Comparison of Different Current Collector Materials for In Situ Lithium Deposition with Slurry-Based Solid Electrolyte Layers
Abstract
1. Introduction
1.1. Lithium Metal Electrode
1.2. SE Layer (Suitable for Production Scale)
2. Experimental
2.1. Materials
2.2. Cell Assembly
2.3. Electrochemical Characterization
2.4. Surface Characterization
3. Results and Discussion
3.1. Variation of the Current Collector Material
3.2. SIL in LPS Layer
3.3. Summary of the Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Al | Aluminum |
| CE | Coulombic efficiency |
| Cu | Copper |
| HNBR | Hydrogenated nitrile-butadiene rubber |
| Li | Lithium |
| Li-In | Lithium-indium |
| Lithium ion | |
| Lithium triglyme bis(trifluoromethanesulfonyl)imide | |
| LPS | Lithium phosphorus sulfide (-Li3PS4) |
| LPSCl | Lithium phosphorus sulfur chloride (Li6PS5Cl) |
| NBR | Nitrile-butadiene rubber |
| Ni | Nickel |
| SE | Solid electrolyte |
| SEs | Solid electrolytes |
| SSE | Solid-state electrolyte |
| SEI | Solid electrolyte interface |
| SIL | Solvate ionic liquid |
| SST | Stainless steel |
| vs. | Versus |
References
- Su, H.; Jiang, Z.; Liu, Y.; Li, J.; Gu, C.; Wang, X.; Xia, X.; Tu, J. Recent progress of sulfide electrolytes for all-solid-state lithium batteries. Energy Mater. 2022, 2, 200005. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Chen, S.; Yang, X.; Zhang, J.; Ma, J.; Meng, Y.; Tao, K.; Li, F.; Geng, J. Aluminum–lithium alloy as a stable and reversible anode for lithium batteries. Electrochim. Acta 2021, 368, 137626. [Google Scholar] [CrossRef]
- Xu, X.Q.; Cheng, X.B.; Jiang, F.N.; Yang, S.J.; Ren, D.; Shi, P.; Hsu, H.; Yuan, H.; Huang, J.Q.; Ouyang, M.; et al. Dendrite—Accelerated thermal runaway mechanisms of lithium metal pouch batteries. SusMat 2022, 2, 435–444. [Google Scholar] [CrossRef]
