Sustainable Alkali-Activated and Geopolymer Materials: What Is the Future for Italy?
Abstract
1. Introduction
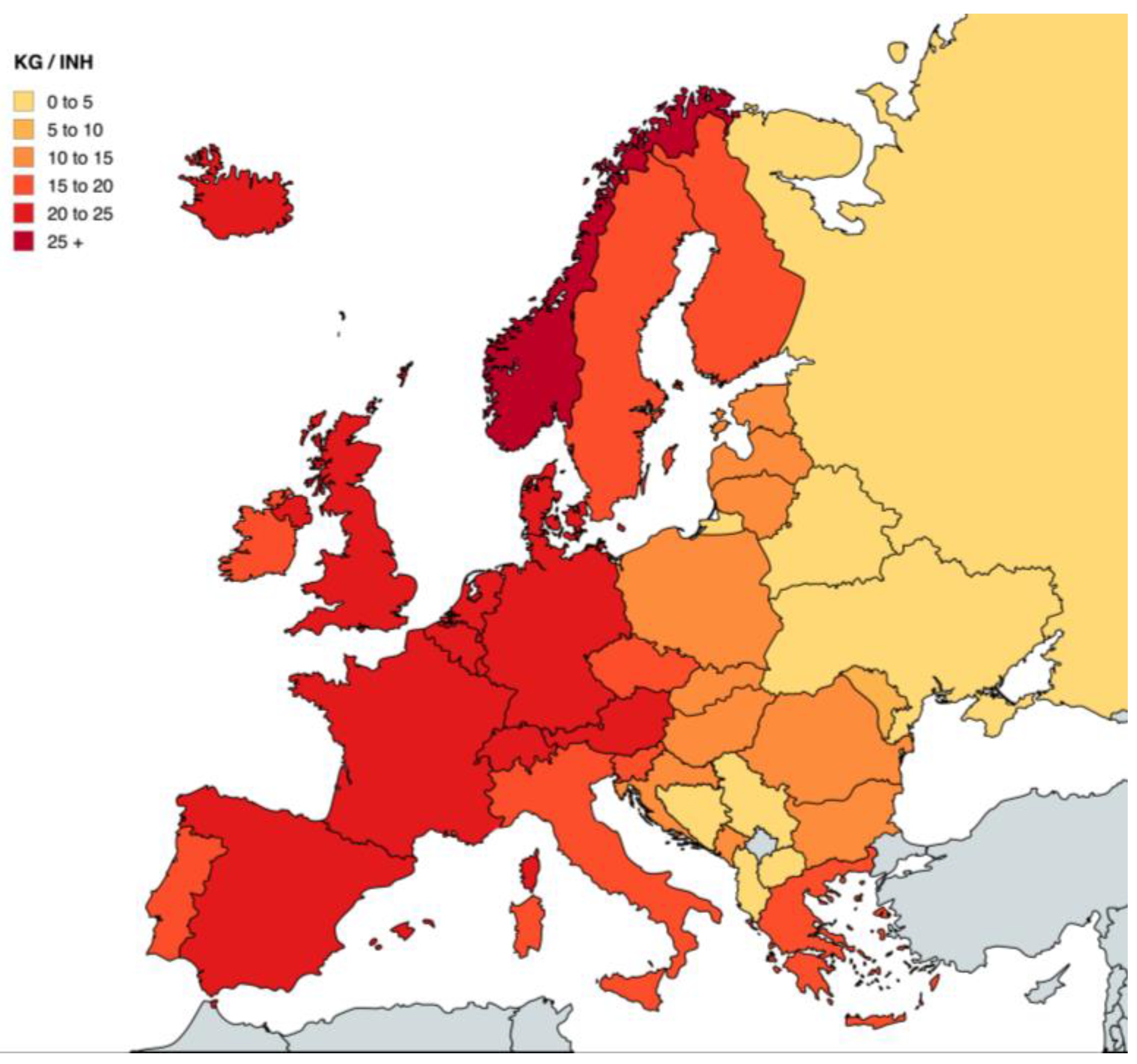
1.1. Global Context
1.2. Policy Drivers in Europe and Italy
1.3. Alkali-Activated Materials (AAMs) and Geopolymers (GPs) as a Technical Solution
1.4. Durability, Environmental Assessment, and Current Research Gaps
1.5. Aims and Structure of This Review
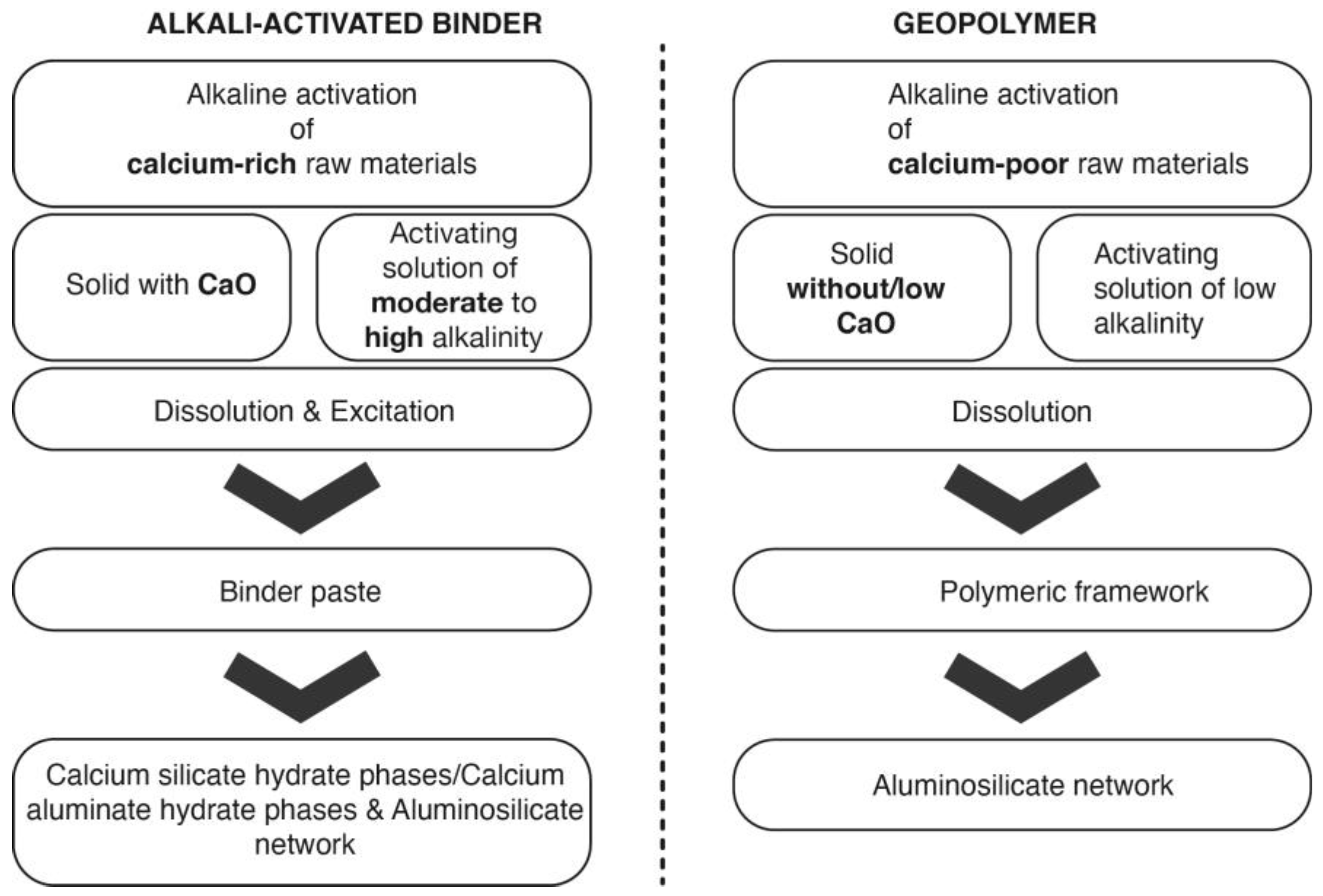
2. Chemistry of the Alkaline Activation of Aluminosilicate Materials
3. Performance Comparison of Geopolymers (GPs) and Alkaline-Activated Materials (AAMs) Versus Ordinary Portland Cement (OPC)
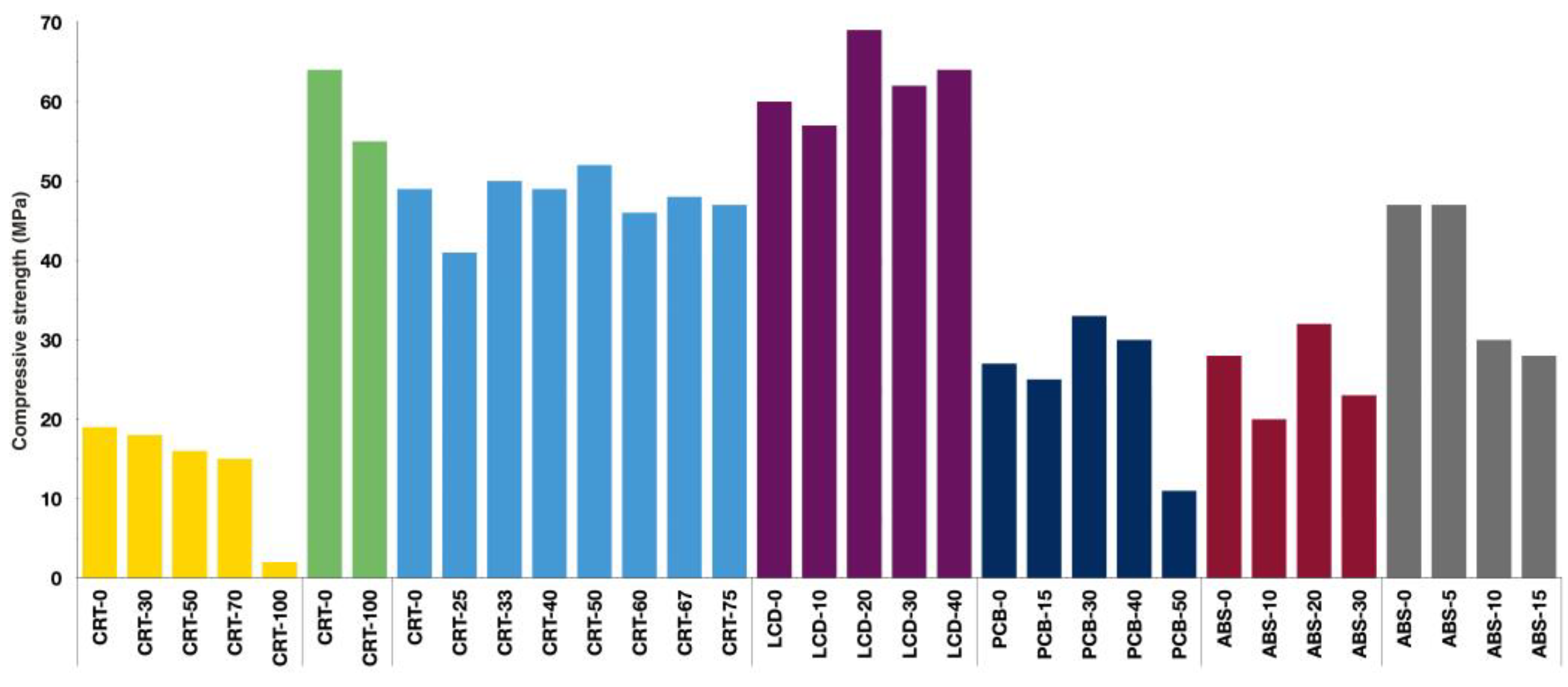
3.1. Mechanical Properties
3.2. Cost and Energy Demand
3.3. Environmental Impact
4. Main Aluminosilicate Sources from Italian Waste
4.1. Construction and Demolition Waste
| Waste | Addition (Wt/Wt) | Activator | Curing | Compressive Strength Max (MPa) | Sample | Ref. | |
|---|---|---|---|---|---|---|---|
| °C | Days | ||||||
| Concrete | 20% MK | NaOH + Na2SiO3 | 60 | 3 | 33 | Paste | [58] |
| Masonry | — | NaOH + Na2SiO3 | 60 | 7 | 50 | Paste Mortar | [59] |
| Concrete | — | NaOH + Na2SiO3 | 90 | 7 | 13 | Paste | [60] |
| Masonry | 58 | ||||||
| Tile | 50 | ||||||
| Masonry | 0% | NaOH + Na2SiO3 | 25 | — | 54 | Paste | [61] |
| 20% OPC | 103 | ||||||
| Concrete | 0% | NaOH + Na2SiO3 | 25 | — | 26 | Paste | [62] |
| 30% OPC | 34 | ||||||
| 10% MK | 46 | ||||||
| Concrete | — | NaOH + Na2SiO3 | 80 | 1 | 8 | Paste | [63] |
| Masonry | 39 | ||||||
| Tile | 58 | ||||||
| Masonry | — | NaOH + Na2SiO3 | 50 | 1 | — | Coating | [64] |
| Ceramic | 15% OPC | NaOH + Na2SiO3 | 25 | — | 58 | Paste | [65] |
| 25 | Mortar | ||||||
| Ceramic | 5% Ca(OH)2 | NaOH + Na2SiO3 | 65 | 3 | 43 | Mortar | [66] |
| Masonry | 0% | NaOH | 25 | — | 7 | Paste | [67] |
| 10% OPC | 41 | ||||||
| 0% | NaOH + Na2SiO3 | 54 | |||||
| 20% OPC | 103 | ||||||
| Ceramic | 5% Ca(OH)2 | NaOH + Na2SiO3 | 65 | 3 | 43 | Mortar | [68] |
| Masonry | 0% | NaOH | 25 | — | 7 | Paste | [69] |
| 30% OPC | 41 | ||||||
| 0% | NaOH + Na2SiO3 | 54 | |||||
| 30% OPC | 103 | ||||||
| Concrete | 0% | NaOH | 7 | ||||
| 30% OPC | 10 | ||||||
| 0% | NaOH + Na2SiO3 | 26 | |||||
| 30% OPC | 34 | ||||||
| Masonry | — | NaOH + Na2SiO3 | 90 | 5 | 36 | Mortar | [70] |
| Masonry | — | NaOH + Na2SiO3 | 25 | — | 42 | Paste | [67] |
| Masonry | 30% GBFS + 10% FA | NaOH + Na2SiO3 | 25 | — | 70 | Paste | [70] |
| Ceramic | 30% GBFS + 10% FA | NaOH + Na2SiO3 | 25 | — | 60 | ||
| Concrete | 10% OPC | NaOH + Na2SiO3 | 25 | 42.6 | Concrete | [57] | |
4.2. Mining Waste
- -
- The widespread presence of unattended mining waste;
- -
- Abandoned structures and processing plants that may represent dangerous areas due to their potential collapse;
- -
- Several underground voids can manifest on the surface, such as problems with sinkholes or groundwater imbalance, resulting in sudden water spills at the surface (including water spills outside the abandoned tunnels).
