Study of the Effect of Temperature to Optimize the Anaerobic Digestion of Slaughterhouse Sludge by Co-Digestion with Slaughterhouse Wastewater
Abstract
1. Introduction
2. Results
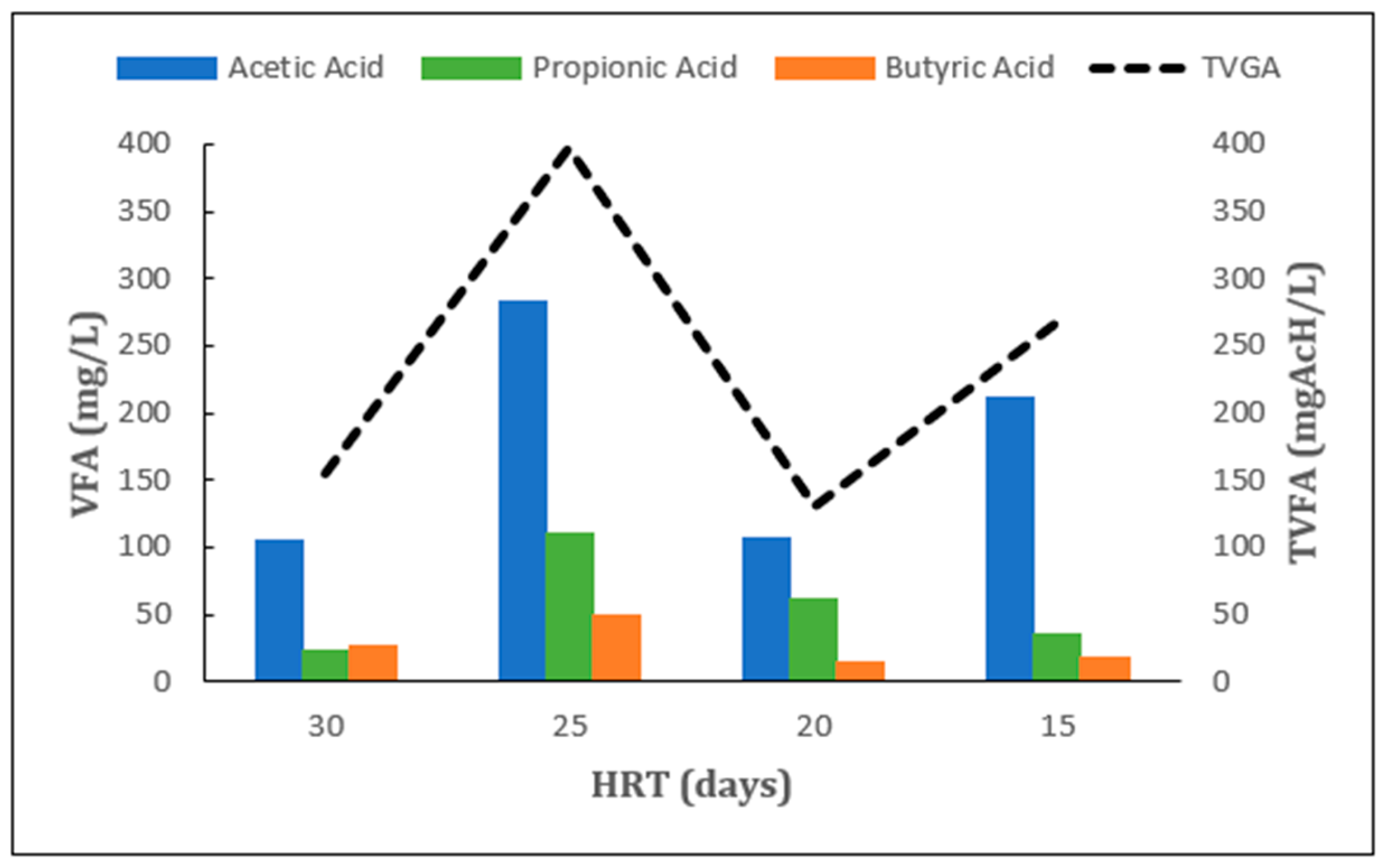
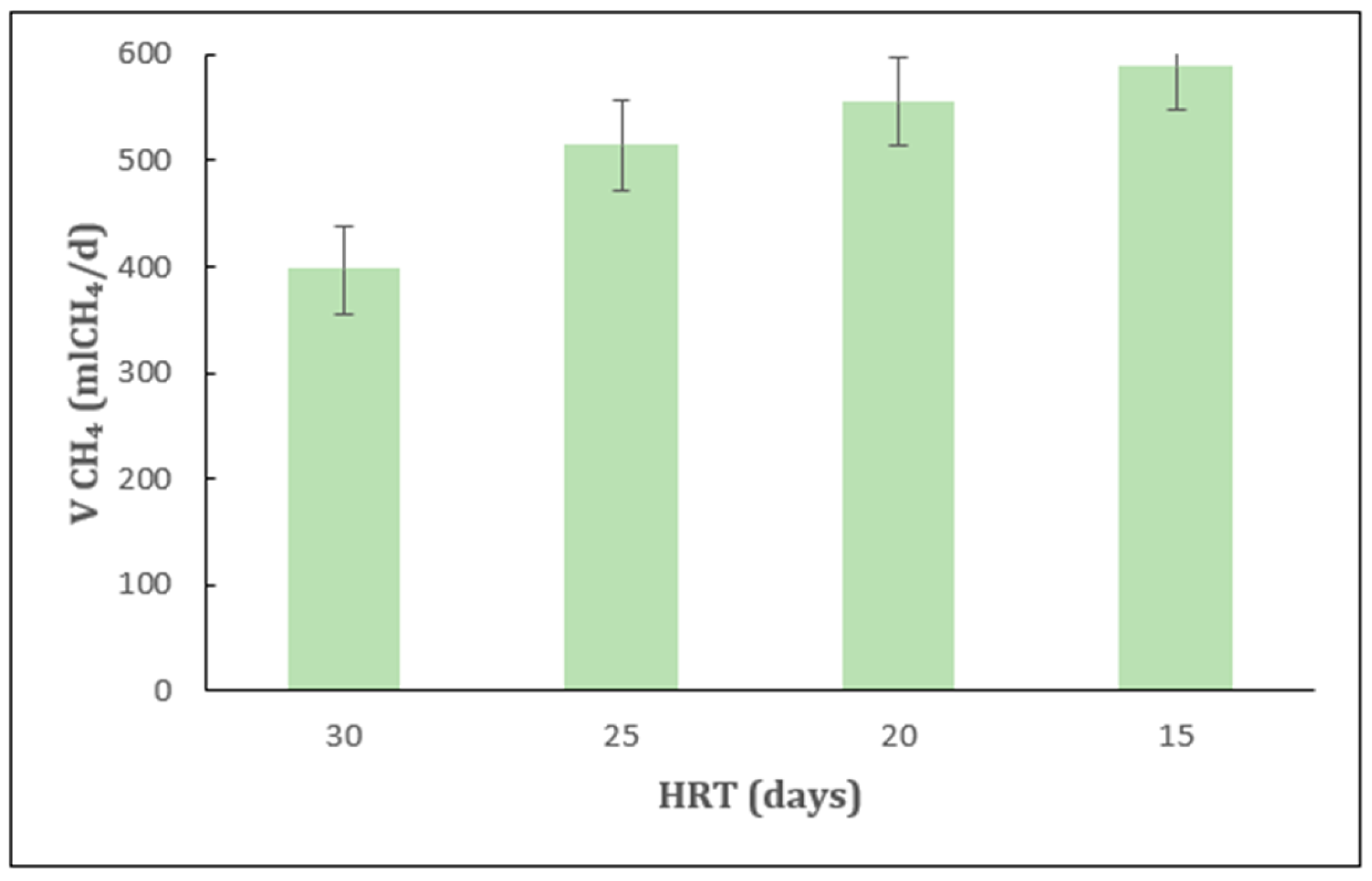
2.1. Effect of HRT Under Mesophilic Conditions
2.2. Effect of HRT Under Thermophilic Conditions
3. Discussion
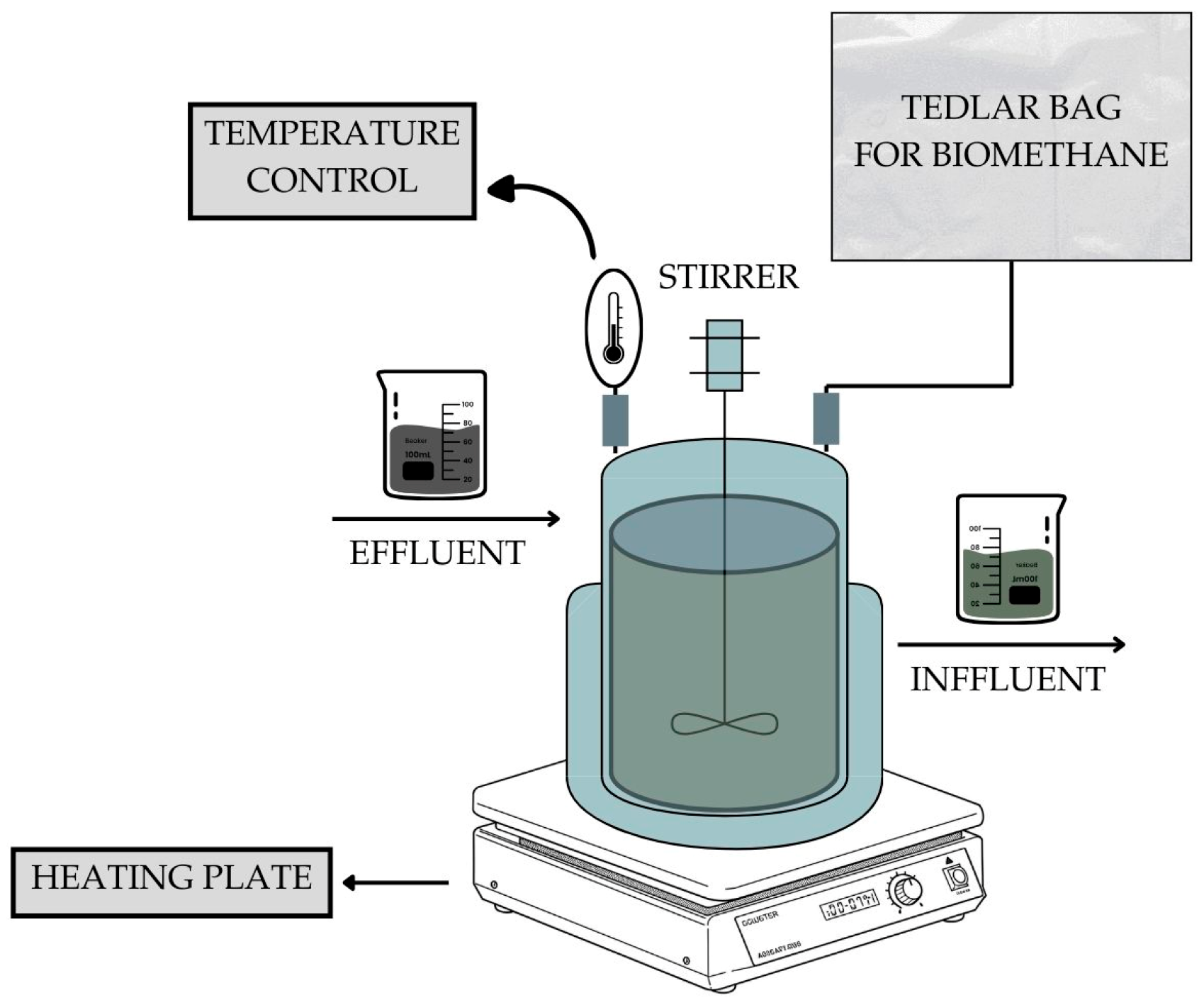
4. Materials and Methods
4.1. Characterization of the Substrates, the Feed and the Inoculum
4.2. Operating Conditions
4.3. Analytical Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| ACoD | Anaerobic Co-Digestion |
| TPACoD | Temperature-Phased Anaerobic Co-Digestion |
| WWTP | Waste Water Treatment Plan |
| CTSR | Continuous Stirred Tank Reactor |
| FID | Flame Ionization Detector |
| VFA | Volatile Fatty Acids |
| TVFA | Total Volatile Fatty Acids |
| LCFA | Long-chain Fatty Acids |
| S | Slaughterhouse Sludge |
| SW | Slaughterhouse Wastewater |
| HRT | Hydraulic Retention Time |
| tCOD | Chemical Oxygen Demand Total |
| sCOD | Chemical Oxygen Demand Soluble |
| TS | Total Solids |
| VS | Volatile Solids |
References
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. The title of the cited article. Biotechnol. Biofuels 2021, 14, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Pirmoradi, N.; Ghaneian, M.T.; Ehrampoush, M.H.; Salmani, M.H.; Hatami, B. The conversion of poultry slaughterhouse wastewater sludge into biodiesel: Process modeling and optimization. Renew. Energy 2021, 178, 1236–1249. [Google Scholar] [CrossRef]
- Sadh, P.K.; Chawla, P.; Kumar, S.; Das, A.; Sulakh, R.K.; Bains, A.; Sridhar, K.; Duhan, J.S.; Sharma, M. Recovery of agricultural waste biomass: A path for circular bioeconomy. Sci. Total Environ. 2023, 870, 161904. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Lam, C.H.; Subramanian, K.; Qin, Z.H.; Mou, J.H.; Jin, M.; Chopra, S.S.; Singh, V.; Ok, Y.S.; et al. Emerging waste valorisation techniques to moderate the hazardous impacts, and their path towards sustainability. J. Hazard. Mater. 2022, 423, 127023. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Biochemical assays of potential methane to test biogas production from dark fermentation of sewage sludge and agricultural residues. Int. J. Hydrogen Energy 2022, 27, 13289–13299. [Google Scholar] [CrossRef]
