Conceptual Recycling Chain for Proton Exchange Membrane Water Electrolyzers—Case Study Involving Review-Derived Model Stack
Abstract
1. Introduction
2. Model Stack of Proton Exchange Membrane Water Electrolyzer
2.1. Catalyst-Coated Membrane
2.1.1. Anode
2.1.2. Cathode
2.1.3. Membrane
2.2. Porous Transport Layer
2.3. Membrane Electrode Assembly Configurations
2.4. Bipolar Plates
2.5. Sealing
2.6. Clamping System
2.7. Stack Layout
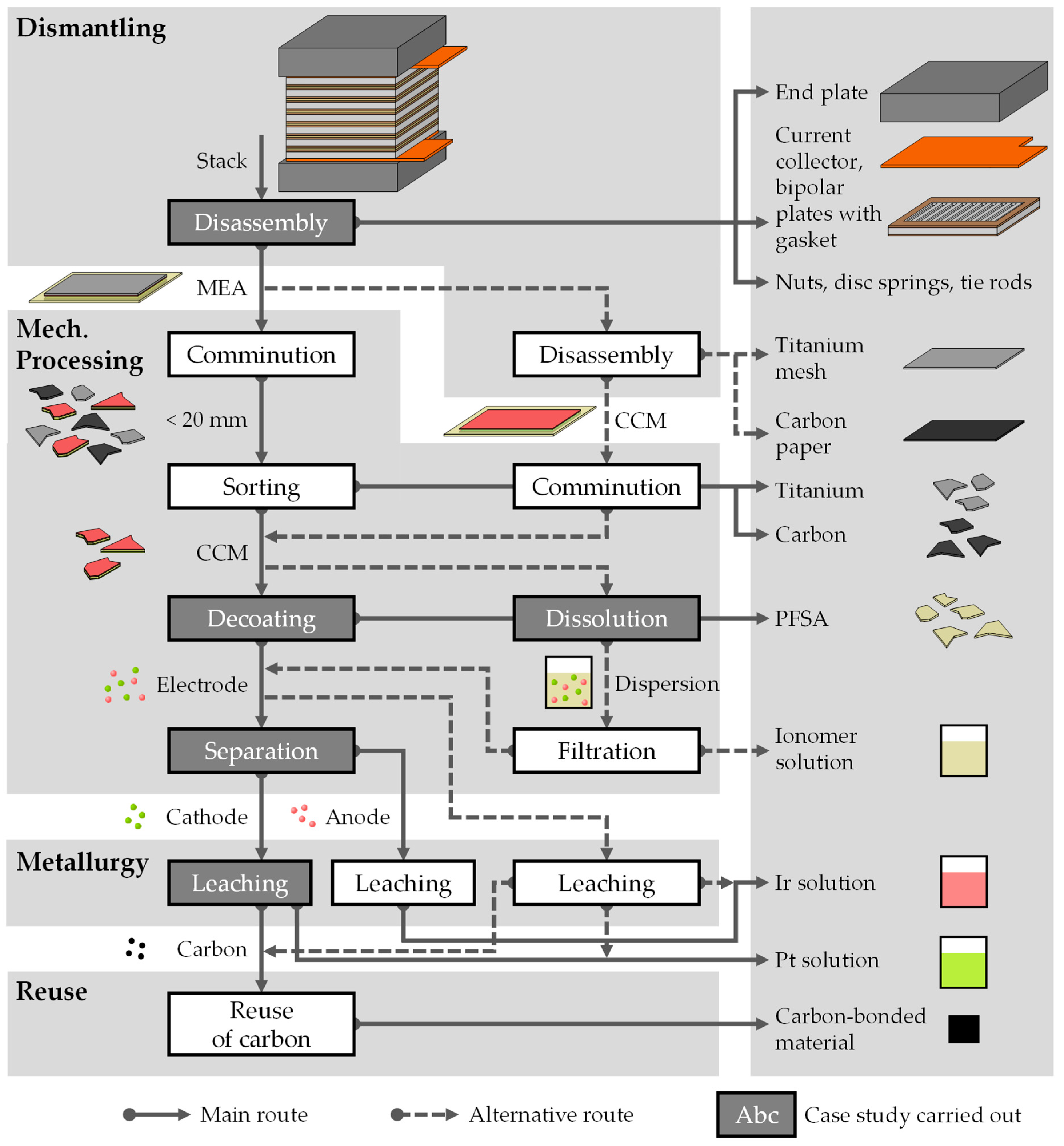
3. Recycling Process
3.1. General Recycling Approaches
- Collection and pre-sorting;
- Pretreatment and disassembly;
- Mechanical and chemical processing;
- The production of secondary (raw) materials.