- -
- About ITL 35 million was due to sulfur from Sicily;
- -
- ITL 12 million was due to lead and zinc ores from Sardinia and Tuscany;
- -
- ITL 10 million was due to Apuan marbles;
- -
- ITL 2 million was due to boric acid from the Larderello dandelions.
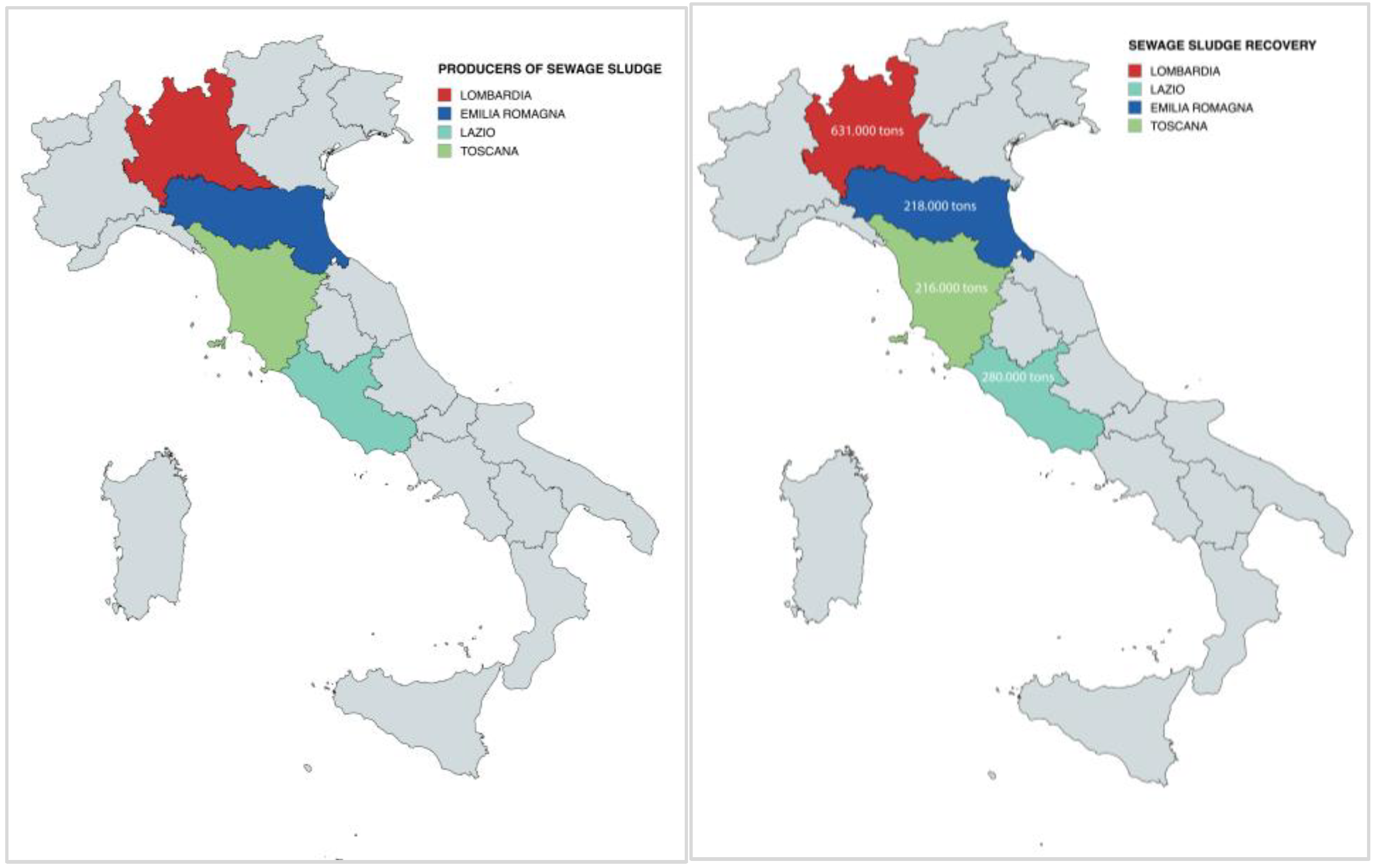
4.3. Sewage Sludge
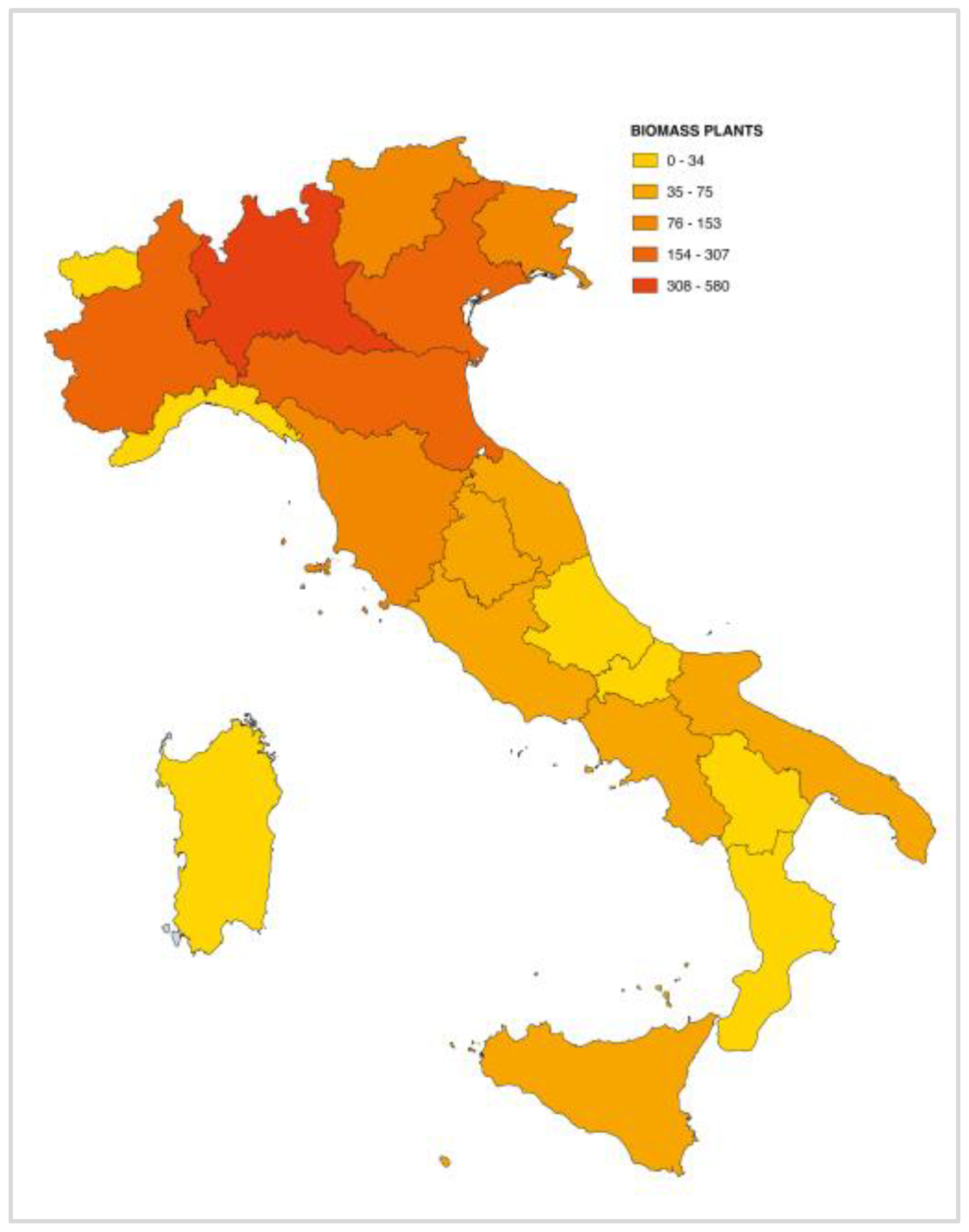
4.4. Biomass Ash
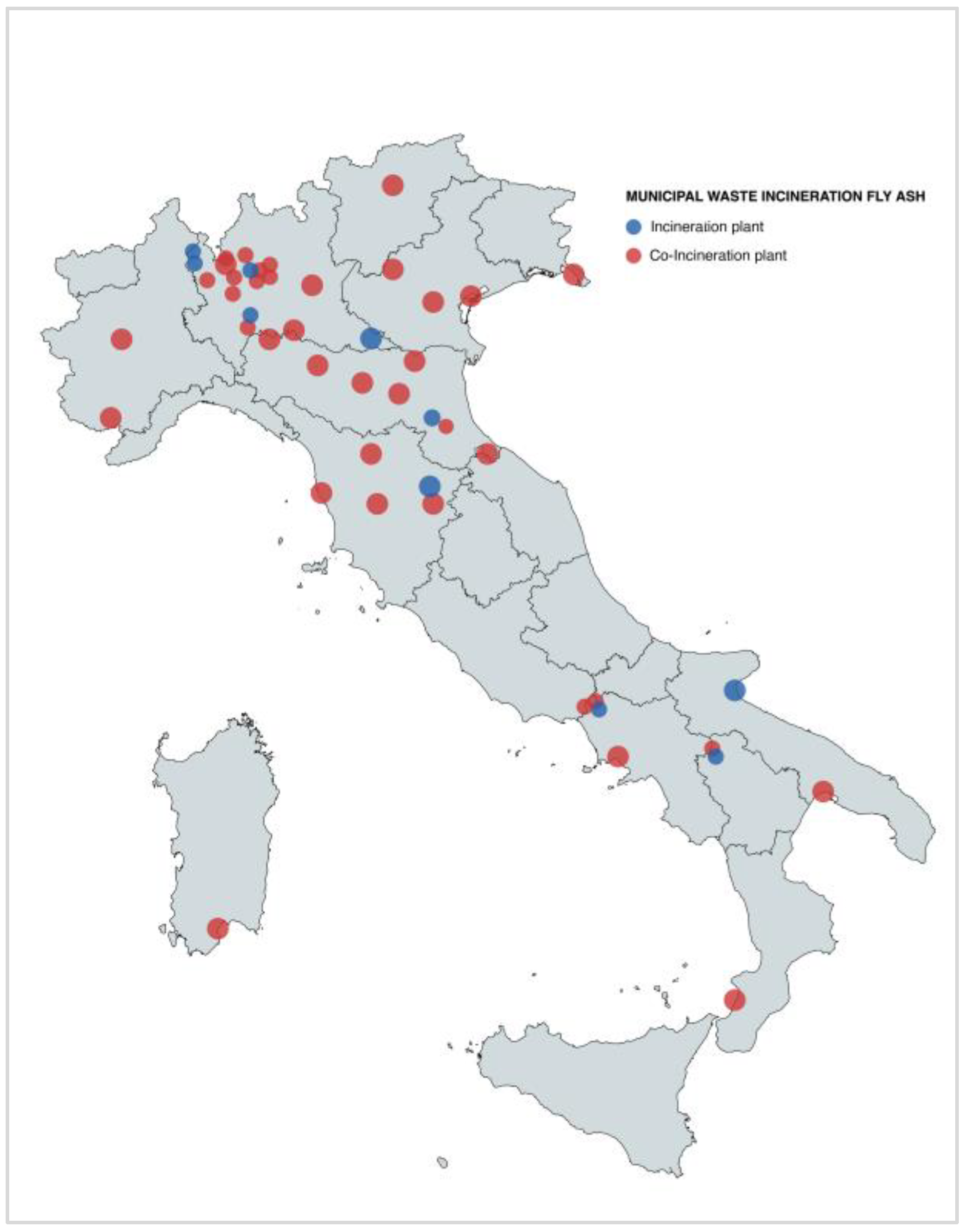
4.5. Municipal Waste Incineration Fly Ash
4.6. Non-Metallic Fractions of Electronic Waste
- To fully evaluate their potential, the properties of e-waste-based GPs should be investigated under various circumstances, including varying e-waste fractions, curing regimes, precursors, aggregates (size and gradation), fibers, etc. Addressing divergent viewpoints over the effectiveness of these items will also result from this.