- Venegas, M.; Leiva, A.M.; Reyes-Contreras, C.; Neumann, P.; Piña, B.; Vidal, G. Presence and fate of micropollutants during anaerobic digestion of sewage and their implications for the circular economy: A short review. J. Environ. Chem. Eng. 2021, 9, 104931. [Google Scholar] [CrossRef]
- Amodeo, C.; Hattou, S.; Buffiere, P.; Benbelkacem, H. Temperature phased anaerobic digestion (TPAD) of organic fraction of municipal solid waste (OFMSW) and digested sludge (DS): Effect of different hydrolysis conditions. Waste Manag. 2021, 126, 21–29. [Google Scholar] [CrossRef]
- Wu, L.J.; Qin, Y.; Hojo, T.; Li, Y.Y. Upgrading of anaerobic digestion of waste activated sludge by a hyper-thermophilic-mesophilic temperature-phased process with a recycle system. RSC Adv. 2015, 5, 68531–68541. [Google Scholar] [CrossRef]
- Algapani, D.E.; Qiao, W.; di Pumpo, F.; Bianchi, D.; Wandera, S.M.; Adani, F.; Dong, R. Long-term bio-H2 and bio-CH4 production from food waste in a continuous two-stage system: Energy efficiency and conversion pathways. Bioresour. Technol. 2018, 248, 204–213. [Google Scholar] [CrossRef]
- Liao, X.; Li, H.; Zhang, Y.; Liu, C.; Chen, Q. Accelerated high-solids anaerobic digestion of sewage sludge using low-temperature thermal pretreatment. Intl. Biodeterior. Biodegrad. 2016, 106, 141–149. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Q.; Wu, S.; Qi, D.; Li, W.; Zuo, Z.; Dong, R. Batch anaerobic co-digestion of pig manure with dewatered sewage sludge under mesophilic conditions. Appl. Energy 2014, 128, 175–183. [Google Scholar] [CrossRef]
- Borowski, S.; Doma’nski, J.; Weatherley, L. Anaerobic co-digestion of swine and poultry manure with municipal sewage sludge. Waste Manag. 2014, 34, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Grosser, A.; Neczaj, E.; Singh, B.R.; Almås, R.; Brattebø, H.; Kacprzak, M. Anaerobic digestion of sewage sludge with grease trap sludge and municipal solid waste as co-substrates. Environ. Res. 2017, 155, 249–260. [Google Scholar] [CrossRef]
