The Effect of the Redox Potential of Aqua Regia and Temperature on the Au, Cu, and Fe Dissolution from WPCBs
†
Abstract
:1. Introduction
2. Experimental
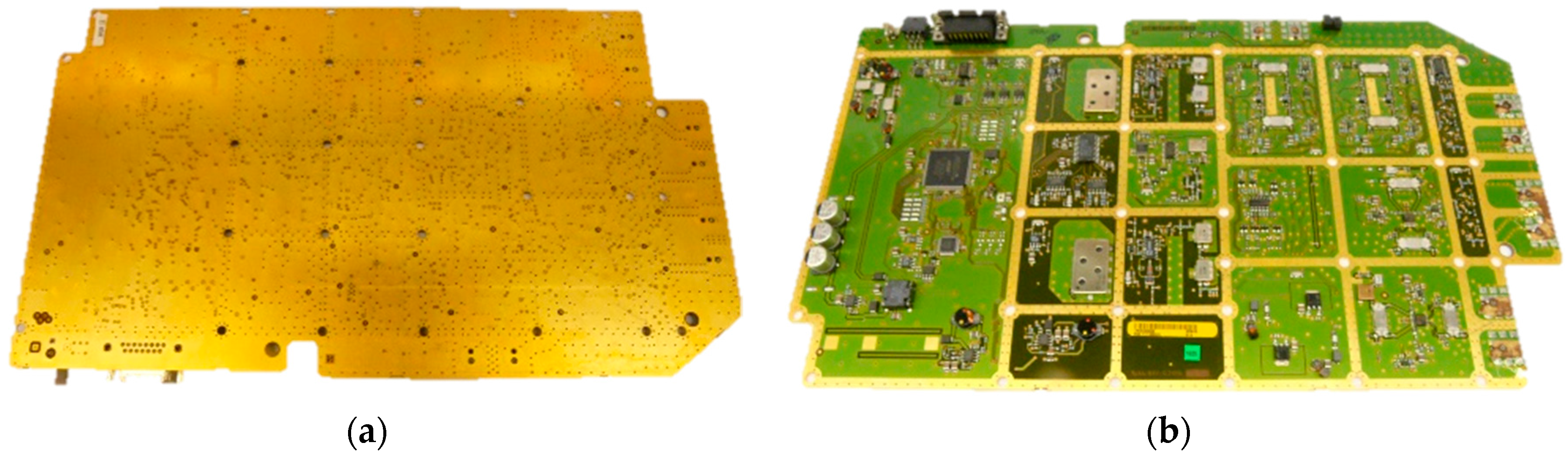
2.1. Material
2.2. Methods
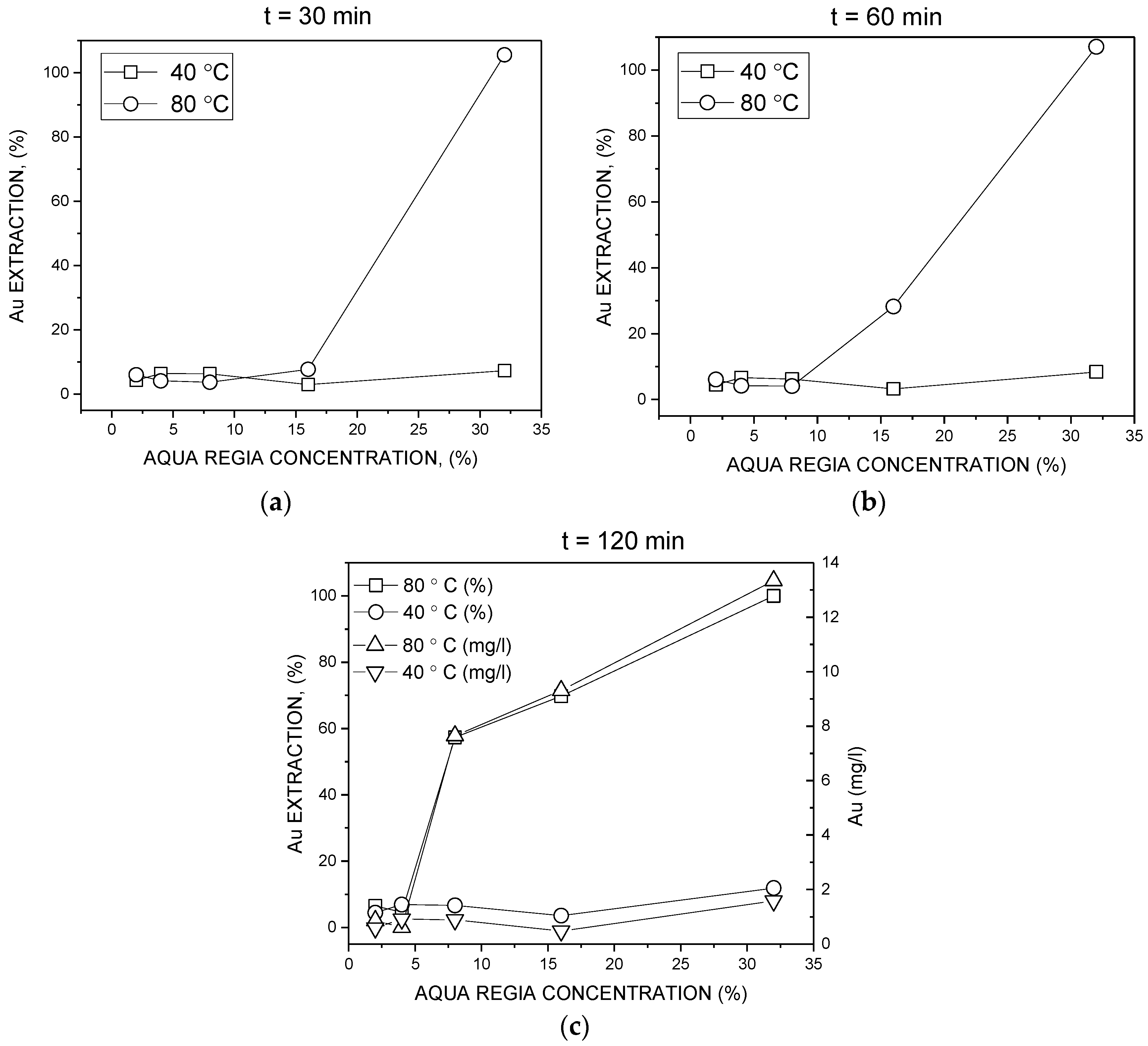
3. Results and Discussion
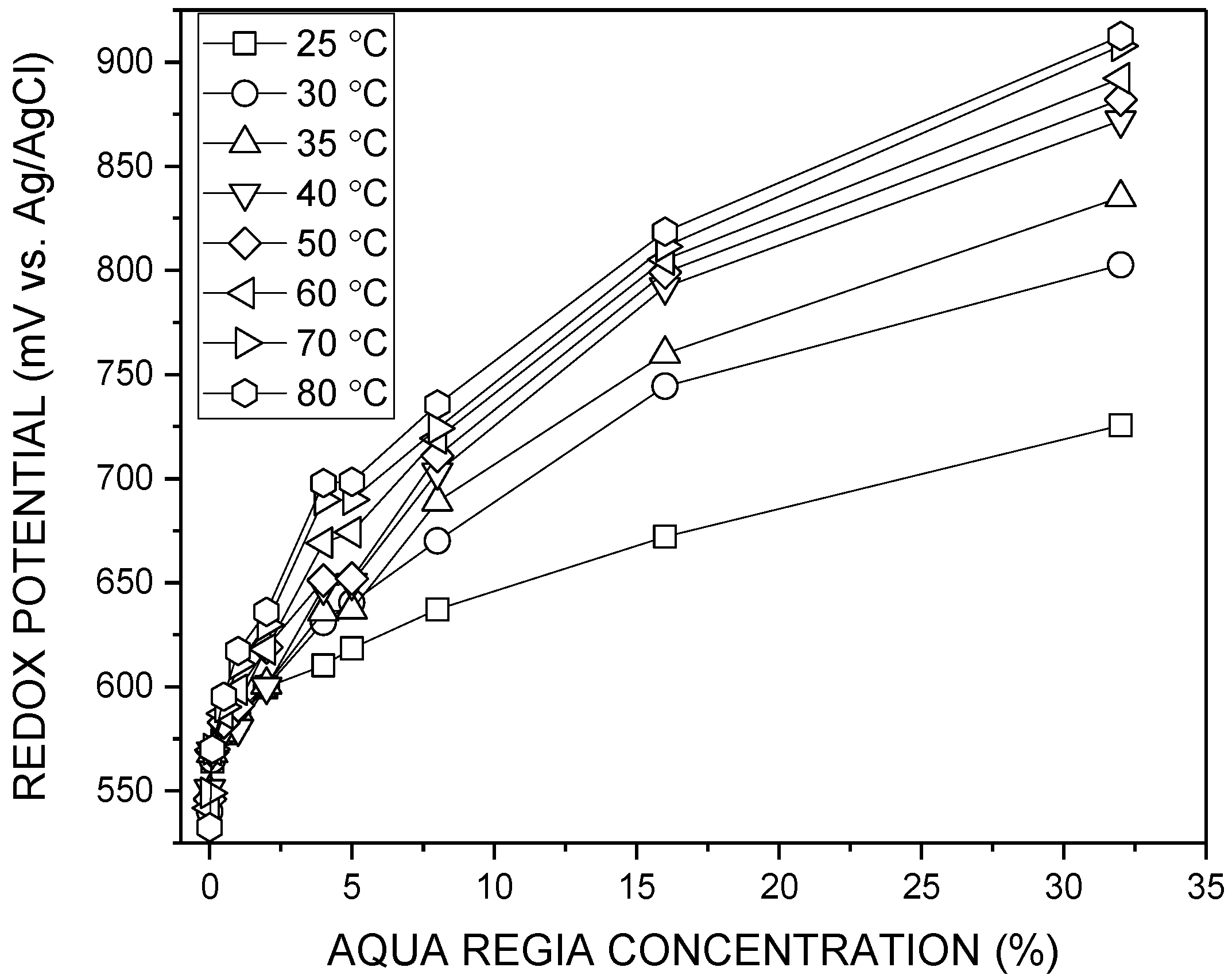
3.1. Redox Potential
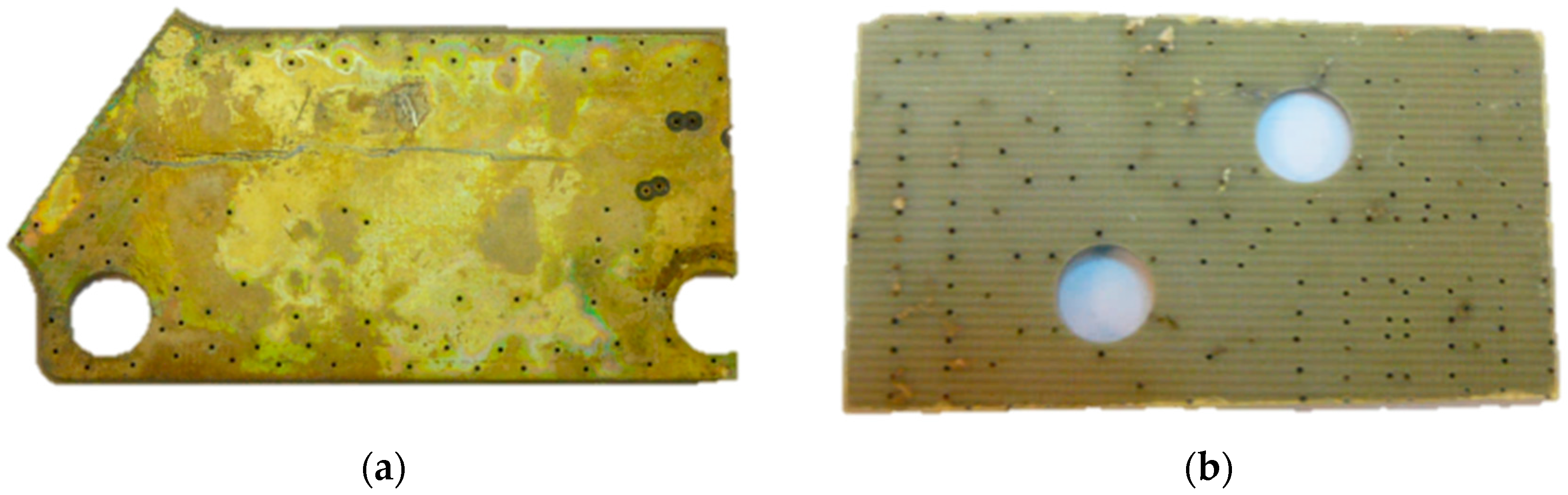
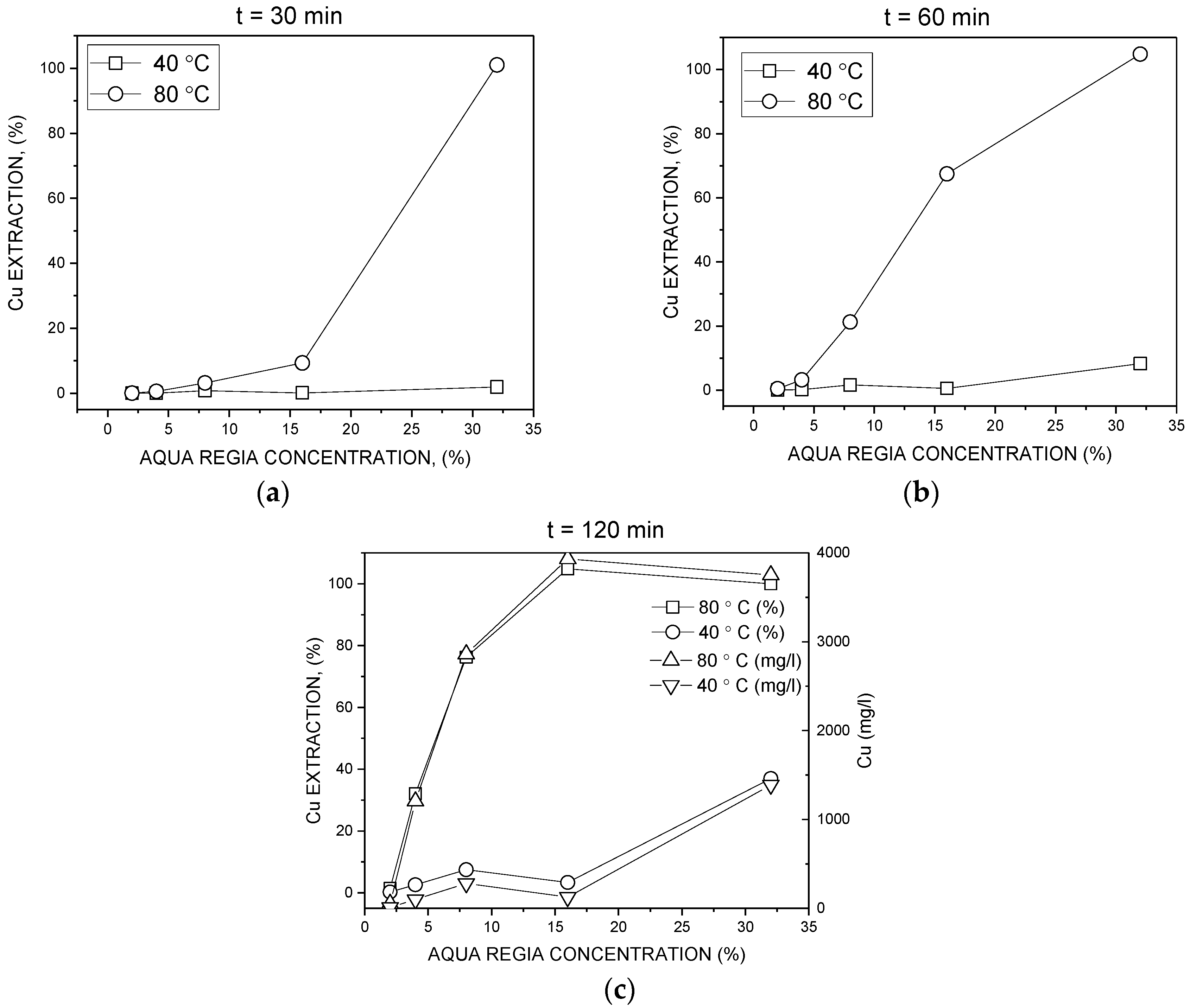
3.2. Batch Leaching
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Commission. Directive 2012/19/EU of the European Parliament and of the Council of 4 of July on Waste Electrical and Electronic Equipment; Official Journal of the European Union: Strasbourg, France, 2012. [Google Scholar]
- Marsden, J.; House, I. The Chemistry of Gold Extraction, 2nd ed.; The Society of Mining, Metallurgy and Exploration: Littleton, CO, USA, 2006; p. 650. ISBN 10-0-87335-240-8. [Google Scholar]
