Multiscale Modelling Methodologies of Lithium-Ion Battery Aging: A Review of Most Recent Developments
Abstract
:1. Introduction
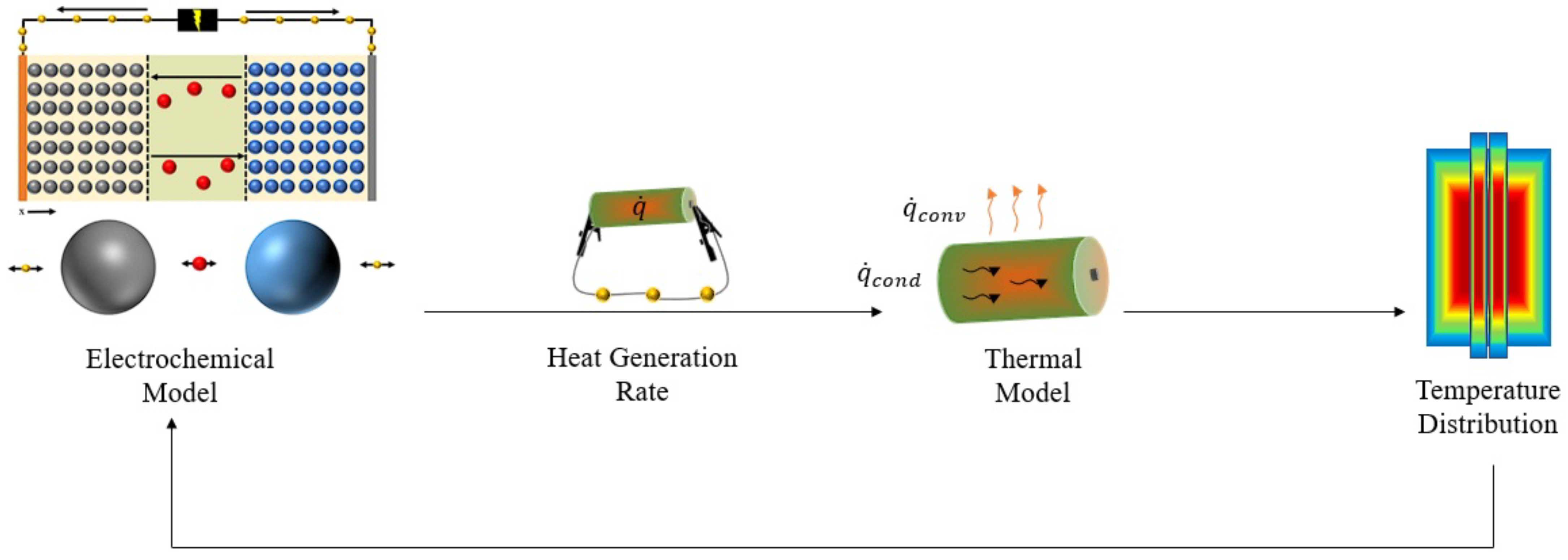
2. Heat Generation in Battery Systems
2.1. Electrode Particle Heat Generation Phenomenon
2.2. Battery Cell Heat Generation Phenomenon
2.3. Battery Pack Heat Generation Phenomenon
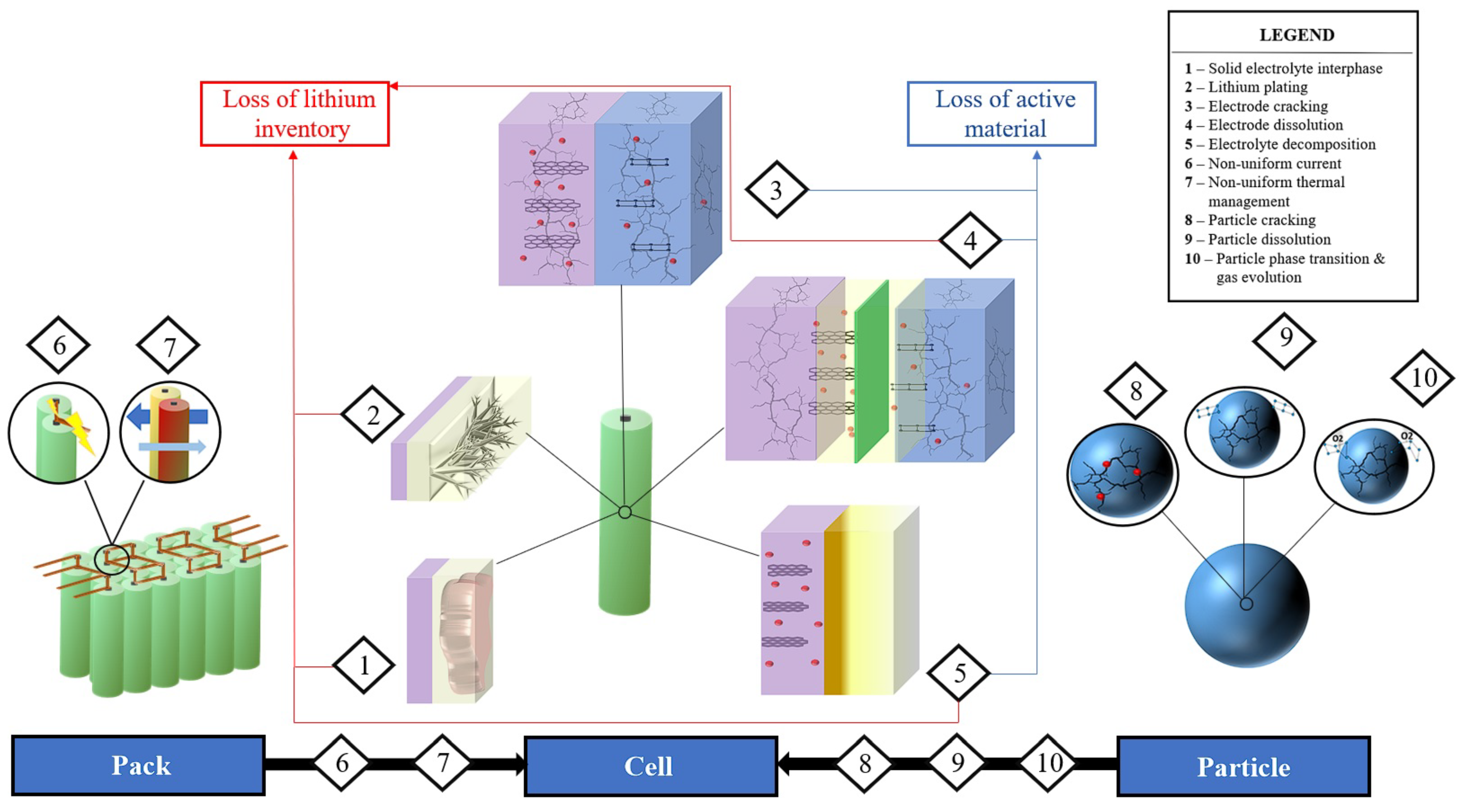
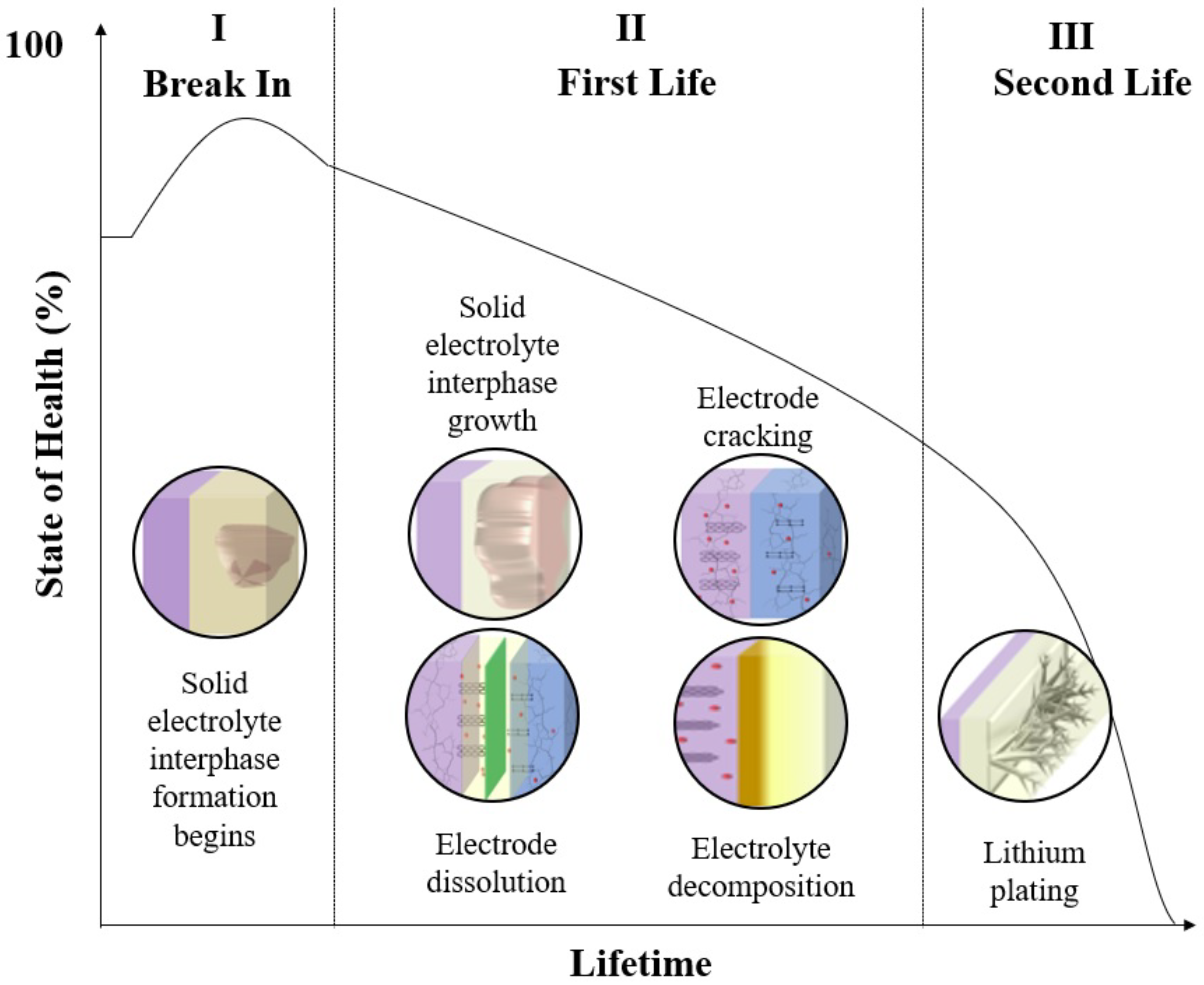
3. Aging Mechanisms
3.1. Electrode Particle Aging Contributing Factors
3.2. Battery Cell Aging Mechanisms
3.2.1. Production-Related Aging Mechanisms
3.2.2. Calendar Aging Mechanisms
3.2.3. Cycle Aging Mechanisms
3.3. Battery Pack Aging Contributing Factors
3.3.1. Cell Spreading
3.3.2. External Stress Factors
3.3.3. User Patterns Impact
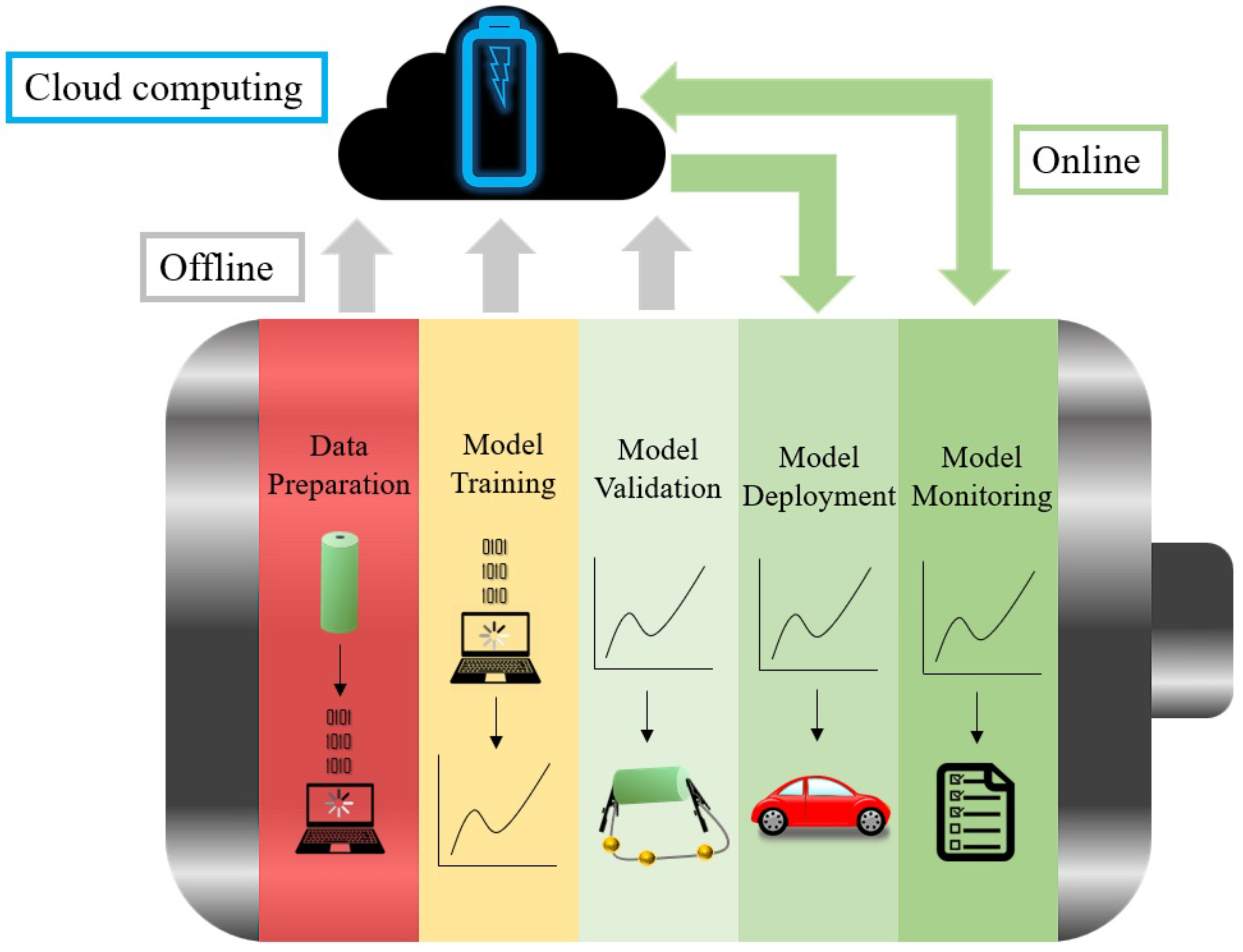
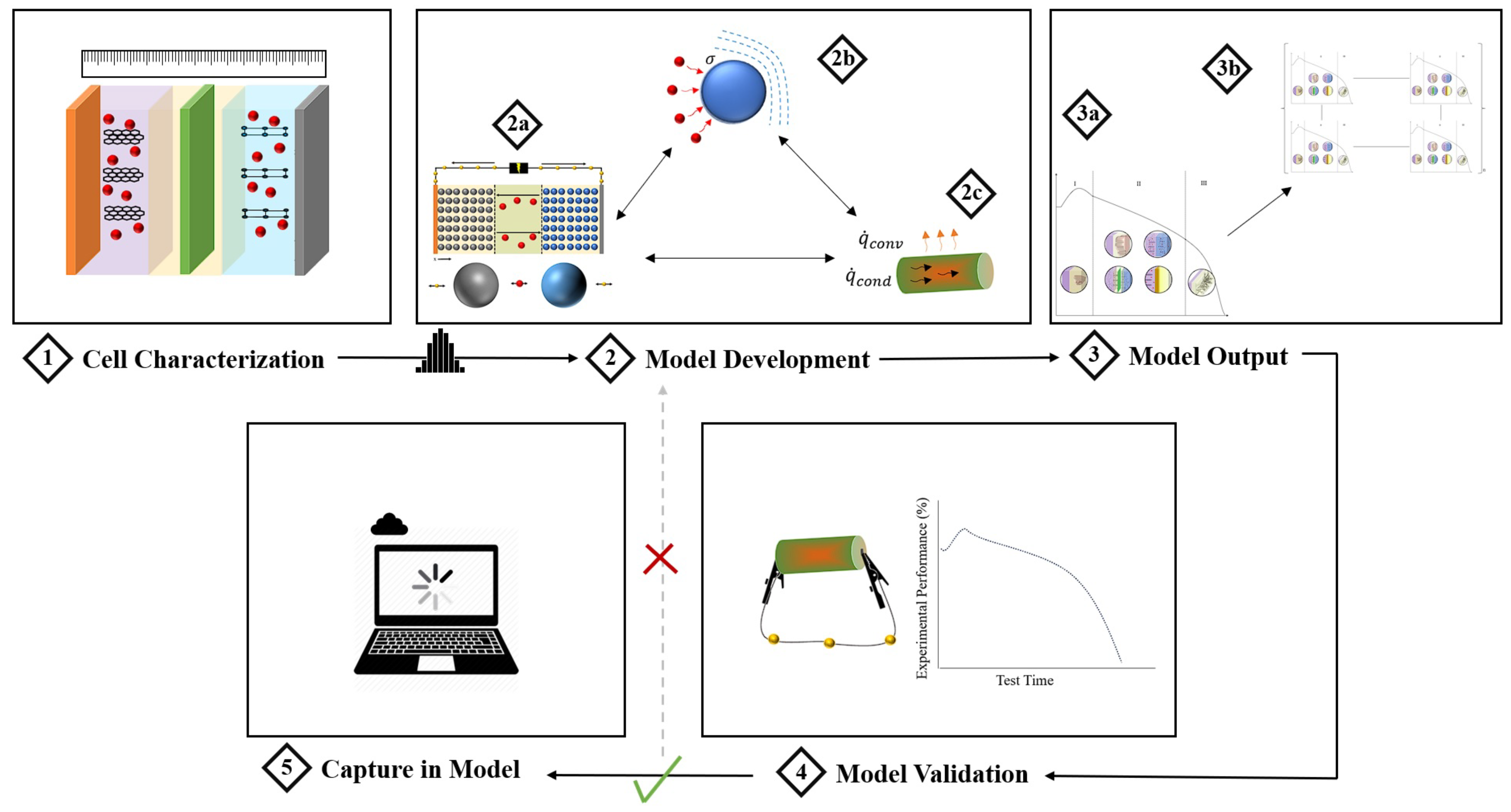
4. Aging Modelling Strategies
- Model the cycle and calendar aging phenomena together;
- Reference electro-chemo-mechanical-related aging phenomena;
- Be scalable to multiple hierarchical scales;
- Validated experimentally under realistic operating conditions;
- Implementable online in a battery management system (BMS).
4.1. Data-Based Models
4.1.1. Empirical Models