- To identify their suitable uses in the building sector, the GPs transformed by e-waste should be grouped according to their performance.
- Another step that might raise manufacturing costs and embodied energy is reprocessing e-waste components to the size of the available geopolymer reagents. To determine if GPs truly provide sustainable e-waste disposal, a thorough lifecycle evaluation and lifecycle cost analysis of e-waste-incorporated GPs should be carried out.
- Except for possible economic, social, regulatory, and policymaking implications, this study briefly focuses on the technological viability of the environmental advantages of GPs derived from e-waste. In order to reveal the potential and hidden constraints of this disposal method, future research should concentrate on evaluating these factors.
4.7. Waste-Derived Activators for GPs and AAMs
5. A Look Beyond Italy: European and UK Perspectives on Regulatory Frameworks
6. Conclusions and Future Remarks
- -
- The collection, separation, and characterization of the aluminosilicate waste most suitable for alkaline activation is a key step to support the development of geopolymers. In this sense, the creation of a structured database for the classification of different wastes could offer an important operational and cognitive tool.
- -
- Deepening the mechanisms of geopolymerization is essential to build a solid theoretical basis to guide the choice of precursors and the optimization of the Si/Al ratio, both in the case of single waste and in mixed streams.
- -
- Understanding the relationship between waste characteristics and the final properties of geopolymers makes it possible to modulate material performance by acting on composition, reactivity, and critical process parameters (such as liquid/solid ratios, mixing modes, and curing conditions).
- -
- Studying chemo-rheological behavior and reaction kinetics as a function of curing time and temperature is crucial to refine formulations and make production scalable, including 3D printing.
- -
- The development of synthesis methods for alternative alkaline activators, obtained under mild conditions, could significantly reduce both costs and environmental impacts.
- -
- Comprehensive LCA studies capable of realistically assessing the ecological footprint of alkaline-activated materials produced from different waste combinations are needed.
- -
- Identifying new application frontiers, beyond conventional use as an alternative to Portland cement, represents a strategic opportunity to expand the impact of geopolymers and alkaline-activated materials.
- -
- Areas such as water treatment, environmental catalysis, or regenerative medicine offer concrete scenarios where their unique properties can be exploited, accelerating their adoption on an industrial scale and enhancing their relevance in high-tech contexts.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- PCA How Cement Is Made. 2019. Available online: https://www.cement.org/cement-concrete/how-cement-is-made (accessed on 26 April 2023).
- Hendriks, C.A.; Worrell, E.; De Jager, D.; Blok KRiemer, P. Emission reduction of greenhouse gases from the cement industry. In Proceedings of the Fourth International Conference on Greenhouse Gas Control Technologies, Interlaken, Switzerland, 30 August–2 September 1998; IEA GHG R&D Programme: Interlaken, Austria, 1998. [Google Scholar]
- Neupane, K. Evaluation of environmental sustainability of one-part geopolymer binder concrete. Clean. Mater. 2022, 6, 100138. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/clima/eu-action/european-green-deal/delivering-european-green-deal_en (accessed on 15 June 2022).
- Provis, J.L.; Duxson, P.; van Deventer, J.S.J. The role of particle technology in developing sustainable construction materials. Adv. Powder Technol. 2010, 21, 2–7. [Google Scholar] [CrossRef]
- Glukhovsky, V.D. Ancient, Modern and Future Concretes. In Proceedings of the First International Conference on Alkaline Cements and Concretes, Kiev, Ukraine, 17–19 May 1994; pp. 1–8. [Google Scholar]
- Barsoum, M.W.; Ganguly, A.; Hug, G. Microstructural Evidence of Reconstituted Limestone Blocks in the Great Pyramids of Egypt. J. Am. Ceram. Soc. 2006, 89, 3788–3796. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer’99. In Proceedings of the 2nd International Conference, Saint-Quentin, France, 30 June–2 July 1999; pp. 9–39. [Google Scholar]
- Davidovits, J. Mineral Polymers and Methods of Making Them. US Patent 4,349,386, 14 September 1982. [Google Scholar]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- ISO 14040/44; Environmental ManagementLife Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- Ricciotti, L.; Occhicone, A.; Ferone, C.; Cioffi, R.; Roviello, G. Eco-design of geopolymer-based materials recycling porcelain stoneware wastes: A life cycle assessment study. Environ. Dev. Sustain. 2024, 26, 4055–4074. [Google Scholar] [CrossRef]
- Jihui, Z. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar]
- Bakhtyar, B.; Kacemi, T.; Nawaz, M.A. A Review on Carbon Emissions in Malaysian Cement Industry. Int. J. Energy Econ. Policy 2017, 7, 282–286. [Google Scholar]
- Ricciotti, L.; Occhicone, A.; Ferone, C.; Cioffi, R.; Tarallo, O.; Roviello, G. Development of Geopolymer-Based Materials withCeramic Waste for Artistic and Restoration Applications. Materials 2022, 15, 8600. [Google Scholar] [CrossRef]
- Ricciotti, L.; Occhicone, A.; Manzi, S.; Saccani, A.; Ferone, C.; Tarallo, O.; Roviello, G. Sustainable materials based on geopolymer polyvinyl acetate composites for Art&Design applications. Polymers 2022, 14, 5461. [Google Scholar]
- Roviello, G.; Chianese, E.; Ferone, C.; Ricciotti, L.; Roviello, V.; Cioffi, R.; Tarallo, O. Hybrid geopolymeric foams for the removal of metallic ions from aqueous waste solutions. Materials 2019, 12, 4091. [Google Scholar] [CrossRef]
- ISPRA. Institute for Environmental Protection and Research Report; ISPRA: Rome, Italy, 2023; p. 393. ISBN 978-88-448-1200-3. [Google Scholar]
- Thapa, V.B.; Waldmann, D. A short review on alkali-activated binders and geopolymer binders. In Vielfalt Im Massivbau—Festschrift Zum 65. Geburtstag Von Prof. Dr. Ing. Jürgen Schnell; Ernst & Sohn: Berlin, Germany, 2018; pp. 576–591. Available online: http://hdl.handle.net/10993/35284 (accessed on 30 April 2025).
- Aversa, R.; Ricciotti, L.; Perrotta, V.; Apicella, A. Thermokinetic and Chemorheology of the Geopolymerization of an Alumina-Rich Alkaline-Activated Metakaolin in Isothermal and Dynamic Thermal Scans. Polymers 2024, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, L.; Apicella, A.; Perrotta, V.; Aversa, R. Geopolymer Materials for Bone Tissue Applications: Recent Advances and Future Perspectives. Polymers 2023, 15, 1087. [Google Scholar] [CrossRef]
- Haq, M.U.; Ricciotti, L. Design and development of geopolymer composite bricks for eco-friendly construction. J. Mater. Sci. 2025, 60, 737–758. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Bernal, S.A.; Van Deventer, J.S.J.; Provis, J.L. Structural evolution of synthetic alkaliactivated CaO-MgO-Na2O-Al2O3-SiO2 materials is influenced by Mg content. Cem. Concr. Res. 2017, 99, 155–171. [Google Scholar] [CrossRef]
- Gómez-Casero, M.A.; Pérez-Villarejo, L.; Castro, E.; Eliche-Quesada, D. Effect of steel slag and curing temperature on the improvement in technological properties of biomass bottom ash based alkali-activated materials. Constr. Build. Mater. 2021, 302, 124205. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S. Effects of curing environment on strength and microstructure of alkali-activated fly ash-slag binder. Constr. Build. Mater. 2020, 235, 117481. [Google Scholar] [CrossRef]
- Jittin, V.; Madhuri, P.; Santhanam, M.; Bahurudeen, A. Influence of preconditioning and curing methods on the durability performance of alkali-activated binder composites. Constr. Build. Mater. 2021, 311, 125346. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different additives on the properties of alkali-activated slag—A guide for Civil Engineer. Constr. Build. Mater. 2013, 47, 29–55. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Wang, Y.S.; Alrefaei, Y.; Dai, J.G. Silico-aluminophosphate and alkali-aluminosilicate geopolymers: A comparative review. Front. Mater. 2019, 6, 106. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Cioffi, R.; Tarallo, O. Synthesis and Characterization of Novel Epoxy Geopolymer Hybrid Composites. Materials 2013, 6, 3943–3962. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Tarallo, O. Fire resistant melamine based organic-geopolymer hybrid composites. Cem. Concr. Compos. 2015, 59, 89–99. [Google Scholar] [CrossRef]
- Ricciotti, L.; Occhicone, A.; Petrillo, A.; Ferone, C.; Cioffi, R.; Roviello, G. Geopolymer-based hybrid foams: Lightweight materials from a sustainable production process. J. Clean. Prod. 2020, 250, 119588. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Asprone, D.; Di Maggio, R.; Cappelletto, E.; Prota, A.; et al. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Molino, A.J.; Menna, C.; Ferone, C.; Cioffi, R.; Tarallo, O. Hybrid Geopolymers from Fly Ash andPolysiloxanes. Molecules 2019, 24, 3510. [Google Scholar] [CrossRef]
- Ricciotti, L.; Molino, A.J.; Roviello, V.; Chianese, E.; Cennamo, P.; Roviello, G. Geopolymer Composites for Potential Applications in Cultural Heritage. Environments 2017, 4, 91. [Google Scholar] [CrossRef]
- Yan, L.; Kasal, B.; Huang, L. A review of recent research on the use of cellulosic fibers, their fiber fabric reinforced cementitious, geo-polymer and polymer composites in civil engineering. Compos. Part B 2016, 92, 94–132. [Google Scholar] [CrossRef]
- Shaikh, F. Review of mechanical properties of short fiber reinforced geopolymer composites. Constr. Build. Mater. 2013, 43, 37–49. [Google Scholar] [CrossRef]
- Sakulich, A. Reinforced geopolymer composites for enhanced material greenness and durability. Sustain. Cities Soc. 2011, 1, 195–210. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.J.; Luo, W.J. A novel waterproof, fast setting and high early strength repair material derived from metakaolin geopolymer. Constr. Build. Mater. 2016, 124, 69–73. [Google Scholar] [CrossRef]
- Salazar, R.A.R.; Jesús, C.; de Gutiérrez, R.M.; Pacheco-Torgal, F. Alkali-activated binary mortar based on natural volcanic pozzolan for repair applications. J. Build. Eng. 2019, 25, 100785. [Google Scholar] [CrossRef]
- Ahmad Zailani, W.W.; Bouaissi, A.; Abdullah, M.M.A.B.; Abd Razak, R.; Yoriya, S.; Mohd Salleh, M.A.A.; Mohd Remy Rozainy, M.A.Z.; Fansuri, H. Bonding strength characteristics of FA-based geopolymer paste as a repair material when applied on OPC substrate. Appl. Sci. 2020, 10, 3321. [Google Scholar] [CrossRef]
- Palomoa, A.; Grutzeck, M.W.; Blancoa, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Panda, B.; Paul, S.C.; Hui, L.J.; Tay, Y.W.D.; Tan, M.J. Additive manufacturing of geopolymer for sustainable built environment. J. Clean. Prod. 2017, 167, 281–288. [Google Scholar] [CrossRef]
- Panda, B.; Paul, S.C.; Mohamed, N.A.N.; Tay, Y.W.D.; Tan, M.J. Measurement of tensile bond strength of 3D printed geopolymer mortar. Measurement 2018, 113, 108–116. [Google Scholar] [CrossRef]
- Panda, B.; Ruan, S.; Unluer, C.; Tan, M.J. Investigation of the properties of alkali-activated slag mixes involving the use of nanoclay and nucleation seeds for 3D printing. Compos. Part B Eng. 2020, 186, 107826. [Google Scholar] [CrossRef]
- Sellami, M.; Barre, M.; Toumi, M. Synthesis, thermal properties and electrical conductivity of phosphoric acid-based geopolymer with metakaolin. Appl. Clay Sci. 2019, 180, 105192. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Wongsa, A.; Srinavin, K.; Chindaprasirt, P. Recycled aggregate high calcium fly ash geopolymer concrete with inclusion of OPC and nano-SiO2. Constr. Build. Mater. 2018, 174, 244–252. [Google Scholar] [CrossRef]
- Shi, W.; Ren, H.; Li, M.; Shu, K.; Xu, Y.; Yan, C.; Tang, Y. Tetracycline removal from aqueous solution by visible-light-driven photocatalytic degradation with low cost red mud wastes. Chem. Eng. J. 2020, 382, 122876. [Google Scholar] [CrossRef]
- Zhang, Y.J.; He, P.Y.; Yang, M.Y.; Kang, L. A new graphene bottom ash geopolymeric composite for photocatalytic H-2 production and degradation of dyeing wastewater. Int. J. Hydrogen Energy 2017, 42, 20589–20598. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Zhang, Y.; Chen, H. A novel electroconductive graphene/fly ash-based geopolymer composite and its photocatalytic performance. Chem. Eng. J. 2018, 334, 2459–2466. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, L.; Ma, G.; Zhao, X.; Zhao, X. Preparation and properties of bio-geopolymer composites with waste cotton stalk materials. J. Clean. Prod. 2020, 245, 118842. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Chen, G.; Wang, L. Experimental study of the feasibility of using anhydrous sodium metasilicate as a geopolymer activator for soil stabilization. Eng. Geol. 2020, 264, 105316. [Google Scholar] [CrossRef]
- Yu, Z.; Song, W.; Li, J.; Li, Q. Improved simultaneous adsorption of Cu (II) and Cr (VI) of organic modified metakaolin-based geopolymer. Arab. J. Chem. 2020, 13, 4811–4823. [Google Scholar] [CrossRef]
- Ellen MacArthur Foundation. Accelerating the Circular Economy through Commercial Deconstruction and Reuse; Ellen MacArthur Foundation: Cowes, UK, 2019; Available online: https://www.ellenmacarthurfoundation.org/topics/built-environment/overview (accessed on 30 April 2025).
