Abstract
A previously pyrometallurgical process, developed to obtain a Pb-Ag alloy and a slag rich in sulfur from the recycling of a mixture of industrial wastes of jarosite and lead paste, was thermodynamically assessed at 1200 °C. The industrial jarosite sourced from a Mexican zinc hydrometallurgical plant corresponded to an ammonium jarosite with a measurable silver content. The specific heat capacity (Cp) of the ammonium jarosite was obtained from TGA and DSC measurements, as well as the thermodynamic functions of enthalpy, entropy, and Gibbs free energy. The Cp was successfully modeled using polynomial regression, with a second-degree polynomial employed to describe the low-temperature behavior. The thermodynamic data generated were input into the thermodynamic software FactSage 8.2 for modeling of the lead paste–ammonium jarosite-Na2CO3-SiC system and represented by stability phase diagrams. The thermodynamic assessment of the pyrometallurgical process predicted compounds formed at high temperatures, showing that a Pb-Ag alloy and a slag rich in Na, S, and Fe (NaFeS2 and NaFeO2) were obtained. The compounds formed evidence of the effective sulfur retention in the slag, which is crucial for mitigating SO2 emissions during high-temperature treatments. The experimental compounds, after solidification, were determined by X-ray diffraction measurements to be Na2Fe(SO4)2 and Na2(SO4), which reasonably match the thermodynamic assessment. The heat capacity of the ammonium jarosite provides essential thermodynamic insights into the compositional complexities of industrial waste, which are particularly relevant for thermodynamic modeling and process optimization in pyrometallurgical systems aimed at metal recovery and residue valorization.
1. Introduction
The increasing generation of industrial metallurgical wastes, such as jarosite residues from the zinc hydrometallurgy process and lead paste from spent lead-acid batteries, presents serious environmental and economic challenges. These by-products, if improperly managed, can lead to heavy metal leaching (e.g., As, Pb, and Cd) and air pollution due to sulfur emissions [1]. However, they also contain valuable elements, such as silver, lead, and zinc, whose recovery could support the principles of a circular economy while mitigating environmental damage. Recent studies highlight that toxic elements in jarosites remain encapsulated even at high temperatures (e.g., higher than 1000 °C), posing long-term risks if landfilled [2,3,4,5,6]. This underscores the urgency of developing processes that simultaneously recover metals and stabilize hazardous components from metallurgical wastes. The electrolytic zinc process requires iron removal in the leaching stage by producing an iron precipitate that could be hematite, goethite, or jarosite. The jarosite process is probably the most cost-efficient removal method, because it has generally been found that ammonium is the lowest cost reagent for precipitation of the bulk of the iron as jarosite [7]. Jarosite, typically with the formula AFe3(SO4)2(OH)6 (where A = K+, Na+, NH4+, etc.), is widely produced during the iron removal stage in zinc hydrometallurgy. Among its various forms, ammonium jarosite has attracted interest for its thermal and structural behaviors, as well as its potential to host recoverable quantities of silver and zinc [8]. On the other hand, lead–acid battery recycling is a growing industry, driven by stricter environmental regulations and demand for recycled lead instead of primary lead. Lead paste, the main residue from lead–acid battery recycling, contains significant quantities of Pb, S, and trace Ag and Sb. Advanced recycling technologies are focused on reusing, recovery, and minimizing hazardous waste and pollutant gas emissions during processing [9]. However, both jarosite and lead paste, due to their mineralogical complexity and hazardous components, are often landfilled, resulting in long-term environmental liabilities [10]. A metallurgical process is a complex multi-phenomena system that involves various physical and chemical reactions during high-temperature smelting. Therefore, a thermodynamic assessment is essential to calculate and investigate the feasibility, direction, and limitations of reactions and the products formed using advanced thermodynamic software such as FactSage or HSC Chemistry Software, among others. Y. Li et al. [11] presented a thermodynamic analysis to explain an innovative lead-recycling process from scrap lead-acid battery paste. The process avoids SO2 generation and emission by using a reductive sulfur-fixing technique. The thermodynamic diagrams obtained allow for establishment of a possible reaction mechanism and path in the PbSO4–Fe2O3–Na2CO3–C smelting system [12]. Stability phase diagrams were obtained with the thermodynamic software FactSage to study the lead paste reduction from lead acid batteries in a silicon carbide crucible with Na2CO3 additions to 1123 K. The carbon of the SiC crucible acts like a reducing agent, while the silicon allows for the lead silicate compounds’ formation to obtain more stable slags [13]. Z. Zulhan et al. [14] focus on sulfur elimination and iron extraction from natrojarosite by the roasting process at different temperatures. A thermodynamic simulation was performed using FactSage to evaluate the phase changes in the natrojarosite residue during heating. Despite natrojarosite data not being available in the current version of the thermochemical database, sodium sulfate (Na2SO4) and ferric sulfate (Fe2(SO4)3), which are stable at room temperature, were considered instead of natrojarosite (NaFe3(SO4)2(OH)6). A thermodynamic analysis was carried out to study the pyrometallurgical extraction of indium and silver from a roasted jarosite residue. It was found that the formation of chlorine compounds showed a high potential for extraction of the minor elements (Ag and In) from roasted jarosite together with zinc at the lowest possible energy input [15]. Thermodynamic studies have reported the heat capacity, standard entropy, and Gibbs free energy of jarosite-group minerals, including ammonium jarosite, providing critical insights into their stability and decomposition pathways [16]. Thermal decomposition studies using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) reveal multi-stage mass losses linked to dehydration (activation energy ~22 kJ/mol), desulfurization (~42 kJ/mol), and sulfate reduction (~46 kJ/mol), with toxic elements retained in hematite-rich residues [2]. Such kinetic parameters are vital for optimizing high-temperature processes. A recent research study demonstrates that pyrometallurgical co-processing of jarosite and lead paste, particularly when assisted by sodium carbonate (Na2CO3), can enable efficient recovery of silver-rich lead alloys while stabilizing sulfur in slag as Na2SO4 or Na2Fe(SO4)2, thereby reducing SO2 emissions [17]. However, these efforts lack a detailed thermodynamic understanding of phase interactions, particularly the role of Na2CO3 as a flux and sulfur-fixing agent, or the influence of silicon carbide (SiC) crucibles on redox equilibria. In this work, a thermodynamic assessment of a pyrometallurgical process previously reported [15], which leads to the recovery of a lead-silver alloy and a slag with high sulfur content from a mixture of two metallurgical wastes, known as jarosite and lead paste, was carried out. Thermodynamic information (heat capacity, entropy, enthalpy, and Gibbs free energy) of the industrial jarosite was generated from its thermal characterization to be incorporated into the FactSage software. The heat capacity was modeled using polynomial regressions, including a second-degree fit at low temperatures, and compared with literature data [16,18] to validate phase transition interpretations. The lead paste–jarosite-Na2CO3-SiC system was thermodynamically studied with the software FactSage, where stability phase diagrams were obtained to 1200 °C. The predicted compounds were compared with those reported by the XRD measurements.
2. Materials and Methods
2.1. Materials
The raw materials used in this study include industrial jarosite sourced from a Mexican zinc hydrometallurgical plant and lead paste obtained from spent lead–acid batteries through a recycling process. Figure 1 shows the raw materials used in the pyrometallurgical trials. Both wastes were dried (100 to 120 °C for 8 to 12 h), homogenized, and classified using standard sieves to ≤90 µm and ≤75 µm for the lead paste and jarosite, respectively. The chemical and mineralogical composition of both wastes was determined by atomic absorption spectrometry (AAS) and X-ray diffraction measurements (XRD), respectively. The dry raw materials were used for reduction pyrometallurgical tests and their characterization by atomic absorption, XRD, and SEM-EDS.

Figure 1.
Metallurgical wastes used for the pyrometallurgical trials.
2.2. Thermal Characterization
The thermal characterization was performed on an as-received industrial jarosite waste, obtained from the zinc hydrometallurgical process without any prior purification or pretreatment. The material exhibited a notably high moisture content and was analyzed in its original state to accurately reflect its thermal behavior under practical processing conditions. The jarosite waste was analyzed using a simultaneous thermogravimetric and differential scanning calorimetry system (TGA/DSC; TA Instruments SDT Q600) (TA Instruments, New Castle, DE, USA). Around 28 mg of sample was placed in alumina crucibles and heated from ambient temperature to 950 °C at a rate of 10 °C/min under a nitrogen flow of 50 mL/min. Mass loss steps and associated thermal events were recorded to identify dehydration, dehydroxylation, ammonium decomposition, and sulfate breakdown. Specific heat capacity (Cp) data were extracted from DSC measurements based on the ASTM E1269 and ISO 11357-4:2021 standards [19,20]. The Cp results were fitted using polynomial regressions across the entire temperature range. Thermodynamic functions, including enthalpy (H), entropy (S), and Gibbs free energy (G), were subsequently derived from Cp(T) and compared with literature data [16,18].
2.3. Pyrometallurgical Trials and Products Characterization
Mixtures of jarosite (15, 20, 30%), lead paste (40, 50, 55%), and Na2CO3 (30%) were melted in silicon carbide (SiC) crucibles with a capacity of 0.5 kg, using an electric resistance furnace. The mixtures (100 g per trial) were smelted at 1200 °C for 2 h, then poured into cast iron molds to separate the metal and slag phases. The furnace temperature was controlled via a PID controller (DwyerOmega, Michigan City, IN, USA) and monitored with a type-K thermocouple. The solidified metallic buttons were cut and analyzed at the center zone while the slag samples were crushed in an agate mortar, homogenized, embedded in epoxy, and polished to be analyzed. Both products were analyzed by X-ray diffraction (XRD; Bruker D8 Advance, Cu Kα radiation, λ = 1.5406 Å) (Bruker, Billerica, MA, USA). Scans were performed from 10° to 80° (2θ) at a step size of 0.02° and a scanning speed of 1°/min. Qualitative chemical analysis was carried out on polished sections from the center zone of the metallic Pb-Ag alloy produced in an SEM Jeol 6300 (JEOL, Peabody, CA, USA) with energy dispersive spectra analysis. Backscattering electrons technique at 20 kV and 10 A was used for image production.