- Deng, J.; Yang, X.; Zhang, G. Simulation study on internal short circuit of lithium ion battery caused by lithium dendrite. Mater. Today Commun. 2022, 31, 103570. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Varzi, A.; Raccichini, R.; Passerini, S.; Scrosati, B. Challenges and prospects of the role of solid electrolytes in the revitalization of lithium metal batteries. J. Mater. Chem. A 2016, 4, 17251–17259. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Han, S.; Wang, J.; Peng, Z.; Chen, L. Unlocking the Energy Capabilities of Lithium Metal Electrode with Solid-State Electrolytes. Joule 2018, 2, 1674–1689. [Google Scholar] [CrossRef]
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide Solid Electrolytes for Lithium Battery Applications. Adv. Energy Mater. 2018, 8, 1800933. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Han, F.; Yao, X.; Wang, C. Lithium/Sulfide All-Solid-State Batteries using Sulfide Electrolytes. Adv. Mater. 2021, 33, e2000751. [Google Scholar] [CrossRef]
- Kim, K.J.; Balaish, M.; Wadaguchi, M.; Kong, L.; Rupp, J.L.M. Solid–State Li–Metal Batteries: Challenges and Horizons of Oxide and Sulfide Solid Electrolytes and Their Interfaces. Adv. Energy Mater. 2021, 11, 2002689. [Google Scholar] [CrossRef]
- Doerrer, C.; Capone, I.; Narayanan, S.; Liu, J.; Grovenor, C.R.M.; Pasta, M.; Grant, P.S. High Energy Density Single-Crystal NMC/Li6PS5Cl Cathodes for All-Solid-State Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 37809–37815. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, e1901131. [Google Scholar] [CrossRef]
- Suci, W.G.; Aliwarga, H.K.; Azinuddin, Y.R.; Setyawati, R.B.; Stulasti, K.N.R.; Purwanto, A. Review of various sulfide electrolyte types for solid-state lithium-ion batteries. Open Eng. 2022, 12, 409–423. [Google Scholar] [CrossRef]
