are distributed in flue dusts, and they can be recovered by hydrometallurgical methods. During electrorefining, As, Sb, and Bi accumulate in the electrolyte and their concentration must be controlled. The levels of Sb and Bi must be only hundreds of mg/L, whereas As can be tolerated in grams per liter level. A high As/(Sb+Bi) molar ratio in the electrolyte prevents the formation of floating slimes that can contaminate the cathode. Lead will precipitate in anode slime. The As, Sb, and Bi are usually removed in the last electrolyte purification stages as impure Cu-As-Sb-Bi deposits. Methods to leach and recover valuable Sb and Bi are beginning to emerge in industrial scale.
5. Discussion
The main reasons to recycle scrap in primary copper production are the control of internal home scrap flows, need of cold material for converter cooling, and recovery of noble metals from old scrap, especially from WEEE. Anode scrap from electrorefining constitutes, on average, less than 13% of the anode weight and a few percent of anodes are rejected after casting [6,19]. These home scrap flows are often not enough for converter cooling purposes, so purchased copper scrap is needed. Purchased scrap lots are usually rather pure, as the alloying elements have little value and the aim is to remove them from copper. The use of old scrap can introduce foreign elements, but their levels are controlled by pyrometallurgical processing. High nickel is the main factor that can affect electrolysis. Metal scrap will not introduce harmful elements As, Sb, Bi, Se, and Te.
The recycling of WEEE in primary copper production is done mainly to recover noble metals. For example, in [29], the total WEEE value distribution was 29% ferrous metal, 26% Cu, 24% Au, 11% Al, 6% PGMs, and 2% for both Sn and Ag. The value distribution in PCBs was 59% Au, 15% PGMs, 15% Cu, 7% Sn, and 4% Ag. The PCBs only constitute 4% of the total WEEE weight but contain 40% of the metal recovery value [29]. WEEE can be fed in pyrometallurgical steps, but flash smelting can take only small-size feed, and the burning of organic material creates excess heat in the converter. Using the metal wheel and carrier metal approach, it can be concluded that Ag, Au, PGMs, Pb, Sn, Zn, As, Sb, and Bi follow copper and can be recovered in further processing stages. Metal oxides are probably lost in slags and REEs that are not soluble in copper are lost. As the PCBs have high levels of Pb, Sb, and Bi compared to As [24], the As level in both anodes and the electrolyte has to be maintained for trouble-free operation.
Metal recycling has always been practiced, but nowadays, the recovery of a certain metal may not be feasible due to technological limitations. In certain cases, it may not be technically possible or economical to recover metals from secondary streams because they are in too low concentrations or in more problematic forms than in natural ores. This results in, for example, the loss of Ge, Ga, and In from WEEE to slags during smelting. Secondary smelting and hydrometallurgical methods are more suitable for recovering small amounts of valuable and specialty metals than primary smelting and refining. The recycling of old scrap can be technologically challenging unless the obsolete products can be dismantled or mechanically treated to separate metal fractions. The contamination of main metal by other materials results in tramp elements that can have a detrimental effect on metal properties. Increasing the Ni, Sb, Bi, and Pb levels from WEEE increases the risk of cathode contamination.
6. Conclusions
The purpose of primary copper production is to produce copper cathodes with less than 65 ppm metallic impurities. This can only be achieved by limiting the concentration of impurities both in the anodes and in the electrolyte. Primary copper smelting, converting, and fire-refining are efficient in collecting valuable minor metals from secondary raw materials. Unfortunately, molten copper will also dissolve unwanted impurities. Fortunately, the copper electrorefining process is efficient in removing unwanted metals as long as their concentrations can be kept low enough by previous process stages and purification of the electrolyte.
The elements from raw materials charged into copper production exit in slags, offgas dusts, copper cathodes, anode slime, and electrolyte purification products. Today, minor elements in slags are lost. Dusts, anode slime, and electrolyte purification residues are treated to recover valuable metals, mainly the noble metals Se and Te, but also Sb, Bi, Ni, and As.
Acknowledgments
This research has been performed within the project System Integrated Metal Production (SIMP) of DIMECC (Digital, Internet, Materials & Engineering Co-Creation) in Tampere, Finland.
Author Contributions
The paper is originally written as plenary presentation for the 6th International Conference Quo Vadis Recycling. All the authors have contributed in collection and reviewing the literature material. J.A. wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest
References
- ICSG World Copper Factbook. Available online: http://www.icsg.org (accessed on 2 March 2017).
- Brininstool, M. USGS Minerals Yearbook 2014. Copper [Advance Release]. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/copper/myb1-2014-coppe.pdf (accessed on 2 March 2017).
- USGS Historical Global Statistics for Mineral and Material Commodities. Data Series 896; 2015 version. Available online: https://minerals.usgs.gov/minerals/pubs/historical-statistics/global/ (accessed on 2 October 2017).
- Kelly, T.D.; Matos, G.R. USGS Copper End-Use Statistics; Historical Statistics for Mineral and Material Commodities in the United States; Data Series 140. Available online: https://minerals.usgs.gov/minerals/pubs/historical-statistics/ (accessed on 2 October 2017).
- Graedel, T.E.; Van Beers, D.; Bertram, M.; Fuse, K.; Gordon, R.B.; Gritsinin, A.; Kapur, A.; Klee, J.R.; Lifset, R.J.; Memon, L.; et al. Multilevel Cycle of Anthropogenic Copper. Environ. Sci. Technol. 2004, 38, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M.; King, M.; Sole, K.; Davenport, W. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; p. 455. ISBN 978-0-08-096789-9. [Google Scholar]
- ISRI Scrap Specifications Circular. 2017. Available online: http://www.scrap2.org/specs/files/assets/common/downloads/publication.pdf (accessed on 2 October 2017).