- Montañes, R.; Perez, M.; Solera, R. Anaerobic mesophilic co-digestion of sewage sludge and sugar beet pulp lixiviation in batch reactors: Effect of pH control. Chem. Eng. J. 2014, 255, 492–499. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Ye, H.; Wang, Y.; Luo, W.; Chang, J.S.; Li, Q.; He, N. Anaerobic co-digestion of sewage sludge and food waste for hydrogen and VFA production with microbial community analysis. Waste Manag. 2018, 78, 789–799. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in biogas production: Pretreatment and co-digestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Lee, E.; Oliveira, D.S.B.L.; Oliveira, L.S.B.L.; Jimenez, E.; Kim, Y.; Wang, M.; Ergas, S.J.; Zhang, Q. Comparative environmental and economic life cycle assessment of high solids anaerobic co-digestion for biosolids and organic waste management. Water Res. 2020, 171, 115443. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, W.; Luo, W.; Fang, H.; Lv, H.; Liu, R.; Niu, Q. Anaerobic co-digestion of chicken manure and cardboard waste: Focusing on methane production, microbial community analysis and energy evaluation. Bioresour. Technol. 2021, 321, 124429. [Google Scholar] [CrossRef]
- Valentino, F.; Munarin, G.; Biasiolo, M.; Cavinato, C.; Bolzonella, D.; Pavan, P. Enhancing volatile fatty acids (VFA) production from food waste in a two-phases pilot-scale anaerobic digestion process. J. Environ. Chem. Eng. 2021, 9, 106062. [Google Scholar] [CrossRef]
- Valenti, F.; Zhong, Y.; Sun, M.; Porto, S.M.C.; Toscano, A.; Dale, B.E.; Sibilla, F.; Liao, W. Anaerobic co-digestion of multiple agricultural residues to enhance biogas production in southern Italy. Waste Manag. 2018, 78, 151–157. [Google Scholar] [CrossRef]
- ANICE 2023. Available online: https://www.anice.es/industrias/el-sector/el-sector-carnico-espanol_171_1_ap.html (accessed on 31 October 2024).