2.4. Thermodynamic Modeling
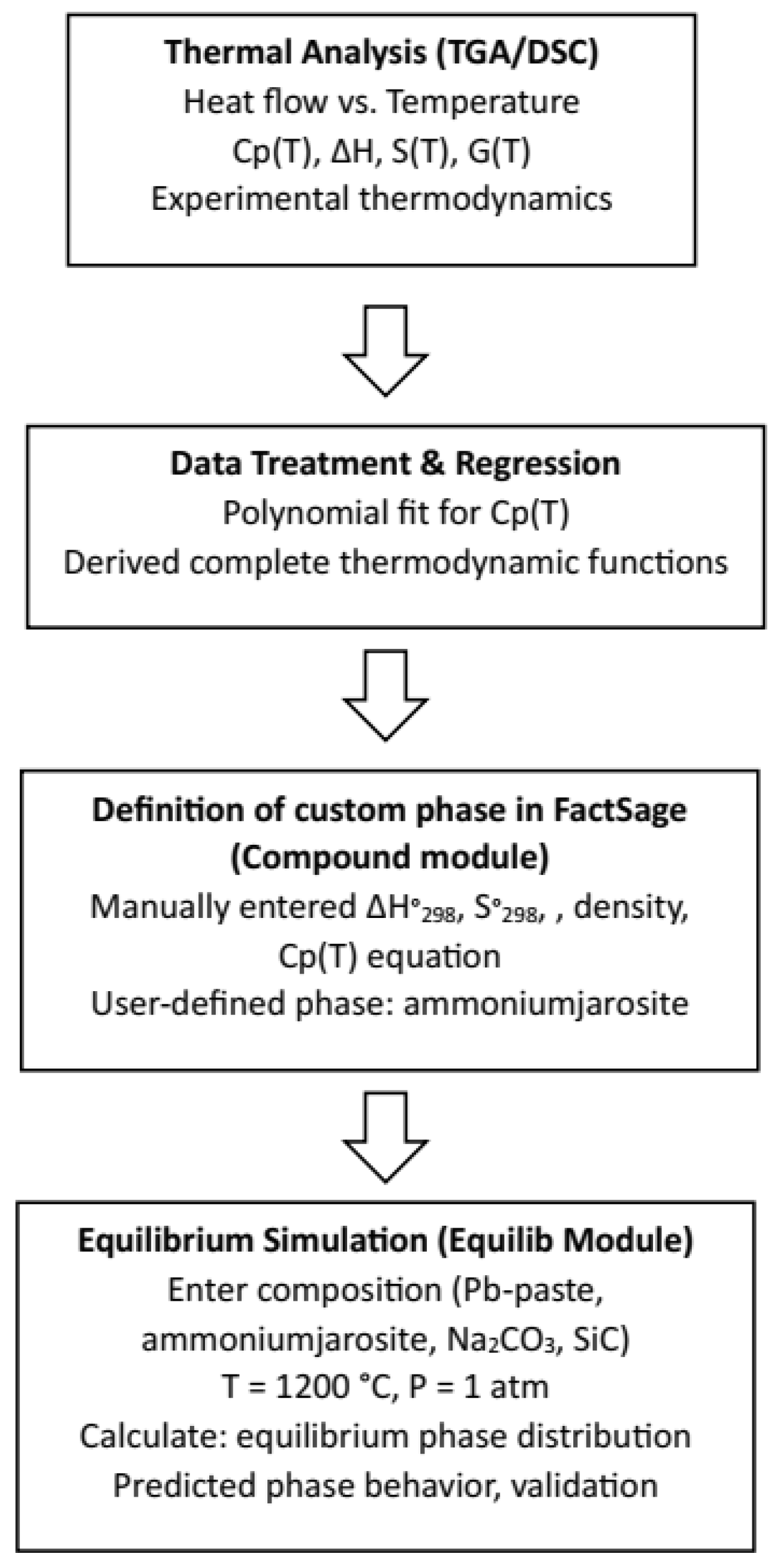
The thermodynamic software FactSage 8.2 (Facility for the analysis of chemical thermodynamics) [21] with the module Equilib was used to determine the concentration of the different chemical species once they reached the chemical equilibrium state. The user gives the initial amount of chemical species, the temperature, and the pressure of the system (usually 1 atm), and then the program calculates the most stable species with the Gibbs free energy minimization method. FactSage does not include jarosite in its database, so this phase was manually incorporated into a database defined using the module Compound. Figure 2 illustrates the detailed workflow followed in this study to bridge the experimental thermodynamic data and generate equilibrium phase diagrams using the FactSage software.
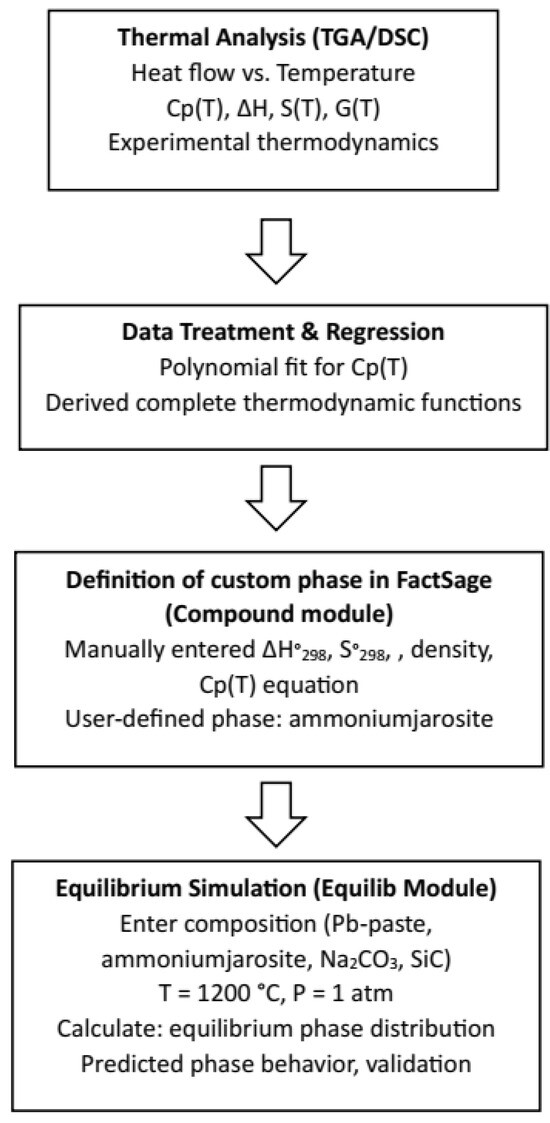
Figure 2.
Flowchart for the obtention of the stability phase diagrams.
The process began with thermal analysis (TGA/DSC) of industrial ammonium jarosite, from which the heat flow as a function of temperature was obtained. Based on these data, the heat capacity (Cp(T)) was derived and modeled using polynomial regression. Subsequently, the entropy (S(T)), enthalpy increment (H(T)−H°), and Gibbs free energy (G(T)) were calculated through analytical integration. The phase properties were introduced based on the experimental thermodynamic data generated in this work. Specifically, the heat capacity equation, standard enthalpy (ΔH°298), and standard entropy (S°298) were manually implemented into FactSage using the Compound module, since ammonium jarosite is not present in the default databases. Once the phase definition was completed, the Equilib module was used to perform equilibrium simulations under pyrometallurgical conditions (T = 1200 °C, P = 1 atm) for mixtures of lead paste, ammonium jarosite, sodium carbonate, and silicon carbide. The resulting phase distributions and stability diagrams were analyzed and compared with experimental outcomes to validate the thermodynamic model and predict silver recovery behavior during processing. The simulations accounted for the experimentally determined compositions of lead paste and jarosite, as well as the potential redox influence of SiC crucibles. This methodology allowed for improved prediction of phase stability, metal-slag separation, and sulfur retention mechanisms within the system [22].
3. Results and Discussion
3.1. Chemical and Mineralogical Characterization
Table 1 shows the chemical composition of the industrial jarosite and lead paste determined by atomic absorption spectrometry.

Table 1.
Chemical composition of the metallurgical wastes (wt. %).
The lead paste is predominantly composed of Pb and S. On the other hand, the jarosite residue contains significant amounts of Fe, S, Ca, and Zn, along with trace amounts of Ag. The presence of ammonium indicates that during the removal of iron in the leaching of zinc, ammonium-jarosite was precipitated.
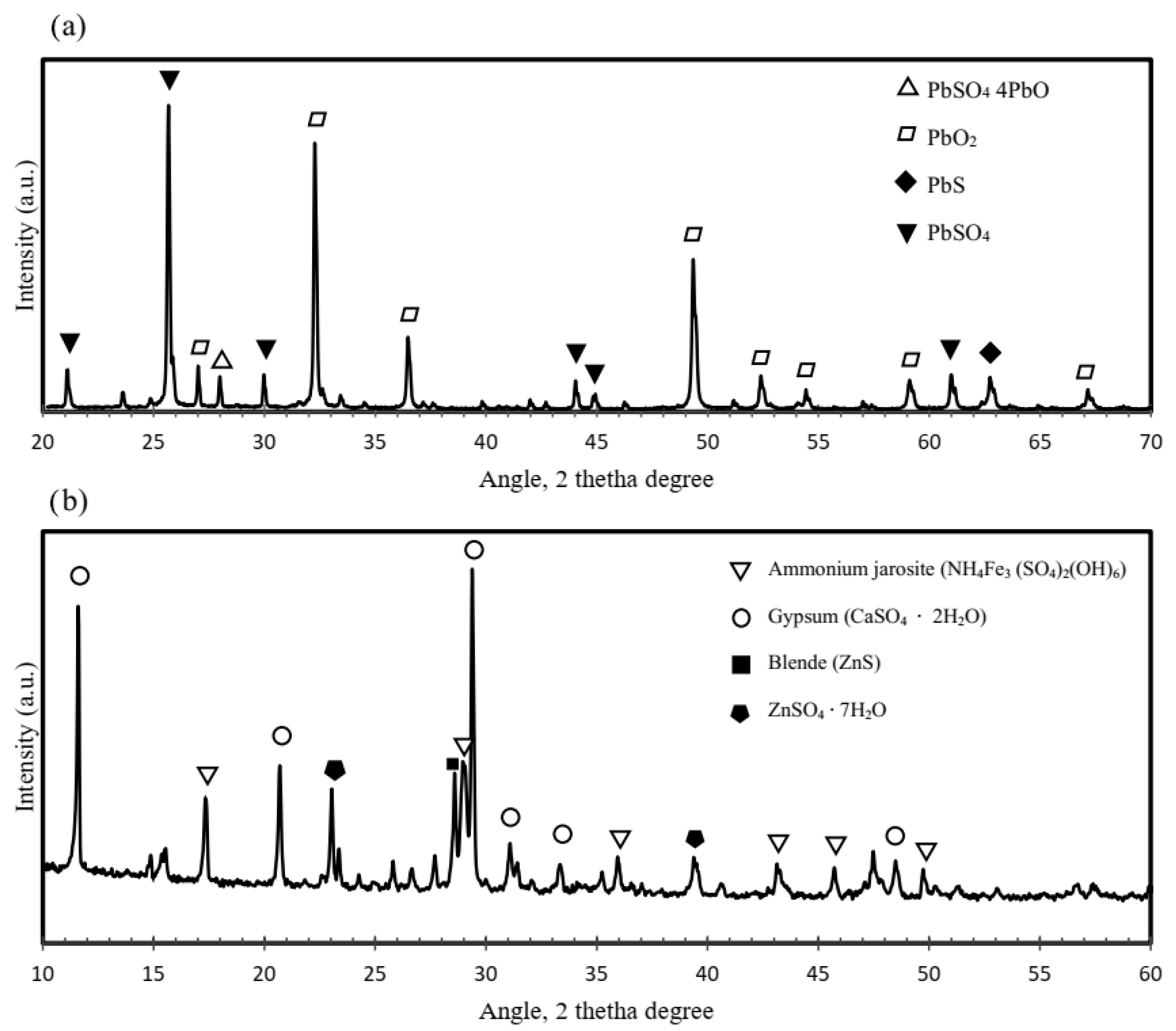
Figure 3 shows the mineralogical composition of the jarosite and lead paste determined by XRD.
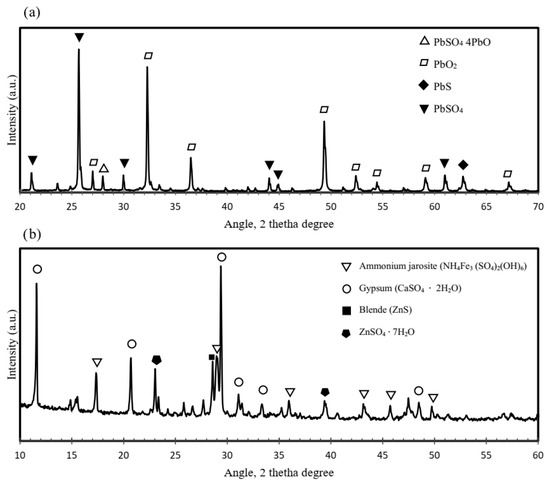
Figure 3.
X-ray pattern diffractions for (a) lead paste and (b) jarosite wastes.
The lead paste primarily consists of galena (PbS) and lead oxide (PbO2), with minor amounts of lead sulfate (PbSO4) and lead oxide sulfate (PbSO4·4PbO). In contrast, the jarosite residue is composed mainly of ammonium jarosite ((NH4)2Fe3(SO4)2(OH)6), confirming its mineral identity and providing a baseline for the subsequent thermal analysis. It must be stressed that the Pb content reported in Table 1 corresponds to the total elemental lead as determined by AAS. This value includes all Pb-bearing compounds present in the lead paste, such as PbSO4, PbO, and PbS, identified by the XRD technique. The absence of metallic Pb peaks is consistent with the chemical nature of the lead paste, which derives from oxidized residues of spent lead–acid batteries and does not contain metallic lead.