- Oh, P.; Yun, J.; Choi, J.H.; Saqib, K.S.; Embleton, T.J.; Park, S.; Lee, C.; Ali, J.; Ko, K.; Cho, J. Development of High-Energy Anodes for All-Solid-State Lithium Batteries Based on Sulfide Electrolytes. Angew. Chem. (Int. Ed. Engl.) 2022, 61, e202201249. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, B.; Shen, Y.; Wu, J.; Zhao, Z.; Zhong, C.; Hu, W. Confronting the Challenges in Lithium Anodes for Lithium Metal Batteries. Adv. Sci. 2021, 8, e2101111. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, R.; Li, Y.; Liu, Y.; Xin, C.; Richter, F.H.; Nan, C.W. Interfacial challenges for all-solid-state batteries based on sulfide solid electrolytes. J. Mater. 2021, 7, 209–218. [Google Scholar] [CrossRef]
- Otoyama, M.; Suyama, M.; Hotehama, C.; Kowada, H.; Takeda, Y.; Ito, K.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Visualization and Control of Chemically Induced Crack Formation in All-Solid-State Lithium-Metal Batteries with Sulfide Electrolyte. ACS Appl. Mater. Interfaces 2021, 13, 5000–5007. [Google Scholar] [CrossRef]
- Jiang, W.; Yan, L.; Zeng, X.; Meng, X.; Huang, R.; Zhu, X.; Ling, M.; Liang, C. Adhesive Sulfide Solid Electrolyte Interface for Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2020, 12, 54876–54883. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Wang, Y.; Dai, L.; Yuan, C. Cell failures of all-solid-state lithium metal batteries with inorganic solid electrolytes: Lithium dendrites. Energy Storage Mater. 2020, 33, 309–328. [Google Scholar] [CrossRef]
- Singer, C.; Schnell, J.; Reinhart, G. Scalable Processing Routes for the Production of All–Solid–State Batteries—Modeling Interdependencies of Product and Process. Energy Technol. 2021, 9, 2000665. [Google Scholar] [CrossRef]
- Wang, M.J.; Carmona, E.; Gupta, A.; Albertus, P.; Sakamoto, J. Enabling lithium-free manufacturing of pure lithium metal solid-state batteries through in situ plating. Nat. Commun. 2020, 11, 5201. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Adams, B.D.; Zheng, J.