- Tam, V.W.Y.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Vieira, C.S.; Pereira, P.M. Use of recycled construction and demolition materials in geotechnical applications: A review. Resour. Conserv. Recycl. 2015, 103, 192–204. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Valencia-Saavedra, W.; Mejía de Gutiérrez, R. Construction and Demolition Waste (CDW) Recycling—As Both Binder and Aggregates—In Alkali-Activated Materials: A Novel Re-Use Concept. Sustainability 2020, 12, 5775. [Google Scholar] [CrossRef]
- Lampris, C.; Lupo, R.; Cheeseman, C. Geopolymerisation of silt generated from construction and demolition waste washing plants. Waste Manag. 2009, 29, 368–373. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.; Borrachero, M.; Monzó, J.; Cheeseman, C.; Payá, J. Properties and microstructure of alkali-activated red clay brick waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef]
- Robayo, R.A.; Mulford, A.; Munera, J.; De Gutiérrez, R.M. Alternative cements based on alkali-activated red clay brick waste. Constr. Build. Mater. 2016, 128, 163–169. [Google Scholar] [CrossRef]
- Vásquez, A.; Cárdenas, V.; Robayo, R.A.; De Gutiérrez, R.M. Geopolymer based on concrete demolition waste. Adv. Powder Technol. 2016, 27, 1173–1179. [Google Scholar] [CrossRef]
- Zaharaki, D.; Galetakis, M.; Komnitsas, K. Valorization of construction and demolition (C&D) and industrial wastes through alkali activation. Constr. Build. Mater. 2016, 121, 686–693. [Google Scholar]
- Sassoni, E.; Pahlavan, P.; Franzoni, E.; Bignozzi, M.C. Valorization of brick waste by alkali-activation: A study on the possible use for masonry repointing. Ceram. Int. 2016, 42, 14685–14694. [Google Scholar] [CrossRef]
- Murillo, L.M.; Delvasto, S.; Suárez, M.G. A study of a hybrid binder based on alkali-activated ceramic tile wastes and portland cement. In Sustainable and Nonconventional Construction Materials Using Inorganic Bonded Fiber Composites; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 291–311. [Google Scholar]
- Reig, L.; Sanz, M.; Borrachero, M.; Monzó, J.; Soriano, L.; Payá, J. Compressive strength and microstructure of alkali-activated mortars with high ceramic waste content. Ceram. Int. 2017, 43, 13622–13634. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Rivera, J.F.; De Gutiérrez, R.M. Alkali-activated building materials made with recycled construction and demolition wastes. Constr. Build. Mater. 2017, 149, 130–138. [Google Scholar] [CrossRef]
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of alkali activator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. Part B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Fořt, J.; Vejmelková, E.; Koňáková, D.; Alblová, N.; Čáchová, M.; Keppert, M.; Rovnaníková, P.; Cerny, R. Application of waste brick powder in alkali activated aluminosilicates: Functional and environmental aspects. J. Clean. Prod. 2018, 194, 714–725. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Yehualaw, M.D.; Vo, D.-H.; Huynh, T.-P. Development of high-strength alkali-activated pastes containing high volumes of waste brick and ceramic powders. Constr. Build. Mater. 2019, 218, 519–529. [Google Scholar] [CrossRef]
- Mohanty, M.; Dhal, N.; Patra, P.; Das, B.; Reddy, P. Phytoremediation: A Novel Approach for Utilization of Iron-Ore Wastes, Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- BRGM. Management of Mining, Quarrying and Ore-Processing Waste in the European Union; Report No. BRGM/RP-50319-FR; European Commission: Brussels, Belgium, 2001; p. 79. Available online: https://ec.europa.eu/environment/pdf/waste/studies/mining/0204finalreportbrgm.pdf (accessed on 30 April 2025).
- Lu, Z.; Cai, M. Disposal methods on solid wastes from mines in transition from open-pit to underground mining. Procedia Environ. Sci. 2012, 16, 715–721. [Google Scholar] [CrossRef]
- Franks, D.M.; Boger, D.V.; Cote, C.M.; Mulligan, D.R. Sustainable development principles for the disposal of mining and mineral processing wastes. Resour. Policy 2011, 36, 114–122. [Google Scholar] [CrossRef]
- Singh, S.; Sukla, L.B.; Goyal, S.K. Mine waste & circular economy. Mater. Today Proc. 2020, 30, 332–339. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Production of eco-friendly bricks from copper mine tailings through geopolymerization. Constr. Build. Mater. 2012, 29, 323–331. [Google Scholar] [CrossRef]
- Zhang, L.; Ahmari, S.; Zhang, J. Synthesis and characterization of fly ash modified mine tailings-based geopolymers. Constr. Build. Mater. 2011, 25, 3773–3781. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, L.; Ramey, D.; Waterman, B.; Ormsby, S. Utilization of aluminum sludge (AS) to enhance mine tailings-based geopolymer. J. Mater. Sci. 2015, 50, 1370–1381. [Google Scholar] [CrossRef]
- Caballero, E.; Sánchez, W.; Ríos, C.A. Synthesis of Geopolymers from Alkaline Activation of Gold Mining Wastes. Ing. Compet. 2014, 16, 317–330. Available online: https://revistaingenieria.univalle.edu.co/index.php/ingenieria_y_competitividad/article/view/3735 (accessed on 30 April 2025).
- .Kiventerä, J.; Golek, L.; Yliniemi, J.; Ferreira, V.; Deja, J.; Illikainen, M. Utilization of sulphidic tailings from gold mine as a raw material in geopolymerization. Int. J. Miner. Process. 2016, 149, 104–110. [Google Scholar] [CrossRef]
- Ansiero, J.P.J.; Opiso, E.M.; Banda, M.H.T.; Tabelin, C.B. Potential utilization of artisanal gold-mine tailings as geopolymeric source material: Preliminary investigation. SN Appl. Sci. 2019, 1, 35. [Google Scholar] [CrossRef]
- Huang, B.; Feng, Q.; An, D.; Zhang, J. Use of mine tailings as precast construction materials through alkali activation. Min. Metall. Explor. 2020, 37, 251–265. [Google Scholar] [CrossRef]
- Niu, F.S.; Zhou, S.S.; Liu, S.X.; Zhang, J.X. Study of manufacture process and properties of tailings and slag based geopolymers. In Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2011; Volume 156, pp. 803–807. [Google Scholar] [CrossRef]
- Kuranchie, F.A.; Shukla, S.K.; Habibi, D. Utilisation of iron ore mine tailings for the production of geopolymer bricks. Int. J. Min. Reclamat. Environ. 2016, 30, 92–114. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Ramos, F.C.R.; Caetano, T.R.; Panzerra, T.H.; Santos, H. Reuse of iron ore tailings in the production of geopolymer mortars. REM Int. Eng. J. 2019, 72, 581–587. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Ren, D. Fresh properties, compressive strength and microstructure of fly ash geopolymer paste blended with iron ore tailing under thermal cycle. Constr. Build. Mater. 2016, 118, 76–88. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Investigations about the effect of aggregates on strength and microstructure of geopolymeric mine waste mud binders. Cement Concr. Res. 2007, 37, 933–941. [Google Scholar] [CrossRef]
- Kastiukas, G.; Zhou, X.; Castro-Gomes, J. Preparation conditions for the synthesis of alkali-activated binders using tungsten mining waste. J. Mater. Civ. Eng. 2017, 29, 04017181. [Google Scholar] [CrossRef]
- Sedira, N.; Castro-Gomes, J.; Magrinho, M. Red clay brick and tungsten mining waste-based alkali-activated binder: Microstructural and mechanical properties. Constr. Build. Mater. 2018, 190, 1034–1048. [Google Scholar] [CrossRef]
- Sedira, N.; Castro-Gomes, J. Effects of EAF-Slag on alkali-activation of tungsten mining waste: Mechanical properties. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2019; Volume 274. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, Y.; Bao, S. Preparation of Geopolymers from Vanadium Tailings by Mechanical Activation. Constr. Build. Mater. 2017, 145, 236–242. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, Y.; Chen, T. Thermal stability of a silica-rich vanadium tailing based geopolymer. Constr. Build. Mater. 2013, 38, 43–47. [Google Scholar] [CrossRef]
- Liu, D.; Cui, X.; Di, Y.; Li, F. Preparation of vanadium tailings based geopolymer by mechano−alkali activation. Conserv. Util. Miner. Resour. 2025, 1–8. [Google Scholar] [CrossRef]
- Wang, A.; Liu, H.; Hao, X.; Wang, Y.; Liu, X.; Li, Z. Geopolymer synthesis using garnet tailings from molybdenum mines. Minerals 2019, 9, 48. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Effect of elevated temperature on the properties of geopolymer synthesized from calcined ore-dressing tailing of bauxite and groundgranulated blast furnace slag. Constr. Build. Mater. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Hertel, T.; Blanpain, B.; Pontikes, Y. A proposal for a 100% use of bauxite residue towards inorganic polymer mortar. J. Sustain. Metall. 2016, 2, 394–404. [Google Scholar] [CrossRef]
- Moukannaa, S.; Loutou, M.; Benzaazoua, M.; Vitola, L.; Alami, J.; Hakkou, R. Recycling of phosphate mine tailings for the production of geopolymers. J. Clean. Prod. 2018, 185, 891–903. [Google Scholar] [CrossRef]
- Moukannaa, S.; Nazari, A.; Bagheri, A.; Loutou, M.; Sanjayan, J.G.; Hakkou, R. Alkaline fused phosphate mine tailings for geopolymer mortar synthesis: Thermal stability, mechanical and microstructural properties. J. Non-Cryst. Solids 2019, 511, 76–85. [Google Scholar] [CrossRef]
- Dabbebi, R.; de Aguiar, J.B.; Camões, A.; Samet, B.; Baklouti, S. Effect of the calcinations temperatures of phosphate washing waste on the structural and mechanical properties of geopolymeric mortar. Constr. Build. Mater. 2018, 185, 489–498. [Google Scholar] [CrossRef]
- Mabroum, S.; Aboulayt, A.; Taha, Y.; Benzaazoua, M.; Semlal, N.; Hakkou, R. Elaboration of geopolymers based on clays by-products from phosphate mines for construction applications. J. Clean. Prod. 2020, 261, 121317. [Google Scholar] [CrossRef]
- Hamdane, H.; Tamraoui, Y.; Mansouri, S.; Oumam, M.; Bouih, A.; El Ghailassi, T.; Boulif, R.; Manoun, B.; Hannache, H. Effect of alkali-mixed content and thermally untreated phosphate sludge dosages on some properties of metakaolin based geopolymer material. Mater. Chem. Phys. 2020, 122938. [Google Scholar] [CrossRef]
- Herselman, J.E.; Snyman, H.G. Guidelines for the utilisation and disposal of wastewater sludge. Water Res. Comm. 2008. [Google Scholar]
- Matheri, A.N.; Eloko, N.S.; Ntuli, F.; Catherine, J. Influence of pyrolyzed sludge use as an adsorbent in removal of selected trace metals from wastewater treatment. Case Stud. Chem. Environ. Eng. 2020, 2, 100018. [Google Scholar] [CrossRef]
- Hong, J.; Li, X. Environmental assessment of sewage sludge as secondary raw material in cement production e a case study in China. Waste Manag. 2011, 31, 1364–1371. [Google Scholar] [CrossRef]