- Font-I-Furnols, M.; Guerrero, L. Spanish perspective on meat consumption and consumer attitudes. Meat Sci. 2022, 191, 108874. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, C.; Cavinato, C.; Cecchi, F.; Bolzonella, D. Anaerobic co-digestion of winery waste and waste activated sludge: Assessment of process feasibility. Water Sci. Technol. 2014, 69, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Golbaz, S.; Farzadkia, M.; Vanani, A.; Emamjomeh, M. Livestock slaughterhouses waste man-agement in urban environment. Int. J. Hum. Cap. Urban Manag. 2017, 2, 163–170. [Google Scholar]
- Shende, A.D.; Pophali, G.R. Anaerobic treatment of slaughterhouse wastewater: A review. Environ. Sci. Pollut. Res. Int. 2021, 28, 35–55. [Google Scholar] [CrossRef]
- Agabo-Garcia, C.; Solera, R.; Perez, M. Anaerobic sequential batch reactor for co-digestion of slaughterhouse residues: Wastewater and activated sludge. Energy 2022, 255, 124575. [Google Scholar] [CrossRef]
- Rodriguez-Abalde, A.; Flotats, X.; Fernandez, B. Optimization of the anaerobic co-digestion of pasteurized slaughterhouse waste, pig slurry and glycerine. Waste Manag. 2017, 61, 521–528. [Google Scholar] [CrossRef]
- Guamán-Marquines, C.W.; Mendoza-Loor, R.J.; Gómez-Salcedo, Y.; Baquerizo-Crespo, R.J. Assessment of the start-up of tubular reactors on a laboratory scale for the anaerobic digestion of slaughterhouse wastewater. Int. J. Thermofluids 2023, 19, 100378. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C.; Mehrvar, M. Treatment of actual slaughterhouse wastewater by combined anaerobic–aerobic processes for biogas generation and removal of organics and nutrients: An optimization study towards a cleaner production in the meat processing industry. J. Clean Prod. 2017, 141, 278–289. [Google Scholar] [CrossRef]
- Moukazis, I.; Pellera, F.; Gidarakos, E. Slaughterhouse by-products treatment using anaerobic digestion. Waste Manag. 2018, 71, 652–662. [Google Scholar] [CrossRef]