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.; Hu, J.; Zhang, J.G. Anode-Free Rechargeable Lithium Metal Batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Heubner, C.; Maletti, S.; Auer, H.; Hüttl, J.; Voigt, K.; Lohrberg, O.; Nikolowski, K.; Partsch, M.; Michaelis, A. From Lithium–Metal toward Anode–Free Solid–State Batteries: Current Developments, Issues, and Challenges. Adv. Funct. Mater. 2021, 31, 2106608. [Google Scholar] [CrossRef]
- Homann, G.; Meister, P.; Stolz, L.; Brinkmann, J.P.; Kulisch, J.; Adermann, T.; Winter, M.; Kasnatscheew, J. High-Voltage All-Solid-State Lithium Battery with Sulfide-Based Electrolyte: Challenges for the Construction of a Bipolar Multicell Stack and How to Overcome Them. ACS Appl. Energy Mater. 2020, 3, 3162–3168. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A review of current collectors for lithium-ion batteries. J. Power Source 2021, 485, 229321. [Google Scholar] [CrossRef]
- Shinzo, S.; Higuchi, E.; Chiku, M.; Hayashi, A.; Inoue, H. High-Rate Lithium Metal Plating and Stripping on Solid Electrolytes Using a Porous Current Collector with a High Aperture Ratio. ACS Appl. Energy Mater. 2021, 4, 12613–12622. [Google Scholar] [CrossRef]
- Shinzo, S.; Higuchi, E.; Chiku, M.; Hayashi, A.; Inoue, H. Suppression of Dendritic Growth on Li Negative Electrode for All-Solid-State Rechargeable Battery. ECS Meet. Abstr. 2020, MA2020-02, 980. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, M.; Cheng, Z.; Jiang, H.; Yang, J.; Wang, P.; He, P.; Zhou, H. Carbon-free and binder-free Li-Al alloy anode enabling an all-solid-state Li-S battery with high energy and stability. Sci. Adv. 2022, 8, eabn4372. [Google Scholar] [CrossRef]
- Wang, R.; Li, W.; Liu, L.; Qian, Y.; Liu, F.; Chen, M.; Guo, Y.; Liu, L. Carbon black/graphene-modified aluminum foil cathode current collectors for lithium ion batteries with enhanced electrochemical performances. J. Electroanal. Chem. 2019, 833, 63–69. [Google Scholar] [CrossRef]
