Selective Ammonium Recovery from Livestock and Organic Solid Waste Digestates Using Zeolite Tuff: Efficiency and Farm-Scale Prospects
Abstract
1. Introduction
- (i)
- Faster NH4+ adsorption kinetics in digestates with a higher initial NH4+ concentration.
- (ii)
- A negative effect of total solids content and other ions present in the digestates on the NH4+ adsorption process.
- (iii)
- A greater reduction in NH4+ in digestates that have undergone preliminary treatment processes, such as clarification and microfiltration.
- (iv)
- Higher daily nitrogen recovery in digestates with a higher initial NH4+ concentration.
2. Materials and Methods
2.1. The Digestate
2.2. The Zeolitic Tuff
2.3. Analytical Techniques
2.4. Experimental Setup
2.4.1. NH4+-N Adsorption Isotherm
2.4.2. NH4+-N Adsorption Kinetics
3. Results and Discussions
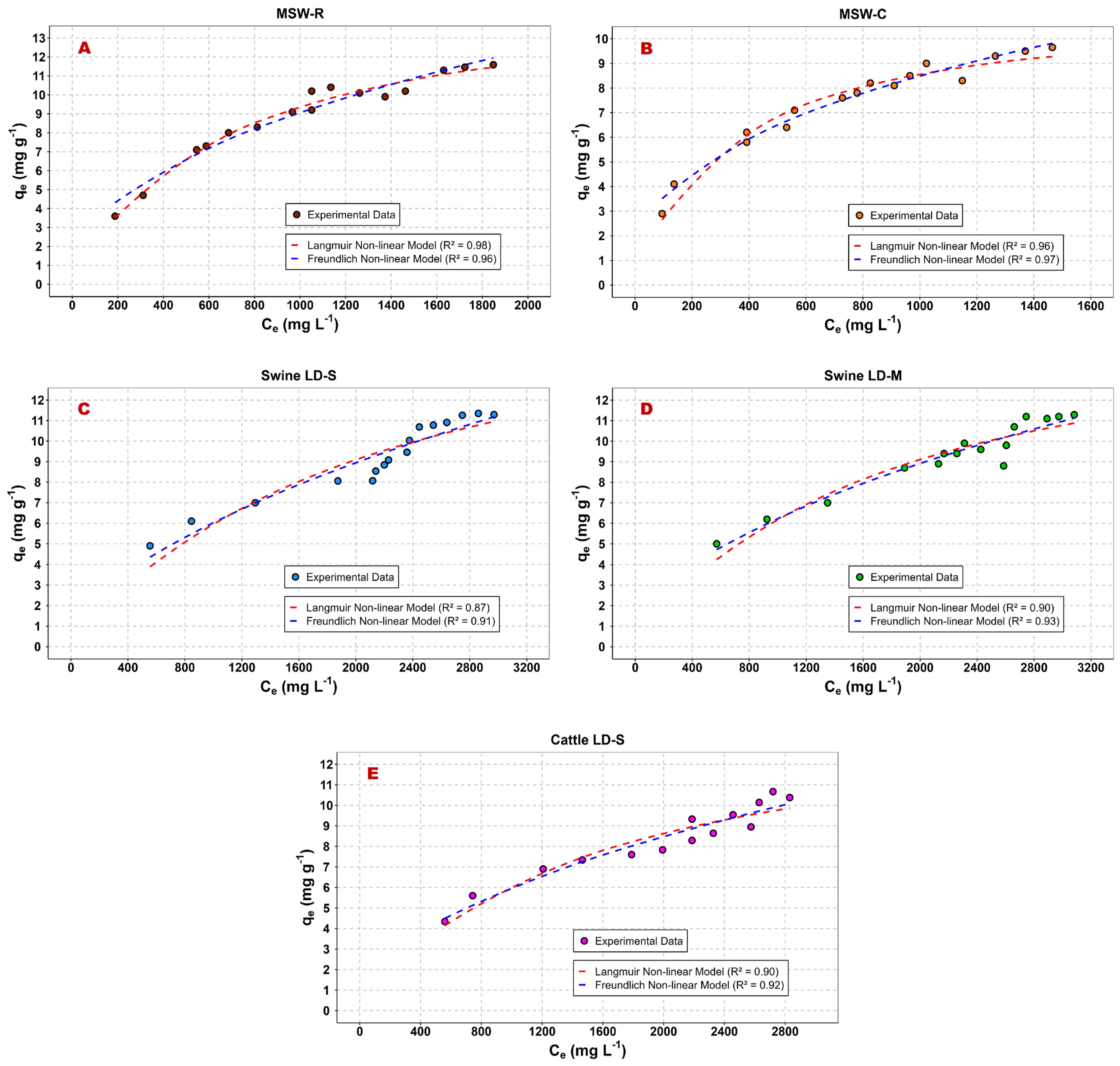
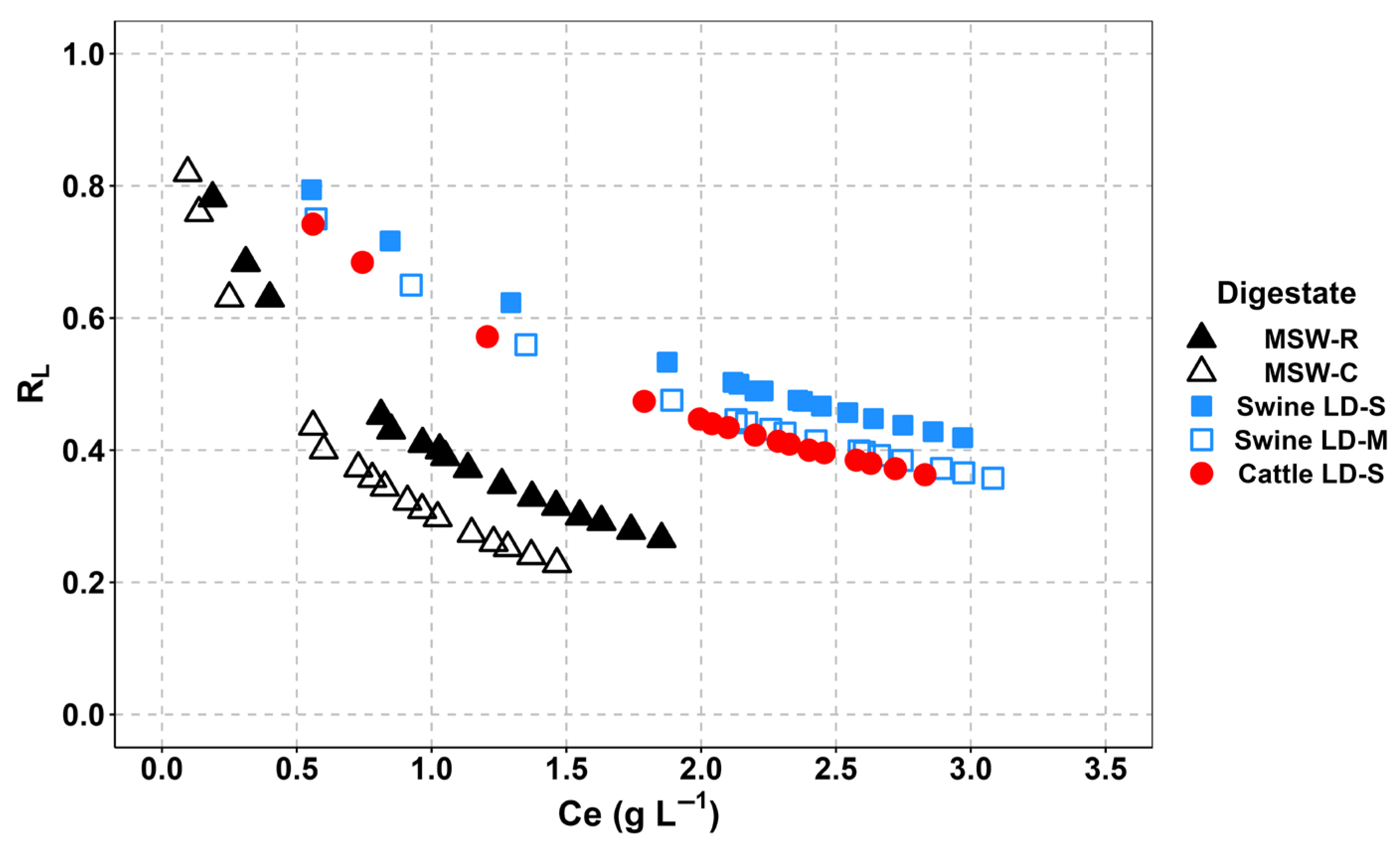
3.1. Equilibrium Adsorption Isotherms
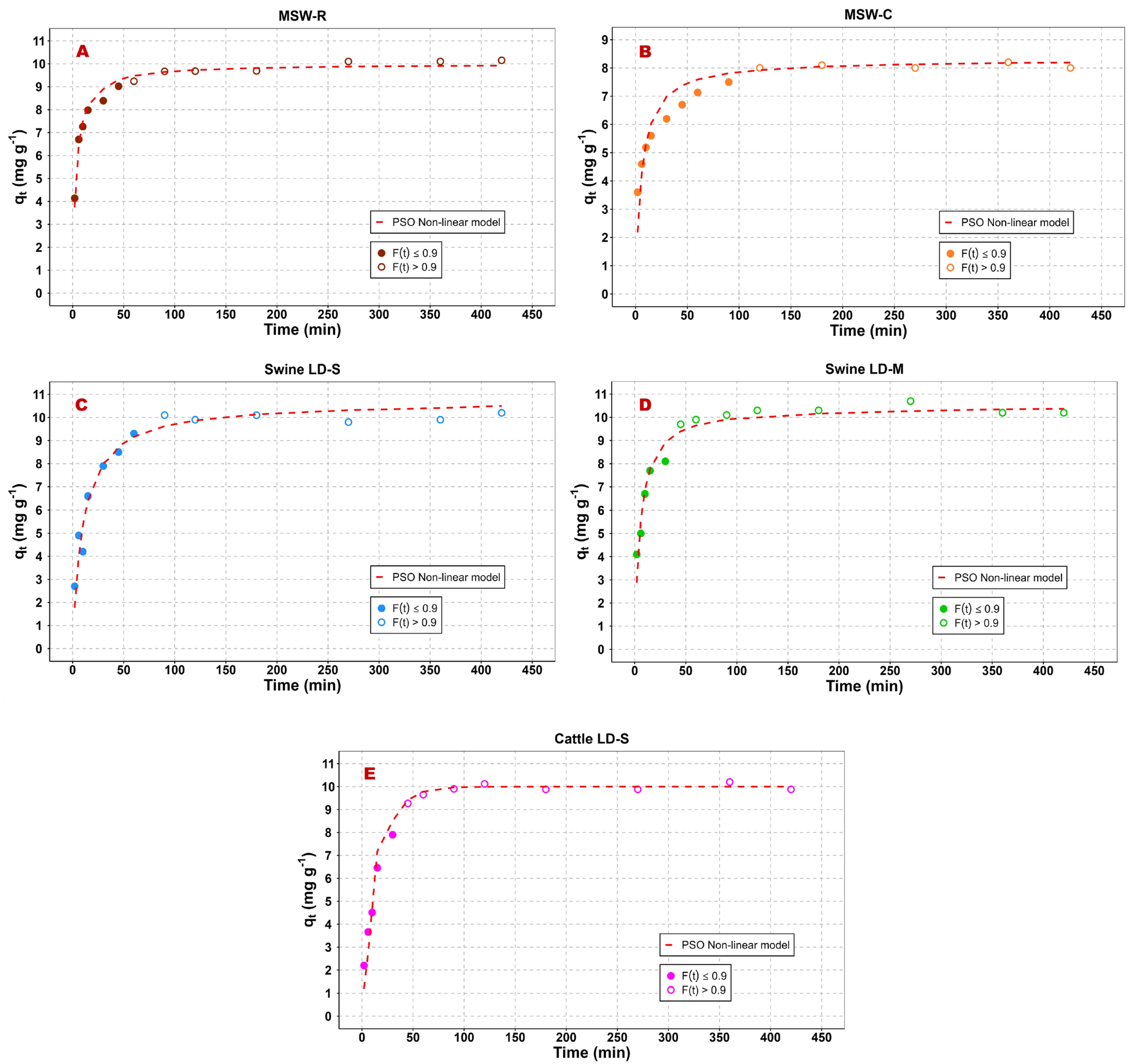
3.2. Adsorption Kinetics
3.3. Parameters Affecting Ammonium Adsorption
3.3.1. Mineralogical Composition
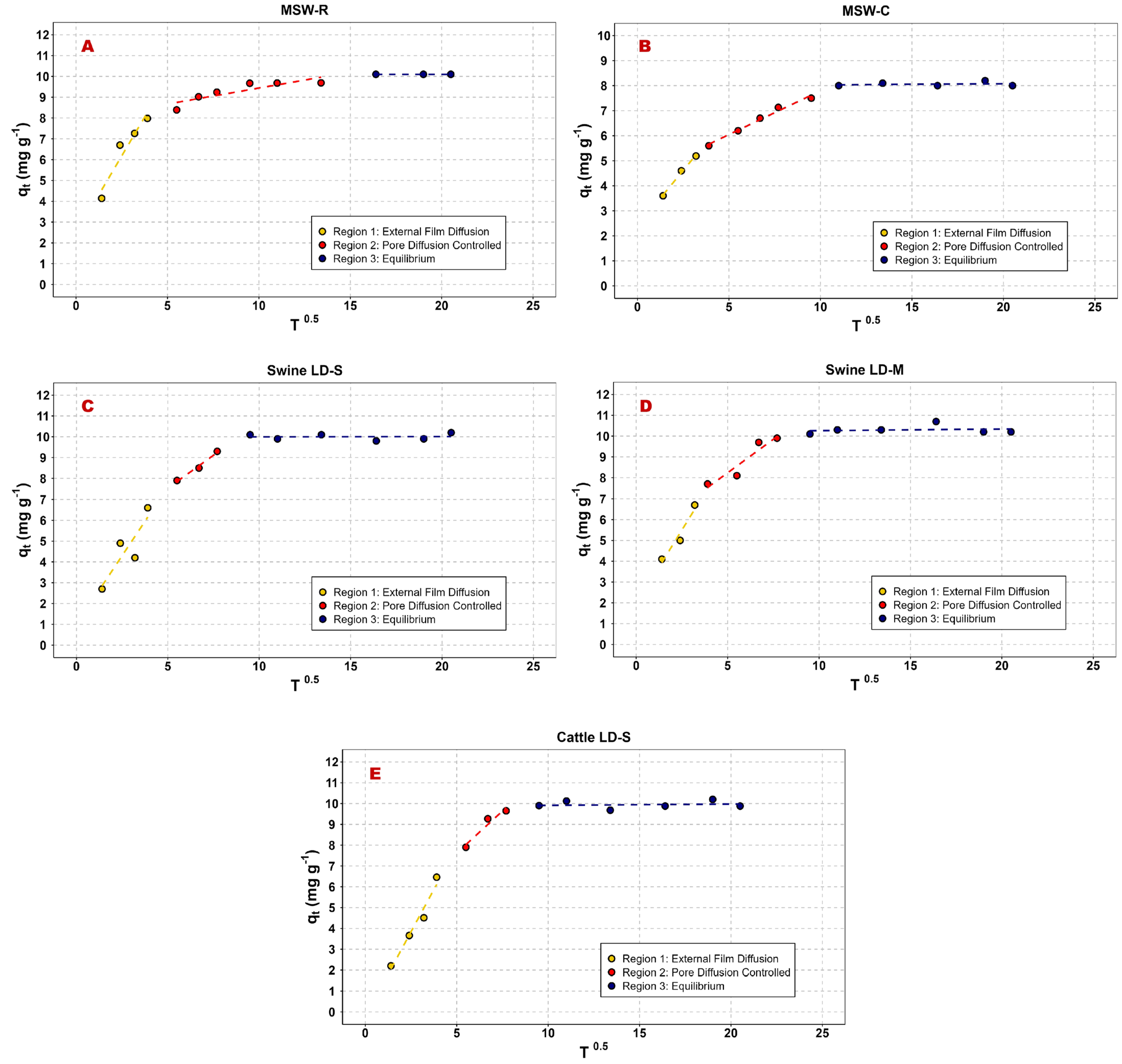
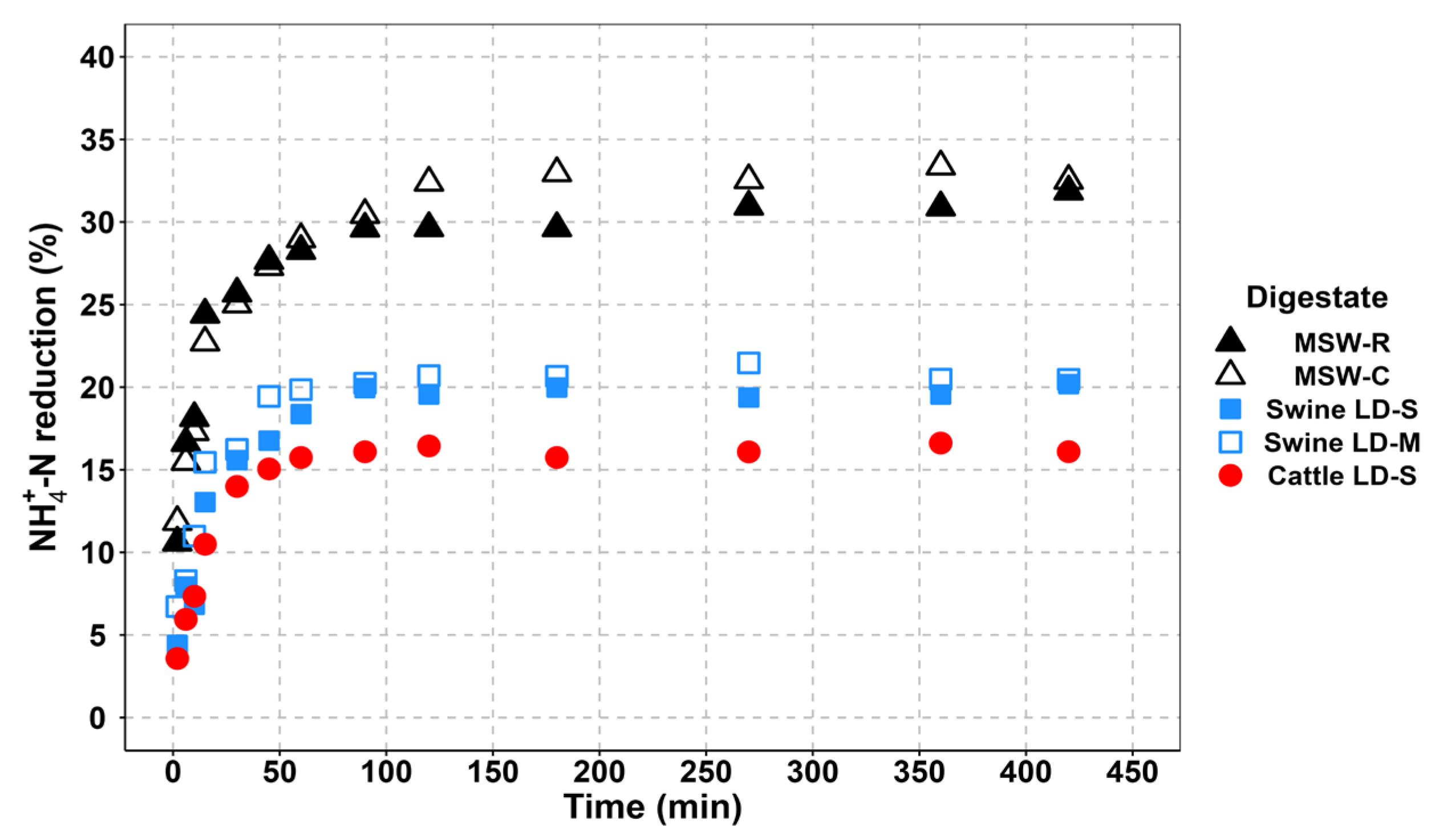
