Research Progress in Thermal Runaway Vent Gas Characteristics of Li-Ion Battery
Abstract
1. Introduction
2. Li-Ion Battery Thermal Runaway
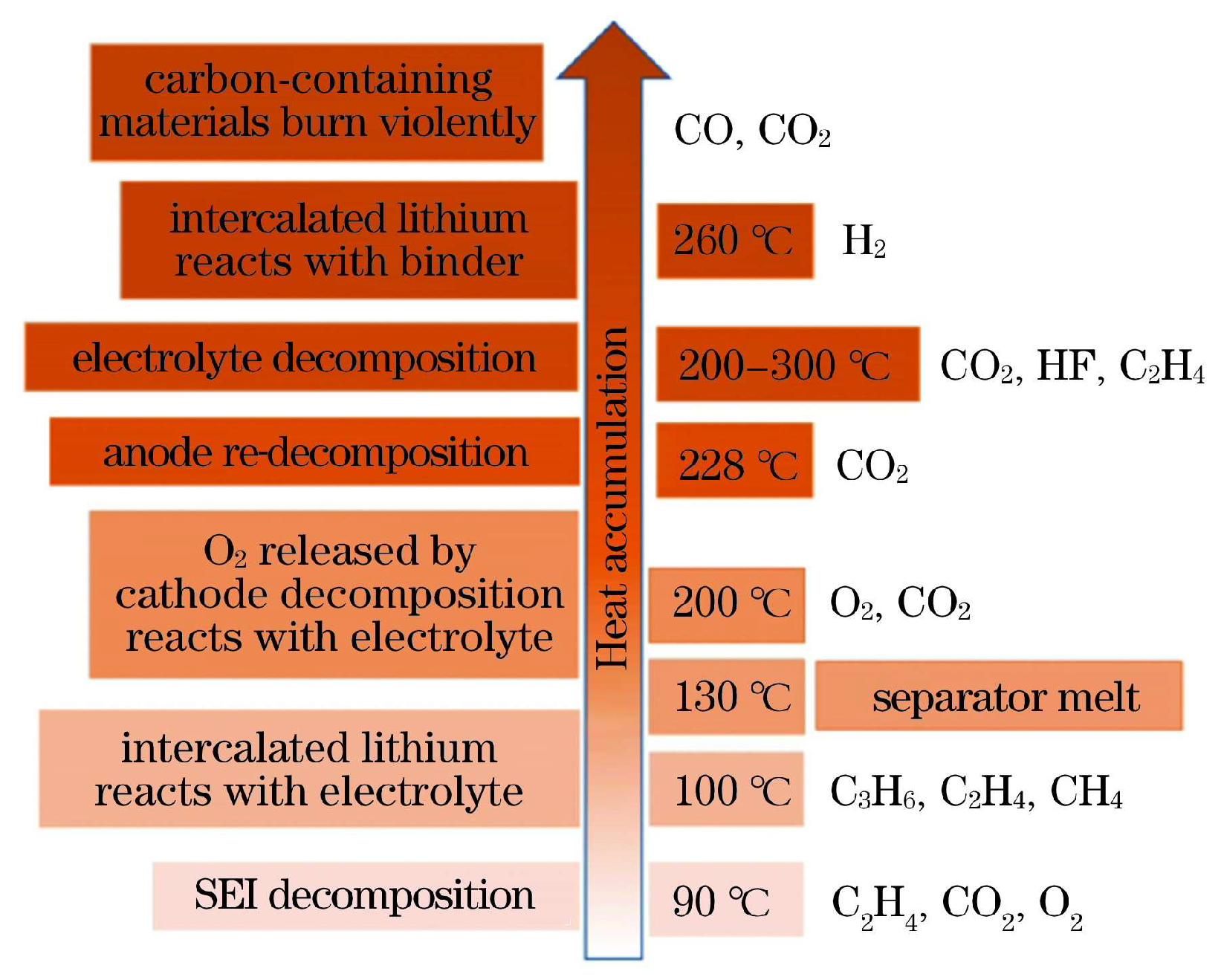
2.1. Li-Ion Battery Thermal Runaway Mechanism
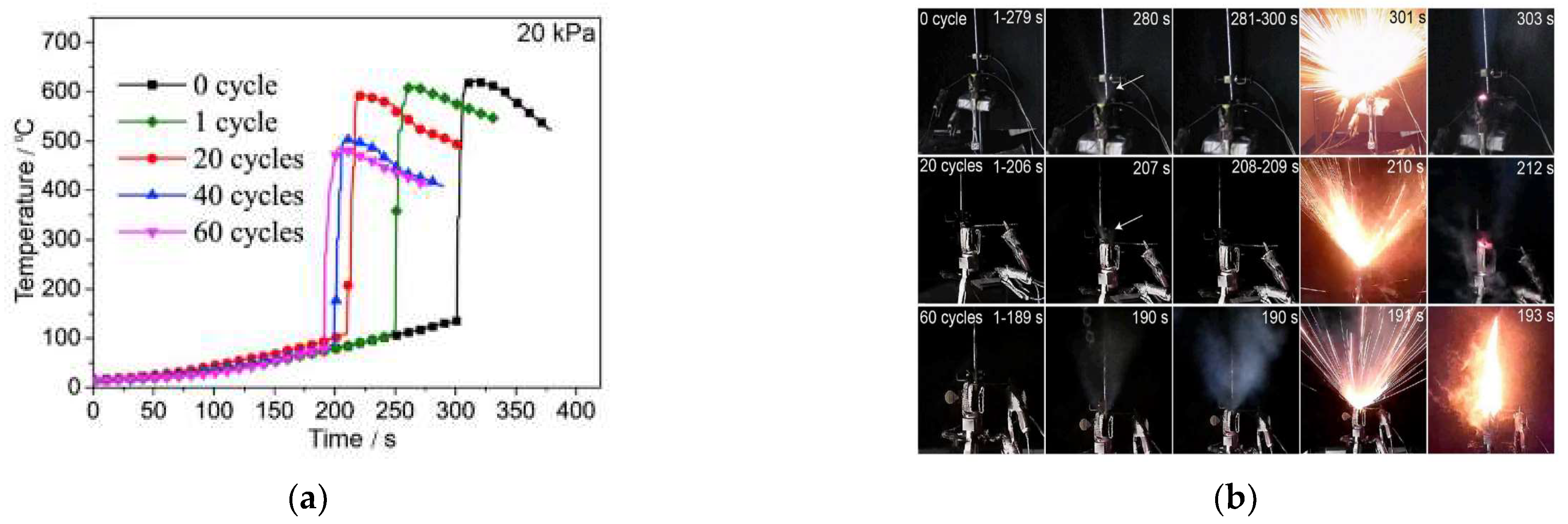
2.2. Aging Li-Ion Battery Thermal Runaway
3. Li-Ion Battery Thermal Runaway Gas Production
3.1. Gas-Production Mechanism and Composition and Content
3.2. Influencing Factors of Gas Production
3.2.1. Electrode Material and Electrolyte
3.2.2. The State of Charge
3.2.3. Battery Aging
3.3. Hazard Characteristics of Gas Production
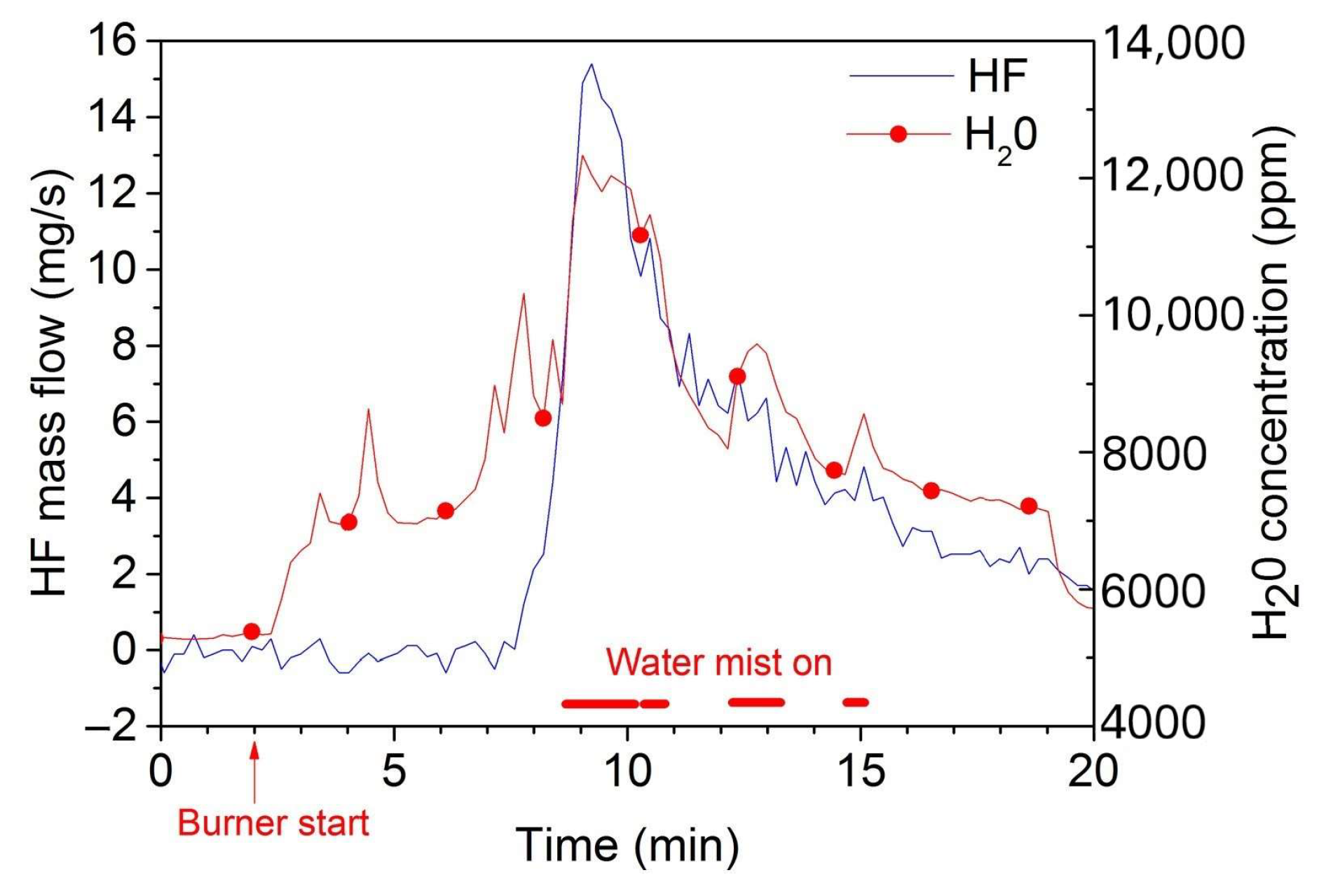
3.3.1. Gas Toxicity Hazards
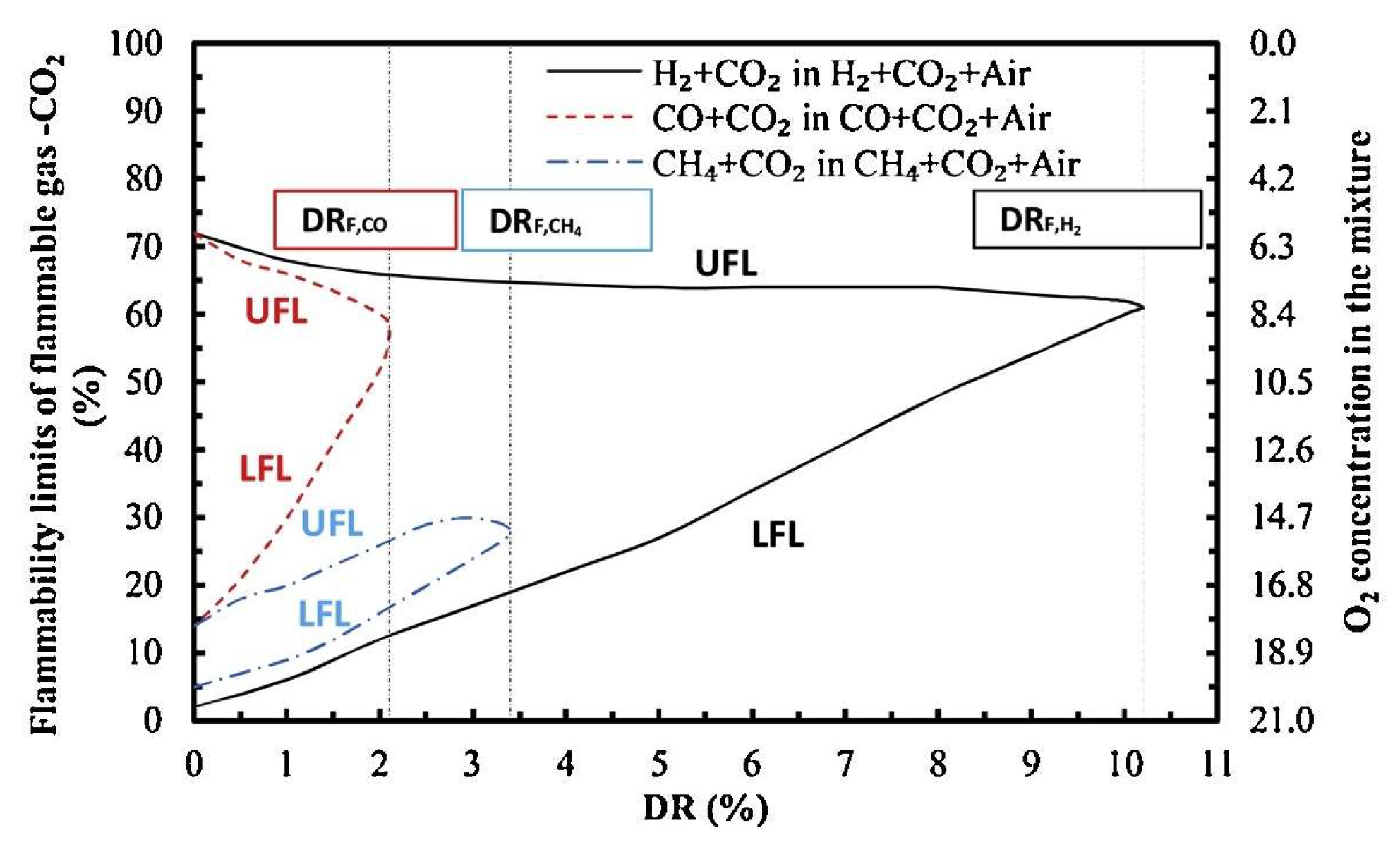
3.3.2. Gas Explosive Hazards
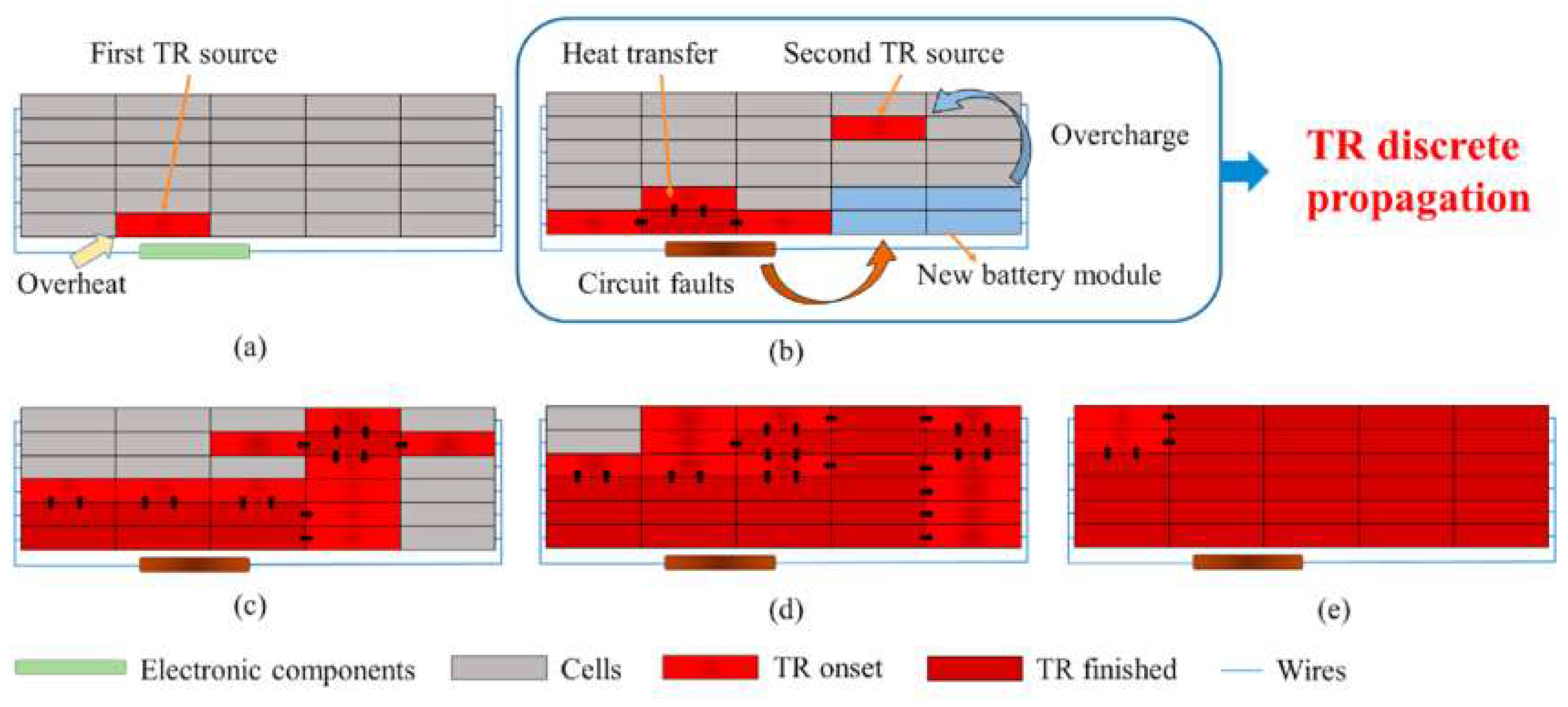
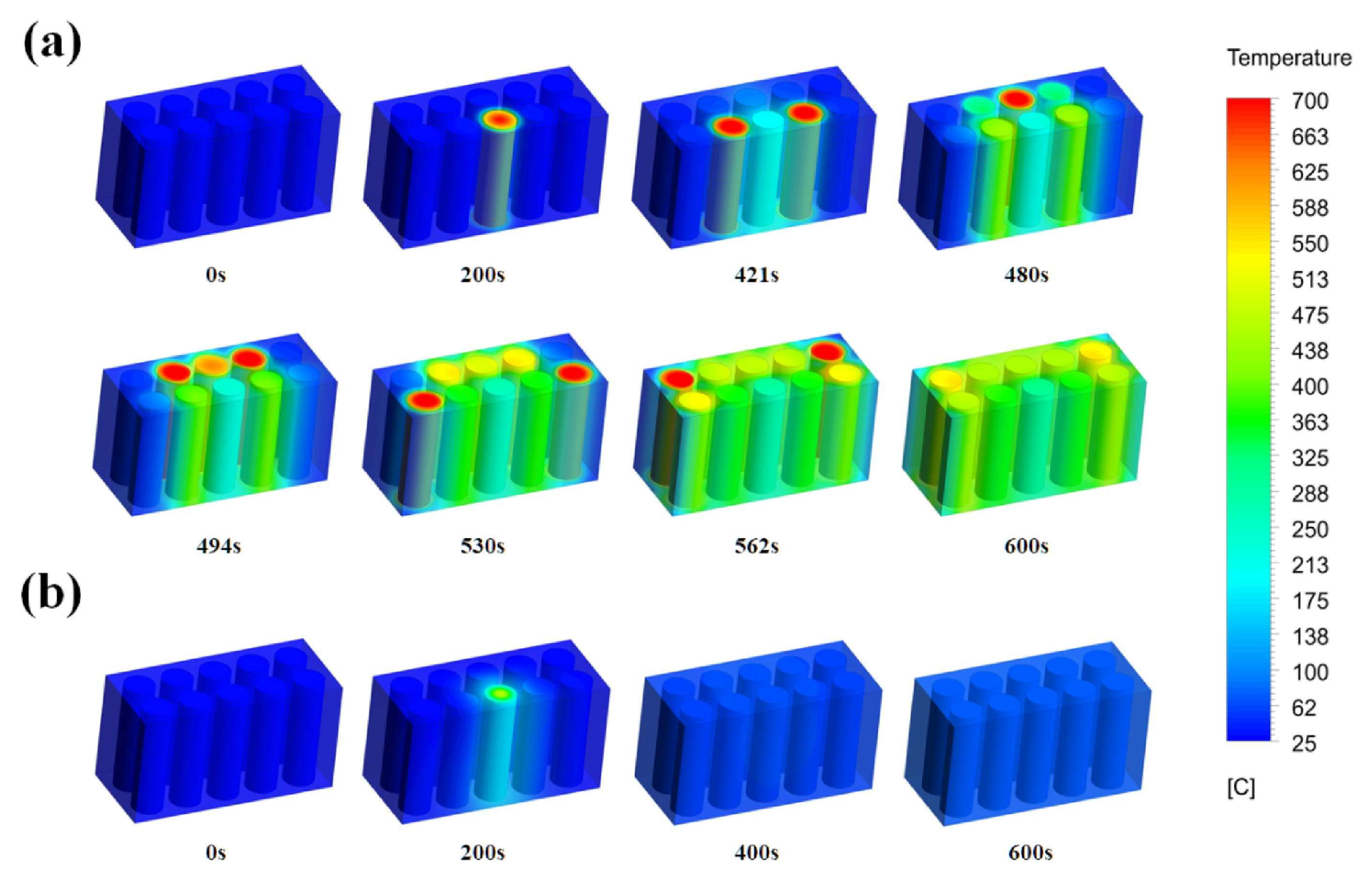
4. Fire Separation
4.1. Insulation Technology
