Abstract
Polyethylene terephthalate (PET) products are ubiquitous in daily life, offering convenience but posing significant environmental challenges due to their persistence and the difficulty of recycling them. Improper disposal of waste PET contributes to severe pollution and resource loss. Chemical degradation has emerged as one of the most effective methods for recovering and reusing waste PET. This article introduces a catalytic glycolysis strategy for efficient and environmentally sustainable PET recycling using potassium-rich biomass, specifically banana peels. The study demonstrated that K2O and K2CO3, derived from calcined banana peels, significantly catalyze the glycolysis of PET. Under optimal conditions, complete degradation of PET was achieved within 1.5 h at 180 °C, without additional chemical reagents. Product distribution confirmed that high-purity bis(2-hydroxyethyl) terephthalate could be obtained. The interaction between K2CO3 and ethylene glycol plays a critical role in determining the competition between glycolysis and alkaline hydrolysis. Furthermore, Density Functional Theory calculations provided valuable insights into the transesterification process during glycolysis. The reaction system also demonstrated excellent compatibility with colored PET products. This study successfully realized the simultaneous recycling of post-consumer PET and banana peels, offering a novel and sustainable approach to waste valorization.
1. Introduction
The rapid increase in global plastic consumption, particularly polyethylene terephthalate (PET), has resulted in significant environmental challenges, rendering PET recycling an urgent and essential pursuit. PET is extensively utilized in packaging, textiles, and construction due to its versatility, durability, and cost effectiveness, substantially contributing to plastic waste accumulation [1]. Despite its utility, PET’s non-biodegradable nature leads to long-term environmental pollution, with substantial amounts ending up in landfills or entering into ecosystems, posing threats to marine life and human health [2]. The growing demand for PET, driven by its extensive applications, has exacerbated waste management issues. For instance, global PET bottle recycling rates remain low, with only about 30% of waste PET being recycled, while the rest contributes to environmental degradation [3]. This underscores the urgent need for effective recycling strategies to mitigate the environmental impact of waste PET [4]. Technological advancements in PET recycling, including mechanical [5], pyrolytic [6], chemical [7,8], and biological methods, provide promising solutions. Chemical recycling, in particular, has gained attention for its ability to depolymerize PET into its monomers, facilitating the production of high-quality recycled PET (rPET) suitable for food-grade applications.
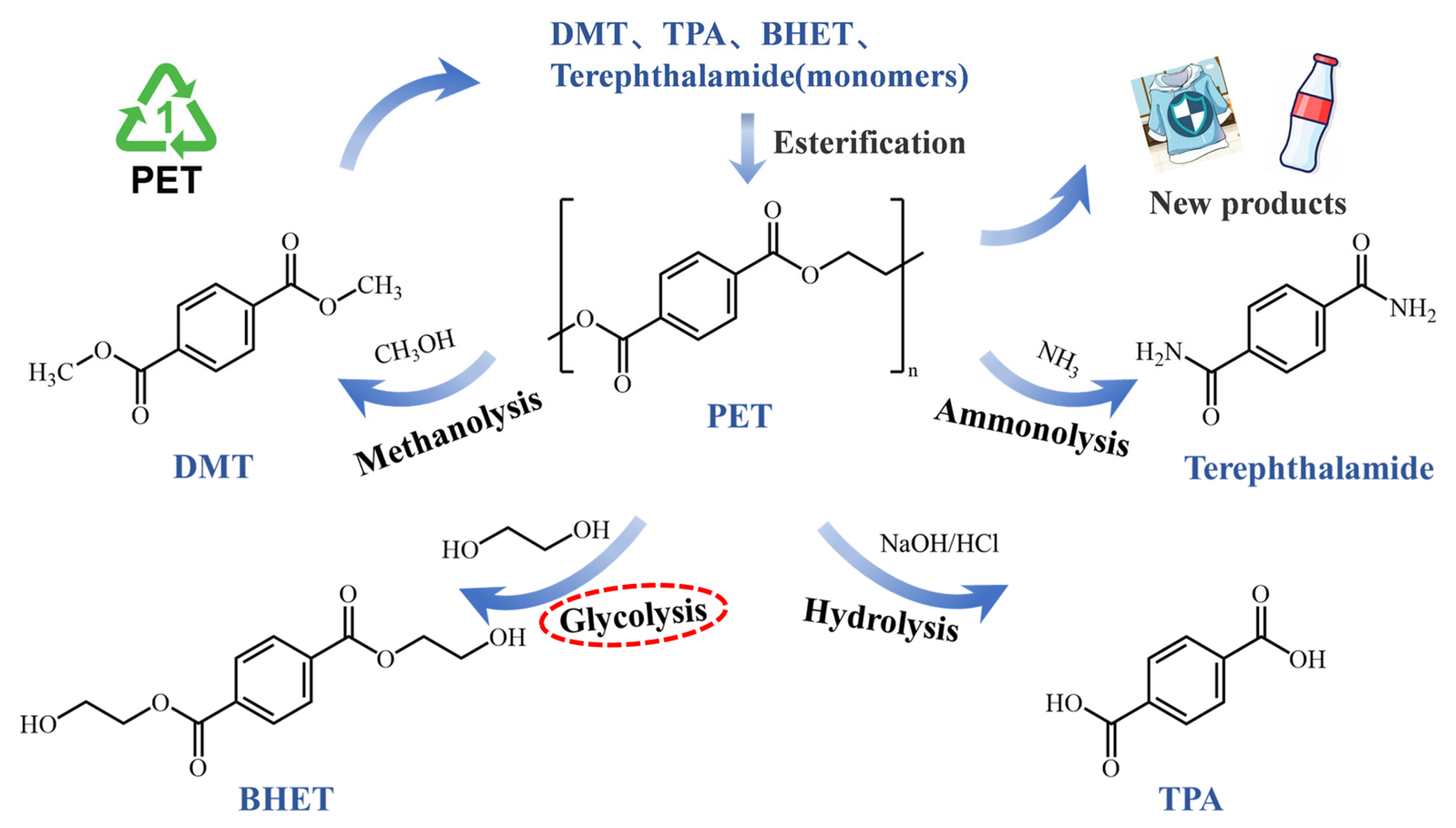
Chemical recovery involves the depolymerization of PET under the influence of chemical reagents, producing oligomers, intermediates, or monomers, such as terephthalic acid (TPA), dimethyl terephthalate (DMT), bis(2-hydroxyethyl) terephthalate (BHET), and ethylene glycol (EG), and further transforming it into value-added products [9,10]. Documented chemical recycling processes for waste PET typically include ammonolysis [11], hydrolysis [12], glycolysis [13], and methanolysis [14], as illustrated in Figure 1. However, challenges, such as high costs, energy consumption, and contamination during chemical recycling, persist, highlighting the need for further innovation and investment in sustainable recycling technologies.
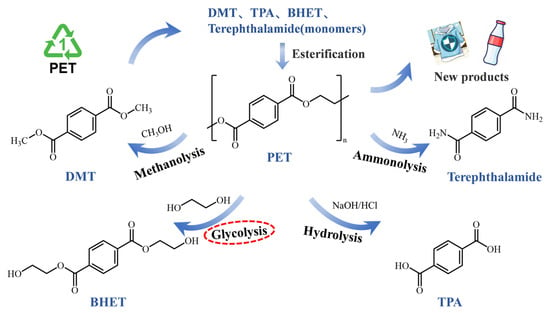
Figure 1.
Various approaches to chemical recycling of PET.
Among these, glycolysis has attracted considerable interest in waste PET management, with EG serving as both solvent and reactant. This is primarily attributed to its favorable reaction conditions, use of solvents with low volatility, and the potential for continuous processing compared to other depolymerization methods [15]. The primary product of glycolysis is BHET, which can be directly utilized in the final polymerization steps [16]. However, the slow reaction rate of glycolysis remains a major limitation. Recent research has been focused on developing efficient and rapid PET glycolysis systems using catalysts, which can be classified as heterogeneous and homogeneous [17]. Heterogeneous catalysts for PET glycolysis have been proposed due to their ability to facilitate catalyst recycling and produce high-purity products, including bulk catalysts [18], metal–organic frameworks (MOFs) [19], and nanoparticles [20]. Homogeneous catalysts have been reported as effective catalytic systems for their high reactivity and selectivity. Metal salt catalysts, such as Zn(OAc)2, are the most commonly used industrial catalysts in glycolysis. To enhance glycolysis reaction kinetics, ionic liquids [21], deep eutectic solvents (DESs) [22,23], and co-solvents [24] have been explored for application in glycolysis by various researchers. While the addition of these catalysts has shown promising catalytic performance, they also introduce environmental concerns, complex synthesis steps, and economic burdens. López et al. [25] were the first to successfully use Na2CO3 as a glycolysis catalyst, achieving performance comparable to Zn(OAc)2. Light-element metal salts, such as alkali metal salts, have emerged as a more attractive alternative to traditional catalytic systems in glycolysis. Sert et al. [26] optimized the reaction parameters of PET glycolysis catalyzed by K2CO3-EG, demonstrating its superiority. Meanwhile, changes in the reaction solution, alkaline hydrolysis induced by the addition of carbonate, applicability to actual PET products, and computational simulations of the reaction mechanism in the presence of carbonate are rarely reported. Sustainable, environmentally friendly, and efficient methods for catalytic glycolysis are of increasing concern, meaning that reactivity enhancement is no longer the sole consideration [27,28,29].
In recent years, increasing attention has been directed toward the use of bio-based catalysts across various fields, including organic synthesis [30], hydrogenation [31], and transesterification [32], to reduce the environmental footprint. As an alternative to traditional heavy metal catalysts, bio-based materials can provide comparable catalytic performance while reducing both costs and environmental impact. Bio-based catalysts also show excellent performance in the catalytic chemical recovery of PET, as summarized in Table 1. Banana peels possess substantial catalytic potential in specific reactions, as well. When banana peels are discarded without recycling, they result in the wasting of valuable resources and contribute to the accumulation of agricultural waste [33]. Recent studies have highlighted that processed banana peels can be used in the production of biodiesel [34], the preparation of nitrogen-doped porous carbon with enhanced electrocatalytic activity [35], and the alkaline hydrolysis of PET [36]. The most common method of banana peel reutilization is as a fertilizer, owing to its high potassium content. Additionally, the potassium-rich nature of banana peels enables their use as a bio-based and sustainable catalyst, demonstrating excellent catalytic performance, particularly in ester exchange reactions [37]. Promoting a sustainable polymer production system through polymer recycling and the adoption of bio-based alternatives to petroleum-derived materials will significantly reduce both carbon footprints and economic costs [38].