- : capacity degradation at non-ambient conditions;
- : capacity degradation at ambient temperature T;
- : average SOC, deviation from average SOC;
- : charge;
- : empirically derived coefficients.
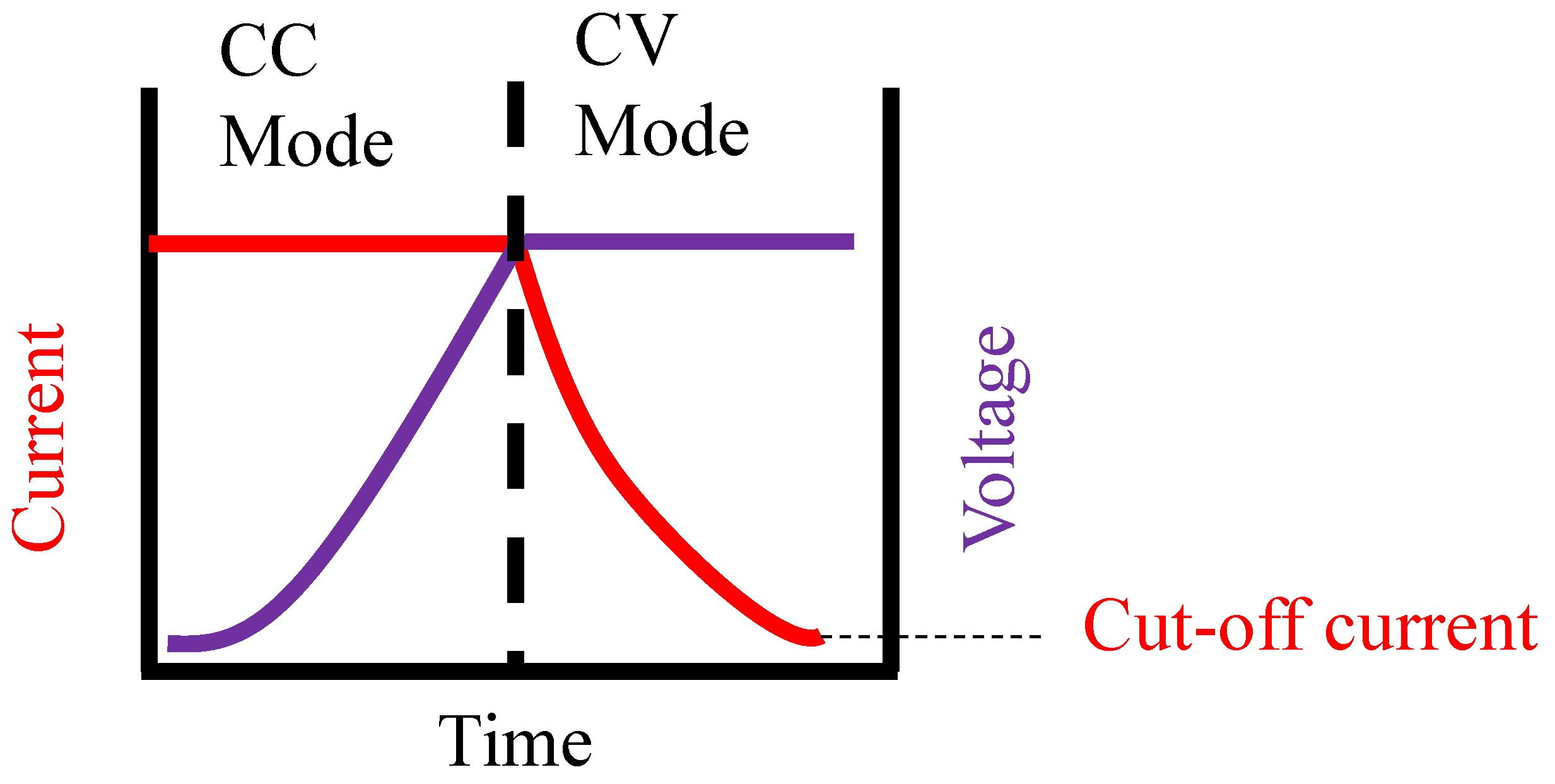
4.1.2. Equivalent Circuit Models
4.1.3. Machine Learning Models
4.1.4. Deep Learning Models
4.1.5. Statistical Models
4.2. Multiphysics-Based Modelling
4.2.1. Pseudo-Two-Dimensional Model
4.2.2. Single-Particle Model and Other Simplified Electrochemical Models
- Discrete-time realization algorithm (DRA) that converts functions to discrete-time unit pulse responses, and then uses either the Ho–Kalman or the eigensystem realization algorithm to generate an ROM;
- Continuous time realization algorithm (CRA) that converts the function to a continuous time state space model and then to a discrete-time state space model;
- Hybrid realization algorithm (HRA) that converts functions to discrete-time frequency response and then reduces the model order;
- Lagrange interpolation realization algorithm (LRA) that converts functions to a continuous state space model which approximates function values followed by a reduction in order and conversion to a discrete state space model.
