Abstract
An ultrasonic-enhanced hydrogen peroxide water-washing process was developed to recover lead from raw flue dust (RFD) under neutral conditions. At optimal parameters (40 °C, 30 min, 4 mL H2O2, liquid-to-solid ratio 2:1, 240 W ultrasound), the Pb mass fraction in the solid residue increased from 41.68% in the RFD to 68.11%, accompanied by a Pb recovery rate of 97.1%. These values are significantly higher than those obtained under identical conditions without ultrasound (64.07% and 95.93%, respectively). Ultrasound promotes de-agglomeration and generates •OH radicals that accelerate the oxidation of PbSO3 to insoluble PbSO4 while concurrently removing impurity cadmium. This research offers a green and efficient alternative to traditional lead recovery methods, fostering sustainable development in the metallurgical industry.
1. Introduction
In the process of lead smelting, a large amount of raw flue dust (RFD) is generated, which contains approximately 50% lead, significantly higher than the grade of typical lead ores (2–5%). As a representative heavy metal pollutant, improper disposal of lead poses serious environmental risks [1,2]. By employing effective processes to recover lead from RFD into the lead smelting system not only reduces the environmental burden but also provides a secondary resource, enhancing resource utilization efficiency and supporting sustainable development [3,4].
Currently, the primary methods for lead recovery from RFD are pyrometallurgical and hydrometallurgical [5,6]. The pyrometallurgical method separates lead through high temperature roasting by exploiting differences in component volatility, yet it suffers from significant limitations including excessive energy consumption and substantial environmental pollution [7]. Recovery of lead through hydrometallurgical technology is the mainstream choice nowadays, the core principle of which is to dissolve impurities such as cadmium, zinc, and iron into the liquid phase while retaining lead in the solid phase, thus realizing the separation of lead from impurities. Among them, cadmium is treated as the main impurity due to its high content (about 10–20%) and toxicity [8,9,10]. In the past studies, acid washing has been the dominant process due to its simplicity of operation and low energy consumption [11,12,13]. Chen et al. used sulfuric acid as an oxidizing agent to separate lead and other metals in the process of soot leaching, which produces acidic wastewater requiring further treatment [14]. Thus, this approach generates a large amount of acidic wastewater that is difficult to treat, imposing economic and environmental burdens [15]. Additionally, partial lead dissolution alongside impurities results in significant lead loss.
To address the drawbacks of acid leaching, a neutral water washing process has been proposed. Although this method can reduce the loss of lead and minimize the generation of acidic wastewater, the impurity removal efficiency was found to be low in the practical operation of the enterprise, and the content of cadmium in the solid phase was still up to more than 4%, which led to unsatisfactory separation of lead impurities. Li et al. [16] achieved good separation of valent metallic elements from flue dust by pressurized oxidative leaching. However, such a method requires the redesign and modification of a closed high-pressure leach vessel and presents certain safety hazards. Guo et al. [17] demonstrated that microwave radiation selectively accelerated the dissolution of arsenic in copper smelting flue dust while reducing acid consumption, but the effect under neutral conditions was not studied. Recently, researchers have introduced oxidants such as hydrogen peroxide (H2O2) during water washing [13,18]. This method converts lead sulfite into insoluble lead sulfate while transforming sparingly soluble impurities (e.g., cadmium sulfite) into soluble forms that enter the solution, thereby improving lead recovery and reducing impurity content in the lead residue. However, the limited oxidizing ability of these oxidants results in incomplete phase transformation, preventing lead recovery rates from reaching ideal levels. In addition, due to the phenomenon of encapsulation and agglomeration, impurities are encapsulated inside and are difficult to remove. This observation suggests a potential strategy: transforming lead into an insoluble phase while converting impurities such as cadmium into soluble forms through intensive oxidation. This approach holds promise for achieving efficient lead recovery under neutral conditions.
Ultrasonic waves, which are mechanical waves with frequencies over 20 kHz, have unique cavitation and mechanical effects [19,20]. They can significantly enhance processes such as leaching, removing impurities, and mixing in physical and chemical reactions [21,22]. The cavitation bubbles generated during ultrasonic treatment collapse violently, producing localized regions of high temperature and pressure, which can significantly enhance chemical reaction rates [23]. This makes ultrasonic technology particularly effective in breaking down complex structures and promoting reactions [19,20,21]. When combined with oxidants, ultrasound can promote the generation of free radicals, thereby enhancing oxidation reactions [24,25]. For instance, studies have demonstrated that ultrasonic-enhanced oxidation can effectively improve the leaching efficiency of metals from various ores and industrial wastes by increasing the reaction rate and oxidation efficiency [26]. The synergistic effect of ultrasonic treatment and oxidants can lead to more complete oxidation of metal sulfides and other compounds, facilitating their separation and recovery [27]. Furthermore, ultrasonic technology can help in the dispersion of reactants, preventing the aggregation of particles and improving the contact efficiency between reactants [28,29,30]. In lead recovery from RFD, good particle dispersion facilitates impurity removal and offers more reaction sites for oxidants, promoting oxidation and boosting lead recovery efficiency. However, the effectiveness of ultrasonic H2O2 synergistic systems in lead recovery from RFD under neutral conditions, and the intensification mechanism of ultrasonic, have not been reported in the literature.
To address these challenges, this study develops an efficient method for lead recovery from RFD through ultrasonic-enhanced H2O2 water washing under neutral conditions. RFD is analyzed to determine its elemental composition and the forms of phases present. The impacts of various factors, including washing time, H2O2 dosage, liquid–solid ratio, ultrasonic power, and temperature on lead recovery, are investigated to identify optimal process conditions. The study also employs multiple analytical techniques to demonstrate the enhancement effect of ultrasound on lead recovery and to clarify its mechanism in enhancing the oxidation process. Consequently, an efficient and environmentally friendly lead recovery process is established. This process is anticipated to improve economic benefits for enterprises, promote a green and sustainable transformation in the metallurgical industry, and contribute to eco-friendly societal development.
2. Results and Discussion
2.1. Analysis of Raw Flue Dust
X-ray fluorescence (XRF) analysis of the RFD reveals a complex elemental composition (as shown in Table 1). The results show that lead (Pb) is the major component, making up 41.68% of the sample by weight. Alongside lead, significant amounts of cadmium (Cd, 14.33%) and sulfur (S, 7.1%) are also present. Trace elements such as arsenic (As), silicon (Si), calcium (Ca), and others collectively highlight the intricate makeup of RFD. This diversity of elements suggests the presence of multiple lead and cadmium compounds, which can affect lead recovery efficiency during water washing.

Table 1.
Different elements and their contents in raw flue dust by XRF.
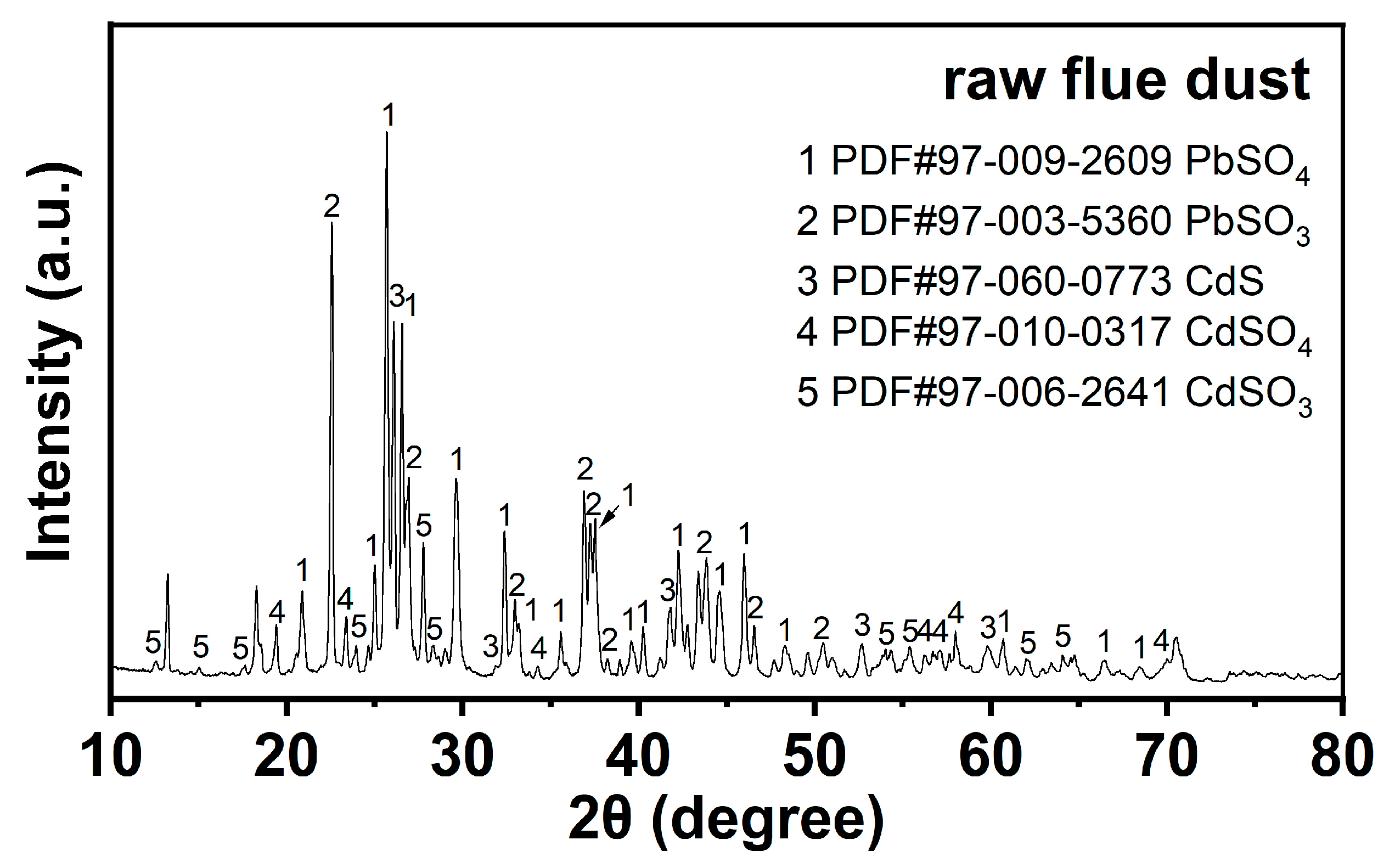
The X-ray diffraction (XRD) pattern of RFD confirms the presence of various crystalline phases, as shown in Figure 1. The peaks in the pattern correspond to cadmium sulfite (CdSO3), cadmium sulfate (CdSO4), cadmium sulfide (CdS), lead sulfite (PbSO3), and lead sulfate (PbSO4) [11]. The coexistence of PbSO3 and PbSO4 indicates that lead is present in both slightly soluble and insoluble forms. During water washing, the slight solubility of PbSO3 may lead to some lead being lost into the solution, thereby reducing the recovery rate. Similarly, the presence of CdSO3 and CdS implies that these compounds, which are not very soluble, can remain in the solid phase after water washing, acting as impurities that affect the purity of the recovered lead.
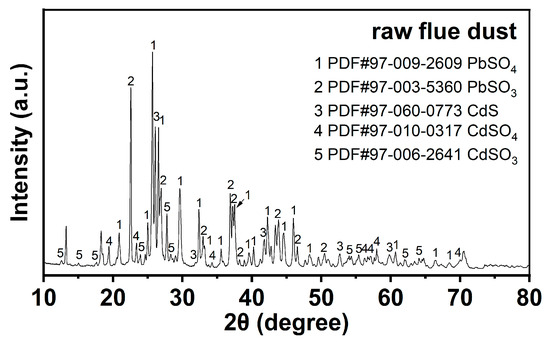
Figure 1.