3.2. Process Chain for PEMWE Recycling and Proof of Principle Tests
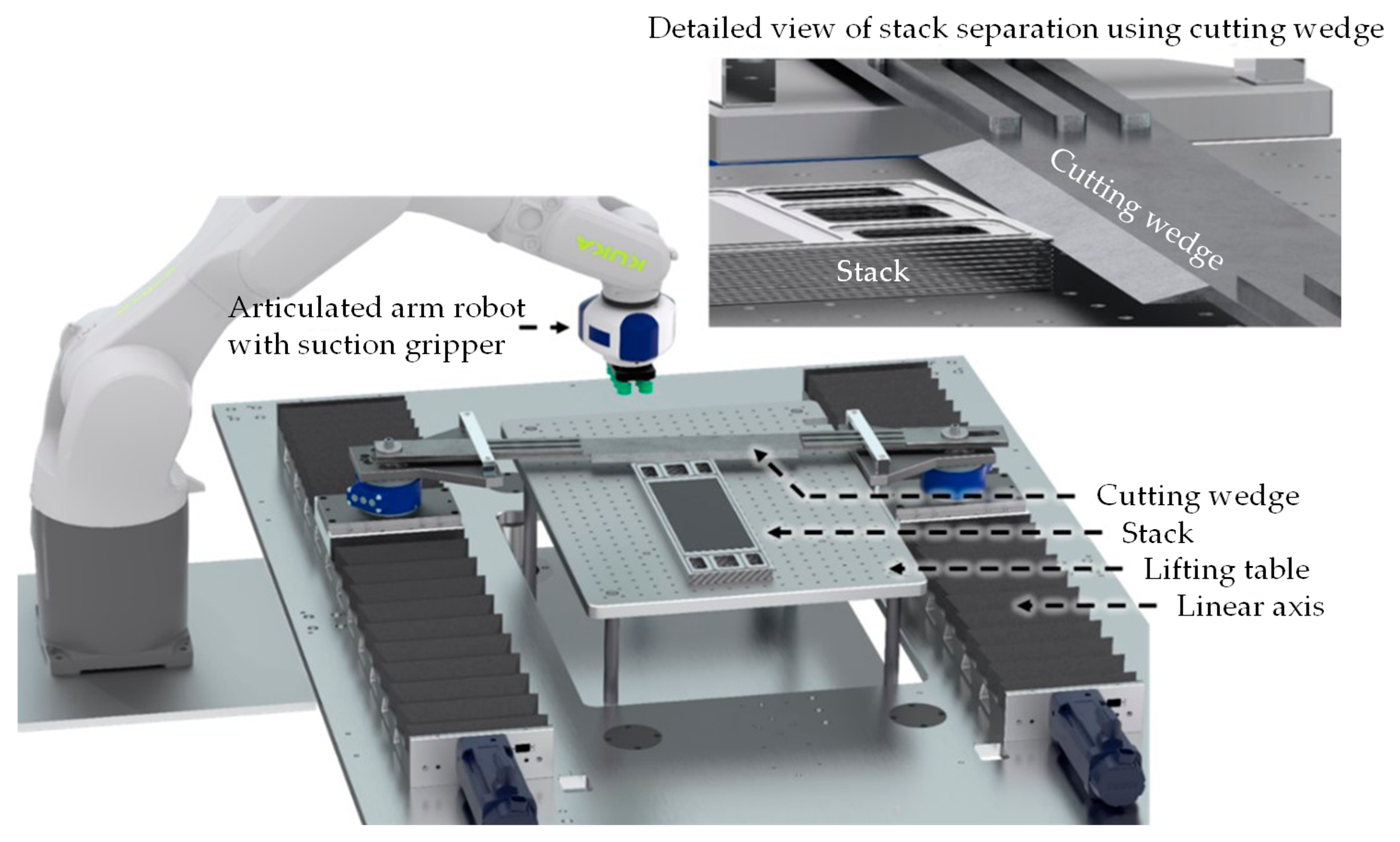
3.2.1. Disassembly into Individual Stack Components
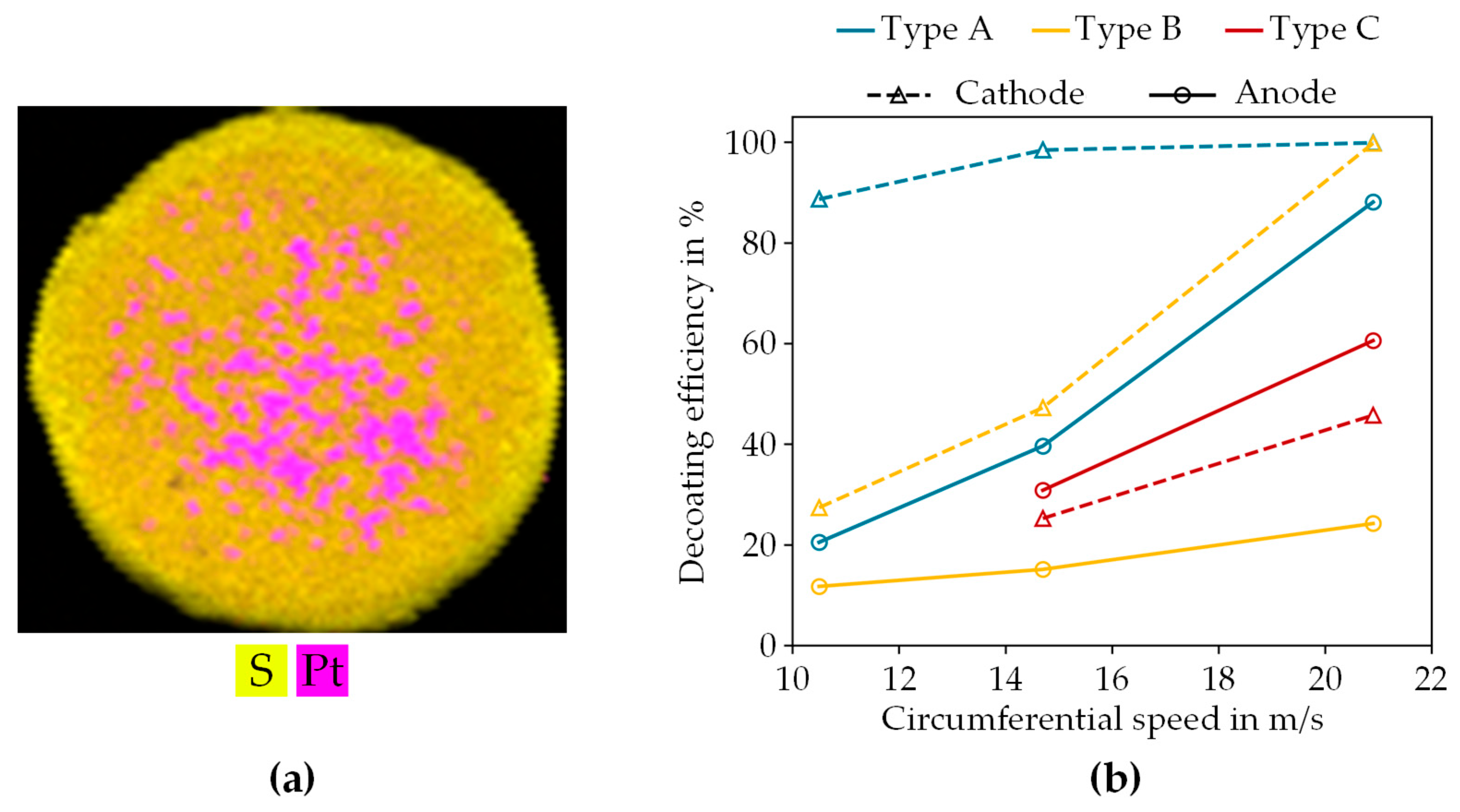
3.2.2. Decoating of Catalyst-Coated Membrane
3.2.3. Dissolution of Catalyst-Coated Membrane
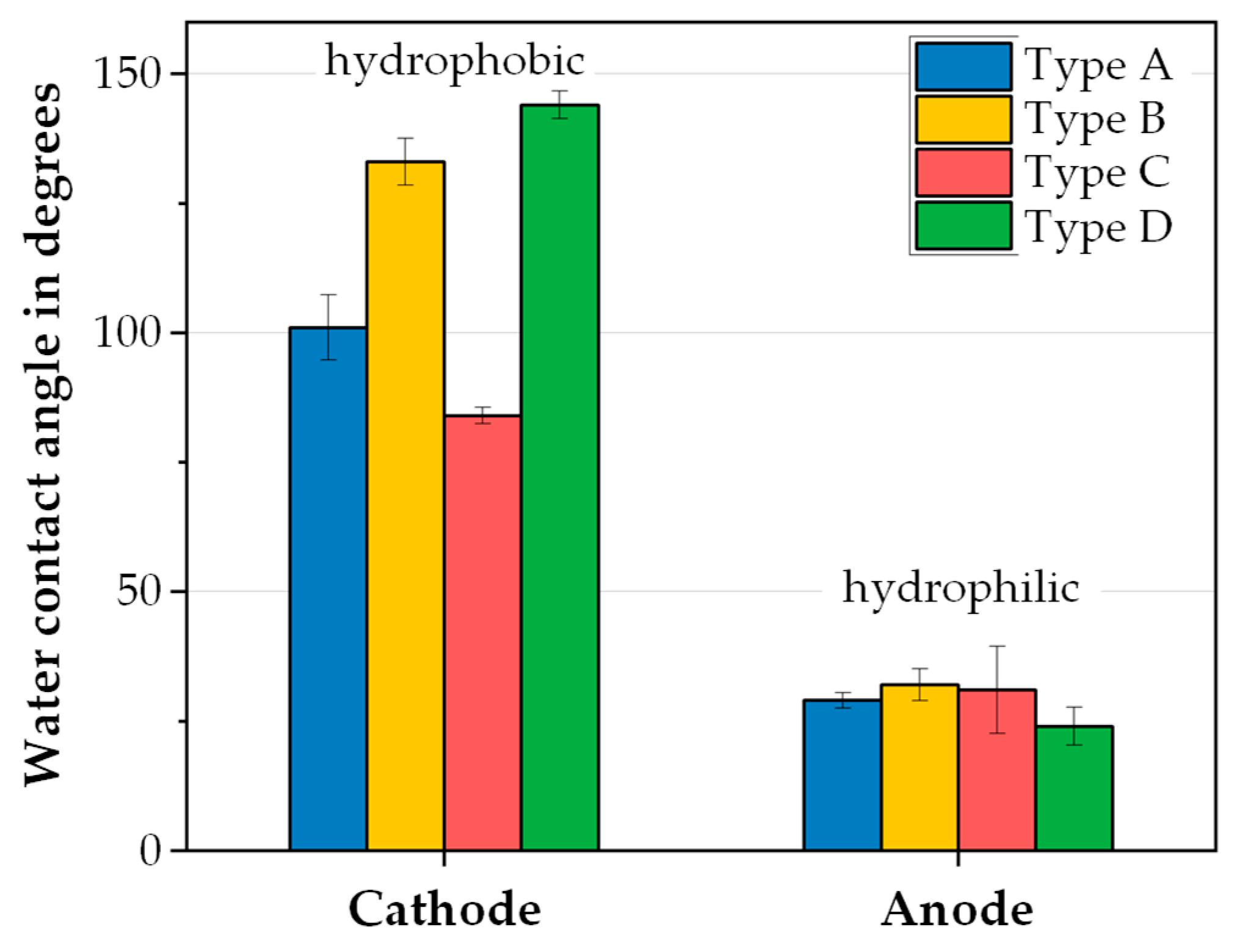
3.2.4. Separation of Anode and Cathode Materials
3.2.5. Leaching of Separated Platinum Catalyst from CCM Material
4. Conclusions
- During disassembly, adhesive joints were found that were not caused by the manufacturing process but by the operation of the electrolyzer at high pressures. These joints can be broken by an automated non-destructive cutting tool.