- Yang, W.; Huang, R.; Ni, Z.; Cheng, H.; Zhou, S.; Wang, Y.; Li, X.; Zhang, Y.; Zhang, Y. Application and research of current collector for lithium-sulfur battery. Ionics 2022, 28, 1713–1738. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; He, Q.; Ma, Y. Advanced Current Collectors for Alkali Metal Anodes. Chem. Res. Chin. Univ. 2020, 36, 386–401. [Google Scholar] [CrossRef]
- Ates, T.; Keller, M.; Kulisch, J.; Adermann, T.; Passerini, S. Development of an all-solid-state lithium battery by slurry-coating procedures using a sulfidic electrolyte. Energy Storage Mater. 2019, 17, 204–210. [Google Scholar] [CrossRef]
- Oh, D.Y.; Nam, Y.J.; Park, K.H.; Jung, S.H.; Kim, K.T.; Ha, A.R.; Jung, Y.S. Slurry–Fabricable Li + –Conductive Polymeric Binders for Practical All–Solid–State Lithium–Ion Batteries Enabled by Solvate Ionic Liquids. Adv. Energy Mater. 2019, 9, 1802927. [Google Scholar] [CrossRef]
- Fan, X.; Ji, X.; Han, F.; Yue, J.; Chen, J.; Chen, L.; Deng, T.; Jiang, J.; Wang, C. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery. Sci. Adv. 2018, 4, eaau9245. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations. ACS Appl. Mater. Interfaces 2015, 7, 23685–23693. [Google Scholar] [CrossRef]
- Jabbari, V.; Yurkiv, V.; Rasul, M.G.; Phakatkar, A.H.; Mashayek, F.; Shahbazian-Yassar, R. In situ formation of stable solid electrolyte interphase with high ionic conductivity for long lifespan all-solid-state lithium metal batteries. Energy Storage Mater. 2023, 57, 1–13. [Google Scholar] [CrossRef]
- Byeon, Y.W.; Kim, H. Review on Interface and Interphase Issues in Sulfide Solid-State Electrolytes for All-Solid-State Li-Metal Batteries. Electrochem 2021, 2, 30. [Google Scholar] [CrossRef]
- Loeffler, B.N.; Bresser, D.; Passerini, S.; Copley, M. Secondary Lithium-Ion Battery Anodes: From First Commercial Batteries to Recent Research Activities. Johns. Matthey Technol. Rev. 2015, 59, 34–44. [Google Scholar] [CrossRef]