Table 1.
Catalytic chemical recovery of PET by bio-based catalyst.
Hence, in this study, we developed a sustainable and effective strategy for PET glycolysis by using banana peel ash (BPA) as a potassium-rich biomass catalyst. BPA demonstrated remarkably high catalytic performance, comparable to conventional catalysts. Under the optimal conditions of 2 g PET, 20 mL EG, 0.2 g BPA, and atmospheric pressure at 180°C for 1.5 h, PET conversion and BHET yield reached 100% and 65.11%, respectively. Product distribution and the effective catalytic components of BPA were systematically investigated. Moreover, the relationship between the characteristic changes in the reaction solution and competition between PET glycolysis and alkaline hydrolysis was elucidated in this research. Finally, the reaction mechanism was proposed based on Density Functional Theory (DFT) calculations. This article offers novel insight into the concurrent treatment of wastes in waste recycling processes.
2. Results and Discussion
2.1. Catalytic Glycolysis of PET
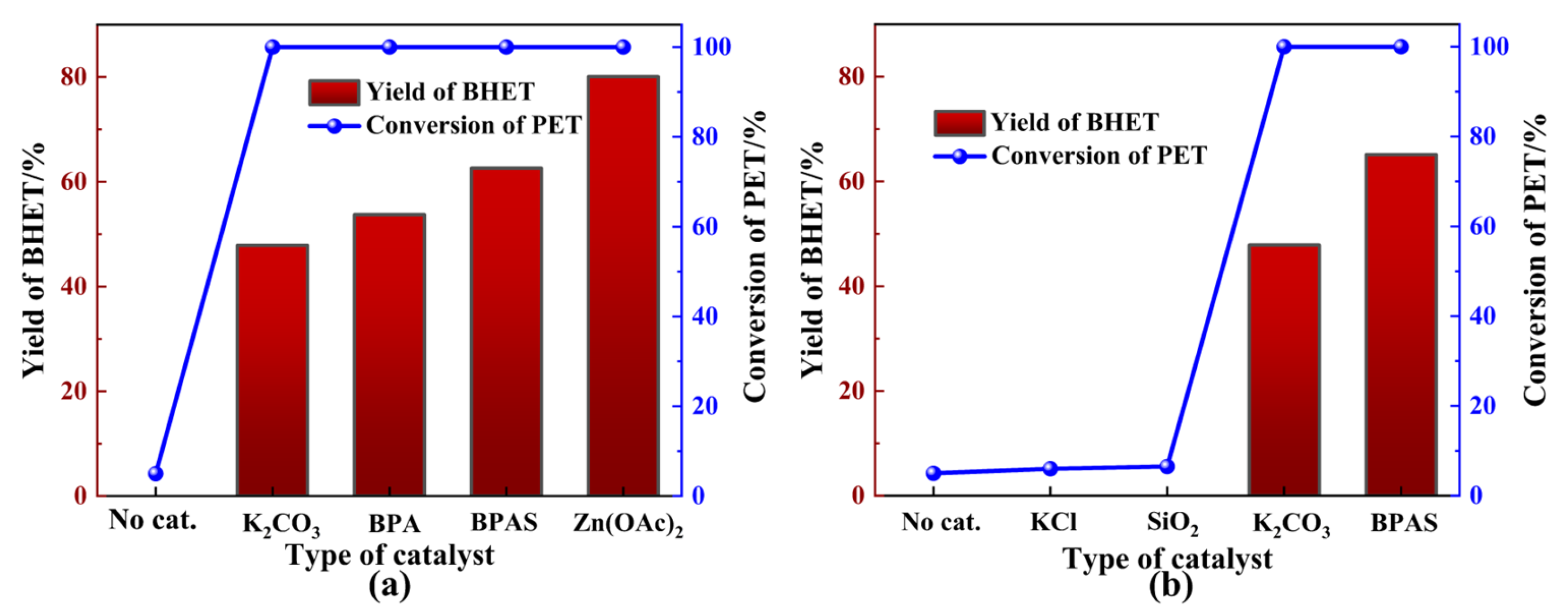
The initial study focused on the effect of various catalysts on PET glycolysis. Figure 2a shows the catalytic performance of different catalysts during PET glycolysis.
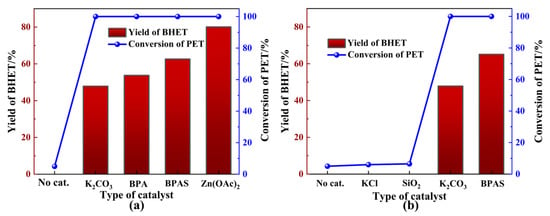
Figure 2.
Catalytic glycolysis of PET. (a) Potassium-rich biomass. (b) Specific components in BPA. General reaction conditions: 2 g PET, 0.2 g catalyst, 20 mL EG, 180 °C, 1.5 h.
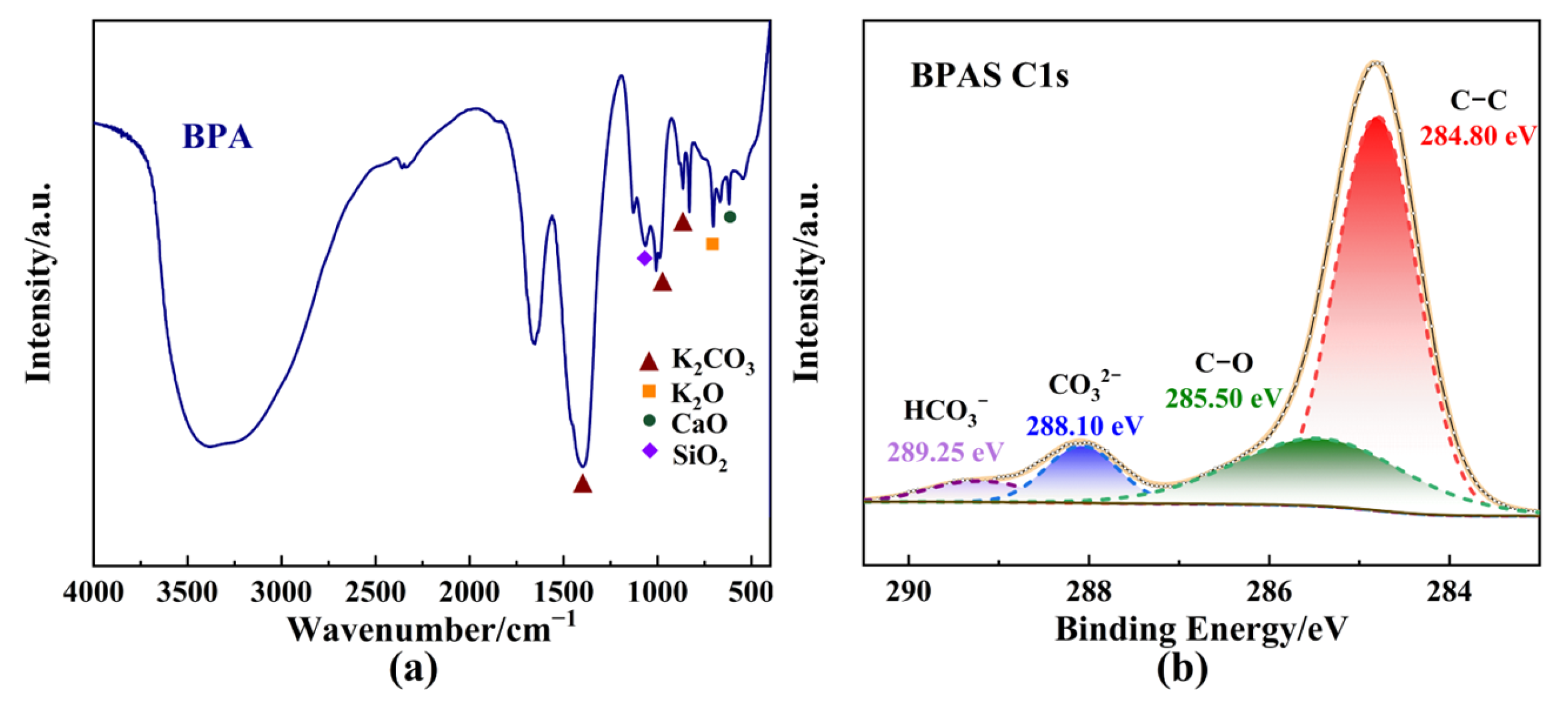
The results indicated that no significant BHET production occurred in the absence of catalysts. Surprisingly, the addition of specific amounts of BPA markedly enhanced the catalytic efficiency of PET glycolysis. BPA demonstrated catalytic activity comparable to K2CO3, which has been reported as an efficient and environmentally friendly catalyst for PET glycolysis (Figure 2a). Meanwhile, BPA could exhibit equivalent depolymerization activity but a lower BHET yield to Zn(OAc)2, which is employed as the most common catalyst in the glycolysis of PET. To further explore the catalytic components of BPA in PET glycolysis, a series of experiments was conducted on the diverse chemical compositions of BPA. X-ray fluorescence spectrometer (XRF) analysis of the catalyst, as shown in Table 2, revealed that the proportion of K2O in BPA was 70.29%. The inherent alkalinity of metal oxides can exhibit excellent catalytic performance. Additionally, the adsorption of CO2 from the atmosphere onto the surface of metal oxides like K2O will induce the generation of K2CO3 [43], which is also likely to be the source of BPA’s catalytic activity. From Figure 2b, a slight difference in PET conversion can be observed, except for K2CO3, indicating that K2CO3 might be the active catalytic component in BPA. The Fourier transform infrared spectroscopy (FTIR) spectrum of BPA (Figure 3a) showed the characteristic peaks for carbonates at 1643 cm−1, 1393 cm−1, 982 cm−1, and 870 cm−1, which correspond to the presence of carbonate stretching and bending vibrations [42,44], further supporting the presence of K2CO3. Metal oxides were also observed in the FTIR spectrum at 704 cm−1 and 602 cm−1, which is attributed to the stretching frequencies of K-O and Ca-O, respectively [44]. X-ray diffraction (XRD) patterns of BPA were consistent with a mixture of KCl, K2CO3, and K2CO3·1.5 H2O (Figure S1) [36].

Table 2.
Chemical content of BPA.