- Dry mechanical decoating of the CCM showed that the electrode layers can be liberated from the membrane material by mechanical stress. Depending on the type of CCM and the electrode side, up to 99% decoating efficiency was achieved.
- Alternatively to mechanical decoating, the membrane coating of the CCM was dissolved at high temperatures and pressures, which enabled us to recover the valuable membrane material as an ionomer dispersion. More than 99% iridium and platinum recovery has been achieved in small-scale experiments.
- A separation process based on the wetting properties of the mixed materials can be used to separate mechanically liberated iridium-containing anode material from the platinum-containing cathode material.
- The platinum-containing catalyst on the cathode side can be dissolved with high efficiency using standard metallurgical processes. However, these involve highly corrosive chemicals, so a more environmentally friendly alternative was sought and tested on carbon-supported platinum. Using hydrochloric acid in combination with organic acids and other oxidizing agents and salts, leaching efficiencies of >70% could be achieved even before optimization.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPP | Bipolar plate |
| CCM | Catalyst-coated membrane |
| EoL | End of Life |
| GDL | Gas diffusion layer |
| LGDL | Liquid–gas diffusion layer |
| LLPS | Liquid–liquid phase/particle separation |
| PEM | Proton exchange membrane |
| PEMFC | PEM fuel cell |
| PEMWE | PEM water electrolyzer |
| PGM | Platinum-group metal |
| PFSA | Perfluorosulfonic acid |
| PTE | Porous transport electrodes |
| PTFE | Polytetrafluoroethylene |
| PTL | Porous transport layer |
| µXRF | Micro-X-ray fluorescence spectroscopy |
References
- IEA. Global Hydrogen Review 2023; IEA: Paris, France, 2023. [Google Scholar]
- Hebling, C.; Ragwitz, M.; Fleiter, T.; Groos, U.; Härle, D.; Held, A.; Jahn, M.; Müller, N.; Pfeifer, T.; Plötz, P.; et al. Eine Wasserstoff-Roadmap für Deutschland; Fraunhofer-Institut für System- und Innovationsforschung ISI: Karlsruhe, Germany; Fraunhofer-Institut für Solare Energiesysteme ISE: Freiburg, Germany; unter Beteiligung von Fraunhofer-Institut für Mikrostruktur von Werkstoffen und Systemen IMWS: Halle, Germany; Fraunhofer-Institut für Keramische Technologien und Systeme IKTS: Dresden, Germany, 2019. [Google Scholar]
- Minke, C.; Suermann, M.; Bensmann, B.; Hanke-Rauschenbach, R. Is iridium demand a potential bottleneck in the realization of large-scale PEM water electrolysis? Int. J. Hydrogen Energy 2021, 46, 23581–23590. [Google Scholar] [CrossRef]
- IEA. Electrolysers. Available online: https://www.iea.org/energy-system/low-emission-fuels/electrolysers (accessed on 23 May 2025).
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis–A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Bernt, M.; Schröter, J.; Möckl, M.; Gasteiger, H.A. Analysis of Gas Permeation Phenomena in a PEM Water Electrolyzer Operated at High Pressure and High Current Density. J. Electrochem. Soc. 2020, 167, 124502. [Google Scholar] [CrossRef]
- Moschovi, A.M.; Zagoraiou, E.; Polyzou, E.; Yakoumis, I. Recycling of Critical Raw Materials from Hydrogen Chemical Storage Stacks (PEMWE), Membrane Electrode Assemblies (MEA) and Electrocatalysts. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1024, 012008. [Google Scholar] [CrossRef]
- Aricò, A.S.; Siracusano, S.; Briguglio, N.; Baglio, V.; Di Blasi, A.; Antonucci, V. Polymer electrolyte membrane water electrolysis: Status of technologies and potential applications in combination with renewable power sources. J. Appl. Electrochem. 2013, 43, 107–118. [Google Scholar] [CrossRef]
- Bareiß, K.; de la Rua, C.; Möckl, M.; Hamacher, T. Life cycle assessment of hydrogen from proton exchange membrane water electrolysis in future energy systems. Appl. Energy 2019, 237, 862–872. [Google Scholar] [CrossRef]
- Valente, A.; Martín-Gamboa, M.; Iribarren, D.; Dufour, J. Deliverable 2.2 Existing End-of-Life Technologies Applicable to FCH Products; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Mo, J.; Steen, S.; Han, B.; Kang, Z.; Terekhov, A.; Zhang, F.-Y.; Retterer, S.T.; Cullen, D.A. Investigation of titanium felt transport parameters for energy storage and hydrogen/oxygen production. In Proceedings of the 13th International Energy Conversion Engineering Conference, Orlando, FL, USA, 27–29 July 2015. [Google Scholar]
- Toray. Torayca® Carbon Paper for Fuel Cells. Available online: https://toray-cfe.com/en/products/carbon-paper/ (accessed on 29 February 2024).
- TANAKA Precious Metals. Electrocatalysts for Fuel Cell/Water Electrolysis (PEM Type). Available online: https://tanaka-preciousmetals.com/en/products/detail/pefcs/?nav=func (accessed on 9 September 2024).
- Mott. Porous Transport Layers for Electrolysis. Available online: https://mottcorp.com/product/gas-diffusion-transport-layers/porous-transport-layers/#:~:text=For%20customers%20working%20on%20electrolysis,resistance%20to%20bending%20and%20cracking (accessed on 29 February 2024).
- GORE. GORE® PEM for Water electrolysis. Available online: https://www.gore.com/system/files/2024-02/GORE-PEM-Water-Electrolysis-Datasheet-EN.pdf (accessed on 29 February 2024).
- Freudenberg. Freundenberg Gas Diffusion Layers-Technical Data. Available online: https://www.fuelcellstore.com/spec-sheets/freudenberg-gdl-technical-data.pdf (accessed on 29 February 2024).
- fumatech. Membranes for PEM Water Electrolysis (PEMWE)). Available online: https://www.fumatech.com/en/products/membranes-pem-water-electrolysis/ (accessed on 29 February 2024).
- DuPontTM. Nafion® PFSA Membranes-Product information. Available online: http://www.hesen.cn/userfiles/bochi/file/117%E3%80%81115%E5%8F%82%E6%95%B0.pdf (accessed on 29 February 2024).
- SGL CARBON. SIGRACET® Fuel Cell Components. Available online: https://www.sglcarbon.com/data/pdf/SIGRACET-Whitepaper.pdf (accessed on 29 February 2024).