4.2.3. Thermal Modelling
4.3. Combined Modelling Techniques
5. Battery Testing Strategies
5.1. Test Types
- Formation or characterization tests to diagnose cell initial prognostics and variations;
- Duty cycle test to test the battery under the desired condition;
- Reference performance test (RPT) to characterize parameter evolution for the battery.
5.1.1. Characterization Tests
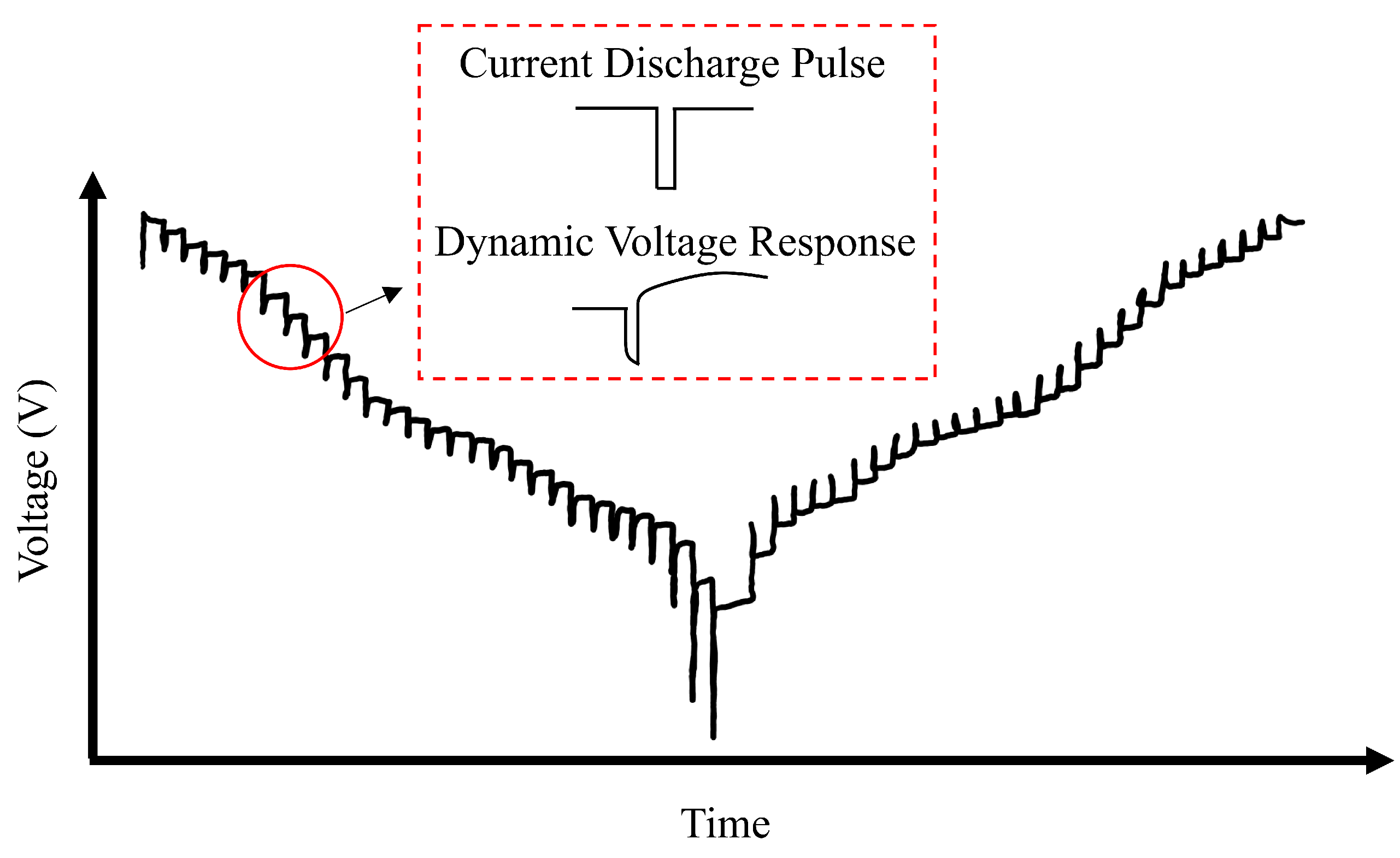
5.1.2. Aging Emulation Testing
5.2. Aging Phenomena Diagnosis Techniques
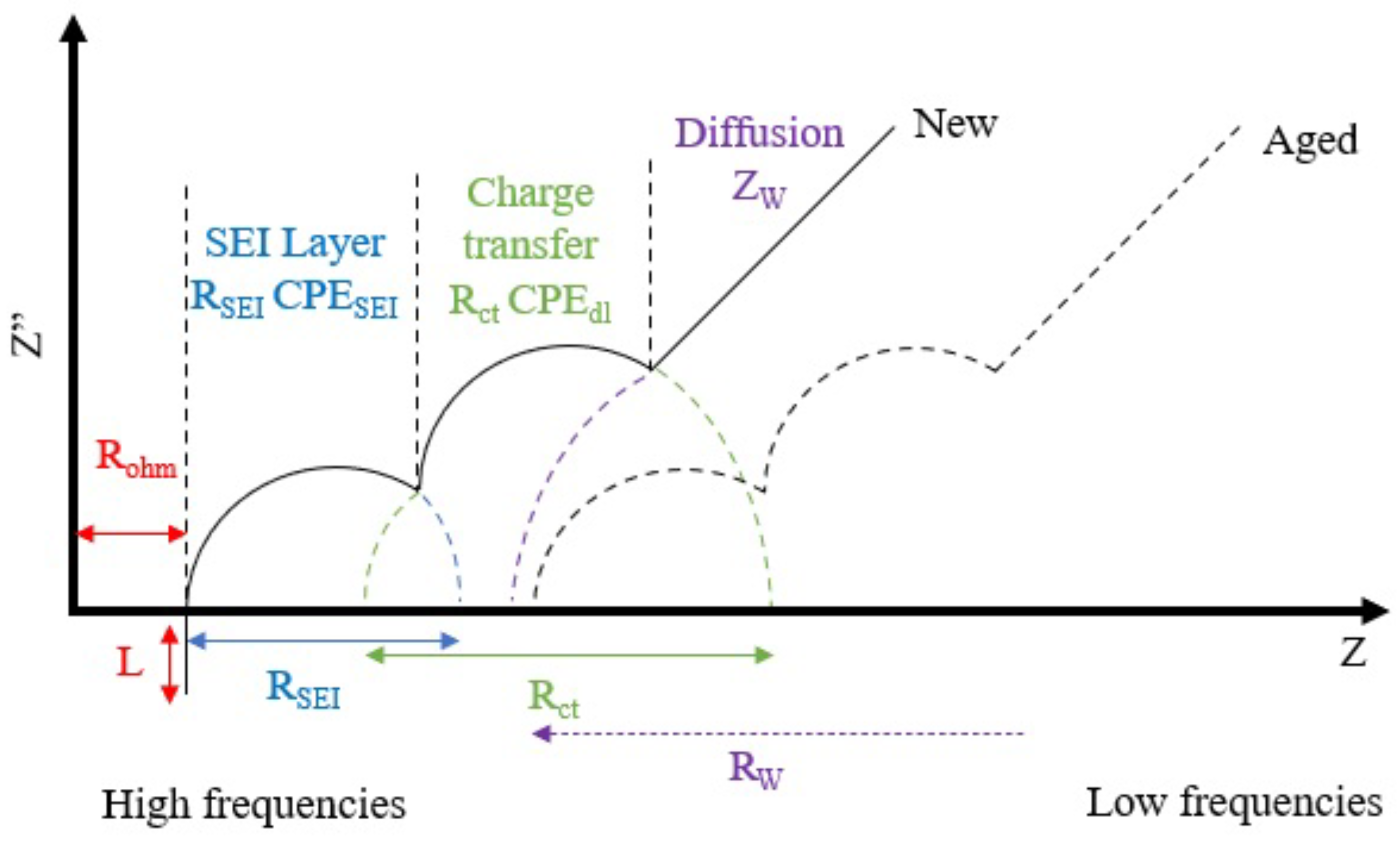
5.2.1. Electrochemical Impedance Spectroscopy
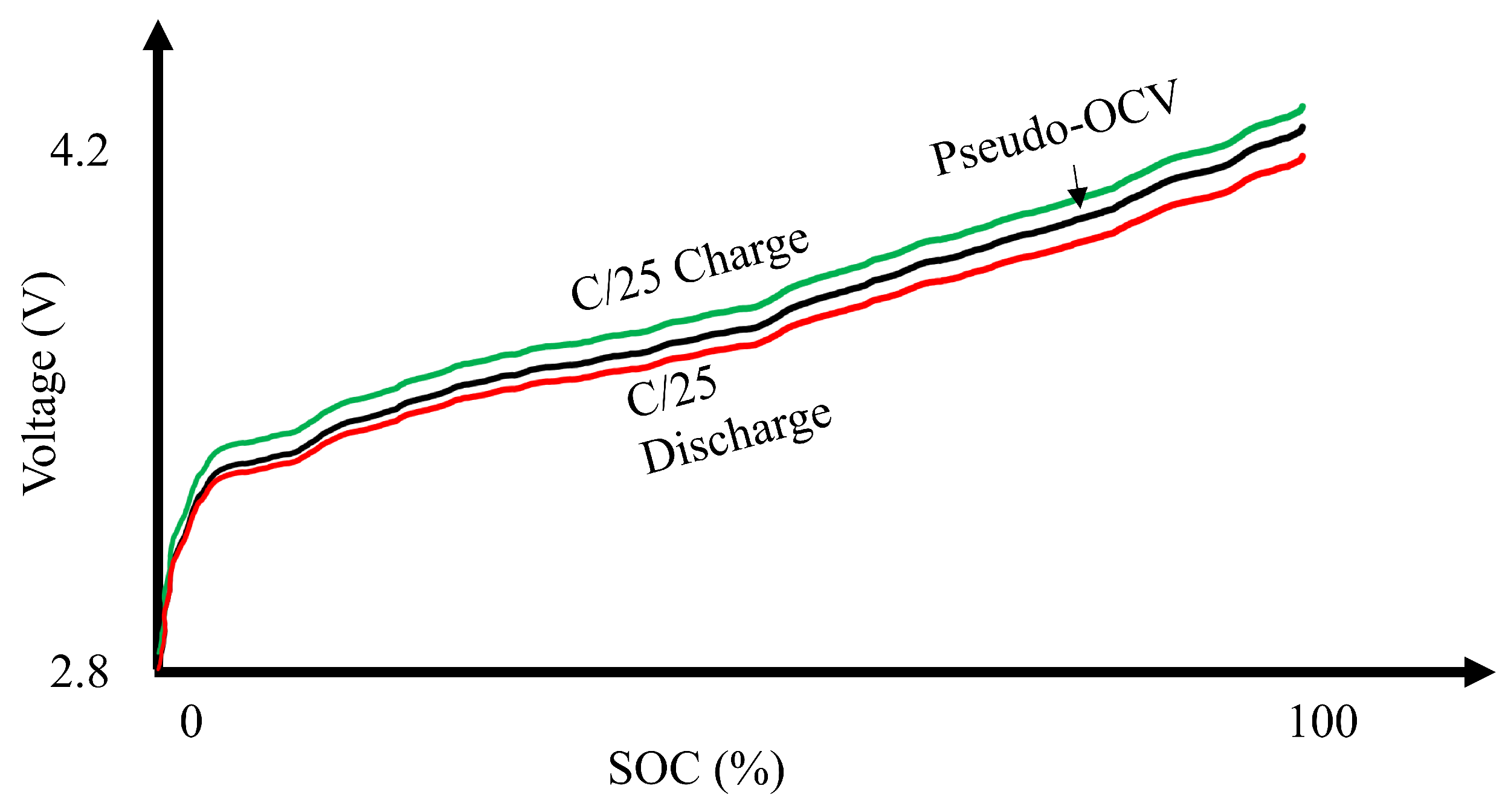
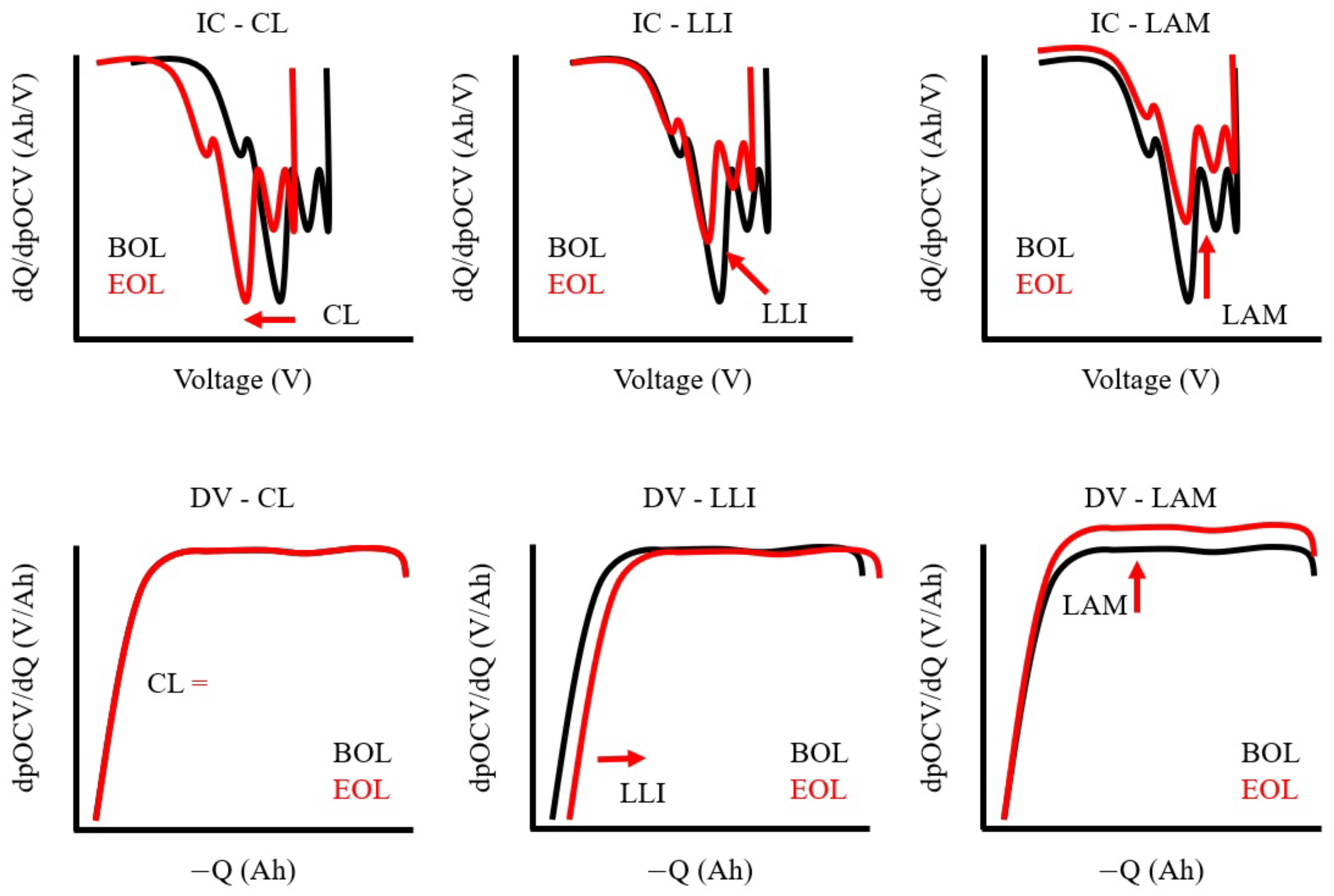
5.2.2. Incremental Capacity Differential Voltage Analysis
6. Overview and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANN | Artificial neural network |
| BMS | Battery Management System |
| BOL | Beginning of life |
| BTMS | Battery thermal management system |
| CRA | Continuous realization algorithm |
| CEI | Cathodic electrolyte interphase |
| CC-CV | Constant current - constant voltage |
| CNN | Convolutional neural network |
| DOD | Depth of discharge |
| DRA | Discrete realization algorithm |
| DST | Dynamic stress tests |
| ECM | Equivalent circuit model |
| EIS | Electrochemical impedance spectroscopy |
| EMD | Empirical mode decomposition |
| EOL | End of life |
| EV | Electric vehicles |
| FFLSRA | Forgetting factor recursive least squares algorithm |
| FUDS | Federal urban driving schedules |
| GM | General Motors |
| HRA | Hybrid realization algorithm |