XRD analysis results for raw flue dust.
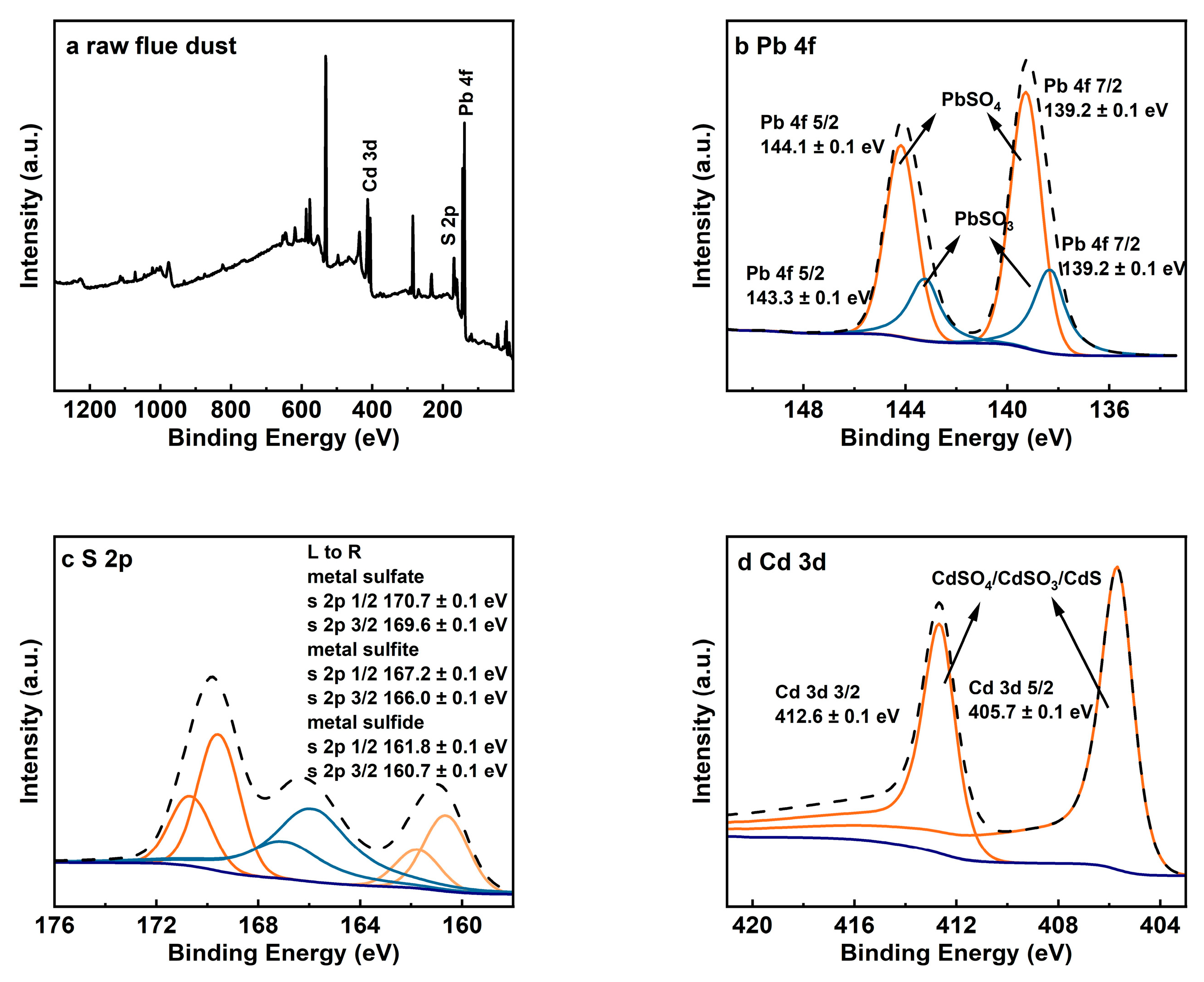
X-ray photoelectron spectroscopy (XPS) analysis provides further insight into the chemical states of elements in RFD, as shown in Figure 2. The Pb 4f spectrum (Figure 2b) shows peaks at binding energies of 139.2 ± 0.1 eV and 143.3 ± 0.1 eV, which are characteristic of lead sulfate (PbSO4). Additionally, peaks at 144.1 ± 0.1 eV and 148.3 ± 0.1 eV indicate the presence of lead sulfite (PbSO3) [8]. The S 2p spectrum (Figure 2c) reveals multiple peaks, with those at 170.7 ± 0.1 eV and 169.6 ± 0.1 eV corresponding to sulfate (SO42−), and peaks at 167.2 ± 0.1 eV and 166.0 ± 0.1 eV corresponding to sulfite (SO32−) [13]. Peaks at 161.8 ± 0.1 eV and 160.7 ± 0.1 eV suggest the presence of sulfur in metallic sulfides. The Cd 3d spectrum (Figure 2d) shows peaks at 405.7 ± 0.1 eV and 412.6 ± 0.1 eV [8,31]. Since the binding energies of cadmium compounds are close, combining S 2p spectral analysis shows cadmium exists as sulfides, sulfites, and sulfates. These results match the XRD findings and highlight the challenges of extracting lead from RFD due to the various compounds that can impact recovery efficiency.
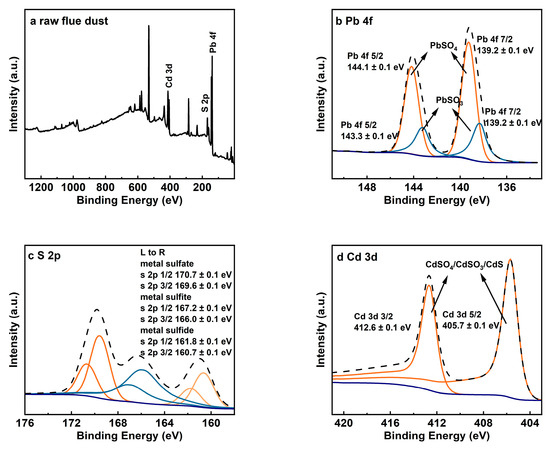
Figure 2.
XPS analysis results for raw flue dust: (a) XPS spectrum; (b) Pb 4f; (c) S 2p; (d) Cd 3d.
XRF, XRD, and XPS analyses reveal that RFD has a complex composition. Lead exists as both insoluble PbSO4 and slightly soluble PbSO3. PbSO3 can dissolve somewhat during water washing, risking lead loss. Some cadmium compounds are hard to dissolve and usually end up as impurities in the solid phase. Given the mix of elements and compounds in RFD, advanced and efficient recovery methods are needed to improve lead recovery and ensure the final product’s quality.
2.2. Performance Comparison of Various Leaching Methods
To preliminarily verify if ultrasound and H2O2 addition promote lead recovery from RFD, exploratory experiments were conducted under room temperature (20 °C) conditions with a 10 min washing time and a liquid–solid ratio of 2:1, and the results are presented in Figure 3. The experimental setups included conventional water washing (CW), conventional oxidative water washing (CWO), ultrasonic water washing (UW), and ultrasonic oxidative water washing (UWO). During CWO and UWO, 5 mL of H2O2 was added. For UW and UWO, ultrasonic power at 120 W was applied.
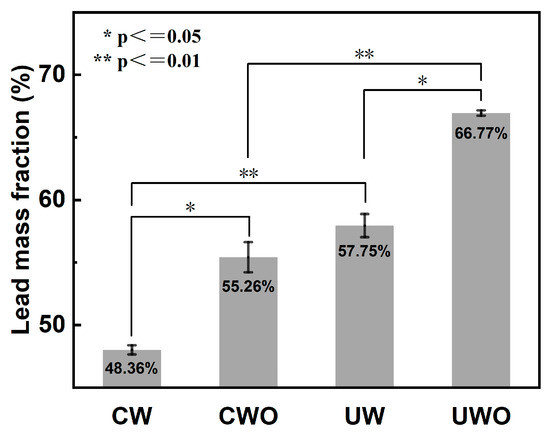
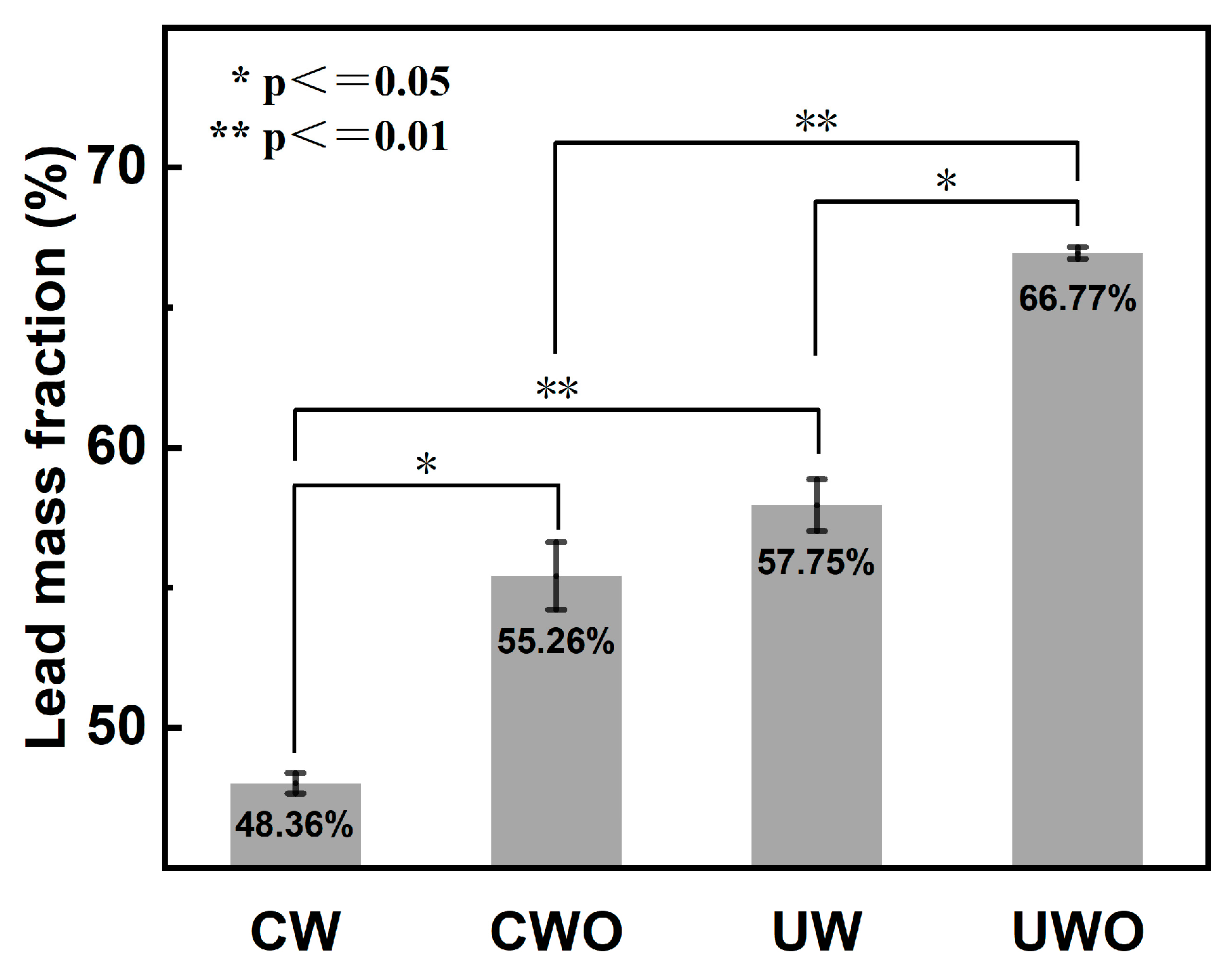
Figure 3.