- Holzapfel, P.; Bühler, M.; Van Pham, C.; Hegge, F.; Böhm, T.; McLaughlin, D.; Breitwieser, M.; Thiele, S. Directly coated membrane electrode assemblies for proton exchange membrane water electrolysis. Electrochem. Commun. 2020, 110, 106640. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. End of life of fuel cells and hydrogen products: From technologies to strategies. Int. J. Hydrogen Energy 2019, 44, 20965–20977. [Google Scholar] [CrossRef]
- Steinmüller, H.; Tichler, R.; Reiter, G.; Koppe, M.; Harasek, M.; Haider, M.; Gawlik, W.; Haas, R. Power to Gas–Eine Systemanalyse. Markt- und Technologiescouting und -Analyse (Endbericht 2014); 2014. [Google Scholar]
- Chatterjee, S.; Peng, X.; Intikhab, S.; Zeng, G.; Kariuki, N.N.; Myers, D.J.; Danilovic, N.; Snyder, J. Nanoporous Iridium Nanosheets for Polymer Electrolyte Membrane Electrolysis. Adv. Energy Mater. 2021, 11, 2101438. [Google Scholar] [CrossRef]
- Taie, Z.; Peng, X.; Kulkarni, D.; Zenyuk, I.V.; Weber, A.Z.; Hagen, C.; Danilovic, N. Pathway to Complete Energy Sector Decarbonization with Available Iridium Resources using Ultralow Loaded Water Electrolyzers. ACS Appl. Mater. Interfaces 2020, 12, 52701–52712. [Google Scholar] [CrossRef]
- Mirshekari, G.; Ouimet, R.; Zeng, Z.; Yu, H.; Bliznakov, S.; Bonville, L.; Niedzwiecki, A.; Capuano, C.; Ayers, K.; Maric, R. High-performance and cost-effective membrane electrode assemblies for advanced proton exchange membrane water electrolyzers: Long-term durability assessment. Int. J. Hydrogen Energy 2021, 46, 1526–1539. [Google Scholar] [CrossRef]
- Yu, H.; Bonville, L.; Jankovic, J.; Maric, R. Microscopic insights on the degradation of a PEM water electrolyzer with ultra-low catalyst loading. Appl. Catal. B Environ. 2020, 260, 118194. [Google Scholar] [CrossRef]
- Rozain, C.; Mayousse, E.; Guillet, N.; Millet, P. Influence of iridium oxide loadings on the performance of PEM water electrolysis cells: Part II–Advanced oxygen electrodes. Appl. Catal. B Environ. 2016, 182, 123–131. [Google Scholar] [CrossRef]
- Ayers, K.; Danilovic, N.; Ouimet, R.; Carmo, M.; Pivovar, B.; Bornstein, M. Perspectives on Low-Temperature Electrolysis and Potential for Renewable Hydrogen at Scale. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Linkov, V.; Bladergroen, B.J. Membrane electrode assemblies with low noble metal loadings for hydrogen production from solid polymer electrolyte water electrolysis. Int. J. Hydrogen Energy 2013, 38, 9601–9608. [Google Scholar] [CrossRef]
- Pantò, F.; Siracusano, S.; Briguglio, N.; Aricò, A.S. Durability of a recombination catalyst-based membrane-electrode assembly for electrolysis operation at high current density. Appl. Energy 2020, 279, 115809. [Google Scholar] [CrossRef]
- Xu, W.; Scott, K. The effects of ionomer content on PEM water electrolyser membrane electrode assembly performance. Int. J. Hydrogen Energy 2010, 35, 12029–12037. [Google Scholar] [CrossRef]
- Pham, C.V.; Escalera-López, D.; Mayrhofer, K.; Cherevko, S.; Thiele, S. Essentials of High Performance Water Electrolyzers–From Catalyst Layer Materials to Electrode Engineering. Adv. Energy Mater. 2021, 11, 2101998. [Google Scholar] [CrossRef]
- Bernt, M.; Siebel, A.; Gasteiger, H.A. Analysis of Voltage Losses in PEM Water Electrolyzers with Low Platinum Group Metal Loadings. J. Electrochem. Soc. 2018, 165, F305–F314. [Google Scholar] [CrossRef]
- Padgett, E.; Bender, G.; Haug, A.; Lewinski, K.; Sun, F.; Yu, H.; Cullen, D.A.; Steinbach, A.J.; Alia, S.M. Catalyst Layer Resistance and Utilization in PEM Electrolysis. J. Electrochem. Soc. 2023, 170, 084512. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Millet, P.; Fateev, V.N. Evaluation of carbon-supported Pt and Pd nanoparticles for the hydrogen evolution reaction in PEM water electrolysers. J. Power Sources 2008, 177, 281–285. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Wang, J.; Zhong, C.-J.; Liu, C.-J. Highly Active and Stable Pt-Pd Alloy Catalysts Synthesized by Room-Temperature Electron Reduction for Oxygen Reduction Reaction. Adv. Sci. 2017, 4, 1600486. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Rohstoffagentur (DERA) in der Bundesanstalt für Geowissenschaften und Rohstoffe (BGR). Preismonitor Dezember 2024; DERA: Berlin, Germany, 2024.
- Stähler, M.; Stähler, A.; Scheepers, F.; Carmo, M.; Lehnert, W.; Stolten, D. Impact of porous transport layer compression on hydrogen permeation in PEM water electrolysis. Int. J. Hydrogen Energy 2020, 45, 4008–4014. [Google Scholar] [CrossRef]
- Briguglio, N.; Pantò, F.; Siracusano, S.; Aricò, A.S. Enhanced performance of a PtCo recombination catalyst for reducing the H2 concentration in the O2 stream of a PEM electrolysis cell in the presence of a thin membrane and a high differential pressure. Electrochim. Acta 2020, 344, 136153. [Google Scholar] [CrossRef]
- Wittstock, R.; Pehlken, A.; Wark, M. Challenges in Automotive Fuel Cells Recycling. Recycling 2016, 1, 343–364. [Google Scholar] [CrossRef]
- Lettenmeier, P. Entwicklung und Integration Neuartiger Komponenten für Polymerelektrolytmembran- (PEM) Elektrolyseure. Ph.D. Dissertation, Universität Stuttgart, Stuttgart, Germany, 2018. [Google Scholar]
- Inamuddin, D.; Mohammad, A.; Asiri, A.M. Organic-Inorganic Composite Polymer Electrolyte Membranes: Preparation, Properties, and Fuel Cell Applications; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Bruton, T.A.; Blum, A. Proposal for coordinated health research in PFAS-contaminated communities in the United States. Environ. Health 2017, 16, 120. [Google Scholar] [CrossRef]
- Klose, C.; Saatkamp, T.; Münchinger, A.; Bohn, L.; Titvinidze, G.; Breitwieser, M.; Kreuer, K.D.; Vierrath, S. All-Hydrocarbon MEA for PEM Water Electrolysis Combining Low Hydrogen Crossover and High Efficiency. Adv. Energy Mater. 2020, 10, 1903995. [Google Scholar] [CrossRef]
- Wegner, R.; Fokkens, E.; Holdt, H.; Bukowsky, H.; Trautmann, M.; Nettesheim, S.; Jakubith, S.; Scholz, P.; Mollenhauer, T.; Theuring, S.; et al. reACT–Rückgewinnung und Wiedereinsatz von Edelmetallen aus Brennstoffzellen. In Recycling und Rohstoffe; Thomé-Kozmiensky, K.J., Goldmann, D., Eds.; TK Verlag Karl Thomé-Kozmiensky: Neuruppin, Germany, 2012; Volume 5, pp. 429–441. [Google Scholar]
- Haque, N.; Giddey, S.; Saha, S.; Sernia, P. Recyclability of Proton Exchange Membrane Electrolysers for Green Hydrogen Production. In Proceedings of the New Directions in Mineral Processing, Extractive Metallurgy, Recycling and Waste Minimization, San Diego, CA, USA, 19–23 March 2023; pp. 137–150. [Google Scholar]
- Bessarabov, D. (Invited) Membranes with Recombination Catalyst for Hydrogen Crossover Reduction: Water Electrolysis. ECS Trans. 2018, 85, 17–25. [Google Scholar] [CrossRef]
- Trinke, P.; Haug, P.; Brauns, J.; Bensmann, B.; Hanke-Rauschenbach, R.; Turek, T. Hydrogen Crossover in PEM and Alkaline Water Electrolysis: Mechanisms, Direct Comparison and Mitigation Strategies. J. Electrochem. Soc. 2018, 165, F502–F513. [Google Scholar] [CrossRef]
- Ma, L.; Zimmerer, N.; Schäfer, J.; Quarz, P.; Heckmann, T.; Scharfer, P.; Schabel, W.; Fleischer, J. Investigation on a micro-environment concept for MEA production process supported by numerical simulations. In Proceedings of the FC3-2nd Fuel Cell Conference Chemnitz 2022-Saubere Antriebe, Effizient Produziert: Wissenschaftliche Beiträge und Präsentationen der zweiten Brennstoffzellenkonferenz, Chemnitz, Germany, 31 May–1 June 2022; von Unwerth, T., Drossel, W.-G., Eds.; 2022. [Google Scholar]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Scott, K.; Puthiyapura, V. Polymer electrolyte membrane water electrolyser with Aquivion® short side chain perfluorosulfonic acid ionomer binder in catalyst layers. Int. J. Hydrogen Energy 2012, 37, 13243–13248. [Google Scholar] [CrossRef]
