Abstract
Improper management of anaerobic pig sludge poses significant environmental and health risks. Converting this waste into biochar to enhance methane production during anaerobic digestion (AD) presents an environmentally sound and circular solution, especially when the biochar is produced through co-pyrolysis with lignocellulosic biomass residues. This study first determined the co-pyrolysis biomass ratio (anaerobic sludge to lignocellulosic biomass) that caused the highest increase on methane yield. Subsequently, the effects of biochar dosage (6, 12, 18, 24, and 30 g/L) and particle size (0.5–1 cm, 212–355 µm, and <53 µm) on methane production were assessed. Biochar derived from up to 25 % anaerobic pig sludge increased methane yield by 74.49 ± 1.25 % without compromising its catalytic effect. Methane yield was significantly affected by both biochar dosage and particle size, with best results observed at dosages of 12–18 g/L. These findings highlight the feasibility of the co-pyrolysis of anaerobic pig sludge and lignocellulosic residues as an attractive circular solution for integrated waste management and energy production.
1. Introduction
1.1. Opportunities for Circular Management of Pig Farming Waste
Pig farming is a crucial economic activity in Mexico, providing an affordable source of nutrition for approximately 40 % of the population. Mexico ranks among the world’s leading producers of pig meat, with an annual output of 1.32 million tonnes. In 2021, Yucatan stood among the top five national producers, contributing 155,497 tonnes per year [1]. Despite its economic relevance, pig farming in Yucatan raises substantial environmental concerns, particularly its adverse impact on the region’s karstic aquifers, which serve as the primary freshwater resource [2]. Improper disposal of manure leads to deposition of heavy metals (Cu, Zn, As, and Cd) and an excess of nutrients (N, P, K, Ca, and Mg) in soil and water, presenting significant risks to both human and animal health [3,4,5].
There are various methods for manure treatment available, including composting, oxidation ponds, and anaerobic digestion (AD). Among these, AD is the most cost-effective, as it generates biogas, which contains methane, a renewable energy source. However, AD faces challenges such as prolonged start-up periods, odor emissions, and, in some cases, insufficient removal of nutrients and pathogens [6]. Additionally, the anaerobic pig sludge (APS), a waste byproduct of AD, requires further stabilization before disposal, which accounts for approximately 50 % of the total operation and maintenance costs [7]. Small- and medium-scale pig farms often resort to dehydrating APS on drying beds and applying it as a biofertilizer [8]. However, this practice risks nutrient leaching and organic pollution of the karstic aquifers [2].
In recent years, the Mexican government has introduced legislation aimed at supporting circular-economy principles. Among these, the General Circular Economy Law promotes material reuse, reducing environmental impacts and minimizing waste generation [9]. Also, the General Law for Waste Prevention and Comprehensive Management requires that livestock and wastewater treatment residues be recycled, treated, or, when uneconomical, disposed of in controlled sites [10]. However, current APS management practices frequently fail to meet these regulations. Hence, this study examines the technical feasibility of an alternative strategy for APS management, tailored for rural contexts, including challenging environments such as karstic soils. From a circular economy perspective, APS can be transformed into valuable products, reducing the need for waste disposal and reintegrating materials into pig farms. One approach is the production of biochar (BC), a versatile material with numerous applications. Notably, BC is widely used as soil amendment, either applied directly or mixed with compost or mineral fertilizers, to improve the physicochemical and biological properties of soils [11,12,13,14]. However, a potential circular solution in pig farming involves using BC derived from APS as a biogas enhancer during the AD of pig manure.
1.2. Factors Affecting Methane Production in Anaerobic Digestion
The AD process relies on four key microbial interactions: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. During hydrolysis, complex polymers are broken down by hydrolytic bacteria into simpler compounds, which acidogenic bacteria convert into volatile fatty acids (VFAs) and alcohols. The intermediates are further metabolized by acetogenic bacteria into acetate, hydrogen, and carbon dioxide—key substrates for methanogenic archaea, which produce methane. Advances in AD technology include, among many, improvements in reactor design, feedstock optimization, and process control strategies. Current research focuses on microbial community resilience, integration of AD with other waste treatment technologies, and optimization of biogas purification for sustainable energy applications [15,16]. Additionally, recent studies highlight the importance of direct interspecies electron transfer (DIET), where electrons are transferred directly between microorganisms or via conductive materials, enhancing methane production efficiency [15].
AD depends on the syntrophic metabolisms of bacteria and methanogenic archaea. As a catabolic process facilitated by diverse functional microorganisms and their extracellular enzymes, its efficiency depends on the operational stability of the anaerobic system, which in turn depends on the composition and activity of the functional microbial community [17]. Also, AD is sensitive to inhibitory factors, such as the accumulation of volatile fatty acids (VFAs), high concentrations of ammonia, metals, and toxins, all of which can interfere with the microbial community’s metabolism [18,19,20]. Biochar can mitigate these inhibition factors by absorbing and adsorbing many of these species, given its inherent properties of high porosity, large surface area, and buffering capacity [21,22]. BC also creates habitats for microbial colonization in four ways: providing a porous structure to adhere to, supplementing nutrients and trace elements, alleviating environmental stress, and accelerating electron transfer [21,23].
There is a large body of research supporting the abovementioned interactions [24,25,26]. BC supplementation promotes microbial population and diversity in digesters. The proliferation of genera such as Syntrophobacter, Clostridia, Petrimonas, and Paraclostridium has been observed and correlated to improvements in biogas production [23]. These bacteria belong to the group of electroactive microorganisms that can release intracellular electrons. Furthermore, BC can enhance the growth of electrophilic methanogens Methanotrix, Methanosarcina, and Methanocullens, which can directly accept extracellular electrons [27,28,29].
1.3. Catalytic Effect of Biochar on Anaerobic Digestion
Research indicates that BC can enhance methane production by facilitating electron transfer, promoting the growth of methanogenic microorganisms, and stabilizing the microenvironmental pH [7,30]. For instance, BC derived from lignocellulosic biomass at 500 °C and applied at a dosage of 10 g/L increased methane yields by up to 172 % [31]. Using BC derived from lignocellulosic feedstock mixed with pig manure at dosages between 7 and 14 g/L has been shown to enhance methane yields by 6.46–28.6 % in non-agitated AD systems [32,33]. While the degree of improvement varied depending on the feedstock, studies consistently report a general positive effect on AD performance [12,34,35]. For instance, BC produced from agricultural residues has been reported to enhance the degradation of COD and TS in the AD of pig manure [33,36,37]. These improvements are primarily attributed to a significant reduction in volatile fatty acid (VFA) accumulation, the presence of essential trace metals in the BC (Mn, Co, Ni, Zn, and Fe), and synergistic interactions between BC and anaerobic microorganisms [32,33,36,38].
However, BC derived from sludges has less surface area and, in general, poorer catalytic properties than BC from lignocellulosic materials. Therefore, to use APS, it is necessary to co-pyrolyze it with a better-performing biomass. Key factors influencing BC performance in AD include particle size and substrate/inoculum (S/I) ratios. Methane yield is inversely proportional to the S/I ratio at values lower than three [39], with an optimum at 1.5 ± 0.32 for pig manure AD [30,32,33,38]. There is less agreement on the behavior of particle size. Some studies conclude that powdered BC (<53 µm) exhibits a stronger enhancing effect due to its larger surface area [40,41,42]. However, others report reduced increments on methane yields with powdered BC, attributing this to undesirable compounds observed on the BC surface, or agglutination of the smaller particles [43,44,45]. In contrast, granular BC (0.5 mm–1 cm) appears to increase methane yields, with some studies associating this positive effect with the stimulated growth of Methanosarcina spp. [41,46,47]. Larger particle sizes (>1 cm), however, show minimal catalytic effects, likely due to the flotation of BC particles [35,48].
Discrepancies in results often arise from variations in biomass sources, which significantly influence the physicochemical properties of BC and its interactions within the AD process. BC derived from APS has shown a 20 % lower catalytic effect on methane yields compared to lignocellulosic BC. This was attributed to a higher ash content and lower surface area in the APS-derived BC [5]. Co-pyrolysis of APS with lignocellulosic residues may address these limitations, enhancing BC properties while recycling APS. While no studies have specifically investigated APS co-pyrolysis, research on sewage sludge co-pyrolysis with lignocellulosic materials (e.g., walnut shells and pine sawdust) indicates notable increases in BC surface area, ranging from 50 % to 391 %, using mixing ratios between 3:1 and 1:3 [49]. Similar improvements were observed with pine sawdust, wheat straw, and rice straw mixed with sewage sludge [50,51,52]. The present study is the first to explore APS co-pyrolysis with lignocellulosic residues.
1.4. Potential Feedstock for Co-Pyrolysis of APS-Derived Biochar
BC production can be achieved through various pyrolysis methods, including slow (hours), intermediate (seconds to minutes), and fast (seconds) processes. Slow pyrolysis (0.1–1 K/min) favors higher BC yields [53], resulting in greater fixed carbon content but reduced surface area [54,55]. Conversely, both intermediate (1–10 K/s) and fast (10–200 K/s) pyrolysis produce BC with larger surface areas (>100 m2/g) but lower fixed carbon content [56,57]. Table 1 summarizes the physicochemical properties of BC obtained through different pyrolysis methods.

Table 1.
Reported physicochemical properties of BC by process type. Elaborated with data from the literature [49,50,51,52,53,54,55,56,57,58,59].
Large-scale pyrolysis systems require significant investment, with costs exceeding USD 500,000 and maintenance expenses typically ranging between USD 600 and 900 per tonne of BC. These high costs render such systems impractical for rural areas [60]. In contrast, the Kon-Tiki flame-curtain pyrolyzer (KTK) is a low-cost, user-friendly alternative capable of producing BC with properties comparable to intermediate pyrolysis (see Table 1). This makes the KTK a feasible option for sub-industrial BC production in rural communities, including those near pig farms in the Global South [58,59,60].
In Mexico, pruning waste is one of the most abundant organic residues, accounting for approximately 7.9 % of total waste generation [61]. Mismanagement of these residues often leads to greenhouse gas emissions (primarily methane) and proliferation of disease-carrying pests like flies, cockroaches, and rats. Furthermore, the decomposition of pruning waste increases the risk of zoonotic and vector-borne disease transmission, while producing leachates that pollute soil and water [61,62]. Such issues are particularly evident in rural areas, where proper waste management strategies are lacking, and practices like open burning or dumping are common [63].
The pyrolysis of organic waste presents a solution with significantly lower environmental impact. A life cycle analysis of BC production using a KTK pyrolyzer estimated contributions to atmospheric carbon sequestration, offsetting greenhouse gases emissions generated during its production [64]. This aligns with circularity principles by reducing waste and closing resource loops.
All of the above provides context for the research questions of this study, regarding the circularity of pig farming: (1) Can BC derived from APS and residual lignocellulosic biomass significantly enhance methane yield in the AD of pig manure in low-efficiency digesters? (2) If so, what is the optimal ratio of APS-to-lignocellulosic biomass for maximizing methane yield? (3) What are the best BC dosage and particle size to achieve the highest methane yield?
This research represents the first report on the co-pyrolysis of APS and residual lignocellulosic biomass using a decentralized approach tailored for rural communities. It investigates the application of the resulting BC as a methane enhancer in the AD of pig manure. Residual lignocellulosic biomass sourced from tree pruning and APS collected from a local farm were co-pyrolyzed to produce BC, with variations in dosage and particle size analyzed for their effect on methane yield. The findings highlight the feasibility of this process as an innovative waste management strategy for APS, rooted in the principles of circular economy.
2. Results and Discussions
2.1. Biochar Physiochemical Properties
Biochar (BC) samples were produced through co-pyrolysis of three different mixtures of APS and lignocellulosic biomass. The samples were coded according to the mass percentage of lignocellulosic biomass (100, 75, and 50). It was hypothesized that the addition of APS would increase the ash, nitrogen (N), and volatile matter contents in the BC, while reducing surface area and fixed carbon content. The experiments aimed to determine the maximum proportion of APS that would not compromise BC quality beyond acceptable limits.
The average BC yield across all samples was 18 ± 6.37 % dry basis. This yield was mainly influenced by pyrolysis temperature, as it is known that higher temperatures lead to lower biochar yields and higher carbon content (see Table 1). Table 2 presents the physicochemical properties of the BC samples. As expected, samples with higher APS proportions were associated with increased volatile matter and ash contents, alongside reduced fixed carbon content.