- Reuter, M.A.; Hudson, C.; van Schaik, A.; Heiskanen, K.; Meskers, C.; Hagelüken, C. Metal Recycling: Opportunities, Limits, Infrastructure; A Report of the Working Group on the Global Metal Flows to the International Resource Panel, United Nations Environment Programme: Nairobi, Kenya, 2013; p. 316. ISBN 978-92-807-3267-2. [Google Scholar]
- Reuter, M.A.; Kojo, I.V. Copper: A Key Enabler of Resource Efficiency. World Metall. ERZMETALL 2014, 67, 46–53. [Google Scholar]
- SIMP-System Integrated Metals Processing, Personal Communication. Available online: https://www.dimecc.com/dimecc-services/simp-system-integrated-metals-processing/ (accessed on 16 February 2017).
- Wang, S.; Davenport, W.; Siegmund, A.; Yao, S.; Gonzales, T.; Walters, G.; George, D. Copper Smelting: 2016 World Copper Smelter Data; Paper PY1-1, Copper 2016; The Mining and Materials Processing Institute of Japan: Kobe, Japan, 2016; p. 8. [Google Scholar]
- Sohn, H.Y.; Malfliet, A.; Scheunis, L.; Jones, P.T.; Blanpain, B. Copper Production. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 3, pp. 534–624. ISBN 978-0-08-096988-6. [Google Scholar]
- Avarmaa, K.; O’Brien, H.; Johto, H.; Taskinen, P. Equilibrium Distribution of Precious Metals Between Slag and Copper Matte at 1250–1350 °C. J. Sustain. Metall. 2015, 1, 216–228. [Google Scholar] [CrossRef]
- Larouche, P. Minor Elements in Copper Smelting and Electrorefining. Master’s Thesis, McGill University, Montréal, QC, Canada, 2001; p. 165. [Google Scholar]
- Taskinen, P.; Seppälä, K.; Laulumaa, J.; Poijärvi, J. Oxygen pressure in the Outokumpu flash smelting furnace—Part 1: Copper flash smelting settler. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2001, 110, C94–C100. [Google Scholar] [CrossRef]
- Von Gleich, A.; Ayres, R.U.; Gössling-Reisemann, S. (Eds.) Sustainable Metals Management; Springer: Dordrecht, The Netherlands, 2006; p. 607. ISBN 978-1-4020-4539-4. [Google Scholar]
- Nakajima, K.; Takeda, O.; Miki, T.; Matsubae, K.; Nagasaka, T. Thermodynamic Analysis for the Controllability of Elements in the Recycling Process of Metals. Environ. Sci. Technol. 2011, 45, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Azakami, T.; Yazawa, A. Activity measurements of liquid copper binary alloys. Can. Metall. Quat. 1976, 15, 111–122. [Google Scholar] [CrossRef]
- Moats, M.; Wang, S.; Filzwieser, A.; Siegmund, A.; Davenport, W.; Robinson, T. Survey of Copper Electrorefining Operations; Paper EL1-1, Copper 2016; The Mining and Materials Processing Institute of Japan: Kobe, Japan, 2016; p. 10. [Google Scholar]
- Zeng, W.; Wang, S.; Free, M.L. Experimental and Simulation Studies of Electrolyte Flow and Slime Particle Transport in a Pilot Scale Copper Electrorefining Cell. J. Electrochem. Soc. 2016, 163, E111–E122. [Google Scholar] [CrossRef]
- Hiskey, B.J. Mechanism and Thermodynamics of Floating Slimes Formation; T.T. Chen Symposium; Wiley: Hoboken, NJ, USA, 2012; pp. 101–112. [Google Scholar]
- Glöser, S.; Soulier, M.; Tercero Espinoza, L.A. Dynamic Analysis of Global Copper Flows. Global Stocks, Postconsumer Material Flows, Recycling Indicators, and Uncertainty Evaluation. Environ. Sci. Technol. 2013, 47, 6564–6572. [Google Scholar] [CrossRef] [PubMed]
- Baldé, C.P.; Wang, F.; Kuehr, R.; Huisman, J. The Global E-Waste Monitor—2014; United Nations University, IAS—SCYCLE: Bonn, Germany, 2015; p. 79. ISBN 978-92-808-4556-3. [Google Scholar]
- Huisman, J. Review of Directive 2002/96 on Waste Electrical and Electronic Equipment (WEEE). Available online: http://ec.europa.eu/environment/waste/weee/pdf/final_rep_unu.pdf (accessed on 22 March 2017).
- Lane, D.J.; Cook, N.J.; Grano, S.R.; Ehrig, K. Selective leaching of penalty elements from copper concentrates: A review. Miner. Eng. 2016, 98, 110–121. [Google Scholar] [CrossRef]
- Hagelüken, C. Improving metal returns and eco-efficiency in electronics recycling. In Proceedings of the IEEE International Symposium on Electronics and the Environment, San Francisco, CA, USA, 8–11 May 2006; pp. 218–223. [Google Scholar]
- Delbeke, K.; Rodriguez, P.H. Copper Concentrates—Environmental and Human Health hazard classification. European Copper Institute, 2014; p. 22. Available online: http://copperalliance.eu/docs/default-source/reach-documents/cu_conc_technical-summary-_20141030-internet-3.pdf (accessed on 8 October 2017).
- Moats, M.S.; Wang, S.; Kim, D. A Review of the Behavior and Deportment of Lead, Bismuth, Antimony and Arsenic in Copper Electrorefining; T.T. Chen Symposium; Wiley: Hoboken, NJ, USA, 2012; pp. 3–21. [Google Scholar]
- Golev, A.; Corder, G.D. Quantifying metal values in e-waste in Australia: The value chain perspective. Miner. Eng. 2017, 107, 81–87. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).