- Carmo, M.; Keeley, G.P.; Holtz, D.; Grube, T.; Robinius, M.; Müller, M.; Stolten, D. PEM water electrolysis: Innovative approaches towards catalyst separation, recovery and recycling. Int. J. Hydrogen Energy 2019, 44, 3450–3455. [Google Scholar] [CrossRef]
- Alipour Moghaddam, J.; Parnian, M.J.; Rowshanzamir, S. Preparation, characterization, and electrochemical properties investigation of recycled proton exchange membrane for fuel cell applications. Energy 2018, 161, 699–709. [Google Scholar] [CrossRef]
- Kutter, M.; Greve, C.; Maier, M.; Schilling, M.; Mauel, A.; Hilgert, A.; Hoffmann, H.; Hagemeier, W.; Rosin, A.; Muggli, M.; et al. Recycling of perfluorosulfonic acid-based membranes and their Re-application in PEM fuel cells. J. Membr. Sci. 2024, 693, 122370. [Google Scholar] [CrossRef]
- Höh, M.A. Poröse Transportschichten für die Polymerelektrolytmembran-Wasserelektrolyse. Ph.D. Dissertation, RWTH Aachen University, Jülich, Germany, 2017. [Google Scholar]
- Liu, C.; Shviro, M.; Gago, A.S.; Zaccarine, S.F.; Bender, G.; Gazdzicki, P.; Morawietz, T.; Biswas, I.; Rasinski, M.; Everwand, A.; et al. Exploring the Interface of Skin-Layered Titanium Fibers for Electrochemical Water Splitting. Adv. Energy Mater. 2021, 11, 2002926. [Google Scholar] [CrossRef]
- Maier, M.; Smith, K.; Dodwell, J.; Hinds, G.; Shearing, P.R.; Brett, D.J.L. Mass transport in PEM water electrolysers: A review. Int. J. Hydrogen Energy 2022, 47, 30–56. [Google Scholar] [CrossRef]
- Borgardt, E.; Panchenko, O.; Hackemüller, F.J.; Giffin, J.; Bram, M.; Müller, M.; Lehnert, W.; Stolten, D. Mechanical characterization and durability of sintered porous transport layers for polymer electrolyte membrane electrolysis. J. Power Sources 2018, 374, 84–91. [Google Scholar] [CrossRef]
- Borgardt, E. Mechanische Eigenschaften von katalysatorbeschichteten Membranen für die Polymer-Elektrolyt-Membran Elektrolyse. Ph.D. Thesis, RWTH Aachen, Jülich, Germany, 2021. [Google Scholar]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and perspectives of key materials for PEM electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Shirvanian, P.; van Berkel, F. Novel components in Proton Exchange Membrane (PEM) Water Electrolyzers (PEMWE): Status, challenges and future needs. A mini review. Electrochem. Commun. 2020, 114, 106704. [Google Scholar] [CrossRef]
- Bekaert. Porous Transport Layers for Electrochemical Production of Hydrogen and Green Molecules. Available online: https://www.bekaert.com/en/product-catalog/metal-fibers/engineered-solutions/porous-transport-layers (accessed on 29 February 2024).
- Rakousky, C.; Reimer, U.; Wippermann, K.; Carmo, M.; Lueke, W.; Stolten, D. An analysis of degradation phenomena in polymer electrolyte membrane water electrolysis. J. Power Sources 2016, 326, 120–128. [Google Scholar] [CrossRef]
- Kurzweil, P.; Dietlmeier, O.K. Elektrochemische Speicher, 1st ed.; Springer: Wiesbaden, Germany, 2015; pp. XIX, 579. [Google Scholar]
- Grigoriev, S.A.; Millet, P.; Volobuev, S.A.; Fateev, V.N. Optimization of porous current collectors for PEM water electrolysers. Int. J. Hydrogen Energy 2009, 34, 4968–4973. [Google Scholar] [CrossRef]
- Panchenko, O.; Carmo, M.; Rasinski, M.; Arlt, T.; Manke, I.; Müller, M.; Lehnert, W. Non-destructive in-operando investigation of catalyst layer degradation for water electrolyzers using synchrotron radiography. Mater. Today Energy 2020, 16, 100394. [Google Scholar] [CrossRef]
- Bach, M.; Gehre, P.; Biermann, H.; Aneziris, C.G. Recycling of carbon fiber composites in carbon-bonded alumina refractories. Ceram. Int. 2020, 46, 12574–12583. [Google Scholar] [CrossRef]
- Stähler, A.; Stähler, M.; Scheepers, F.; Lehnert, W.; Carmo, M. Scalable Implementation of Recombination Catalyst Layers to Mitigate Gas Crossover in PEM Water Electrolyzers. J. Electrochem. Soc. 2022, 169, 034522. [Google Scholar] [CrossRef]
- cmc Klebetechnik. Adhesive Film for PEM Fuel Cells and PEM Electrolysis Cells. Available online: https://www.cmc.de/en/wasserstofftechnik-brennstoffzellen (accessed on 29 February 2024).
- Mayyas, A.T.; Ruth, M.; Pivovar, B.S.; Bender, G.; Wipke, K.B. Manufacturing Cost Analysis for Proton Exchange Membrane Water Electrolyzers; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2019.
- Langemann, M.; Fritz, D.L.; Müller, M.; Stolten, D. Validation and characterization of suitable materials for bipolar plates in PEM water electrolysis. Int. J. Hydrogen Energy 2015, 40, 11385–11391. [Google Scholar] [CrossRef]
- Teuku, H.; Alshami, I.; Goh, J.; Masdar, M.S.; Loh, K.S. Review on bipolar plates for low-temperature polymer electrolyte membrane water electrolyzer. Int. J. Energy Res. 2021, 45, 20583–20600. [Google Scholar] [CrossRef]
- Kellenberger, A.; Vaszilcsin, N.; Duca, D.; Dan, M.L.; Duteanu, N.; Stiber, S.; Morawietz, T.; Biswas, I.; Ansar, S.A.; Gazdzicki, P.; et al. Towards Replacing Titanium with Copper in the Bipolar Plates for Proton Exchange Membrane Water Electrolysis. Materials 2022, 15, 1628. [Google Scholar] [CrossRef]
- Moreno Soriano, R.; Rojas, N.; Nieto, E.; de Guadalupe González-Huerta, R.; Sandoval-Pineda, J.M. Influence of the gasket materials on the clamping pressure distribution in a PEM water electrolyzer: Bolt torques and operation mode in pre-conditioning. Int. J. Hydrogen Energy 2021, 46, 25944–25953. [Google Scholar] [CrossRef]
- Höller, S. Water electrolysis stack for generating hydrogen and oxygen from water. U.S. Patent Application 18/558,387, 7 November 2024. [Google Scholar]
- Selamet, Ö.F.; Acar, M.C.; Mat, M.D.; Kaplan, Y. Effects of operating parameters on the performance of a high-pressure proton exchange membrane electrolyzer. Int. J. Energy Res. 2013, 37, 457–467. [Google Scholar] [CrossRef]
- Borgardt, E.; Giesenberg, L.; Reska, M.; Müller, M.; Wippermann, K.; Langemann, M.; Lehnert, W.; Stolten, D. Impact of clamping pressure and stress relaxation on the performance of different polymer electrolyte membrane water electrolysis cell designs. Int. J. Hydrogen Energy 2019, 44, 23556–23567. [Google Scholar] [CrossRef]
- Hydrogen Europe. Hydrogen Europe Position Paper on PFAS; HYDROGEN EUROPE: Brussels, Belgium, 2023. [Google Scholar]
- Selamet, O.F.; Ergoktas, M.S. Effects of bolt torque and contact resistance on the performance of the polymer electrolyte membrane electrolyzers. J. Power Sources 2015, 281, 103–113. [Google Scholar] [CrossRef]
- Smolinka, T.; Lehner, F.; Kiemel, S. Studie IndWEDe-Industrialisierung der Wasserelektrolyse in Deutschland: Chancen und Herausforderungen für Nachhaltigen Wasserstoff für Verkehr, Strom und Wärme; Nationale Organisation Wasserstoff- und Brennstoffzellentechnologie–NOW GmbH: Berlin, Germany, 2018. [Google Scholar]
- Caparrós Mancera, J.J.; Segura Manzano, F.; Andújar, J.M.; Vivas, F.J.; Calderón, A.J. An Optimized Balance of Plant for a Medium-Size PEM Electrolyzer: Design, Control and Physical Implementation. Electronics 2020, 9, 871. [Google Scholar] [CrossRef]
- Selamet, Ö.F.; Becerikli, F.; Mat, M.D.; Kaplan, Y. Development and testing of a highly efficient proton exchange membrane (PEM) electrolyzer stack. Int. J. Hydrogen Energy 2011, 36, 11480–11487. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Di Blasi, A.; Briguglio, N.; Stassi, A.; Ornelas, R.; Trifoni, E.; Antonucci, V.; Aricò, A.S. Electrochemical characterization of single cell and short stack PEM electrolyzers based on a nanosized IrO2 anode electrocatalyst. Int. J. Hydrogen Energy 2010, 35, 5558–5568. [Google Scholar] [CrossRef]
- SIEMENS. SILYZER 300-The Next Paradigm of PEM Electrolysis; SIEMENS: Munich, Germany, 2018. [Google Scholar]
- Bosch GmbH. Hybrion PEM Electrolysis Stack Brochure. Available online: https://www.bosch-hydrogen-energy.com/pem-electrolysis/pem-elektrolysis-stacks/ (accessed on 13 June 2025).