Comparative lead mass fraction in the solid phase under four conditions (conventional water washing (CW), conventional oxidative water washing (CWO), ultrasonic water washing (UW), and ultrasonic oxidative water washing (UWO), error bars represent standard deviation (n = 3). Statistical significance: * p ≤ 0.05, ** p ≤ 0.01).
Statistical significance of lead mass fraction between groups was assessed using a one-sample t-test, and each ultrasound-enhanced group was compared with the conventional group. All experiments were replicated three times and a p-value ≤ 0.05 was considered statistically significant. The differences between CW and CWO (* p ≤ 0.05) and UW and UWO (* p ≤ 0.05) were significant, and it is noteworthy that the differences between CW and UW (** p ≤ 0.01) and CWO and UWO (** p ≤ 0.01) were even more significant.
For CW, the lead mass fraction in the solid phase after washing is 48.36%. The relatively low lead mass fraction in CW may be due to two factors. Firstly, partial lead is lost to the solution, especially in the form of soluble lead compounds such as lead sulfite (PbSO3). Secondly, certain impurities that are difficult to remove with water washing alone remain in the solid phase, diluting the lead mass fraction. When H2O2 is added to conventional water washing (CWO), the lead mass fraction in the solid phase increases to 55.26%. This improvement occurs because the addition of H2O2 promotes the oxidation of lead sulfite to lead sulfate (PbSO4), which has lower solubility and remains in the solid phase, reducing lead loss. Moreover, H2O2 may convert some impurities into more soluble forms, enabling their removal from the solid phase, thereby increasing the lead mass fraction. For UW without H2O2, the lead mass fraction in the solid phase further increases to 57.75%. Ultrasound effectively disperses particles and prevents aggregation, improving the contact between water and solid particles and promoting the dissolution of soluble impurities. The mechanical effects of ultrasound also help in removing impurities more effectively, resulting in a higher lead mass fraction in the solid phase. The most effective method is ultrasonic water washing with H2O2 (UWO), which yields a lead mass fraction of 66.77%. The combination of ultrasound and H2O2 creates a synergistic effect [32]. The ultrasonic cavitation enhances the oxidation reaction by generating free radicals and increasing the contact efficiency between reactants [26]. This dual action of ultrasound and H2O2 maximizes lead retention in the solid phase and impurity removal, resulting in the highest lead mass fraction.
In summary, both H2O2 addition and ultrasonic application enhance lead recovery from RFD. The combination of the two proves to be the most effective approach, highlighting the necessity of studying ultrasonic oxidative water washing for improving lead recovery efficiency.
2.3. Optimization of Ultrasonic-Enhanced H2O2 Water Washing Conditions
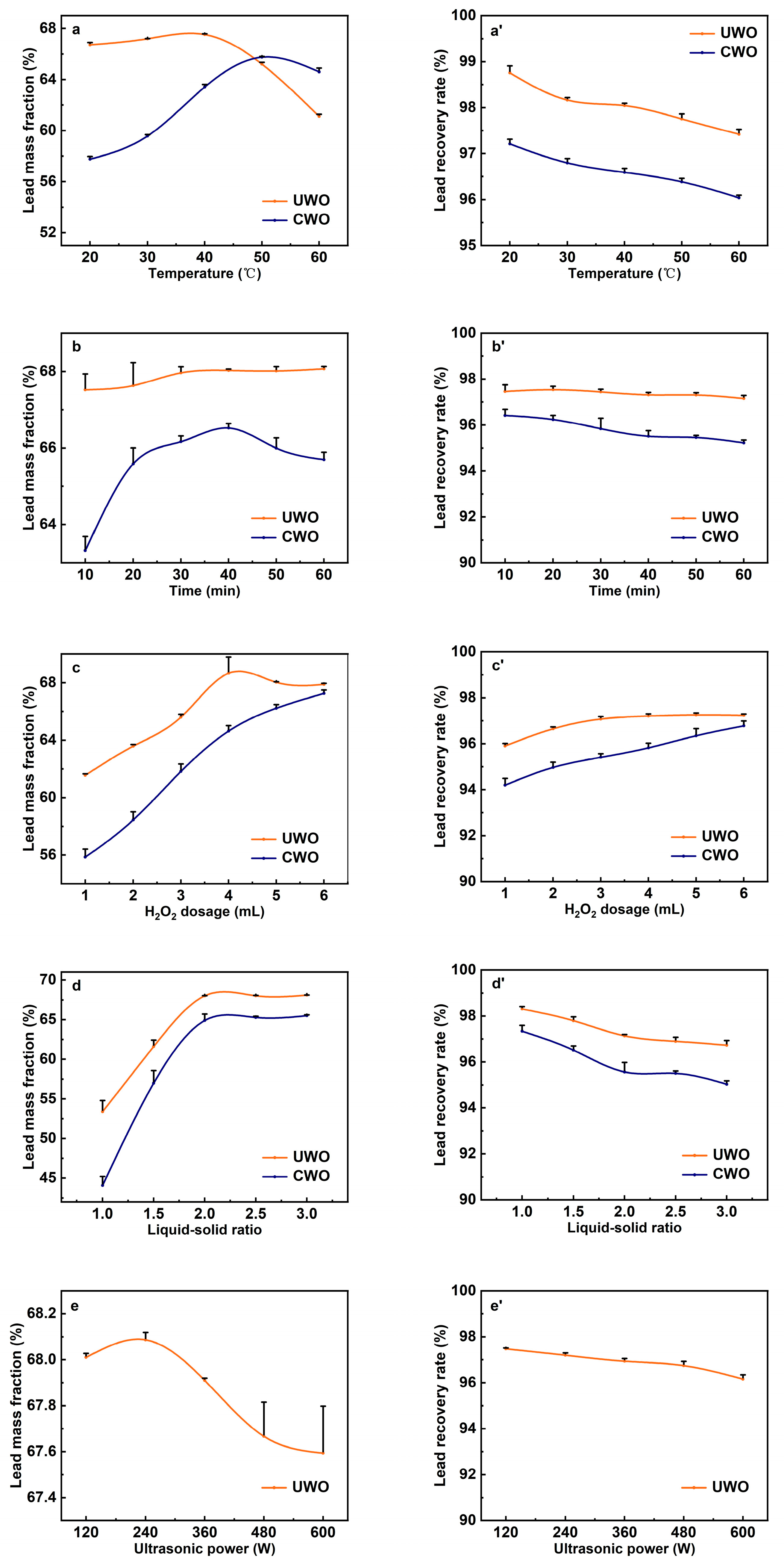
To optimize the ultrasonic-enhanced H2O2 water washing process for lead recovery from flue dust, the effects of temperature, time, H2O2 dosage, liquid–solid ratio, and ultrasonic power on lead recovery efficiency were investigated, as shown in Figure 4. The lead mass fraction in the solid phase after washing was analyzed, and the lead recovery rate was calculated based on the difference in lead mass fraction in the solid phase before and after water washing.
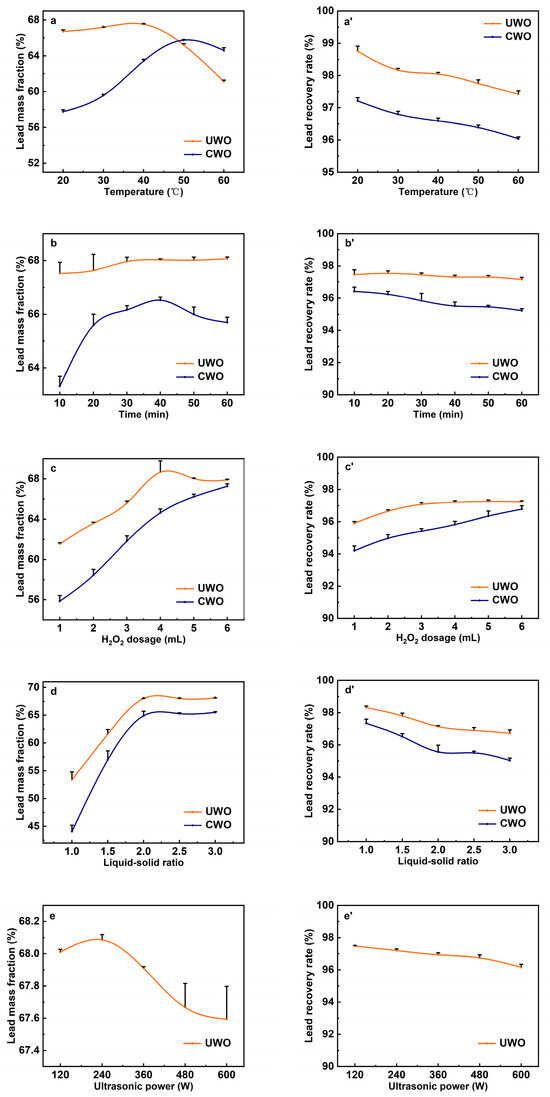
Figure 4.
The effect of various conditions on the lead mass fraction and recovery rate in the solid phase after ultrasonic-enhanced (UWO) and conventional (CWO) oxidative water washing (data are presented as mean ± standard deviation, n = 3): (a,a’) temperature; (b,b’) time; (c,c’) H2O2 dosage; (d,d’) liquid–solid ratio; (e,e’) ultrasonic power.
The effect of the water washing temperature was investigated at a washing time of 10 min, a H2O2 dosage of 5 mL, a liquid–solid ratio of 2:1, and an ultrasonic power of 120 W, with the results shown in Figure 4a. A comparison between UWO and CWO reveals that the lead mass fraction in the solid phase initially increases then decreases with temperature, while lead recovery rate declines slowly. These trends are due to the interplay of thermodynamic and kinetic effects [33]. For UWO, at low temperatures, the limited molecular kinetic energy slows chemical reactions. As temperature rises, increased molecular collisions and energy enhance the oxidation of lead sulfite to lead sulfate by H2O2 and promote impurity dissolution. Consequently, the lead mass fraction in the solid phase rises with temperature, peaking at 67.53% around 40 °C due to accelerated reaction rates and improved oxidation efficiency. However, when the temperature exceeds approximately 40 °C, the lead mass fraction begins to decline. This is because higher temperatures accelerate the decomposition of H2O2 into water and oxygen (2H2O2 → 2H2O + O2), reducing the H2O2 concentration and oxidizing capacity in the solution, which leads to incomplete oxidation of lead sulfite, resulting in more lead being lost to the solution [34]. Additionally, the solubility of certain lead compounds, such as lead sulfate, increases with temperature, further contributing to lead loss in the solution. Under low temperature conditions (<50 °C), the localized high temperatures and pressures generated by ultrasonic cavitation enhance the reaction rates and oxidation efficiency, even at lower bulk temperatures. This means that UWO can achieve higher lead mass fractions at lower temperatures compared to CWO. Furthermore, the mechanical effects of ultrasonic waves help disperse particles and increase the contact efficiency between reactants, ensuring more thorough oxidation and impurity removal. However, at high temperatures (>50 °C), the lead loss is more pronounced in UWO compared to CWO. This is because the local high temperatures and pressures produced by ultrasonic cavitation can synergistically intensify the negative impact of bulk temperature on H2O2 decomposition and lead solubility. High temperatures also increase energy consumption. Thus, the optimal temperature is 40 °C. At this temperature, UWO achieves a lead mass fraction of 67.53% and a recovery rate of 98.03%. For CWO, the lead mass fraction is 63.57% and the recovery rate is 96.67%.