- Salehiyoun, A.R.; Di Maria, F.; Sharifi, M.; Norouzi, O.; Zilouei, H.; Aghbashlo, M. Anaerobic co-digestion of sewage sludge and slaughterhouse waste in existing wastewater digesters. Renew. Energy 2020, 145, 2503–2509. [Google Scholar]
- Sganzerla, W.G.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Techno-economic assessment of bioenergy and fertilizer production by anaerobic digestion of brewer’s spent grains in a biorefinery concept. J. Clean Prod. 2021, 297, 126600. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Z.; Li, C. Improvement of solid-state anaerobic digestion of yard waste by co-digestion and pH adjustment. Waste Biomass Valori. 2017, 9, 211–221. [Google Scholar] [CrossRef]
- Latif, M.A.; Mehta, C.M.; Batstone, D.J. Influence of low pH on continuous anaerobic digestion of waste activated sludge. Water Res. 2017, 113, 42–49. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, R.; Liu, F.; Yong, X.; Wu, X.; Zheng, T.; Jiang, M.; Jia, H. Biogas production and microbial community shift through neutral pH control during the anaerobic digestion of pig manure. Bioresour. Tecnnol. 2016, 217, 44–49. [Google Scholar] [CrossRef]
- Xing, B.S.; Han, Y.; Cao, S.; Wang, X.C. Effects of long-term acclimatization on the optimum substrate mixture ratio and substrate to inoculum ratio in anaerobic codigestion of food waste and cow manure. Bioresour. Technol. 2020, 317, 123994. [Google Scholar] [CrossRef]
- Aboudi, K.; Greses, S.; González-Fernández, C. Hydraulic Retention Time as an Operational Tool for the Production of Short-Chain Carboxylates via Anaerobic Fermentation of Carbohydrate-Rich Waste. Molecules 2023, 28, 6635. [Google Scholar] [CrossRef]
- Tsegaye, D.; Khan, M.M.; Leta, S. Optimization of Operating Parameters for Two-Phase Anaerobic Digestion Treating Slaughterhouse Wastewater for Biogas Production: Focus on Hydrolytic–Acidogenic Phase. Sustainability 2023, 15, 5544. [Google Scholar] [CrossRef]
- Iglesias-López, M.E.; Fernández, N.; Borja, D.; García-Morales, J.L. Enhancing Anaerobic Digestion with an UASB Reactor of the Winery Wastewater for Producing Volatile Fatty Acid Effluent Enriched in Caproic Acid. Fermentation 2023, 9, 958. [Google Scholar] [CrossRef]