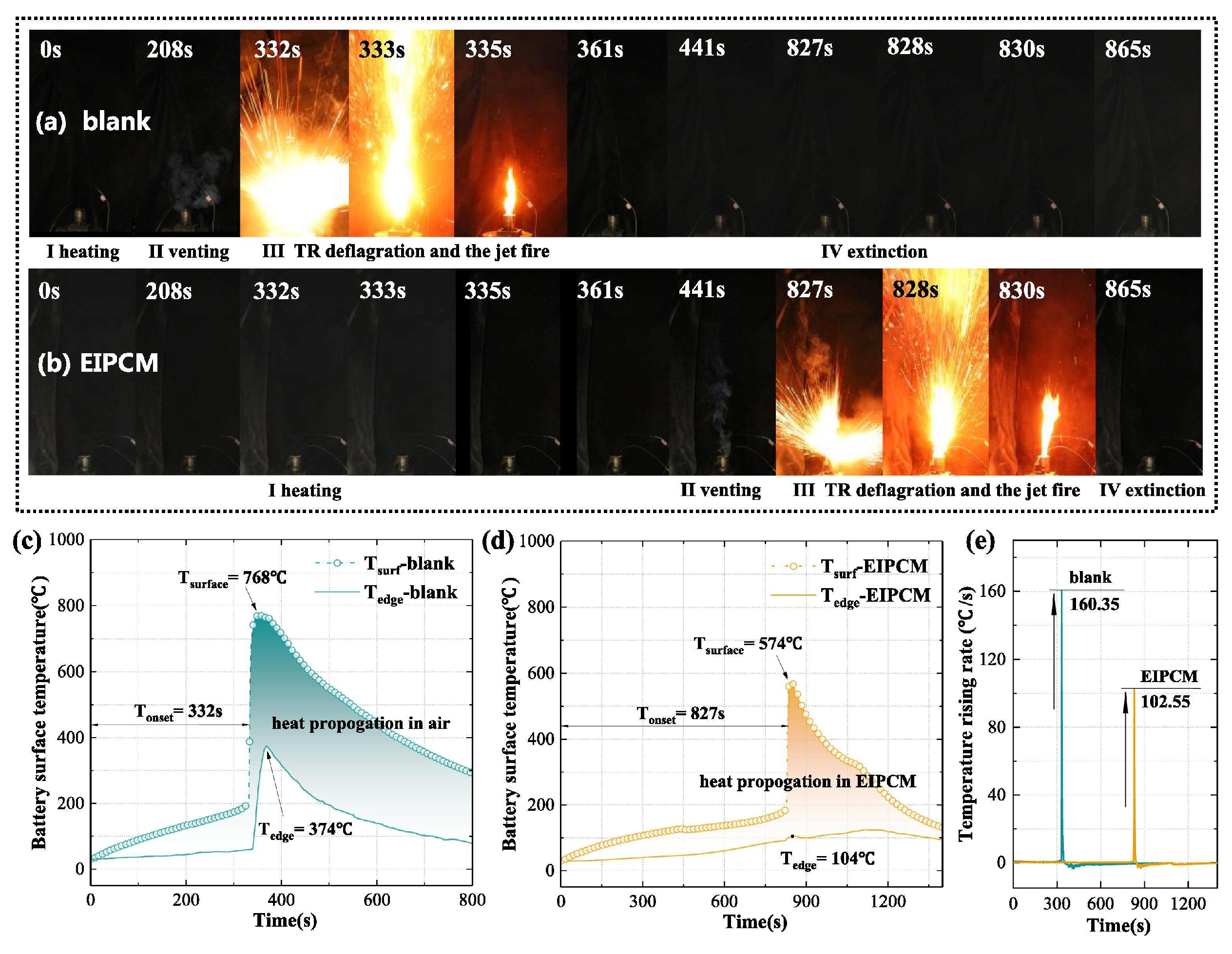
4.2. Phase-Change Material
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Huang, L.; Liu, L.; Lu, L.; Feng, X.; Han, X.; Li, W.; Zhang, M.; Li, D.; Liu, X.; Sauer, D.U.; et al. A review of the internal short circuit mechanism in lithium-ion batteries: Inducement, detection and prevention. Int. J. Energy Res. 2021, 45, 15797–15831. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, S.; Li, K.; Zhang, G.; Habetler, T.G. A survey of methods for monitoring and detecting thermal runaway of lithium-ion batteries. J. Power Sources 2019, 436, 226879. [Google Scholar] [CrossRef]
- Mallick, S.; Gayen, D. Thermal behaviour and thermal runaway propagation in lithium-ion battery systems A critical review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Tran, M.