3.2. Thermal Behavior
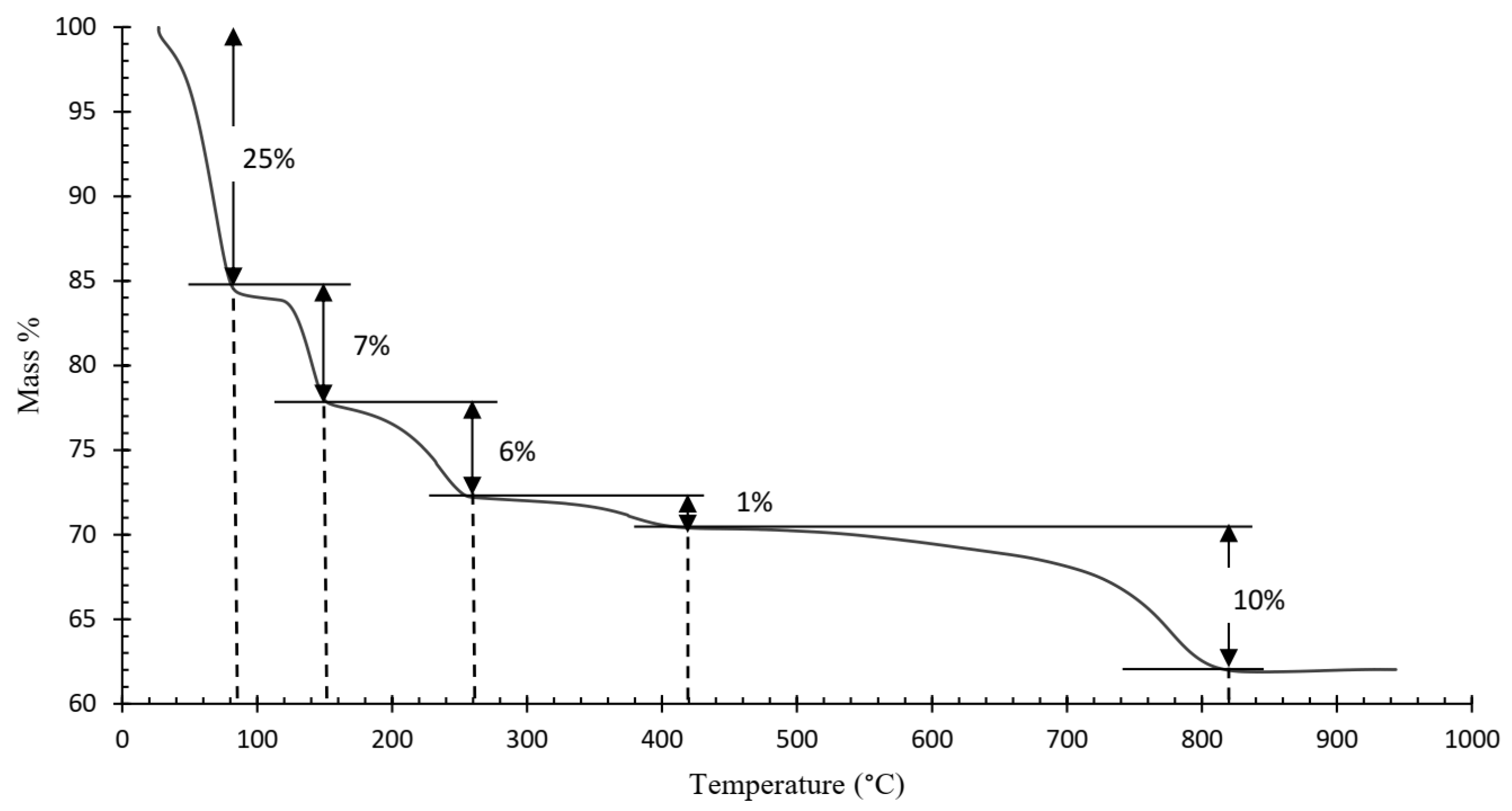
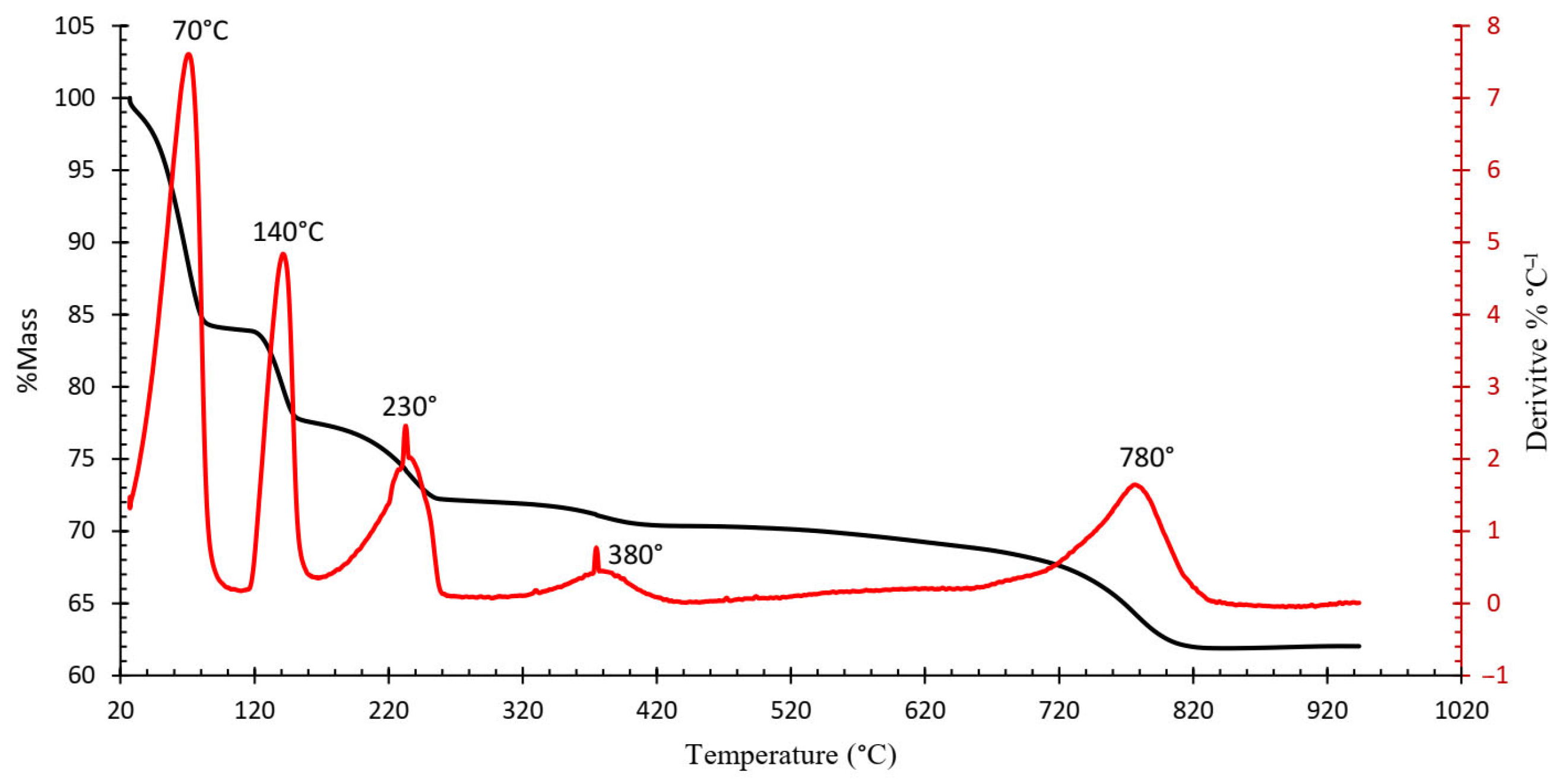
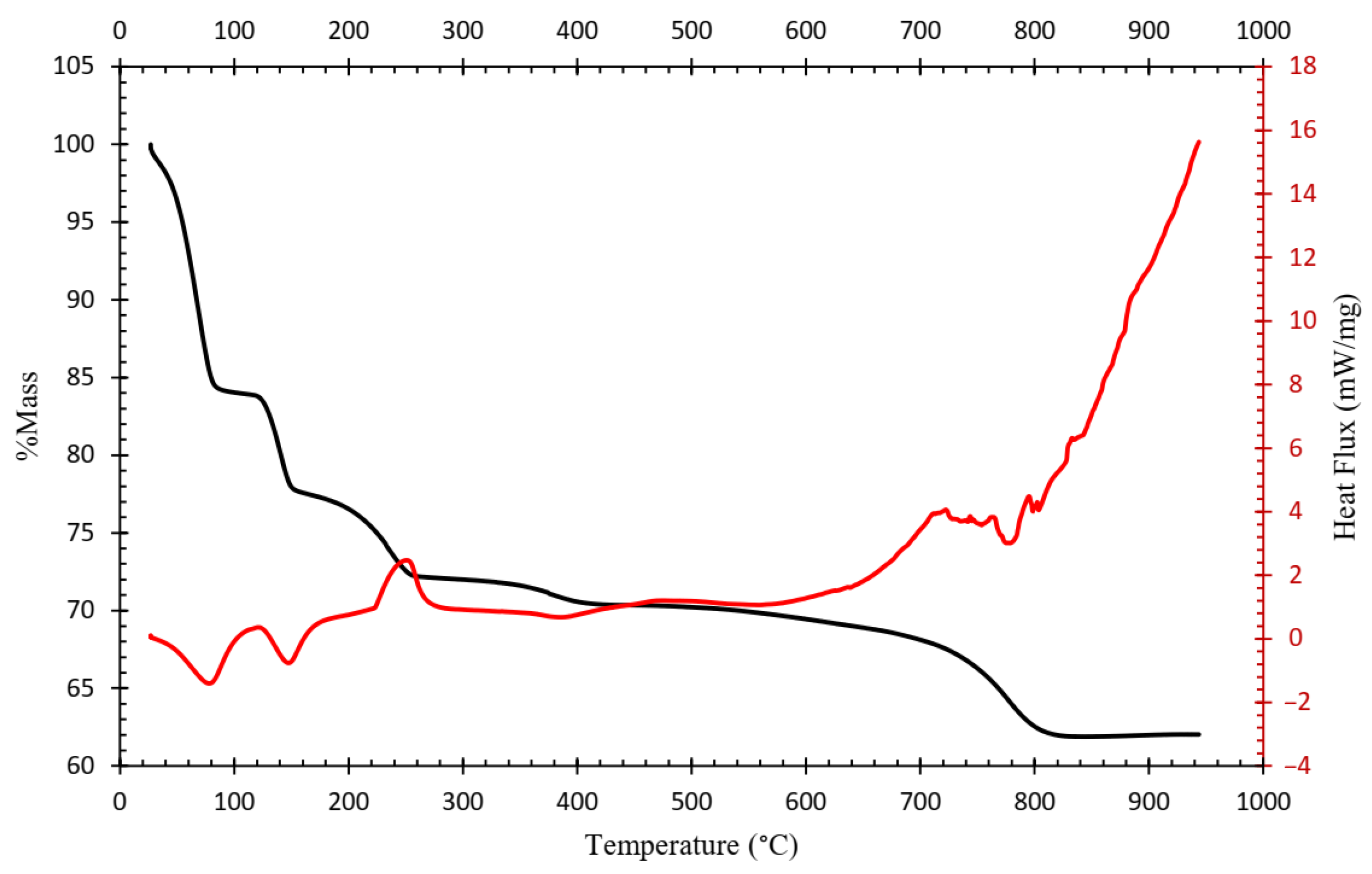
Figure 4 shows the resulting TGA curve of the ammonium jarosite sample. A complex multi-step decomposition pathway with five distinct mass loss events is identified. The corresponding derivative thermogravimetric (DTG) curve (Figure 5) reveals five major peaks, each representing the temperature at which the maximum mass loss rate occurs within defined thermal intervals [23].