| IC-DV | Incremental capacity/differential voltage |
| ISPM+ | Improved SPM |
| KRR | Kernel ridge regression |
| LAM | Loss of active material |
| LRA | Lagrange interpolation realization algorithm |
| LCO | Lithium Cobalt Oxide |
| LIB | Lithium-ion battery |
| LLI | Loss of lithium inventory |
| LFP | Lithium Iron Phosphate |
| LMO | Lithium Manganese Oxide |
| LSTM | Long short term memory |
| LTO | Lithium Titante Oxide |
| NCA | Nickel Cobalt Aluminum Oxide |
| NEDC | New european driving cycle |
| NMC | Nickel Manganese Cobalt Oxide |
| OCV | Open circuit voltage |
| OEM | Original equipment manufacturer |
| P2D | Pseudo 2D model |
| PCM | Phase change material |
| PDE | Partial differential equation |
| PHEV | Plug in hybrid electric vehicle |
| pOCV | Pseudo OCV |
| RDE | Remaining discharge energy |
| RMSE | Root mean squared error |
| RNN | Recurrent neural network |
| ROM | Reduced order model |
| RPT | Reference performance test |
| SEI | Solid electrolyte interphase |
| SPM | Single particle model |
| SOC | State of charge |
| SOH | State of health |
| SVM | Support vector machine |
| TEMA | Transport technology and mobility |
| V2G | Vehicle to grid |
| WLTP | World harmonized light duty vehicle test procedures |
References
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Riley, C. The great electric car race is just beginning. CNN Business. Available online: https://www.cnn.com/interactive/2019/08/business/electric-cars-audi-volkswagen-tesla/ (accessed on 15 December 2021).