-K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A Review of Lithium-Ion Battery Thermal Runaway Modeling and Diagnosis Approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Sun, F.; Wang, Z. An Overview on Thermal Safety Issues of Lithium-ion Batteries for Electric Vehicle Application. IEEE Access 2018, 6, 23848–23863. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, J. Research progress of water mist fire extinguishing technology and its application in battery fires. Process Saf. Environ. Prot. 2021, 149, 559–574. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.; Xing, X.; Wang, Z.; Zheng, J. Review of Research on Flue Gas Components Produced by Thermal Runawayof Lithium-ion Batteries. Mod. Chem. Res. 2021, 20–21. [Google Scholar]
- Cui, X.; Cong, X.; Zhao, L. Research progress in thermalrunaway gases and explosion hazards of Li-ion battery. Battery Bimon. 2021, 51, 407–411. [Google Scholar]
- Li, H.; Tang, X.; Shao, D.; Liang, J. Research progress in thermal runaway gas of Li-ion battery. Battery Bimon. 2023, 53, 98–102. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Han, X.; Lu, L.; Ouyang, M. Recent Progress on Evolution of Safety Performance of Lithium-ion Battery during Aging Process. Energy Storage Sci. Technol. 2018, 7, 10. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, M.; Huang, Q.; Weng, J.; Wang, Z.; Wang, J. A Review on the Thermal Hazards of the Lithium-Ion Battery and the Corresponding Countermeasures. Appl. Sci. 2019, 9, 2483. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Jia, Y.; Uddin, M.; Li, Y.; Xu, J. Thermal runaway propagation behavior within 18650 lithium-ion battery packs: A modeling study. J. Energy Storage 2020, 31, 101668. [Google Scholar] [CrossRef]