- Veit, H.M.; Bernardes, A.M.; Ferreira, J.Z.; Tenório, J.A.S.; de Fraga Malfatti, C. Recovery of copper from printed circuit boards scraps by mechanical processing and electrometallurgy. J. Hazard. Mater. 2006, 137, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Yannopoulos, J.C. The Extractive Metallurgy of Gold; Van Nostrand Reinhold: New York, NY, USA, 1991; p. 281. ISBN 0-442-31797-2. [Google Scholar]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Fray, D.J. Recovery of high purity precious metals from printed circuit boards. J. Hazard. Mater. 2009, 167, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Lekka, M.; Masavetas, I.; Benedetti, A.V.; Moutsatsou, A.; Fedrizzi, L. Gold recovery from waste electrical and electronic equipment by electrodeposition: A feasibility study. Hydrometallurgy 2015, 157, 97–106. [Google Scholar] [CrossRef]
- Sheng, P.P.; Etsell, T.H. Recovery of gold from computer circuit board scrap using aqua regia. Waste Manag. Res. 2007, 25, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Saulny, M. The Behavior of Printed Circuit Boards in Knife Shredder and Its Effects on the Recycling Process. Master’s Thesis, Aalto University, Espoo, Finland, 2015. [Google Scholar]
- Lampinen, M.; Seisko, S.; Forsström, O.; Laari, A.; Aromaa, J.; Lundström, M.; Koiranen, T. Mechanism and kinetics of gold leaching by cupric chloride. Hydrometallurgy 2017, 169, 103–111. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling of electronic scrap at Umicore’s integrated metals smelter and refinery. Erzmetall 2006, 59, 152–161. [Google Scholar]

| Au | Ag | Cu | Fe | Sn | Al | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | 372 | 570 | 250,000 | 37,300 | 11,670 | 49,800 | 8830 | 30,000 | 14,600 |