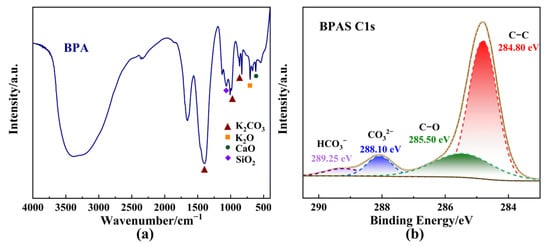
Figure 3.
Characterization of catalysts. (a) FTIR spectrum of BPA. (b) XPS C1s spectrum of BPAS.
To minimize the impact of insoluble components on the glycolysis reaction, after BPA dissolved completely in EG at 180 °C, an EG solution containing banana peel ash soluble (BPAS) was obtained through filtration and subsequently employed to catalyze PET glycolysis. Additionally, BPAS was obtained through filtration and distillation to remove the insoluble components and EG, respectively. From Figure 2a, BPA exhibited poorer catalytic performance than BPAS due to mass transfer resistance caused by the insoluble components in BPA, such as SiO2 and KCl. BPAS, in contrast, demonstrated catalytic activity, supported by surface electronic state characterization and elemental composition analysis of BPAS via X-ray photoelectron spectroscopy (XPS). The binding energy of all species was calibrated to the C1s feature at 284.80 eV. The C1s spectrum of BPAS displayed four distinct carbon species, which could be deconvoluted into four peaks corresponding to C-O (285.50 eV) and carbonate groups (288.10 eV) in Figure 3b. Moreover, the presence of K2CO3 was confirmed through deconvolution of the K2p and O1s spectra (Figure S2). Based on the above test results and analysis, K2O and formed K2CO3 through adsorption might be the main catalytic sources of BPA and, ultimately, exist in BPAS.
Notably, the catalytic performance of 0.2 g BPA was equivalent to that of 0.2 g K2CO3, with greater BHET yield. This discrepancy was attributed to the alkaline hydrolysis of BHET, facilitated by the increased alkalinity of the reaction mixture. Further research should be conducted to investigate this in more detail. Based on the overall catalytic performance and product separation, subsequent exploratory experiments were carried out using an EG solution containing BPAS to catalyze the glycolysis of PET instead of BPA.
2.2. Optimal Experiment
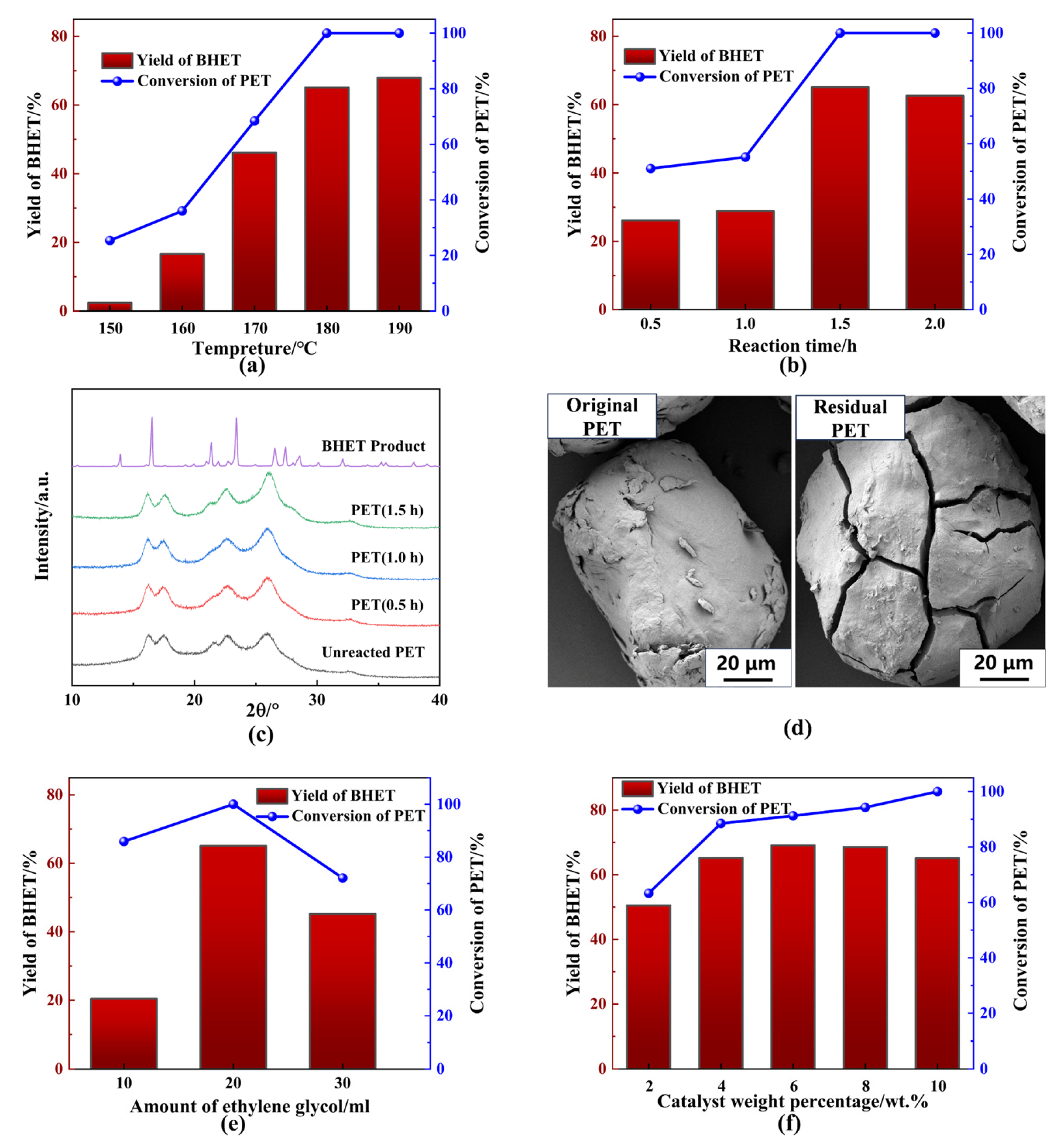
To investigate the effects of various experimental conditions on PET glycolysis, 2 g PET powder was introduced into a specified amount of EG to initiate the glycolysis reaction in the presence of a catalyst. The results from these experiments are presented as functions of various variables in Figure 4. Temperature plays a crucial role in PET glycolysis, as it is an endothermic process. At relatively low temperatures, overcoming the energy barrier is difficult, making PET glycolysis inefficient below 150 °C, as demonstrated in Figure 4a. Both PET conversion and BHET yield increased significantly as the temperature rose from 150 °C to 190 °C. As anticipated, increasing the reaction time improved PET conversion and BHET yield, which reached 100% and 65.11%, respectively, after 1.5 h (Figure 4b). No further significant increase in BHET yield was observed beyond this point, indicating that the system reached equilibrium in terms of product transformation within 1.5 h. XRD analysis of the residual PET at different reaction times revealed minimal changes in the spectrum compared to the initial PET (Figure 4c). However, a minor increase in the intensity of the characteristic peak at 2θ = 25.80°, which was indicative of a polyester configuration [15], could be observed with longer reaction times, suggesting breakdown of ester groups and slight changes in the crystal structure due to the glycolysis of PET. To further investigate the changes in the PET surface, scanning electron microscope (SEM) images (Figure 4d) were captured. The smooth surface of the original PET particles remained intact during the early stages of glycolysis. However, irregular cracks appeared on the PET surface and gradually deepened as the reaction progressed. These cracks allowed the catalytic solvent mixture to penetrate deeper into the polymer, facilitating the glycolysis reaction. The SEM results indicated that the glycolysis process occurred both on the surface and within the cracks of the PET particles.
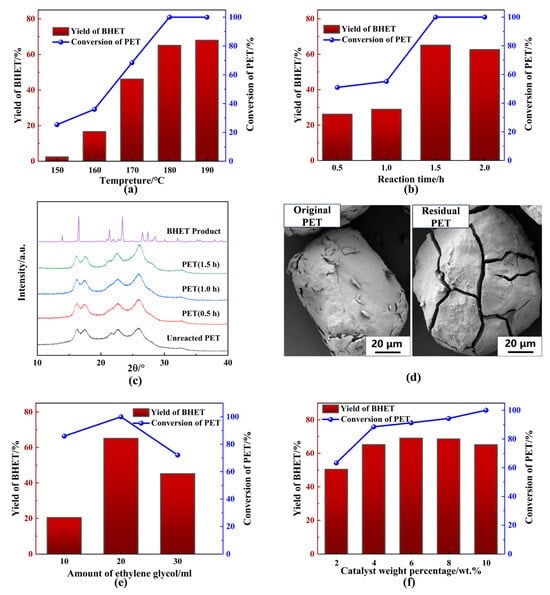
Figure 4.
Influence of reaction conditions on catalytic glycolysis by BPAS. (a) Temperature. (b) Reaction time. (c) XRD spectrum of residual PET. (d) SEM of the original PET and residual PET. (e) Amount of ethylene glycol. (f) Catalyst weight percentage. General reaction conditions: 2 g PET, 0.2 BPA, 20 mL EG, 180 °C, 1.5 h.
Next, the effect of EG volume on PET glycolysis at 180 °C was examined, and the results are shown in Figure 4d. When 10 mL EG was used, PET conversion decreased to 85.90% due to insufficient reactant supply. The BHET yield was especially low under these conditions, as a larger proportion of oligomers formed through the re-polymerization of BHET monomers or incomplete depolymerization of PET. At 30 mL EG, a decrease in catalyst concentration resulted in lower glycolysis activity, and both PET conversion and BHET yield exhibited a general decline. These results highlight the critical role of EG volume in PET glycolysis. The molar ratio of EG to catalyst should be precisely controlled to achieve complete PET conversion and high BHET selectivity. Furthermore, the effect of catalyst weight percentage was also explored (Figure 4f). Complete degradation of PET could not be achieved at a lower catalyst percentage than 10 wt.% within 1.5 h, although higher BHET selectivity was obtained. This may be attributed to the possibility that the lower catalyst percentage caused a decrease in the alkalinity of the solution such that the glycolysis rate was relatively low. Meanwhile, the conversion balance between BHET monomer and oligomer, as well as the alkaline hydrolysis of BHET, would be affected by the increasing alkalinity of the solution. In consideration of complete conversion of PET, 10 wt.% BPA was selected as the best amount of catalyst. Based on these findings, 180 °C, 1.5 h, 2 g PET, 0.2 g BPA, and a ratio of mBPA to vEG = 0.01 were identified as optimal conditions for subsequent exploratory experiments.