Figure 4b demonstrates the effect of water washing time on lead recovery at a washing temperature of 40 °C, a dosage of 5 mL of H2O2, a liquid–solid ratio of 2:1, and an ultrasonic power of 120 W. The variations in lead mass fraction between UWO and CWO at different washing times were compared. The results indicate that in the UWO process, the lead mass fraction increases slightly and tends to stabilize around 68.02% after 30 min. The increase is due to the enhanced oxidation of lead sulfite to lead sulfate and more efficient impurity removal as time progresses. The stabilization after 30 min suggests that the reaction approaches equilibrium, with most lead sulfite converted to lead sulfate and impurities dissolved. In contrast, the CWO process shows a more pronounced increase initially but starts to decline after reaching a peak of 66.17% at 30 min. This decline is likely due to the insufficient oxidizing ability of H2O2 over time. Without continuous oxidation, unoxidized lead sulfite remains slightly dissolved and is gradually lost to the solution as water washing time extends [35]. Additionally, prolonged water washing may enhance the dissolution of certain lead compounds, contributing to the decrease in lead mass fraction [36]. The consistent cavitation and mechanical effects of ultrasound maintain reaction efficiency, ensuring continuous oxidation and impurity removal, thereby achieving higher and more stable lead mass fraction compared to CWO. The optimized washing time is 30 min. At this point, under UWO, the lead mass fraction is 68.02% and the recovery rate is 97.43%. Under CWO, these values are 66.17% and 95.98%, respectively.
The effects of H2O2 dosage were examined under conditions of 40 °C washing temperature, a washing time of 30 min, a liquid–solid ratio of 2:1, and 120 W ultrasonic power. As shown in Figure 4c, the lead mass fraction in the solid phase and the lead recovery rate for both UWO and CWO increase with the dosage of H2O2. UWO consistently outperformed CWO, highlighting ultrasonic enhancement. Insufficient dosage leads to incomplete oxidation, causing some of the sparingly soluble lead sulfite to enter the solution, while other sparingly soluble impurities like cadmium sulfite remain in the solid phase. Both factors contribute to a lower lead mass fraction in the solid phase. As the dosage of H2O2 increases, its oxidizing ability also enhances, thereby promoting the conversion of lead sulfite into lead sulfate and improving the removal rate of impurities. This explains why, in both methods, the mass fraction of lead increases with the addition of more H2O2. The reaction primarily depends on H2O2 to oxidize lead sulfite and other sulfite impurities. In UWO, ultrasonic cavitation induces local high temperatures and pressures, accelerating H2O2 decomposition and generating more free radicals [37]. These radicals enhance the oxidation reaction, making UWO more efficient than CWO at the same H2O2 dosage [38]. The mechanical effects of ultrasound also improve reactant mixing and contact, further boosting oxidation efficiency. Therefore, UWO achieves a higher lead mass fraction than CWO. After a certain H2O2 dosage, the lead mass fraction in UWO stabilizes. Excess H2O2 decomposes into water and oxygen, reducing its effective concentration. At this point, the reaction rate no longer rises with additional H2O2. For UWO, the optimal H2O2 dosage is about 4 mL, achieving a lead mass fraction of 68.01% and a recovery rate of 97.11%. For CWO, the corresponding values are 64.19% and 95.89%. Beyond this dosage, increases in lead mass fraction and recovery rate are minimal, indicating diminishing returns.
The effects of the liquid–solid ratio were examined under conditions of 40 °C washing temperature, 30 min washing time, 4 mL H2O2 dosage, and 120 W ultrasonic power, with results presented in Figure 4d. As the liquid–solid ratio increased, the lead mass fraction initially rose and then gradually declined in both methods, with UWO consistently outperforming CWO in lead recovery efficiency. This trend can be attributed to the increased liquid–solid ratio providing a more favorable environment for ultrasonic cavitation. The additional water serves as a medium that facilitates the formation and growth of cavitation bubbles, which, when subjected to ultrasonic waves, undergo violent collapse, generating localized high temperatures and pressures [39,40]. These extreme conditions significantly augment the kinetic energy of molecules within the solution, thereby intensifying molecular collisions and interactions. The heightened molecular activity accelerates the oxidation of lead sulfite to lead sulfate and expedites the dissolution of impurities, resulting in a higher lead mass fraction in the solid phase. Furthermore, the intense mixing by ultrasonic waves disrupts particle aggregates, thereby increasing the surface area available for reaction [41]. As a result, UWO achieves superior lead recovery compared to CWO at equivalent liquid–solid ratios. After the liquid–solid ratio reaches around 2, further increases have minimal effect on the lead mass fraction. Excess water dilutes the reactants, lowering their concentration and slowing the reaction. The increased volume also enhances the solubility of unoxidized lead, reducing the lead recovery rate. Thus, the optimal liquid–solid ratio is 2. At this ratio, UWO yields a lead mass fraction of 68.03% and a recovery rate of 97.43%, while CWO gives 64.07% and 95.93%, respectively.
Figure 4e illustrates the lead mass fraction in the solid phase under UWO across varying ultrasonic powers. The lead mass fraction initially increases with ultrasonic power, reaching a maximum of 68.11% at 240 W before decreasing as power continues to rise. However, the lead recovery rate exhibits a gradual decline. This trend can be attributed to the dual effects of ultrasonic cavitation and its impact on the reaction environment. At lower ultrasonic powers, the cavitation effect is relatively mild. As the power increases, the intensity of cavitation increases, leading to more frequent and violent collapse of cavitation bubbles [32]. This generates localized high temperatures and pressures within the solution. These extreme conditions significantly enhance molecular kinetic energy, promoting more frequent and energetic molecular collisions [42,43]. This heightened molecular activity accelerates the oxidation of lead sulfite to lead sulfate and improves the dissolution of impurities, resulting in a higher lead mass fraction in the solid phase. The mechanical effects of ultrasonic waves also play a crucial role. Ultrasound induces intense mixing and breaks up particle aggregates, increasing the surface area available for reaction [44]. This enhanced contact between reactants ensures more thorough oxidation and impurity removal, further contributing to the increase in lead mass fraction. At 240 W, the ultrasonic power is optimal, maximizing the beneficial effects of cavitation and mechanical action. However, when the ultrasonic power exceeds 240 W, the lead mass fraction begins to decline. This can be attributed to several factors. First excessive ultrasonic power can lead to the over-dissolution of lead compounds. Intense cavitation at high power may enhance the dissolution of lead sulfite, enabling it to overcome the solubility barrier and enter the solution. This causes lead loss from the solid phase and explains why the lead recovery rate declines gradually. Second, high ultrasonic power can cause the premature decomposition of hydrogen peroxide. The localized high temperatures and pressures generated by intense cavitation can accelerate the decomposition of H2O2 into water and oxygen. This reduces the effective concentration of H2O2 available for the oxidation reaction, leading to incomplete oxidation of lead sulfite and a subsequent decrease in lead mass fraction. Thus, the optimal ultrasonic power is 240 W. At this condition, the lead mass fraction in UWO reaches 68.11%, with a lead recovery rate of 97.19%.
2.4. Effects of Ultrasound on Particle Agglomeration and Encapsulation in Lead Recovery
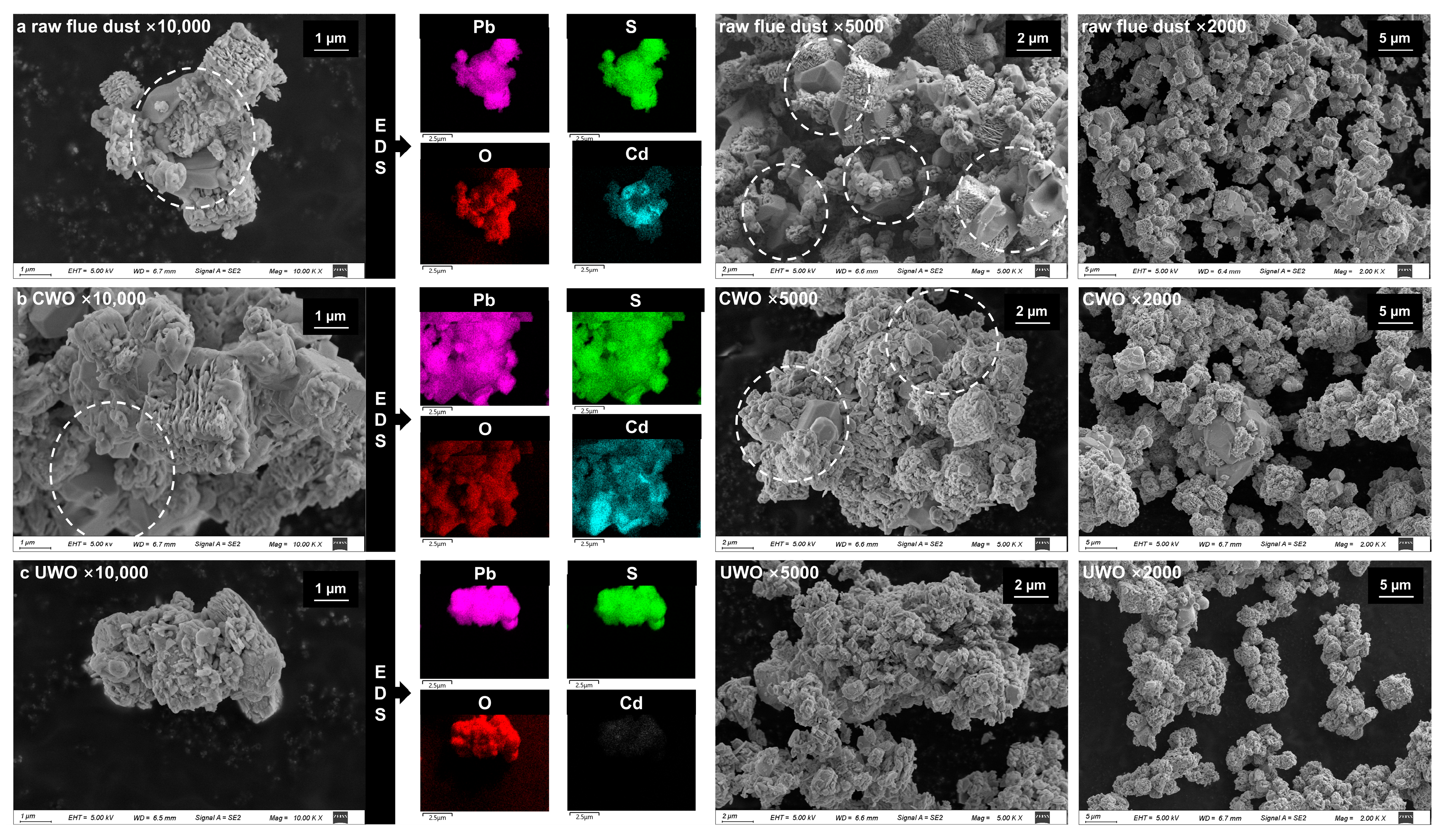
The SEM-EDS and particle size analysis were conducted on the solid phases of RFD, CWO, and UWO under optimal conditions, and the results are shown in Figure 5 and Figure 6, respectively.