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. 2008. Available online: https://eur-lex.europa.eu/eli/dir/2008/98/oj/eng (accessed on 31 May 2024).
- Al Assadi, A.; Goes, D.; Baazouzi, S.; Staudacher, M.; Malczyk, P.; Kraus, W.; Nägele, F.; Huber, M.F.; Fleischer, J.; Peuker, U.; et al. Challenges and prospects of automated disassembly of fuel cells for a circular economy. Resour. Conserv. Recycl. Adv. 2023, 19, 200172. [Google Scholar] [CrossRef]
- Martens, H.; Goldmann, D. Recyclingtechnik; Springer: Wiesbaden, Germany, 2016; pp. XXIII, 556. [Google Scholar]
- Kumar, A.; Holuszko, M.; Espinosa, D.C.R. E-waste: An overview on generation, collection, legislation and recycling practices. Resour. Conserv. Recycl. 2017, 122, 32–42. [Google Scholar] [CrossRef]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef]
- Melke, J.; Maletzko, A.; Gomez Villa, E.D.; Bornet, A.; Wiberg, G.K.H.; Arenz, M.; Sandig-Predzymirska, L.; Thiere, A.; Charitos, A.; Stelter, M.; et al. Recycalyse–New Sustainable and Recyclable Catalytic Materials for Proton Exchange Membrane Electrolysers. Chem. Ing. Tech. 2024, 96, 126–142. [Google Scholar] [CrossRef]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Werner, D.M.; Mütze, T.; Peuker, U.A. Influence of Pretreatment Strategy on the Crushing of Spent Lithium-Ion Batteries. Metals 2022, 12, 1839. [Google Scholar] [CrossRef]
- Vanegas, P.; Peeters, J.R.; Cattrysse, D.; Tecchio, P.; Ardente, F.; Mathieux, F.; Dewulf, W.; Duflou, J.R. Ease of disassembly of products to support circular economy strategies. Resour. Conserv. Recycl. 2018, 135, 323–334. [Google Scholar] [CrossRef]
- Khatib, F.N.; Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; El-Hassan, Z.; Durrant, A.; Thompson, J.; Olabi, A.G. Material degradation of components in polymer electrolyte membrane (PEM) electrolytic cell and mitigation mechanisms: A review. Renew. Sustain. Energy Rev. 2019, 111, 1–14. [Google Scholar] [CrossRef]
- Millet, P.; Ranjbari, A.; de Guglielmo, F.; Grigoriev, S.A.; Auprêtre, F. Cell failure mechanisms in PEM water electrolyzers. Int. J. Hydrogen Energy 2012, 37, 17478–17487. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, X.Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- European Environment Agency. Accelerating the Circular Economy in Europe-State and Outlook 2024. 2024. Available online: https://www.eea.europa.eu/en/analysis/publications/accelerating-the-circular-economy (accessed on 31 May 2024).
- DIN/TS 54405:2020-12; Construction Adhesives-Guideline for Separation and Recycling of Adhesives and Substrates from Bonded Joints. DIN Media: Berlin, Germany, 2020.
- Bharti, A.; Natarajan, R. Recovery of expensive Pt/C catalysts from the end-of-life membrane electrode assembly of proton exchange membrane fuel cells. RSC Adv. 2020, 10, 35057–35061. [Google Scholar] [CrossRef]
- Arlt, C.-R.; Brekel, D.; Franzreb, M. Continuous fractionation of nanoparticles based on their magnetic properties applying simulated moving bed chromatography. Sep. Purif. Technol. 2021, 259, 118123. [Google Scholar] [CrossRef]
- Rhein, F.; Scholl, F.; Nirschl, H. Magnetic seeded filtration for the separation of fine polymer particles from dilute suspensions: Microplastics. Chem. Eng. Sci. 2019, 207, 1278–1287. [Google Scholar] [CrossRef]
- Giesler, J.; Pesch, G.R.; Weirauch, L.; Schmidt, M.-P.; Thöming, J.; Baune, M. Polarizability-Dependent Sorting of Microparticles Using Continuous-Flow Dielectrophoretic Chromatography with a Frequency Modulation Method. Micromachines 2020, 11, 38. [Google Scholar] [CrossRef]
