Organic Acid Leaching of Black Mass with an LFP and NMC Mixed Chemistry
Abstract
1. Introduction
2. Method and Materials
2.1. Materials
2.2. Methods
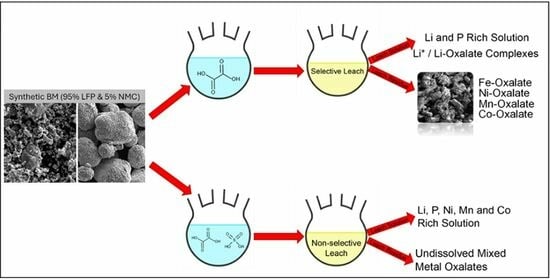
2.2.1. General Leaching Experiments
2.2.2. Screening Experiments
2.2.3. Optimisation Experiments
2.3. Analytical Techniques
3. Results and Discussion
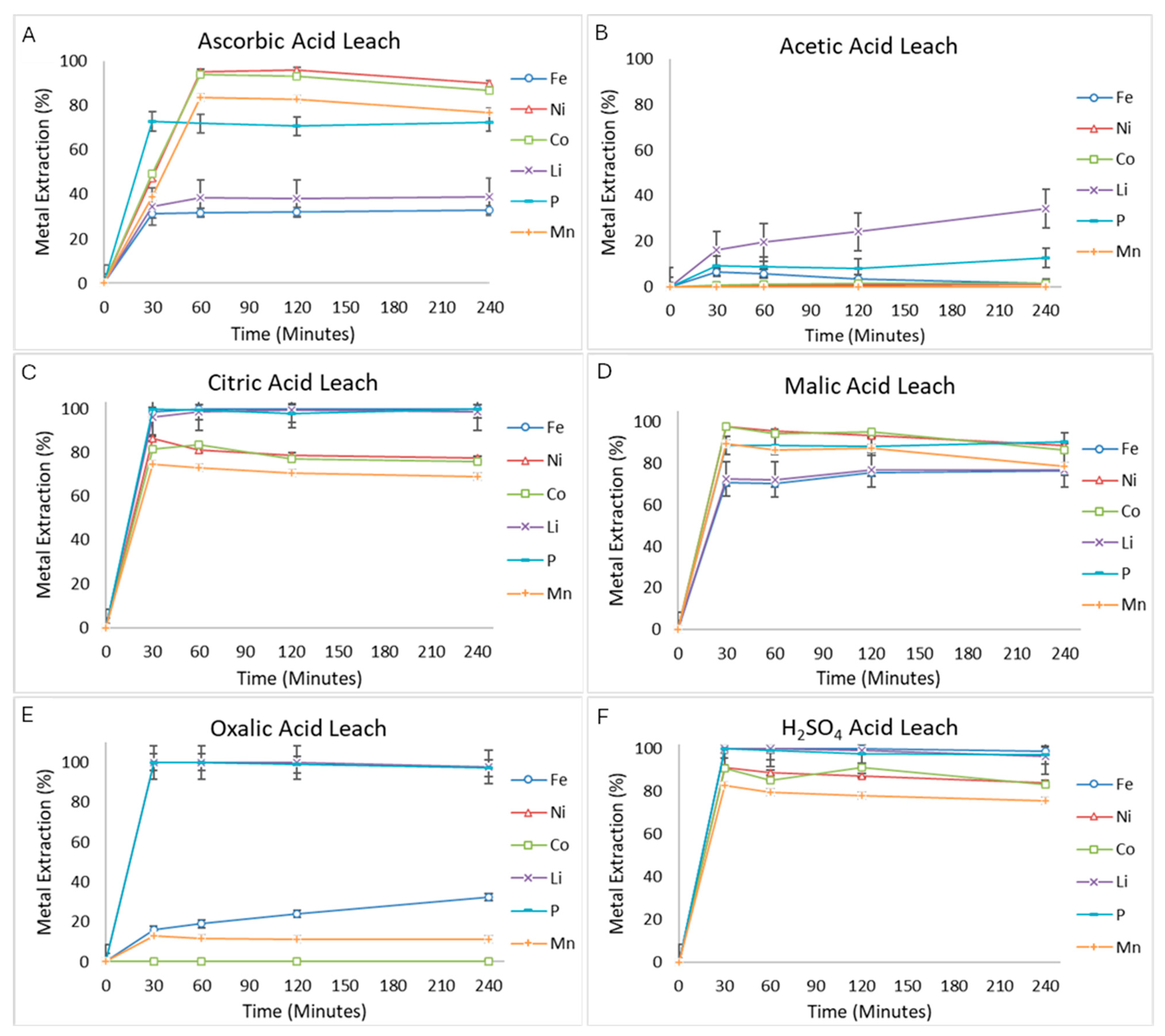
3.1. Lixiviant Screening
3.2. Optimisation of Oxalic Acid Leach—Synthetic Sample
3.2.1. Effect of Concentration