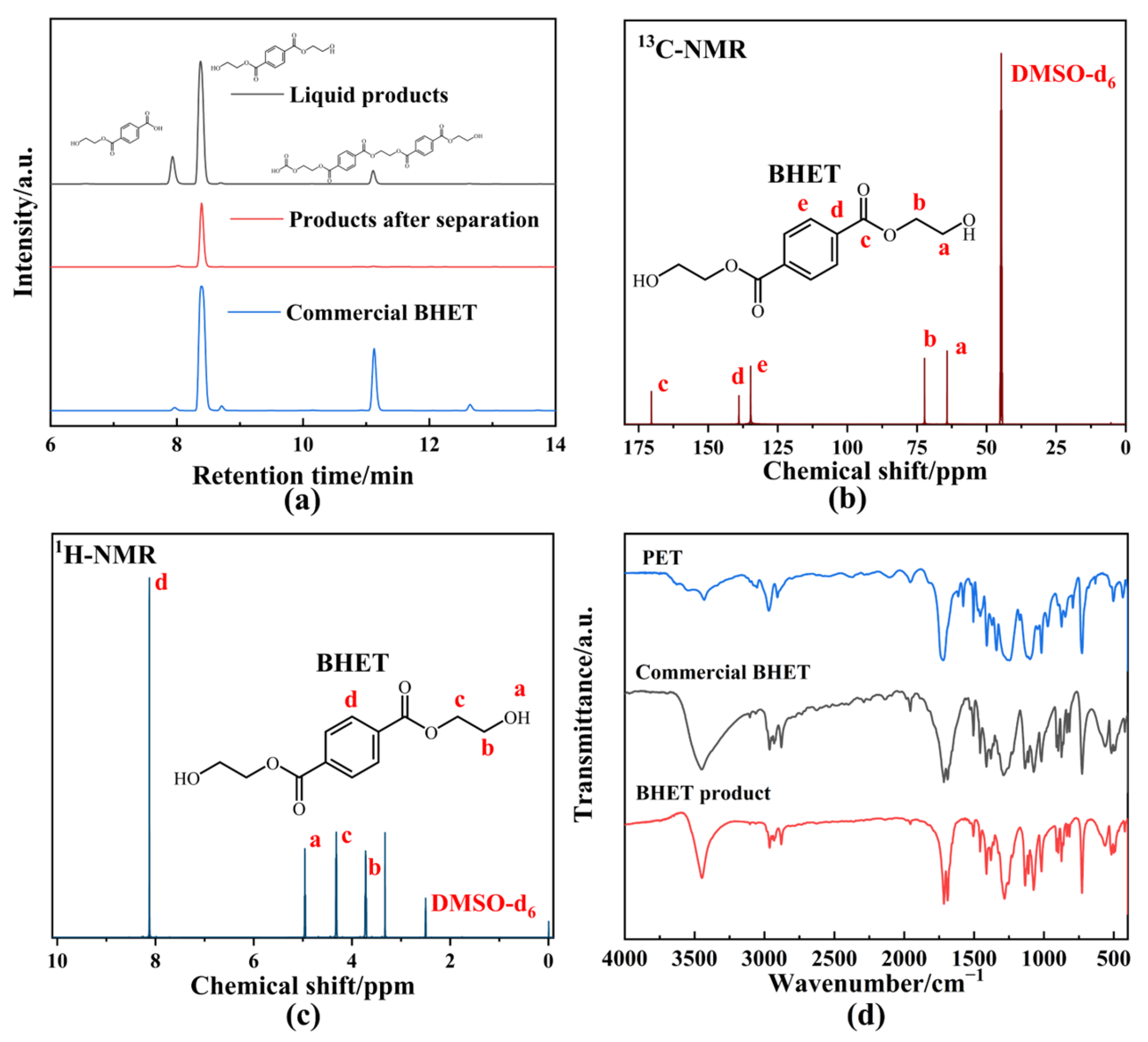
Product distribution for the optimal experiment is shown in Figure 5a. Three main product peaks were identified in the unseparated liquid products, with retention time (RT) corresponding to mono(2-hydroxyethyl) terephthalate (MHET, 7.933 min, Figure S3), BHET (8.373 min), and acidic substances (11.320 min), confirmed through High-performance liquid chromatography–electrospray ionization–quadrupole time of flight/mass spectrometry (HPLC-ESI-QTOF/MS) analysis. After condensation–crystallization, only a single chromatographic peak (RT = 8.373 min) appeared, indicating the successful separation of BHET from the mixture (Figure S4). Compared to the HPLC chromatogram of commercial BHET, no oligomers were detected in the separated BHET. To further confirm the purity of the BHET product and eliminate the matrix effect, additional characterizations were performed. The 13C-NMR (Figure 5b), 1H-NMR (Figure 5c), and FTIR (Figure 5d) spectra of the BHET product were consistent with the standard BHET. Therefore, the retention time of 8.373 min in the HPLC chromatogram confirmed that the product was BHET with a high purity level. The XRD spectrum (Figure S5) of the BHET product displayed sharper and narrower diffraction peaks compared to PET, indicating that the crystallinity of the product had increased, which was indicative of successful depolymerization. Although the BHET yield of BPAS was lower than that of other heavy metal catalysts [45] or co-solvents [24], the use of waste and green reagents in this system allowed for depolymerization performance comparable to Zn(OAc)2, demonstrating the potential for a more environmentally friendly approach to PET glycolysis.
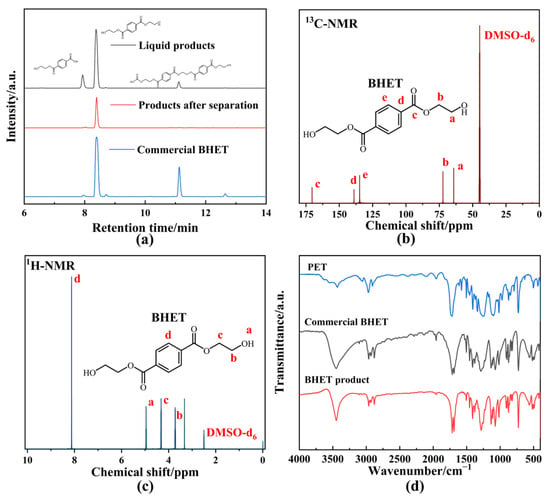
Figure 5.
(a) HPLC chromatogram of glycolysis product. (b) 13C-NMR spectrum of BHET product. (c) 1H-NMR spectrum of BHET product. (d) FTIR spectrum of BHET product and PET. The letters represented the corresponding chemical shift of C or H atoms located in different chemical environments in BHET product.
2.3. Competition Between Glycolysis and Alkaline Hydrolysis
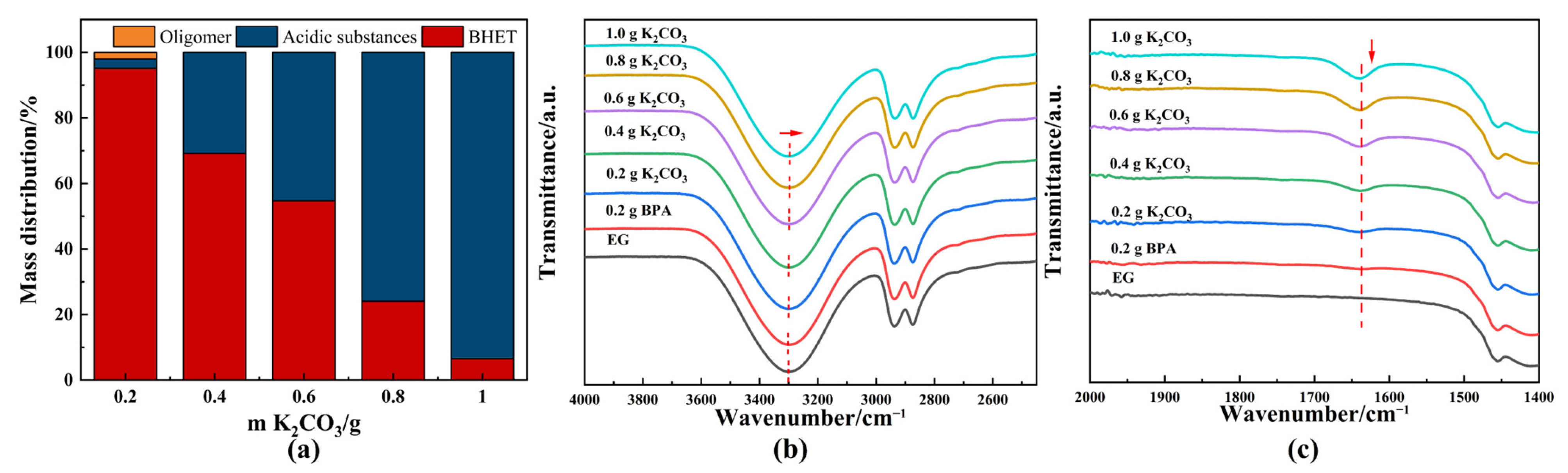
DESs consist of a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD), displaying unique properties, such as low vapor pressure, low toxicity, and dissolution characteristics similar to those of ionic liquids [46]. K2CO3-based DESs have been reported as environmentally friendly solvents widely utilized in fields like carbon dioxide capture [47] and PET depolymerization [26] due to their favorable properties. The molar ratio of EG to K2CO3 significantly influenced the balance between glycolysis and hydrolysis, as well as product distribution. To further elaborate on the competition between glycolysis and alkaline hydrolysis, K2CO3 (HBA)-EG (HBD) solutions with different concentrations were synthesized through heating at 180 °C for 0.5 h and employed to catalyze glycolysis of PET. Figure 6a shows the product distribution for various amounts of K2CO3 in 20 mL EG. As the proportion of K2CO3 increased, alkaline hydrolysis products became more dominant, while BHET yield decreased. This shift was attributed to the increased basicity of the reaction medium (Table S1). Additionally, the products derived from solvent acidification were analyzed using HPLC-ESI-QTOF/MS. A significant amount of TPA was produced, but only a few oligomeric–acidic substances were detected (Figures S6–S8), many of which were difficult to reuse as desired products. Recycling these oligomeric products required additional acid and resulted in the generation of water.