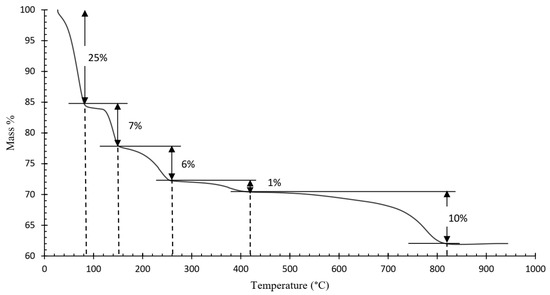
Figure 4.
Thermogravimetric analysis (TGA) of the industrial ammonium jarosite sample.
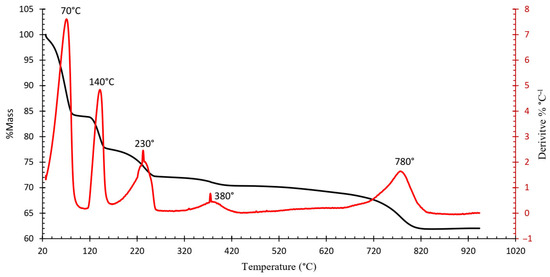
Figure 5.
Thermogravimetric—Derivative Thermogravimetry (TGA-DTG) of the industrial ammonium jarosite sample.
TGA-DTG curves indicated that the decomposition of ammonium jarosite occurs in five steps recorded at 70, 140, 230, 380, and 780 °C. The mass losses associated with these temperature ranges are 25, 7, 6, 1, and 10%, respectively, resulting in a total mass loss of 49%. The first event is associated with the removal of adsorbed water (up to ~70 °C), followed by an initial dehydroxylation process (~140 °C), further dehydroxylation and partial decomposition of ammonium groups occur at around 230 °C, at a higher temperature ~380 °C, the onset of sulfate breakdown with combined evolution of ammonia and additional water molecules, then a Final mass loss corresponding to complete sulfate decomposition and formation of a hematite-rich residue (~780 °C).
Complementary TGA–DSC analysis shown in Figure 6 evidenced three endothermic reactions and two exothermic reactions in the temperature range of 0 to 950 °C. The endothermic events correspond to water loss and dehydroxylation processes, while the exothermic events may be associated with the crystallization or reduction reactions occurring during sulfate decomposition, including the iron oxidation.
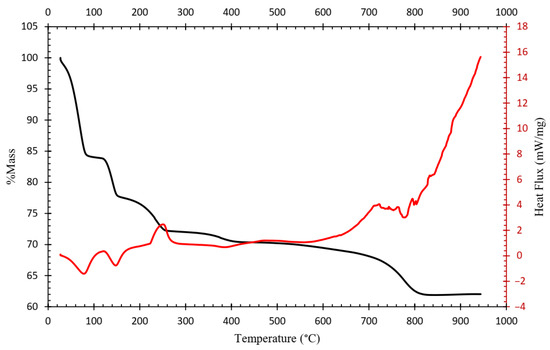
Figure 6.
Thermogravimetric-differential scanning calorimetry (TGA-DSC) of the industrial ammonium jarosite.
3.3. Heat Capacity and Thermodynamic Functions
The specific heat capacity (Cp) of ammonium jarosite was determined from the heat flow signal obtained during the differential scanning calorimetry (DSC) measurements, following a classical principles of thermodynamics. The method is based on the relationship between the heat capacity and the rate of heat flow into the sample under a controlled heating program. The molar heat capacity at constant pressure is defined by the Equation (1).
where is the number of moles of the sample and is the derivative of heat absorbed concerning temperature. Since DSC provides the heat flow (HF), defined as the thermal power in watts (J/s), and assuming a constant heating rate (), the derivative of heat concerning temperature is represented with Equation (2).
Equation (3) is obtained by substituting Equation (2) into Equation (1).
This approach is supported by the ASTM E1269 and ISO 11357-4:2021 standards, which outline the procedure for calculating specific heat capacity using DSC data under controlled heating rates and describe a comparable methodology for deriving Cp values from heat flow measurements, respectively. Both standards [19,20] provide a well-established thermophysical procedure for determining the specific heat capacity of materials using DSC measurements.
Considering Equations (1)–(3) and the heat flow data from DSC measurements, heat capacity can be determined for the ammonium jarosite sample as follows: The ammonium jarosite mass was 28 mg, for a molar mass of 179.86 g/mol for NH4Fe3(SO4)2(OH)6, therefore, the number of moles was 5.83 × 10−5 mol. The heating rate used in the DSC experiments was 15 °C/min, equivalent to . Therefore, the heat capacity is computed with Equation (4) for each temperature.
The ammonium jarosite is a key waste produced by a Mexican enterprise that produces zinc from a hydrometallurgical process; therefore, the determination of its heat capacity (Cp) is of particular relevance given the current lack of thermodynamic data for this waste. To avoid the endo and exothermic effects that distort the heat flow signal, particularly within the 300–500 K range, a baseline correction procedure was applied. A continuous baseline was constructed across the temperature range, excluding regions associated with thermal events such as phase transitions or decomposition. This baseline was then subtracted from the raw heat flow data on a point-by-point basis, effectively isolating the true heat capacity of the stable solid phase.
Following this correction, a new Cp(T) curve was generated and fitted using a fifth-degree polynomial regression in the range from 300 to 1200 K. This refined curve minimizes the possibility of negative Cp values, producing a smoother and consistent profile across the entire temperature range. The resulting equation captures the progressive increase in heat capacity with temperature, reflecting both vibrational and configurational contributions as expected for complex inorganic materials.
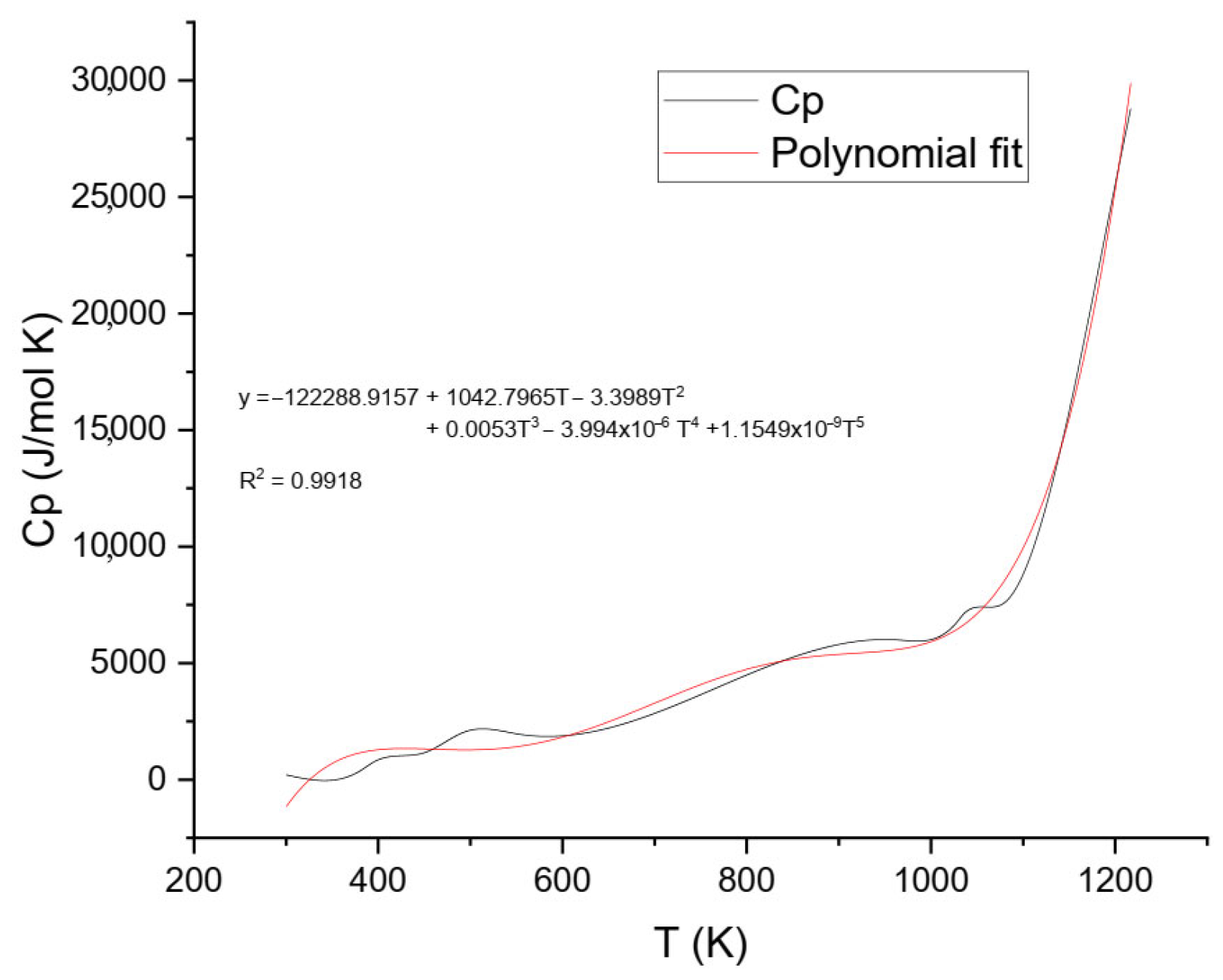
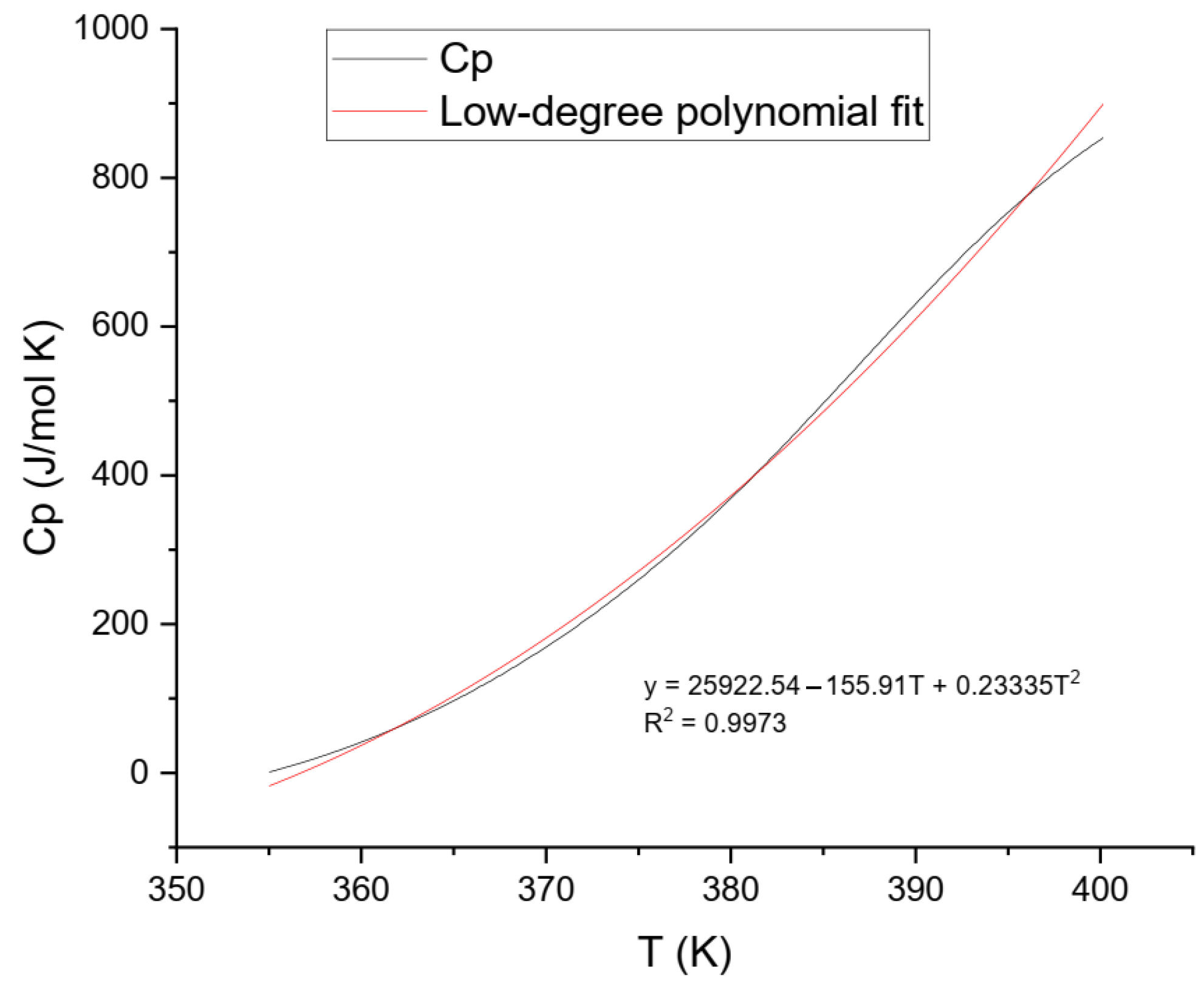
The final Cp values were plotted against temperature and analyzed using two approaches: (1) a fifth-degree polynomial model over the entire temperature range (Figure 7), and (2) a lower-order model applied to the low-temperature segment to better represent solid-phase behavior (Figure 8).