3.2.2. Effect of Temperature
3.2.3. Possible Leaching Reactions
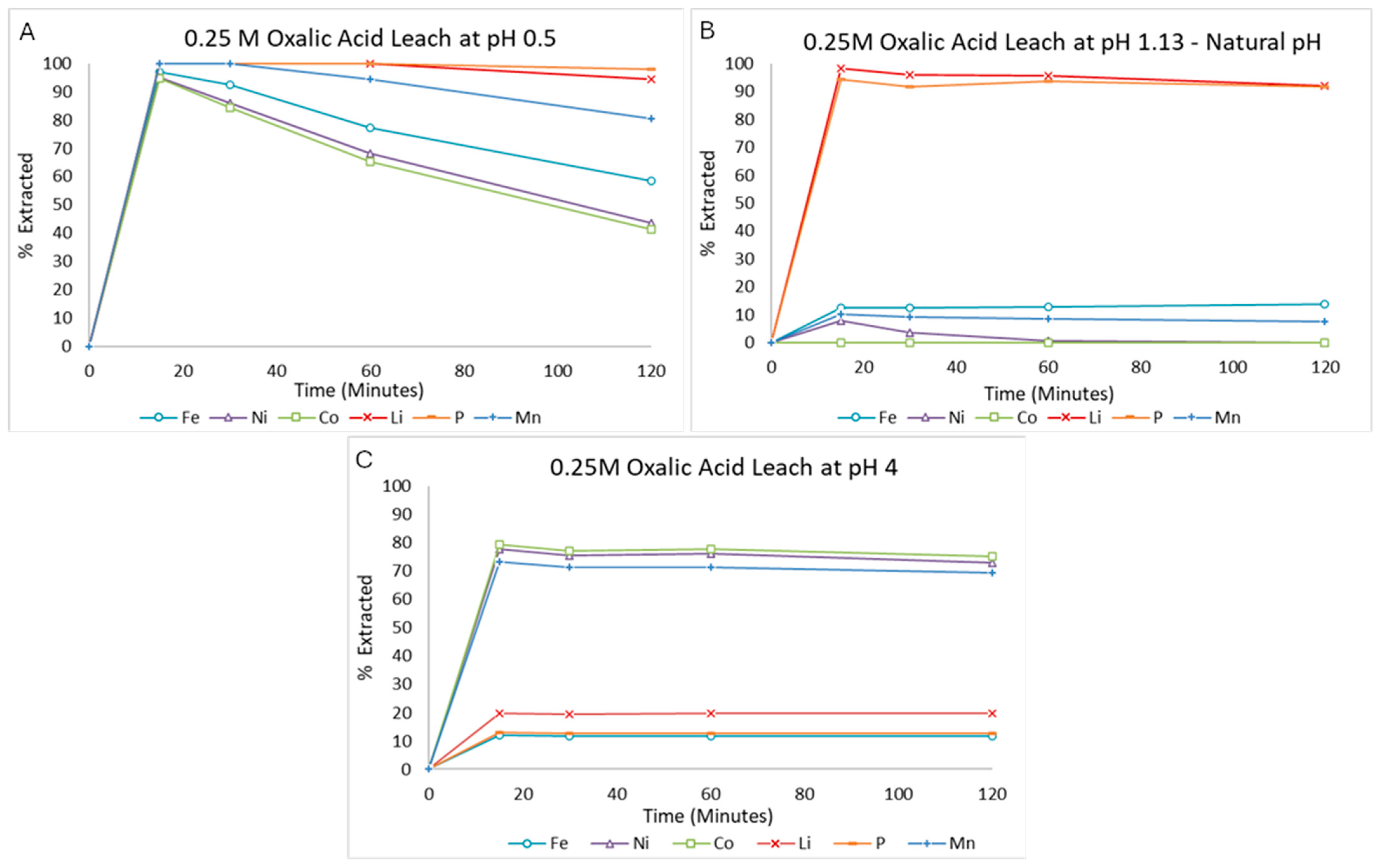
3.2.4. Changing pH
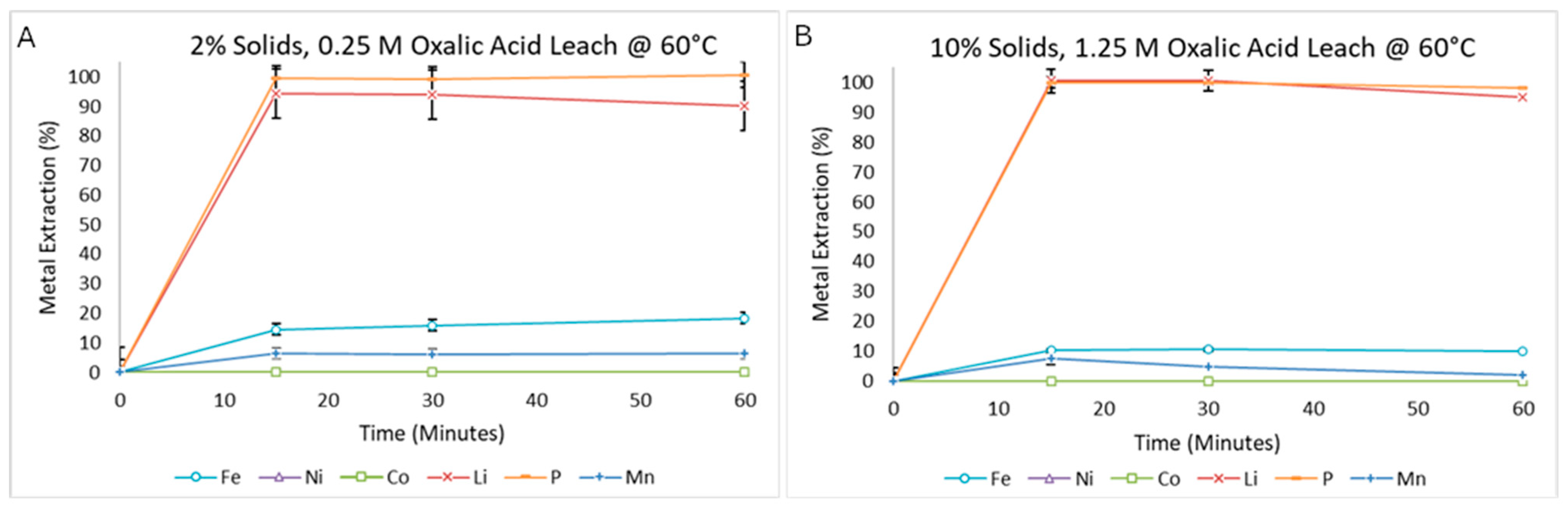
3.2.5. Effect of Solid–Liquid Ratio
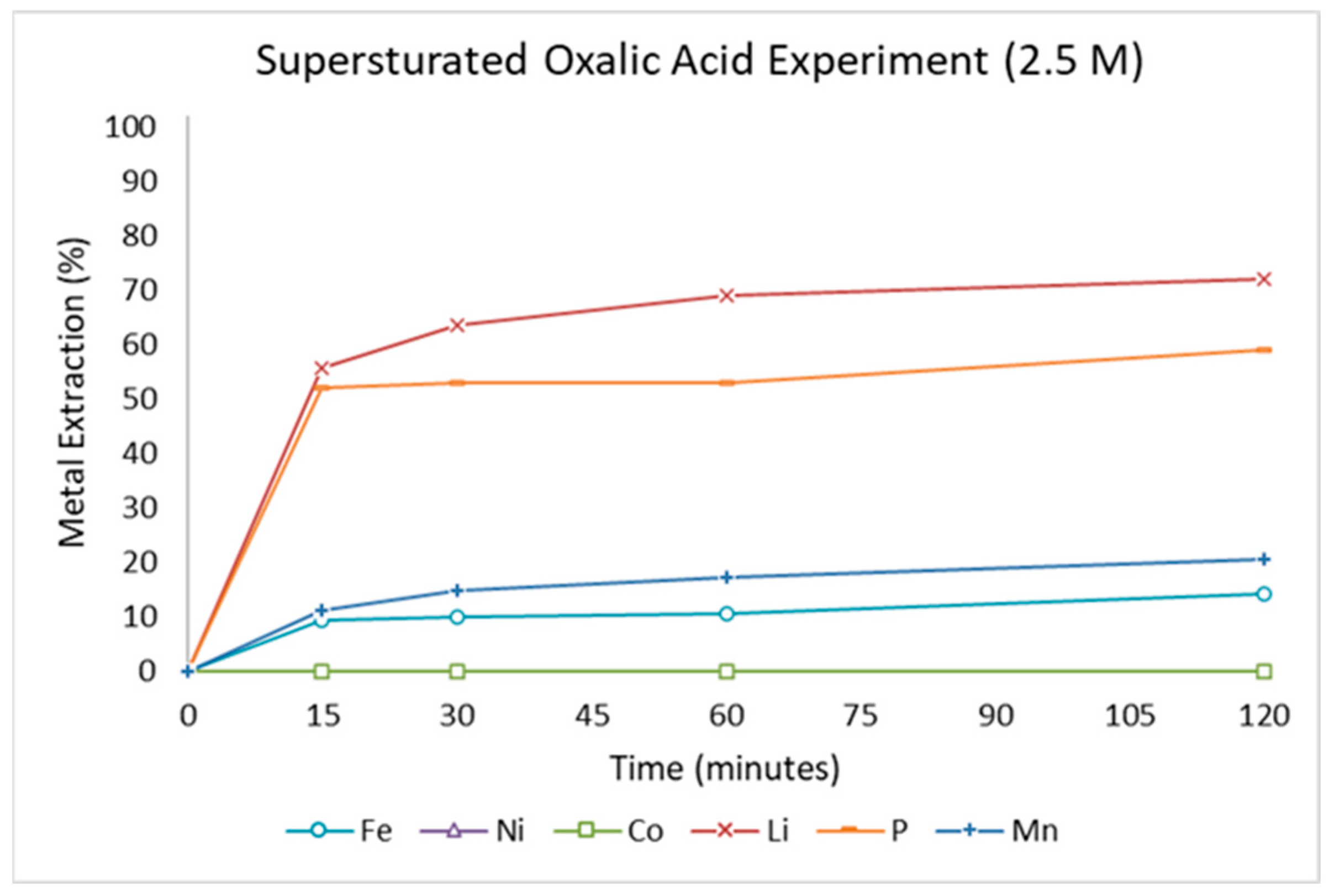