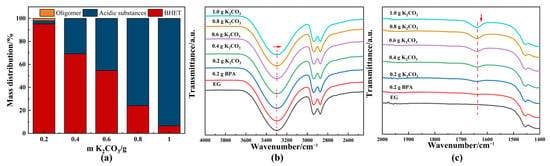
Figure 6.
(a) Product distribution of catalytic glycolysis of PET using different amounts of K2CO3. FTIR spectrum of different concentrations of EG-K2CO3 solution: (b) 4000–2500 cm−1; (c) 2000–1400 cm−1. General reaction conditions: 2 g PET, EG-K2CO3 solution, 180 °C, 2 h. EG-K2CO3 solution was obtained through heating at 180 °C for 0.5 h. The direction of the arrow indicated the trend of peak shift and intensity change.
The preferential occurrence of glycolysis versus hydrolysis is closely linked to the chemical properties of the DESs. K2CO3 is crucial for promoting deprotonation of EG to initiate the glycolysis reaction, as evidenced by a slight but important shift in the hydroxyl absorption peak in the FTIR spectrum (Figure 6b). The formation of EG-based carbonate was confirmed by a new band at 1650 cm−1 [48], corresponding to the asymmetric stretching mode of C=O in the FTIR spectrum (Figure 6c). The interaction between a high concentration of carbonate and the hydroxyl group of EG leads to the production of hydroxide ions (OH−) [48]. If this OH− is not eliminated, it can contribute to hydrolysis reactions, resulting in the formation of acidic products (Figure 6a). Consequently, acidic substances could replace BHET as the primary product. Although the presence of OH− would accelerate the PET depolymerization reaction, hydrolysis, which is contrary to the view of “green chemistry”, was not desired in this study.
2.4. Reaction Mechanism
To gain a more comprehensive understanding of the mechanism of PET catalytic glycolysis using BPA, the interaction between EG and BPA and the key reaction pathway were investigated using DFT calculations. Due to the large molecular weight of PET, ethylene glycol dibenzoate was employed as a model compound to simulate the breakdown of the PET ester bond, thereby reducing computational complexity. Because K2CO3 was identified as the partial catalytic source of BPA, K2CO3 was used in the corresponding calculations instead of BPA.
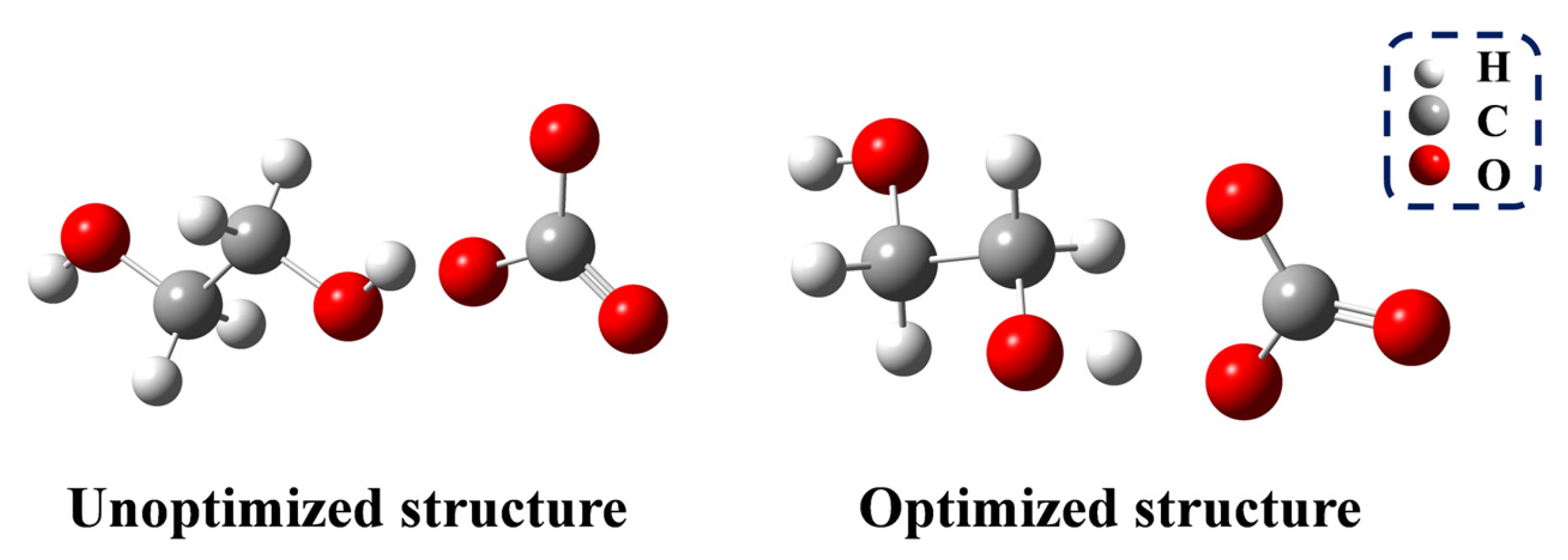
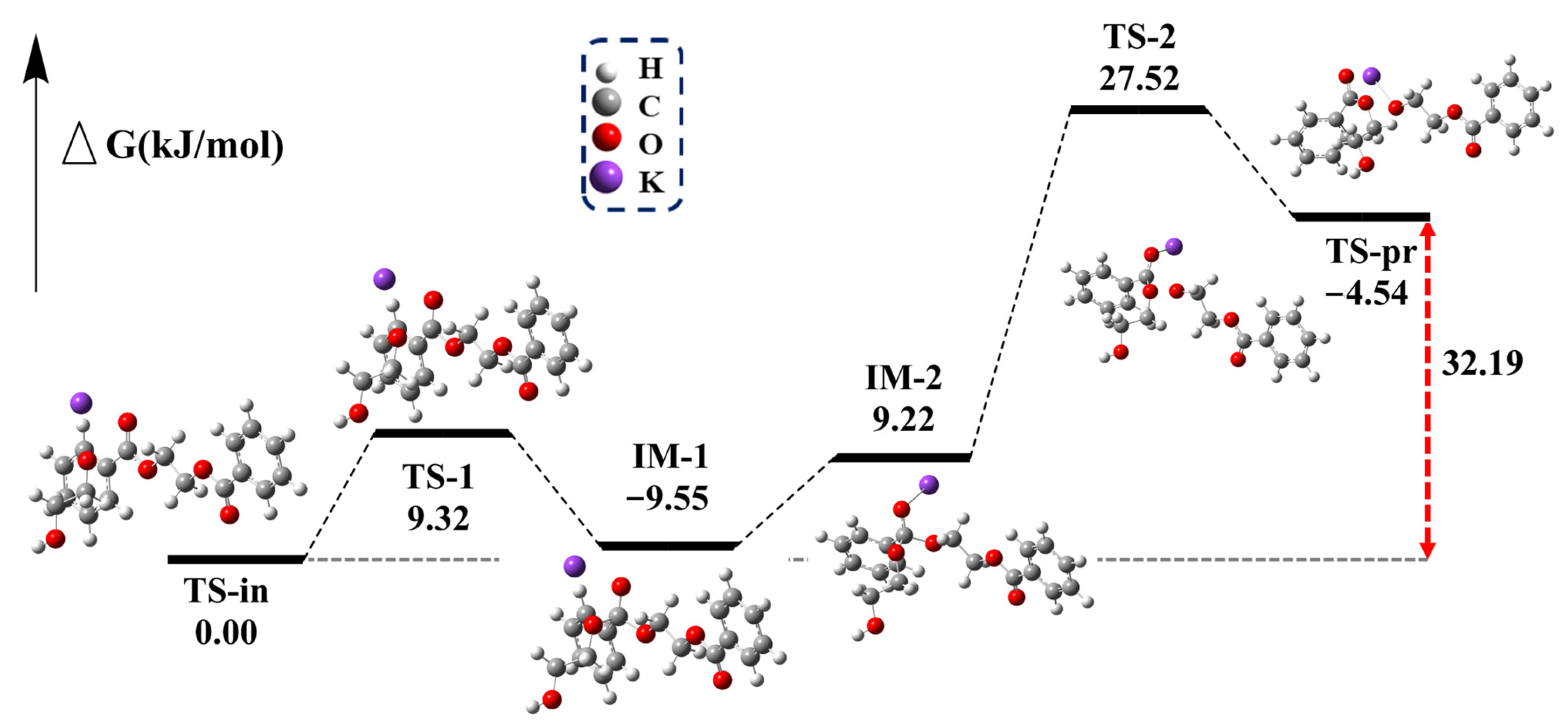
The glycolysis of PET is a transesterification reaction that proceeds via a nucleophilic addition–elimination mechanism (Figure S9). The presence of nucleophiles is essential for initiating the reaction. In the first and most important step, CO32− acts as an HBA, deprotonating EG to form HOCH2CH2O−. The O-H bond length of EG changes from 0.96 Å to 1.04 Å (Figure 7), thereby confirming the deprotonation of EG. The following reaction pathway is shown in Figure 8. In the second step, the electron-deficient carbon atom of the carbonyl group is attacked by HOCH2CH2O−, resulting in the formation of the transition state TS-1. The dihedral angle between the ester oxygen, the carbonyl carbon, and the phenyl ring carbon changes to 151.18°, indicating the formation of a tetrahedral structure, which ultimately leads to the addition reaction and the formation of IM-1.
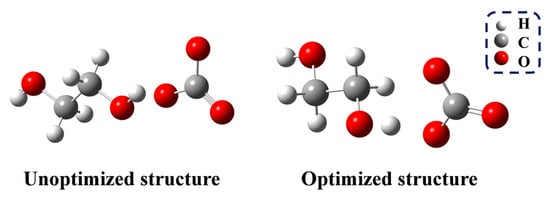
Figure 7.
Unoptimized and optimized structure of EG and CO32−.
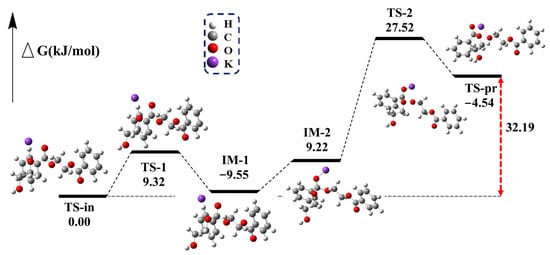
Figure 8.
Reaction pathway and relative Gibbs free energy curves for the attack of HOCH2CH2O− and K on ester bonds of ethylene glycol dibenzoate.