Table 2.
Biochar samples’ physicochemical properties.
The ash content in the BC samples was within the lower range typically reported for sewage sludge derived BC (42.2–67.3%) [5,50], despite the addition of organic matter in samples 50 and 75. The increase in ash content is likely due to the mineral composition of the APS, which in turn is closely associated with their diet and feed supplements. Surface area measurements revealed no significant difference between samples 100 and 75, but a notable 45 % reduction was observed in sample 50. Previous studies on sewage sludge pyrolysis [5,49] have linked reductions in surface area and higher ash content in the biomass to pyrolysis temperature. While higher pyrolysis temperatures generally increase surface area under controlled conditions, the lack of temperature control in the KTK results in non-homogeneous volatilization across the kiln height. This leads to wider pore size distributions and reduced surface areas [65]. Consequently, all BC samples presented surface areas below the 100–200 m2/g typically reported for industrially produced BC [60]. Nevertheless, even with reduced surface areas, BC is still expected to produce a catalytic effect on methane yields during AD [66,67,68].
Ultimate analysis of the BC samples indicated a higher N-content in the co-pyrolyzed samples. This was expected, as APS is a N-rich substrate. Conversely, the C content did not show a significant increase, contrary to findings in another study [49], where higher lignocellulosic content correlated with increased C levels. This suggests incomplete carbonization of the feedstock materials in the KTK, likely due to the temperature gradients inherent to its operation. The lower BC yields observed further support this conclusion.
The BC samples were alkaline, with pH values ranging from 10.37 in sample 100 to 9.95 in sample 50. This alkalinity is attributed to two phenomena during pyrolysis: the release of alkaline salts from the raw materials; and the formation of organic nitrogen species, such as pyridine-like compounds. Additionally, the reduction in oxygen content during pyrolysis likely decreases acid functional groups on the BC surface, aligning with the observed oxygen content measurements [52].
Electrical conductivity (EC), an indicator of salinity, was lower in the samples with higher APS content. The EC of lignocellulosic BC was 1252.5 µS, decreasing by approximately 16 % in the APS-containing samples.
Table 3 summarizes the metal content of the BC samples, including heavy metals. All BC samples complied with the International Biochar Initiative (IBI) limits for soil remediation. However, the addition of APS led to increased concentrations of specific metals, notably chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn). These metals are commonly found in pig diets, explaining their presence in APS [69]. Although cadmium (Cd) and chromium (Cr) concentrations above 170 and 775 mg/L, respectively, have been reported as problematic for AD [70], the low concentration of these metals in the BC samples suggest they will not adversely impact AD’s performance or any other BC applications.

Table 3.
Metal content in biochar samples (all values are in mg/kg).
2.2. Effect of Biochar Addition on Methane Yield
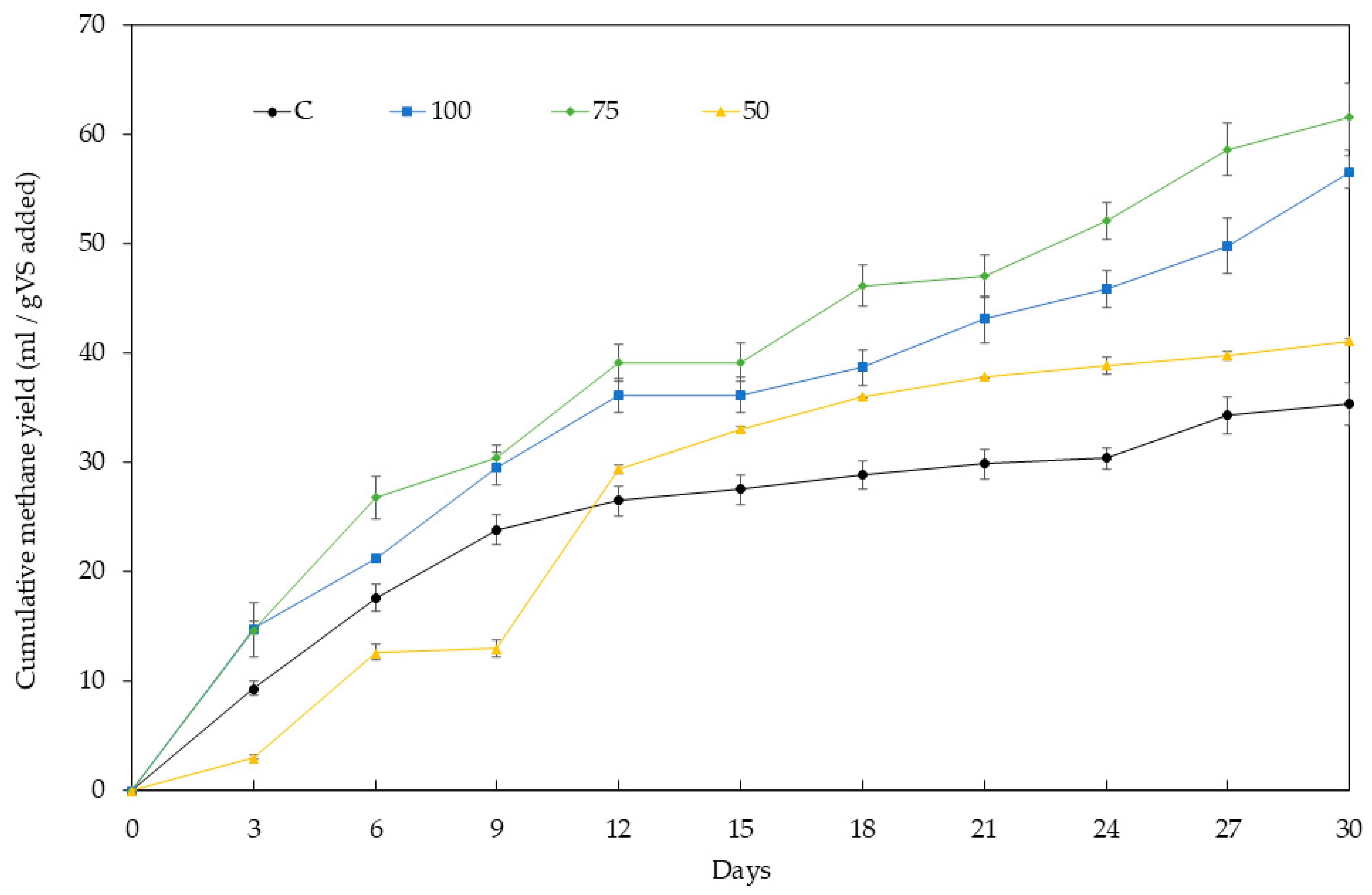
Figure 1 illustrates the cumulative methane yields observed in the AD experiments. Samples 75 and 100 exhibited substantial improvements in methane yield, with increments of 60–75 %, significantly higher than the control. Statistical analysis (p < 0.05) revealed no significant differences between these two samples. In contrast, sample 50 exhibited a smaller increase in methane yield (16.4 %), which correlates with its lower surface area. Reduced surface area may limit the BC’s ability to immobilize microorganisms [70], thus decreasing its catalytic effect. These findings are consistent with previous reports of methane yield enhancements ranging from 11 % to 100 % [34,47,71].

Figure 1.
Effect of biochar addition to cumulative methane yield. Sample codes refer to the percentage of lignocellulosic biomass in the co-pyrolysis mixture with APS.
A 3-day lag phase was observed exclusively in sample 50, likely due to differences in the surface area of the samples [35]. Previous research [72] indicates that lag phases are shorter when BC provides a greater surface area available for anaerobic bacteria to colonize. In this study, samples 75 and 100 had significantly larger surface areas (Table 2) and, consequently, no observable lag phase [47].
In these experiments, the total chemical oxygen demand (COD) reduction was 51.6–64.7 %, larger than the 41.2 % reduction observed in the control. The BC is not directly used as a substrate by anaerobic microorganisms [31], but rather the observed methane yield enhancement in the presence of BC is due to an increased degradation of the substrate (raw pig wastewater). Previous studies have shown that BC promotes inter-species electron transfer in the AD process. This is attributed to the presence of redox-active functional groups on the BC surface, which serve as electron acceptors or mediators for electron transfer, ultimately enhancing methane yields [23,73]. The functional groups that have been identified on BC’s surface and their corresponding mechanisms are [73] (a) reversible redox reactions between quinone moiety and hydroquinone moiety; (b) phenolic moiety as an electron donor and precursor to quinone; (c) redox reactions between hydroxyl and carboxyl groups on the branched carbon chain; (d) electron transfer by a graphite-like sheet in biochar; and (e) environmental persistent free-radicals donating electrons. These phenomena are likely to be present in these experiments given the observation of increased electric conductivity on the samples as the fixed carbon content increases (see Table 2).
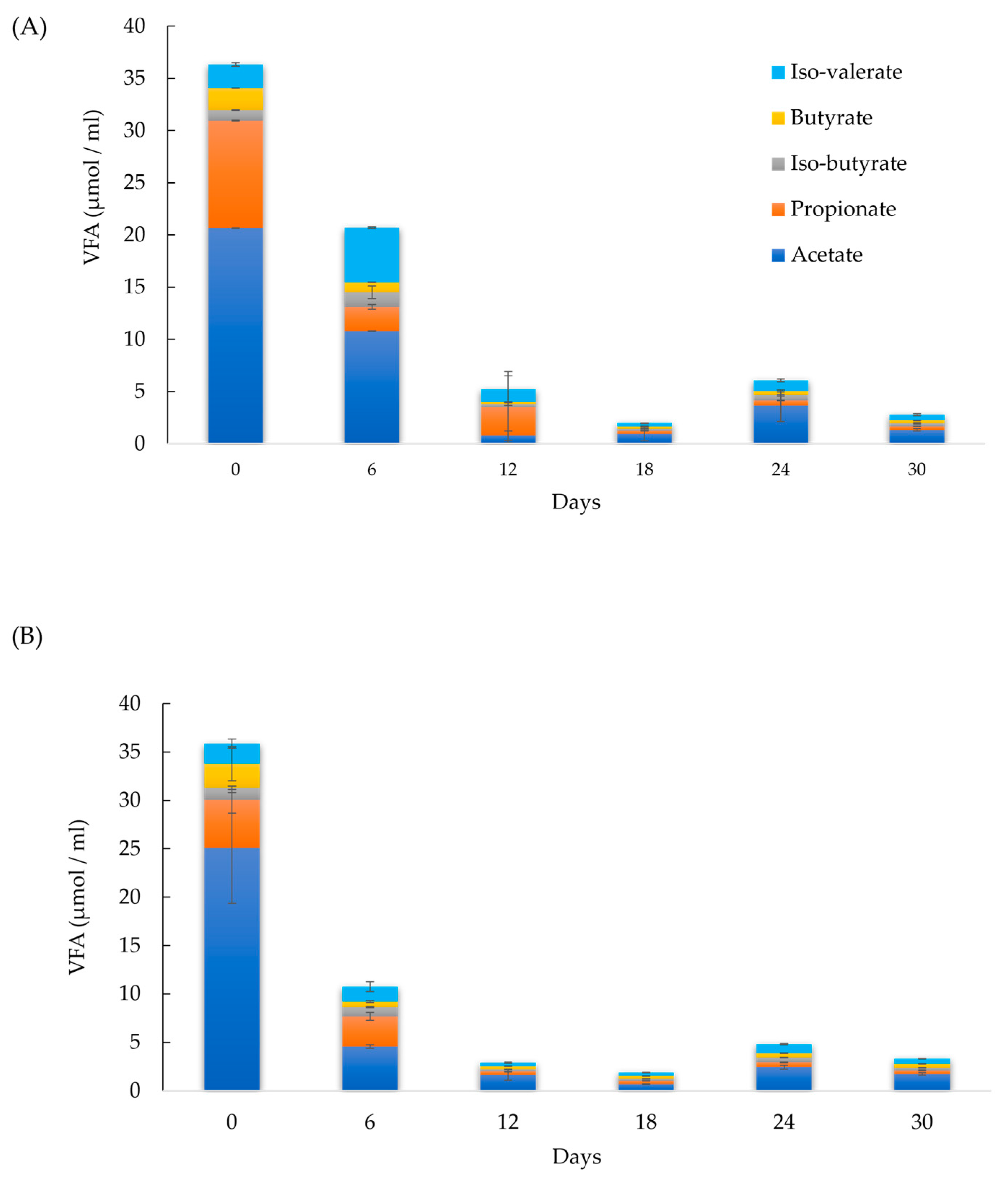
Furthermore, the presence of trace metals in BC, such as Mn, Co, Ni, Zn, and Fe (Table 3), has been shown to reduce volatile fatty acid (VFA) accumulation during AD [5,33]. A deficiency in these metal elements can destabilize the digester by promoting VFA accumulation and thus reducing methane yield [33]. This study corroborates these findings, with the acetate concentration in sample 75 decreasing by 81 % within the first six days—a significantly larger reduction compared to the control (50 %). From days 12 to 30, acetate remained the predominant VFA in sample 75, with concentrations ranging from 0.7 to1.7 µmol/mL, which were 57 % lower than the control. Propionate concentrations in sample 75 were ≤ 0.3 µmol/mL, compared to 0.3–2.7 µmol/mL in the control. This consistent trend across all AD experiments indicates that BC promotes acetate fermentation by methanogenic microorganisms. Figure 2A,B display the VFA measurements for the control group and sample 75, respectively.