- Zhao, Y.; Patel, Y.; Zhang, T.; Offer, G.J. Modeling the Effects of Thermal Gradients Induced by Tab and Surface Cooling on Lithium Ion Cell Performance. J. Electrochem. Soc. 2018, 165, A3169–A3178. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Keil, P.; Schuster, S.F.; Wilhelm, J.; Travi, J.; Hauser, A.; Karl, R.C.; Jossen, A. Calendar Aging of Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1872–A1880. [Google Scholar] [CrossRef]
- Galatro, D.; Romero, D.A.; Freitez, J.A.; Da Silva, C.; Trescases, O.; Amon, C.H. Modeling degradation of lithium-ion batteries considering cell-to-cell variations. J. Energy Storage 2021, 44, 103478. [Google Scholar] [CrossRef]
- Wu, B.; Widanage, W.D.; Yang, S.; Liu, X. Battery digital twins: Perspectives on the fusion of models, data and artificial intelligence for smart battery management systems. Energy AI 2020, 1, 100016. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Zhang, X.; Soc, J.E.; Zhang, X.; Sastry, A.M.; Shyy, W. Intercalation-Induced Stress and Heat Generation within Single Lithium-Ion Battery Cathode Particles Intercalation-Induced Stress and Heat Generation within Single Lithium-Ion Battery Cathode Particles. J. Electrochem. Soc. 2008, 155, A542–A552. [Google Scholar] [CrossRef]
- Galatro, D.; Al-Zareer, M.; Da Silva, C.; Romero, D.A.; Amon, C.H. Thermal behavior of Lithium-ion batteries: Aging, heat generation, thermal management and failure. Front. Heat Mass Transf. 2020, 14, 1–18. [Google Scholar] [CrossRef]
- Feng, F.; Hu, X.; Hu, L.; Hu, F.; Li, Y.; Zhang, L. Propagation mechanisms and diagnosis of parameter inconsistency within Li-Ion battery packs. Renew. Sustain. Energy Rev. 2019, 112, 102–113. [Google Scholar] [CrossRef]
- Kang, D.; Lee, P.Y.; Yoo, K.; Kim, J. Internal thermal network model-based inner temperature distribution of high-power lithium-ion battery packs with different shapes for thermal management. J. Energy Storage 2020, 27, 101017. [Google Scholar] [CrossRef]
- Atalay, S.; Sheikh, M.; Mariani, A.; Merla, Y.; Bower, E.; Widanage, W.D. Theory of battery ageing in a lithium-ion battery: Capacity fade, nonlinear ageing and lifetime prediction. J. Power Sources 2020, 478, 229026. [Google Scholar] [CrossRef]
- Qi, Y.; Harris, S.J. In Situ Observation of Strains during Lithiation of a Graphite Electrode. J. Electrochem. Soc. 2010, 157, A741–A747. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, E.; Zhang, K.; Wang, X.; Borzenets, V.; Sun, Z.; Pianetta, P.; Yu, X.; Liu, Y.; Yang, X.Q.; et al. In situ Visualization of State-of-Charge Heterogeneity within a LiCoO2 Particle that Evolves upon Cycling at Different Rates. Acs Energy Lett. 2017, 2, 1240–1245. [Google Scholar] [CrossRef]
- Xu, R.; Sun, H.; de Vasconcelos, L.S.; Zhao, K. Mechanical and Structural Degradation of LiNixMnyCozO2 Cathode in Li-Ion Batteries: An Experimental Study. J. Electrochem. Soc. 2017, 164, A3333–A3341. [Google Scholar] [CrossRef]
- Trevisanello, E.; Ruess, R.; Conforto, G.; Richter, F.H.; Janek, J. Polycrystalline and Single Crystalline NCM Cathode Materials—Quantifying Particle Cracking, Active Surface Area, and Lithium Diffusion. Adv. Energy Mater. 2021, 11, 2003400. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, J.; Lu, W. A Comprehensive Experimental and Modeling Study on Dissolution in Li-Ion Batteries. J. Electrochem. Soc. 2019, 166, A1340–A1354. [Google Scholar] [CrossRef]
- Zhuo, M.; Offer, G.; Marinescu, M. Degradation model of high-nickel positive electrodes: Effects of loss of active material and cyclable lithium on capacity fade. J. Power Sources 2023, 556, 232461. [Google Scholar] [CrossRef]
- Kim, D.H.; Song, J.H.; Jung, C.H.; Eum, D.; Kim, B.; Hong, S.H.; Kang, K. Stepwise Dopant Selection Process for High-Nickel Layered Oxide Cathodes. Adv. Energy Mater. 2022, 12, 2200136. [Google Scholar] [CrossRef]
- Bank, T.; Feldmann, J.; Klamor, S.; Bihn, S.; Sauer, D.U. Extensive aging analysis of high-power lithium titanate oxide batteries: Impact of the passive electrode effect. J. Power Sources 2020, 473, 228566. [Google Scholar] [CrossRef]
- Logan, E.R.; Hebecker, H.; Eldesoky, A.; Luscombe, A.; Johnson, M.B.; Dahn, J.R. Performance and Degradation of LiFePO4/Graphite Cells: The Impact of Water Contamination and an Evaluation of Common Electrolyte Additives. J. Electrochem. Soc. 2020, 167, 130543. [Google Scholar] [CrossRef]
- Werner, D.; Paarmann, S.; Wetzel, T. Calendar aging of li-ion cells—Experimental investigation and empirical correlation. Batteries 2021, 7, 28. [Google Scholar] [CrossRef]
- Schmitt, J.; Maheshwari, A.; Heck, M.; Lux, S.; Vetter, M. Impedance change and capacity fade of lithium nickel manganese cobalt oxide-based batteries during calendar aging. J. Power Sources 2017, 353, 183–194. [Google Scholar] [CrossRef]
- Sieg, J.; Schmid, A.U.; Rau, L.; Gesterkamp, A.; Storch, M.; Spier, B.; Birke, K.P.; Sauer, D.U. Fast-charging capability of lithium-ion cells: Influence of electrode aging and electrolyte consumption. Appl. Energy 2022, 305, 117747. [Google Scholar] [CrossRef]
- Rechkemmer, S.K.; Zang, X.; Zhang, W.; Sawodny, O. Calendar and cycle aging study of a commercial LiMn2O4 cell under consideration of influences by cell progress. J. Energy Storage 2020, 30, 101547. [Google Scholar] [CrossRef]
- Chahbaz, A.; Meishner, F.; Li, W.; Ünlübayir, C.; Uwe Sauer, D. Non-invasive identification of calendar and cyclic ageing mechanisms for lithium-titanate-oxide batteries. Energy Storage Mater. 2021, 42, 794–805. [Google Scholar] [CrossRef]
- Zhao, Y.; Stein, P.; Bai, Y.; Al-Siraj, M.; Yang, Y.; Xu, B.X. A review on modeling of electro-chemo-mechanics in lithium-ion batteries. J. Power Sources 2019, 413, 259–283. [Google Scholar] [CrossRef]
- Yang, X.G.; Leng, Y.; Zhang, G.; Ge, S.; Wang, C.Y. Modeling of lithium plating induced aging of lithium-ion batteries: Transition from linear to nonlinear aging. J. Power Sources 2017, 360, 28–40. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Q.; Ma, M.; Zhao, C.; Sun, J.; Wang, Q. Aging mechanisms and thermal stability of aged commercial 18650 lithium ion battery induced by slight overcharging cycling. J. Power Sources 2020, 445, 227263. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Venet, P.; Dube, Y.; Sari, A. Lithium-Ion Battery Aging Experiments at Subzero Temperatures and Model Development for Capacity Fade Estimation. IEEE Trans. Veh. Technol. 2016, 65, 4328–4343. [Google Scholar] [CrossRef]
- Gao, T.; Bai, J.; Ouyang, D.; Wang, Z.; Bai, W.; Mao, N.; Zhu, Y. Effect of aging temperature on thermal stability of lithium-ion batteries: Part A – High-temperature aging. Renew. Energy 2023, 203, 592–600. [Google Scholar] [CrossRef]
- Spotte-Smith, E.W.C.; Petrocelli, T.B.; Patel, H.D.; Blau, S.M.; Persson, K.A. Elementary Decomposition Mechanisms of Lithium Hexafluorophosphate in Battery Electrolytes and Interphases. Acs Energy Lett. 2023, 8, 347–355. [Google Scholar] [CrossRef]
- Zhu, J.; Dewi Darma, M.S.; Knapp, M.; Sørensen, D.R.; Heere, M.; Fang, Q.; Wang, X.; Dai, H.; Mereacre, L.; Senyshyn, A.; et al. Investigation of lithium-ion battery degradation mechanisms by combining differential voltage analysis and alternating current impedance. J. Power Sources 2020, 448, 28–30. [Google Scholar] [CrossRef]
- Leonardi, S.G.; Aloisio, D.; Brunaccini, G.; Stassi, A.; Ferraro, M.; Antonucci, V.; Sergi, F. Investigation on the ageing mechanism for a lithium-ion cell under accelerated tests: The case of primary frequency regulation service. J. Energy Storage 2021, 41, 102904. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity fading of ni-rich li[NixCoyMn1-x-y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Preger, Y.; Barkholtz, H.M.; Fresquez, A.; Campbell, D.L.; Juba, B.W.; Romàn-Kustas, J.; Ferreira, S.R.; Chalamala, B. Degradation of Commercial Lithium-Ion Cells as a Function of Chemistry and Cycling Conditions. J. Electrochem. Soc. 2020, 167, 120532. [Google Scholar] [CrossRef]
- Martinez-Laserna, E.; Sarasketa-Zabala, E.; Villarreal Sarria, I.; Stroe, D.I.; Swierczynski, M.; Warnecke, A.; Timmermans, J.M.; Goutam, S.; Omar, N.; Rodriguez, P. Technical Viability of Battery Second Life: A Study from the Ageing Perspective. IEEE Trans. Ind. Appl. 2018, 54, 2703–2713. [Google Scholar] [CrossRef]
- Braco, E.; San Martín, I.; Berrueta, A.; Sanchis, P.; Ursúa, A. Experimental assessment of cycling ageing of lithium-ion second-life batteries from electric vehicles. J. Energy Storage 2020, 32, 101695. [Google Scholar] [CrossRef]
- Casals, L.C.; García, B.A.; Aguesse, F.; Iturrondobeitia, A. Second life of electric vehicle batteries: Relation between materials degradation and environmental impact. Int. J. Life Cycle Assess. 2017, 22, 82–93. [Google Scholar] [CrossRef]
- Zheng, Y.; Ouyang, M.; Lu, L.; Li, J. Understanding aging mechanisms in lithium-ion battery packs: From cell capacity loss to pack capacity evolution. J. Power Sources 2015, 278, 287–295. [Google Scholar] [CrossRef]
- Zilberman, I.; Schmitt, J.; Ludwig, S.; Naumann, M.; Jossen, A. Simulation of voltage imbalance in large lithium-ion battery packs influenced by cell-to-cell variations and balancing systems. J. Energy Storage 2020, 32, 101828. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Wang, L.; Wang, Z.; Guo, H. Dependency analysis and degradation process-dependent modeling of lithium-ion battery packs. J. Power Sources 2019, 414, 318–326. [Google Scholar] [CrossRef]
- Yuksel, T.; Litster, S.; Viswanathan, V.; Michalek, J.J. Plug-in hybrid electric vehicle LiFePO4 battery life implications of thermal management, driving conditions, and regional climate. J. Power Sources 2017, 338, 49–64. [Google Scholar] [CrossRef]