- Ahn, S.; Rudolph, M. Development of Fine Particle Mechanical Separation Processes with Representative Catalyst Materials for Recycling PEM Water Electrolyzers Exploiting their Wetting Characteristics. ChemCatChem 2023, 16, e202300931. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Ahn, S.; Patil, S.; Rudolph, M. Ultrafine Particle Recycling—Efficiency of the Hydrophobic Double Emulsion Technique for the Selective Agglomeration and Froth Flotation of Ultrafine Cathode Catalyst Particles from PEM Water Electrolyzers. ACS Eng. Au 2024, 5, 57–65. [Google Scholar] [CrossRef]
- Machunsky, S.; Peuker, U.A. Liquid-Liquid Interfacial Transport of Nanoparticles. Phys. Sep. Sci. Eng. 2007, 2007, 034832. [Google Scholar] [CrossRef]
- Heilmann, C.; Ditscherlein, L.; Peuker, U.A. Influence of Hydrolyzed Metal Ions and Surfactants on the Phase Transfer of Al2O3, SiO2, and SnO2. Langmuir 2024, 40, 2543–2550. [Google Scholar] [CrossRef]
- Olbrich, W.; Kadyk, T.; Sauter, U.; Eikerling, M. Review—Wetting Phenomena in Catalyst Layers of PEM Fuel Cells: Novel Approaches for Modeling and Materials Research. J. Electrochem. Soc. 2022, 169, 054521. [Google Scholar] [CrossRef]
- Ahn, S.; Rudolph, M. Impact of ionomer containing particles on the selective mechanical separation processes of PEM water electrolyzer recycling for PGM recovery. In Proceedings of the International Mineral Processing Congress, Washington, DC, USA, 29 September–3 October 2024. [Google Scholar]
- Madhav, D.; Wang, J.; Keloth, R.; Mus, J.; Buysschaert, F.; Vandeginste, V. A Review of Proton Exchange Membrane Degradation Pathways, Mechanisms, and Mitigation Strategies in a Fuel Cell. Energies 2024, 17, 998. [Google Scholar] [CrossRef]
- Granados-Fernández, R.; Montiel, M.A.; Díaz-Abad, S.; Rodrigo, M.A.; Lobato, J. Platinum Recovery Techniques for a Circular Economy. Catalysts 2021, 11, 937. [Google Scholar] [CrossRef]
- Sverdrup, H.U.; Ragnarsdottir, K.V. A system dynamics model for platinum group metal supply, market price, depletion of extractable amounts, ore grade, recycling and stocks-in-use. Resour. Conserv. Recycl. 2016, 114, 130–152. [Google Scholar] [CrossRef]
- Saguru, C.; Ndlovu, S.; Moropeng, D. A review of recent studies into hydrometallurgical methods for recovering PGMs from used catalytic converters. Hydrometallurgy 2018, 182, 44–56. [Google Scholar] [CrossRef]
- Asamoah-Bekoe, Y. Investigation of the Leaching of the Platinum Group Metal Concentrate in Hydrochloric Acid Solution by Chlorine. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 1998. [Google Scholar]
- Wilkens, M.; Peuker, U.A. Grundlagen und aktuelle Entwicklungen der Filterkuchenwaschung. Chem. Ing. Tech. 2012, 84, 1873–1884. [Google Scholar] [CrossRef]
- Sauer, F.; Löwer, E.; Henn, H.; Peuker, U.; Hoffner, B. Displacement Washing of Filter Cakes With a Fine Particle Top Layer. Chem. Eng. Technol. 2025, 48, e202400394. [Google Scholar] [CrossRef]

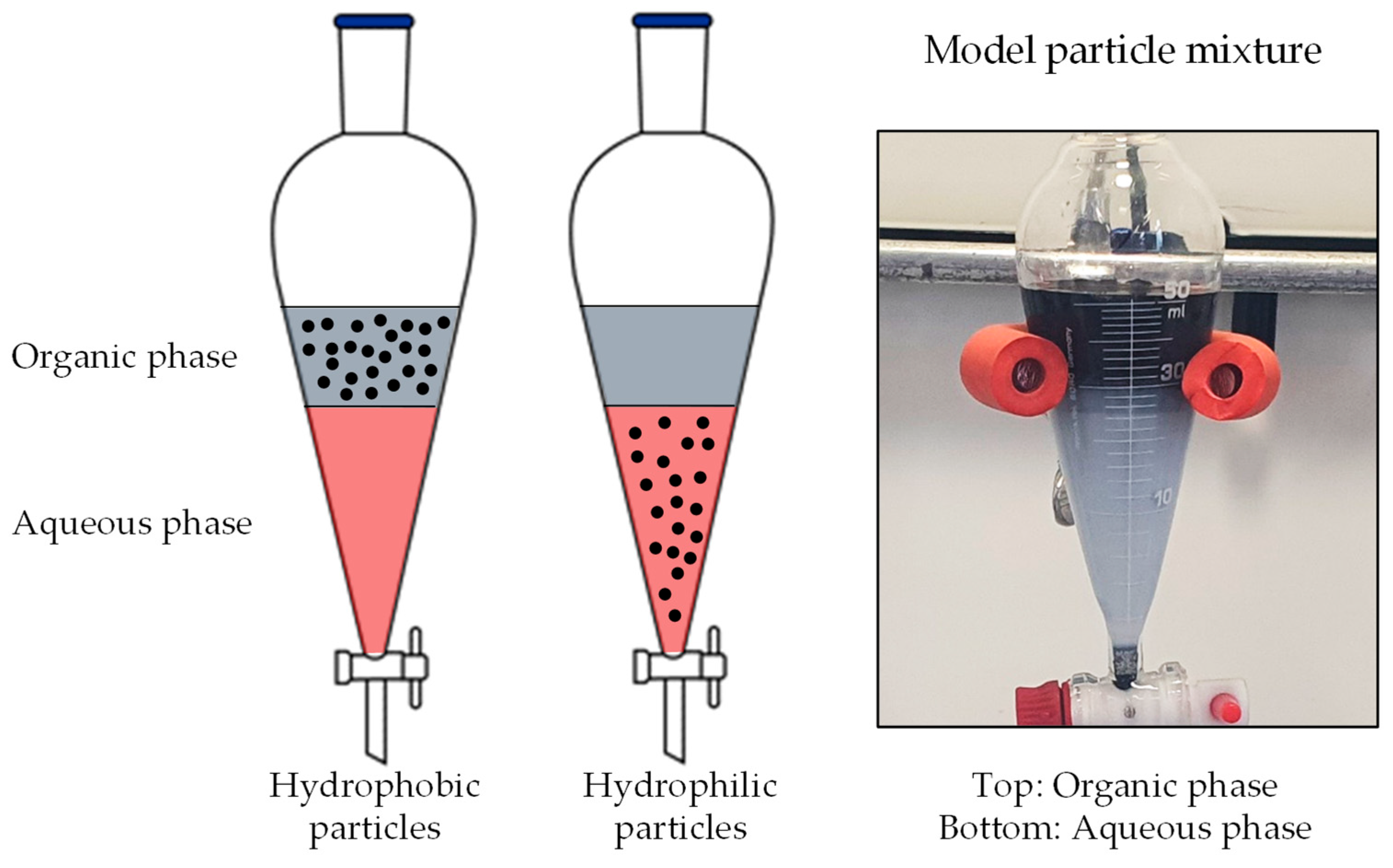
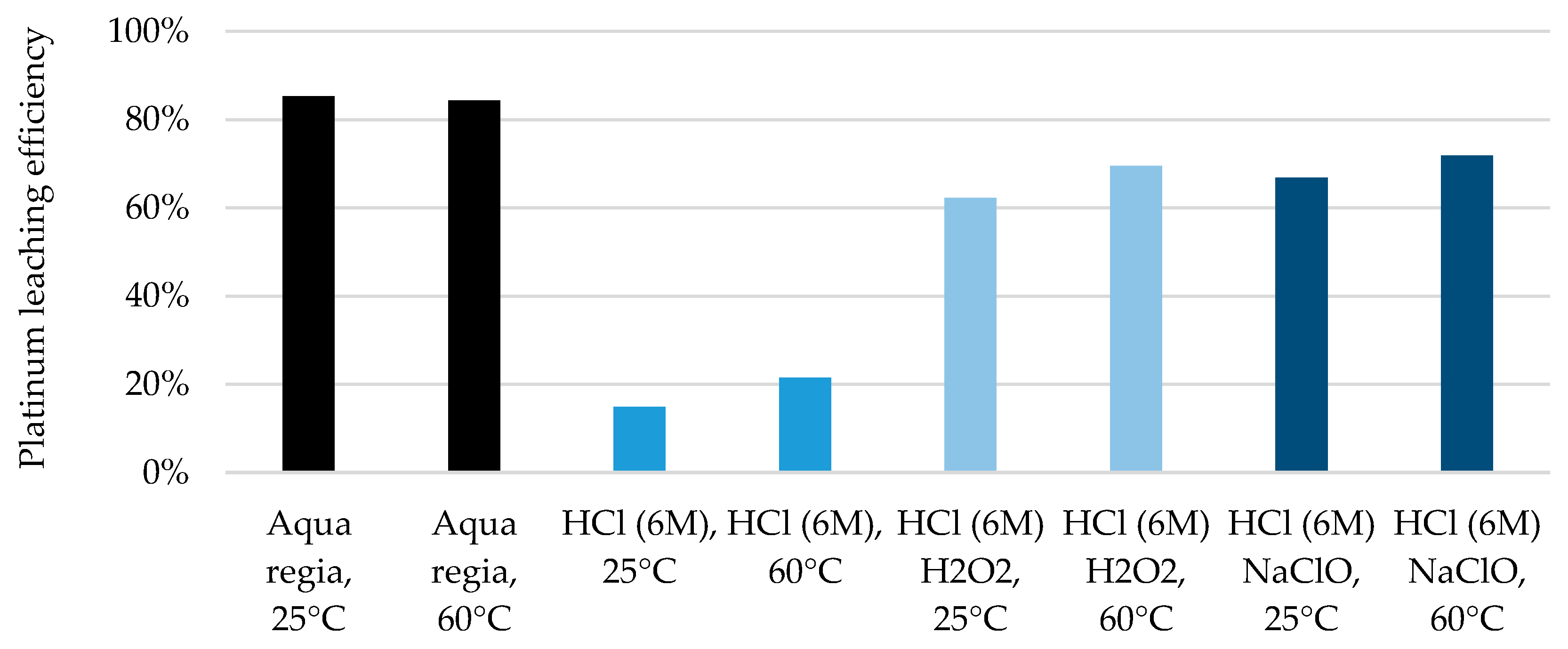
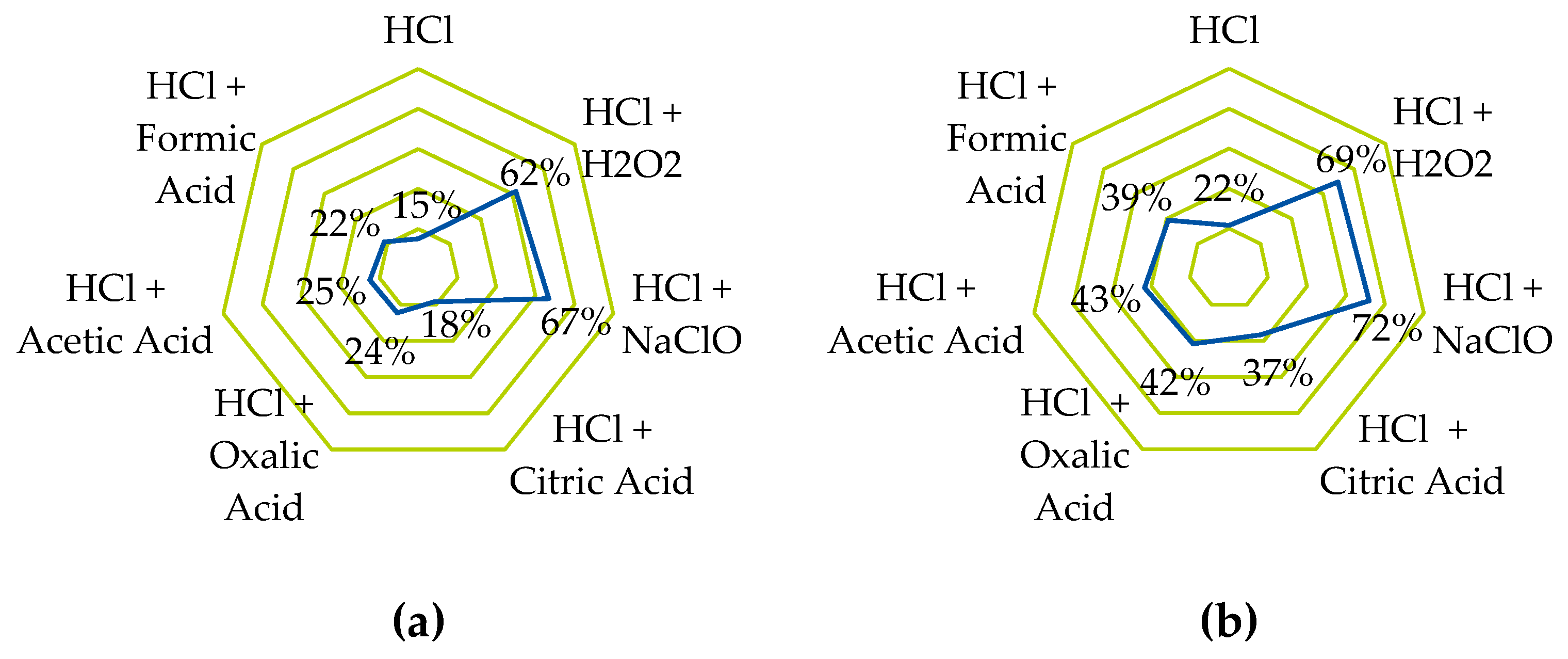
| Components | Different. Properties | PEMWE Manifestations | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Membrane and Catalyst Layer | membrane material | perfluorosulfonic acid (PFSA) | hydrocarbon | … | ||||||
| membrane thickness | 51 µm | ↔ | 127 µm | ↔ | 254 µm | … | ||||
| anode material | IrO2 | Ir black | IrRuO2 | Ir mixed metal | … | |||||
| anode loading | … | 0.3 mgIr cm−2 | ↔ | 1 mgIr cm−2 | ↔ | 3 mgIr cm−2 | … | |||
| anode layer thickness | … | 2 µm | ↔ | 12 µm | … | |||||
| cathode material | Pt black | Pt carbon-supported | Pd | … | ||||||