3.3.2. Contact Time
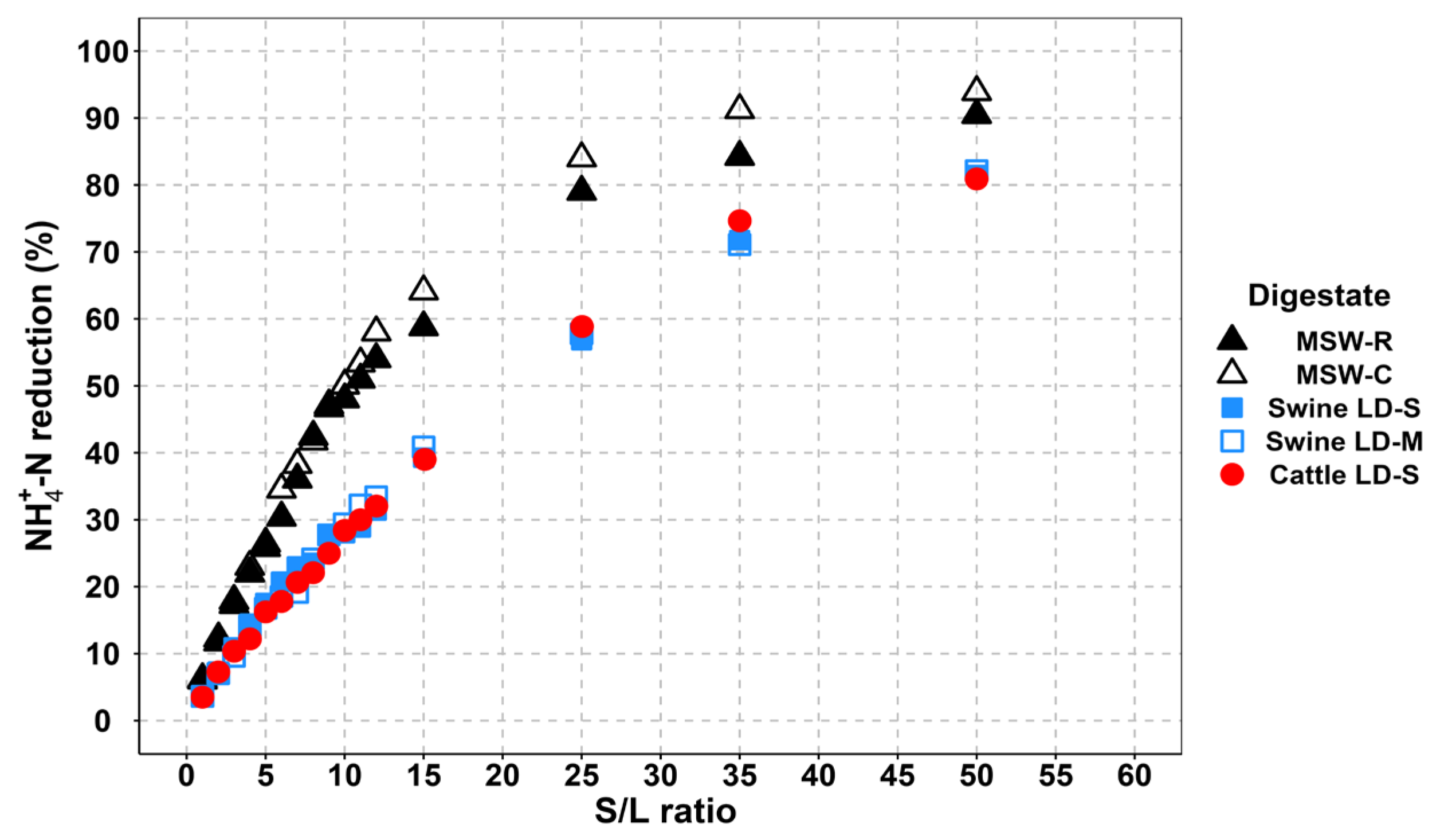
3.3.3. Solid–Liquid Ratio
3.3.4. Digestate Composition: NH4+ Initial Concentration, Total Solids Content, and Competing Ions
3.3.5. Effects of Digestate Pre-Treatments
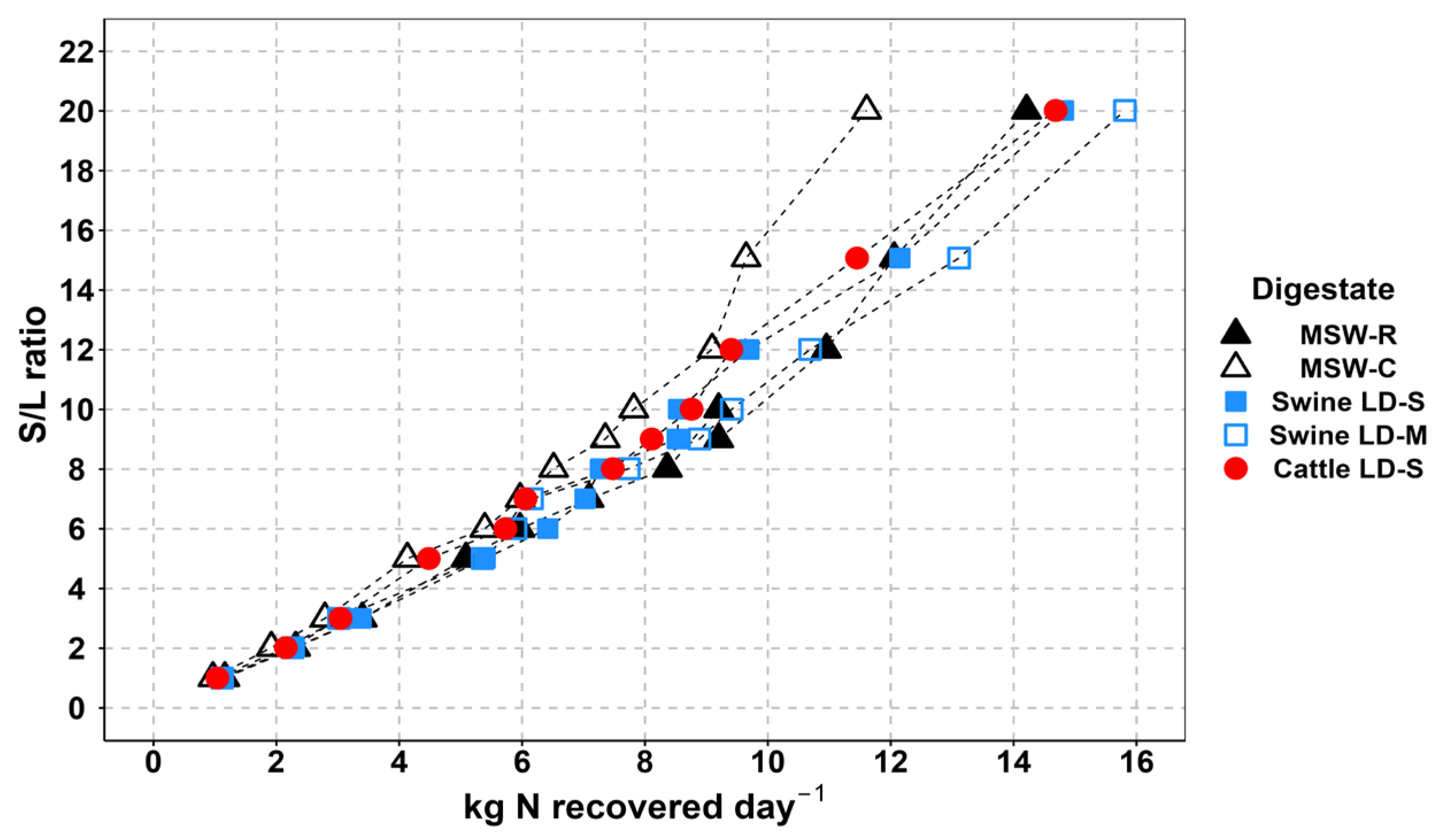
3.4. Estimation of Farm-Scale Daily Nitrogen Recovery Rates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. How to Feed the World in 2050. In Proceedings of the Expert Meeting on How to Feed the World in 2050, Rome, Italy, 24–26 June 2009. [Google Scholar]
- Jadhav, D.A.; Ghosh Ray, S.; Ghangrekar, M.M. Third generation in bio-electrochemical system research—A systematic review on mechanisms for recovery of valuable by-products from wastewater. Renew. Sustain. Energy Rev. 2017, 76, 1022–1031. [Google Scholar] [CrossRef]
- Kim, D.; Min, K.J.; Lee, K.; Yu, M.S.; Park, K.Y. Effects of pH, molar ratios and pre-treatment on phosphorus recovery through struvite crystallization from effluent of anaerobically digested swine wastewater. Environ. Eng. Res. 2017, 22, 12–18. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Rosinger, C.; Huber, S.; Mentler, A.; Pines, E.D.; Faccini, B.; Keiblinger, K.M. Potential for agricultural recycling of struvite and zeolites to improve soil microbial physiology and mitigate CO2 emissions. Geoderma 2025, 453, 117149. [Google Scholar] [CrossRef]
- Kim, T.; Nam, Y.; Lee, M. Nitrogen and Phosphorus Removal from Livestock Wastewater by Zeolite Ion Exchange and Ionizing Radiation. World Acad. Sci. Eng. Technol. 2009, 54, 9–13. [Google Scholar]
- Longhurst, R.D.; Roberts, A.H.C.; O’Connor, M.B. Farm dairy effluent: A review of published data on chemical and physical characteristics in New Zealand. N. Z. J. Agric. Res. 2000, 43, 7–14. [Google Scholar] [CrossRef]
- Tambone, F.; Orzi, V.; Zilio, M.; Adani, F. Measuring the organic amendment properties of the liquid fraction of digestate. Waste Manag. 2019, 88, 21–27. [Google Scholar] [CrossRef]
- Makdi, M.; Tomcsik, A.; Orosz, V. Digestate: A New Nutrient Source—Review. In Biogas; InTech: Vienna, Austria, 2012. [Google Scholar] [CrossRef]
- Ferretti, G.; Galamini, G.; Medoro, V.; Coltorti, M.; Di Giuseppe, D.; Faccini, B. Impact of Sequential Treatments with Natural and Na-Exchanged Chabazite Zeolite-Rich Tuff on Pig-Slurry Chemical Composition. Water 2020, 12, 310. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Rossi, G.; Beni, C.; Benedetti, A.; Felici, B.; Neri, U. Effect of Mineral or OFMSW Digestate Fertilization on Ryegrass and Nitrogen Leaching. Agronomy 2023, 13, 1316. [Google Scholar] [CrossRef]
- Hickey, C.W.; Quinn, J.M.; Davies-Colley, R.J. Effluent Characteristics of Dairy Shed Oxidation Ponds and Their Potential Impacts on Rivers. N. Z. J. Mar. Freshw. Res. 1989, 23, 569–584. [Google Scholar] [CrossRef]
- Hooda, P.S.; Edwards, A.C.; Anderson, H.A.; Miller, A. A Review of Water Quality Concerns in Livestock Farming Areas. Sci. Total Environ. 2000, 250, 143–167. [Google Scholar] [CrossRef]
- Jongbloed, A.W.; Lenis, N.P. Environmental Concerns about Animal Manure. J. Anim. Sci. 1998, 76, 2641–2648. [Google Scholar] [CrossRef]
- Martinez, J.; Guiziou, F.; Peu, P.; Gueutier, V. Influence of Treatment Techniques for Pig Slurry on Methane Emissions during Subsequent Storage. Biosyst. Eng. 2003, 85, 347–354. [Google Scholar] [CrossRef]