Due to the strong electronegativity of both the carbonyl oxygen and the ethylene glycol oxygen, electron-deficient potassium ions are attracted to these atoms. This interaction causes the potassium ion to move between the carbonyl oxygen and the ethylene glycol oxygen, stabilizing the charge distribution during the addition reaction. A similar interaction mechanism occurs during the departure of the remaining PET chains, leading to the formation of IM-2. As HOCH2CH2O− attaches to the carbonyl carbon, the remaining PET chains begin to depart. The distance between the carbonyl carbon and the PET ester oxygen changes from 1.49 Å to 2.16 Å, indicating a tendency for the bond to break at the transition state TS-2. Finally, the remaining PET is completely removed in the form of potassium alkoxide, and the Gibbs free energy for the entire process is calculated to be 32.19 kJ/mol.
2.5. Glycolysis of Colored Waste PET
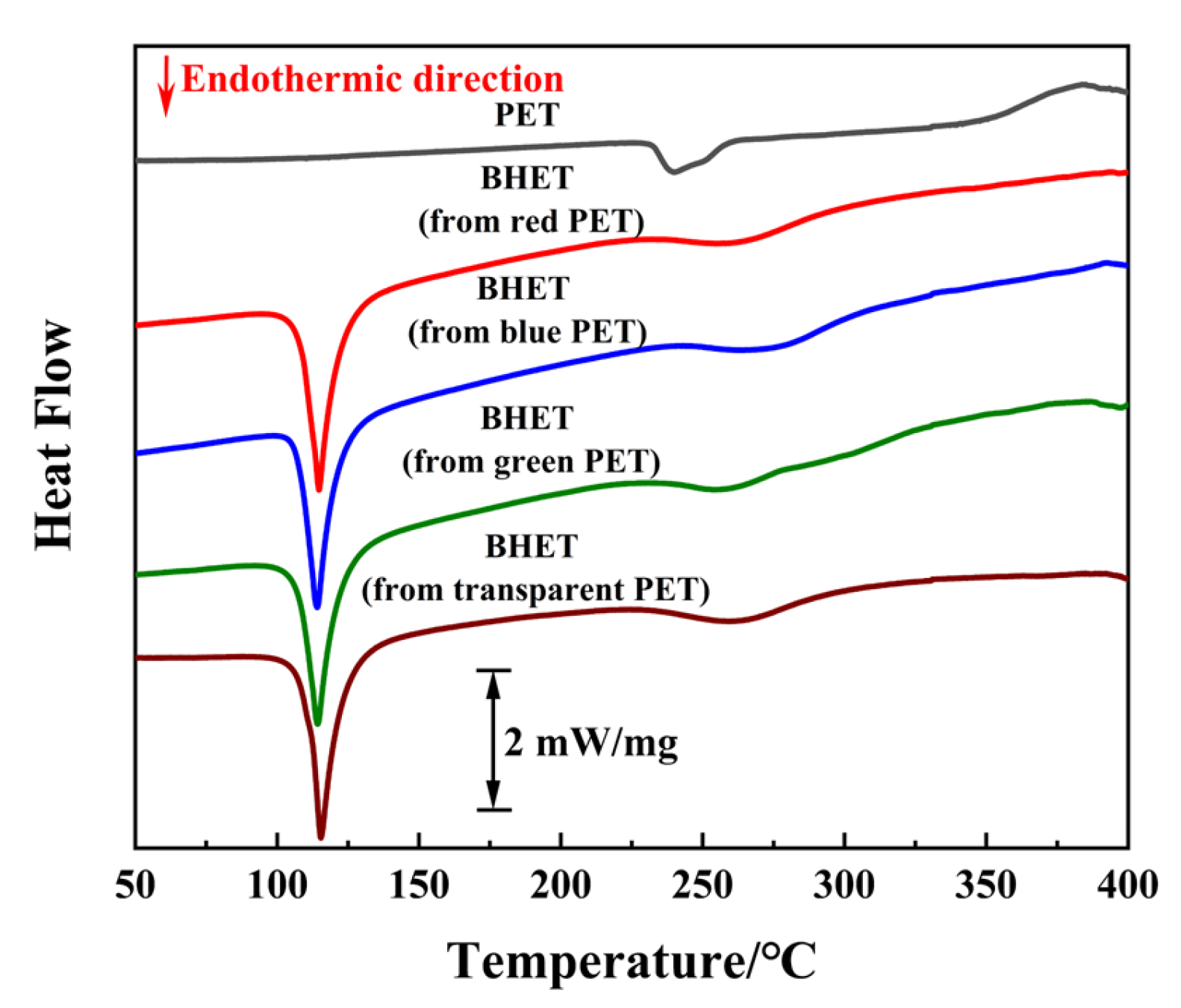
To verify the feasibility of this reaction system for real PET products, several colored waste beverage bottles with a thickness of 0.11 mm were selected as experimental materials. These bottles were cleaned, labels were removed, and they were cut into 5 mm × 5 mm pieces under the optimal reaction conditions. The conversion and BHET yield for each waste PET product are shown in Table 3. Despite the less efficient mass transfer process for PET flakes compared to PET particles, complete conversion was achieved within 3 h. A relatively high BHET yield was obtained, even with the interference of additives, such as dyes and other organic impurities. Differential scanning calorimetry (DSC) was used to further assess the purity of the BHET product derived from various colored PET bottles, as shown in Figure 9. The high purity of BHET, which is free from contaminants, such as MHET, oligomers, or additives inherent in waste PET, was confirmed by a single peak at approximately 105 °C in the DSC profiles [49]. Notably, the BHET product obtained through condensation–crystallization was noticeably less colored compared to the initial waste PET flakes. This indicates that the BPA-EG system is tolerant of colored waste PET and can extract and decolorize dyes in PET.

Table 3.
Different colored flake PET and glycolysis products under optimal conditions for 3 h.
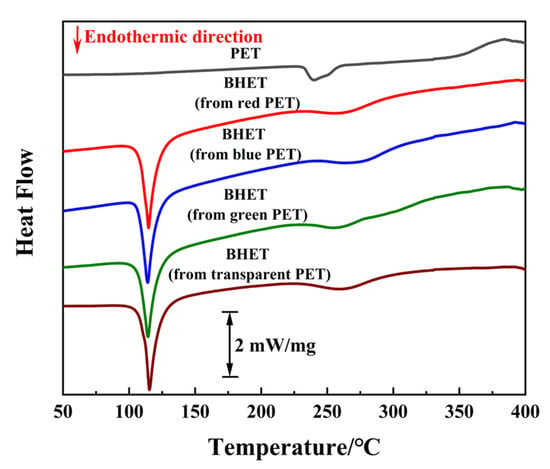
Figure 9.
DSC curve of PET and BHET derived from different colored PET.
In this approach, from raw materials (post-consumer PET) to the final product (BHET), the process involved only EG, with no additional chemical reagents. The catalyst and experimental materials were derived from everyday waste. This green and effective method for PET catalytic glycolysis also contributes to waste reduction by reusing waste PET materials. The combined recycling of waste PET and biomass reuse, such as potassium-rich banana peels, suggests a promising path for greener methods in the recovery of waste PET.
3. Materials and Methods
3.1. Materials
PET powder (Dongguan Narui New Material Co., Ltd., Guangdong, China), potassium carbonate (K2CO3, 99.7%, Tianjin Dengfeng Chemical Reagent Factory, Tianjin, China), zinc acetate (Zn(OAc)2, 99.7%, Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China), deionized water, glycol (EG, 99%, Tianjin Jingdong Tianzheng Precision Chemical Reagent Factory, Tianjin, China), potassium chloride (KCl, 99.5%, Tianjin Bodi Chemical Co., Ltd., Tianjin, China), silicon dioxide (SiO2, 99.7%, Tianjin Tianli Chemical Reagent Co., Ltd., Tianjin, China), and bis(2-hydroxyethyl) terephthalate (BHET, 85%, Aladdin) were purchased and did not undergo any purification before use. A post-consumer PET bottle and Jianong bananas were purchased from Uni-President China Holdings LTD and a local supermarket, respectively.
3.2. Preparation of BPA Catalyst
Fresh banana peels were collected, washed thoroughly with deionized water, and cut into small pieces. These pieces were then dried in the oven at 120 °C for 48 h to remove all moisture. After drying, the banana peels were calcined in a muffle furnace from room temperature up to 550 °C with a heating rate of 10 °C/min and kept at 550 °C for 2 h. The resulting grayish BPA was then collected and stored in a desiccator at room temperature. In this work, 73.50 g dried fresh banana peels could be converted into 12.24 g BPA after calcination.
3.3. Catalytic Glycolysis of PET with BPA
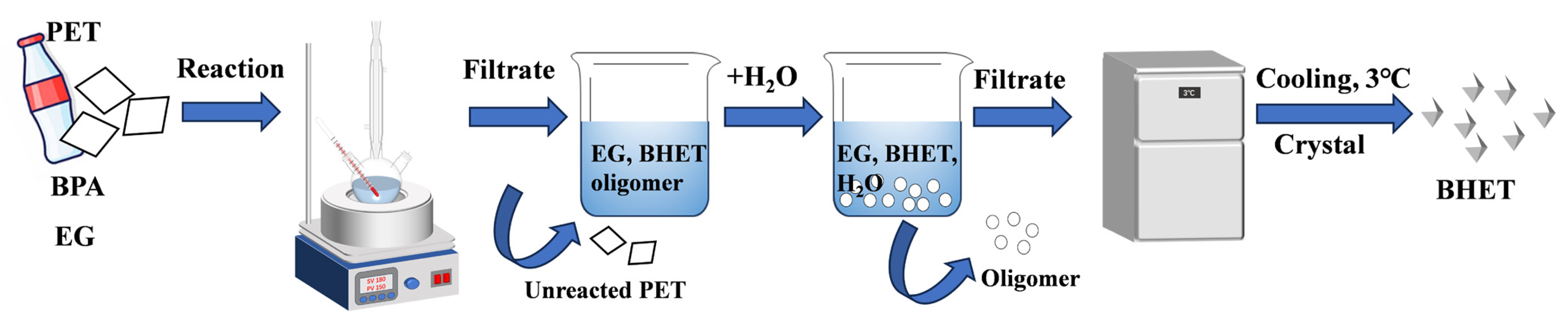
In a typical procedure (Scheme 1), 0.2 g BPA catalyst was dissolved in a round-bottom flask containing 20 mL EG, and the mixture was preheated to the specified temperature using an oil bath. Next, 2 g PET powder was added to the solution to initiate the glycolysis reaction at 180 °C under magnetic stirring at 300 rpm. Once the reaction was complete, the round-bottom flask was rapidly cooled in an ice-water bath. After cooling, the unreacted PET was separated through filtration using a sand core funnel and washed with deionized water. The unreacted PET was then dried in the oven at 60 °C for 24 h, and PET conversion was calculated using Equation (1). Subsequently, 50 mL hot deionized water was added to the filtrate to dissolve the BHET, and the mixture was allowed to cool to room temperature. The insoluble oligomers were separated from the BHET that was soluble in water via filtration. The filtrate containing BHET was then cooled in a refrigerator at 3 °C for 24 h to allow the BHET to crystallize completely. The crystallized BHET was filtered, transferred to a beaker, and dried in an oven at 60 °C for 6 h.