3.2.6. Supersaturated Leaching System
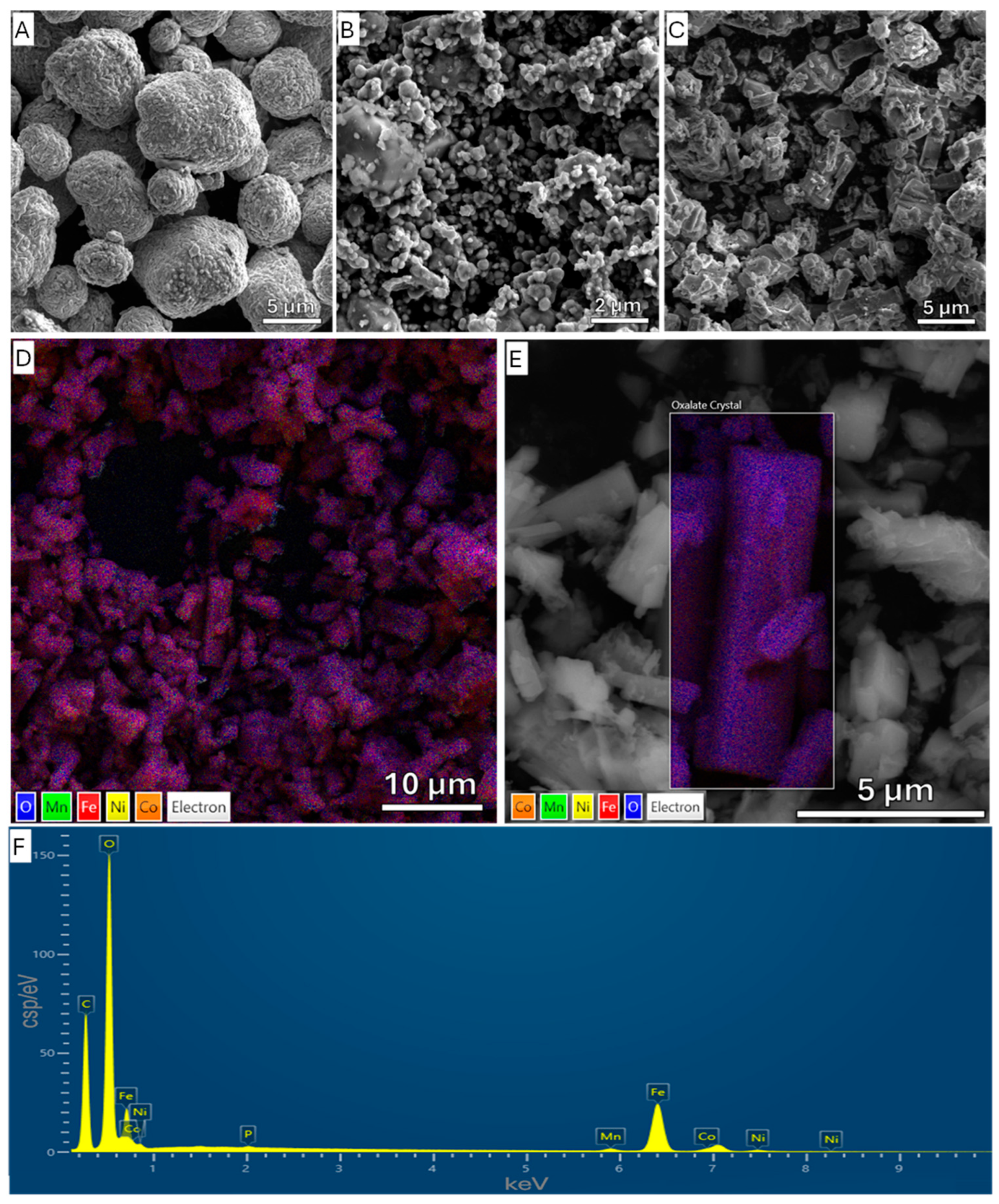
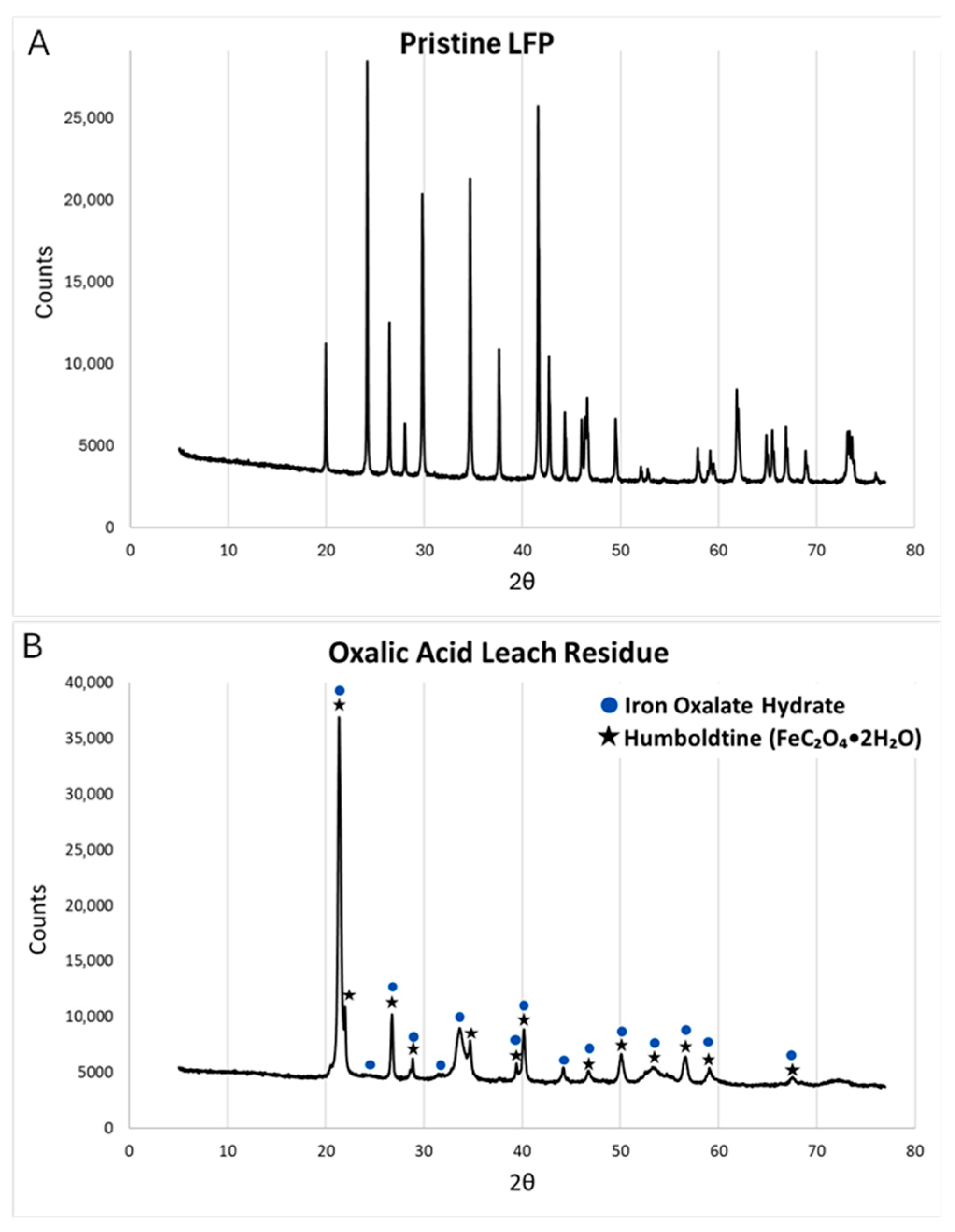
3.2.7. Analysis of Residue Material
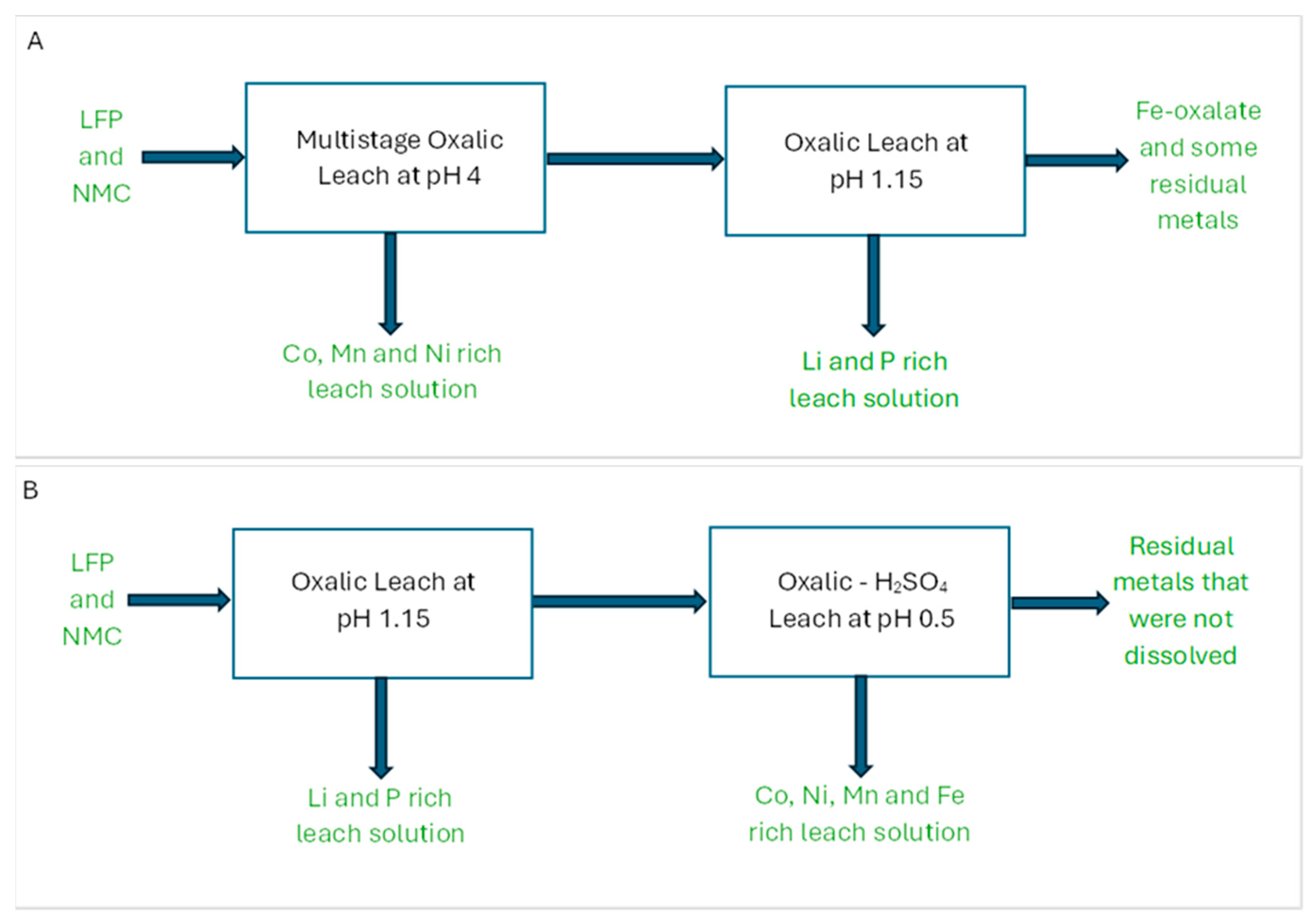
3.2.8. Applications
4. Conclusions
- The proposed process should be applied to industrially sourced black mass, as it has so far only been demonstrated on a simplified system using pristine LFP and NMC materials. Testing with real-world samples is necessary to evaluate practical applicability.