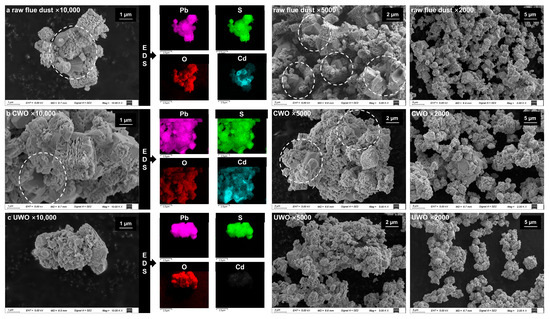
Figure 5.
SEM images and EDS analysis results for different samples under various conditions and magnifications: (a) raw flue dust; (b) conventional oxidative water washing (CWO); (c) ultrasonic-enhanced oxidative water washing (UWO).
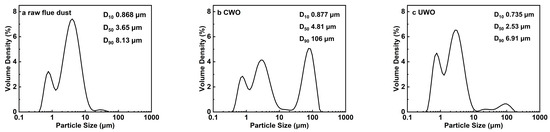
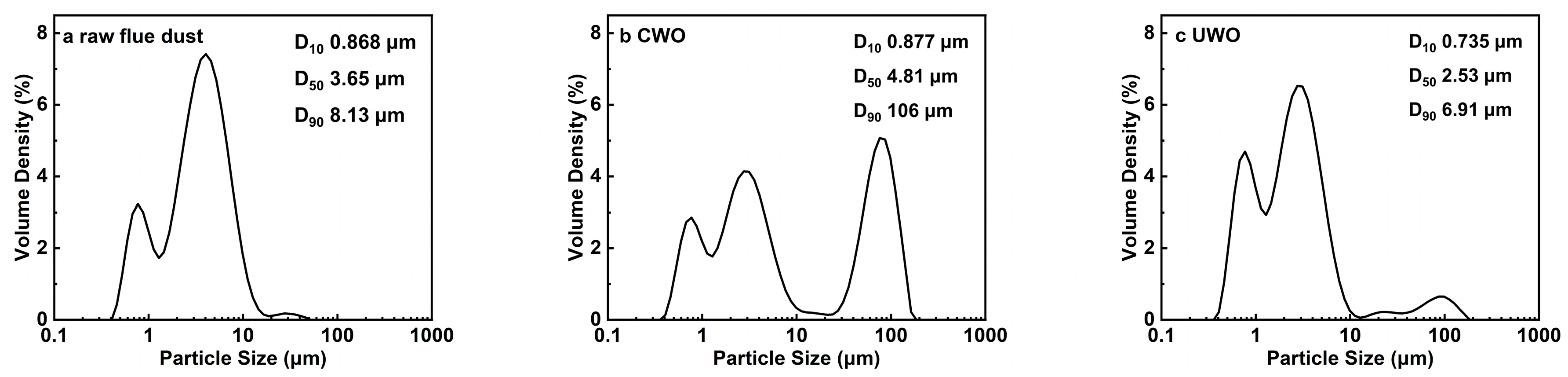
Figure 6.
Particle size distribution of different samples: (a) raw flue dust; (b) conventional oxidative water washing (CWO); (c) ultrasonic-enhanced oxidative water washing (UWO).
Figure 5a reveals the complex morphology of RFD, where particles aggregate and accumulate with one another to form complex structures. These aggregated structures result in impurities (e.g., cadmium) being encapsulated inside. CWO (Figure 5b) relies on mechanical agitation, which is insufficient to effectively penetrate and disperse these complex structures. A large number of impurities remain entrapped inside, limiting their exposure during oxidation or dissolution, resulting in a lower lead mass fraction in the final lead residue. Ultrasound introduces mechanical and cavitation effects that can significantly enhance the water washing process. The mechanical energy generated by ultrasound disrupts particle agglomeration and breaks them into smaller, more homogeneous particles. This is evident in the SEM-EDS images of UWO-treated samples (Figure 5c), which show a more dispersed and less aggregated particle morphology compared to CWO. The cavitation effect further contributes to this phenomenon by generating localized shock waves and micro-jets that effectively disperse the aggregated particles and expose the impurities encapsulated inside, which makes them more susceptible to chemical reactions and removal during the water washing process.
Laser diffractometer was used to determine the particle size distribution of different samples, each sample was repeated three times and averaged, the results are shown in Figure 6. Particle size distribution analysis further illustrates the intensifying effect of UWO. The D10, D50, and D90 of CWO were 0.877, 4.81, and 106 µm, respectively, which suggests that mechanical agitation not only fails to effectively disperse particles but also increases the particle size and significantly widens the distribution of particle sizes (0.3~110 µm). This means aggravated particle aggregation. Wang et al. [45], in their study on the replacement of high concentration of cadmium by aluminum powder, found that conventional stirring could not effectively disperse the aluminum powder but instead promoted the degree of aggregation. The D10, D50, and D90 of UWO were 0.735, 2.53, and 6.91 µm, respectively. This indicates that UWO not only reduces the average particle size but also narrows the particle size distribution, resulting in a more homogeneous particle population and lower degree of aggregation. The smaller and more homogeneous particles in the UWO-treated samples resulted in a larger surface area available for chemical interaction, which led to increased reactivity and improved lead recovery efficiency and purity.
2.5. Phase Transition Under Ultrasound
To further demonstrate the mechanisms of sonication enhancement, XPS was performed on the raw material and the solids from UWO and CWO, with the results shown in Figure 7.
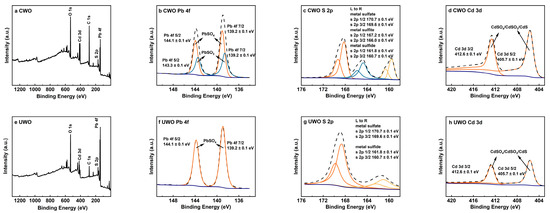
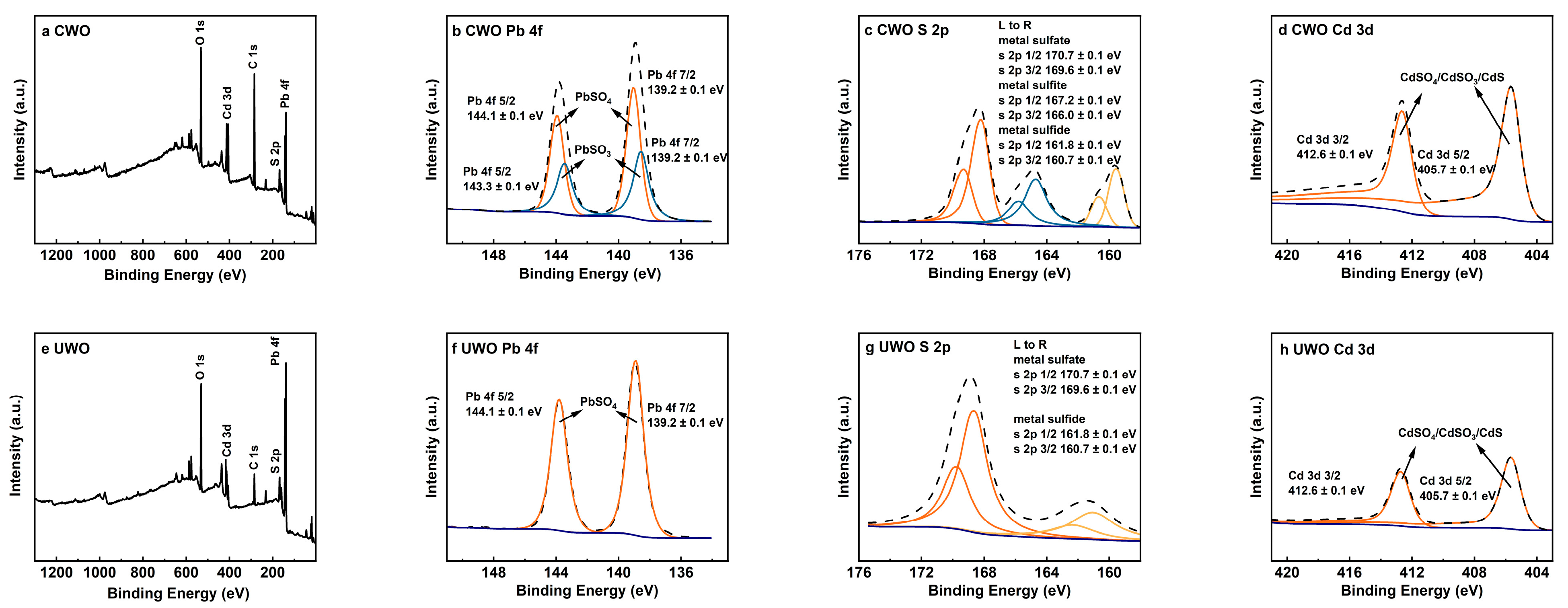
Figure 7.
XPS analysis results for solids obtained from different treatments under optimal conditions: (a–d) conventional oxidative water washing; (e–h) ultrasonic-enhanced oxidative water washing (UWO).
The Pb 4f spectra (Figure 7b,f) reveal that the UWO exhibits more pronounced and sharper peaks at 139.2 ± 0.1 eV (Pb 4f 7/2) and 143.3 ± 0.1 eV (Pb 4f 5/2), corresponding to the typical signals of PbSO4. These peaks are significantly purer and more distinct in UWO than in CWO, indicating a more thorough transformation of lead species into PbSO4. In contrast, CWO shows additional peaks at 144.1 ± 0.1 eV and 148.3 ± 0.1 eV, corresponding to PbSO3, which are significantly weakened in UWO. This confirms that UWO effectively promotes the oxidation of PbSO3 to PbSO4, minimizing lead loss during water washing. The S 2p spectra (Figure 7c,g) further confirm this phase transformation. In UWO, the signals for metal sulfides (S 2p 1/2 at 161.8 ± 0.1 eV and S 2p 3/2 at 160.7 ± 0.1 eV) are substantially weakened compared to CWO. This indicates a significant reduction in sulfide species, consistent with their oxidation to sulfates under UWO conditions. This aligns with the transformation of metal sulfides like CdS to more oxidized forms such as CdSO4, which are more water-soluble and can be efficiently removed during water washing. The Cd 3d spectra (Figure 7d,h) also reflect the beneficial effects of UWO, with markedly reduced Cd signals compared to CWO. This suggests a decrease in cadmium-containing compounds in the solid phase after UWO treatment, likely due to their oxidation to more soluble forms like CdSO4, which can be effectively removed during the water washing stage.