- Nhubu, T.; Mbohwa, C.; Muzenda, E. Eutrophication Impact Potential of Solid Waste Management Options in Harare. In Wastes: Solutions, Treatments and Opportunities III, Proceedings of the 5th International Conference Wastes 2019, Lisbon, Portugal, 4–6 September 2019; CRC Press: London, UK, 2019; pp. 360–366. [Google Scholar] [CrossRef]
- Policastro, G.; Cesaro, A. Composting of Organic Solid Waste of Municipal Origin: The Role of Research in Enhancing Its Sustainability. Int. J. Environ. Res. Public Health 2023, 20, 1312. [Google Scholar] [CrossRef]
- Ross, C.; Donnison, A. Campylobacter and Farm Dairy Effluent Irrigation. N. Z. J. Agric. Res. 2003, 46, 255–262. [Google Scholar] [CrossRef]
- Martinez, J.; Dabert, P.; Barrington, S.; Burton, C. Livestock Waste Treatment Systems for Environmental Quality, Food Safety, and Sustainability. Bioresour. Technol. 2009, 100, 5527–5536. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Medoro, V.; Tescaro, N.; Faccini, B. Isotherms, Kinetics, and Thermodynamics of NH4+ Adsorption in Raw Liquid Manure by Using Natural Chabazite Zeolite-Rich Tuff. Water 2020, 12, 2944. [Google Scholar] [CrossRef]
- European Commission. Farm to Fork Strategy; DG SANTE/Unit Food Inf. Compos. Food Waste. 2020. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 22 March 2025).