- Keil, P.; Jossen, A. Aging of Lithium-Ion Batteries in Electric Vehicles: Impact of Regenerative Braking EVS28 International Electric Vehicle Symposium and Exhibition 2. World Electr. Veh. J. 2015, 7, 41–51. [Google Scholar] [CrossRef]
- Mueller, S.; Rohr, S.; Schmid, W.; Lienkamp, M. Analysing the influence of driver behaviour and tuning measures on battery aging and residual value of electric vehicles. In Proceedings of the EVS 2017—30th International Electric Vehicle Symposium and Exhibition, Stuttgart, Germany, 9–11 October 2017; pp. 1–12. [Google Scholar]
- Liu, H.; Chen, F.; Tong, Y.; Wang, Z.; Yu, X.; Huang, R. Impacts of driving conditions on ev battery pack life cycle. World Electr. Veh. J. 2020, 11, 17. [Google Scholar] [CrossRef]
- Jafari, M.; Gauchia, A.; Zhao, S.; Zhang, K.; Gauchia, L. Electric Vehicle Battery Cycle Aging Evaluation in Real-World Daily Driving and Vehicle-to-Grid Services. IEEE Trans. Transp. Electrif. 2017, 4, 122–134. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, Z.; Ren, Y.; Tao, L.; Lu, C.; Tian, J.; Hu, D.; Wang, Y.; Su, Y.; Chong, J.; et al. A modified reliability model for lithium-ion battery packs based on the stochastic capacity degradation and dynamic response impedance. J. Power Sources 2019, 423, 40–51. [Google Scholar] [CrossRef]
- Maheshwari, A.; Paterakis, N.G.; Santarelli, M.; Gibescu, M. Optimizing the operation of energy storage using a non-linear lithium-ion battery degradation model. Appl. Energy 2020, 261, 114360. [Google Scholar] [CrossRef]
- Suri, G.; Onori, S. A control-oriented cycle-life model for hybrid electric vehicle lithium-ion batteries. Energy 2016, 96, 644–653. [Google Scholar] [CrossRef]
- De Gennaro, M.; Paffumi, E.; Martini, G.; Giallonardo, A.; Pedroso, S.; Loiselle-Lapointe, A. A case study to predict the capacity fade of the battery of electrified vehicles in real-world use conditions. Case Stud. Transp. Policy 2020, 8, 517–534. [Google Scholar] [CrossRef]
- Xu, B.; Oudalov, A.; Ulbig, A.; Andersson, G.; Kirschen, D.S. Modeling of lithium-ion battery degradation for cell life assessment. IEEE Trans. Smart Grid 2018, 9, 1131–1140. [Google Scholar] [CrossRef]
- Redondo-Iglesias, E.; Venet, P.; Pelissier, S. Modelling lithium-ion battery ageing in electric vehicle applications—calendar and cycling ageing combination effects. Batteries 2020, 6, 14. [Google Scholar] [CrossRef]
- Galatro, D.; Silva, C.D.; Romero, D.A.; Trescases, O.; Amon, C.H. Challenges in data-based degradation models for lithium-ion batteries. Int. J. Energy Res. 2020, 44, 3954–3975. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Xie, S.; Song, Z.; Hu, L.; Hou, C. Adaptively coordinated optimization of battery aging and energy management in plug-in hybrid electric buses. Appl. Energy 2019, 256, 113891. [Google Scholar] [CrossRef]
- Du, R.; Hu, X.; Xie, S.; Hu, L.; Zhang, Z.; Lin, X. Battery aging- and temperature-aware predictive energy management for hybrid electric vehicles. J. Power Sources 2020, 473, 228568. [Google Scholar] [CrossRef]
- Nikolian, A.; Jaguemont, J.; de Hoog, J.; Goutam, S.; Omar, N.; Van Den Bossche, P.; Van Mierlo, J. Complete cell-level lithium-ion electrical ECM model for different chemistries (NMC, LFP, LTO) and temperatures (−5 °C to 45 °C)—Optimized modelling techniques. Int. J. Electr. Power Energy Syst. 2018, 98, 133–146. [Google Scholar] [CrossRef]
- Su, J.; Lin, M.; Wang, S.; Li, J.; Coffie-Ken, J.; Xie, F. An equivalent circuit model analysis for the lithium-ion battery pack in pure electric vehicles. Meas. Control 2019, 52, 193–201. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, D.; Cheng, J.; Wang, B.; Luk, P.C.K. An improved Thevenin model of lithium-ion battery with high accuracy for electric vehicles. Appl. Energy 2019, 254, 113615. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Arias, S.; Krishna, M.; Worwood, D.; Barai, A.; Widanalage, D.; Marco, J. Quantifying cell-to-cell variations of a parallel battery module for different pack configurations. Appl. Energy 2021, 282, 115859. [Google Scholar] [CrossRef]
- Escobar, C.; Gong, Z.; Da Silva, C.; Trescases, O.; Amon, C.H. Effect of Cell-to-Cell Thermal Imbalance and Cooling Strategy on Electric Vehicle Battery Performance and Longevity. In Proceedings of the 2022 21st IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (iTherm), San Diego, CA, USA, 31 May–1 June 2022; pp. 1–9. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, X.; Xia, S.; Zhang, K.; Wei, C.; Bak, S.; Shadike, Z.; Liu, X.; Yang, Y.; Xu, R.; et al. High-Voltage Charging-Induced Strain, Heterogeneity, and Micro-Cracks in Secondary Particles of a Nickel-Rich Layered Cathode Material. Adv. Funct. Mater. 2019, 1900247, 1–11. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Yang, H.; Ye, C.; Mao, Y.; Wang, J.; Shi, S.; Yang, J.; Zhang, W. Rationalizing the interphase stability of Li|doped-Li7La3Zr2O12 via automated reaction screening and machine learning. J. Mater. Chem. 2019, 12, 19961–19969. [Google Scholar] [CrossRef]
- Roman, D.; Saxena, S.; Robu, V.; Pecht, M.; Flynn, D. Machine learning pipeline for battery state-of-health estimation. Nat. Mach. Intell. 2021, 3, 447–456. [Google Scholar] [CrossRef]
- Li, Y.; Zou, C.; Berecibar, M.; Nanini-Maury, E.; Chan, J.C.; van den Bossche, P.; Van Mierlo, J.; Omar, N. Random forest regression for online capacity estimation of lithium-ion batteries. Appl. Energy 2018, 232, 197–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Zhang, Y.; Wang, J.; Stimming, U.; Lee, A.A. Identifying degradation patterns of lithium ion batteries from impedance spectroscopy using machine learning. Nat. Commun. 2020, 11, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Jeong, S.; Kwak, E.; Kim, J.-h.; Oh, K.Y. Integrated framework for SOH estimation of lithium-ion batteries using multiphysics features. Energy 2022, 238, 121712. [Google Scholar] [CrossRef]
- Li, H.; Ji, W.; Zhang, P.; Zhao, J. Safety boundary of power battery based on quantitative lithium deposition. J. Energy Storage 2022, 52, 104789. [Google Scholar] [CrossRef]
- Chen, B.R.; Kunz, M.R.; Tanim, T.R.; Dufek, E.J. A machine learning framework for early detection of lithium plating combining multiple physics-based electrochemical signatures. Cell Rep. Phys. Sci. 2021, 2, 100352. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, J.; Pan, D.; Peng, Y.; Peng, X. Lithium-ion battery remaining useful life estimation with an optimized Relevance Vector Machine algorithm with incremental learning. Meas. J. Int. Meas. Confed. 2015, 63. [Google Scholar] [CrossRef]
- Ni, Y.; Xu, J.; Zhu, C.; Pei, L. Accurate residual capacity estimation of retired LiFePO4 batteries based on mechanism and data-driven model. Appl. Energy 2022, 305, 117922. [Google Scholar] [CrossRef]
- Johnen, M.; Pitzen, S.; Kamps, U.; Kateri, M.; Dechent, P.; Sauer, D.U. Modeling long-term capacity degradation of lithium-ion batteries. J. Energy Storage 2021, 34, 102011. [Google Scholar] [CrossRef]
- Weng, C.; Feng, X.; Sun, J.; Peng, H. State-of-health monitoring of lithium-ion battery modules and packs via incremental capacity peak tracking. Appl. Energy 2016, 180, 360–368. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, M.; Pecht, M. Remaining useful life estimation of lithium-ion cells based on k-nearest neighbor regression with differential evolution optimization. J. Clean. Prod. 2020, 249, 119409. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, X.; Lin, X.; Che, Y.; Xu, L.; Guo, W. Data-driven state of charge estimation for lithium-ion battery packs based on Gaussian process regression. Energy 2020, 205, 118000. [Google Scholar] [CrossRef]
- Severson, K.A.; Attia, P.M.; Jin, N.; Perkins, N.; Jiang, B.; Yang, Z.; Chen, M.H.; Aykol, M.; Herring, P.K.; Fraggedakis, D.; et al. Data-driven prediction of battery cycle life before capacity degradation. Nat. Energy 2019, 4, 383–391. [Google Scholar] [CrossRef]
- Fei, Z.; Yang, F.; Tsui, K.L.; Li, L.; Zhang, Z. Early prediction of battery lifetime via a machine learning based framework. Energy 2021, 225, 120205. [Google Scholar] [CrossRef]
- Kong, J.-z.; Yang, F.; Zhang, X.; Pan, E.; Peng, Z.; Wang, D. Voltage-temperature health feature extraction to improve prognostics and health management of lithium-ion batteries. Energy 2021, 223, 120114. [Google Scholar] [CrossRef]
- Diao, W.; Naqvi, I.H.; Pecht, M. Early detection of anomalous degradation behavior in lithium-ion batteries. J. Energy Storage 2020, 32, 101710. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y. Li-ion Battery Electrode Health Diagnostics using Machine Learning. In Proceedings of the American Control Conference, Denver, CO, USA, 1–3 July 2020; pp. 1137–1142. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, J.; Chu, S.q.; Li, J.; Zhang, K.; Yuan, Q.; Pianetta, P.; Li, L.; Jung, K.; Liu, Y. Understanding the Mesoscale Degradation in Nickel-Rich Cathode Materials through Machine-Learning-Revealed Strain—Redox Decoupling. Acs Energy Lett. 2021, 6, 687–693. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Yang, Y.; Mu, L.; Wei, C.; Yu, X.; Zhao, K.; Cloetens, P.; Lin, F.; Liu, Y.; et al. Machine-learning-revealed statistics of the particle-carbon/binder detachment in lithium-ion battery cathodes. Nat. Commun. 2020, 11, 2310. [Google Scholar] [CrossRef]
- Bhowmik, A.; Castelli, I.E.; Garcia-lastra, J.M.; Bj, P.; Winther, O.; Vegge, T. A perspective on inverse design of battery interphases using multi-scale modelling, experiments and generative deep learning. Energy Storage Mater. 2019, 21, 446–456. [Google Scholar] [CrossRef]