- Juala, R.; Ballim, Y.; Mulopo, J. Assessment of local sewage sludge ash as a supplementary cementitious material e effects of incineration temperature and cooling rate of the ash. J. S. Afr. Inst. Civ. Eng. 2019, 1, 37–47. [Google Scholar] [CrossRef]
- Mejdi, M.; Saillio, M.; Chaussadent, T.; Divet, L.; Tagnit-Hamou, A. Hydration mechanisms of sewage sludge ashes used as cement replacement. Cement Concr. Res. 2020, 135, 106115. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, W.; Zeng, G.; Wang, C.; Fan, X. Pyrolysis characteristics and kinetics of sewage sludge for different sizes and heating rates. J. Therm. Anal. 2012, 107, 1015–1022. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, S.; Wang, C.; Hou, J.; Guo, P.; Lin, Z. Study of heavy metal in sewage sludge and in Chinese cabbage grown in soil amended with sewage sludge. Afr. J. Biotechnol. 2008, 7, 1329–1334. [Google Scholar]
- Mu, L.; Zhao, C.; Zhao, L.; Chen, B.; Xu, Z.; Yang, Z.; Shang, Y.; Yin, H. Mineralogical composition evolution and thermogravimetric characteristics of sewage sludge ash at different ashing temperatures. Energy Fuels 2018, 32, 12617–12629. [Google Scholar] [CrossRef]
- Chen, M.; Blanc, D.; Gautier, M.; Mehu, J.; Gourdon, R. Environmental and technical assessments of the potential utilization of sewage sludge ashes (SSAs) as secondary raw materials in construction. Waste Manag. 2013, 33, 1268–1275. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, C.; Huang, B.; Lu, X. Development of alkali activated cementitious material from sewage sludge ash: Two-part and one-part geopolymer. J. Clean. Prod. 2023, 384, 135547. [Google Scholar] [CrossRef]
- Istuque, D.B.; Reig, L.; Moraes, J.C.B.; Akasaki, J.L.; Borrachero, M.V.; Soriano, L.; Paya, J.; Malmonge, J.A.; Tashima, M.M. Behaviour of metakaolin-based geopolymers incorporating sewage sludge ash (SSA). Mater. Lett. 2016, 180, 192–195. [Google Scholar] [CrossRef]
- Santos, G.Z.; Melo Filho, J.A.; Pinheiro, M.; Manzato, L. Synthesis of water treatment sludge ash-based geopolymers in an Amazonian context. J. Environ. Manag. 2019, 249, 109328. [Google Scholar] [CrossRef] [PubMed]
- Nimwinya, E.; Arjharn, W.; Horpibulsuk, S.; Phoo-Ngernkham, T.; Poowancum, A. A sustainable calcined water treatment sludge and rice husk ash geopolymer. J. Clean. Prod. 2016, 119, 128–134. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Camões, A.; Branco, F.G. Synergetic Effect of Biomass Fly Ash on Improvement of High-Volume Coal Fly Ash Concrete Properties. Constr. Build. Mater. 2022, 314, 125680. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Hasler, P.; Nussbaumer, T. Particle Size Distribution of the Fly Ash from Biomass Combustion. In Proceedings of the 10th European Conference and Technology Exhibition on Biomass for Energy and Industry, Rimpar Carmen, Germany, 8–11 June 1998; James & James (Science Publishers) Ltd.: London, UK, 1998; pp. 8–11. [Google Scholar]
- Chen, H.; Liu, Z.; Chen, X.; Chen, Y.; Dong, Z.; Wang, X.; Yang, H. Comparative Pyrolysis Behaviors of Stalk, Wood and Shell Biomass: Correlation of Cellulose Crystallinity and Reaction Kinetics. Bioresour. Technol. 2020, 310, 123498. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef] [PubMed]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Progress, Current Thinking and Challenges in Geopolymer Foam Concrete Technology. Cem. Concr. Compos. 2021, 116, 103886. [Google Scholar] [CrossRef]
- Jindal, B.B. Investigations on the Properties of Geopolymer Mortar and Concrete with Mineral Admixtures: A Review. Constr. Build. Mater. 2019, 227, 116644. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Tobaldi, D.M.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. Synthesis of Porous Biomass Fly Ash-Based Geopolymer Spheres for Efficient Removal of Methylene Blue from Wastewaters. J. Clean. Prod. 2019, 207, 350–362. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Porous Biomass Fly Ash-Based Geopolymers withTailored Thermal Conductivity. J. Clean. Prod. 2016, 119, 99–107. [Google Scholar] [CrossRef]
- Rossi, A.D.; Simão, L.; Ribeiro, M.J.; Novais, R.M.; Labrincha, J.A.; Hotza, D.; Moreira, R.F.P.M. In-Situ Synthesis of Zeolites by Geopolymerization of Biomass Fly Ash and Metakaolin. Mater. Lett. 2019, 236, 644–648. [Google Scholar] [CrossRef]
- Saeli, M.; Senff, L.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Novel Biomass Fly Ash-Based Geopolymeric Mortars Using Lime Slaker Grits as Aggregate for Applications in Construction: Influence of Granulometry and Binder/Aggregate Ratio. Constr. Build. Mater. 2019, 227, 116643. [Google Scholar] [CrossRef]
- Rossi, A.D.; Simão, L.; Ribeiro, M.J.; Hotza, D.; Moreira, R.F.P.M. Study of Cure Conditions Effect on the Properties of Wood Biomass Fly Ash Geopolymers. J. Mater. Res. Technol. 2020, 9, 7518–7528. [Google Scholar] [CrossRef]
- Bouzar, B.; Mamindy-Pajany, Y. Immobilization Study of As, Cr, Mo, Pb, Sb, Se and Zn in Geopolymer Matrix: Application to Shooting Range Soil and Biomass Fly Ash. Int. J. Environ. Sci. Technol. 2023, 20, 11891–11912. [Google Scholar] [CrossRef]
- Abdulkareem, O.A.; Ramli, M.; Matthews, J.C. Production of Geopolymer Mortar System Containing High Calcium Biomass Wood Ash as a Partial Substitution to Fly Ash: An Early Age Evaluation. Compos. Part B Eng. 2019, 174, 106941. [Google Scholar] [CrossRef]
- Jurado-Contreras, S.; Bonet-Martínez, E.; Sánchez-Soto, P.; Gencel, O.; Eliche-Quesada, D. Synthesis and Characterization of Alkali-Activated Materials Containing Biomass Fly Ash and Metakaolin: Effect of the Soluble Salt Content of the Residue. Arch. Civ. Mech. Eng. 2022, 22, 121. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Bonet-Martínez, E.; Eliche-Quesada, D.; Sánchez-Soto, P.J.; Rincón-López, J.M.; Castro-Galiano, E. Biomass Fly Ash and Aluminium Industry Slags-Based Geopolymers. Mater. Lett. 2018, 229, 6–12. [Google Scholar] [CrossRef]
- Rossi, A.D.; Ribeiro, M.J.; Labrincha, J.A.; Novais, R.M.; Hotza, D.; Moreira, R.F.P.M. Effect of the Particle Size Range of Construction and Demolition Waste on the Fresh and Hardened-State Properties of Fly Ash-Based Geopolymer Mortars with Total Replacement of Sand. Process Saf. Environ. Prot. 2019, 129, 130–137. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Seabra, M.P.; Bajare, D.; Labrincha, J.A. Novel Porous Fly Ash-Containing Geopolymers for PH Buffering Applications. J. Clean. Prod. 2016, 124, 395–404. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R.; Davidovits, M. Geopolymeric Cement Based on Fly Ash and Harmless to Use. U.S. Patent No. US8202362B2, 19 June 2012. [Google Scholar]
- Król, M.; Rozek, P.; Chlebda, D.; Mozgawa, W. Influence of Alkali Metal Cations/Type of Activator on the Structure of Alkali-Activated Fly Ash—ATR-FTIR Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 33–37. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L. Quantitative Study of the Reactivity of Fly Ash in Geopolymerization by FTIR. J. Sustain. Cem. Based Mater. 2012, 1, 154–166. [Google Scholar] [CrossRef]
- Svoboda, P.; Skvara, F.; Dolezal, J.; Dvoracek, K.; Lucuk, K. Fly-Ash Concrete Compositon, Method of Preparation by GeoPolymeric Reaction of Activated Fly-Ash and Its Use. Patent EP1801084A1, 27 June 2007. [Google Scholar]
- Saeli, M.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Mix Design and Mechanical Performance of Geopolymeric Binders and Mortars Using Biomass Fly Ash and Alkaline Effluent from Paper-Pulp Industry. J. Clean. Prod. 2019, 208, 1188–1197. [Google Scholar] [CrossRef]
- Songpiriyakij, S.; Pulngern, T.; Pungpremtrakul, P.; Jaturapitakkul, C. Anchorage of Steel Bars in Concrete by Geopolymer Paste. Mater. Des. 2011, 32, 3021–3028. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Development of Geopolymers as Substitutes for Traditional Ceramics for Bricks with Chamotte and Biomass Bottom Ash. Materials 2021, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Mishra, J.; Mustakim, S.M.; Adesina, A.; Kaze, C.R.; Das, D. Sustainable Utilization of Ultrafine Rice Husk Ash in Alkali Activated Concrete: Characterization and Performance Evaluation. J. Sustain. Cem. Based Mater. 2022, 11, 100–112. [Google Scholar] [CrossRef]
- Diamond, S. Particle Morphologies in Fly Ash. Cem. Concr. Res. 1986, 16, 569–579. [Google Scholar] [CrossRef]
- Memon, S.A.; Khan, S.; Wahid, I.; Shestakova, Y.; Ashraf, M. Evaluating the Effect of Calcination and Grinding of Corn Stalk Ash on Pozzolanic Potential for Sustainable Cement-Based Materials. Adv. Mater. Sci. Eng. 2020, 2020, 1619480. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of Fly Ashes. Potential Reactivity as Alkaline Cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.; Van Deventer, J.; Lukey, G. The Characterisation of Source Materials in Fly Ash-Based Geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Sharko, A.; Louda, P.; Nguyen, V.V.; Buczkowska, K.E.; Stepanchikov, D.; Ercoli, R.; Kascak, P.; Le, V.S. Multicriteria Assessment for Calculating the Optimal Content of Calcium-Rich Fly Ash in Metakaolin-Based Geopolymers. Ceramics 2023, 6, 525–537. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer Foam Concrete: An Emerging Material for Sustainable Construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Billong, N.; Kinuthia, J.; Oti, J.; Melo, U.C. Performance of Sodium Silicate Free Geopolymers from Metakaolin (MK) and Rice Husk Ash (RHA): Effect on Tensile Strength and Microstructure. Constr. Build. Mater. 2018, 189, 307–313. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, H.; Zhang, Z.; Wu, Q.; Du, J. Investigation of the Waterproof Property of Alkali-Activated Metakaolin Geopolymer Added with Rice Husk Ash. J. Clean. Prod. 2019, 230, 603–612. [Google Scholar] [CrossRef]
- Liu, H.; Jing, W.; Qin, L.; Duan, P.; Zhang, Z.; Guo, R.; Li, W. Thermal Stability of Geopolymer Modified by Different Silicon Source Materials Prepared from Solid Wastes. Constr. Build. Mater. 2022, 315, 125709. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A Critical Review of Geopolymer Properties for Structural Fire-Resistance Applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Das, S.K.; Mishra, J.; Singh, S.K.; Mustakim, S.M.; Patel, A.; Das, S.K.; Behera, U. Characterization and Utilization of Rice Husk Ash (RHA) in Fly Ash–Blast Furnace Slag Based Geopolymer Concrete for Sustainable Future. Mater. Today Proc. 2020, 33, 5162–5167. [Google Scholar] [CrossRef]
- World Bank. Solid Waste Management; The World Bank Group: Washington, DC, USA, 2019; Available online: https://www.worldbank.org/en/topic/urbandevelopment/brief/solid-waste-management (accessed on 30 April 2025).