- Faccini, B.; Di Giuseppe, D.; Ferretti, G.; Coltorti, M.; Colombani, N.; Mastrocicco, M. Natural and NH4+-Enriched Zeolitite Amendment Effects on Nitrate Leaching from a Reclaimed Agricultural Soil (Ferrara Province, Italy). Nutr. Cycl. Agroecosyst. 2018, 110, 327–341. [Google Scholar] [CrossRef]
- Damodara, A.K.; Parameswaran, P. Ammonia Adsorption and Recovery from Swine Wastewater Permeate Using Naturally Occurring Clinoptilolite. J. Water Process Eng. 2021, 43, 102234. [Google Scholar] [CrossRef]
- Malferrari, D.; Laurora, A.; Brigatti, M.F.; Coltorti, M.; Di Giuseppe, D.; Faccini, B.; Passaglia, E.; Vezzalini, M.G. Open-field experimentation of an innovative and integrated zeolitite cycle: Project definition and material characterization. Rend. Lincei 2013, 24, 141–150. [Google Scholar] [CrossRef]
- Faccini, B. ; ZeoLIFE. Water Pollution Reduction and Water Saving Using a Natural Zeolitite Cycle; Aracne: Canterano, Italy, 2018. [Google Scholar]
- Ferretti, G.; Faccini, B.; Antisari, L.V.; Di Giuseppe, D.; Coltorti, M. 15N Natural Abundance, Nitrogen and Carbon Pools in Soil-Sorghum System Amended with Natural and NH4+-Enriched Zeolitites. Appl. Sci. 2019, 9, 4524. [Google Scholar] [CrossRef]
- Ali, S.; Nakhli, A.; Delkash, M. Application of Zeolites for Sustainable Agriculture: A Review on Water and Nutrient Retention. Water Air Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Ferretti, G.; Faccini, B.; Blasi, E.; Passeri, N.; Bianchini, G.; Coltorti, M. Is it possible to cultivate corn in a sustainable way using a quarry waste? Period. Mineral. 2016, 85, 179–183. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of Natural Zeolites on Agriculture and Food Production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Ferretti, G.; Rosinger, C.; Diaz-Pines, E.; Faccini, B.; Coltorti, M.; Keiblinger, K.M. Soil Quality Increases with Long-Term Chabazite-Zeolite Tuff Amendments in Arable and Perennial Cropping Systems. J. Environ. Manag. 2024, 354, 120303. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Rosinger, C.; Huber, S.; Medoro, V.; Mentler, A.; Díaz-Pinés, E.; Gorfer, M.; Faccini, B.; Keiblinger, K.M. Recycling nitrogen from liquid digestate via novel reactive struvite and zeolite minerals to mitigate agricultural pollution. Chemosphere 2023, 317, 137881. [Google Scholar] [CrossRef]
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The Use of Zeolites as an Addition to Fertilisers—A Review. Catena 2022, 213, 106125. [Google Scholar] [CrossRef]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended Nomenclature for Zeolite Minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Mineral. Mag. 1998, 62, 533–571. [Google Scholar] [CrossRef]
- Dyer, A.; Zubair, M. Ion-Exchange in Chabazite. Microporous Mesoporous Mater. 1998, 22, 135–150. [Google Scholar] [CrossRef]
- Chen, H.F.; Lin, Y.J.; Chen, B.H.; Yoshiyuki, I.; Liou, S.Y.H.; Huang, R.T. A Further Investigation of NH4+ Removal Mechanisms by Using Natural and Synthetic Zeolites in Different Concentrations and Temperatures. Minerals 2018, 8, 499. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Yan, B.; Yang, L. Ammonium Removal from Aqueous Solutions by Using Natural Chinese (Chende) Zeolite as Adsorbent. J. Hazard. Mater. 2010, 175, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Tanner, C.C. Ammonium Removal from Wastewaters Using Natural New Zealand Zeolites. N. Z. J. Agric. Res. 1998, 41, 427–446. [Google Scholar] [CrossRef]
- Wasielewski, S.; Rott, E.; Minke, R.; Steinmetz, H. Evaluation of Different Clinoptilolite Zeolites as Adsorbent for Ammonium Removal from Highly Concentrated Synthetic Wastewater. Water 2018, 10, 584. [Google Scholar] [CrossRef]
- Young, H.D. Statistical Treatment of Experimental Data; McGraw-Hill B. Company, Inc.: New York, NY, USA, 1962. [Google Scholar]
- Gualtieri, A.F.; Gatta, G.D.; Arletti, R.; Artioli, G.; Ballirano, P.; Cruciani, G.; Guagliardi, A.; Malferrari, D.; Masciocchi, N.; Scardi, P. Quantitative Phase Analysis Using the Rietveld Method: Towards a Procedure for Checking the Reliability and Quality of the Results. Period. Mineral. 2019, 88, 147–151. [Google Scholar] [CrossRef]
- Detho, A.; Memon, A.A.; Memon, A.H.; Almohana, A.I.; Daud, Z.; Rosli, M.A. Sorption Kinetics, Isotherm Studies and Mechanism of Removal of Organic and Inorganic by Adsorption onto Renewable Biomineral. Water Air Soil Pollut. 2023, 234, 22. [Google Scholar] [CrossRef]
- Guo, X.; Zeng, L.; Li, X.; Park, H.S. Ammonium and Potassium Removal for Anaerobically Digested Wastewater Using Natural Clinoptilolite Followed by Membrane Pretreatment. J. Hazard. Mater. 2008, 151, 125–133. [Google Scholar] [CrossRef]
- Eberle, S.; Börnick, H.; Stolte, S. Granular natural zeolites: Cost-effective adsorbents for the removal of ammonium from drinking water. Water 2022, 14, 939. [Google Scholar] [CrossRef]
- Halim, A.A.; Aziz, H.A.; Johari, M.A.M.; Ariffin, K.S. Comparison study of ammonia and COD adsorption on zeolite, activated carbon and composite materials in landfill leachate treatment. Desalination 2010, 262, 31–35. [Google Scholar] [CrossRef]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Zeolite as a potential medium for ammonium recovery and second cheese whey treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.J.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef]
- Taddeo, R.; Prajapati, S.; Lepistö, R. Optimizing ammonium adsorption on natural zeolite for wastewaters with high loads of ammonium and solids. J. Porous Mater. 2017, 24, 1545–1554. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, H.M.; Zhang, D. Removal of ammonium from greywater using natural zeolite. Desalination 2011, 277, 15–23. [Google Scholar] [CrossRef]
- Zhou, H.D.; Wang, C.Y.; Wang, Q.; Xu, B.X.; Zhu, G. Efficiency, mechanism and application prospect of ammonium adsorption and desorption over a sodium-acetate-modified synthetic zeolite. RSC Adv. 2024, 14, 17843–17854. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language Environment for Statistical Computing, Version 2023-3.0-386; The R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 22 March 2025).