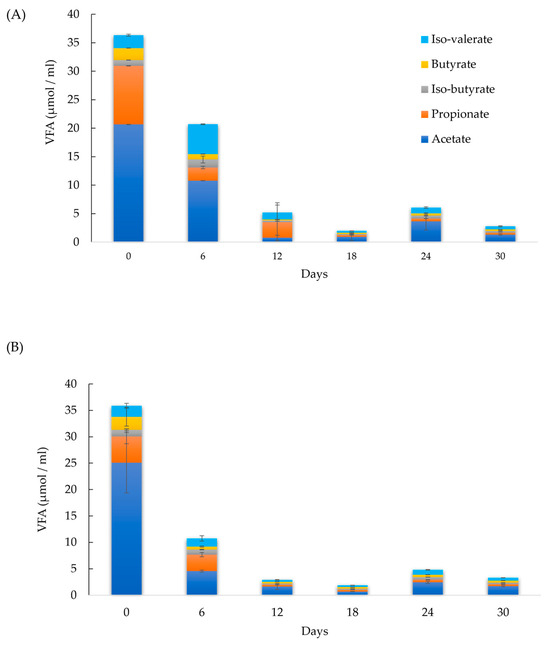
Figure 2.
Volatile fatty acid (VFA) measurements in (A) control and (B) sample 75.
The low methane yields (35–62 mL/g VS) observed after 30 days are typical for AD of pig manure in non-agitated systems. This is primarily due to poor mass transfer and an inadequate S/I ratio [37]. Of course, in fully mixed systems, methane yields are expected to be 2–6 times higher (165–380 mL/g VS) under comparable conditions of organic load and BC dosage [32,33,38]. However, incorporating agitation into the rural devices that are the aim of this study is not feasible. This limitation underscores the significance of the improvements in methane production achieved through the addition of co-pyrolysis BC within the scope of this study.
2.3. Effect of Biochar Addition on pH, CODs, and VS
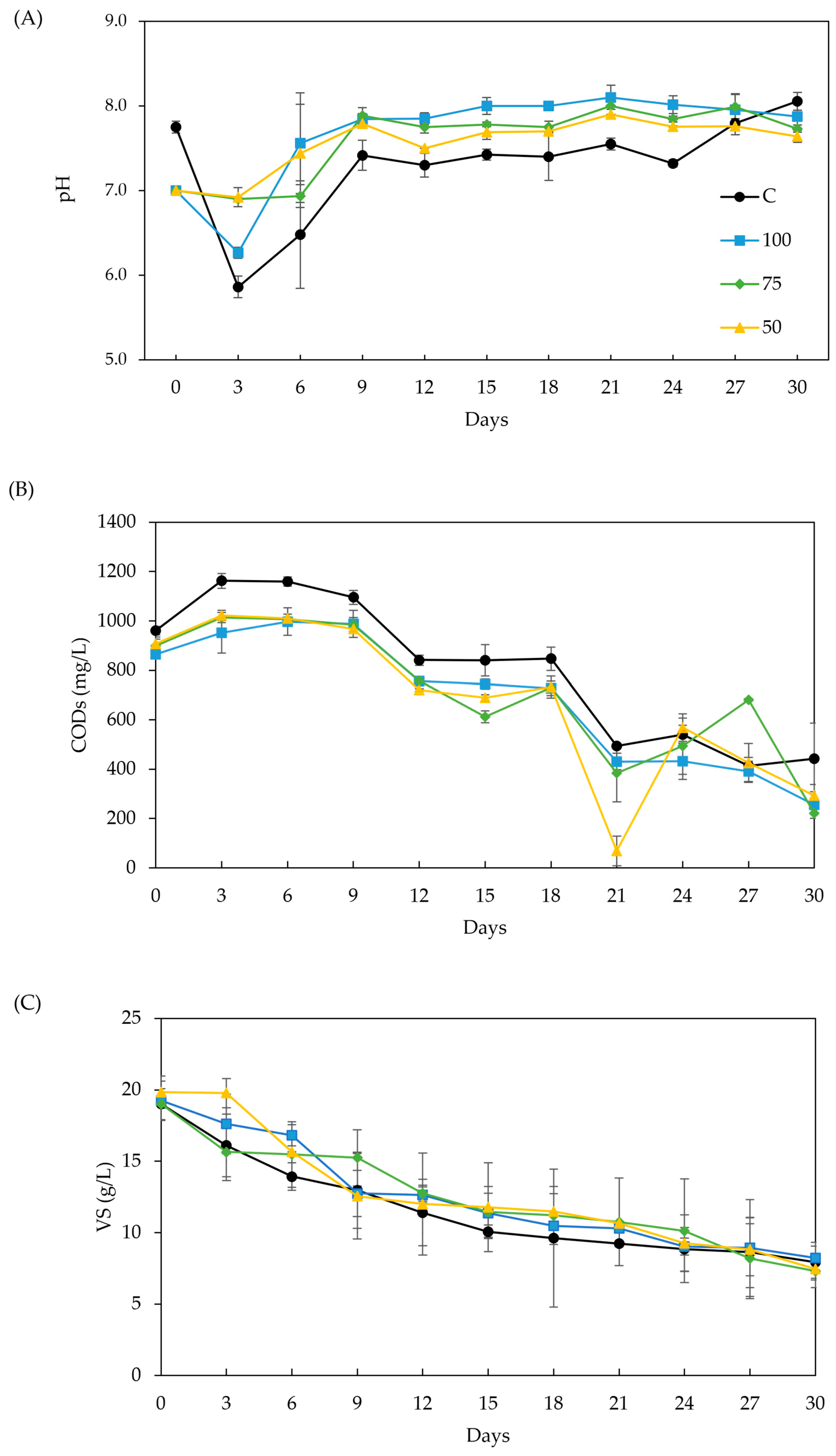
Figure 3A–C show the effects of BC addition on pH, soluble COD (CODs), and VS, respectively. The ideal pH range for AD is 6.6–7.6. The pH value is affected mainly by the VFA concentrations. As anaerobic microorganisms consumed the VFA (as shown in Figure 2), the pH increased. The alkaline nature of BC makes it act as a buffer, promoting a lower pH reduction in the first 3 days, compared to the control, and stabilizing the digester at a higher pH value [5,33] (Figure 3A). This trend is clearer in samples 50 and 75, which accumulated significantly more acetate and propionate in the first 6 days. The pH dropped below 6.6 in the control, resulting in significantly lower methane production (p < 0.05), 35–37 % less than in samples 100 and 75 (see Figure 1). A study on fruit waste digestion with sewage sludge BC [66] also correlated a similar pH drop to a decline in methane yield.
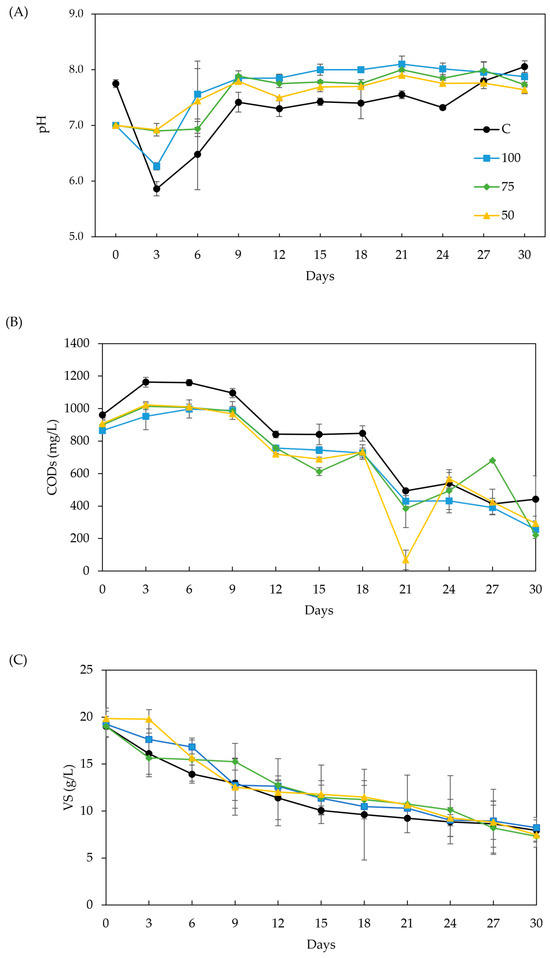
Figure 3.
Effect of biochar addition to AD process stability: (A) pH, (B) VS, and (C) CODs.
CODs provides a more reliable measure for monitoring the digestion process compared to total COD, as the latter can be affected by the carbon contributed by the added BC in the medium. Variations in CODs reflect fluctuations in the degree of hydrolysis and the presence of organic compounds within the reactors [35]. As shown in Figure 3B, CODs initially increased by 10–76 % during the first 6 days, followed by a sharp decline. The CODs’ removal was not significantly different among the treatments, but it was significantly larger (p < 0.05) than the control. This trend indicates enhanced hydrolysis and faster degradation of organic matter in BC treatments. Such behavior may be attributed to the BC’s ability to enhance enzyme activity, including protease, alpha-amylase, phosphate acetyl transferase, adenylate kinase, and coenzyme F420, all of which facilitate organic degradation and methane production [74]. Also, the control exhibited slower VFA reduction and higher CODs values before stabilizing. Throughout the experiment, acetate and propionate were the predominant VFAs, with BC promoting rapid acetate consumption by methanogens and adsorbing long-chain VFA (like n-valerate), thus enhancing methane production [5,30]. CODs is a direct indicator of VFA levels in the digestate [39], with the data showing strong correspondence. Previous studies have demonstrated that the appropriate addition of BC supports the abundance of functional genes responsible for the metabolism of carbohydrates, proteins, and lipids. This suggests that BC promotes rapid bio-utilization of organic substrates, thereby improving the efficiency of hydrolysis and methanogenesis [19,75].
Figure 3C shows the trends of VS concentration along the AD. While it was expected to find a directly proportional correlation of VS removal and the accumulated methane production [76], there were no significant differences in VS removal across treatments. Another study [34] also reports this lack of correlation between methane production and VS removal and attributed it to a masking effect on VS removal by the growth of microbial communities on the BC surface as the methane yield increased. While this effect could not be verified in this work, the results were enough to select sample 75 for further quantification of the methane enhancement varying BC dose and particle size.