- Further investigation is needed to better understand the observed shift in metal dissolution with changes in pH.
- Additives such as amine-based chelating agents could be explored to improve the dissolution of Co, Ni, and Mn in the oxalic acid-leaching system.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mossali, E.; Picone, N.; Gentilini, L.; Rodriguez, O.; Perez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.D.S.; Tenório, J.A.S.; Junior, A.B.B.; Espinosa, D.C.R. Circular Recycling Strategies for LFP Batteries: A Review Focusing on Hydrometallurgy Sustainable Processing. Metals 2023, 13, 543. [Google Scholar] [CrossRef]
- Islam, M.T.; Huda, N.; Baumber, A.; Hossain, R.; Sahajwalla, V. Waste battery disposal and recycling behavior: A study on the Australian perspective. Environ. Sci. Pollut. Res. Int. 2022, 29, 58980–59001. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, E.; Muryanta, W.A.; Anggraini, A.G.; Sudibyo, S.; Amin, M.; Al Muttaqii, M. Tannic acid as a novel and green leaching reagent for cobalt and lithium recycling from spent lithium-ion batteries. J. Mater. Cycles Waste Manag. 2022, 24, 927–938. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Sharma, P.; Pandey, A.; Jang, M.; Jeon, B.H.; Varjani, S.; Kim, S.H. Recycling of cathode material from spent lithium-ion batteries: Challenges and future perspectives. J. Hazard. Mater. 2022, 429, 128312. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Cho, C.W.; Yun, Y.S. Organic acid-based linear free energy relationship models for green leaching of strategic metals from spent lithium-ion batteries and improvement of leaching performance. J. Hazard. Mater. 2022, 423, 127214. [Google Scholar] [CrossRef] [PubMed]
- Gerold, E.; Schinnerl, C.; Antrekowitsch, H. Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling 2022, 7, 4. [Google Scholar] [CrossRef]
- Meshram, P.; Mishra, A.; Abhilash; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef] [PubMed]
- Refly, S.; Floweri, O.; Mayangsari, T.R.; Sumboja, A.; Santosa, S.P.; Ogi, T.; Iskandar, F. Regeneration of LiNi1/3Co1/3Mn1/3O2 Cathode Active Materials from End-of-Life Lithium-Ion Batteries through Ascorbic Acid Leaching and Oxalic Acid Coprecipitation Processes. ACS Sustain. Chem. Eng. 2020, 8, 16104–16114. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Schmitz, D.; Prasetyo, H.; Birich, A.; Yeetsorn, R.; Friedrich, B. Co-Precipitation of Metal Oxalates from Organic Leach Solution Derived from Spent Lithium-Ion Batteries (LIBs). Metals 2024, 14, 80. [Google Scholar] [CrossRef]
- Roshanfar, M.; Golmohammadzadeh, R.; Rashchi, F. An environmentally friendly method for recovery of lithium and cobalt from spent lithium-ion batteries using gluconic and lactic acids. J. Environ. Chem. Eng. 2019, 7, 102794. [Google Scholar] [CrossRef]
- Esmaeili, M.; Rastegar, S.O.; Beigzadeh, R.; Gu, T. Ultrasound-assisted leaching of spent lithium ion batteries by natural organic acids and H2O2. Chemosphere 2020, 254, 126670. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Recovery of cobalt as cobalt oxalate from spent lithium ion batteries by using glycine as leaching agent. J. Environ. Chem. Eng. 2016, 4, 2378–2383. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, C.; Zhou, T.; Li, H.; Dai, A. Recovery of valuable metals from LiNi0.5Co0.2Mn0.3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leachant. Waste Manag. 2019, 85, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Sun, Y.; Li, S.; Tian, Y.; Yang, Z.; Fan, X.; Zhang, W. A novel process to recycle spent LiFePO4 for synthesizing LiFePO4/C hierarchical microflowers. Electrochim. Acta 2016, 190, 134–140. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Cao, H.; Zhao, C.; Lin, X.; Ning, P.; Zhang, Y.; Jin, W.; Sun, Z. A Closed-Loop Process for Selective Metal Recovery from Spent Lithium Iron Phosphate Batteries through Mechanochemical Activation. ACS Sustain. Chem. Eng. 2017, 5, 9972–9980. [Google Scholar] [CrossRef]