- Zahedi, S.; Solera, R.; Perez, M. An eco-friendly way to valorize winery wastewater and sewage sludge: Anaerobic co-digestion. Biomass Bioenergy 2020, 142, 105779. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.K.; Shin, H.S. Acidification kinetics and microbial community changes in a thermophilic anaerobic acid digester. Water Res. 2004, 38, 3214–3222. [Google Scholar]
- Demirel, B.; Scherer, P. Production of methane from sugar beet silage without manure addition by a two-stage anaerobic digestion system. Biomass Bioenergy 2011, 35, 3418–3424. [Google Scholar]
- Li, Y.; Ni, J.; Cheng, H.; Zhu, A.; Guo, G.; Qin, Y.; Li, Y.Y. Methanogenic performance and microbial community during thermophilic digestion of food waste and sewage sludge in a 415 high-solid anaerobic membrane bioreactor. Bioresour. Technol. 2021, 342, 125938. [Google Scholar] [CrossRef] [PubMed]
- Van Lier, J.B. Limitation of thermophilic anaerobic wastewater treatment and the consequences for process design. Antonie Leeuwenhoek 1996, 69, 1–14. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, Y.; Speece, R.E. Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res. 2002, 36, 4369–4385. [Google Scholar] [CrossRef]
- Maibaum, C.; Kuehn, V. Thermophilic and mesophilic operation of an anaerobic treat-ment of chicken slurry together with organic residual substances. Water Sci. Technol. 1999, 40, 231–236. [Google Scholar] [CrossRef]
- Speece, R.E.; Boonyakitsombut, S.; Kim, M.; Azbar, N.; Ursillo, P. Overview of anaerobic treatment: Thermophilic and propionate implications. Water Environ. Res. 2006, 78, 460–473. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Liu, Y.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef]
- Cirne, D.G.; Paloumet, X.; Capellazzi, S.B.; Praderas, A.G.; Ferrer, J. Effect of temperature on anaerobic digestion of pig slurry. Water Res. 2007, 41, 4501–4509. [Google Scholar]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Colprim, J.; Pérez, E.I. A critical review on anaerobic digestion of organic solid wastes. Bioresour. Technol. 2014, 160, 115–127. [Google Scholar]