- Waste Generation and Treatment (env_wasgt), Reference Metadata in Euro SDMX Metadata Structure (ESMS). Available online: https://ec.europa.eu/eurostat/cache/metadata/en/env_wasgt_esms.htm (accessed on 30 April 2025).
- Krautwurst, N.; Nicoleau, L.; Dietzsch, M.; Lieberwirth, I.; Labbez, C.; FernandezMartinez, A.; Van Driessche, A.E.S.; Barton, B.; Leukel, S.; Tremel, W. Twostep nucleation process of calcium silicate hydrate, the nanobrick of cement. Chem. Mater. 2018, 30, 2895–2904. [Google Scholar] [CrossRef]
- Dou, X.; Ren, F.; Minh Quan, N.; Ahamed, A.; Yin Ke Chan, W.; Chang, V. Review of MSWI bottom ash utilization from perspectives of collective characterization, treatment and existing application. Renew. Sustain. Energy Rev. 2017, 79, 24–38. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Lynn, C.J.; Dhir, R.K. Use of municipal solid waste incineration bottom ashes in alkali activated materials, ceramics and granular applications: A review. Waste Manag. 2017, 68, 207–220. [Google Scholar] [CrossRef]
- Bosio, A.; Zacco, A.; Borgese, L.; Rodella, N.; Colombi, P.; Benassi, L.; Depero, L.E.; Bontempi, E. A sustainable technology for Pb and Zn stabilization based on the use of only waste materials: A green chemistry approach to avoid chemicals and promote CO2 sequestration. Chem. Eng. J. 2014, 253, 377–384. [Google Scholar] [CrossRef]
- Huang, T.; Liu, L.; Zhou, L.; Yang, K. Operating optimization for the heavy metal removal from the municipal solid waste incineration fly ashes in the three-dimensional Chock tar electrokinetics. Chemosphere 2018, 204, 294–302. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.; Huang, Y.; Shimaoka, T.; Wang, H.; Wang, Y.; Ma, L.; Zhang, D. Evaluation of chemical speciation and environmental risk levels of heavy metals during varied acid corrosion conditions for raw and solidified/stabilized MSWI fly ash. Waste Manag. 2019, 87, 407–416. [Google Scholar] [CrossRef]
- Li, Y.; Min, X.; Ke, Y.; Liu, D.; Tang, C. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef]
- Ferreira, C.; Ribeiro, A.; Ottosen, L. Possible applications for municipal solid waste fly ash. J. Hazard. Mater. 2003, 96, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C.W. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, W.; Gao, X. Solidification and immobilization of MSWI fly ash through aluminate geopolymerization: Based on partial charge model analysis. Waste Manag. 2016, 58, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Z.; Ma, B.; Wu, B. Effect of MSWI fly ash and incineration residues on cement performances. J. Wuhan Univ. Technol. 2010, 25, 312–315. [Google Scholar] [CrossRef]
- Lee, W.K.; van Deventer, J.S.J. Structural reorganisation of class F fly ash in alkaline silicate solutions. Colloids Surf. A 2002, 211, 49–66. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Dimas, D.; Giannopoulou, I.; Panias, D. Polymerization in sodium silicate solutions: A fundamental process in geopolymerization technology. J. Mater. Sci. 2009, 44, 3719–3730. [Google Scholar] [CrossRef]
- Almalkawi, A.T.; Balchandra, A.; Soroushian, P. Potential of using industrial wastes for production of geopolymer binder as green construction materials. Constr. Build. Mater. 2019, 220, 516–524. [Google Scholar] [CrossRef]
- He, J.; Jie, Y.; Zhang, J.; Yu, Y.; Zhang, G. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]
- Guo, X.; Hu, W.; Shi, H. Microstructure and self-solidification/stabilization (S/S) of heavy metals of nano-modified CFA-MSWIFA composite geopolymers. Constr. Build. Mater. 2014, 56, 81–86. [Google Scholar] [CrossRef]
- Zhong, M.F.; Zhang, Z.J.; Zhou, J.; Zhou, X.T. Synthesis and mechanical properties of nano-alumina reinforced acid-activated metakaolinite-based geopolymer. J. Guizhou Univ. 2009, 26, 103–105. [Google Scholar]
- Chen, L.; Wang, L.; Cho, D.; Tsang, D.; Tong, L.; Zhou, Y.; Yang, J.; Hu, Q.; Poon, C. Sustainable stabilization/solidification of municipal solid waste incinerator fly ash by incorporation of green materials. J. Clean. Prod. 2019, 222, 335–343. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, Q.; Qiu, W.; Xue, Y.; Li, N. Microstructure and strength of alkali-activated bricks containing municipal solid waste incineration (MSWI) fly ash developed as. Constr. Mater. Sustain. 2019, 11, 1283. [Google Scholar] [CrossRef]
- Lind, T.; Hokkinen, J.; Jokiniemi, J. Fine particle and trace element emissions from waste combustion—Comparison of fluidized bed and grate firing. Fuel Process. Technol. 2007, 88, 737–746. [Google Scholar] [CrossRef]
- Lach, M.; Mierzwinski, D.; Korniejenko, K.; Mikuła, J.; Hebda, M. Geopolymers as a material suitable for immobilization of fly ash from municipal waste incineration plants. J. Air Waste Manag. 2018, 68, 1190–1197. [Google Scholar] [CrossRef]
- Lei, Z.; Wei, W.; Shi, Y. The effects of alkaline dosage and Si/Al ratio on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere 2010, 79, 665–671. [Google Scholar]
- Tian, X.; Rao, F.; León-Patiño, C.A. Effects of aluminum on the expansion and microstructure of alkali-activated MSWI fly ash-based pastes. Chemosphere 2020, 240, 124986. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Kirkelund, G. Electrodialytic remediation of municipal solid waste incineration fly ash as pre-treatment before geopolymerisation with coal fly ash. J. Hazard. Mater. 2021, 412, 125220. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, C.; Wang, W.; Shi, Y.; Gao, X. Immobilization of MSWI fly ash through geopolymerization: Effects of water-wash. Waste Manag. 2011, 31, 311–317. [Google Scholar] [CrossRef]
- Casanova, S.; Silva, R.V.; Brito, J.; Pereira, M.F.C. Mortars with alkali-activated municipal solid waste incinerator bottom ash and fine recycled aggregates. J. Clean. Prod. 2021, 289, 125707. [Google Scholar] [CrossRef]
- Ajorloo, M.; Ghodrat, M.; Scott, J.; Strezov, V. Heavy Metals Removal/Stabilization from Municipal Solid Waste Incineration Fly Ash: A Review and Recent Trends. J. Mater. Cycles Waste Manag. 2022, 24, 1693–1717. [Google Scholar] [CrossRef]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L.; Ren, J. Utilisation of municipal solid waste incinerator (MSWI) fly ash with metakaolin for preparation of alkali-activated cementitious material. J. Hazard. Mater. 2021, 402, 23451. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zheng, Z.; Sun, Y.; Chen, L.; Jin, Z. Resistance of metakaolin-MSWI fly ash based geopolymer to acid and alkaline environments. J. Non-Cryst. Solids 2016, 450, 116–122. [Google Scholar] [CrossRef]
- Liu, K.; Tan, Q.; Yu, J.; Wang, M. A global perspective on e-waste recycling. Circ. Econ. 2023, 2, 100028. [Google Scholar] [CrossRef]
- Nadarajan, P.; Vafaei-Zadeh, A.; Hanifah, H.; Thurasamay, R. Sustaining the environment through e-waste recycling: An extended valence theory perspective. Aslib J. Inf. Manag. 2024, 76, 1059–1087. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukhopadhyay, A.; Bhattacharjee, P. A global perspective on E-waste: From cradle to grave. In Electronic Waste Management: Policies, Processes, Technologies, and Impact; The Royal Society of Chemistry: Cambridge, UK; Wiley: Hoboken, NJ, USA, 2023; pp. 66–80. [Google Scholar]
- Das, S.; Gupta, B.; Sarkar, A. Diverse technological initiatives for E-waste management and its impact on ecosystem. In Conversion of Electronic Waste in to Sustainable Products; Springer: Berlin/Heidelberg, Germany, 2022; pp. 79–102. [Google Scholar]
- Thakur, P.; Kumar, S. Evaluation of e-waste status, management strategies, and legislations. Int. J. Environ. Sci. Technol. 2022, 19, 6957–6966. [Google Scholar] [CrossRef]
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor 2017; United Nations University (UNU): Bonn, Germany; International Telecommunication Union (ITU) &: Geneva, The Switzerland; International Solid Waste Association (ISWA): Vienna, Austria, 2017. [Google Scholar]
- Agarwal, S.; Darbar, S.; Saha, S.; Choudhury, M.; Singh, R.P. Chapter 10 – E-Waste Management Using Different Cost-Effective, Eco-Friendly Biological Techniques: An Overview. In Waste Management and Resource Recycling in the Developing World; Singh, P., Verma, P., Singh, R., Ahamad, A., Batalhão, A.C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 205–235. [Google Scholar] [CrossRef]
- Lin, S.; Ali, M.U.; Zheng, C.; Cai, Z.; Wong, M.H. Toxic chemicals from uncontrolled e-waste recycling: Exposure, body burden, health impact. J. Hazard. Mater. 2022, 426, 127792. [Google Scholar] [CrossRef]
- Sharma, D.; Nema, A.; Prasad, R.; Sweta, K.; Sonaviya, D.R.; Karmakar, S. Global E-waste management: Consolidated information showcasing best available practices. In Global E-Waste Management Strategies and Future Implications; Elsevier: London, UK, 2023; pp. 289–314. [Google Scholar]
- Verma, P.; Dhurvey, P.; Sundramurthy, V.P. Potential assessment of E-waste plastic in metakaolin based geopolymer using petrography image analysis. Adv. Mater. Sci. Eng. 2022, 2022, 7790320. [Google Scholar] [CrossRef]
- Long, W.-J.; Lin, C.; Ye, T.-H.; Dong, B.; Xing, F. Stabilization/solidification of hazardous lead glass by geopolymers. Constr. Build. Mater. 2021, 294, 123574. [Google Scholar] [CrossRef]
- Gao, X.; Yao, X.; Xie, R.; Li, X.; Cheng, J.; Yang, T. Performance of flash-based geopolymer mortars with waste cathode ray tubes glass fine aggregate: A comparative study with cement mortars. Constr. Build. Mater. 2022, 344, 128243. [Google Scholar] [CrossRef]
- Wielgus, N.; Górski, M.; Kubica, J. Discarded Cathode Ray Tube glass as an alternative for aggregate in a metakaolin-based geopolymer. Sustainability 2021, 13, 479. [Google Scholar] [CrossRef]
- Shiu, H.S.; Lin, K.L.; Chao, S.J.; Hwang, C.L.; Cheng, T.W. Effects of foam agent on characteristics of thin-film transistor liquid crystal display waste glass-metakaolin-based cellular geopolymer. Environ. Prog. Sustain. Energy 2014, 33, 538–550. [Google Scholar] [CrossRef]
- Janardhanan, T.; Ramasamy, V. Improvements in the Microstructural and Mechanical Properties of Geopolymer Concrete Containing NMFs of E-Wastes as Partial Replacement of Aggregates. Eur. J. Environ. Civ. Eng. 2017, 1–12. [Google Scholar] [CrossRef]
- Sundar, M.L.; Raj, S. Study on characteristics of geopolymer concrete with E-waste. J. Mech. Civ. Eng. 2017, 14, 21–27. [Google Scholar] [CrossRef]
- Wei, X.; Sun, Y.; Su, Y.; Shen, X.; Tang, Y.; Yan, F.; Zhang, Z. Structural evolution of geopolymers incorpo-rated with heavy metals: Solidification mechanism of Pb2+ and Cd2+. J. Phys. Chem. C 2023, 127, 19563–19573. [Google Scholar] [CrossRef]
- Fan, J.; Yan, J.; Zhou, M.; Xu, Y.; Lu, Y.; Duan, P.; Zhu, Y.; Zhang, Z.; Li, W.; Wang, A. Heavy metals immobilization of ternary geopolymer based on nickel slag, lithium slag and metakaolin. J. Hazard. Mater. 2023, 453, 131380. [Google Scholar] [CrossRef]