| cathode loading | … | 0.1 mgPt cm−2 | ↔ | 0.5 mgPt cm−2 | ↔ | 1 mgPt cm−2 | … | |||
| cathode layer thickness | … | 8 µm | ↔ | 21 µm | … | |||||
| Porous Transport Layer | LGDL material | Ti | … | |||||||
| LGDL thickness | … | 100 µm | ↔ | 250 µm | ↔ | 2.000 µm | ↔ | 3.175 µm | … | |
| LGDL specification | mesh | felt | foam | grid | sintered Ti powder | … | ||||
| LGDL porosity | … | 30% | ↔ | 50% | … | |||||
| LGDL coating material | Au | Pt | Ir | … | none | |||||
| GDL material | carbon | … | ||||||||
| GDL thickness | … | 110 µm | ↔ | 370 µm | … | |||||
| GDL specification | felt | paper | nonwovens | … | ||||||
| GDL porosity | … | 75% | ↔ | 80% | … | |||||
| Membrane Electrode Assembly Configurations | frame/subgasket structure | yes | no | |||||||
| one-sided around CCM | two-sided around CCM | |||||||||
| GDL enclosed | GDL overlaid | |||||||||
| frame/subgasket material | PI | PEN | PEEK | PPS | PTFE | … | ||||
| Bipolar Plates | bipolar plate material | Ti | stainless steel | graphite | Al | … | ||||
| bipolar plate coating | yes | no | ||||||||
| bipolar plate coating material | Pt | Au | Ti | Ir | Nb | … | ||||
| bipolar plate thickness | … | 300 µm | ↔ | 3.000 µm | … | |||||
| flow field | integrated flow field | flow field as separate component | ||||||||
| Sealing Elements | material | PTFE | silicone | Teflon | FKM | EPDM | PVD | PFA | rubber | … |
| thickness | … | 250 µm | ↔ | 1.640 µm | … | |||||
| production/joining method | injection molding | screen-printing | dispensing | … | ||||||
| sealing concept | sealing on MEA | sealing on bipolar plate | ||||||||
| Clamping System | end plate material | Al | stainless steel | … | ||||||
| fixation/compression elements | tie rod | tension bands | ||||||||
| position of compression elements | inside | outside | ||||||||
| Stack Layout and Active Area | stack format | rectangular/quadratic | circular | |||||||
| size stack area | … | 0.0025 m2 | ↔ | 0.7 m2 | ↔ | 1 m2 | … | |||
| CCM | Platinum Recovery in Residue (wt-%) | Iridium Recovery in Residue (wt-%) |
|---|---|---|
| Type #1—Spent PEMWE | >99% | >99% |
| Type #2—Spent PEMFC | >99% | Not contained |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staudacher, M.; Goes, D.; Ahn, S.; Vrucak, D.; Gießmann, T.; Bauer-Siebenlist, B.; Leißner, T.; Rudolph, M.; Fleischer, J.; Friedrich, B.; et al. Conceptual Recycling Chain for Proton Exchange Membrane Water Electrolyzers—Case Study Involving Review-Derived Model Stack. Recycling 2025, 10, 121. https://doi.org/10.3390/recycling10030121
Staudacher M, Goes D, Ahn S, Vrucak D, Gießmann T, Bauer-Siebenlist B, Leißner T, Rudolph M, Fleischer J, Friedrich B, et al. Conceptual Recycling Chain for Proton Exchange Membrane Water Electrolyzers—Case Study Involving Review-Derived Model Stack. Recycling. 2025; 10(3):121. https://doi.org/10.3390/recycling10030121
Chicago/Turabian StyleStaudacher, Malena, Dominik Goes, Sohyun Ahn, Dzeneta Vrucak, Tim Gießmann, Bernhard Bauer-Siebenlist, Thomas Leißner, Martin Rudolph, Jürgen Fleischer, Bernd Friedrich, and et al. 2025. "Conceptual Recycling Chain for Proton Exchange Membrane Water Electrolyzers—Case Study Involving Review-Derived Model Stack" Recycling 10, no. 3: 121. https://doi.org/10.3390/recycling10030121
APA StyleStaudacher, M., Goes, D., Ahn, S., Vrucak, D., Gießmann, T., Bauer-Siebenlist, B., Leißner, T., Rudolph, M., Fleischer, J., Friedrich, B., & Peuker, U. A. (2025). Conceptual Recycling Chain for Proton Exchange Membrane Water Electrolyzers—Case Study Involving Review-Derived Model Stack. Recycling, 10(3), 121. https://doi.org/10.3390/recycling10030121