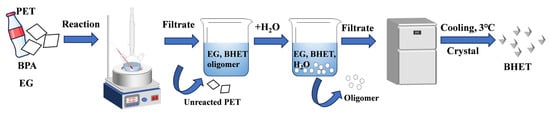
Scheme 1.
Catalytic glycolysis of PET by BPA.
The dried product was weighed to calculate the BHET yield using Equation (2). Finally, 1.5 mol/L HCl was added to the filtrate, and the pH was adjusted to 3 to precipitate any acidic substances.
where , , and represent PET’s initial mass, unreacted PET’s mass, and BHET products generated by condensation–crystallization, respectively. and represent the relative molecular mass of PET (192) and BHET (256), respectively.
3.4. Characterization
A Bruker AVANCE III 500 NMR spectrometer (Bruker, Billerica, Switzerland) was employed to characterize the structure and purity of the main products. The determination frequency was set to 500 MHz, and the spectra were obtained in a deuterated dimethyl sulfoxide (DMSO-d6) solution. FTIR (Bruker, EQUINOX55, Bruker Optics, Ettlingen, Germany) was used to characterize the functional groups of EG influenced by the presence of BPA. The sample was prepared by mixing the sample with KBr at a ratio of 1:100. The infrared scanning range was set to 4000–400 cm−1, and the step size was set to 2 cm−1. The surface electronic states and elemental composition of BPAS in EG were analyzed through XPS (ESCALAB250Xi, Thermo Fisher Scientific, Waltham, MA, USA). The surface morphology of residual PET after glycolysis was analyzed using a field emission SEM 5000 from CIQTEK company (Hefei, China). Because PET was not conductive, the sample needed to be sprayed with gold during the sample preparation process. The gold spraying current was 30 mA, and the time was 40 s. HPLC-ESI-QTOF/MS (Agilent G6546A, Agilent Technologies, Santa Clara, CA, USA) with a UV detector (λmax = 254 nm) and a C18 column was used to analyze the product’s distribution and test the purity of the product. The mobile phase consisted of 0.2% formic acid solution and acetonitrile (90:10 v/v), and the flow rate was set to 0.3 mL/min. The proportion of acetonitrile increased to 20% and 100% at 5 and 15 min, respectively. The column oven temperature was kept constant at 35 °C. DSC (STA 449 F5 Jupiter thermogravimetric analyzer, NETZSCH, Selb, Germany) was utilized to explore the thermal properties of the BHET product from 50 °C to 400 °C using a heating ramp of 10 °C/min. XRD (Smart Lab 9 KW, Rigaku, Tokyo, Japan) was applied to study the composition of the catalyst and the change in the PET crystal structure with a scanning rate of 10°/min. An XRF (Axios) from PANalytical B.V. (Almelo, The Netherlands) was adopted to investigate the chemical content of BPA with the gas-flow detector and an SST-mAX tube.
3.5. Computational Details
All of the DFT calculations were carried out using Gaussian 09 software [50] and the B3LYP/6-31+**method for geometry optimization and frequency calculations with GD3 [51] dispersion corrections. The Integral Equation Formalism Polarizable continuum model (IEFPCM) was adopted for the solvent environment for EG. For each transition state (TS), the intrinsic reaction coordinate (IRC) was calculated to verify the corresponding reactants and products.
4. Conclusions
The chemical recovery of PET has gained significant attention as a solution to plastic waste pollution in line with the principles of the circular economy. Glycolysis offers an effective method for obtaining monomers from waste PET, operating under relatively mild reaction conditions and imposing a lower environmental burden compared to other chemical recycling methods. The development of bio-based catalysts can further reduce carbon footprints and economic costs associated with heavy metal catalysts while maintaining comparable catalytic performance. In response to the growing demand for sustainable PET recycling processes, this study presents an environmentally friendly and efficient approach for catalytic glycolysis using bio-based materials for selectively producing BHET. In this system, calcined BPA derived from potassium-rich biomass, banana peels, served as a catalyst, demonstrating excellent catalytic performance. Complete degradation of 2 g PET and high BHET yields were achieved within 1.5 h at 180 °C. This study introduces an environmentally sustainable and efficient method for the targeted decomposition of PET into glycolysis products, offering a novel perspective on both PET recycling and biomass utilization.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/recycling10030085/s1, Figure S1: XRD spectrum of BPA catalyst; Figure S2: XPS K2ps and O1s spectrum of BPAS; Figure S3: Mass spectrum of MHET (RT = 7.933 min); Figure S4: Mass spectrum of BHET (RT = 8.373 min); Figure S5: XRD spectrum of BHET product; Figure S6: HPLC chromatogram of acidic substances; Figure S7: Mass spectrum of TPA (RT = 6.538 min); Figure S8: Mass spectrum of acidic substances (RT = 11.320 min); Figure S9: Reaction mechanism of addition–elimination and optimized cartesian coordinates for PET glycolysis; Table S1: The pH of different solutes in 20 mL EG; Table S2: The acid, hydroxyl number values, and molecular weight of BHET.
Author Contributions
Z.B. (Zhe Bai): conceptualization; methodology; software; validation; formal analysis; visualization; investigation; data curation; writing—original draft. Z.B. (Zhixian Bao): methodology; formal analysis. H.H.: conceptualization; funding acquisition; supervision; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 22078053) and the Innovation Team Support Program in Key Areas of Dalian Science and Technology Bureau (2019RT10).
Data Availability Statement
All data can be used upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kim, D.H.; Han, D.O.; In Shim, K.; Kim, J.K.; Pelton, J.G.; Ryu, M.H.; Joo, J.C.; Han, J.W.; Kim, H.T.; Kim, K.H. One-Pot Chemo-bioprocess of PET Depolymerization and Recycling Enabled by a Biocompatible Catalyst, Betaine. ACS Catal. 2021, 11, 3996–4008. [Google Scholar] [CrossRef]
- Enayati, M.; Mohammadi, S.; Bouldo, M.G. Sustainable PET Waste Recycling: Labels from PET Water Bottles Used as a Catalyst for the Chemical Recycling of the Same Bottles. ACS Sustain. Chem. Eng. 2023, 11, 16618–16626. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, C.; Purbey, R.; Bora, D.; Chetia, P.; Maheswari, R.U.; Duarah, R.; Dutta, K.; Sadiku, E.R.; Varaprasad, K.; Jayaramudu, J. A Review on Sustainable PET Recycling: Strategies and Trends. Mater. Today Sustain. 2024, 27, 100936. [Google Scholar] [CrossRef]
- Adamides, E.D.; Syrigos, A.D. A Systems Firm-Centered Perspective on the Environmental Assessment of Recyclable PET and Glass Soft Drink Containers. Recycling 2024, 9, 78. [Google Scholar] [CrossRef]
- Santomasi, G.; Todaro, F.; Petrella, A.; Notarnicola, M.; Thoden van Velzen, E.U. Mechanical Recycling of PET Multi-Layer Post-Consumer Packaging: Effects of Impurity Content. Recycling 2024, 9, 93. [Google Scholar] [CrossRef]
- Amoh, P.O.; Elkady, M.; Nasr, M.; Shokry, H. Green Valorization of Waste Plastics to Graphene as an Upcycled Eco-Friendly Material for Advanced Gas Sensing. Recycling 2024, 9, 38. [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Chemical Recycling of Waste Plastics for New Materials Production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Iwuozor, K.O.; Emenike, E.C.; Ajala, O.J.; Ogunniyi, S.; Muritala, K.B. Thermochemical Co-conversion of Biomass-plastic Waste to Biochar: A Review. Green Chem. Eng. 2024, 5, 31–49. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, Y.; Furukawa, S.; Xia, J.; Sun, C.; Hülsey, M.J.; Wang, H.; Guo, Y.; Liu, X.; Yan, N. Towards the Circular Economy: Converting Aromatic Plastic Waste Back to Arenes over a Ru/Nb2O5 Catalyst. Angew. Chem. Int. Ed. 2021, 60, 5527–5535. [Google Scholar] [CrossRef]
- Muringayil Joseph, T.; Azat, S.; Ahmadi, Z.; Moini Jazani, O.; Esmaeili, A.; Kianfar, E.; Haponiuk, J.; Thomas, S. Polyethylene terephthalate (PET) Recycling: A Review. Case Stud. Chem. Environ. Eng. 2024, 9, 100673. [Google Scholar] [CrossRef]
- Liang, J.; Fu, J.; Lin, H.; Chen, J.; Peng, S.; Sun, Y.; Xu, Y.; Kang, S. Valorization of Polyethylene Terephthalate Wastes to Terephthalamide via Catalyst-free Ammonolysis. J. Ind. Eng. Chem. 2024, 132, 578–587. [Google Scholar] [CrossRef]
- Chen, H.; Hu, H. Solvent System with Improved Hydroxide Reactivity for Mild and High-Efficiency PET Alkaline Hydrolysis. Ind. Eng. Chem. Res. 2023, 62, 12925–12934. [Google Scholar] [CrossRef]