- Deocaris, C.C.; De Osio, L.P. Fitting Henry’s Adsorption Isotherm Model in R Using PUPAIM Package. Asia Pac. J. Res. 2020, 1, 47–51. [Google Scholar]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Karaçetin, G.; Sivrikaya, S.; Imamoğlu, M. Adsorption of methylene blue from aqueous solutions by activated carbon prepared from hazelnut husk using zinc chloride. J. Anal. Appl. Pyrolysis 2014, 110, 270–276. [Google Scholar] [CrossRef]
- Jha, V.K.; Hayashi, S. Modification on natural clinoptilolite zeolite for its NH4+ retention capacity. J. Hazard. Mater. 2009, 169, 29–35. [Google Scholar] [CrossRef]
- Khan, A.A.; Singh, R.P. Adsorption thermodynamics of carbofuran on Sn (IV) arsenosilicate in H+, Na+ and Ca2+ forms. Colloids Surf. 1987, 24, 33–42. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Magalong, J.R.; DelaCruz, J.; Bumatay, J.; Deocaris, C. PUPAK: Parameter Estimation, and Plot Visualization of Adsorption Kinetic Models. 2022. Available online: https://cran.r-project.org/web/packages/PUPAK/index.html (accessed on 22 March 2025).
- Lagergen, S. About the Theory of So-Called Adsorption of Soluble Substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Weber, J.; Walter, J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar]
- Cheng, Z.; Ding, W. Ammonium removal from water by natural and modified zeolite: Kinetic, equilibrium, and thermodynamic studies. Desalin. Water Treat. 2015, 55, 978–985. [Google Scholar] [CrossRef]
- Tang, H.; Xu, X.; Wang, B.; Lv, C.; Shi, D. Removal of Ammonium from Swine Wastewater Using Synthesized Zeolite from Fly Ash. Sustainability 2020, 12, 3423. [Google Scholar] [CrossRef]
- Haghseresht, F.; Lu, G.Q. Adsorption characteristics of phenolic compounds onto coal-reject-derived adsorbents. Energy Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Huang, H.; Yang, L.; Xue, Q.; Liu, J.; Hou, L.; Ding, L. Removal of ammonium from swine wastewater by zeolite combined with chlorination for regeneration. J. Environ. Manag. 2015, 160, 333–341. [Google Scholar] [CrossRef]
- Ivanova, E.; Karsheva, M.; Koumanova, B. Adsorption of ammonium ions onto natural zeolite. J. Univ. Chem. Technol. Metall. 2010, 45, 295–302. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Moussavi, G.; Talebi, S.; Farrokhi, M.; Sabouti, R.M. The investigation of mechanism, kinetic and isotherm of ammonia and humic acid co-adsorption onto natural zeolite. Chem. Eng. J. 2011, 171, 1159–1169. [Google Scholar] [CrossRef]
- Shaban, M.; AbuKhadra, M.R.; Nasief, F.M.; Abd El-Salam, H.M. Removal of ammonia from aqueous solutions, groundwater, and wastewater using mechanically activated clinoptilolite and synthetic zeolite-A: Kinetic and equilibrium studies. Water Air Soil Pollut. 2017, 228, 11. [Google Scholar] [CrossRef]
- Leggo, P.J.; Cochemé, J.-J.; Demant, A.; Lee, W.T. The role of argillic alteration in the zeolitization of volcanic glass. Mineral. Mag. 2001, 65, 653–663. [Google Scholar]
- Holder, C.F.; Schaak, R.E. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Demir, A.; Günay, A.; Debik, E. Ammonium removal from aqueous solution by ion-exchange using packed bed natural zeolite. Water SA 2002, 28, 329–335. [Google Scholar] [CrossRef]
- Farkaš, A.; Rožić, M.; Barbarić-Mikočević, Ž. Ammonium exchange in leakage waters of waste dumps using natural zeolite from the Krapina region, Croatia. J. Hazard. Mater. 2005, 117, 25–33. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Passaglia, E.; Galli, E. Ion exchange selectivity of phillipsite. Stud. Surf. Sci. Catal. 2002, 142, 1705–1712. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Z.; Vogt, T.; Lee, Y. Chabazite structures with Li+, Na+, Ag+, K+, NH4+, Rb and Cs as extra-framework cations. Microporous Mesoporous Mater. 2016, 221, 253–263. [Google Scholar] [CrossRef]
- Proskynitopoulou, V.; Garagounis, I.; Vourros, A.; Dimopoulos, P.; Lorentzou, S.; Zouboulis, A. Nutrient recovery from digestate: Pilot test experiments. J. Environ. Manag. 2024, 353, 120166. [Google Scholar] [CrossRef]
- Dal Verme, M.; Lipari, D.; Lucido, G.; Maio, V.; Surace, V.; Liberatore, P. Statistical Report 2021: Renewable Energy Sources in Italy; GSE—Gestore dei Servizi Energetici: Rome, Italy, 2021. [Google Scholar]
- Exploring Digestate’s Contribution to Healthy Soils; European Biogas Association: Brussels, Belgium, 2022.