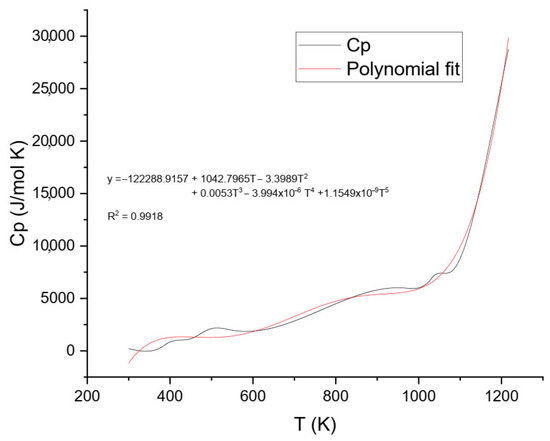
Figure 7.
Heat Capacity (Cp) data for ammonium jarosite and respective polynomial Fit (temperature range of 300–1200 K).
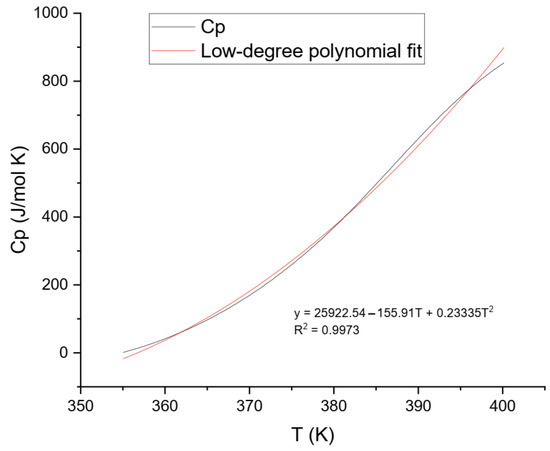
Figure 8.
Heat Capacity (Cp) data for ammonium jarosite and respective lower polynomial fit for low temperatures (temperature range of 355–400 K).
The polynomial approach is supported by both empirical practice and theoretical models of solid-state thermodynamics. Low-order polynomial functions are widely applied to approximate the Cp of inorganic compounds including jarosites which exhibit deviations due to structural disorder and anharmonic lattice vibrations over moderate temperature ranges (300–500 K) [24,25,26].
Figure 7 shows the experimental heat capacity (Cp) of ammonium jarosite in the temperature range from 300 to 1200 K. The data revealed that the Cp values increased gradually with temperature throughout the studied range, consistent with typical solid-state vibrational behavior. After applying baseline correction to exclude transient thermal effects, the resulting Cp values were modeled using a fifth-degree polynomial regression (Equation (5)), capturing the intrinsic temperature dependence of the stable phase without interference from decomposition events.
This higher-order polynomial provides flexibility to describe complex thermal behavior over a broad temperature range. Such mathematical treatment is well-established in the literature for modeling the heat capacity of inorganic solids, especially when extended ranges or transitions are involved [27,28]. For example, Guyot and Richet applied polynomial regression to describe Cp(T) for perovskite CaTiO3 and detect thermally activated structural transitions [27], while King and Weller demonstrated the use of high-order polynomials to capture phase transformations in vanadate ceramics [28].
The fifth-degree fit of ammonium jarosite yielded a high correlation coefficient (R2 = 0.9918), confirming its adequacy for describing the experimental data. The continuous nature of the fitted curve enabled its direct integration to derive fundamental thermodynamic functions, including entropy S(T), enthalpy increment H(T)−H(0), and Gibbs free energy G(T). The obtained parameters reflect the intrinsic thermal behavior of ammonium jarosite and reveal the influence of both temperature and compositional features.
The sample used in this work was an industrial ammonium jarosite waste. The waste is presented as a fine-grained, clay-like solid, distinct from highly crystalline synthetic jarosites typically investigated in thermodynamic studies. Due to its industrial origin, the sample likely contains structural imperfections, substitutional ions (e.g., Pb2+, Zn2+), and potential interlayer water. These features can significantly influence vibrational entropy and thermal properties. However, at elevated temperatures, particularly above ~620 K, where the onset of thermal decomposition is observed in TGA mass losses associated with the release of water and sulfate groups, also contribute to changes in the measured heat capacity and derived functions. Therefore, both lattice anharmonicity and the evolving phase assemblage should be considered when interpreting the high-temperature thermodynamic behavior of industrial ammonium jarosite.
Comparisons with literature values, such as those reported by Majzlan et al. for synthetic jarosite variants [24], indicate that the ammonium jarosite fits are in close agreement in the low to moderate temperature range (300–400 K), where the crystal structure remains largely intact. However, above 700 K, slight deviations are observed, consistent with the progressive thermal decomposition of jarosite into hematite, amorphous iron oxides, and sulfate species. Previous studies have shown that ammonium jarosite is thermodynamically stable only up to approximately 600–650 K under atmospheric pressure [2], beyond which irreversible breakdown processes dominate. Therefore, the lower-temperature region of the Cp(T) curve (300–650 K) accurately describes the jarosite phase in its structurally preserved state, while values at higher temperatures are influenced by decomposition events. Nonetheless, this high-temperature data remains critically important for modeling energy balances in pyrometallurgical processes, particularly those involving lead paste and sodium carbonate, where jarosite is intentionally decomposed to recover valuable metals and stabilize sulfur in a slag phase.
Figure 8 illustrates the low-temperature behavior of ammonium jarosite, evaluated in the range of 355–400 K. After baseline correction of the raw heat flow data, the resulting heat capacity (Cp) values exhibited a smooth and monotonic increase with temperature, consistent with the expected vibrational behavior of crystalline solids in the absence of phase transitions. To represent this trend, the data were fitted using a second-degree polynomial regression model, expressed as Equation (6).
Equation (6) effectively captures the lattice vibrational contributions and provides a reliable approximation of the Cp behavior within the low-to-moderate temperature regime. Although the Debye model predicts a Cp ∝ T3 behavior for non-metallic crystalline solids in the low-temperature limit (T ≪ θ_D), real materials such as industrial jarosites often exhibit deviations due to lattice imperfections, cation substitutions, interstitial water, and anharmonic phonon interactions [25].
Empirical studies show that low-degree polynomial models are widely used to approximate Cp over experimental ranges above cryogenic temperatures (e.g., 300–500 K) [2]. This methodology is consistent with established practices in calorimetric thermodynamics, particularly for minerals [26].
The fitted parameters yielded a high-quality match to the experimental data (R2 = 0.9973), with no negative values or discontinuities. The obtained curve (Equation (6)) closely aligns with the behavior reported by Majzlan et al. [24] for synthetic hydronium jarosite, particularly in the 298–340 K range. The deviations are attributed to the industrial origin of the sample used in this study, which was sourced from a Mexican zinc hydrometallurgical plant. This material contains other compounds and structural heterogeneities, which are known to affect vibrational heat capacity, especially at low temperatures where phonon modes are highly sensitive to lattice disorder.
The polynomial Cp expression obtained from the low-temperature data provides a reliable foundation for incorporation into thermochemical modeling platforms. Because standard databases such as FactSage do not include detailed thermodynamic entries for ammonium jarosite, the fitted function enables the definition of user-customized compound properties.
Table 2 shows the heat capacity equations reported for jarosites. It is observed from Table 2 that the heat capacity (Cp) for the ammonium jarosite sample (Equation (6)) exhibits a distinctive trend when compared to previously reported models [16,24]. Although the linear term (in T) shows a negative coefficient in Equation (6), which is unusual for crystalline materials, this difference is mainly attributed to the structural nature of the sample; however, other variations are due to the measurement conditions or potential overfitting in the regression model. Despite these discrepancies, the magnitude of the Cp values fits within a reasonable range for jarosite-type minerals. Notably, the previously published models converge around average Cp values between 270 and 290 J/mol·K at 298 K, which provides a valuable benchmark to validate Equation (6).

Table 2.
Comparison of Heat Capacity (Cp) Equations for Jarosites.
The experimentally Cp values obtained for the ammonium jarosite provide a solid foundation for calculating entropy, enthalpy, and Gibbs free energy, enabling accurate assessment of phase equilibria, decomposition pathways, and energy balances during high-temperature processing.
From the polynomial fit of the experimental heat capacity data (Equation (5)) over the temperature range of 300–1200 K, three fundamental thermodynamic functions were calculated: entropy S(T), enthalpy increment H(T)−H(0), and Gibbs free energy G(T). These functions were derived through the integration of the Cp (T) function using the standard thermodynamic expressions represented in Equations (7)–(9).
where T0 = 298.15 K is the reference temperature.
Table 3 shows the entropy, enthalpy increment, and Gibbs free energy for different temperatures. These thermodynamic properties are essential for modeling equilibrium phase transformations in the lead paste–jarosite–Na2CO3 system and are subsequently incorporated into FactSage to obtain stability phase diagrams.

Table 3.
Thermodynamic functions of ammonium jarosite.
It is observed from Table 3 that the entropy increases steadily with temperature, reflecting the accumulation of accessible microstates due to vibrational mode activation and increasing lattice disorder. At 1200 K, the entropy reaches approximately 4873.01 J mol−1 K−1, consistent with entropy values observed for similarly structured jarosite-type minerals [16,24]. In addition, the enthalpy increment H(T)−H(0) also grows nonlinearly with temperature, it requires a significant thermal energy of 4.41 × 106 J mol−1 to reach 1200 K under constant pressure conditions.
The Gibbs free energy considers the thermal and entropic effects and shows a typical decrease when temperature is increased. At 1200 K, the Gibbs free energy is −1195.73 J mol−1. The negative value reflects the tendency of jarosite to undergo decomposition under such conditions, a result that aligns well with previous thermal stability assessments [2].
Table 4 shows the thermodynamic properties evaluated from the obtaining of the Cp (Equation (6)) and those reported in [16,24].

Table 4.
Comparison of Thermodynamic Properties at 298.15 K.
From Table 4 it is shown that the values obtained in this work for the specific heat (Cp), entropy (S°), standard enthalpy of formation (ΔHf°), and Gibbs free energy of formation (ΔGf°) for the industrial ammonium jarosite fits with those reported in the literature for synthetic potassium jarosite by Majzlan et al. [24]. The overall agreement validates the experimental procedure and the mathematical treatment applied to the Cp(T) data. Specifically, a second-degree polynomial regression was employed to model the Cp behavior at low temperatures (355–400 K), while a fifth-degree polynomial regression was applied over the full temperature range to enable accurate integration of Cp(T) for deriving H(T), S(T), and G(T).