| Sample 2 | 421 | 245 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Aqua Regia Concentration | Redox Test Series | Batch Leaching of WPCB, Test Series | Chloride [Cl−], M |
|---|---|---|---|
| 0.01% | R1 (25–80 °C) | 0.003 | |
| 0.1% | R2 (25–80 °C) | 0.026 | |
| 0.5% | R3 (25–80 °C) | 0.129 | |
| 1% | R4 (25–80 °C) | 0.257 | |
| 2% | R5 (25–80 °C) | L1 (40–80 °C) | 0.515 |
| 4% | R6 (25–80 °C) | L2 (40–80 °C) | 1.030 |
| 5% | R7 (25–80 °C) | 1.286 | |
| 8% | R8 (25–80 °C) | L3 (40–80 °C) | 2.058 |
| 16% | R9 (25–80 °C) | L4 (40–80 °C) | 4.116 |
| 32% | R10 (25–80 °C) | L5 (40–80 °C) | 8.233 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elomaa, H.; Seisko, S.; Junnila, T.; Sirviö, T.; Wilson, B.P.; Aromaa, J.; Lundström, M.
The Effect of the Redox Potential of Aqua Regia and Temperature on the Au, Cu, and Fe Dissolution from WPCBs
. Recycling 2017, 2, 14.
https://doi.org/10.3390/recycling2030014
Elomaa H, Seisko S, Junnila T, Sirviö T, Wilson BP, Aromaa J, Lundström M.
The Effect of the Redox Potential of Aqua Regia and Temperature on the Au, Cu, and Fe Dissolution from WPCBs
. Recycling. 2017; 2(3):14.
https://doi.org/10.3390/recycling2030014
Elomaa, Heini, Sipi Seisko, Tero Junnila, Tuomas Sirviö, Benjamin P. Wilson, Jari Aromaa, and Mari Lundström.
2017. "The Effect of the Redox Potential of Aqua Regia and Temperature on the Au, Cu, and Fe Dissolution from WPCBs
" Recycling 2, no. 3: 14.
https://doi.org/10.3390/recycling2030014
Elomaa, H., Seisko, S., Junnila, T., Sirviö, T., Wilson, B. P., Aromaa, J., & Lundström, M.
(2017). The Effect of the Redox Potential of Aqua Regia and Temperature on the Au, Cu, and Fe Dissolution from WPCBs
. Recycling, 2(3), 14.
https://doi.org/10.3390/recycling2030014