- Long, W.-J.; Li, H.-D.; Ma, H.; Fang, Y.; Xing, F. Green alkali-activated mortar: Sustainable use of discarded cathode-ray tube glass powder as precursor. J. Clean. Prod. 2019, 229, 1082–1092. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Guo, X.; You, Y.; Saha, N.; Wu, S.; Scheuermann, A.; Ren, C.; Huang, L. Co-solidification of bauxite residue and coal ash into indurated monolith via ambient geopolymerisation for in situ environmental application. J. Hazard. Mater. 2022, 422, 126925. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. Comparison of alkali and silica sources in one-part alkali-activated blast furnace slag mortar. J. Clean. Prod. 2018, 187, 171–179. [Google Scholar] [CrossRef]
- Heath, A.; Paine, K.; McManus, M. Minimising the global warming potential of clay based geopolymers. J. Clean. Prod. 2014, 78, 75–83. [Google Scholar] [CrossRef]
- Vinai, R.; Soutsos, M. Production of sodium silicate powder from waste glass cullet for alkali activation of alternative binders. Cement Concr. Res. 2019, 116, 45–56. [Google Scholar] [CrossRef]
- Aphane, M.E.; Doucet, F.J.; Kruger, R.A.; Petrik, L.; van der Merwe, E.M. Preparation of sodium silicate solutions and silica nanoparticles from South African coal fly ash. Waste Biomass Valorization 2020, 11, 4403–4417. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; de Gutiérrez, R.M.; Provis, J.L.; Delvasto, S. Activation of metakaolin/slag blends using alkaline solutions based on chemically modified silica fume and rice husk ash. Waste Biomass Valorization 2012, 3, 99–108. [Google Scholar] [CrossRef]
- Alam, Q.; Hendrix, Y.; Thijs, L.; Lazaro, A.; Schollbach, K.; Brouwers, H. Novel low temperature synthesis of sodium silicate and ordered mesoporous silica from incineration bottom ash. J. Clean. Prod. 2019, 211, 874–883. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A.; Shultz, J. A simple method for production of pure silica from rice hull ash. Bioresour. Technol. 2000, 73, 257–262. [Google Scholar] [CrossRef]
- Kamseu, E.; Moungam, L.B.À.; Cannio, M.; Billong, N.; Chaysuwan, D.; Melo, U.C.; Leonelli, C. Substitution of sodium silicate with rice husk ash-NaOH solution in metakaolin based geopolymer cement concerning reduction in global warming. J. Clean. Prod. 2017, 142, 3050–3060. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.; Lazaro, A.; Brouwers, H. Investigation on a green olivine nano-silica source based activator in alkali activated slag-fly ash blends: Reaction kinetics, gel structure and carbon footprint. Cement Concr. Res. 2017, 100, 129–139. [Google Scholar] [CrossRef]
- Poulesquen, A.; Frizon, F.; Lambertin, D. Rheological behavior of alkali-activated metakaolin during geopolymerization. J. Non-Cryst. Solids 2011, 357, 3565–3571. [Google Scholar] [CrossRef]
- Mellado, A.; Catalán, C.; Bouzón, N.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Carbon footprint of geopolymeric mortar: Study of the contribution of the alkaline activating solution and assessment of an alternative route. RSC Adv. 2014, 4, 23846–23852. [Google Scholar] [CrossRef]
- Passuello, A.; Rodríguez, E.D.; Hirt, E.; Longhi, M.; Bernal, S.A.; Provis, J.L.; Kirchheim, A.P. Kirchheim, Evaluation of the potential improvement in the environmental footprint of geopolymers using waste-derived activators. J. Clean. Prod. 2017, 166, 680–689. [Google Scholar] [CrossRef]
- Tong, K.T.; Vinai, R.; Soutsos, M.N. Use of Vietnamese rice husk ash for the production of sodium silicate as the activator for alkali-activated binders. J. Clean. Prod. 2018, 201, 272–286. [Google Scholar] [CrossRef]
- Samarakoon, M.H.; Ranjith, P.G.; Duan, W.H.; De Silva, V.R.S. Properties of one-part fly ash/slag-based binders activated by thermally-treated waste glass/NaOH blends: A comparative study. Cement Concr. Compos. 2020, 112, 103679. [Google Scholar] [CrossRef]
- Murtaza, M.; Kamal, M.S.; Mahmoud, M. Application of a novel and sustainable silicate solution as an alternative to sodium silicate for clay swelling inhibition. ACS Omega 2020, 5, 17405–17415. [Google Scholar] [CrossRef]
- Elkatatny, S.; Jafarov, T.; Al-Majed, A.; Mahmoud, M. Formation damage avoidance by reducing invasion with sodium silicate-modified water-based drilling fluid. Energies 2019, 12, 1485. [Google Scholar] [CrossRef]
- Mainier, F.B.; Figueiredo, A.A.M.; de Freitas, A.E.R.; de Alencar Junior, A.A.M. The use of sodium silicate as a corrosion inhibitor in a saline drilling fluid: A nonaggressive option to the environment. J. Environ. Protect. 2016, 7, 2025. [Google Scholar] [CrossRef]
- Liu, W.Q.; Hu, R.J.; Yue, M.; Yin, Y.X.; Zhang, D.T. Preparation and properties of isotropic Nd-Fe-B bonded magnets with sodium silicate binder. J. Magn. Magn. Mater. 2017, 435, 187–193. [Google Scholar] [CrossRef]
- Liu, W.; Xi, W.; Hu, R.; Yue, M.; Yin, Y.; Guo, J.; Zhang, D.; Zhang, H. Preparation and characterization of sodium silicate/epoxy resin composite bonded Nd-Fe-B magnets with high performance. J. Rare Earths 2019, 37, 1083–1087. [Google Scholar] [CrossRef]
- Pham, L.T.; Hatzignatiou, D.G. Rheological evaluation of a sodium silicate gel system for water management in mature, naturally-fractured oilfields. J. Petrol. Sci. Eng. 2016, 138, 218–233. [Google Scholar] [CrossRef]
- Ren, X.; Hu, X.; Xue, D.; Li, Y.; Shao, Z.; Dong, H.; Cheng, W.; Zhao, Y.; Xin, L.; Lu, W. Novel sodium silicate/polymer composite gels for the prevention of spontaneous combustion of coal. J. Hazard. Mater. 2019, 371, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-W.; Xiao, Y.; Zhong, K.-Q.; Shu, C.-M.; Lü, H.-F.; Deng, J.; Wu, S. Overview of commonly used materials for coal spontaneous combustion prevention. Fuel 2020, 275, 117981. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Cheng, W.; Shao, Z.; Xue, D.; Zhao, Y.; Lu, W. A novel high-toughness, organic/inorganic double-network fire-retardant gel for coal-seam with high ground temperature. Fuel 2020, 263, 116779. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium waterglass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S. Ranjbar, Synthesis of sodium waterglass from white rice husk ash as an activator to produce metakaolin-based geopolymer cements. J. Build. Eng. 2016, 6, 252–261. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Tran, T.T.; Tran, A.T.H.; Le, O.T.K.; Sagadevan, S.; Mohd Kaus, N.H. Effect of Red Mud and Rice Husk Ash-Based Geopolymer Composites on the Adsorption of Methylene Blue Dye in Aqueous Solution for Wastewater Treatment. ACS Omega 2023, 8, 41258–41272. [Google Scholar] [CrossRef] [PubMed]
- Mejía, J.M.; De Gutiérrez, R.M.; Montes, C. Rice husk ash and spent diatomaceous earth as a source of silica to fabricate a geopolymeric binary binder. J. Clean. Prod. 2016, 118, 133–139. [Google Scholar] [CrossRef]
- Mejía, J.M.; de Gutiérrez, R.M.; Montes, C. Rice husk ash as a source of silica in alkali-activated fly ash and granulated blast furnace slag systems. Mater. Construcción 2013, 63, 361–375. [Google Scholar] [CrossRef]
- Geraldo, R.H.; Fernandes, L.F.; Camarini, G. Water treatment sludge and rice husk ash to sustainable geopolymer production. J. Clean. Prod. 2017, 149, 146–155. [Google Scholar] [CrossRef]
- Bouzòn, N.; Payà, J.; Borrachero, M.; Soriano, L.; Tashima, M.M..; Monzò, J. Refluxed rice husk ash/NaOH suspension for preparing alkali activated binders. Mater. Lett. 2014, 115, 72–74. [Google Scholar] [CrossRef]
- Basgöz, Ö.; Güler, Ö. The unusually formation of porous silica nano-stalactite structure by high temperature heat treatment of SiO2 aerogel synthesized from rice hull. Ceram. Int. 2020, 46, 370–380. [Google Scholar] [CrossRef]
- El-Naggar, M.R.; El-Dessouky, M.I. Re-use of waste glass in improving properties of metakaolin-based geopolymers: Mechanical and microstructure examinations. Construct. Build. Mater. 2017, 132, 543–555. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Palomo, J.G.; Maroto, F.P. Sodium silicate solutions from dissolution of glasswastes. Statistical analysis. Mater. Construcción 2014, 64, e014. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass in the geopolymer preparation. Mechanical and microstructural characterization. J. Clean. Prod. 2015, 90, 397–408. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Tognonvi, M.T.; Tagnit-Hamou, F. Puertas, Durability of alkali-activated slag concretes prepared using waste glass as alternative activator. ACI Mater. J. 2015, 112, 791. [Google Scholar]
- König, K.; Traven, K.; Pavlin, M.; Ducman, V. Evaluation of locally available amorphous waste materials as a source for alternative alkali activators. Ceram. Int. 2020, 47, 4864–4873. [Google Scholar] [CrossRef]
- Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Paya, J.; Monzo, J.M.; Borrachero, M.V. Effect of nanosilica-based activators on the performance of an alkali-activated fly ash binder. Cement Concr. Compos. 2013, 35, 1–11. [Google Scholar] [CrossRef]
- Autef, A.; Joussein, E.; Gasgnier, G.; Rossignol, S. Role of the silica source on the geopolymerization rate: A thermal analysis study. J. Non-Cryst. Solids 2013, 366, 13–21. [Google Scholar] [CrossRef]
- Abdul Rahim, R.H.; Rahmiati, T.; Azizli, K.A.; Man, Z.; Nuruddin, M.F.; Ismail, L. Comparison of Using NaOH and KOH Activated Fly Ash-Based Geopolymer on the Mechanical Properties. Mater. Sci. Forum 2014, 803, 179–184. [Google Scholar] [CrossRef]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterization. Cement Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Solid reactant-based geopolymers from rice hull ash and sodium aluminate. Waste Biomass Valorization 2017, 8, 2131–2140. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.J.G.; Brouwers, H.J.H.; Kühne, H.-C. Synthesizing one-part geopolymers from rice husk ash. Constr. Build. Mater. 2016, 124, 961–966. [Google Scholar] [CrossRef]
- Circular Construction Economy Transition Agenda. Available online: https://circulairebouweconomie.nl/about-us/?utm (accessed on 1 May 2025).
- FONA—Forschung Für Nachhaltige Entwicklung. Available online: https://www.fona.de/de/ (accessed on 30 April 2025).
- Réglementation Environnementale RE2020. Available online: https://www.ecologie.gouv.fr/politiques-publiques/reglementation-environnementale-re2020 (accessed on 30 April 2025).
- Horizon 2020 Project: ReSHEALience. Available online: https://uhdc.eu/ (accessed on 30 April 2025).
- Boverket—The Swedish National Board of Housing, Building and Planning. Available online: https://www.government.se/government-agencies/boverket--the-swedish-national-board-of-housing-building-and-planning/ (accessed on 30 April 2025).
- Part Z—Proposed Amendment to the UK Building Regulations. Available online: https://part-z.uk/ (accessed on 30 April 2025).
- BSI Flex 350—Guidelines for Alternative Low-Impact Binders. Available online: https://knowledge.bsigroup.com/products/alternative-binder-systems-for-lower-carbon-concrete-code-of-practice-11 (accessed on 30 April 2025).
- Future Homes Standard—Standard Expected in 2025. Available online: https://www.gov.uk/government/consultations/the-future-homes-standard-changes-to-part-l-and-part-f-of-the-building-regulations-for-new-dwellings (accessed on 30 April 2025).