The Cp and the thermodynamic functions obtained from the TGA –DSC measurements of the industrial ammonium jarosite were incorporated into the FactSage thermodynamic software to study the interaction of the lead paste with ammonium jarosite and sodium carbonate to obtain stability phase diagrams and determine the compounds formed in the reduction trials carried out to 1200 °C.
3.4. Reduction Trials of the Lead Paste–Jarosite-Na2CO3 Mixtures
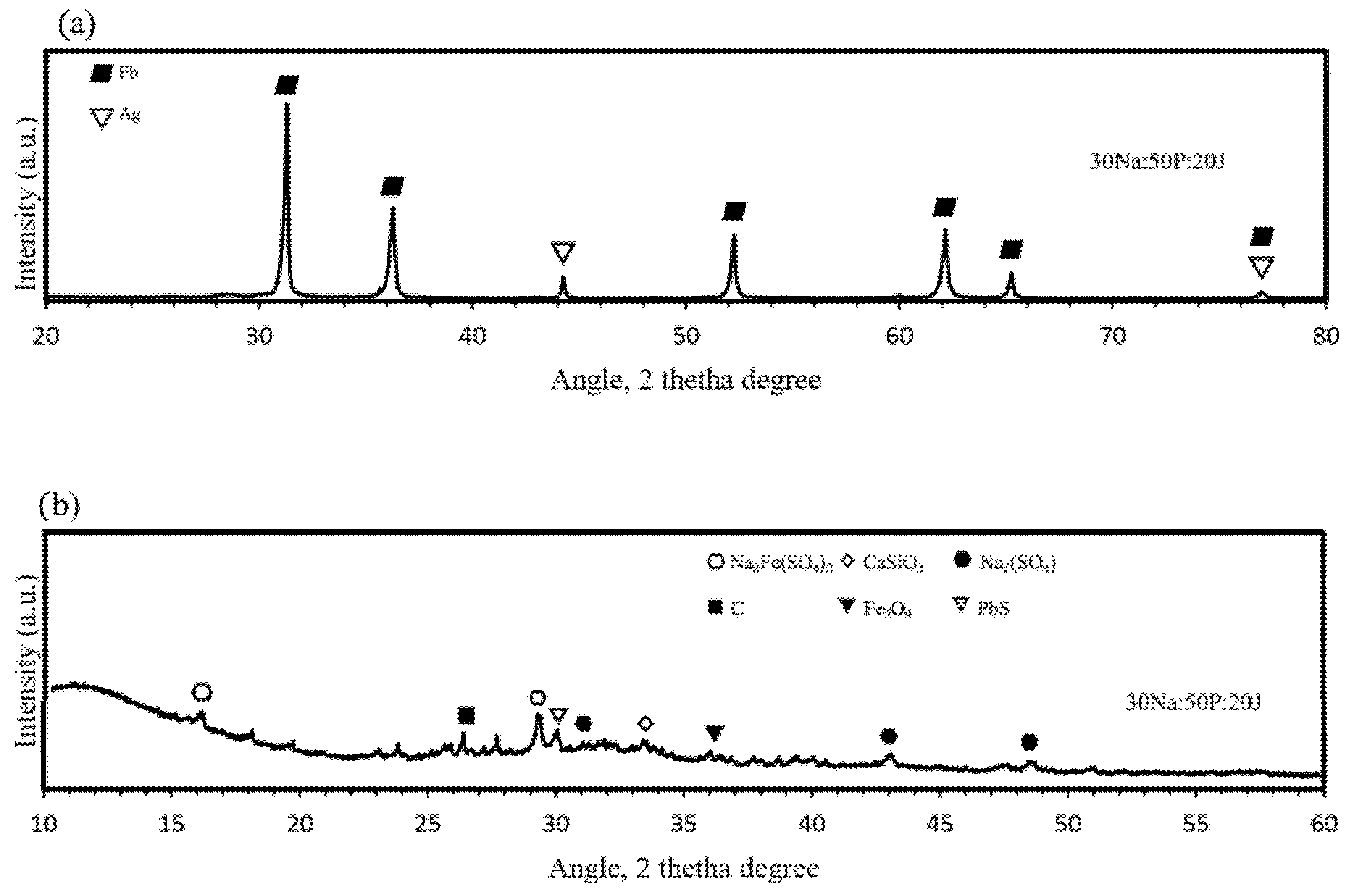
Pyrometallurgical trials were carried out by melting optimized mixtures of jarosite, lead paste, and Na2CO3 in SiC crucibles at 1200 °C for 2 h. Upon cooling and pouring into cast iron molds, two distinct phases were obtained: a dense, Pb-Ag alloy and a lighter, glassy slag. The highest metallic phase recovery was achieved by mixing 50% lead paste with 20% ammonium jarosite and 30% Na2CO3 (30Na:50P:20J) with a grade of silver of 80 ppm. Samples of the metallic button and the slag obtained were analyzed by the X-ray diffraction technique and results are shown in Figure 9.
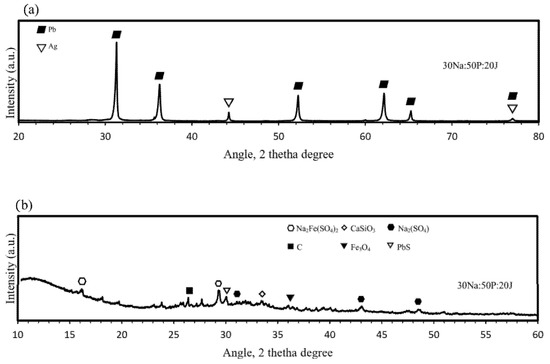
Figure 9.
X-ray diffraction patterns of (a) the metallic button and (b) the slag phase for the Lead paste– ammonium jarosite-Na2CO3 reduction trials.
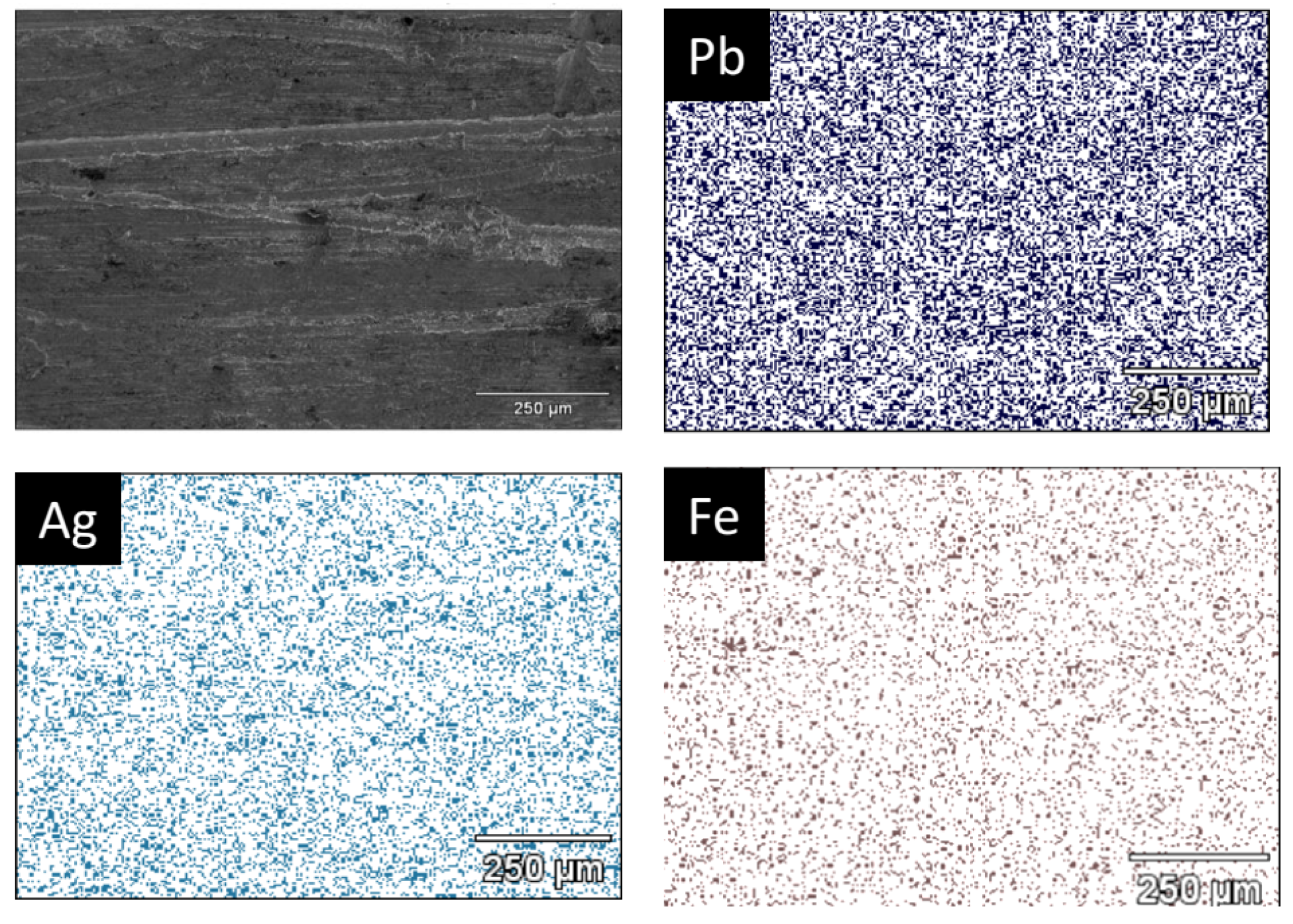
It is observed from Figure 9 that the metallic button is constituted of lead and silver, forming a Pb-Ag alloy for the trials developed, while the slag is a mixture of several compounds obtained from the interaction of the raw materials with the SiC crucible [29]. The main compounds observed are wollastonite (CaSiO3), magnetite (Fe3O4), sodium sulfate (Na2(SO4)), sodium ferrisulfate (Na2Fe(SO4)2), carbon, and galena (PbS). This observation confirms the dual role of Na2CO3 as a fluxing agent and as a sulfur fixer, thereby reducing potential SO2 emissions during processing [15]. The XRD pattern of the metallic button, obtained from a polished central section, displays characteristic reflections of Pb and a Pb-Ag solid solution. Because of the low silver concentration in the raw materials (<0.0143 wt%), distinct Ag peaks are not easily detectable by XRD. Therefore, SEM–EDS analysis was performed on the same section to confirm the elemental distribution. As shown in Figure 10, the EDS technique reveals a homogeneous presence of Ag dispersed throughout the Pb matrix, indicating that Ag was successfully recovered, forming a solid solution with lead. Iron was also detected in minor amounts, likely originating from the partial reduction of iron-bearing residues.
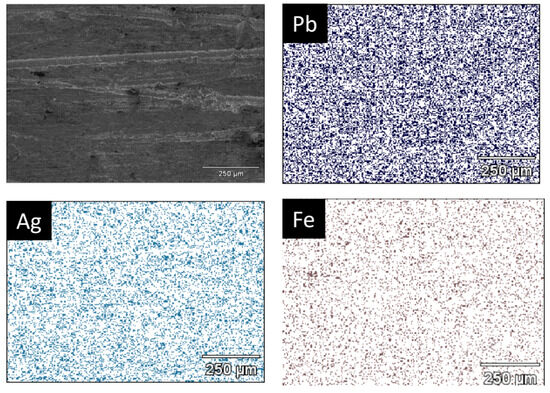
Figure 10.
SEM micrograph from the metallic sample of trial 30Na:50P:20J showing the presence of Pb, Ag, and Fe for the EDS technique.