- Ni, S.; Yang, X.; Li, T. Fabrication of porous Ni3S2/Ni nanostructured electrode and its application in lithium ion battery. Mater. Chem. Phys. 2012, 132, 1103–1107. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Wang, X.L.; Xia, X.H.; Zhong, J.; Tu, J.P. Fabrication of highly ordered porous nickel phosphide film and its electrochemical performances toward lithium storage. J. Alloys Compd. 2011, 509, 157–160. [Google Scholar] [CrossRef]
- WANG, J.; CHOU, S.; CHEW, S.; SUN, J.; FORSYTH, M.; MACFARLANE, D.; LIU, H. Nickel sulfide cathode in combination with an ionic liquid-based electrolyte for rechargeable lithium batteries. Solid State Ionics 2008, 179, 2379–2382. [Google Scholar] [CrossRef]
- Lee, Y.J.; Reddy, B.S.; Hong, H.A.; Kim, K.W.; Cho, S.J.; Ahn, H.J.; Ahn, J.H.; Cho, K.K. Synthesis and electrochemical properties of nickel sulfide/carbon composite as anode material for lithium–ion and sodium–ion batteries. Int. J. Energy Res. 2022, 46, 16883–16895. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Tu, J.P.; Wang, X.L.; Huang, X.H.; Yuan, Y.F.; Xia, X.H.; Zeng, Z.Y. Electrochemical performances of nanostructured Ni3P–Ni films electrodeposited on nickel foam substrate. J. Power Source 2008, 185, 519–525. [Google Scholar] [CrossRef]
- Hayashi, A.; Inoue, A.; Tatsumisago, M. Electrochemical performance of NiP2 negative electrodes in all-solid-state lithium secondary batteries. J. Power Source 2009, 189, 669–671. [Google Scholar] [CrossRef]
- Bang, H.J.; Kim, S.; Prakash, J. Electrochemical investigations of lithium-aluminum alloy anode in Li/polymer cells. J. Power Source 2001, 92, 45–49. [Google Scholar] [CrossRef]
- Kwon, G.D.; Moyen, E.; Lee, Y.J.; Joe, J.; Pribat, D. Graphene-Coated Aluminum Thin Film Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 29486–29495. [Google Scholar] [CrossRef]