2.4. Effect of Biochar Dose and Particle Size on Methane Yield
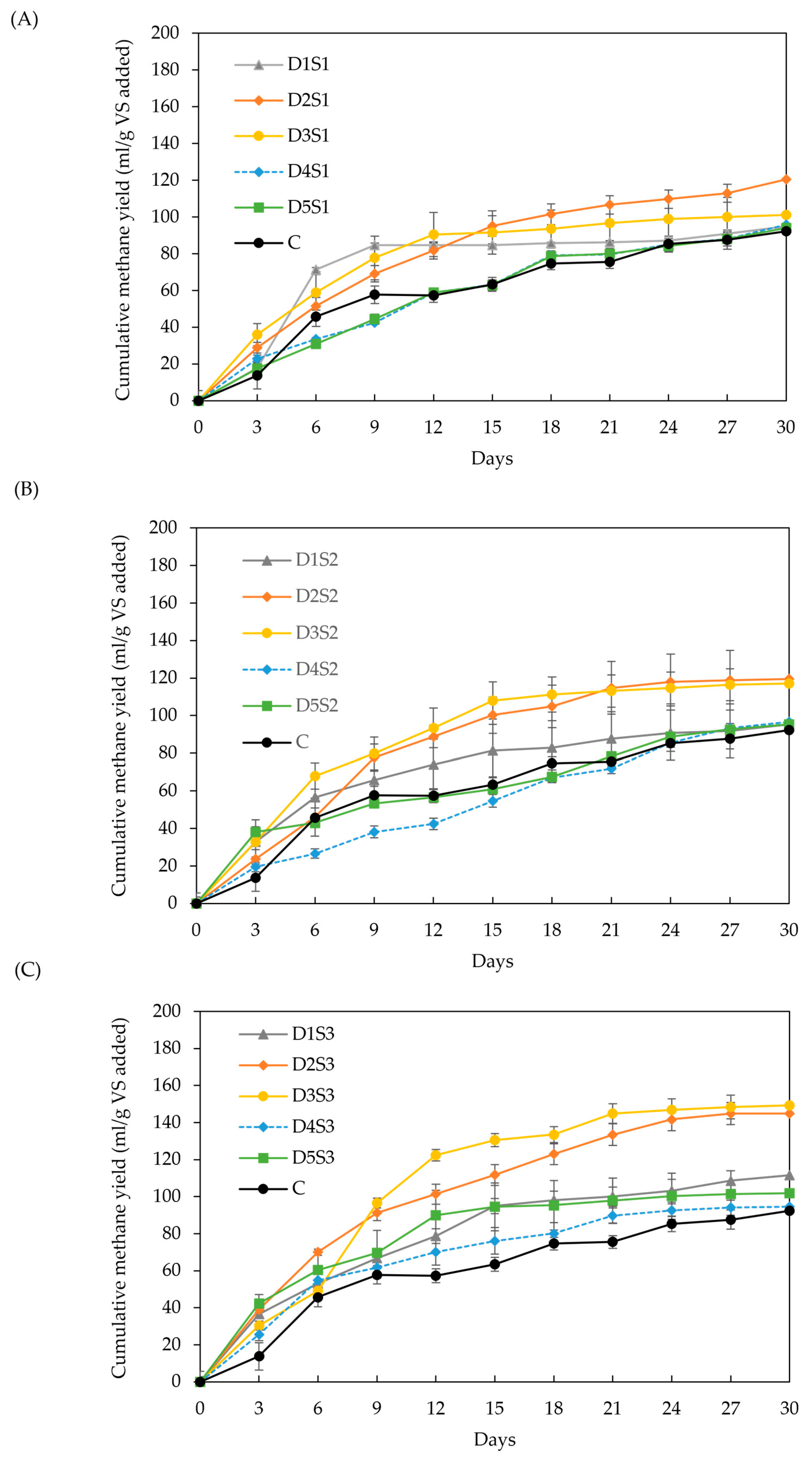
Figure 4A–C show the cumulative methane yields for various BC doses and particle sizes, as described in Section 3.2.2. Both BC dose and particle size had a significant impact on methane yield (p > 0.05). Cumulative yield increased by 10–62 % relative to the control for doses D2 and D3, irrespective of particle size. These results exceed those reported in the literature under comparable conditions [32,33], likely due to factors such as feedstock variation and an improved balance between surface area and microbial adhesion sites, which enhance methanogenesis efficiency at these dose range [35,46]. In contrast, doses D1, D4, and D5 showed no significant difference compared to the control for particle sizes S2 and S3. Smaller particles, such as S1, showed a tendency to clump together, reducing the effective surface area for microbial adhesion [77].
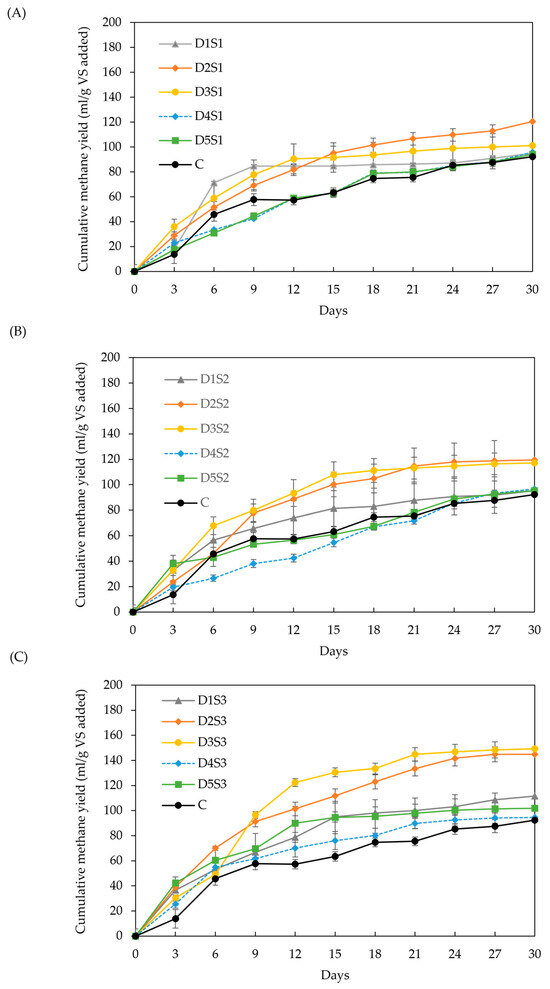
Figure 4.
Effect of biochar dose and particle size on methane yield: (A) particle size S1 (<53 µm), (B) particle size S2 (212–355 µm), and (C) particle size S3 (0.5–1 cm). Doses D1 to D5 are 6, 12, 18, 24, and 30 g/L, respectively.
Across the three particle sizes, increasing the BC dose (D1: 6 g/L up to D5: 30 g/L) produced a consistent trend: intermediate doses D2 and D3 grouped within a homogeneous group, significantly outperforming D1, D4, D5, and the control. Higher BC doses are expected to provide more adsorption sites for CO2 removal, thereby increasing methane content in the biogas. However, excessive BC concentrations can inhibit mass transfer, as reported in another study [35]. Other research recommended BC doses of 10–15 g/L, with higher doses (16.6 g/L) suitable for systems with organic loads between 8.3 and 33.3 g VS/L [34,35]. This study identified optimal doses between 16 and 18 g/L, depending on particle size, resulting in methane yield enhancements of 30 % (S1), 29 % (S2), and 62 % (S3).
The effect of BC particle size on methane yield is inconsistent in the literature. Smaller particles are generally expected to enhance methane yield due to their larger surface area, as observed in the particle size range of 75–150 µm for BC derived from fruitwoods [41]. However, other studies [47,78], including this one, observe the opposite trend. Small particle sizes, such as powder BC, can lead to ion imbalance, enzyme inhibition, oxidative stress, and microbial competition for BC pore sites [35,79]. Moreover, BC-amended digesters may reduce microbial community diversity due to selective microbial enrichment. While granular BC (>1 mm) has been shown to favor the growth of Methanosarcina spp., thereby improving methane yields, smaller particles tend to support fewer methanogenic communities, resulting in less gaseous mass transfer and methane production. This phenomenon is known as cationic toxicity and can also occur at high BC doses [35,41,46,47].
In this study, the lowest methane-yield increments were observed at the highest BC doses (24 and 30 g/L) combined with the smallest particle sizes (<53 µm and 212–355 µm). Additionally, the lowest COD values (Figure 5) were recorded for particle size S3, which corresponded to the highest methane yields. These findings support the observation of a reduced enhancement effect when smaller BC particles are used.

Figure 5.
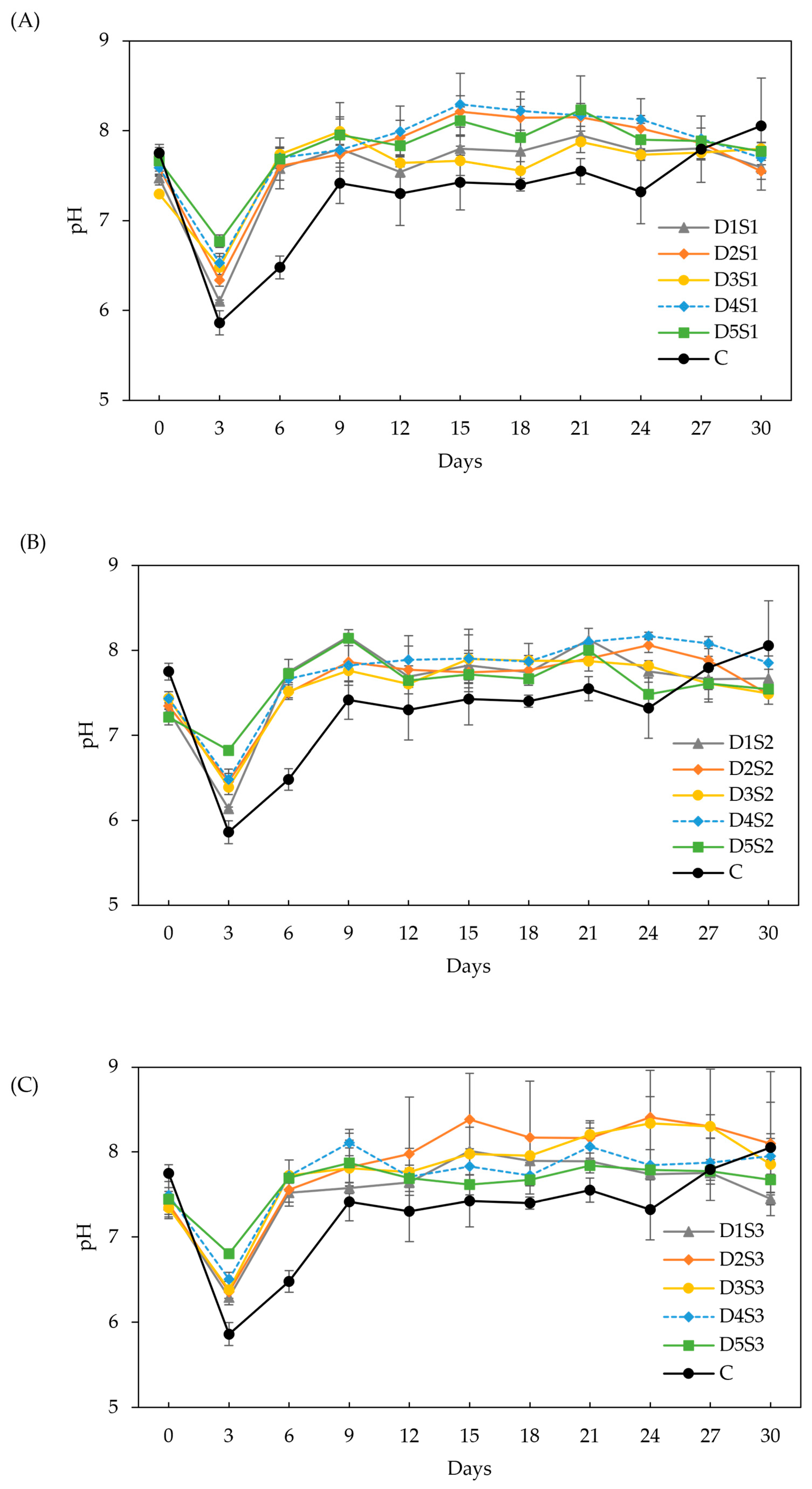
Effect of biochar dose and particle size on pH. (A) particle size S1 (<53 µm), (B) particle size S2 (212–355 µm), (C) particle size S3 (0.5–1 cm). Doses D1 to D5 are 6, 12, 18, 24, and 30 g/L, respectively.
In general, the organic load in pig farm effluents exhibits continuous variations from 0.48 to 21.5 g/L as volatile suspended solids (VSS) and depends mainly on the type of farm and animal age. The highest organic loads, between 10.13 and 21.5 g/L as VSS, are typically associated with fattening farms [80]. Considering the organic load utilized in this study (as described in Section 3.2.1), the results suggest that BC addition at doses between 12 and 18 g/L with particle size S3 represents a suitable strategy for methane enhancement in fattening farms.
2.5. Effect of Biochar Dose and Particle Size on pH, CODs and VS
Figure 5, Figure 6 and Figure 7 show the effects of BC dose and particle size on pH, CODs, and VS, respectively.