3.5. Thermodynamic Assessment
The thermodynamic functions experimentally obtained for the ammonium jarosite were incorporated into the FactSage 8.2 thermodynamic software, and the Equilib and Phase Diagram modules were used to obtain the stability phase diagrams of Figure 11 and Figure 12. The system considered for the thermodynamic modeling was a ternary mixture composed of lead paste (primarily PbO and PbSO4), sodium carbonate (Na2CO3), and ammonium jarosite (NH4Fe3(SO4)2(OH)6) contained in a CSi crucible. A representative mixture ratio was defined using 30% Na2CO3, 50% lead paste, and 20% ammonium jarosite, denoted as 30Na:50P:20J, which corresponds to the reduction trial where the highest alloy recovery was obtained. The SiC content was considered in the range from 0 to 5%. During the reduction trials, the system collects a sufficient amount of SiC to develop chemical reactions with the components in the mixture where the carbon in the crucible acts as a reducing agent while the silicon allows the formation of lead silicates [13]. The reaction considered for the thermodynamic modeling based on the mixture ratio and the chemical composition of the raw materials is represented as Equation (10).
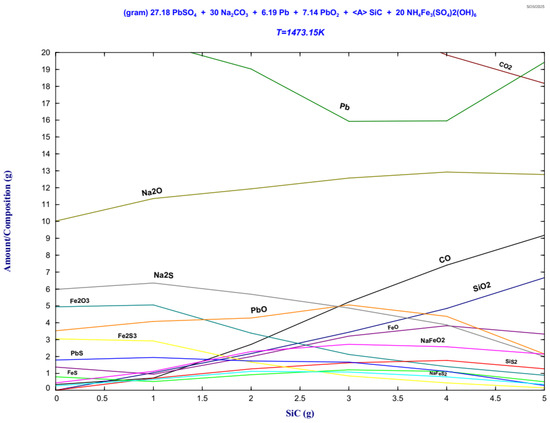
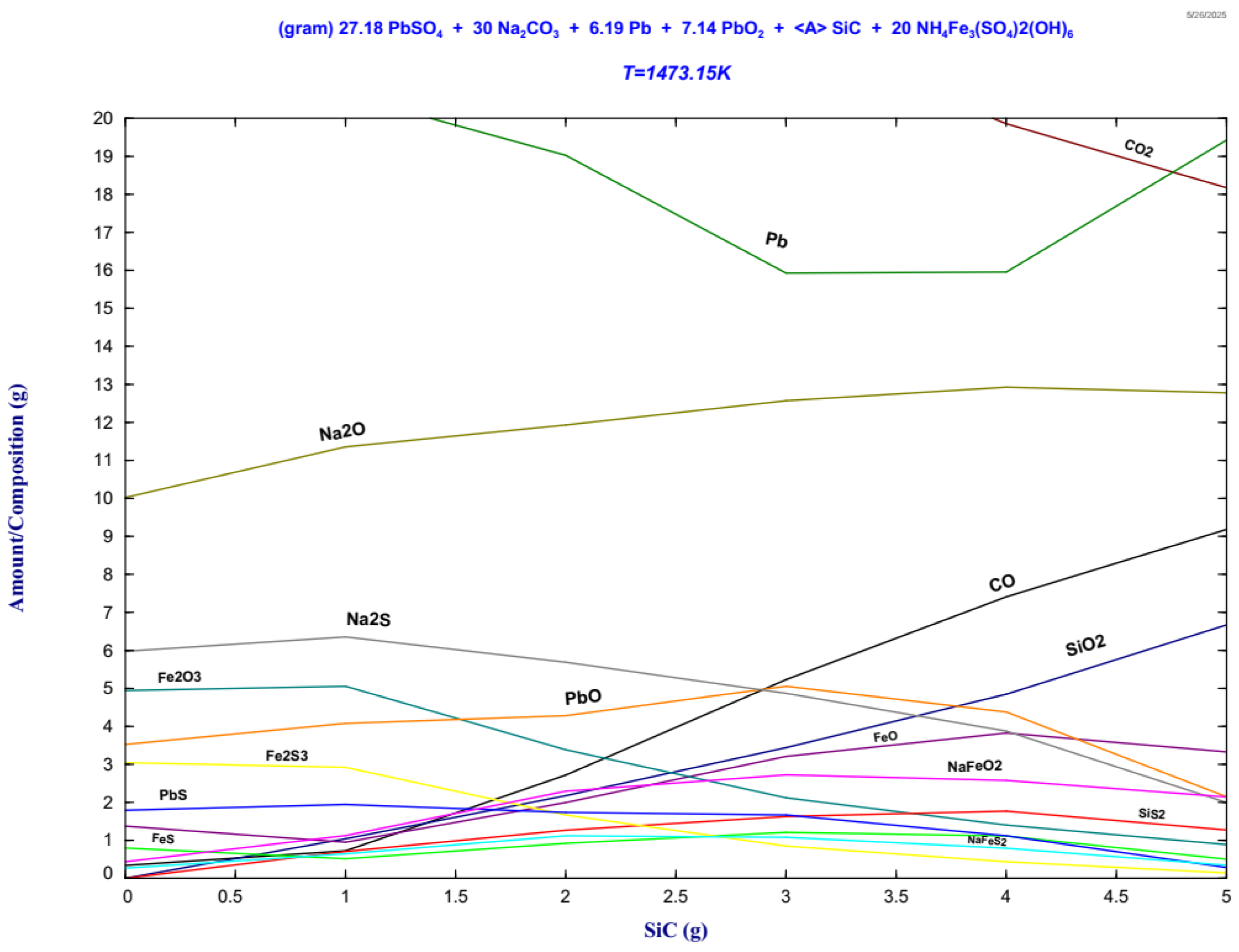
Figure 11.
Stability phase diagram of the lead paste–ammonium jarosite-Na2CO3-SiC mixture for the 30Na:50P:20J sample for the slag phase.
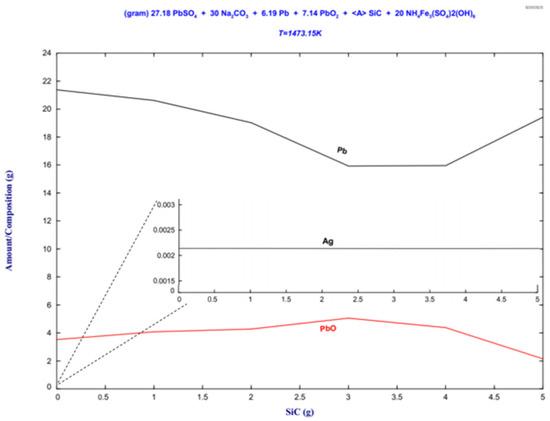
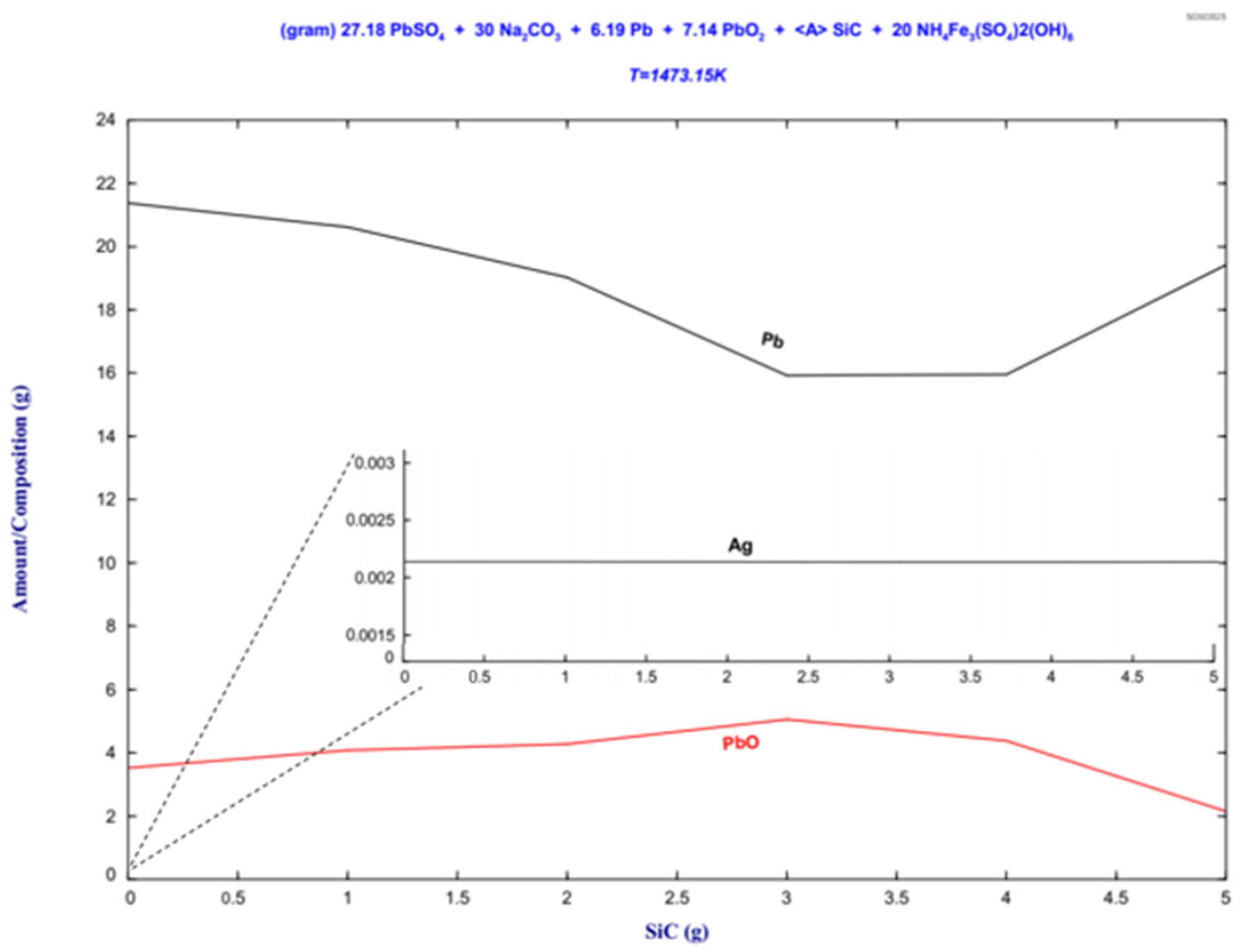
Figure 12.
Stability phase diagram of the lead paste–ammonium jarosite-Na2CO3-SiC mixture for the 30Na:50P:20J sample for the metallic phase.
The thermodynamic modeling of reaction (10) was carried out at 1473.15 K under atmospheric pressure.
It is observed from Figure 11 the formation of two principal equilibrium domains: (i) a metallic phase predominantly composed of lead (Pb), and (ii) a sulfur rich slag phase. The metallic phase originates from the thermal decomposition and carbothermic reduction of Pb compounds (PbSO4, PbS) present in the lead paste.
The stability phase diagram of Figure 12 shows the formation of metallic lead from the PbO reduction. The remarkable affinity between lead and silver in the liquid state facilitates the uptake of the silver present in the jarosite by the lead contained in the lead paste, forming a Pb-Ag alloy. The silver contained in the alloy reported by the thermodynamic evaluation indicates a content of 0.00214 g. The highest lead recovery is reached for a SiC content of 1.5 g.
The Pb-Ag alloy prediction is confirmed by the X-ray diffraction patterns of the metallic button in Figure 9a and the EDS results reported in Figure 10, where a Pb–Ag solid solution was detected.