| Property | OPC | GPs/AAMs |
|---|---|---|
| Compressive strength (MPa) | 20–60 | 40–90 |
| Acid/sulphate resistance | Moderate to poor | High |
| Fire resistance | <300 °C | Up to 800 °C |
| Raw material origin | Virgin (limestone, clay) | Industrial by-products, waste-derived |
| Production temperature | ~1450 °C | Ambient to <100 °C |
| CO2 emissions (tCO2/t binder) | ~0.9 | 0.2–0.6 |
| Cost variability | Stable, standardized | Variable (dependent on activator type) |
| Standardization and codes | Fully established | Limited, under development |
| Waste | Mineralogical Composition | Waste Activation | Activator | Si/Al | Curing (°C) | Curing (Days) | Compressive Strength (MPa) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CT | Qtz, Alb, San, Gyp | — | NaOH | 7.78 | 90 | 7 | 22 | [76] |
| CT + FA | Qtz, Alb, San, Gyp | — | NaOH | 1.89 | 60 | 7 | 21.02 | [77] |
| CT + AS | Qtz, Alb, San | — | NaOH | 2.71 | 90 | 7 | 44.8 | [78] |
| GT | Qtz, Musc, Pyr, Alu, Goe | — | NaOH—Na2SiO3 | — | 70 | 28 | 50 | [79] |
| GT | Qtz, Gyp, Pyr, Alb, Dol | — | NaOH | 16.10 | Ambient | 28 | 3.05 | [80] |
| GT + GGBFS | Qtz, Gyp, Pyr, Alb, Dol | — | NaOH | — | Ambient | 28 | 25 | [80] |
| GT | Qtz, Cal, Verm, Musc | — | Lime sludge, NaOH | — | Ambient | 7 | 5.95 | [81] |
| GT | Qtz, Feld, Plag, Musc | — | NaOH—Ca(OH)2, Al2O3 | — | 170 | 3 | 40 | [82] |
| IT + Slag | Qtz | — | NaOH—Na2SiO3 | — | 30 | 7 | 63.79 | [83] |
| IT | Qtz, Bir, Goe, Alu, Sod | — | Na2SiO3 | — | 80 | 7 | 50.53 | [84] |
| IT + MK | Qtz, Hem | — | NaOH—Na2SiO3 | — | 45 | 7 | 49.5 | [85] |
| IT + FA | Qtz, Ant, Alb, Amp, Cal, Dol, Chl, Hem, Gyp | — | NaOH—Na2SiO3 | — | Ambient | 28 | 50 | [86] |
| TT | Qtz, Musc | Thermal | NaOH—Na2SiO3, Ca(OH)2 | 5.05 | Ambient | 28 | 75 | [87] |
| TT + WG | Qtz, Alb, Musc, Na-Al-Si | Thermal | NaOH—Na2SiO3 | — | 80 | 28 | 22 | [88] |
| TT + BW | Qtz, Clc, Musc | Thermal | NaOH—Na2SiO3 | 1.36 | 60 | 28 | 59 | [89] |
| TT + Slag | Qtz, Clc, Musc | Thermal | NaOH—Na2SiO3 | 5.02 | 60 | 90 | 30.1 | [90] |
| VT + MK | Qtz, Feld, Dio | Alkaline roasting | NaOH | 1.78 | 60 | 7 | 55.7 | [91] |
| VT + FA | Qtz, Feld, Dio | Dry milling | Na2SiO3 | 3.03 | Ambient | 7 | 42 | [92] |
| VT + MK | Qtz, Feld, Hem, Plas | Mechanical activation | Na2SiO3 | — | Ambient | 14 | 25 | [93] |
| Mo+MK | Cal, Gro, And, Alm, Qtz | — | NaOH—Na2SiO3 | — | Ambient | 3 | 46 | [94] |
| BT+GGBFS | Kln, Dio, Musc, Qtz, Cor | Thermal | NaOH—Na2SiO3 | 1.22 | Ambient | 3 | 56 | [95] |
| BT | Ca–Al–Si, Cal, Can, Dia, Goe, Hem, Qtz | Thermal | K2SiO3 | — | 60 | 3 | 40 | [96] |
| PS+FA | Fap, Dol, Cal, Qtz | — | NaOH—Na2SiO3 | 3.50 | 83.33 | 14.50 | 62 | [97] |
| PS+MK | Fap, Dol, Cal, Qtz | — | NaOH—Na2SiO3 | 3.46 | 83.33 | 14.50 | 53 | [97] |
| PS+MK | Fap, Dol, Cal, Qtz | Alkali fusion | NaOH—Na2SiO3 | — | 60 | 28 | 40 | [98] |
| PW | Heu, Qtz, Cal, Gyp, Pal, Fap | Thermal | NaOH—Na2SiO3 | — | Ambient | 28 | 10 | [99] |
| PWR | Qtz, Dol, Mont, Fap | Thermal | NaOH—Na2SiO3 | 3.78 | Ambient | 28 | 25 | [100] |
| PS+MK | Fap, Dol, Cal, Pal, Qtz | — | NaOH—Na2SiO3, KOH | 2.08 | 60 | 28 | 46.86 | [101] |
| Fly Ash Source | Aluminosilicate Source | Application | Ref |
|---|---|---|---|
| Paper waste | Metakaolin. | Wastewater treatment. | [122] |
| Paper waste | Metakaolin. | Board and wall panels. | [123] |
| Co-generation plant (BA) | Metakaolin. | Filtration and separation. | [124] |
| Kraft pulp mill (BFA) | Metakaolin. | Construction and masonry. | [125] |
| Wood biomass (BA) | Metakaolin. | Reducing the cost of the geopolymer. | [126] |
| Mixed waste from Hauts-de-France (BFA) | Metakaolin and shooting range soil (SRS). | Immobilization of heavy metal. | [127] |
| Wood biomass (BWA) | Fly ash. | Economic and environmental benefits. | [128] |
| Mix of pine pruning, forest residues | Metakaolin. | Building materials, bricks. | [129] |
| Olive and forest pruning (FBA) | Metakaolin; aluminum industry slangs (AIS). | Partial substitutes for metakaolin and Portland cement. | [130] |
| Burder eucalyptus biomass | Metakaolin; aluminum; construction and demolition waste. | Applications in building, replacing conventional mortars. | [131] |
| Wood biomass | Metakaolin. | pH regulators for biogas reactors or wastewater treatment. | [132] |
| Aluminosilicate | Activator | Liquid/Solid | Curing | Compressive Strength (MPa) | Ref. | |
|---|---|---|---|---|---|---|
| Parameters | Days | |||||
| MSWI FA 100% | NaOH 15 M, Na2SiO3 3 M solutions; Si/Al molar ratio is 2.0 | 0.25 | Room Temp. | 7 | 21.04 | [176] |
| MSWI FA 50%, CFA 50% | Molar ratio (NaOH/Na2SiO3) = 1 NaOH 4.45M | 0.3 | Step 1: 60°C—6 h | 7 | 10.51 | [177] |
| Step 2: room temp. | ||||||
| MSWI FA 15%, CFA 85% | NaOH 8M | 0.37 | Ambient temp. and RH | 28 | 15.69 | [178] |
| MSWI FA 5.5%, SF 9.2%, GBFS 39.9%, Sand 14.4%, Fiber 1.1% | NaOH 3.0%, Na2SiO3 13.6% | 0.5 | Step 1: 80 °C—2 h | 28 | 73.57 | [179] |
| Step 2: room temp. | ||||||
| Water-washed MSWI FA 100% | NaOH (10 M) and Na2SiO3 (0.8M) solutions | 0.21 | Ambient temp. and RH | 130 | 25.94 | [179] |
| CFA 88.5%, MSWI FA 10.5%, nano-SiO2 1.5% | Na2SiO3 43.5, adjust with naOH to 1.5 M. | 0.3 | Ambient temp. and RH | 28 | 57.19 | [170] |
| MSWI FA 60%, NSFA 40% | NaOH (10 M) | 0.5 | 20 °C RH 60% | 28 | 27.36 | [171] |
| MSWI FA 30%, RM 70% | Na2SiO3, solution with 7.91% Na2O, 23.72% SiO2 | 0.5 | Ambient temp. and RH | 28 | 9.95 MPa | [180,181] |
| CFA 21.3%, MSWI BA 5.3%, Sand 73% | NaOH 13%, Na2SiO4 13% | 0.65 | Step 1: 60°—48 h | 28 | 53.0 | [182] |
| Step 2: 60°—50% RH | ||||||
| MSWI FA 90% Metakaolin 10% | NaOH 9.04%, Na2SiO4 16.91% | 0.65 | 20 °C—RH 90% | 90 | 20.51 | [183] |
| MSWI FA 40% Metakaolin 60% | NaOH 9.2%, Na2SiO4 34% | 0.85 | 20° C—RH > 60% | 28 | 40 | [184] |
| Silica Characteristics of Raw Material | Extraction Conditions | |||||||
|---|---|---|---|---|---|---|---|---|
| Silica Source | SiO2 (%) a | LOI (%) | Average Particle Size (μm) | Alkaline Source | Molarity of Alkaline Solution (mol/L) | Temperature (°C) | Duration | Ref. |
| RHA and WG | 83.05 and 68.70 | 0.55 | <90 | NaOH pellets | NR b | 100 | 2 h | [228] |
| RHA | 93.49 | NR | NR | NaOH pellets | NR | 80 | 2 h | [229] |
| RHA | 90.5 | 3.8 | 6.82 | NaOH pellets | 1.0, 2.0, 3.2, 4.9, and 6.5 | Room temp., 60, 80, 90 and 100 | 1, 3, 5, 7, and 15 h | [225] |
| RHA | NR | NR | <45 μm | NaOH granules | 8, 10, and 12 | Room temp. | 40 min | [220] |
| RHA | 97.3 | NR | 9.87 | NaOH | NR | 100 | 1 h | [224] |
| RHA and silica fume | 68 c–95.51 | <2 | 9.2 and 64.1 | NaOH | NR | Room temp. | 10 min | [217] |
| RHA | 91.5 | 6 d | 25 and 32 | NaOH | 2, 4 and 6 | Room temp. | 15 min | [230] |
| RHA | 90.91 | 5.10 | 4.6 | NaOH | NR | Room temp. | 24 h | [231] |
| RHA | ~94 | 2.8–3.5 | 36.9–39.5 | NaOH | NR | Room temp. | NR | [232] |
| RHA | 89.51 | 6.95 | 20.4 | NaOH | NR | Room temp. and 90 | 30 min | [233] |
| RHA | 85.58 | 6.99 | 20.3 and 62.3 | NaOH | NR | 100 | 5–240 min | [234] |
| WG | 82.52 | 0.41 | <38 and 38–125 | NaOh pellets | NR | 550 | NR | [235,236] |
| WG | 69.9–71.4 | NR | <45, 45–90 and >125 | NaOH and NaOH/Na2CO3 | 4 | 22 and 80 | 10 min and 2, 4, and 6 h | [237] |
| RHA | 56.22 | 40.42 | 8 | NaAlO2 | N/A | Room temp. | N/A | [238] |
| RHA | 88.49 | 2.48 | 11.1 | NaAlO2 | N/A | Room temp. | N/A | [239] |
| RHA and microsilica | 88.46 and 94.25 | 9.22 and 2.31 | 23, 30, 172 and 199 | NaOH | 0.1, 1 and 10 | Room temp. | 4 h | [213] |
| WG | 72.37 | NR | <90 | NaOH flakes | 10 | 120 | 4 h and 24 h | [240] |
| WG | 71.51 | 0.27 | 12 | NaOH | NR | 150, 250, 330 and 450 | 1, 2, and 4 h | [215] |
| Nanosilica | NR | NR | NR | NaOH and KOH | NR | NR | NR | [241] |
| Inceneration bottom ash | 58.8 | 2.7 | >125 | NaOH | NR | 20, 75 and 90 | 24, 48, and 72 h | [218] |
| Silica, quartz | 99.9 and 98.6 | NR | 0.14 and 90 | KOH pellets | NR | Room temp. | NR | [242] |
| Country | Key Policy/Programme | Focus Area | Reference |
|---|---|---|---|
| The Netherlands | Transition Agenda for Circular Construction; Betonakkoord | Circular procurement; CO2 reduction targets; recycled materials. | [247] |
| Germany | FONA Programme; DIN 18998 (in progress) | Waste valorization; AAM standardization; sustainable cements. | [248] |
| France | RE2020; E+C- program | LCA-based regulation; prefabricated low-carbon elements. | [249] |
| Spain | Horizon projects: ReSHEALience | Durability in extreme environments; infrastructure applications. | [250] |
| Sweden | Klimatdeklaration Law | Carbon declarations for new buildings; public procurement incentives. | [251] |
| Denmark | Municipal carbon-based procurement initiatives | Eligibility thresholds for CO2 in public tenders. | [251] |
| United Kingdom | Part Z; BSI Flex 350; Future Homes Standard | Lifecycle carbon assessment; low-carbon standards; sustainable materials. | [252,253,254] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciotti, L.; Lucariello, D.; Perrotta, V.; Apicella, A.; Aversa, R. Sustainable Alkali-Activated and Geopolymer Materials: What Is the Future for Italy? Recycling 2025, 10, 140. https://doi.org/10.3390/recycling10040140
Ricciotti L, Lucariello D, Perrotta V, Apicella A, Aversa R. Sustainable Alkali-Activated and Geopolymer Materials: What Is the Future for Italy? Recycling. 2025; 10(4):140. https://doi.org/10.3390/recycling10040140
Chicago/Turabian StyleRicciotti, Laura, Daniele Lucariello, Valeria Perrotta, Antonio Apicella, and Raffaella Aversa. 2025. "Sustainable Alkali-Activated and Geopolymer Materials: What Is the Future for Italy?" Recycling 10, no. 4: 140. https://doi.org/10.3390/recycling10040140
APA StyleRicciotti, L., Lucariello, D., Perrotta, V., Apicella, A., & Aversa, R. (2025). Sustainable Alkali-Activated and Geopolymer Materials: What Is the Future for Italy? Recycling, 10(4), 140. https://doi.org/10.3390/recycling10040140