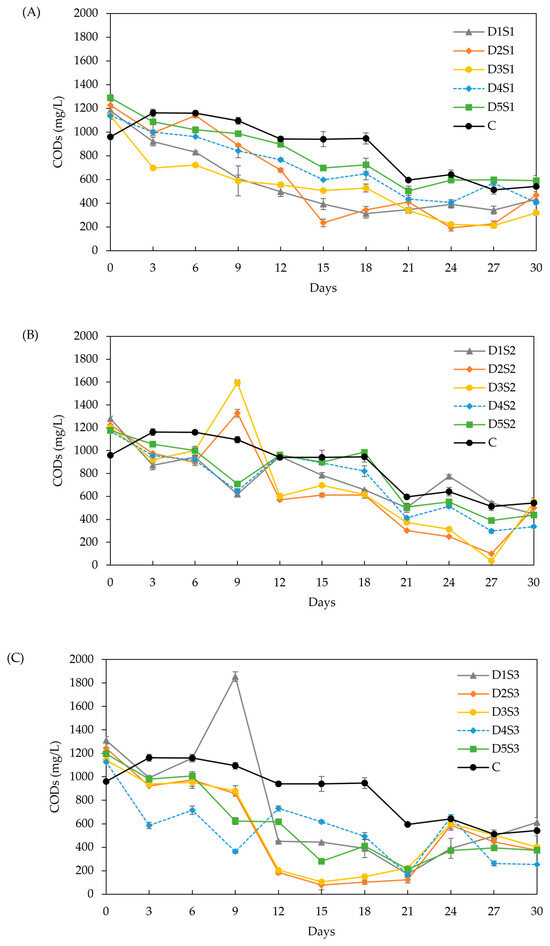
Figure 6.
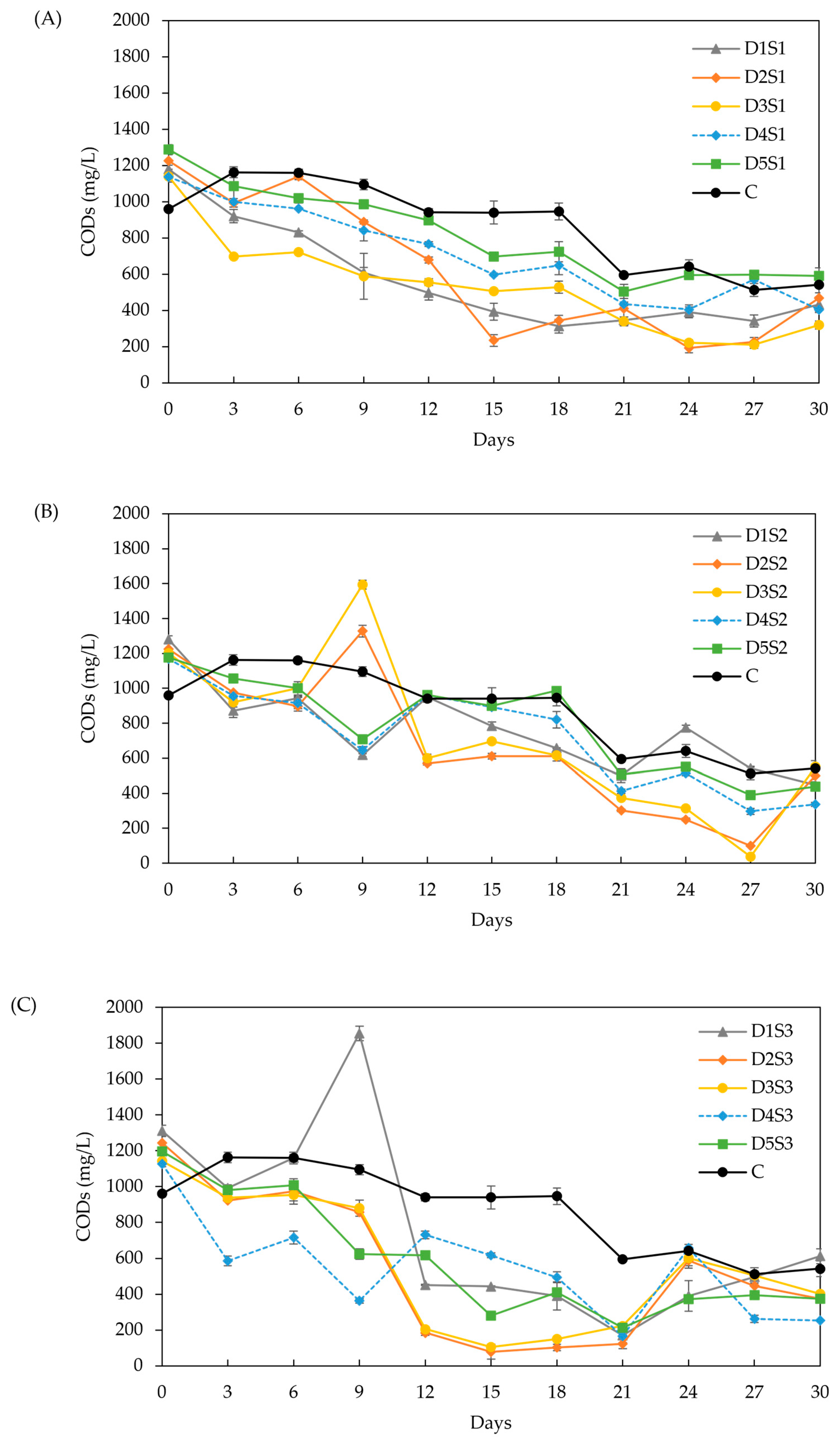
Effect of biochar dose and particle size on CODs: (A) particle size S1 (<53 µm), (B) particle size S2 (212–355 µm), and (C) particle size S3 (0.5–1 cm). Doses D1 to D5 are 6, 12, 18, 24, and 30 g/L, respectively.
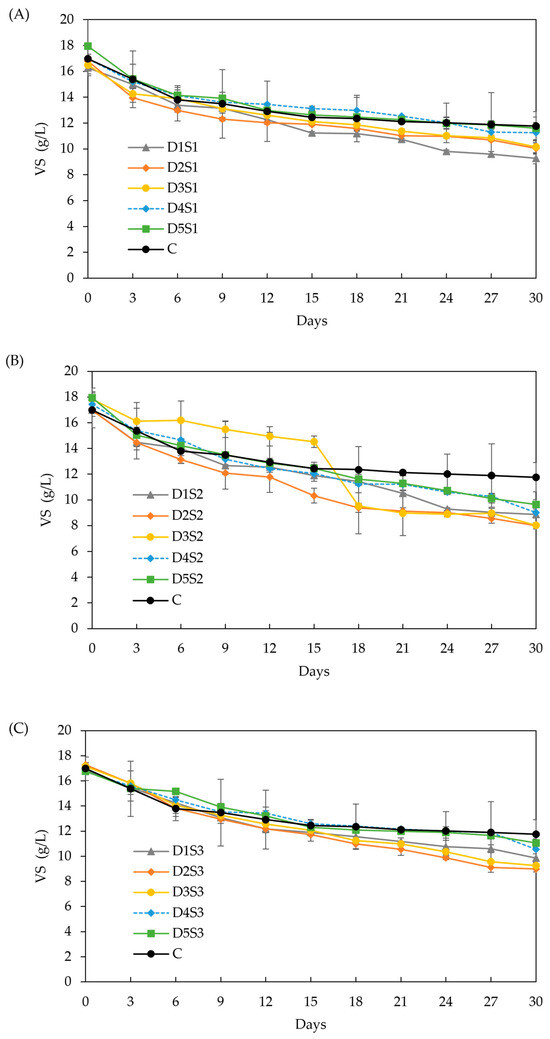
Figure 7.
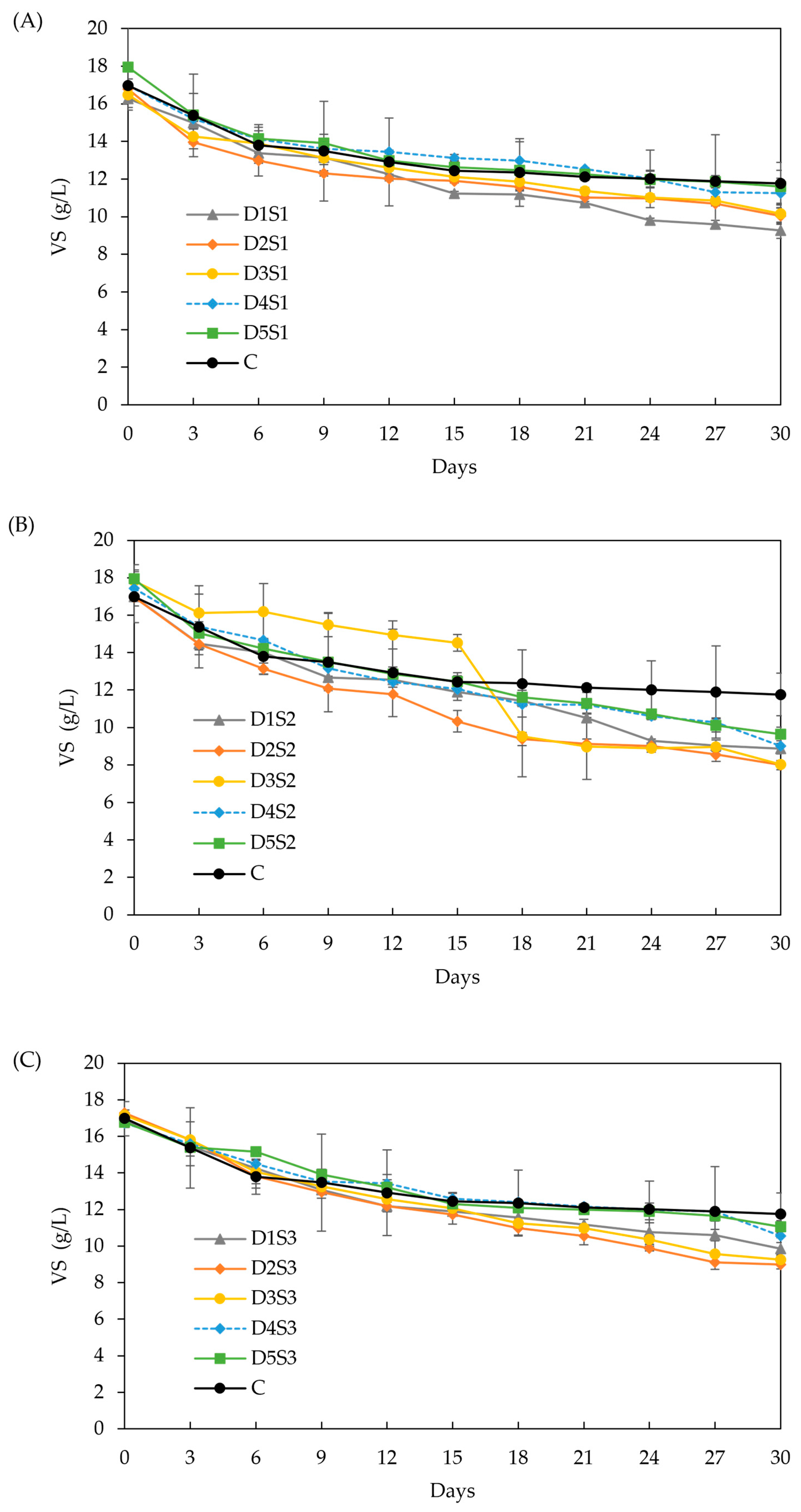
Effect of biochar dose and particle size on VS. (A) particle size S1 (<53 µm), (B) particle size S2 (212–355 µm), and (C) particle size S3 (0.5–1 cm). Doses D1 to D5 are 6, 12, 18, 24, and 30 g/L, respectively.
pH (Figure 5): Like previous experiments, pH dropped during the first three days, followed by a significant increase on day 6. The buffer capacity of BC increased at higher doses. In the long term, pH stabilized and remained constant throughout the experiment, with no significant differences (p > 0.05) between BC treatments. In contrast, the control group showed a gradual increase in pH over time. These results suggest that all BC doses and particle sizes have sufficient buffer capacity to regulate VFA accumulation, supporting previous findings [32], where no significant differences were observed in pH across a wide range of BC particle sizes.