- Sharma, N.; Peterson, V.K. Overcharging a lithium-ion battery: Effect on the LixC 6 negative electrode determined by in situ neutron diffraction. J. Power Sources 2013, 244, 695–701. [Google Scholar] [CrossRef]
- Yi, L.; Xu, H. Research in light transmission characteristics of 1-dimensional photonic crystal. Opt.-Int. J. Light Electron Opt. 2012, 123, 314–318. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Gu, Z.; Zhu, Y.; Fu, Y.; Tao, Z.; Zhang, Q. Analysis of the thermal stability of a battery under overcharge and over-discharge. J. Phys. Conf. Ser. 2021, 2009, 12022. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, P.; Ping, P.; Du, Y.; Li, K.; Sun, J. Combustion behavior of lithium iron phosphate battery induced by external heat radiation. J. Loss Prev. Process Ind. 2017, 49, 961–969. [Google Scholar] [CrossRef]
- Peng, P.; Jiang, F. Thermal safety of lithium-ion batteries with various cathode materials: A numerical study. Int. J. Heat Mass Transf. 2016, 103, 1008–1016. [Google Scholar] [CrossRef]
- Yi, W.; Saurabh, S.; Yinjiao, X.; Youren, W.; Chuan, L.; Winco, Y.; Michael, P. Analysis of Manufacturing-Induced Defects and Structural Deformations in Lithium-Ion Batteries Using Computed Tomography. Energies 2018, 11, 925. [Google Scholar]
- Röder, P.; Stiaszny, B.; Ziegler, J.C.; Baba, N.; Lagaly, P.; Wiemhöfer, H.-D. The impact of calendar aging on the thermal stability of a LiMn2O4–Li(Ni1/3Mn1/3Co1/3)O2/graphite lithium-ion cell. J. Power Sources 2014, 268, 315–325. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A comparative study of commercial lithium ion battery cycle life in electrical vehicle: Aging mechanism identification. J. Power Sources 2014, 251, 38–54. [Google Scholar] [CrossRef]