- Zhou, L.; Qin, E.; Huang, H.; Wang, Y.; Li, M. PET Glycolysis to BHET Efficiently Catalyzed by Stable and Recyclable Pd-Cu/γ-Al2O3. Molecules 2024, 29, 4305. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Cheng, X.; Rong, C.; Feng, J.; Feng, X.; Xin, J.; Zhou, Q.; Li, Y.; Xu, J.; et al. Efficient Methanolysis of PET Catalyzed by Nonmetallic Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2024, 63, 12373–12384. [Google Scholar] [CrossRef]
- Javed, S.; Fisse, J.; Vogt, D. Kinetic Investigation for Chemical Depolymerization of Post-Consumer PET Waste Using Sodium Ethoxide. Ind. Eng. Chem. Res. 2023, 62, 4328–4336. [Google Scholar] [CrossRef]
- Imran, M.; Kim, B.-K.; Han, M.; Cho, B.G.; Kim, D.H. Sub- and Supercritical Glycolysis of Polyethylene Terephthalate (PET) into the Monomer Bis(2-hydroxyethyl) Terephthalate (BHET). Polym. Degrad. Stab. 2010, 95, 1686–1693. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, T.; Hwang, H.; Sung, Y.; Kim, B.H. Development of Glycolysis Catalysts for PET Wastes Including Polyester Textiles. Fibers Polym. 2025, 26, 1–17. [Google Scholar] [CrossRef]
- Chiao, Y.-W.; Liao, W.; Krisbiantoro, P.A.; Yu, B.-Y.; Wu, K.C.W. Waste-battery-derived Multifunctional Zinc Catalysts for Glycolysis and Decolorization of Polyethylene Terephthalate. Appl. Catal. B Environ. 2023, 325, 122302. [Google Scholar] [CrossRef]
- Han, N.; Lee, K.; Lee, J.; Jo, J.H.; An, E.J.; Lee, G.; Chi, W.S.; Lee, C. Dual-porous ZIF-8 Heterogeneous Catalysts with Increased Reaction Sites for Efficient PET Glycolysis. Chemosphere 2024, 364, 143187. [Google Scholar] [CrossRef]
- Jo, Y.; Kim, E.J.; Kim, J.; An, K. Efficient Fe3O4 Nanoparticle Catalysts for Depolymerization of Polyethylene Terephthalate. Green Chem. 2023, 25, 8160–8171. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Zhou, L.; Zhang, P.; Wang, Z.; Chen, X. Synergistic Catalysis of Ionic Liquids and Metal Salts for Facile PET Glycolysis. Eur. Polym. J. 2023, 201, 112578. [Google Scholar] [CrossRef]
- Li, F.; Yao, X.; Ding, R.; Bao, Y.; Zhou, Q.; Yan, D.; Li, Y.; Xu, J.; Xin, J.; Lu, X. Directional Glycolysis of Waste PET Using Deep Eutectic Solvents for Preparation of Aromatic-based Polyurethane Elastomers. Green Chem. 2024, 26, 9802–9813. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, X.; Geng, Y.; Zhou, Q.; Lu, X.; Zhang, S. Deep Eutectic Solvents as Highly Active Catalysts for the Fast and Mild Glycolysis of Poly(ethylene Terephthalate)(PET). Green Chem. 2015, 17, 2473–2479. [Google Scholar] [CrossRef]
- Liu, B.; Lu, X.; Ju, Z.; Sun, P.; Xin, J.; Yao, X.; Zhou, Q.; Zhang, S. Ultrafast Homogeneous Glycolysis of Waste Polyethylene Terephthalate via a Dissolution-Degradation Strategy. Ind. Eng. Chem. Res. 2018, 57, 16239–16245. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Flores-Giraldo, L.; Gutiérrez-Ortiz, J.I. Kinetics of Catalytic Glycolysis of PET Wastes with Sodium Carbonate. Chem. Eng. J. 2011, 168, 312–320. [Google Scholar] [CrossRef]
- Sert, E.; Yılmaz, E.; Atalay, F.S. Chemical Recycling of Polyethlylene Terephthalate by Glycolysis Using Deep Eutectic Solvents. J. Polym. Environ. 2019, 27, 2956–2962. [Google Scholar] [CrossRef]
- Yunita, I.; Putisompon, S.; Chumkaeo, P.; Poonsawat, T.; Somsook, E. Effective Catalysts Derived from Waste Ostrich Eggshells for Glycolysis of Post-consumer PET Bottles. Chem. Pap. 2019, 73, 1547–1560. [Google Scholar] [CrossRef]
- Javed, S.; Vogt, D. Development of Eco-Friendly and Sustainable PET Glycolysis Using Sodium Alkoxides as Catalysts. ACS Sustain. Chem. Eng. 2023, 11, 11541–11547. [Google Scholar] [CrossRef]
- Schlüter, M.; Enomoto, R.; Makino, S.; Weihs, L.; Stamm, C.L.; Wohlgemuth, K.; Held, C. Boosting the Kinetics of PET Glycolysis. React. Chem. Eng. 2024, 9, 3038–3046. [Google Scholar] [CrossRef]
- Laskar, K.; Bhattacharjee, P.; Gohain, M.; Deka, D.; Bora, U. Application of Bio-based Green Heterogeneous Catalyst for the Synthesis of Arylidinemalononitriles. Sustain. Chem. Pharm. 2019, 14, 100181. [Google Scholar] [CrossRef]
- Paganelli, S.; Brugnera, E.; Di Michele, A.; Facchin, M.; Beghetto, V. Chitosan as a Bio-Based Ligand for the Production of Hydrogenation Catalysts. Molecules 2024, 29, 2083. [Google Scholar] [CrossRef]
- Sölle, B.; Shaukat, U.; Rossegger, E.; Schlögl, S. Synthesis and Characterization of Bio-based Transesterification Catalysts for Green 3D-printable Dynamic Photopolymers. Polym. Chem. 2023, 14, 4994–5003. [Google Scholar] [CrossRef]
- Bishnoi, S.; Sharma, S.; Agrawal, H. Exploration of the Potential Application of Banana Peel for Its Effective Valorization: A Review. Indian J. Microbiol. 2023, 63, 398–409. [Google Scholar] [CrossRef]
- Soares Dias, A.P.; Pedra, I.; Salvador, É.; Rijo, B.; Costa Pereira, M.F.; Serralha, F.; Nogueira, I. Biodiesel Production over Banana Peel Biochar as a Sustainable Catalyst. Catalysts 2024, 14, 266. [Google Scholar] [CrossRef]
- Xu, C.; Li, G.; Ning, Y.; Zhou, M.; Hu, Z. Banana Peel Derived Nitrogen-doped Porous Carbon with Enhanced Electrocatalytic Activity for Complete Oxidation of Methanol Under Room Temperature. Sens. Actuators B Chem. 2021, 344, 130112. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, S.; Zhang, M.; Yu, Y.; Qin, T.; Tang, L.; Liu, Y.; Wu, W.; Mei, Q. Green Recycling of Waste PET Plastic Monomers by Banana Peel Extract. Chem. Eng. J. 2023, 474, 145697. [Google Scholar] [CrossRef]
- Tarigan, J.B.; Perangin-angin, S.; Simanungkalit, S.R.; Zega, N.P.; Sitepu, E.K. Utilization of Waste Banana Peels as Heterogeneous Catalysts in Room-temperature Biodiesel Production Using a Homogenizer. RSC Adv. 2023, 13, 6217–6224. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R.S.; Patil, M.; John, J. Navigating the Labyrinth of Polymer Sustainability in the Context of Carbon Footprint. Coatings 2024, 14, 774. [Google Scholar] [CrossRef]
- Laldinpuii, Z.T.; Lalmuanpuia, C.; Lalhmangaihzuala, S.; Khiangte, V.; Pachuau, Z.; Vanlaldinpuia, K. Biomass Waste-derived Recyclable Heterogeneous Catalyst for Aqueous Aldol Reaction and Depolymerization of PET Waste. New J. Chem. 2021, 45, 19542–19552. [Google Scholar] [CrossRef]
- Lalhmangaihzuala, S.; Laldinpuii, Z.; Lalmuanpuia, C.; Vanlaldinpuia, K. Glycolysis of Poly(Ethylene Terephthalate) Using Biomass-Waste Derived Recyclable Heterogeneous Catalyst. Polymers 2021, 13, 37. [Google Scholar] [CrossRef]
- Shingwekar, D.; Laster, H.; Kemp, H.; Mellies, J.L. Two-Step Chemo-Microbial Degradation of Post-Consumer Polyethylene Terephthalate (PET) Plastic Enabled by a Biomass-Waste Catalyst. Bioengineering 2023, 10, 1253. [Google Scholar] [CrossRef]
- Laldinpuii, Z.; Lalhmangaihzuala, S.; Pachuau, Z.; Vanlaldinpuia, K. Depolymerization of Poly(ethylene terephthalate) Waste with Biomass-waste Derived Recyclable Heterogeneous Catalyst. Waste Manag. 2021, 126, 1–10. [Google Scholar] [CrossRef]
- Pathak, G.; Das, D.; Rajkumari, K.; Rokhum, S.L. Exploiting Waste: Towards a Sustainable Production of Biodiesel Using Musa Acuminata Peel Ash as a Heterogeneous Catalyst. Green Chem. 2018, 20, 2365–2373. [Google Scholar] [CrossRef]
- Changmai, B.; Sudarsanam, P.; Rokhum, S.L. Biodiesel Production Using a Renewable Mesoporous Solid Catalyst. Ind. Crops Prod. 2020, 145, 111911. [Google Scholar] [CrossRef]
- Esquer, R.; García, J.J. Metal-catalysed Poly(Ethylene) terephthalate and polyurethane degradations by glycolysis. J. Organomet. Chem. 2019, 902, 120972. [Google Scholar] [CrossRef]
- Liu, B.; Fu, W.; Lu, X.; Zhou, Q.; Zhang, S. Lewis Acid–Base Synergistic Catalysis for Polyethylene Terephthalate Degradation by 1,3-Dimethylurea/Zn(OAc)2 Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2019, 7, 3292–3300. [Google Scholar] [CrossRef]
- Ghaedi, H.; Kalhor, P.; Zhao, M.; Clough, P.T.; Anthony, E.J.; Fennell, P.S. Potassium Carbonate-based Ternary Transition Temperature Mixture (Deep Eutectic Analogues) for CO2 Absorption: Characterizations and DFT Analysis. Front. Environ. Sci. Eng. 2021, 16, 92. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, M.; Dong, X.; Yang, D. The Reaction between K2CO3 and Ethylene Glycol in Deep Eutectic Solvents. Molecules 2024, 29, 4113. [Google Scholar] [CrossRef]
- Mohammadi, S.; Bouldo, M.G.; Enayati, M. FeCl3-Doped Cobalt Ferrite as an Efficient Magnetic Catalyst for PET Glycolysis Depolymerization. J. Polym. Environ. 2024, 32, 5738–5749. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Rev. A.02; ScienceOpen, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and eAccurate ab eInitio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).