CODs (Figure 6): The initial CODs value was around 1250 mg/L for all treatments, with a general decline observed over time and occasional increases that correlated well with pH variations. The decrease in CODs was faster than in the control group. Doses D2 and D3 resulted in the lowest CODs, with no significant difference (p > 0.05) between them. This correlates with the higher methane yields and lower VFA concentrations (approximately 3.5 µmol/mL from days 12–30). Particle size S1 exhibited higher propionate concentrations throughout the experiment (3.3–1.2 µmol/mL), indicating that powder BC is less effective in promoting acetate fermentation for methane production [48]. Particle size S3 showed the lowest total VFA concentrations (4.5–2.3 µmol/mL), with acetate making up 60–40 % of the total VFA. These results align with other studies [29,72], which demonstrated that both powder and granular BC prevented VFA accumulation, particularly propionic acid, leading to a 13.3 % increase in methane yield. The low CODs observed with S3 further support the enhanced VFA conversion to methane.
VS (Figure 7): All BC treatments achieved significantly higher VS removal than the control (30.75 %). The highest VS removal was observed in treatments D2-S2 and D2-S3 (55.01% and 47.97%, respectively), which coincided with the highest methane yield enhancement (62 %). However, no statistically significant differences were found among most treatments, making it difficult to directly correlate higher VS removal with enhanced methane yield. Likewise, the absence of significant differences in total VS removal prevents establishing a clear link to the effect of BC dose or particle size. Like the preliminary experiment, methane yield improvements may occur without proportional VS removal because microbial communities growing on the BC surface can contribute to methane production while masking VS reductions, as demonstrated in earlier research [81].
3. Materials and Methods
3.1. Biochar, Anaerobic Pig Sludge, and Wastewater Obtention
Residual biomass samples were sourced from urban pruning residues in the metropolitan area of Yucatan. Tree species included Leucaena leucocephala, Piscidia piscipula, Terminalia catappa, Cocos nucifera, and Albizia lebbeck. Branches with diameters ≤5 cm were selected, and lengths were adjusted to approximately 1 m to fit the pyrolyzer. A quartering process was applied to obtain a representative biomass sample for pyrolysis.
APS was collected from the sludge blowdown exit of the primary anaerobic reactor at a local pig farm. The farm operates with pigs at multiple stages of production, with a diet predominantly based on cereals, mainly corn. This specific feeding regime is expected to influence the physicochemical properties of APS, reported in Table 4. The APS was press-filtered, and the resulting solid material was left in the sun to achieve a moisture content of ≤18 %. This moisture adjustment was necessary to ensure complete carbonization during pyrolysis.

Table 4.
Physicochemical properties of anaerobic pig sludge and raw wastewater used as inoculum and substrate in each digester.
Pyrolysis was performed using a Kon-Tiki open-flame pyrolyzer (KTK), following a standard operating procedure detailed elsewhere [60]. Three mixed biomass samples (coded 100, 50, and 25) were separately pyrolyzed. The KTK operation began by igniting a fire at the KTK base, followed by the addition of biomass layers. Combustion of the upper layers provided heat and consumed oxygen in the lower layers, where pyrolysis occurred. The process of adding APS–biomass layers for co-pyrolysis with the lignocellulosic residues was the following: to ensure APS carbonization, lignocellulosic biomass was added first to maintain the upper flame, while APS was subsequently added to fill gaps within the kiln. The layering process was repeated until each sample was fully pyrolyzed. The temperature gradient in KTK kilns ranges between 600 and 900 °C [60], with gas recirculation at the kiln’s mouth, enhancing combustion of pyrolysis vapors and incomplete combustion products [64].
The APS used as inoculum in the AD experiment was collected from the same source as the feedstock APS prior to press-filtering. Raw wastewater, used as the substrate for the AD experiments, was obtained from the farm’s main pumping station and refrigerated at 4 °C to prevent premature degradation of organic matter.
3.2. Anaerobic Digestion Experiments
The ability of BC to improve methane production during AD depends on several factors, as discussed in the Introduction. In this study, three key variables were assessed: (1) the proportion of APS in the pyrolysis feedstock, (2) BC particle size, and (3) BC dosage in the digester. The APS proportion was evaluated during the first experimental stage, while the latter two variables were studied simultaneously in a 3 × 5 factorial experiment.
Experimental conditions were designed to replicate rural domestic-scale biodigesters, which are typically bag-type systems without mechanical agitation. Hence, experiments were conducted in batch reactors with daily manual agitation, an approach previously employed by several authors to evaluate the effects of BC on biogas and methane production [30,33,34,37,81].
3.2.1. Variation in the APS Proportion in the Pyrolysis Feedstock
Three BC samples (100, 75, and 50) were compared to a negative control (no BC addition, coded as “C”). The experiments were conducted in 500 mL Erlenmeyer flasks equipped with rubber screw caps, maintaining a working volume of 450 mL. Flasks were placed in a temperature-controlled water bath to sustain a mesophilic range of 37 ± 1 °C. A solution of deionized water, substrate, and inoculum was prepared to achieve a S/I ratio of 1.5 on VS basis [34]. Table 4 summarizes the physicochemical properties of the APS inoculum and raw wastewater substrate in the experiments after the S/I adjustment. It also includes reference data from a standard multistage pig farm in Mexico [80].
The BC dose was set to 12 g/L [35], with a particle size range of 0.5–1 cm [35,40] for all treatments except the control. Prior to their addition to the biodigesters, the BC samples were hydrated with 50 mL of deionized water for 24 h to prevent flotation.
A blank reactor containing only inoculum and deionized water was set up under identical operational conditions to quantify the biogas production solely attributable to the inoculum degradation. To maintain anaerobic conditions, nitrogen gas was bubbled into each reactor for five minutes before starting the experiments.
The AD experiments were conducted over 30 days in all reactors. Parameters monitored every third day included biogas volume, methane content, chemical oxygen demand (COD, total and soluble fraction), pH, and VS. VFAs were measured every sixth day.
All treatments were performed in duplicate unless otherwise specified. A one-way analysis of variance (ANOVA) was conducted using Statgraphics Centurion 19® to determine statistically significant differences in the measured variables at a 5% confidence level (p < 0.05).
3.2.2. Variation in Biochar Particle Size and Dose
The BC samples were ground using a ball mill and sieved to obtain three particle sizes, coded as S1 (<53 µm), S2 (212–355 µm), and S3 (0.5–1 cm). These particle size ranges were selected based on the literature to ensure observable changes in biogas and methane productions [35,40,41,47]. Five BC doses (6, 12, 18, 24, and 30 g/L) were also tested, coded D1 to D5, respectively. A 3 × 5 factorial experimental design was employed, resulting in 15 treatments conducted in duplicate unless otherwise noted. A multifactorial ANOVA was performed using Statgraphics® software at a 5 % confidence level (p < 0.05) to assess the statistical significance of treatment effects.
3.3. Analytical Methods
The analytical methods are explained in detail in the following sections. All analyses of BC samples were performed in triplicate.
3.3.1. Proximate Analyses, pH, and Electrical Conductivity (EC)
Proximate analyses (moisture, ash, volatile matter, and fixed carbon) were conducted using gravimetric techniques on 1 g samples with a particle size range of 850–149 µm [82]: moisture content was determined by drying samples at 105 °C for two hours in a convection oven; measured using a FB1310M muffle furnace (ThermoFisher Scientific, Waltham, MA, USA), volatile matter was determined by heating samples at 900 °C for 10 min, while ash content was measured at 650 °C for six hours with the furnace slightly open for oxygen entry; and fixed carbon was calculated by difference. Electrical conductivity and pH were determined using a multiparameter tester (Oaklon PCSTestr 35, Environmental Express, Charleston, SC, USA). Five-gram samples (ground to <2 mm) were mixed with 50 mL of deionized water, shaken for 1 h, rested for 30 min, and then measured.
3.3.2. Ultimate Analysis (C, N, S, and H)
Ultimate analysis was performed using a Flash 2000 elemental analyzer equipped with a MAS 300 autosampler (ThermoFisher Scientific, Waltham, MA, USA). Samples of 3–4 mg of sample (ground to <300 µm) were placed in tin containers for all element determinations except oxygen, which was measured in silver containers. Vanadium pentoxide (V2O5) was used as a catalyst for sulfur analysis.
3.3.3. Surface Area and Porosity
Surface area and porosity were determined using a BET Surface Area Analyzer (Quantachrome Nova 2200e, SpectraLab, Markham, ON, Canada). Samples of 100 mg (ground to <2 mm) were degassed at 300 °C for five hours prior to analysis. Adsorption/desorption points (30 total) ranged from 0.005 to 0.990 P/P0, using nitrogen as the adsorbate at −195.15 °C (77 K).
3.3.4. Heavy Metal Analysis
Heavy metal analysis was conducted using an Agilent 4200 MP-AES plasma spectrometer (Agilent, Santa Clara, CA, USA). A 1 g sample was reduced to ash and subjected to acid digestion for 30 min (1:4 nitric acid and hydrochloric acid solution). The digested solution was diluted to 100 mL of deionized water, filtered through a 0.22 µm filter, and analyzed.
3.3.5. Liquid Sample Analysis
For AD experiments, triplicate analyses were performed for TS, VS, TSS, COD, and CODs, as well as pH [83]. TS, VS, and TSS were measured using gravimetric techniques. COD and CODs were determined with the close reflux method with Hach® reaction tubes. pH was measured using a Thermo Orion 410 (Weber Scientific, Hamilton, NY, USA) pH analyzer.
COD and VS contributions from BC were also quantified in digesters containing deionized water and BC at identical doses and particle sizes than the test experiments, and the results were subtracted from AD sample measurements.
3.3.6. Volatile Fatty Acids
Volatile fatty acids were analyzed in the soluble fraction obtained by spin-drying at 12,000 rpm for 15 min and filtration through a 0.45 µm filter. VFAs (acetate, propionate, isobutyrate, butyrate, and isovalerate) were measured using a gas chromatograph (GC) Agilent 7890A (Agilent, Santa Clara, CA, USA) equipped with a flame ionization detector and a Nukol fused silica capillary column (15 m × 0.53 mm × 0.5 µm). A SUPELCO 46975-U VFA mix was used as a reference standard. Operating conditions were taken from another study [84].
3.3.7. Methane Content in Biogas
The methane content in biogas was determined using the same GC setup and operating conditions as in the VFA analysis, with a TG-Bond Q column (ThermoFisher Scientific, Waltham, MA, USA), 30 m × 0.32 mm × 10 µm. Methane gas (>99.97 %) was used as the reference standard. The injector temperature was set to 250 °C, the detector to 300 °C, and the oven to 250 °C for 2 min. Manual injections were conducted in split mode (50:1), using helium as the carrier gas (1 mL/min flow rate), with a post-run flow rate of 6.5 mL/min. Hydrogen (30 mL/min) and air (250 mL/min) served as fuel and utility gases, respectively.
4. Conclusions
This work is the first to study the co-pyrolysis of APS with residual lignocellulosic biomass and its potential for enhancing methane yields in pig manure AD. The results demonstrated that methane yield increased by up to 74 % in non-agitated AD systems relative to the control—a substantially higher increment compared to reports using BC derived from the co-pyrolysis of sewage sludge. The best co-pyrolysis mixture was found to be 25 % APS and 75 % lignocellulosic biomass. Increasing APS proportion in the co-pyrolysis mixture will hinder the ability of the resulting BC to enhance AD. The experimental conditions emulated rural practices by incorporating manual operation of Kon-Tiki kilns, the use of pig manure from local farms, and pruning residues from small parcels, as well as non-agitated anaerobic digesters. All of these were used to foster the circularity of waste management in rural areas without access to advanced pyrolysis or AD technology.
Both BC dose and particle size significantly influenced methane yield and process stability. The best performance was achieved with granular BC (0.5–1 cm) at doses between 12 and 18 g/L. In contrast, smaller particle sizes combined with higher BC doses led to particle agglomeration and cationic toxicity, which ultimately reduced methane improvement. While further research is necessary to understand the interactions between BC dose and particle size at a fundamental level, the findings presented here justify moving to demonstration-scale trials.
Additionally, the BC analysis demonstrated that the BC from APS co-pyrolysis has low metallic element content, reducing concerns about its ultimate disposal on soils or leaching to water basins. This highlights the potential of APS co-pyrolysis as a safe waste management strategy.