- Ward, A.J.; Lewis, A.; Greenman, J. Optimisation of electrical power generation during anaerobic digestion of dairy farm slurry. Bioresour. Technol. 2008, 99, 7928–7934. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2009, 35, 403–418. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. Biogas production from anaerobic co-digestion of dairy manure with food waste: A review. Waste Manag. 2013, 33, 2329–2346. [Google Scholar]
- Kim, J.; Park, S.C.; Lee, C. Effects of temperature and hydraulic retention time on thermophilic two-phase anaerobic digestion. J. Biosci. Bioeng. 2006, 102, 328–333. [Google Scholar] [CrossRef]
- Tian, H.; Gui, L.; Zhu, M.; Zhang, Y. Temperature-phased anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2018, 82, 1252–1263. [Google Scholar]
- Luo, G.; Angelidaki, I.; Mathiesen, B.V. Optimal design and operation of integrated biogas plants for combined heat and power production. Appl. Energy 2010, 87, 1395–1402. [Google Scholar]
- Methods, S. Standard methods for the examination of water and wastewater. Water Res. 2012, 16, 1495–1496. [Google Scholar]


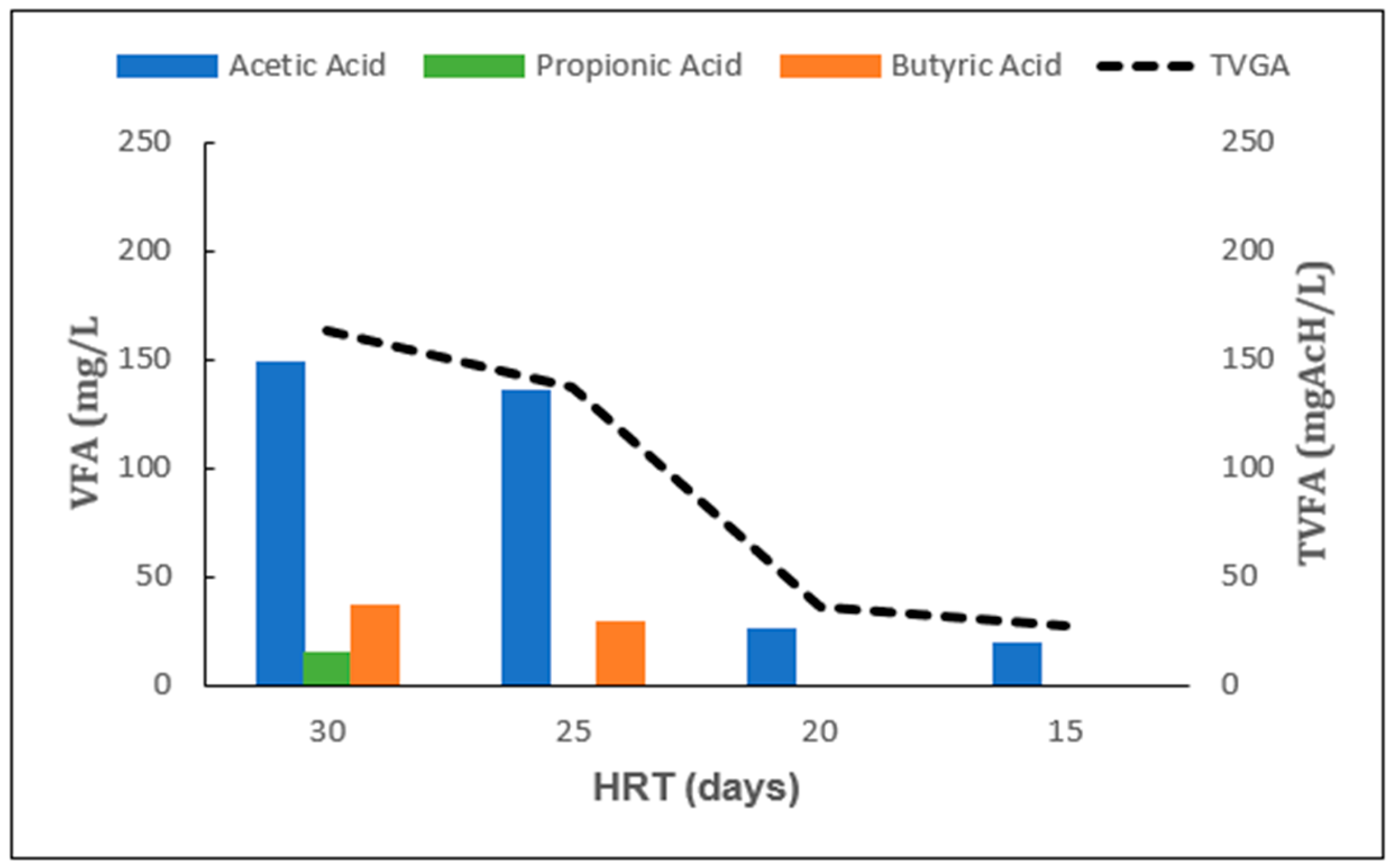
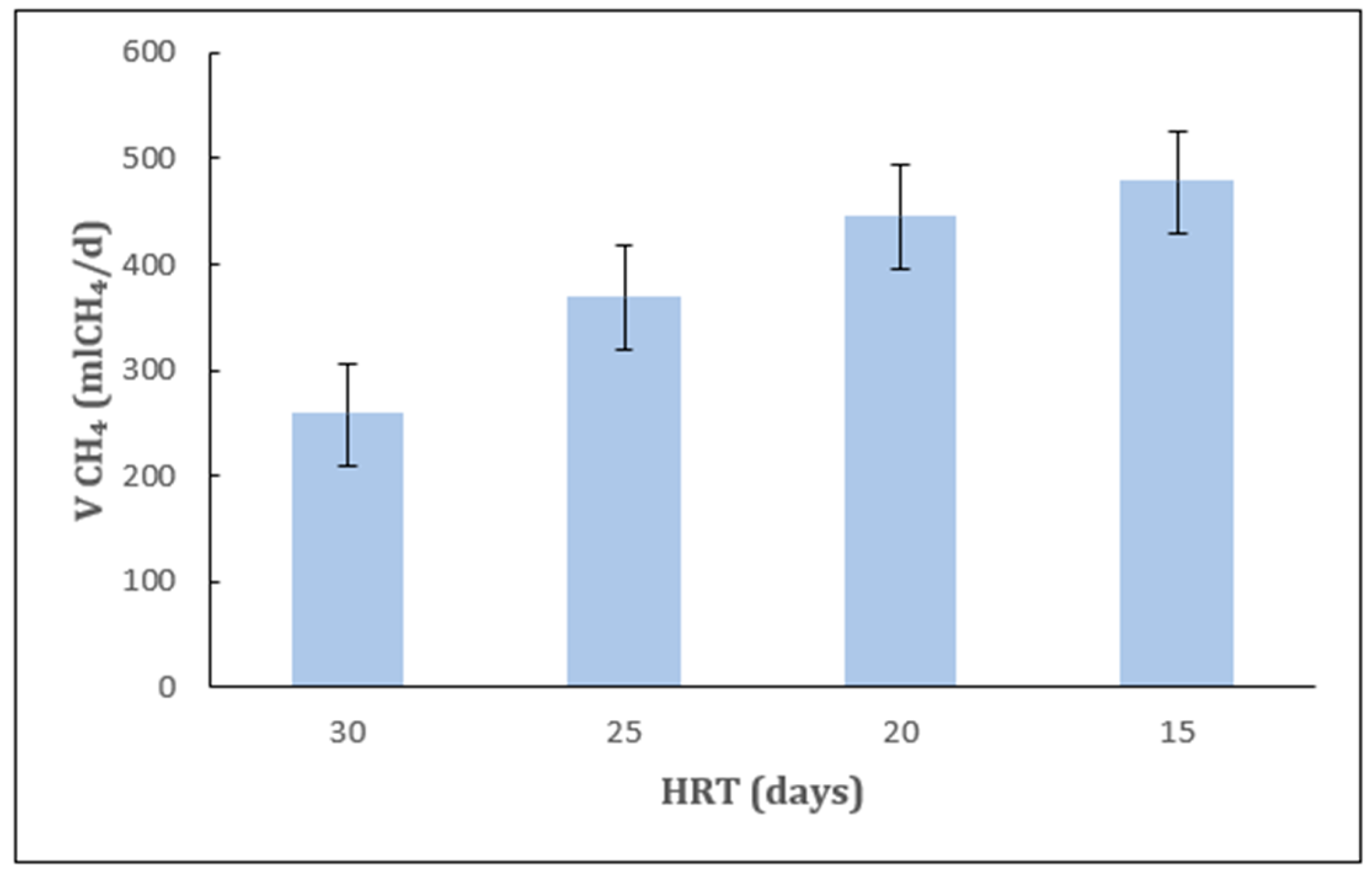
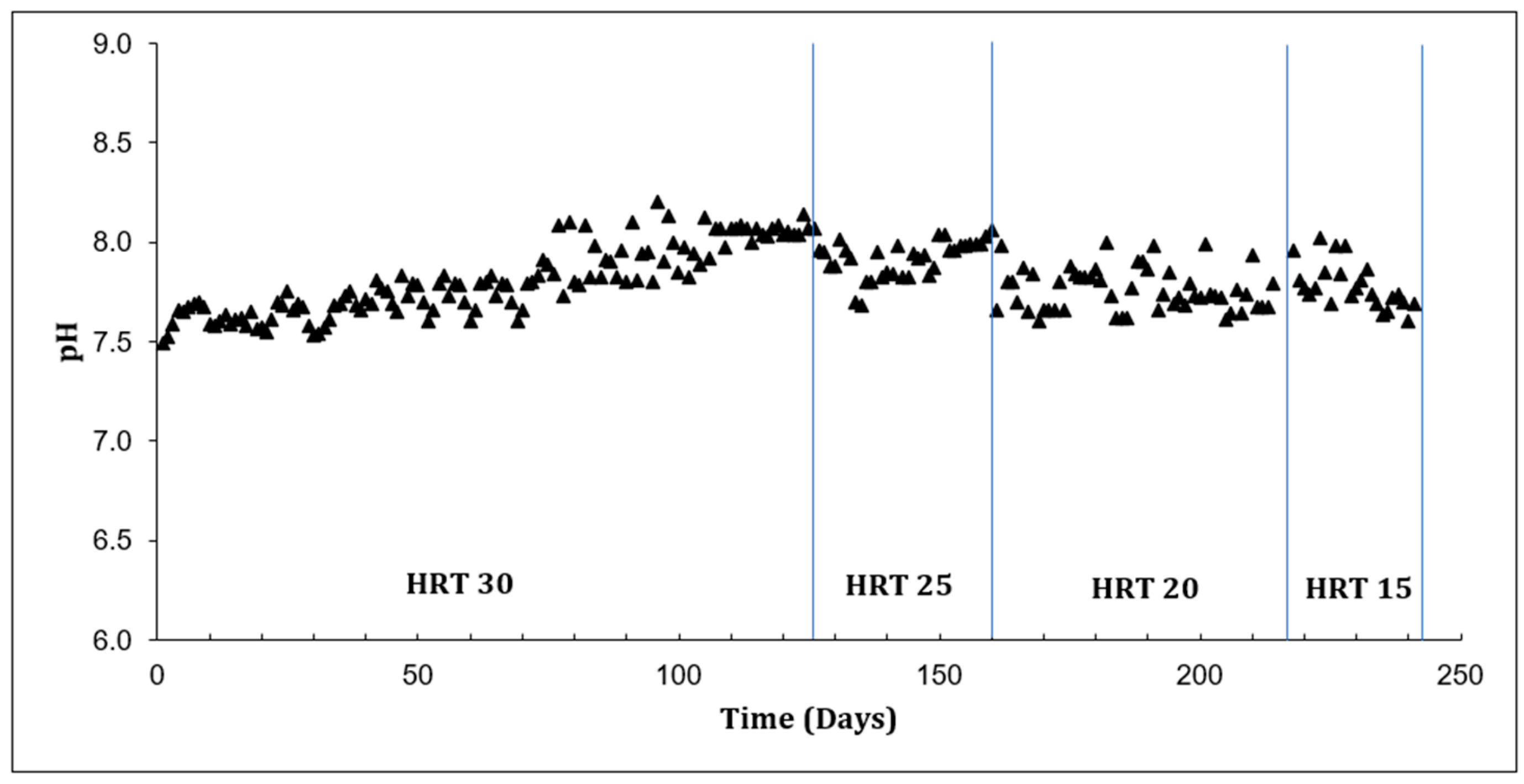
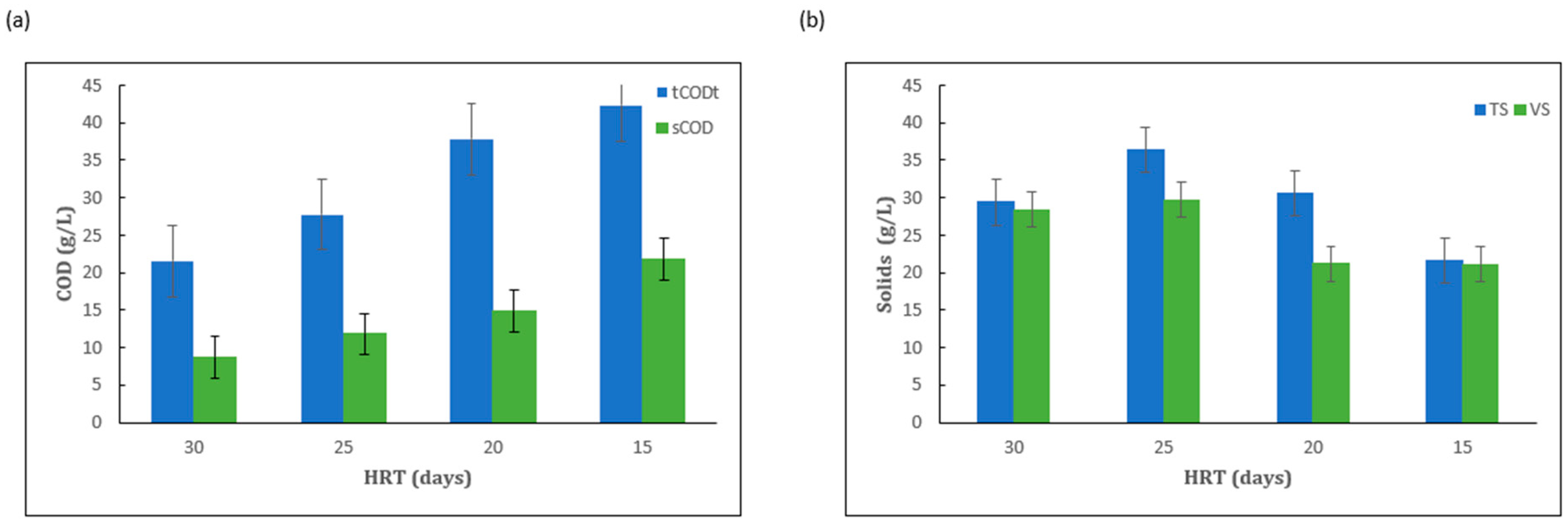
| HRT (d) | tCOD (%) | TS (%) | VS (%) |
|---|---|---|---|
| 30 | 32.72 ± 4.43 | 18.03 ± 3.40 | 31.89 ± 4.42 |
| 25 | 37.23 ± 1.72 | 33.84 ± 2.96 | 32.30 ± 1.89 |
| 20 | 39.21 ± 2.27 | 43.75 ± 3.29 | 37.00 ± 1.50 |
| 15 | 56.28 ± 2.32 | 52.24 ± 1.33 | 41.67 ± 1.67 |
| HRT (d) | Biomethane Yields (mL CH4/gVSadded) |
|---|---|
| 30 | 8.27 ± 0.04 |
| 25 | 8.46 ± 0.04 |
| 20 | 8.78 ± 0.4 |
| 15 | 9.54 ± 0.4 |
| HRT (d) | tCOD (%) | TS (%) | VS (%) |
|---|---|---|---|
| 30 | 38.33 ± 1.88 | 46.32 ± 3.35 | 41.45 ± 4.10 |
| 25 | 44.03 ± 2.48 | 47.36 ± 2.47 | 46.73 ± 2.74 |
| 20 | 48.68 ± 3.14 | 55.26 ± 3.31 | 52.89 ± 3.55 |