- Rehnlund, D.; Lindgren, F.; Böhme, S.; Nordh, T.; Zou, Y.; Pettersson, J.; Bexell, U.; Boman, M.; Edström, K.; Nyholm, L. Lithium trapping in alloy forming electrodes and current collectors for lithium based batteries. Energy Environ. Sci. 2017, 10, 1350–1357. [Google Scholar] [CrossRef]
- Senoh, H.; Takeuchi, T.; Kageyama, H.; Sakaebe, H.; Yao, M.; Nakanishi, K.; Ohta, T.; Sakai, T.; Yasuda, K. Electrochemical characteristics of aluminum sulfide for use in lithium secondary batteries. J. Power Source 2010, 195, 8327–8330. [Google Scholar] [CrossRef]
- Heim, F.; Kreher, T.; Birke, K.P. The Influence of Micro-Structured Anode Current Collectors in Combination with Highly Concentrated Electrolyte on the Coulombic Efficiency of In-Situ Deposited Li-Metal Electrodes with Different Counter Electrodes. Batteries 2020, 6, 20. [Google Scholar] [CrossRef]
- Oh, D.Y.; Nam, Y.J.; Park, K.H.; Jung, S.H.; Cho, S.J.; Kim, Y.K.; Lee, Y.G.; Lee, S.Y.; Jung, Y.S. Excellent Compatibility of Solvate Ionic Liquids with Sulfide Solid Electrolytes: Toward Favorable Ionic Contacts in Bulk-Type All-Solid-State Lithium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1500865. [Google Scholar] [CrossRef]
- Shin, M.; Gewirth, A.A. Incorporating Solvate and Solid Electrolytes for All–Solid–State Li 2 S Batteries with High Capacity and Long Cycle Life. Adv. Energy Mater. 2019, 9, 1900938. [Google Scholar] [CrossRef]
- Santhosha, A.L.; Medenbach, L.; Buchheim, J.R.; Adelhelm, P. The Indium–Lithium Electrode in Solid–State Lithium–Ion Batteries: Phase Formation, Redox Potentials, and Interface Stability. Batter. Supercaps 2019, 2, 524–529. [Google Scholar] [CrossRef]
- Reddy, M.V.; Julien, C.M.; Mauger, A.; Zaghib, K. Sulfide and Oxide Inorganic Solid Electrolytes for All-Solid-State Li Batteries: A Review. Nanomaterials 2020, 10, 1606. [Google Scholar] [CrossRef]
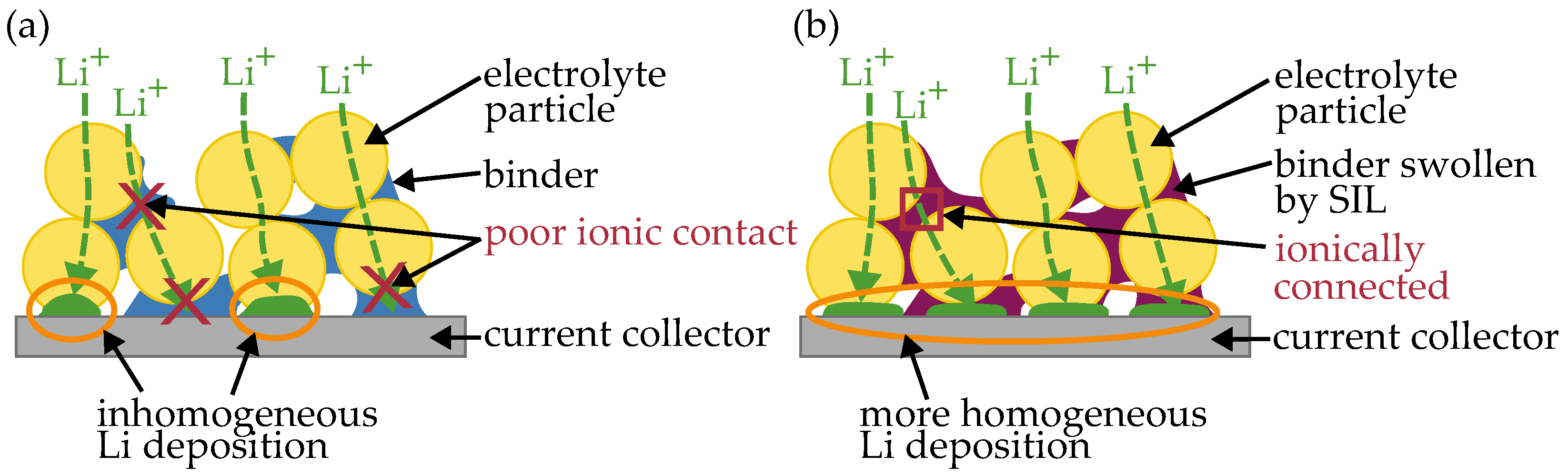

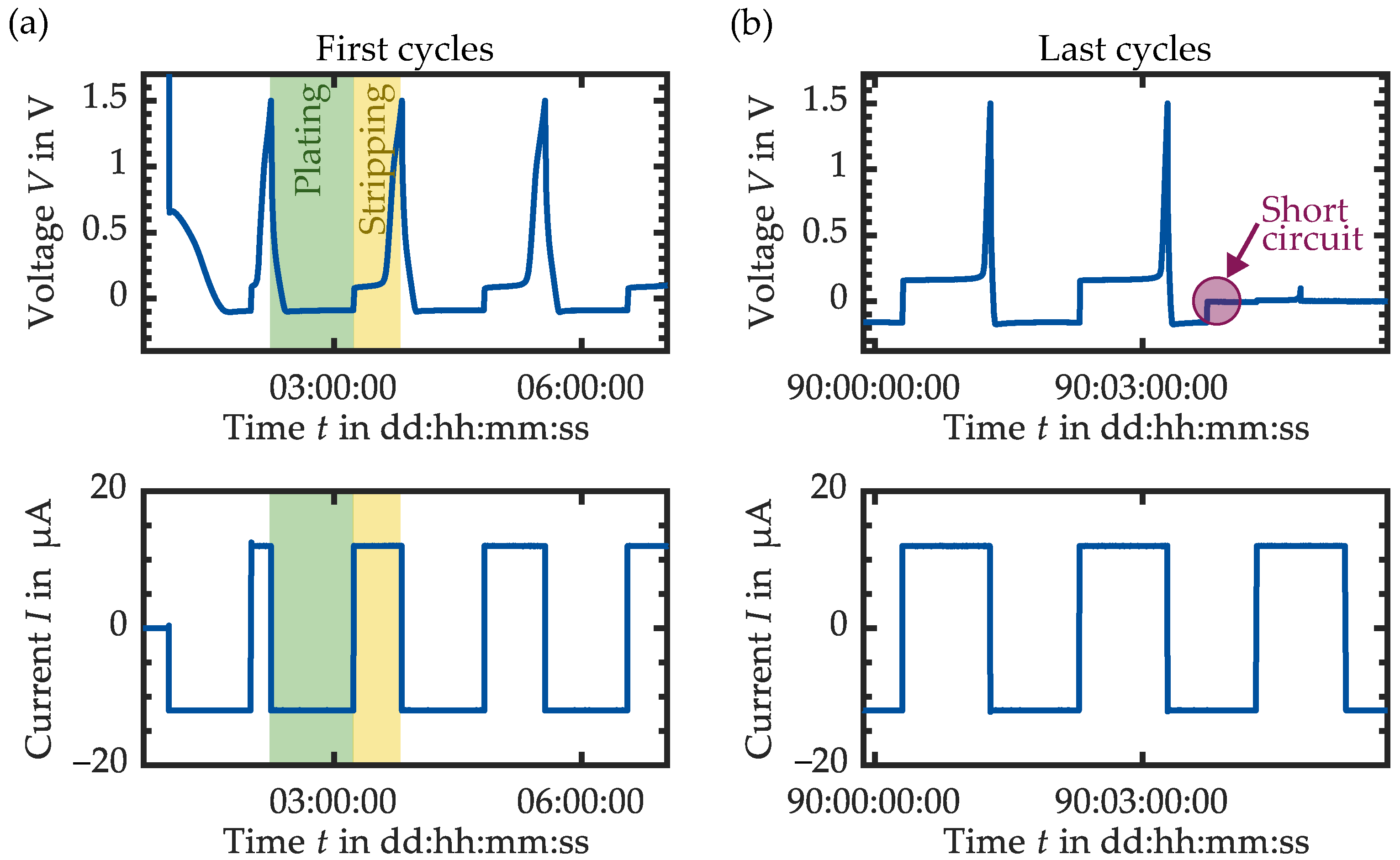

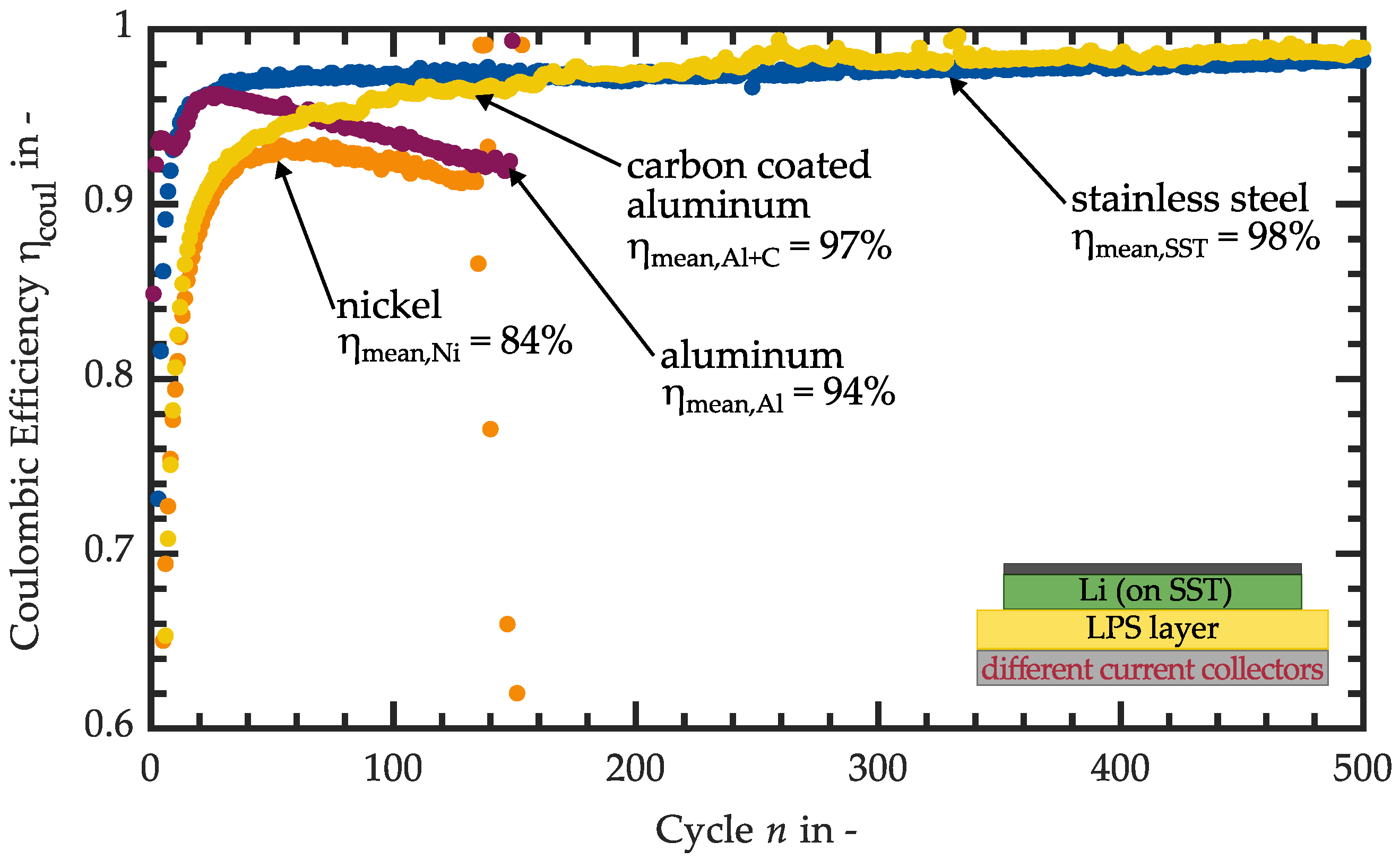
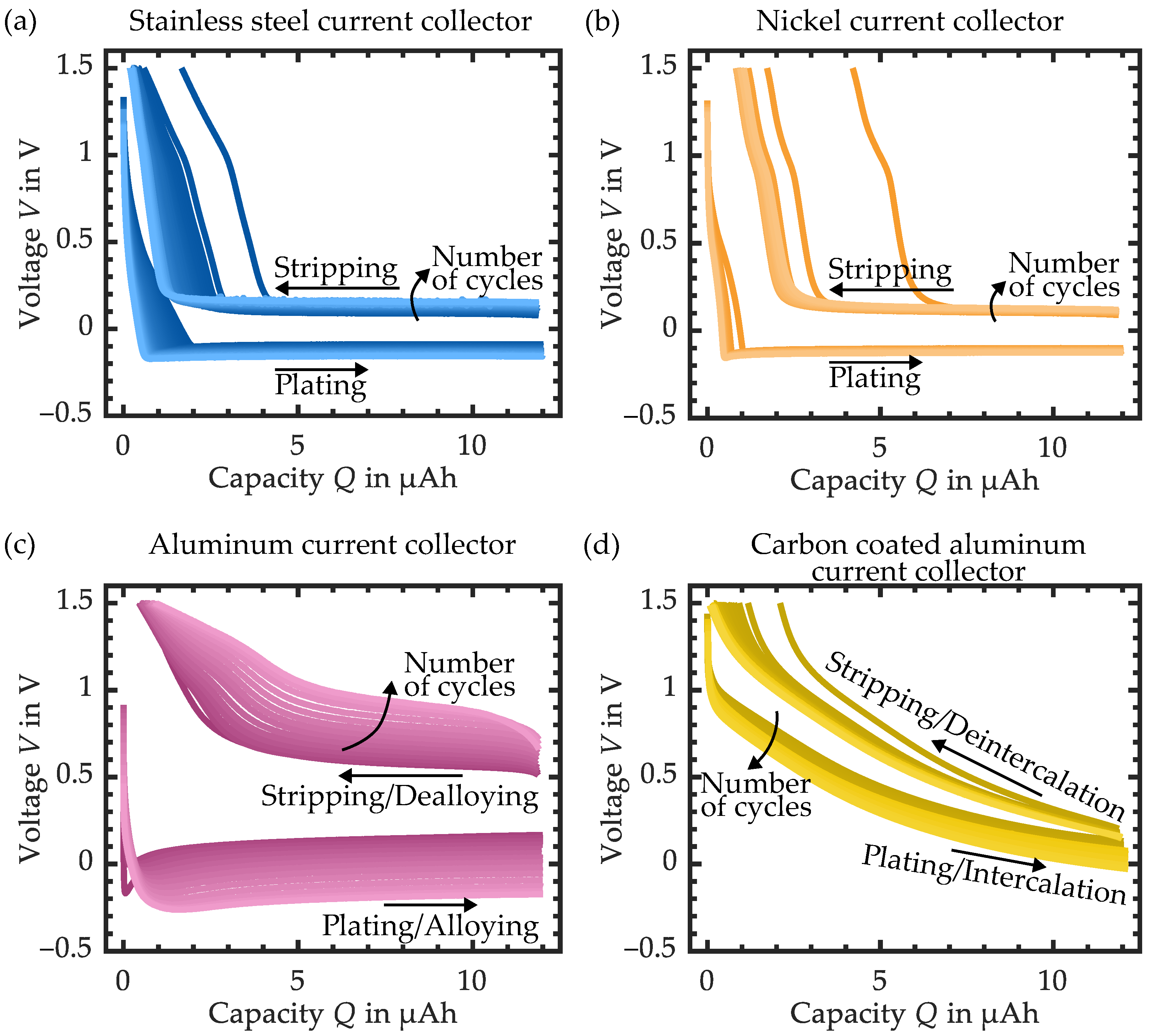