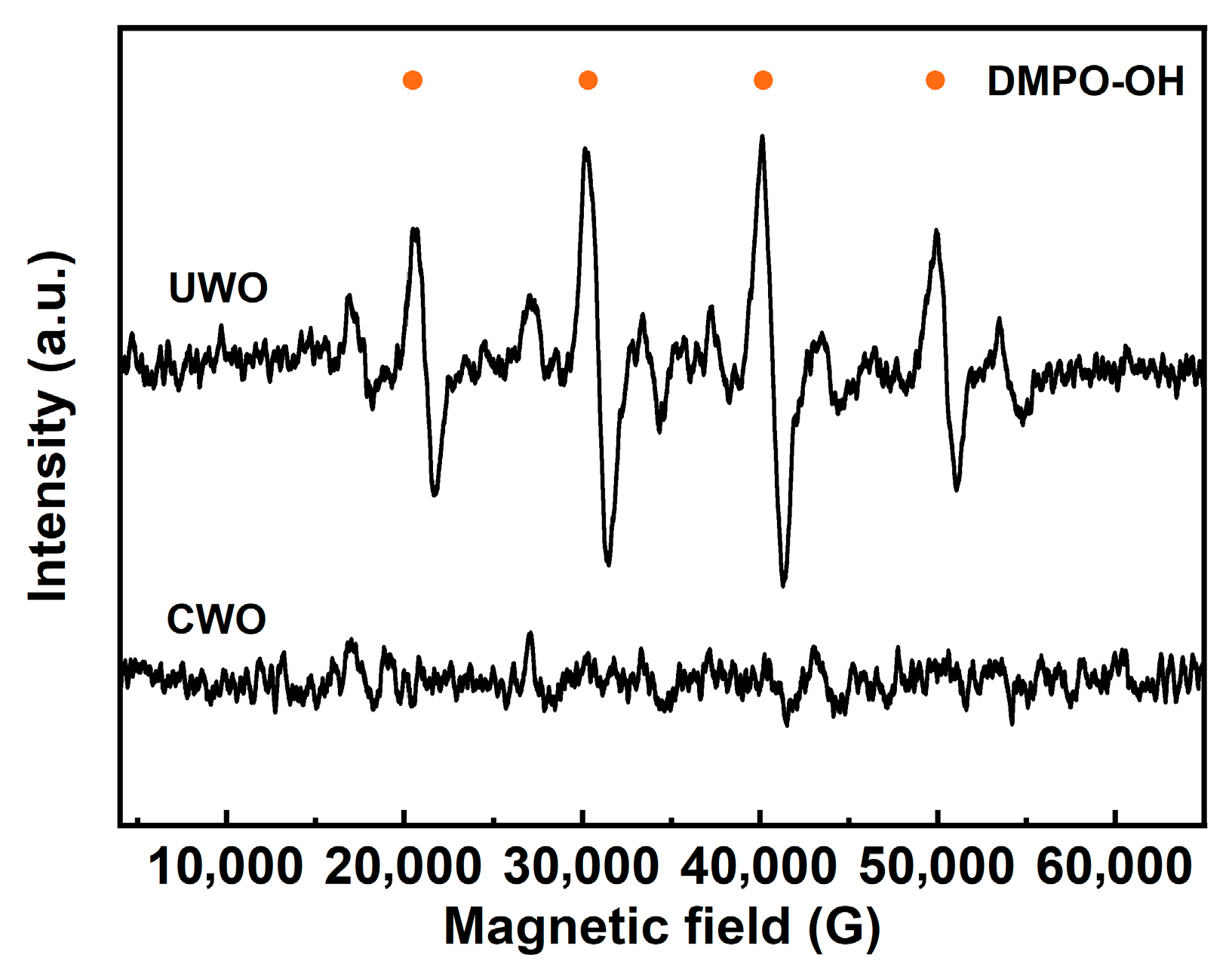
EPR analysis (Figure 8) provides further insight into the oxidation mechanism of UWO. EPR, or Electron Paramagnetic Resonance, is a technique used to detect and quantify free radicals. The sample (UWO, CWO) was mixed with DMPO, and analysis was conducted within 2 min of mixing to capture transient •OH radicals, forming a stable DMPO-OH adduct. This adduct exhibits a characteristic 1:2:2:1 quadruplet EPR spectrum. The EPR spectrum of UWO shows distinct signals corresponding to DMPO-OH radicals, indicating the generation of hydroxyl radicals during the process. These highly reactive •OH radicals, stronger oxidants than H2O2 alone, are likely formed by the ultrasonic cavitation effect on H2O2. They play a crucial role in driving the oxidation of PbSO3 to PbSO4 and transforming Cd-containing compounds into more soluble forms, thereby enhancing their removal.
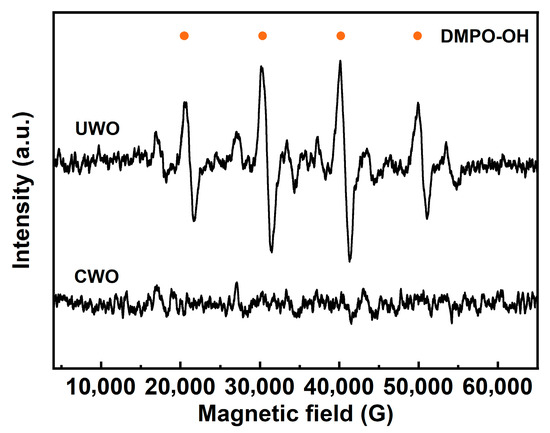
Figure 8.
Comparison of EPR results under different conditions.
2.6. Analysis of the Final Solid Phase
To evaluate the quality of solids obtained from different treatments, a series of analyses were conducted on the solids from optimized CWO and UWO processes.
XRF analysis (Table 2) shows that UWO significantly boosts the lead mass fraction in the solid phase to 66.83%, up from 41.68% in RFD. Simultaneously, the cadmium mass fraction drops sharply from 14.33% to 0.23%. Similar reductions are observed for other elements like sulfur, zinc, iron, and various trace elements. This indicates UWO’s high effectiveness in selectively enriching lead and removing multiple impurities, which is critical for enhancing the quality of the lead residue.

Table 2.
Comparison of the content of different elements in different samples (wt. %).
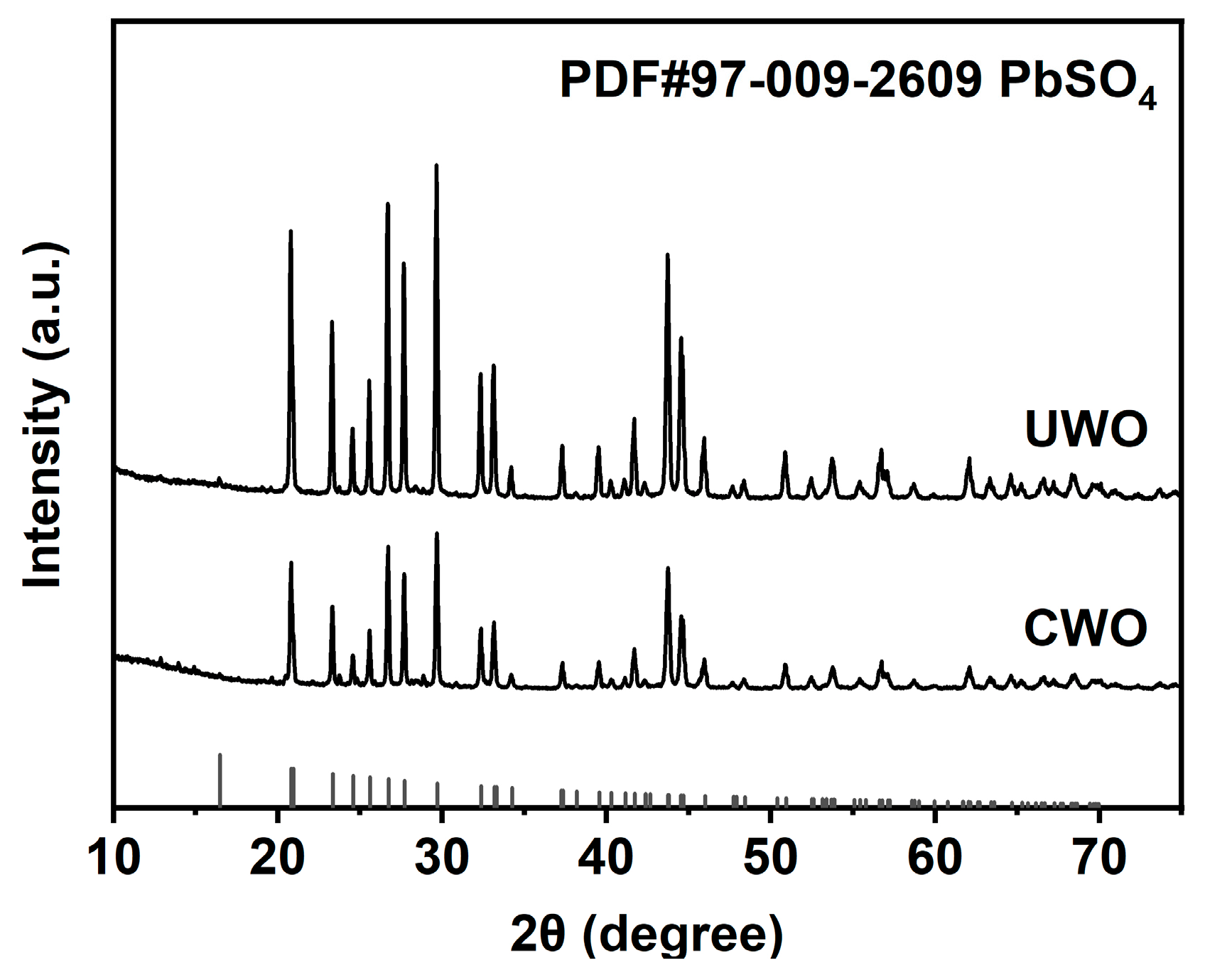
XRD analysis (Figure 9) further confirms the phase structure of the UWO-treated solid. Its XRD pattern closely matches the standard PDF card for PbSO4 (PDF#97-009-2609), with well-defined peaks indicating high-purity crystalline PbSO4. The absence of significant peaks from other phases, such as PbSO3 or cadmium compounds, demonstrates that UWO effectively transforms PbSO3 to PbSO4 and removes cadmium impurities.
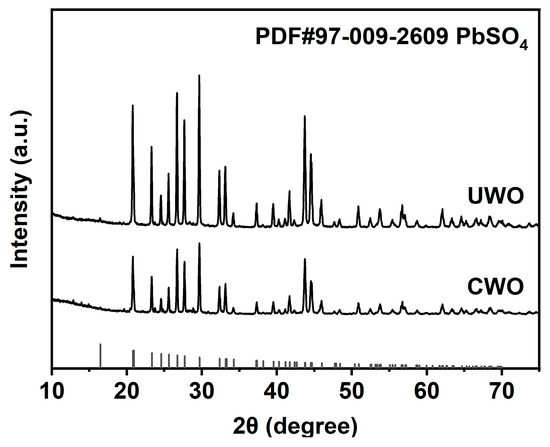
Figure 9.
XRD analysis results for solids obtained from different treatments under optimal conditions.