| 15 | 43.04 ± 2.14 | 67.73 ± 5.65 | 65.84 ± 3.75 |
| HRT (d) | Biomethane Yields (mL CH4/gVSadded) |
|---|---|
| 30 | 11.61 ± 0.04 |
| 25 | 13.24 ± 0.03 |
| 20 | 16.50 ± 0.04 |
| 15 | 23.02 ± 0.06 |
| Parameters | Slaughterhouse Sludge (S) | Slaughterhouse Wastewater (SW) | Feed Mixture (S-SW) | Inoculum |
|---|---|---|---|---|
| pH | 7.21 ± 0.36 | 7.23 ± 0.36 | 7.60 ± 0.38 | 8.33 ± 0.42 |
| tCOD (g/L) | 106.16 ± 0.02 | 4.77 ± 0.04 | 55.59 ± 0.03 | 19.08 ± 0.01 |
| sCOD (g/L) | 12.57 ± 0.02 | 2.93 ± 0.06 | 7.21 ± 0.06 | 3.04 ± 0.02 |
| TS (g/L) | 120.69 ± 7.62 | 1.98 ± 0.11 | 56.52 ± 2.98 | 8.06 ± 0.18 |
| VS (g/L) | 94.64 ± 5.84 | 1.97 ± 0.38 | 44.51 ± 2.57 | 5.56 ± 0.24 |
| TVFA (mgAcH/L) | 8.59 ± 1.23 | 159.02 ± 22.72 | 86.52 ± 12.36 | 89.12 ± 12.73 |
| C/N | 48.79 ± 0.05 | 30.43 ± 0.07 | 5.06 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candel, M.; Ballesteros, L.; Fernandez-Rodriguez, J.; Perez, M.; Solera, R. Study of the Effect of Temperature to Optimize the Anaerobic Digestion of Slaughterhouse Sludge by Co-Digestion with Slaughterhouse Wastewater. Recycling 2025, 10, 47. https://doi.org/10.3390/recycling10020047
Candel M, Ballesteros L, Fernandez-Rodriguez J, Perez M, Solera R. Study of the Effect of Temperature to Optimize the Anaerobic Digestion of Slaughterhouse Sludge by Co-Digestion with Slaughterhouse Wastewater. Recycling. 2025; 10(2):47. https://doi.org/10.3390/recycling10020047
Chicago/Turabian StyleCandel, Maria, Laura Ballesteros, Juana Fernandez-Rodriguez, Montserrat Perez, and Rosario Solera. 2025. "Study of the Effect of Temperature to Optimize the Anaerobic Digestion of Slaughterhouse Sludge by Co-Digestion with Slaughterhouse Wastewater" Recycling 10, no. 2: 47. https://doi.org/10.3390/recycling10020047
APA StyleCandel, M., Ballesteros, L., Fernandez-Rodriguez, J., Perez, M., & Solera, R. (2025). Study of the Effect of Temperature to Optimize the Anaerobic Digestion of Slaughterhouse Sludge by Co-Digestion with Slaughterhouse Wastewater. Recycling, 10(2), 47. https://doi.org/10.3390/recycling10020047