- Lee, S.; Han, S.; Han, K.H.; Kim, Y.; Agarwal, S.; Hariharan, K.S.; Oh, B.; Yoon, J. Diagnosing various failures of lithium-ion batteries using artificial neural network enhanced by likelihood mapping. J. Energy Storage 2021, 40, 102768. [Google Scholar] [CrossRef]
- Ruan, H.; Chen, J.; Ai, W.; Wu, B. Generalised diagnostic framework for rapid battery degradation quantification with deep learning. Energy AI 2022, 9, 100158. [Google Scholar] [CrossRef]
- Chun, H.; Yoon, K.; Kim, J.; Han, S. Improving aging identifiability of lithium-ion batteries using deep reinforcement learning. IEEE Trans. Transp. Electrif. 2022, 9, 995–1007. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, R.; He, H.; Pecht, M.G. Long short-term memory recurrent neural network for remaining useful life prediction of lithium-ion batteries. IEEE Trans. Veh. Technol. 2018, 67, 5695–5705. [Google Scholar] [CrossRef]
- Assefi, M.; Hooshmand, A.; Hosseini, H.; Sharma, R. Battery Degradation Temporal Modeling Using LSTM Networks. In Proceedings of the-17th IEEE International Conference on Machine Learning and Applications, ICMLA 2018, Orlando, FL, USA, 17–20 December 2019; pp. 853–858. [Google Scholar] [CrossRef]
- Liu, K.; Shang, Y.; Ouyang, Q.; Widanage, W.D. A Data-Driven Approach with Uncertainty Quantification for Predicting Future Capacities and Remaining Useful Life of Lithium-ion Battery. IEEE Trans. Ind. Electron. 2021, 68, 3170–3180. [Google Scholar] [CrossRef]
- Azkue, M.; Lucu, M.; Martinez-Laserna, E.; Aizpuru, I. Calendar ageing model for Li-Ion batteries using transfer learning methods. World Electr. Veh. J. 2021, 12, 145. [Google Scholar] [CrossRef]
- Tang, X.; Liu, K.; Wang, X.; Gao, F.; MacRo, J.; Widanage, W.D. Model Migration Neural Network for Predicting Battery Aging Trajectories. IEEE Trans. Transp. Electrif. 2020, 6, 363–374. [Google Scholar] [CrossRef]
- Takyi-Aninakwa, P.; Wang, S.; Zhang, H.; Li, H.; Xu, W.; Fernandez, C. An optimized relevant long short-term memory-squared gain extended Kalman filter for the state of charge estimation of lithium-ion batteries. Energy 2022, 260, 125093. [Google Scholar] [CrossRef]
- Song, L.; Zhang, K.; Liang, T.; Han, X.; Zhang, Y. Intelligent state of health estimation for lithium-ion battery pack based on big data analysis. J. Energy Storage 2020, 32, 101836. [Google Scholar] [CrossRef]
- Che, Y.; Deng, Z.; Li, P.; Tang, X.; Khosravinia, K.; Lin, X.; Hu, X. State of health prognostics for series battery packs: A universal deep learning method. Energy 2022, 238, 121857. [Google Scholar] [CrossRef]
- Shu, X.; Shen, J.; Li, G.; Zhang, Y.; Chen, Z.; Liu, Y. A Flexible State-of-Health Prediction Scheme for Lithium-Ion Battery Packs with Long Short-Term Memory Network and Transfer Learning. IEEE Trans. Transp. Electrif. 2021, 7, 2238–2248. [Google Scholar] [CrossRef]
- Shen, S.; Sadoughi, M.; Chen, X.; Hong, M.; Hu, C. A deep learning method for online capacity estimation of lithium-ion batteries. J. Energy Storage 2019, 25, 100817. [Google Scholar] [CrossRef]
- Khaleghi, S.; Karimi, D.; Beheshti, S.H.; Hosen, M.S.; Behi, H.; Berecibar, M.; Van Mierlo, J. Online health diagnosis of lithium-ion batteries based on nonlinear autoregressive neural network. Appl. Energy 2021, 282, 116159. [Google Scholar] [CrossRef]
- Smiley, A.J.; Harrison, W.K.; Plett, G.L. Postprocessing the outputs of an interacting multiple-model Kalman fi lter using a Markovian trellis to estimate parameter values of aged Li-ion cells. J. Energy Storage 2020, 27, 101043. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Fan, Q.; Lund, P.D.; Hong, J. Improving the state of charge estimation of reused lithium-ion batteries by abating hysteresis using machine learning technique. J. Energy Storage 2020, 32, 101678. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Fang, Q.; Dai, H.; Cao, Y.; Wei, X. An online SOC and capacity estimation method for aged lithium-ion battery pack considering cell inconsistency. J. Energy Storage 2020, 29, 101250. [Google Scholar] [CrossRef]
- Tang, X.; Liu, K.; Wang, X.; Liu, B.; Gao, F.; Widanage, W.D. Real-time aging trajectory prediction using a base model-oriented gradient-correction particle filter for Lithium-ion batteries. J. Power Sources 2019, 440, 227118. [Google Scholar] [CrossRef]
- Meng, J.; Azib, T.; Yue, M. Early-Stage end-of-Life prediction of lithium-Ion battery using empirical mode decomposition and particle filter. Proc. Inst. Mech. Eng. Part J. Power Energy 2023, 237, 1090–1099. [Google Scholar] [CrossRef]
- Kannan, V.; Fisher, A.; Birgersson, E. Monte Carlo assisted sensitivity analysis of a Li-ion battery with a phase change material. J. Energy Storage 2021, 35, 102269. [Google Scholar] [CrossRef]
- Rogers, D.J.; Aslett, L.J.; Troffaes, M.C. Modelling of modular battery systems under cell capacity variation and degradation. Appl. Energy 2021, 283, 116360. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chung, K.J. Lifetime prognosis of lithium-ion batteries through novel accelerated degradation measurements and a combined gamma process and Monte Carlo method. Appl. Sci. 2019, 9, 559. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Wang, X.; Wang, Z.; Zhao, X. Reliability modeling method for lithium-ion battery packs considering the dependency of cell degradations based on a regression model and copulas. Materials 2019, 12, 1054. [Google Scholar] [CrossRef]
- Lai, X.; Huang, Y.; Gu, H.; Han, X.; Feng, X.; Dai, H.; Zheng, Y.; Ouyang, M. Remaining discharge energy estimation for lithium-ion batteries based on future load prediction considering temperature and ageing effects. Energy 2022, 238, 121754. [Google Scholar] [CrossRef]
- Duan, B.; Li, Z.; Gu, P.; Zhou, Z.; Zhang, C. Evaluation of battery inconsistency based on information entropy. J. Energy Storage 2018, 16, 160–166. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety focused modeling of lithium-ion batteries: A review. J. Power Sources 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Torchio, M.; Magni, L.; Gopaluni, R.B.; Braatz, R.D.; Raimondo, D.M. LIONSIMBA: A Matlab Framework Based on a Finite Volume Model Suitable for Li-Ion Battery Design, Simulation, and Control. J. Electrochem. Soc. 2016, 163, A1192–A1205. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Deng, L.; Cui, Z.; Lyu, C.; Wang, L.; Pecht, M. Aging modes analysis and physical parameter identification based on a simplified electrochemical model for lithium-ion batteries. J. Energy Storage 2020, 31, 101538. [Google Scholar] [CrossRef]
- Jokar, A.; Rajabloo, B.; Désilets, M.; Lacroix, M. Review of simplified Pseudo-two-Dimensional models of lithium-ion batteries. J. Power Sources 2016, 327, 44–55. [Google Scholar] [CrossRef]
- Dao, T.S.; Vyasarayani, C.P.; McPhee, J. Simplification and order reduction of lithium-ion battery model based on porous-electrode theory. J. Power Sources 2012, 198, 329–337. [Google Scholar] [CrossRef]
- Smiley, A.; Plett, G.L. An adaptive physics-based reduced-order model of an aged lithium-ion cell, selected using an interacting multiple-model Kalman filter. J. Energy Storage 2018, 19, 120–134. [Google Scholar] [CrossRef]
- Lv, H.; Huang, X.; Kang, L.; Liu, Y. Quantitative Estimation of Turning Point of Ageing Based on a Two-Stage Model for Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 010533. [Google Scholar] [CrossRef]
- Chen, Z.; Danilov, D.L.; Zhang, Q.; Jiang, M.; Zhou, J.; Eichel, R.A.; Notten, P.H. Modeling NCA/C6-Si battery ageing. Electrochim. Acta 2022, 430, 141077. [Google Scholar] [CrossRef]
- Carelli, S.; Bessler, W.G. Coupling Lithium Plating with SEI Formation in a Pseudo-3D Model: A Comprehensive Approach to Describe Aging in Lithium-Ion Cells. J. Electrochem. Soc. 2022, 169, 050539. [Google Scholar] [CrossRef]
- O’Kane, S.E.; Ai, W.; Madabattula, G.; Alonso-Alvarez, D.; Timms, R.; Sulzer, V.; Edge, J.S.; Wu, B.; Offer, G.J.; Marinescu, M. Lithium-ion battery degradation: How to model it. Phys. Chem. Chem. Phys. 2022, 24, 7909–7922. [Google Scholar] [CrossRef]
- Ashwin, T.R.; McGordon, A.; Jennings, P.A. Electrochemical modelling of Li-ion battery pack with constant voltage cycling. J. Power Sources 2017, 341, 327–339. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, D.; Wang, Z.; Ren, Y.; Sun, B.; Feng, Q.; Qian, C. Multiphysical modeling for life analysis of lithium-ion battery pack in electric vehicles. Renew. Sustain. Energy Rev. 2020, 131, 109993. [Google Scholar] [CrossRef]
- Rumpf, K.; Rheinfeld, A.; Schindler, M.; Keil, J.; Schua, T.; Jossen, A. Influence of Cell-to-Cell Variations on the Inhomogeneity of Lithium-Ion Battery Modules. J. Electrochem. Soc. 2018, 165, A2587–A2607. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, X.; Shang, B.; Li, G. Unbalanced discharging and aging due to temperature differences among the cells in a lithium-ion battery pack with parallel combination. J. Power Sources 2016, 306, 733–741. [Google Scholar] [CrossRef]
- Mendoza, H.; Roberts, S.A.; Brunini, V.E.; Grillet, A.M. Mechanical and Electrochemical Response of a LiCoO2 Cathode using Reconstructed Microstructures. Electrochim. Acta 2016, 190, 1–15. [Google Scholar] [CrossRef]
- Lee, Y.K. Effect of transition metal ions on solid electrolyte interphase layer on the graphite electrode in lithium ion battery. J. Power Sources 2021, 484, 229270. [Google Scholar] [CrossRef]
- Lee, Y.K.; Song, J.; Park, J. Multi-scale coupled mechanical-electrochemical modeling for study on stress generation and its impact on multi-layered electrodes in lithium-ion batteries. Electrochim. Acta 2021, 389, 138682. [Google Scholar] [CrossRef]
- Krewer, U.; Röder, F.; Harinath, E.; Braatz, R.D.; Bedürftig, B.; Findeisen, R. Review—Dynamic Models of Li-Ion Batteries for Diagnosis and Operation: A Review and Perspective. J. Electrochem. Soc. 2018, 165, A3656–A3673. [Google Scholar] [CrossRef]