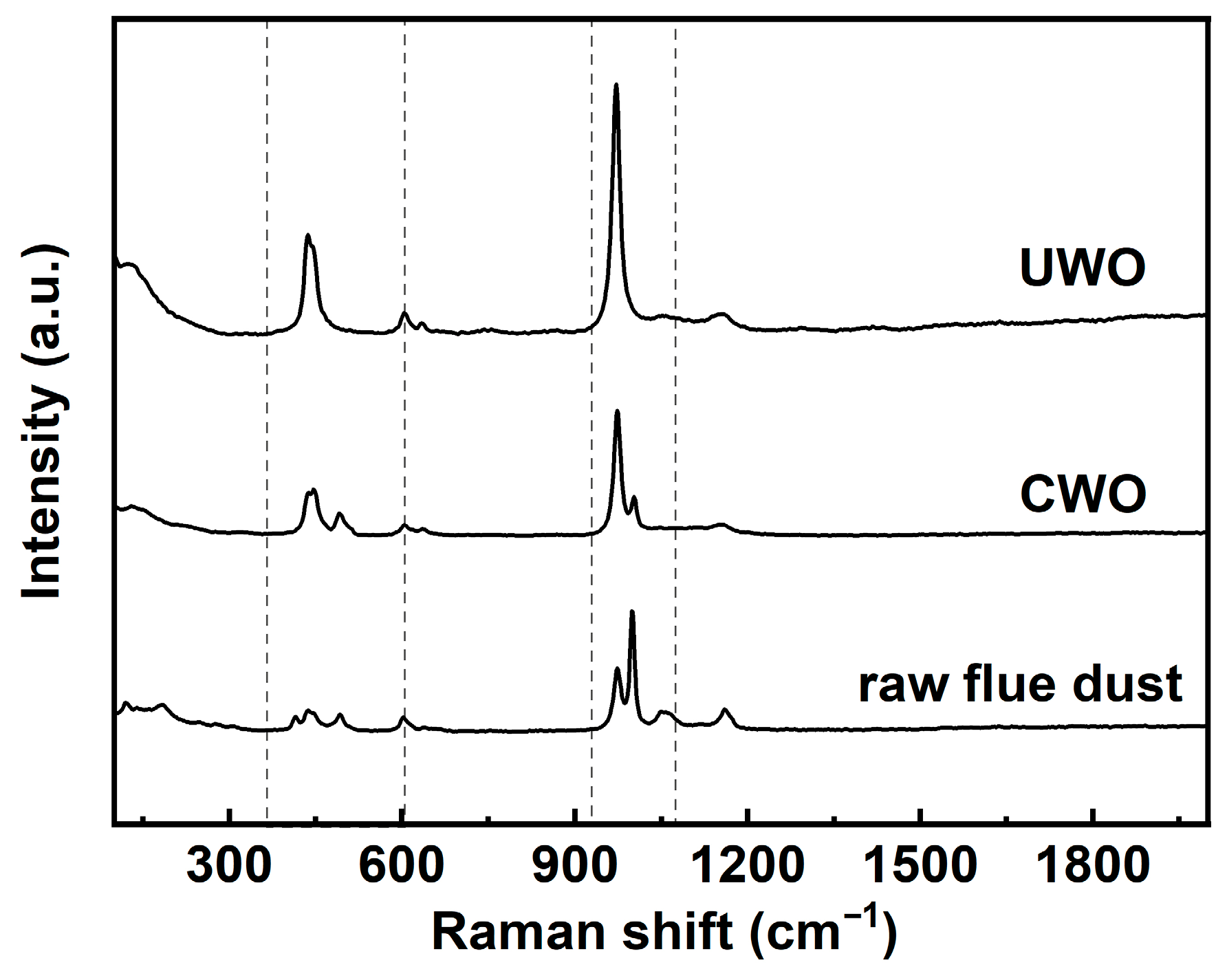
Figure 10 presents the Raman spectral data for three solid phases. In the raw RFD spectrum, a prominent peak is observed in the 980–1090 cm−1 range. This peak corresponds to sulfate ions (SO42−) in compounds like PbSO4 and CdSO4 [40]. It indicates a high content of sulfate compounds in the raw material. Additionally, a broad peak in the 400–600 cm−1 region is attributed to metal–oxygen stretching vibrations in various metal sulfates, reflecting a diverse array of sulfate compounds with different metal–oxygen bonding environments. After CWO, the Raman spectrum shows an increased intensity of the peak in the 980–1090 cm−1 range compared to raw RFD. This suggests a relative increase in the concentration of sulfate compounds such as PbSO4. The elevated intensity implies that the CWO process aids in converting or retaining more lead in the low-solubility sulfate form, which is beneficial for lead recovery. However, the broad peak in the 400–600 cm−1 region remains largely unchanged, indicating that multiple sulfate compounds are still present, which aligns with the higher impurity content in CWO. The Raman spectrum of the UWO sample shows the most significant changes. The peak intensity in the 980–1090 cm−1 ranges increases substantially compared to both raw RFD and CWO samples. This indicates that UWO effectively promotes the formation of sulfate compounds like PbSO4. Moreover, the broad peak in the 400–600 cm−1 region becomes narrower and sharper in the UWO spectrum. This narrowing implies a reduction in the variety of metal sulfate compounds and a more uniform sulfate phase, consistent with previous analyses that UWO enhances lead retention in the solid phase as PbSO4 and improves impurity removal, thereby boosting lead recovery.
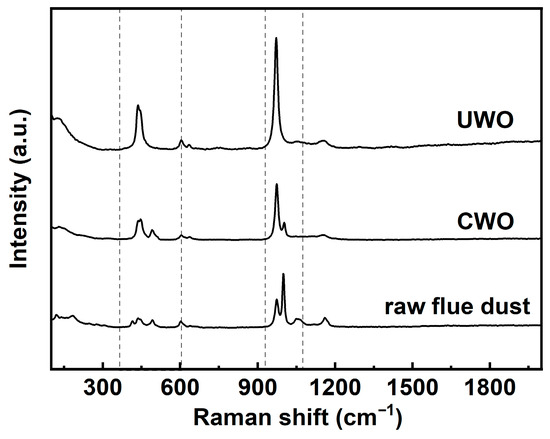
Figure 10.
Raman analysis results for solids obtained from different treatments under optimal conditions.
3. Experimental
3.1. Materials
The raw flue dust used in this study was bottom-blown furnace flue dust from a lead smelting enterprise in Henan Province, China, and the H2O2 used was of analytically pure grade (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). In these experiments, all solutions were prepared using deionized water.
3.2. Experimental Procedure
A total of 50 g of RFD was weighed and placed in a 200 mL beaker with some deionized water, then the beaker was sealed with plastic wrap. An ultrasonic probe (20 kHz, max 1800 W) was positioned vertically in the center of the beaker, 1–1.5 cm above the bottom. The beaker was placed in a water bath and stirred magnetically at 200 rpm. During leaching, H2O2 solution was added at a rate of 10 mL/min. Experiments were conducted under different conditions by adjusting parameters like temperature, time, liquid–solid ratio, ultrasonic power (0 W for conventional water washing), and oxidant dosage. After each experiment, the mixture was transferred to a suction flask and vacuum filter to separate the leachate and residue, which were then characterized with various analytical techniques.
3.3. Analytical Methods
The element content of the samples was determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent 5110, Santa Clara, CA, USA). The physical phases of different samples were studied using a powder X-ray diffractometer (XRD, Rigaku dX 2000, Zhaodao, Japan) with an operating voltage of 40 kV and an operating current of 25 mA, using Cu-Kα as the ray source. The scanning angle of the XRD ranged from 10 to 80°, and the scanning speed was 2°/min. The XRD data were also analyzed using MDI Jade v9.0 software. X-ray photoelectron spectroscopy (XPS, PHI-5300, Chanhassen, MN, USA) was used to analyze the binding energy of the different elements in the samples, with an operating voltage of 12 kV and an operating current of 6 mA, using Al-Kα as the ray source. The results detected by the XPS instrument were analyzed using Avantage v5.9931 software and calibrated at the C1s peak position of 284.8 eV. Qualitative elemental analysis was performed using an X-ray fluorescence spectrometer (XRF, Rigaku ZSX Primus, Akishima, Japan). The laser used was a 532, with a laser energy of 6.0 mW, a 600 g/mm grating, an Olympus 50× objective (Olympus 50×/0.5), an integration time of 35 s, and an accumulation number of 6 times. A scanning electron microscope–energy dispersive spectrometer (SEM-EDS, Zeiss Sigma 300, Jena, Germany) was used to study the micromorphology and elemental distribution of different samples. Particle size and distribution of the samples were measured using a laser particle size analyzer (Malvern Mastersizer 3000+ Ultra, Malvern, UK), with ethanol used as a dispersant. A Raman analyzer (WITec alpha 300R, Ulm, Germany) was used to determine the chemical structure of different samples. Electron paramagnetic resonance (EPR, JEOL JES-FA200, Akishima, Japan) analysis was performed using 5,5-dimethyl-1-pyrrolidine N-oxide (DMPO) as the spin trapping agent. The instrument parameters wre set as follows: operating frequency: 9182 MHz; central magnetic field: 327.0 mT; power: 0.998 mW; scan width: ±5 mT; modulation amplitude: 1.0 G; scan time: 30 s.
The calculation formula for lead recovery rate is as follows:
where represents the mass fraction of lead in the solid phase at the end of the reaction; represents the mass of the solid phase at the end of the reaction; represents the mass fraction of lead in the raw flue dust; and represents the mass of the raw flue dust taken for each experiment.
4. Conclusions
In this study, a method for ultrasonic-enhanced hydrogen peroxide water washing to recover lead from RFD under neutral conditions was developed. The key conclusions are as follows:
- (1)
- The optimized conditions for ultrasonic-enhanced hydrogen peroxide water washing to recover lead from RFD under neutral conditions were determined as follows: a washing temperature of 40 °C, a washing time of 30 min, an H2O2 dosage of 4 mL, a liquid–solid ratio of 2:1, and an ultrasonic power of 240 W. Under these conditions, the lead mass fraction in the UWO-treated lead residue reached 68.11% and a lead recovery rate of 97.19%, significantly higher than that obtained from CWO (lead mass fraction of 64.07%, lead recovery rate of 95.93%).
- (2)
- Ultrasound enhances lead recovery through two key mechanisms: inhibiting aggregation and unwrapping encapsulated particles, promoting phase transformation via hydroxyl radical generation. Its cavitation and mechanical effects break up particle agglomerates, exposing impurities for removal. Meanwhile, ultrasound action on H2O2 generates hydroxyl radicals, boosting oxidation to transform PbSO3 into PbSO4 and Cd compounds into more soluble forms like CdSO4. These synergistic effects improve recovery efficiency and the lead mass fraction in the solid phase after washing.
- (3)
- The UWO process significantly improves the purity and quality of the final lead residue. The lead mass fraction increased significantly, and major impurities such as cadmium were significantly reduced. The treated solids consisted mainly of highly crystalline PbSO4 with reduced formation of other sulfates, resulting in a more homogeneous and lead enrichment.
Ultrasonic technology can significantly improve the efficiency of lead recovery from RFD through a combination of physical destruction and chemical oxidation. This highly efficient process offers a more environmentally friendly and efficient alternative to traditional methods, providing insights into moving the metallurgical industry towards greater sustainability.
Author Contributions
Conceptualization, T.L.; formal analysis, T.W.; resources, L.Z.; writing—original draft preparation, T.W.; writing—review and editing, Y.X., P.D.L., T.L.; supervision, L.Z.; project administration, T.L.; funding acquisition, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the “Yunnan Revitalization Talents Support Plan” High-end Foreign Talents Program, Yunnan Province Key R&D Plan (202403AK140009), the Yunnan Major Scientific and Technological Project (202302AG050008), and the Kunming University of Science and Technology 2023 Top Innovative Talent Project.