In addition to the direct benefit of the increased energy production from AD, this strategy has the potential to reduce water and soil contamination by reducing the direct deposition or treatment of APS into soil. For instance, in the regional context of this research, an annual discharge of approximately 2.5 million kg COD in the state of Yucatan can be avoided.
Author Contributions
Conceptualization, C.O.D.L., D.C.V. and J.C.S.R.; methodology, software formal analysis, and investigation, C.O.D.L., E.A.A.-C., J.M.B.-L., D.E.P.-C. and J.C.S.R.; validation and supervision, J.E.R.E., S.B.-R. and D.C.V.; formal analysis, C.O.D.L. and J.C.S.R.; resources, D.C.V.; data curation, J.C.S.R.; writing—original draft preparation, C.O.D.L.; writing—review and editing, project administration, and funding acquisition, J.C.S.R. and D.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT) graduate studies scholarship CVU-780382.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors want to acknowledge Faculty of Chemical Engineering at Universidad Autonoma de Yucatan and Centro de Investigacion Cientifica de Yucatan for generously providing access to their facilities, equipment, and resources. During the preparation of this manuscript, the authors used ChatGPT-4o and MS Copilot Pro for the purposes of reviewing English grammar and improving conciseness. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AD | anaerobic digestion |
| APS | anaerobic pig sludge |
| BC | biochar |
| COD | chemical oxygen demand |
| CODs | chemical oxygen-demand soluble fraction |
| KTK | Kon-Tiki kiln |
| S/I | substrate-to-inoculum ratio |
| TS | total solids |
| TSS | total suspended solids |
| VFAs | volatile fatty acids |
| VSs | volatile solids |
| VSSs | volatile suspended solids |
References
- Servicio Nacional de Sanidad-Inocuidad y Calidad Agroalimentaria México, Entre Los Principales Productores y Consumidores de Carne de Cerdo En América Latina y El Mundo. Available online: https://www.gob.mx/senasica/prensa/mexico-entre-los-principales-productores-y-consumidores-de-carne-de-cerdo-en-america-latina-y-el-mundo-313553 (accessed on 26 April 2025).
- Méndez, N.R.; Castillo, B.E.; Vázquez, B.E.; Briceño, P.O.; Coronado, P.V.; Pat, C.R.; Garrico, V.P.; Garrido, V.P. Estimación Del Potencial Contaminante de Las Granjas Porcinas y Avícolas Del Estado de Yucatán Estimation of the Polluting Potential of Poultry and Swine Farms in the State of Yucatan. Ing. Rev. Acad. FI-UADY 2009, 13, 13–21. [Google Scholar]
- Díaz-Vázquez, D.; Alvarado-Cummings, S.C.; Meza-Rodríguez, D.; Senés-Guerrero, C.; de Anda, J.; Gradilla-Hernández, M.S. Evaluation of Biogas Potential from Livestock Manures and Multicriteria Site Selection for Centralized Anaerobic Digester Systems: The Case of Jalisco, Mexico. Sustainability 2020, 12, 3527. [Google Scholar] [CrossRef]
- Ramírez-Islas, M.E.; Güereca, L.P.; Sosa-Rodriguez, F.S.; Cobos-Peralta, M.A. Environmental Assessment of Energy Production from Anaerobic Digestion of Pig Manure at Medium-Scale Using Life Cycle Assessment. Waste Manag. 2020, 102, 85–96. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Wang, Y.; Yang, C. Impacts of Different Biochar Types on the Anaerobic Digestion of Sewage Sludge. RSC Adv. 2019, 9, 42375–42386. [Google Scholar] [CrossRef]
- Parra Huertas, R. Anaerobic Digestión: Biotechnological Mechanisms in Waste Water Treatments and Their Application in Food Industry. Prod. Limpia 2015, 10, 142–159. [Google Scholar] [CrossRef]
- Liu, X.; Meng, Q.; Wu, F.; Zhang, C.; Tan, X.; Wan, C. Enhanced Biogas Production in Anaerobic Digestion of Sludge Medicated by Biochar Prepared from Excess Sludge: Role of Persistent Free Radicals and Electron Mediators. Bioresour. Technol. 2022, 347, 126422. [Google Scholar] [CrossRef] [PubMed]
- González, F.T.; Vallejos, G.G.; Silveira, J.H.; Franco, C.Q.; García, J.; Puigagut, J. Treatment of Swine Wastewater with Subsurface-Flow Constructed Wetlands in Yucatán, Mexico: Infuence of Plant Species and Contact Time. Water Sa 2009, 35, 335–342. [Google Scholar] [CrossRef]
- Senado de la República Mexicana. Gaceta Del Senado; 2019. Available online: https://www.senado.gob.mx/65/gaceta_del_senado/documento/101326 (accessed on 26 April 2025).
- Gobierno de México. Ley General Para La Prevención y Gestión Integral de Los Residuos; 2003, pp. 16–17. Available online: https://www.diputados.gob.mx/LeyesBiblio/pdf/LGPGIR.pdf (accessed on 26 April 2025).
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.H.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. Biochar for Soil Amendment. In Char and Carbon Materials Derived from Biomass: Production, Characterization and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–146. ISBN 9780128148945. [Google Scholar]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, Present, and Future of Biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar Organic Fertilizers from Natural Resources as Substitute for Mineral Fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Karim, M.R.; Halim, M.A.; Gale, N.V.; Thomas, S.C. Biochar Effects on Soil Physiochemical Properties in Degraded Managed Ecosystems in Northeastern Bangladesh. Soil. Syst. 2020, 4, 69. [Google Scholar] [CrossRef]
- Liu, X.; Luo, J.; Xu, Q.; Lu, Q.; Ni, B.-J.; Wang, D. Roles and Opportunities of Quorum Sensing in Natural and Engineered Anaerobic Digestion Systems. Water Res. 2025, 275, 123190. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Salvador, A.F.; Pereira, L.; Alves, M.M. Methane Production and Conductive Materials: A Critical Review. Environ. Sci. Technol. 2018, 52, 10241–10253. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, L.; Zhou, X. A Systematical Review of Blackwater Treatment and Resource Recovery: Advance in Technologies and Applications. Resour. Conserv. Recycl. 2023, 197, 107066. [Google Scholar] [CrossRef]
- Zhao, W.; Jeanne Huang, J.; Hua, B.; Huang, Z.; Droste, R.L.; Chen, L.; Wang, B.; Yang, C.; Yang, S. A New Strategy to Recover from Volatile Fatty Acid Inhibition in Anaerobic Digestion by Photosynthetic Bacteria. Bioresour. Technol. 2020, 311, 123501. [Google Scholar] [CrossRef]
- Li, X.; Jin, J.; Zhang, X.; Xu, F.; Zhong, J.; Yin, Z.; Qi, H.; Wang, Z.; Shuai, J. Quantifying the Optimal Strategy of Population Control of Quorum Sensing Network in Escherichia Coli. NPJ Syst. Biol. Appl. 2021, 7, 35. [Google Scholar] [CrossRef]
- Cui, M.-H.; Chen, L.; Zhang, X.-D.; Zhang, Q.; Pan, H.; Liu, L.-Y.; Liu, H.; Wang, A.-J. Recent Advancements on the Migration and Transformation of Hydrophobic Pharmaceutically Active Compounds in Anaerobic Digestion Process. Chem. Eng. J. 2022, 446, 136902. [Google Scholar] [CrossRef]
- Zhao, D.; Yan, B.; Liu, C.; Yao, B.; Luo, L.; Yang, Y.; Liu, L.; Wu, F.; Zhou, Y. Mitigation of Acidogenic Product Inhibition and Elevated Mass Transfer by Biochar during Anaerobic Digestion of Food Waste. Bioresour. Technol. 2021, 338, 125531. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; He, S.; Zhao, Q.; Wei, L. A Review of Biochar in Anaerobic Digestion to Improve Biogas Production: Performances, Mechanisms and Economic Assessments. Bioresour. Technol. 2021, 341, 125797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hu, T.; Ma, H.; Li, D.; Zhao, Q.; Jiang, J.; Wei, L. A Review of Microbial Responses to Biochar Addition in Anaerobic Digestion System: Community, Cellular and Genetic Level Findings. Bioresour. Technol. 2024, 391, 129929. [Google Scholar] [CrossRef]
- Sun, Z.; Feng, L.; Li, Y.; Han, Y.; Zhou, H.; Pan, J. The Role of Electrochemical Properties of Biochar to Promote Methane Production in Anaerobic Digestion. J. Clean. Prod. 2022, 362, 132296. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Ding, C.; Wang, S.; Zhao, C.; Yin, W.; Wang, B.; Yang, R.; Wang, X. Remediation of Lead and Cadmium Co-Contaminated Mining Soil by Phosphate-Functionalized Biochar: Performance, Mechanism, and Microbial Response. Chemosphere 2023, 334, 138938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, F.; Tsui, T.-H.; Yoh, K.; Sun, J.; Loh, K.-C.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Microbial Succession Analysis Reveals the Significance of Restoring Functional Microorganisms during Rescue of Failed Anaerobic Digesters by Bioaugmentation of Nano-Biochar-Amended Digestate. Bioresour. Technol. 2022, 352, 127102. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A.; Thakur, N.; Salama, E.-S. Biochar Addition Augmented the Microbial Community and Aided the Digestion of High-Loading Slaughterhouse Waste: Active Enzymes of Bacteria and Archaea. Chemosphere 2022, 309, 136535. [Google Scholar] [CrossRef]
- Li, Q.; Gao, X.; Liu, Y.; Wang, G.; Li, Y.-Y.; Sano, D.; Wang, X.; Chen, R. Biochar and GAC Intensify Anaerobic Phenol Degradation via Distinctive Adsorption and Conductive Properties. J. Hazard. Mater. 2021, 405, 124183. [Google Scholar] [CrossRef]
- Saif, I.; Thakur, N.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; Usman, M.; et al. Biochar Assisted Anaerobic Digestion for Biomethane Production: Microbial Symbiosis and Electron Transfer. J. Environ. Chem. Eng. 2022, 10, 107960. [Google Scholar] [CrossRef]
- Cheng, Q.; De Los Reyes, F.L.; Call, D.F. Amending Anaerobic Bioreactors with Pyrogenic Carbonaceous Materials: The Influence of Material Properties on Methane Generation. Environ. Sci. 2018, 4, 1794–1806. [Google Scholar] [CrossRef]
- Wang, P.; Peng, H.; Adhikari, S.; Higgins, B.; Roy, P.; Dai, W.; Shi, X. Enhancement of Biogas Production from Wastewater Sludge via Anaerobic Digestion Assisted with Biochar Amendment. Bioresour. Technol. 2020, 309, 123368. [Google Scholar] [CrossRef]
- Yang, S.; Chen, Z.; Wen, Q. Impacts of Biochar on Anaerobic Digestion of Swine Manure: Methanogenesis and Antibiotic Resistance Genes Dissemination. Bioresour. Technol. 2021, 324, 124679. [Google Scholar] [CrossRef]
- Herrmann, C.; Sánchez, E.; Schultze, M.; Borja, R. Comparative Effect of Biochar and Activated Carbon Addition on the Mesophilic Anaerobic Digestion of Piggery Waste in Batch Mode. J. Environ. Sci. Health A Tox Hazard. Subst. Environ. Eng. 2021, 56, 946–952. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic Promotion of Syntrophic Methane Production from Anaerobic Digestion of Complex Organic Wastes by Biochar: Performance and Associated Mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef]
- Zhang, L.; Lim, E.Y.; Loh, K.-C.; Ok, Y.S.; Lee, J.T.E.; Shen, Y.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Biochar Enhanced Thermophilic Anaerobic Digestion of Food Waste: Focusing on Biochar Particle Size, Microbial Community Analysis and Pilot-Scale Application. Energy Convers. Manag. 2020, 209, 112654. [Google Scholar] [CrossRef]
- Sánchez, E.; Herrmann, C.; Maja, W.; Borja, R. Effect of Organic Loading Rate on the Anaerobic Digestion of Swine Waste with Biochar Addition. Environ. Sci. Pollut. Res. 2021, 28, 38455–38465. [Google Scholar] [CrossRef]