| Cell Setup Current Collector SE Layer | @ | @ | Comment | |||
|---|---|---|---|---|---|---|
| Stainless steel LPS | 98% | 98.8% @ n = 1045 | 1095 | mV | No alloy forming with Li or reaction with SEI components | |
| Nickel LPS | 84% | 93.3% @ n = 71 | 136 | mV | No alloy forming with Li, suspected reaction of SEI components with the nickel current collector and decomposition of the latter | |
| Aluminum LPS | 94% | 95% @ n = 25 | 149 | mV | Alloy forming with Li, suspected reaction of SEI components with the Al current collector decomposition of the latter | |
| Carbon-coated aluminum LPS | 97% | 99.3% @ n = 259 | 725 | mV | Intercalation observed | |
| Stainless steel LPS + [Li(G3)]TFSI | 85% | 94.8% @ n = 169 | 169 | mV | No alloy forming with Li, slow formation of SEI suspected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreher, T.; Heim, F.; Pross-Brakhage, J.; Hemmerling, J.; Birke, K.P. Comparison of Different Current Collector Materials for In Situ Lithium Deposition with Slurry-Based Solid Electrolyte Layers. Batteries 2023, 9, 412. https://doi.org/10.3390/batteries9080412
Kreher T, Heim F, Pross-Brakhage J, Hemmerling J, Birke KP. Comparison of Different Current Collector Materials for In Situ Lithium Deposition with Slurry-Based Solid Electrolyte Layers. Batteries. 2023; 9(8):412. https://doi.org/10.3390/batteries9080412
Chicago/Turabian StyleKreher, Tina, Fabian Heim, Julia Pross-Brakhage, Jessica Hemmerling, and Kai Peter Birke. 2023. "Comparison of Different Current Collector Materials for In Situ Lithium Deposition with Slurry-Based Solid Electrolyte Layers" Batteries 9, no. 8: 412. https://doi.org/10.3390/batteries9080412
APA StyleKreher, T., Heim, F., Pross-Brakhage, J., Hemmerling, J., & Birke, K. P. (2023). Comparison of Different Current Collector Materials for In Situ Lithium Deposition with Slurry-Based Solid Electrolyte Layers. Batteries, 9(8), 412. https://doi.org/10.3390/batteries9080412