- Rodríguez, A.; Plett, G.L.; Trimboli, M.S. Comparing four model-order reduction techniques, applied to lithium-ion battery-cell internal electrochemical transfer functions. eTransportation 2019, 1, 100009. [Google Scholar] [CrossRef]
- Lyu, C.; Song, Y.; Zheng, J.; Luo, W.; Hinds, G.; Li, J.; Wang, L. In situ monitoring of lithium-ion battery degradation using an electrochemical model. Appl. Energy 2019, 250, 685–696. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhang, J.; Pecht, M. Lithium-iron-phosphate battery electrochemical modelling under a wide range of ambient temperatures. J. Electroanal. Chem. 2021, 882, 115041. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Tian, J.; Tian, Y. State-of-charge estimation tolerant of battery aging based on a physics-based model and an adaptive cubature Kalman filter. Energy 2021, 220, 119767. [Google Scholar] [CrossRef]
- Ouyang, M.; Feng, X.; Han, X.; Lu, L.; Li, Z.; He, X. A dynamic capacity degradation model and its applications considering varying load for a large format Li-ion battery. Appl. Energy 2016, 165, 48–59. [Google Scholar] [CrossRef]
- Smith, K.; Gasper, P.; Colclasure, A.M.; Shimonishi, Y.; Yoshida, S. Lithium-Ion Battery Life Model with Electrode Cracking and Early-Life Break-in Processes. J. Electrochem. Soc. 2021, 168. [Google Scholar] [CrossRef]
- Zhang, X.; Chumakov, S.; Li, X.; Klinsmann, M.; Kim, S.U.; Linder, C.; Christensen, J. An Electro-chemo-thermo-mechanical Coupled Three-dimensional Computational Framework for Lithium-ion Batteries. J. Electrochem. Soc. 2020, 167, 160542. [Google Scholar] [CrossRef]
- Qi, C.; Zhu, Y.; Gao, F.; Yang, K.; Jiao, Q. Mathematical model for thermal behavior of lithium ion battery pack under overcharge. Int. J. Heat Mass Transf. 2018, 124, 552–563. [Google Scholar] [CrossRef]
- Chen, F.; Huang, R.; Wang, C.; Yu, X.; Liu, H.; Wu, Q.; Qian, K.; Bhagat, R. Air and PCM cooling for battery thermal management considering battery cycle life. Appl. Therm. Eng. 2020, 173, 115154. [Google Scholar] [CrossRef]
- Yamanaka, T.; Takagishi, Y.; Yamaue, T. An Electrochemical-Thermal Model for Lithium-Ion Battery Packs during Driving of Battery Electric Vehicles. J. Electrochem. Soc. 2021, 168, 050545. [Google Scholar] [CrossRef]
- Tran, N.T.; Farrell, T.; Vilathgamuwa, M.; Choi, S.S.; Li, Y. A Computationally Efficient Coupled Electrochemical-Thermal Model for Large Format Cylindrical Lithium Ion Batteries. J. Electrochem. Soc. 2019, 166, A3059–A3071. [Google Scholar] [CrossRef]
- Song, W.; Chen, M.; Bai, F.; Lin, S.; Chen, Y.; Feng, Z. Non-uniform effect on the thermal/aging performance of Lithium-ion pouch battery. Appl. Therm. Eng. 2018, 128, 1165–1174. [Google Scholar] [CrossRef]
- Liu, X.; Ai, W.; Naylor Marlow, M.; Patel, Y.; Wu, B. The effect of cell-to-cell variations and thermal gradients on the performance and degradation of lithium-ion battery packs. Appl. Energy 2019, 248, 489–499. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, Z.; Li, X.; Tian, J. Parallel-connected battery module modeling based on physical characteristics in multiple domains and heterogeneous characteristic analysis. Energy 2022, 239, 122181. [Google Scholar] [CrossRef]
- Jia, Y.; Uddin, M.; Li, Y.; Xu, J. Thermal runaway propagation behavior within 18,650 lithium-ion battery packs: A modeling study. J. Energy Storage 2020, 31, 101668. [Google Scholar] [CrossRef]
- Mevawalla, A.; Panchal, S.; Tran, M.K.; Fowler, M.; Fraser, R. One dimensional fast computational partial differential model for heat transfer in lithium-ion batteries. J. Energy Storage 2021, 37, 102471. [Google Scholar] [CrossRef]
- Abada, S.; Petit, M.; Lecocq, A.; Marlair, G.; Sauvant-Moynot, V.; Huet, F. Combined experimental and modeling approaches of the thermal runaway of fresh and aged lithium-ion batteries. J. Power Sources 2018, 399, 264–273. [Google Scholar] [CrossRef]
- Jia, Y.; Gao, X.; Mouillet, J.B.; Terrier, J.M.; Lombard, P.; Xu, J. Effective thermo-electro-mechanical modeling framework of lithium-ion batteries based on a representative volume element approach. J. Energy Storage 2021, 33, 102090. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Marco, J.; Jennings, P. Combined electrical and electrochemical-thermal model of parallel connected large format pouch cells. J. Energy Storage 2019, 22, 194–207. [Google Scholar] [CrossRef]
- Chalise, D.; Shah, K.; Prasher, R.; Jain, A. Conjugate Heat Transfer Analysis of Thermal Management of a Li-Ion Battery Pack. J. Electrochem. Energy Convers. Storage 2018, 15, 1–8. [Google Scholar] [CrossRef]
- Lamrani, B.; Lebrouhi, B.E.; Khattari, Y.; Kousksou, T. A simplified thermal model for a lithium-ion battery pack with phase change material thermal management system. J. Energy Storage 2021, 44, 103377. [Google Scholar] [CrossRef]
- Kim, M.; Chun, H.; Kim, J.; Kim, K.; Yu, J.; Kim, T.; Han, S. Data-efficient parameter identification of electrochemical lithium-ion battery model using deep Bayesian harmony search. Appl. Energy 2019, 254, 113644. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Wu, W.T. Three-dimensional thermal modeling of Li-ion battery cell and 50 V Li-ion battery pack cooled by mini-channel cold plate. Appl. Therm. Eng. 2019, 147, 829–840. [Google Scholar] [CrossRef]
- Gottapu, M.; Goh, T.; Kaushik, A.; Adiga, S.P.; Bharathraj, S.; Patil, R.S.; Kim, D.; Ryu, Y. Fully coupled simplified electrochemical and thermal model for series-parallel configured battery pack. J. Energy Storage 2021, 36, 102424. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Zhu, J.; Wang, G.; Jiang, J. Co-estimation of state-of-charge, capacity and resistance for lithium-ion batteries based on a high-fidelity electrochemical model. Appl. Energy 2016, 180, 424–434. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, Z.; Ren, Y.; Sun, B.; Yang, D.; Feng, Q. A reliability design method for a lithium-ion battery pack considering the thermal disequilibrium in electric vehicles. J. Power Sources 2018, 386, 10–20. [Google Scholar] [CrossRef]
- Li, W.; Zhu, J.; Xia, Y.; Gorji, M.B.; Wierzbicki, T. Data-Driven Safety Envelope of Lithium-Ion Batteries for Electric Vehicles. Joule 2019, 3, 2703–2715. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Huang, B.; Zhang, H. Numerical investigation on the thermal behavior of cylindrical lithium-ion batteries based on the electrochemical-thermal coupling model. Int. J. Heat Mass Transf. 2022, 199, 123449. [Google Scholar] [CrossRef]
- Tu, H.; Moura, S.; Wang, Y.; Fang, H. Integrating physics-based modeling with machine learning for lithium-ion batteries. Appl. Energy 2023, 329, 120289. [Google Scholar] [CrossRef]
- Appiah, W.A.; Busk, J.; Vegge, T.; Bhowmik, A. Sensitivity analysis methodology for battery degradation models. Electrochim. Acta 2023, 439, 141430. [Google Scholar] [CrossRef]
- Dubarry, M.; Baure, G. Perspective on Commercial Li-ion Battery Testing, Best Practices for Simple and Effective Protocols. Electronics 2020, 9, 152. [Google Scholar] [CrossRef]
- Baure, G.; Dubarry, M. Synthetic vs. Real driving cycles: A comparison of electric vehicle battery degradation. Batteries 2019, 5, 42. [Google Scholar] [CrossRef]
- Pfriem, M.; Gauterin, F. Development of real-world driving cycles for battery electric vehicles. World Electr. Veh. J. 2016, 8, 14–24. [Google Scholar] [CrossRef]
- Wang, D.; Coignard, J.; Zeng, T.; Zhang, C.; Saxena, S. Quantifying electric vehicle battery degradation from driving vs. vehicle-to-grid services. J. Power Sources 2016, 332, 193–203. [Google Scholar] [CrossRef]
- Diao, W.; Saxena, S.; Pecht, M. Accelerated cycle life testing and capacity degradation modeling of LiCoO2-graphite cells. J. Power Sources 2019, 435, 226830. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Uddin, K.; Chouchelamane, G.H.; Widanage, W.D.; Marco, J. A Comparison between Electrochemical Impedance Spectroscopy and Incremental Capacity-Differential Voltage as Li-ion Diagnostic Techniques to Identify and Quantify the Effects of Degradation Modes within Battery Management Systems. J. Power Sources 2017, 360, 301–318. [Google Scholar] [CrossRef]
- Al-Zubaidi R-Smith, N.; Leitner, M.; Alic, I.; Toth, D.; Kasper, M.; Romio, M.; Surace, Y.; Jahn, M.; Kienberger, F.; Ebner, A.; et al. Assessment of lithium ion battery ageing by combined impedance spectroscopy, functional microscopy and finite element modelling. J. Power Sources 2021, 512, 230459. [Google Scholar] [CrossRef]
- Raj, T.; Wang, A.A.; Monroe, C.W.; Howey, D.A. Investigation of Path-Dependent Degradation in Lithium-Ion Batteries**. Batter. Supercaps 2020, 3, 1377–1385. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.A.; Da Silva, C.M.; Amon, C.H. Multiscale Modelling Methodologies of Lithium-Ion Battery Aging: A Review of Most Recent Developments. Batteries 2023, 9, 434. https://doi.org/10.3390/batteries9090434
Ali MA, Da Silva CM, Amon CH. Multiscale Modelling Methodologies of Lithium-Ion Battery Aging: A Review of Most Recent Developments. Batteries. 2023; 9(9):434. https://doi.org/10.3390/batteries9090434
Chicago/Turabian StyleAli, Mir A., Carlos M. Da Silva, and Cristina H. Amon. 2023. "Multiscale Modelling Methodologies of Lithium-Ion Battery Aging: A Review of Most Recent Developments" Batteries 9, no. 9: 434. https://doi.org/10.3390/batteries9090434
APA StyleAli, M. A., Da Silva, C. M., & Amon, C. H. (2023). Multiscale Modelling Methodologies of Lithium-Ion Battery Aging: A Review of Most Recent Developments. Batteries, 9(9), 434. https://doi.org/10.3390/batteries9090434