| MSW | Pig Slurry | Cattle Slurry | ||||
|---|---|---|---|---|---|---|
| Raw | Clarified | Separated | Microfiltered | Separated | ||
| pH | 7.89 ± 0.05 | 8.66 ± 0.00 | 7.62 ± 0.01 | 7.77 ± 0.00 | 7.75 ± 0.01 | |
| EC | mS⋅cm−1 | 25.8 ± 0.4 | 21.7 ± 0.5 | 27.4 ± 1.5 | 27.2 ± 0.7 | 35.8 ± 1.5 |
| TS | g⋅L−1 | 14.1 ± 2.0 | 8.3 ± 0.1 | 32 ± 0.2 | 28.6 ± 0.4 | 31.6 ± 1.9 |
| TKN | mg⋅L−1 | 2514 ± 12 | 1812 ± 22 | 4353 ± 190 | 4100 ± 117 | 3950 ± 79 |
| NH4+-N | mg⋅L−1 | 2031 ± 24 | 1681 ± 37 | 3085 ± 156 | 2993 ± 150 | 3000 ± 91 |
| Na+ | mg⋅L−1 | 1474 ± 90 | 2010 ± 47 | 557 ± 36 | 558 ± 32 | 285 ± 41 |
| Mg2+ | mg⋅L−1 | 188 ± 51 | 102 ±7 | 275 ± 34 | 161 ± 1 | 129 ± 12 |
| K+ | mg⋅L−1 | 1799 ± 125 | 1587 ± 32 | 2044 ± 104 | 2115 ± 81 | 4326 ± 328 |
| Ca2+ | mg⋅L−1 | 1414 ± 412 | 77.4 ± 19.7 | 720 ± 41 | 725 ± 24 | 293 ± 47 |
| Chemical Composition | QPA | CEC | ||
|---|---|---|---|---|
| % | % | cmol+·kg−1 | ||
| SiO2 | 56.79 | Chabazite | 19.0 (3) | 233 |
| TiO2 | 0.42 | Phillipsite | 10.0 (2) | |
| Al2O3 | 16.50 | Mica/Illite/Biotite | 0.7 (2) | |
| Fe2O3 | 3.00 | Sanidine | 13.6 (2) | |
| MnO | 0.14 | Pyroxene | 2.5 (2) | |
| MgO | 0.98 | Quartz | 2.7 (7) | |
| CaO | 4.69 | Plagioclase | 7.0 (1) | |
| Na2O | 1.25 | Other | 44.3 (5) | |
| K2O | 6.21 | |||
| P2O5 | 0.15 | TZC | 29.0 | |
| LOI | 9.86 | |||
| Freundlich | Langmuir | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | R2 | p-Value | KF | n | AIC | R2 | p-Value | qmax | KL | AIC | |
| [K] | [-] | [-] | [mg⋅g−1⋅ (L⋅g−1)1/n] | [-] | [-] | [-] | [-] | [mg⋅g−1] | [L⋅g−1] | [-] | |
| MSW-R | 298.15 | 0.96 | 4.58 × 10−13 | 9.06 | 2.21 | 26.6 | 0.98 | 6.05 × 10−13 | 15.69 | 1.4727 | 16.5 |
| MSW-C | 298.15 | 0.97 | 1.50 × 10−12 | 8.47 | 2.59 | 12.7 | 0.96 | 1.49 × 10−11 | 11.42 | 2.971 | 18.9 |
| Swine LD-S | 298.15 | 0.91 | 9.34 × 10−9 | 6.06 | 1.78 | 33.3 | 0.87 | 1.04 × 10−7 | 18.83 | 0.4673 | 39.5 |
| Swine LD-M | 298.15 | 0.93 | 1.28 × 10−9 | 6.26 | 1.96 | 26.8 | 0.90 | 1.17 × 10−8 | 16.93 | 0.5831 | 32.2 |
| Cattle LD-S | 298.15 | 0.92 | 2.94 × 10−8 | 5.98 | 1.99 | 24.4 | 0.90 | 1.17 × 10−7 | 15.05 | 0.6711 | 28.7 |
| MSW-R | MSW-C | Swine LD-S | Swine LD-M | Cattle LD-S | ||
|---|---|---|---|---|---|---|
| Ke | [-] | 14.8 | 17 | 8 | 8.6 | 7.7 |
| ΔG | [J⋅mol−1] | −6687 | −7024 | −5290 | −5325 | −5075 |
| MODEL | RMSE | MSE | AIC | BIC | K1 | K2 | |
|---|---|---|---|---|---|---|---|
| [-] | [-] | [-] | [-] | [-] | [min−1] | [g·mg−1·min−1] | |
| MSW-R | PFO | 1.1 | 1.2 | 22.1 | 21.7 | 0.159 | |
| PSO | 0.4 | 0.1 | 8.5 | 8.1 | 0.030 | ||
| MSW-C | PFO | 1.4 | 2.0 | 28.7 | 28.6 | 0.071 | |
| PSO | 1.0 | 1.0 | 23.6 | 23.5 | 0.017 | ||
| Swine LD-S | PFO | 1.2 | 1.4 | 26.2 | 26.1 | 0.056 | |
| PSO | 0.7 | 0.4 | 18.1 | 18.0 | 0.009 | ||
| Swine LD-M | PFO | 1.2 | 1.5 | 23.5 | 23.1 | 0.100 | |
| PSO | 0.7 | 0.4 | 16.1 | 15.7 | 0.018 | ||
| Cattle LD-S | PFO | 0.7 | 0.5 | 14.7 | 13.9 | 0.064 | |
| PSO | 0.6 | 0.3 | 12.8 | 12.0 | 0.011 |
| ID Model | ||||
|---|---|---|---|---|
| R2 | KID | C | ||
| [-] | [mg·g−1·min−0.5] | [mg·g−1] | ||
| MSW-R | Region 1 | 0.88 | 1.83 | 1.74 |
| Region 2 | 0.79 | 0.19 | 7.54 | |
| MSW-C | Region 1 | 1.00 | 0.91 | 2.32 |
| Region 2 | 0.98 | 0.35 | 4.31 | |
| Swine LD-S | Region 1 | 0.69 | 1.37 | 0.86 |
| Region 2 | 0.99 | 0.56 | 4.82 | |
| Swine LD-M | Region 1 | 0.85 | 1.47 | 1.84 |
| Region 2 | 0.85 | 0.64 | 5.05 | |
| Cattle LD-S | Region 1 | 0.95 | 1.67 | 0.33 |
| Region 2 | 0.86 | 0.78 | 3.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberghini, M.; Ferretti, G.; Galamini, G.; Botezatu, C.; Faccini, B. Selective Ammonium Recovery from Livestock and Organic Solid Waste Digestates Using Zeolite Tuff: Efficiency and Farm-Scale Prospects. Recycling 2025, 10, 137. https://doi.org/10.3390/recycling10040137
Alberghini M, Ferretti G, Galamini G, Botezatu C, Faccini B. Selective Ammonium Recovery from Livestock and Organic Solid Waste Digestates Using Zeolite Tuff: Efficiency and Farm-Scale Prospects. Recycling. 2025; 10(4):137. https://doi.org/10.3390/recycling10040137
Chicago/Turabian StyleAlberghini, Matteo, Giacomo Ferretti, Giulio Galamini, Cristina Botezatu, and Barbara Faccini. 2025. "Selective Ammonium Recovery from Livestock and Organic Solid Waste Digestates Using Zeolite Tuff: Efficiency and Farm-Scale Prospects" Recycling 10, no. 4: 137. https://doi.org/10.3390/recycling10040137
APA StyleAlberghini, M., Ferretti, G., Galamini, G., Botezatu, C., & Faccini, B. (2025). Selective Ammonium Recovery from Livestock and Organic Solid Waste Digestates Using Zeolite Tuff: Efficiency and Farm-Scale Prospects. Recycling, 10(4), 137. https://doi.org/10.3390/recycling10040137