Data Availability Statement
Data available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Gonzalez-Montero, P.; Iglesias-Gonzalez, N.; Romero, R.; Mazuelos, A.; Carranza, F. Recovery of Zinc and Copper from Copper Smelter Flue Dust. Optimisation of Sulphuric Acid Leaching. Environ. Technol. 2020, 41, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Di, H.; Song, L.; Yang, K.; Zhang, L. Study on Leaching Behaviour of Germanium and Iron in Zinc Oxide Dust from Lead Zinc Smelting. Can. Metall. Q. 2023, 62, 573–580. [Google Scholar] [CrossRef]
- Owusu-Acheaw, Y.; Liu, M.; Ma, G.; Zhang, X.; Du, T.; Xu, J. Recovery of Valuable Metals from Blast Furnace Dust by Iron-Bath Process. J. Sustain. Metall. 2025, 11, 1511–1524. [Google Scholar] [CrossRef]
- Maczek, H.; Kola, R. Recovery of Zinc and Lead from Electric-Furnace Steelmaking Dust at Berzelius. JOM 1980, 32, 53–58. [Google Scholar] [CrossRef]
- Lee, H.; Mishra, B. Recovery of Copper and Precious Metals and Separation of Lead from Flue Dust of Electronic Waste Processing. Miner. Process. Extr. Metall. Rev. 2020, 41, 153–161. [Google Scholar] [CrossRef]
- Xie, B.; Yang, T.; Liu, W.; Zhang, D.; Chen, L. Recovery of Lead from Spent Lead Paste by Pre-Desulfurization and Low-Temperature Reduction Smelting. JOM 2020, 72, 3195–3203. [Google Scholar] [CrossRef]
- Xie, S.; Qin, S.; Su, Z.; Feng, X.; Zhao, L. An Innovative Process for the Direct Recovery of Lead from Waste Lead Paste. J. Electrochem. Soc. 2023, 170, 43501. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Chen, K.; Li, J.; Wang, T.; Mei, M. Recovering Metallic Pb Directly from Lead Smelting Dust by NaOH-Carbon Roasting Process. J. Mater. Res. Technol. 2020, 9, 2744–2753. [Google Scholar] [CrossRef]
- Leclerc, N.; Meux, E.; Lecuire, J.-M. Hydrometallurgical Recovery of Zinc and Lead from Electric Arc Furnace Dust Using Mononitrilotriacetate Anion and Hexahydrated Ferric Chloride. J. Hazard. Mater. 2002, 91, 257–270. [Google Scholar] [CrossRef]
- Geng, X.; Ru, J.; Hua, Y.; Zhang, W. The Recovery of Lead from Spent Lead Acid Battery Paste by Electrodeposition in Deep Eutectic Solvent. J. Sustain. Metall. 2022, 8, 1257–1268. [Google Scholar] [CrossRef]
- Ji, W.; Xie, K.; Yan, S. Separation and Recovery of Heavy Metals Zinc and Lead from Phosphorus Flue Dust by Vacuum Metallurgy. J. Environ. Manag. 2021, 294, 113001. [Google Scholar] [CrossRef]
- Liu, W.; Deng, X.; Zhang, D.; Yang, T.; Chen, L. A Clean Process of Lead Recovery from Spent Lead Paste Based on Hydrothermal Reduction. Trans. Nonferrous Met. Soc. China 2018, 28, 2360–2367. [Google Scholar] [CrossRef]
- Ye, L.; Song, S.; Yang, S.; Chen, Y.; Liu, S. The Recovery of Valuable Components from Secondary Lead Dust by a Multi-Step Hydrometallurgical Process. Hydrometallurgy 2023, 222, 106186. [Google Scholar] [CrossRef]
- Chen, C.; Dai, X.; Wei, X. Production practice of separation and recovery of cadmium from lead fume in oxygen-enriched top-blown zinc co-processing in wet zinc smelting. Min. Metall. 2023, 32, 60–64. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, S.; Ma, Y.; Zhou, J. Removal of Cadmium Ion from Wastewater by Manganese Oxides-Loaded Sludge Biochar. Desalin. Water Treat. 2024, 319, 100563. [Google Scholar] [CrossRef]
- Li, W.; Liu, W.; Jiao, F.; Xie, L.; Qin, W. Comprehensive Recovery of Arsenic and Valuable Metals from Lead Smelting Flue Dust: Process Optimization and Mechanism Investigation. Sep. Purif. Technol. 2025, 353, 128497. [Google Scholar] [CrossRef]
- Guo, L.; Lan, J.; Du, Y.; Zhang, T.C.; Du, D. Microwave-Enhanced Selective Leaching of Arsenic from Copper Smelting Flue Dusts. J. Hazard. Mater. 2020, 386, 121964. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xie, B.; Liu, W.; Zhang, D.; Chen, L. An Environment-Friendly Process of Lead Recovery from Spent Lead Paste. Sep. Purif. Technol. 2020, 233, 116035. [Google Scholar] [CrossRef]
- Rao, M.; Xia, H.; Xu, Y.; Jiang, G.; Zhang, Q.; Yuan, Y.; Zhang, L. Study on Ultrasonic Assisted Intensive Leaching of Germanium from Germanium Concentrate Using HCl/NaOCl. Hydrometallurgy 2024, 230, 106385. [Google Scholar] [CrossRef]
- Devos, C.; Bampouli, A.; Brozzi, E.; Stefanidis, G.D.; Dusselier, M.; Van Gerven, T.; Kuhn, S. Ultrasound Mechanisms and Their Effect on Solid Synthesis and Processing: A Review. Chem. Soc. Rev. 2025, 54, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Xu, L.; Su, X.; Hu, B.; Jia, T.; Mi, L. Experimental Study of the Effect of Mechanical Vibration and Water Velocity on Bubble Management in PEM Electrolysis Cell. Int. J. Hydrogen Energy 2018, 49, 390–403. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.F.; Chen, Y.; Li, X.G.; Wang, Y.H.; Wang, Y.; Ge, Z.T.; Wang, X.; Cai, S.; Yang, X.; et al. Impact of Microbubble Degradation and Flow Velocity on Subharmonic-Aided Pressure Estimation (SHAPE): An Experimental Investigation. Ultrasound Med. Biol. 2024, 50, 1020–1027. [Google Scholar] [CrossRef]
- Meroni, D.; Djellabi, R.; Ashokkumar, M.; Bianchi, C.L.; Boffito, D.C. Sonoprocessing: From Concepts to Large-Scale Reactors. Chem. Rev. 2022, 122, 3219–3258. [Google Scholar] [CrossRef]
- Ye, L.; Zhu, X. Analysis of the Effect of Impact of Near-Wall Acoustic Bubble Collapse Micro-Jet on Al 1060. Ultrason. Sonochem. 2017, 36, 507–516. [Google Scholar] [CrossRef]
- Osterman, A.; Dular, M.; Sirok, B. Numerical Simulation of a Near-Wall Bubble Collapse in an Ultrasonic Field. J. Food Sci. Technol.-Mysore. 2009, 4, 210–221. [Google Scholar] [CrossRef]
- Wood, R.J.; Lee, J.; Bussemaker, M.J. Combined Effects of Flow, Surface Stabilisation and Salt Concentration in Aqueous Solution to Control and Enhance Sonoluminescence. Ultrason. Sonochem. 2019, 58, 104683. [Google Scholar] [CrossRef] [PubMed]
- Vikash; Kumar, V. Ultrasonic-Assisted de-Agglomeration and Power Draw Characterization of Silica Nanoparticles. Ultrason. Sonochem. 2020, 65, 105061. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, A.; Khavari, M.; Subroto, T.; Prentice, P.; Pericleous, K.; Eskin, D.; Durodola, J.; Tzanakis, I. Mechanisms of Ultrasonic De-Agglomeration of Oxides through in-Situ High-Speed Observations and Acoustic Measurements. Ultrason. Sonochem. 2021, 79, 105792. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, H.; Fu, L.; Lin, G.; Zhang, G.; Wang, S.; Zhang, L. Highly Selective Separation of Palladium from Spent Catalysts by Ozonation with Ultrasonic Enhancement in a Low-Acid Medium. Chem. Eng. J. 2023, 467, 143493. [Google Scholar] [CrossRef]
- Guillemin, J.P.; Schaer, E.; Marchal, P.; Lemaître, C.; Nonnet, H.; Ledieu, A. A Mass Conservative Approach to Model the Ultrasonic De-Agglomeration of ZnO Nanoparticle Suspension in Water. Powder Technol. 2012, 219, 59–64. [Google Scholar] [CrossRef]
- Wen, J.; Wu, C.; Bi, X.; Zhang, S.; Ouyang, H.; Ye, J.; Ohnuki, T.; Yu, Q. Soil pH Change Induced by Smelting Activities Affects Secondary Carbonate Production and Long-Term Cd Activity in Subsoils. Appl. Geochem. 2023, 152, 105663. [Google Scholar] [CrossRef]
- Gui, Q.; Hu, Y.; Wang, S.; Zhang, L. Mechanism of Synergistic Pretreatment with Ultrasound and Ozone to Improve Gold and Silver Leaching Percentage. Appl. Surf. Sci. 2022, 576, 151726. [Google Scholar] [CrossRef]
- Tian, J.; Chen, B.; Xia, H.; Yang, W.; Dai, L.; Zhang, L. Ultrasound-Assisted Enhanced Leaching of Lithium and Fluoride Compounds from Waste Cathode Carbon: Comprehensive Recovery and Leaching Mechanism. Sep. Purif. Technol. 2025, 354, 129190. [Google Scholar] [CrossRef]
- Tsuneda, T.; Taketsugu, T. Theoretical Investigations on Hydrogen Peroxide Decomposition in Aquo. Phys. Chem. Chem. Phys. 2018, 20, 24992–24999. [Google Scholar] [CrossRef]
- Lashari, A.A.; Kazi, T.G.; Baig, J.A.; Afridi, H.I.; Lashari, A.; Kandhro, F. An Ultrasound Assisted Modified Solid Phase Micro-Extraction Technique for Enrichment of Cadmium and Lead in Aqueous Extract of Coal Gangue Soil Samples. Geoderma 2023, 437, 116601. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Q.; Wang, D.; Yang, S.; He, K. Release Mechanism of Impurity Potassium in Molybdenum Concentrate Treatment Process. Trans. Nonferrous Met. Soc. China 2023, 33, 917–928. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Jian, L.; Zhou, J.F. Hydrogen Peroxide Treatment Discoloration Industrial Sulfuric Acid. Guangdong Chem. Ind. 2020, 47, 12–13. (In Chinese) [Google Scholar]
- Marković, S.; Mitrić, M.; Starčević, G.; Uskoković, D. Ultrasonic De-Agglomeration of Barium Titanate Powder. Ultrason. Sonochem. 2008, 15, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Hayashida, Y.; Sano, K.; Terasaka, K. Agglomeration and Rapid Ascent of Microbubbles by Ultrasonic Irradiation. Ultrason. Sonochem. 2011, 18, 1193–1196. [Google Scholar] [CrossRef]
- Li, C.; Tang, S.; Xu, Y.; Liu, F.; Li, M.; Zhi, X.; Ma, Y. Ultrasonic-Assisted Activated Carbon Separation Removing Bacterial Endotoxin from Salvia Miltiorrhizae Injection. Ultrason. Sonochem. 2024, 103, 106781. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Shen, X.; Chen, W.; Zhou, Y.; Zhi, X. Ultrasonic-Assisted Supercritical Fluid Separation Removing Plasticizers from Ganoderma Lucidum Spores’ Oil. Ultrason. Sonochem. 2023, 100, 106622. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, S.; Liu, Q.; Chen, L.; Xian, Y.; Wang, Y. Ultrasonic Pretreatment for Enhancing Flotation Separation of Elemental Sulfur and Silver-Bearing Lead Minerals from an Oxidative Pressure Leaching Residue of Zinc Sulfide. Miner. Eng. 2024, 205, 108495. [Google Scholar] [CrossRef]
- Zhong, X.; Huang, C.; Chen, L.; Yang, Q.; Huang, Y. Effect of Ultrasound on the Kinetics of Anti-Solvent Crystallization of Sucrose. Ultrason. Sonochem. 2022, 82, 105886. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ji, H.; Koppala, S.; Zhang, Y.; Song, D.; Yan, Y.; Phan, D.; Le, T.; Zhang, L. Efficient and Eco-Friendly Cadmium Ion Recycling: Ultrasonic Enhancement of Aluminum Powder Replacement for Low-Temperature Industrial Applications. Ultrason. Sonochem. 2024, 102, 106764. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).