- Wu, D.-Y.; Wang, D.-X.; Liu, Z.-Q.; Rao, S.; Zhang, K.-F. Selective recovery of lithium from spent lithium iron phosphate batteries using oxidation pressure sulfuric acid leaching system. Trans. Nonferrous Met. Soc. China 2022, 32, 2071–2079. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Zhai, L.; Zhang, X.; Curtiss, L.; Jin, Y.; Wu, F.; Chen, R.; Amine, K. A facile recovery process for cathodes from spent lithium iron phosphate batteries by using oxalic acid. CSEE J. Power Energy Syst. 2018, 4, 219–225. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Zhang, L.; Wang, Y.; Zhang, H.; Wang, J.; Yan, S.; Hou, P.; Wang, Q.; Zhang, Y.; et al. Study on efficient and synergistic leaching of valuable metals from spent lithium iron phosphate using the phosphoric acid-oxalic acid system. Sep. Purif. Technol. 2022, 303, 122247. [Google Scholar] [CrossRef]
- Kumar, J.; Shen, X.; Li, B.; Liu, H.; Zhao, J. Selective recovery of Li and FePO4 from spent LiFePO4 cathode scraps by organic acids and the properties of the regenerated LiFePO4. Waste Manag. 2020, 113, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Traversy, M.; Choi, Y.; Ghahreman, A. A novel process for multi-stage continuous selective leaching of lithium from industrial-grade complicated lithium-ion battery waste. Sci. Total Environ. 2024, 909, 168533. [Google Scholar] [CrossRef] [PubMed]
- Rouquette, L.M.J.; Petranikova, M.; Vieceli, N. Complete and selective recovery of lithium from EV lithium-ion batteries: Modeling and optimization using oxalic acid as a leaching agent. Sep. Purif. Technol. 2023, 320, 124143. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Y.; Xu, S.; Fan, E.; Xue, Q.; Guan, Y.; Wu, F.; Li, L.; Chen, R. Innovative Application of Acid Leaching to Regenerate Li(NiCoMn)O2 Cathodes from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 5959–5968. [Google Scholar]
- Li, Q.; Fung, K.Y.; Xu, L.; Wibowo, C.; Ng, K.M. Process Synthesis: Selective Recovery of Lithium from Lithium-Ion Battery Cathode Materials. Ind. Eng. Chem. Res. 2019, 58, 3118–3130. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chernyaev, A.; Ossama, M.; Seisko, S.; Lundstrom, M. Leaching of NMC industrial black mass in the presence of LFP. Sci. Rep. 2024, 14, 10818. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Dai, Y.; Hua, D.; Gu, H.; Wang, N. Creative Method for Efficiently Leaching Ni, Co, Mn, and Li in a Mixture of LiFePO4 and LiMO2 Using Only Fe(III). ACS Sustain. Chem. Eng. 2021, 9, 3979–3984. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, J.; Kim, S.K.; Yun, Y.S. Simple, green organic acid-based hydrometallurgy for waste-to-energy storage devices: Recovery of NiMnCoC2O4 as an electrode material for pseudocapacitor from spent LiNiMnCoO2 batteries. J. Hazard. Mater. 2022, 424, 127481. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Manjanna, J.; Pai, K.V.; Vadavi, R.; Keny, S.J.; Tripathi, V.S. Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Vieceli, N.; Benjamasutin, P.; Promphan, R.; Hellström, P.; Paulsson, M.; Petranikova, M. Recycling of Lithium-Ion Batteries: Effect of Hydrogen Peroxide and a Dosing Method on the Leaching of LCO, NMC Oxides, and Industrial Black Mass. ACS Sustain. Chem. Eng. 2023, 11, 9662–9673. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, Y.; Shi, H.; Shao, P.; Yuan, X.; Hu, X.; Zhang, Q.; Zhang, H.; Luo, D.; Wang, C.; et al. Selective recycling of lithium from spent LiNixCoyMn1−x−yO2 cathode via constructing a synergistic leaching environment. J. Environ. Manag. 2024, 352, 120021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, X.; He, T.; Yuan, X.; Li, X.; Shi, H.; Yang, L.; Shao, P.; Wang, C.; Luo, X. Rapid extraction of valuable metals from spent LiNixCoyMn1-x-yO2 cathodes based on synergistic effects between organic acids. Waste Manag. 2023, 165, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gerold, E.; Kadisch, F.; Lerchbammer, R.; Antrekowitsch, H. Bio-metallurgical recovery of lithium, cobalt, and nickel from spent NMC lithium ion batteries: A comparative analysis of organic acid systems. J. Hazard. Mater. Adv. 2024, 13, 100397. [Google Scholar] [CrossRef]
- Duarte, L.M.; Xavier, L.V.; Rossati, K.F.; Oliveira, V.A.D.; Schimicoscki, R.S.; Ávila Neto, C.N.D.; Mendes, G.D.O. Potassium extraction from the silicate rock Verdete using organic acids. Sci. Agric. 2022, 79, e20200164. [Google Scholar] [CrossRef]