- Jiang, B.; Lin, Y.; Lun, Y.; Xu, Z. Optimization of Methane Production in a Swine Manure–Rice Straw Anaerobic Co-Digestion Process with Sycamore Sawdust Biochar Application. Int. J. Environ. Sci. Technol. 2021, 18, 2197–2208. [Google Scholar] [CrossRef]
- Gómez, X.; Meredith, W.; Fernández, C.; Sánchez-García, M.; Díez-Antolínez, R.; Garzón-Santos, J.; Snape, C.E. Evaluating the Effect of Biochar Addition on the Anaerobic Digestion of Swine Manure: Application of Py-GC/MS. Environ. Sci. Pollut. Res. 2018, 25, 25600–25611. [Google Scholar] [CrossRef]
- González-Fernández, C.; García-Encina, P.A. Impact of Substrate to Inoculum Ratio in Anaerobic Digestion of Swine Slurry. Biomass Bioenergy 2009, 33, 1065–1069. [Google Scholar] [CrossRef]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of Eco-Compatible Biochar in Anaerobic Digestion to Relieve Acid Stress and Promote the Selective Colonization of Functional Microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, J.; Zhai, L.; Luo, T.; Mei, Z.; Liu, H. Achievements of Biochar Application for Enhanced Anaerobic Digestion: A Review. Bioresour. Technol. 2019, 292, 122058. [Google Scholar] [CrossRef]
- Xiao, L.; Lichtfouse, E.; Kumar, P.S.; Wang, Q.; Liu, F. Biochar Promotes Methane Production during Anaerobic Digestion of Organic Waste. Environ. Chem. Lett. 2021, 19, 3557–3564. [Google Scholar] [CrossRef]
- Brown, A.E.; Adams, J.M.M.; Grasham, O.R.; Camargo-Valero, M.A.; Ross, A.B. An Assessment of Different Integration Strategies of Hydrothermal Carbonisation and Anaerobic Digestion of Water Hyacinth. Energies 2020, 13, 5983. [Google Scholar] [CrossRef]
- Brown, A.E.; Hammerton, J.M.; Camargo-valero, M.A.; Ross, A.B. Integration of Hydrothermal Carbonisation and Anaerobic Digestion for the Energy Valorisation of Grass. Energies 2022, 15, 3495. [Google Scholar] [CrossRef]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Bray, D.G.; Ross, A.B. The Influence of Biochar Augmentation and Digestion Conditions on the Anaerobic Digestion of Water Hyacinth. Energies 2022, 15, 2524. [Google Scholar] [CrossRef]
- Lü, F.; Luo, C.; Shao, L.; He, P. Biochar Alleviates Combined Stress of Ammonium and Acids by Firstly Enriching Methanosaeta and Then Methanosarcina. Water Res. 2016, 90, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Deng, Y.F.; Wang, F.; Davaritouchaee, M.; Yao, Y.Q. A Review on Biochar-Mediated Anaerobic Digestion with Enhanced Methane Recovery. Renew. Sustain. Energy Rev. 2019, 115, 109373. [Google Scholar] [CrossRef]
- Johnravindar, D.; Patria, R.D.; Lee, J.T.E.; Zhang, L.; Tong, Y.W.; Wang, C.H.; Ok, Y.S.; Kaur, G. Syntrophic Interactions in Anaerobic Digestion: How Biochar Properties Affect Them? Sustain. Environ. 2021, 7, 1945282. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar Produced from the Co-Pyrolysis of Sewage Sludge and Walnut Shell for Ammonium and Phosphate Adsorption from Water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast Co-Pyrolysis of Sewage Sludge and Lignocellulosic Biomass in a Conical Spouted Bed Reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Deng, S.; Tan, H.; Wang, X.; Yang, F.; Cao, R.; Wang, Z.; Ruan, R. Investigation on the Fast Co-Pyrolysis of Sewage Sludge with Biomass and the Combustion Reactivity of Residual Char. Bioresour. Technol. 2017, 239, 302–310. [Google Scholar] [CrossRef]
- Huang, H.J.; Yang, T.; Lai, F.Y.; Wu, G. qiang Co-Pyrolysis of Sewage Sludge and Sawdust/Rice Straw for the Production of Biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, S.; Verma, S.; Sar, T.; Sarsaiya, S.; Ravindran, B.; Liu, T.; Sindhu, R.; Patel, A.K.; Binod, P.; et al. Production and Beneficial Impact of Biochar for Environmental Application: A Comprehensive Review. Bioresour. Technol. 2021, 337, 125451. [Google Scholar] [CrossRef] [PubMed]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A Review On The Comparison Between Slow Pyrolysis And Fast Pyrolysis On The Quality Of Lignocellulosic And Lignin-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, J.S. Production of Biochars by Intermediate Pyrolysis and Activated Carbons from Oak by Three Activation Methods Using CO2. J. Anal. Appl. Pyrolysis 2014, 107, 116–122. [Google Scholar] [CrossRef]
- Kazawadi, D.; Ntalikwa, J.; Kombe, G. A Review of Intermediate Pyrolysis as a Technology of Biomass Conversion for Coproduction of Biooil and Adsorption Biochar. J. Renew. Energy 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Cornelissen, G.; Pandit, N.R.; Taylor, P.; Pandit, B.H.; Sparrevik, M.; Schmidt, H.P. Emissions and Char Quality of Flame-Curtain “Kon Tiki” Kilns for Farmer-Scale Charcoal/Biochar Production. PLoS ONE 2016, 11, e0154617. [Google Scholar] [CrossRef] [PubMed]
- Flesch, F.; Berger, P.; Robles-Vargas, D.; Santos-Medrano, G.E.; Rico-Martínez, R. Characterization and Determination of the Toxicological Risk of Biochar Using Invertebrate Toxicity Tests in the State of Aguascalientes, México. Appl. Sci. 2019, 9, 1706. [Google Scholar] [CrossRef]
- Karananidi, P.; Som, A.M.; Loh, S.K.; Bachmann, R.T. Flame Curtain Pyrolysis of Oil Palm Fronds for Potential Acidic Soil Amelioration and Climate Change Mitigation. J. Environ. Chem. Eng. 2020, 8, 103982. [Google Scholar] [CrossRef]
- Pérez Méndez, M.A.; Martínez Hernández, M.R. Manejo Alternativo de Los Residuos de Jardinería. Kuxulkab’ 2015, 14, 5–11. [Google Scholar] [CrossRef]
- Krystosik, A.; Njoroge, G.; Odhiambo, L.; Forsyth, J.E.; Mutuku, F.; LaBeaud, A.D. Solid Wastes Provide Breeding Sites, Burrows, and Food for Biological Disease Vectors, and Urban Zoonotic Reservoirs: A Call to Action for Solutions-Based Research. Front. Public. Health 2020, 7, 405. [Google Scholar] [CrossRef]
- Tauro, R.; Manrique, S.; Franch-Pardo, I.; Charre-Medellin, J.F.; Ortega-Riascos, C.E.; Soria-González, J.A.; Armendáriz-Arnez, C. Spatial Expansion of Avocado in Mexico: Could the Energy Use of Pruning Residues Offset Orchard GHG Emissions? Environ. Dev. Sustain. 2023, 26, 27325–27350. [Google Scholar] [CrossRef]
- Ramírez López, R.P.; Cabañas Vargas, D.; Aguilera-Cauich, E.A.; Sacramento Rivero, J.C. Life Cycle Assessment of Biochar from Residual Lignocellulosic Biomass Using Kon-Tiki Kilns: Applications in Soil Amendment and Wastewater Filtration. Recycling 2024, 9, 125. [Google Scholar] [CrossRef]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A Review of Biochar Properties and Their Roles in Mitigating Challenges with Anaerobic Digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Rene, E.R.; Dupont, C.; Wongrod, S.; van Hullebusch, E.D. Anaerobic Digestion of Fruit Waste Mixed With Sewage Sludge Digestate Biochar: Influence on Biomethane Production. Front. Energy Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Xu, S.; Duan, Y.; Zou, S.; Liu, H.; Luo, L.; Wong, J.W.C. Evaluations of Biochar Amendment on Anaerobic Co-Digestion of Pig Manure and Sewage Sludge: Waste-to-Methane Conversion, Microbial Community, and Antibiotic Resistance Genes. Bioresour. Technol. 2022, 346, 126400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mao, X.; Pi, L.; Wu, T.; Hu, Y. Adsorptive and Capacitive Properties of the Activated Carbons Derived from Pig Manure Residues. J. Environ. Chem. Eng. 2019, 7, 103066. [Google Scholar] [CrossRef]
- Lan, W.; Yao, C.; Luo, F.; Jin, Z.; Lu, S.; Li, J.; Wang, X.; Hu, X. Effects of Application of Pig Manure on the Accumulation of Heavy Metals in Rice. Plants 2022, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, N.; Lu, G.; Dang, Z. Effects of Pyrolysis Temperature and Holding Time on Physicochemical Properties of Swine-Manure-Derived Biochar. Waste Biomass Valorizat. 2020, 11, 613–624. [Google Scholar] [CrossRef]
- Wang, X.; Deng, S.; Tan, H.; Adeosun, A.; Vujanović, M.; Yang, F.; Duić, N. Synergetic Effect of Sewage Sludge and Biomass Co-Pyrolysis: A Combined Study in Thermogravimetric Analyzer and a Fixed Bed Reactor. Energy Convers. Manag. 2016, 118, 399–405. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of Biochar on the Anaerobic Digestion of Citrus Peel Waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, Y.; Dionysiou, D.D.; Sharma, V.K.; Ma, X. Biochar as a Novel Carbon-Negative Electron Source and Mediator: Electron Exchange Capacity (EEC) and Environmentally Persistent Free Radicals (EPFRs): A Review. Chem. Eng. J. 2022, 429, 132313. [Google Scholar] [CrossRef]
- Qi, Q.; Sun, C.; Zhang, J.; He, Y.; Wah Tong, Y. Internal Enhancement Mechanism of Biochar with Graphene Structure in Anaerobic Digestion: The Bioavailability of Trace Elements and Potential Direct Interspecies Electron Transfer. Chem. Eng. J. 2021, 406, 126833. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, M.; Li, J.; Yao, Y.; Tang, J.; Niu, Q. The Dosage-Effect of Biochar on Anaerobic Digestion under the Suppression of Oily Sludge: Performance Variation, Microbial Community Succession and Potential Detoxification Mechanisms. J. Hazard. Mater. 2022, 421, 126819. [Google Scholar] [CrossRef]
- Husain, A. Mathematical Models of the Kinetics of Anaerobic Digestion—A Selected Review. Biomass Bioenergy 1998, 14, 561–571. [Google Scholar] [CrossRef]
- Sai, Y.W.; Lee, K.M. Enhanced Cellulase Accessibility Using Acid-Based Deep Eutectic Solvent in Pretreatment of Empty Fruit Bunches. Cellulose 2019, 26, 9517–9528. [Google Scholar] [CrossRef]
- Lü, F.; Liu, Y.; Shao, L.; He, P. Powdered Biochar Doubled Microbial Growth in Anaerobic Digestion of Oil. Appl. Energy 2019, 247, 605–614. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for Environmental Sustainability in the Energy-Water-Agroecosystem Nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Garzón-Zúñiga, M.A.; Buelna, G. Caracterización de Aguas Residuales Porcinas y Su Tratamiento Por Diferentes Procesos En México. Rev. Int. De Contam. Ambient. 2014, 30, 65–79. [Google Scholar]
- Shen, R.; Jing, Y.; Feng, J.; Luo, J.; Yu, J.; Zhao, L. Performance of Enhanced Anaerobic Digestion with Different Pyrolysis Biochars and Microbial Communities. Bioresour. Technol. 2020, 296, 122354. [Google Scholar] [CrossRef] [PubMed]
- Camps-Arbestain, M.; Shen, Q.; Wang, T.; van Zwieten, L.; Novak, J. Biochar: A Guide to Analytical Methods; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781486305094. [Google Scholar]
- Greenberg, A.E.; Clescert, L.S.; Eaton, A.D. Standard Methods for the Examination of Water and Watewater; American Public Health Assn: Washington, DC, USA, 1992. [Google Scholar]
- Lizama, A.C.; Figueiras, C.C.; Herrera, R.R.; Pedreguera, A.Z.; Ruiz Espinoza, J.E. Effects of Ultrasonic Pretreatment on the Solubilization and Kinetic Study of Biogas Production from Anaerobic Digestion of Waste Activated Sludge. Int. Biodeterior. Biodegrad. 2017, 123, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).