The slag phase shown in Figure 11 comprises compounds containing sodium, iron, and sulfur. The Na2CO3 added to the mixture is key in the reduction reactions. First, it is decomposed at high temperatures, forming Na2O and CO2. The Na2O is a strong desulfurizing agent that reacts with the sulfur of the ammonium jarosite, forming Na2S and NaFeS2. On the other hand, the carbon from the CSi reacts with the CO2 based on the Boudouard reaction, forming the reducing CO, which aids in the PbO reduction at high temperatures. In addition, it is observed at high CSi contents and oxidizing conditions due to high temperature, the formation of NaFeO2 and SiO2. The iron that is not retained by the Na2O is presented in the slag as FeO and Fe2O3. It is observed a low amount of matte formation comprised of FeS and PbS. It was reported that higher concentrations of sodium carbonate allow the formation of PbS, decreasing the recovery of metallic lead [11,13]. The stability phase diagrams show the thermodynamic equilibrium of the system at 1473 K; however, after solidification and in atmospheric conditions, the slag phases could be slightly modified. The compounds detected after solidification and identified by x-ray diffraction measurements correspond to Na2Fe(SO4)2 and Na2(SO4), where the iron and sulfur are retained by sodium: This matches the thermodynamic evaluation, where NaFeS2 and NaFeO2 were predicted. Small amounts of PbS were identified by both techniques, whereas Fe3O4 was detected by X-ray diffraction and Fe2O3 by FactSage. Wollastonite (CaSiO3) was detected by X-ray diffraction, however, the thermodynamic evaluation does not consider the addition of Ca as CaCO3 contained in the ammonium jarosite, and therefore it was not predicted. The thermodynamic assessment reasonably matches the X-ray diffraction measurements and evidences the formation of Pb-Ag alloy and effective sulfur retention in the slag, which is crucial for mitigating SO2 emissions during high-temperature treatment. The ammonium jarosite waste introduces complexity into the thermodynamic system due to its high thermal stability and chemical composition. The presence of iron sulfates such as Na2Fe(SO4)2 reflects the stability of these species in oxidizing conditions, allowing the partial retention of sulfur in the slag phase, an important consideration for minimizing SO2 emissions during industrial processing. The predicted compounds show excellent qualitative agreement with the experimental outcomes obtained during the high-temperature trials. This congruence reinforces the reliability of thermodynamic modeling as a predictive and process design tool, especially when experimental Cp and phase data are incorporated into custom databases for materials such as industrial jarosite. These findings demonstrate the practical applicability of such simulations in pyrometallurgical process optimization.
4. Conclusions
A thermodynamic assessment was carried out of a mixture of ammonium jarosite and lead paste wastes treated with Na2CO3 as flux, contained in a CSi crucible at 1200 °C. A comprehensive thermodynamic and thermal analysis of an industrial ammonium jarosite waste sourced from a Mexican zinc hydrometallurgical plant was carried out to obtain thermodynamic data, which was incorporated into the database of the thermodynamic software FactSage. Experimental Cp data were obtained from TGA and DSC measurements of the jarosite sample collected over a wide temperature range (300–1200 K). The Cp was successfully represented using polynomial regression models: a second-degree polynomial for the low-temperature region (355–400 K) and a fifth-degree polynomial over the full 300–1200 K range. These models enabled the derivation of key thermodynamic functions, including entropy (S), enthalpy increment (H), and Gibbs free energy (G). The reduction trials of the mixture of industrial wastes allowed the formation of a Pb-Ag alloy and a slag with high sulfur and iron contents. The predicted compounds formed at higher temperatures reasonably match the experimental compounds determined by X-ray diffraction measurements, where a Pb-Ag alloy is formed and a slag rich in Na, S, and Fe is obtained. The stability phase diagrams obtained could be used as an important tool in the recycling of metallurgical wastes such as jarosite and lead paste. In addition, the incorporation of new thermodynamic data of industrial wastes allows the development of robust databases for thermodynamic evaluations.
Author Contributions
Conceptualization, A.C.R.; Data curation, M.G.H.; Formal analysis, J.E.S.V., A.C.R. and J.A.R.S.; Funding acquisition, M.E.F.F.; Investigation, J.E.S.V., R.G.S.A. and T.d.R.J.R.; Methodology, J.E.S.V., M.G.H. and J.C.J.L.; Resources, M.E.F.F., R.G.S.A. and T.d.R.J.R.; Software, J.E.S.V. and J.A.R.S.; Supervision, A.C.R.; Validation, J.A.R.S.; Visualization, R.G.S.A., M.G.H. and J.C.J.L.; Writing—original draft, J.E.S.V. and A.C.R.; Writing—review & editing, A.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors wish to thank the institutions SECIHTI, SNI, COFAA, and SIP-Instituto Politécnico Nacional for their permanent assistance to the Process Metallurgy Group at ESIQIE-Metallurgy and Materials Department. A. Cruz wishes to thank the fund of the government of the state of Hidalgo through the Council of Science, Technology, and Innovation of the State of Hidalgo (CITNOVA).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Olcay, R.H.; Palacios, E.; Reyes, I.; Patiño, F.; Reyes, M.; Pérez, M.; Hernan, I.; Juárez, J.; Flores, M.İ. Behavior of toxic elements in the thermal decomposition of industrial sodium jarosite: A kinetic analysis. React. Kinet. Mech. Catal. 2025, 138, 107–124. [Google Scholar] [CrossRef]
- Frost, R.L.; Wills, R.; Kloprogge, J.; Martens, W. Thermal decomposition of ammonium jarosite (NH4)Fe3(SO4)2(OH)6. J. Therm. Anal. Calorim. 2006, 84, 489–496. [Google Scholar] [CrossRef]
- Jiménez, C.; Flores, M.; Romero, A.; Hernández, A.; López, J.; Cuéllar, L.; Colin, E. Recovery of silver and lead from jarosite residues by roasting and reducing pyrometallurgical processes. Metals 2024, 14, 954. [Google Scholar] [CrossRef]
- Jiménez, C.; Favela, M.; Romero, A.; Hernández, A.; Rodríguez, J.; Cruz, A.; Colin, E. Pyrometallurgical treatment of jarosite residue with a mixture of CaO, SiO2, and CaSi. J. Min. Metall. Sect. B 2024, 60, 205–214. [Google Scholar] [CrossRef]
- Salminen, J.; Nyberg, J.; Imris, M.; Magnus, B. Smelting jarosite and sulphur residue in a plasma furnace. In PbZn 2020, Proceedings of the 9th International Symposium on Lead and Zinc Processing, San Diego, CA, USA, 23–27 February 2020; Springer: Berlin/Heidelberg, Germany, 2020; pp. 391–403. [Google Scholar]
- Rämä, M.; Nurmi, S.; Jokilaakso, A.; Klemettinen, L.; Taskinen, P.; Salminen, J. Thermal processing of jarosite leach residue for a safe disposable slag and valuable metals recovery. Metals 2018, 8, 744. [Google Scholar] [CrossRef]
- Ismael, M.R.; Carvalho, J.M. Iron recovery from sulphate leach liquors in zinc hydrometallurgy. Miner. Eng. 2003, 16, 31–39. [Google Scholar] [CrossRef]
- Sinclair, R.J. The Extractive Metallurgy of Zinc; The Australasian Institute of Mining and Metallurgy: Carlton, VIC, Australia, 2005; Volume 13. [Google Scholar]
- Li, Y.; Wang, Y.; Chen, M.; Huang, T.; Yang, F.; Wang, J. Current status and technological progress in lead recovery from electronic waste. Int. J. Environ. Sci. Technol. 2023, 20, 1037–1052. [Google Scholar] [CrossRef]
- Nowińska, K.; Adamczyk, Z. Zinc and Lead Metallurgical Slags as a Potential Source of Metal Recovery: A Review. Materials 2023, 16, 7295. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Tang, C.; Klemettinen, L.; Rämä, M.; Wan, X.; Jokilaakso, A. A new pyrometallurgical recycling technique for lead battery paste without SO2 generation—A thermodynamic and experimental investigation. In Extraction 2018, Proceedings of the First Global Conference on Extractive Metallurgy, Ottawa, Canada, 26–29 August 2018; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1109–1120. [Google Scholar] [CrossRef]
- Xie, S.; Zhao, B. Phase Equilibrium Studies of Nonferrous Smelting Slags: A Review. Metals 2024, 14, 278. [Google Scholar] [CrossRef]
- Sánchez, A.; Gutiérrez, V.; Cruz, A.; Sánchez, R. Lead production from recycled paste of lead acid batteries with SiC-Na2CO3. Russ. J. Non Ferr. Met. 2016, 57, 316–324. [Google Scholar] [CrossRef]
- Zulhan, Z.; Adzana, Z.; Munawaroh, M.; Yusro, A.H.; Christian, J.D.; Saputri, A.D.; Hidayat, T. Sulfur Removal and Iron Extraction from Natrojarosite Residue of Laterite Nickel Ore Processing by Reduction Roasting. Metals 2023, 13, 52. [Google Scholar] [CrossRef]
- Steinlechner, S.; Antrekowitsch, J. Thermodynamic considerations for a pyrometallurgical extraction of indium and silver from a jarosite residue. Metals 2018, 8, 335. [Google Scholar] [CrossRef]
- Majzlan, J.; Glaskák, P.; Fisher, R.A.; White, M.A.; Johnson, M.B.; Woodfield, B.F.; Boerio-Goates, J. Heat capacity, entropy, and magnetic properties of jarosite-group compounds. Phys. Chem. Miner. 2010, 37, 635–651. [Google Scholar] [CrossRef]
- Sánchez, J.E.; Cruz, A.; Flores, M.A.; Romero, A.; Pérez, M.; Gutiérrez, V.H.; Sánchez, R.G.; Jiménez, J.C. Waste minimization of lead paste and jarosite to recover a silver-rich alloy by the pyrometallurgical route. Recycling 2024, 9, 119. [Google Scholar] [CrossRef]
- Pérez, M.; Delgado, R.; Soto, M.; Reyes, A. Synthesis, thermodynamic, and kinetics of rubidium jarosite decomposition in calcium hydroxide solutions. Metall. Mater. Trans. B 2012, 43, 773–780. [Google Scholar] [CrossRef]
- ASTM E1269-11; Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry*. ASTM International: West Conshohocken, PA, USA, 2011. [CrossRef]
- ISO 11357-4:2021; Plastics—Differential Scanning Calorimetry (DSC)—Part 4: Determination of Specific Heat Capacity*. ISO: Geneva, Switzerland, 2021.
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; Pelton, A.D.; et al. FactSage Thermochemical Software and Databases, version 8.2. CRCT; Polytechnique Montréal and Thermfact Ltd.: Montreal, QC, Canada, 2022. [Google Scholar]
- Jak, E.; Hidayat, T.; Prostakova, V.; Shishin, D.; Shevchenko, M.; Hayes, P.C. Integrated experimental and thermodynamic modelling research for primary and recycling pyrometallurgy. In Proceedings of the EMC 2019, Düsseldorf, Germany, 23–26 June 2019; pp. 587–604. [Google Scholar]
- Kamberović, Ž.; Ranitović, M.; Manojlović, V.; Jevtić, S.; Gajić, N.; Štulović, M. Thermodynamic and kinetic analysis of jarosite Pb–Ag sludge thermal decomposition for hydrometallurgical utilization of valuable elements. J. Therm. Anal. Calorim. 2023, 148, 11799–11810. [Google Scholar] [CrossRef]
- Majzlan, J.; Stevens, R.; Boerio, J.; Woodfield, B.; Navrotsky, A.; Burns, P.; Crawford, M.; Amos, T. Thermodynamic properties, low-temperature heat-capacity anomalies, and single-crystal X-ray refinement of hydronium jarosite, (H3O)Fe3(SO4)2(OH)6. Phys. Chem. Miner. 2004, 31, 518–531. [Google Scholar] [CrossRef]
- Debye, P. Zur Theorie der spezifischen Waerme. Ann. Phys. 1912, 39, 789–839. [Google Scholar] [CrossRef]
- Bowman, S.; Pathak, A.; Agrawal, V.; Sharma, S. A simple method for obtaining heat capacity coefficients of minerals. Am. Mineral. 2024, 109, 624–627. [Google Scholar] [CrossRef]
- Guyot, F.; Richet, P. High-temperature heat capacity and phase transitions of CaTiO3 perovskite. Phys. Chem. Miner. 1993, 20, 141–146. [Google Scholar] [CrossRef]
- King, E.; Weller, M. High-temperature phase transformation and thermodynamic properties of Ca3(VO4)2. J. Am. Ceram. Soc. 2024, 107, 3094–3102. [Google Scholar] [CrossRef]
- Yi, J.; Gao, Z.; Li, S.; San, T.; Kong, X.; Yang, B.; Liu, D.; Xu, B.; Jiang, W. Separation and enrichment of Au and Ag from lead anode slime by a selective oxidation–vacuum volatilization–carbon reduction process. Metals 2024, 14, 693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).