- Saleem, U.; Buvik, V.; Knuutila, H.K.; Bandyopadhyay, S. Recovery of lithium from oxalic acid leachate produced from black mass of spent electric vehicle Li-ion batteries. Chem. Eng. J. Adv. 2024, 20, 100648. [Google Scholar] [CrossRef]
- Georgeta, G.; Marcela, S.; Delia, S.O.; Gratiela, P.A.I. Recovering Of Manganese Cations From Industrial Wastewater Using Oxalate Precipitation. Ann. Univ. Oradea Fascicle Environ. Prot. 2017, 28, 253–260. [Google Scholar]
- Mettke, L.N.; Yagmurlu, B. Optimizing Early-Stage Lithium Recovery: Investigating Oxalic Acid Leaching of Black Mass from End-of-Life NMC 622 Batteries; Lazou, A., Meskers, C., Olivetti, E., Diaz, F., Gökelma, M., Eds.; REWAS; Springer: Cham, Switzerland, 2025. [Google Scholar]
- Liu, Y.; Zhang, C.; Li, B.; Li, H.; Zhan, H. 2015. Extraction and Determination of Total and Soluble Oxalate in Pulping and Papermaking Raw Materials. BioResources 2025, 10, 4580–4587. [Google Scholar]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Zhang, X.; Bian, Y.; Xue, Q.; Wu, J.; Wu, F.; Chen, R. Selective Recovery of Li and Fe from Spent Lithium-Ion Batteries by an Environmentally Friendly Mechanochemical Approach. ACS Sustain. Chem. Eng. 2018, 6, 11029–11035. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhao, J.; Xia, J.; Wang, L.; Lai, W.Y.; Pang, H.; Huang, W. Room temperature synthesis of cobalt-manganese-nickel oxalates micropolyhedrons for high-performance flexible electrochemical energy storage device. Sci. Rep. 2015, 5, 8536. [Google Scholar] [CrossRef] [PubMed]



| Battery Chemistry | Conditions | Extraction Efficiencies | Feed Materials | Reference |
|---|---|---|---|---|
| NMC | 0.6 M Oxalic acid S:L ratio of 50 g/L 60 °C for 60 min | 100% Al 98.8% Li <0.5% of Ni and Co 1.5% Mn | Dismantled electric vehicle LiBs (NMC 111)—they are crushed, sieved, and undergo magnetic separation to produce a NMC-, graphite-, Cu- and Al-containing black mass | [23] |
| NMC | 0.6 M Oxalic acid S:L ratio of 20 g/L 30 min at 70 °C | >98.5% Co, Mn and Ni | Manually dismantled 18,650 NMC cells. Dimethyl carbonate is used to remove electrolyte and N-methyl-2-pyrrolidone to separate cathodic material from Al foil. The cathode material is calcined at 700 °C | [24] |
| NMC | 1 M Oxalic acid S:L ratio of 10 g/L 95 °C for 12 h | NMC 111—100% Li, 0.26% Ni, 1.12% Co and 22.8% Mn NMC 532—95.4% Li, 0.12% Ni, 0.52% Co and 24% Mn NMC 811—89.6% Li, 0.13% Ni, 0.17% Co and 20.6% Mn. | Pristine cathode samples | [25] |
| LCMO | 93.6% Li, 0% Ni, 1.8% Co and 30% Mn | |||
| LMO | 100% Li, 0% Ni, 0% Co and 26.2% Mn | |||
| LCO | 1M Oxalic acid S:L ratio of 50 g/L 80 °C for 120 min | >98% of LiCoO2 Li in solution Co-oxalate in residue | Spent LiBs from phones—they are dismantled, and the cathodes undergo vacuum pyrolysis. Cathode material is separated from Al foil | [26] |
| LCO | 1M Oxalic acid Stirring rate 400 rpm S:L ratio of 15 g/L 95 °C for 150 min. | 98% Li 97% Co (insoluble oxalate) | Consumer electronics—crushed and sieved (−1.43 mm) | [27] |
| LFP | 0.3 M Oxalic acid S:L ratio of 60 g/L 80 °C for 60 min | 98% Li 92% Fe (precipitated as FeC2O4·2H2O) | Spent 123-18650 (LFP) batteries from electronics. The anode and cathode are manually separated, and then the cathodic material is pretreated using NMP, followed by calcination and a final grinding step | [19] |
| LFP | 0.65 M H3PO4 0.33 M Oxalic acid S:L ratio of 40 g/L 70 °C for 51 min. | 98.24% Fe 97.72% Li | Cathodic material from spent LFP batteries | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, M.S.; Beh, C.C.; Oraby, E.; Eksteen, J. Organic Acid Leaching of Black Mass with an LFP and NMC Mixed Chemistry. Recycling 2025, 10, 145. https://doi.org/10.3390/recycling10040145
Henderson MS, Beh CC, Oraby E, Eksteen J. Organic Acid Leaching of Black Mass with an LFP and NMC Mixed Chemistry. Recycling. 2025; 10(4):145. https://doi.org/10.3390/recycling10040145
Chicago/Turabian StyleHenderson, Marc Simon, Chau Chun Beh, Elsayed Oraby, and Jacques Eksteen. 2025. "Organic Acid Leaching of Black Mass with an LFP and NMC Mixed Chemistry" Recycling 10, no. 4: 145. https://doi.org/10.3390/recycling10040145
APA StyleHenderson, M. S., Beh, C. C., Oraby, E., & Eksteen, J. (2025). Organic Acid Leaching of Black Mass with an LFP and NMC Mixed Chemistry. Recycling, 10(4), 145. https://doi.org/10.3390/recycling10040145