- Huang, H. Study on Safety of Lithium-Ion Batteries. Ph.D. Thesis, Graduate School of Chinese Academy of Sciences, Beijing, China, 2005. [Google Scholar]
- Huang, H.; Xie, J. Over-charge performances of lithium ion batteries after different cycles. Chin. J. Power Sources 2005, 4–7, 633. [Google Scholar]
- Zhang, L. Study on Influencing Factors of Safety for Lithium Battery. Master’s Thesis, Yanshan University, Qinghuangdao, China, 2012. [Google Scholar]
- Xu, Z.; Li, X.; Jia, L.; Chen, B.; Dai, Z.; Zheng, L. Effect of overcharge cycle on capacity attenuation and safety of lithium-ion batteries. Energy Storage Sci. Technol. 2022, 11, 3978–3986. [Google Scholar]
- Friesen, A.; Horsthemke, F.; Monnighoff, X.; Brunklaus, G.; Krafft, R.; Borner, M.; Risthaus, T.; Winter, M.; Schappacher, F.M. Impact of cycling at low temperatures on the safety behavior of 18650-type lithium ion cells: Combined study of mechanical and thermal abuse testing accompanied by post-mortem analysis. J. Power Sources 2016, 334, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, Y. Research on the Toxicity of Gases of Thermal Runaway Released from Ternary Lithium-ion Batteries Featuring Cyclic Aging Process. J. Beijing Univ. Aeronaut. Astronaut. 2022, 1–10. [Google Scholar]
- Larsson, F.; Bertilsson, S.; Furlani, M.; Albinsson, I.; Mellander, B.-E. Gas explosions and thermal runaways during external heating abuse of commercial lithium-ion graphite-LiCoO2 cells at different levels of ageing. J. Power Sources 2018, 373, 220–231. [Google Scholar] [CrossRef]
- Boerner, M.; Friesen, A.; Gruetzke, M.; Stenzel, Y.P.; Brunklaus, G.; Haetge, J.; Nowak, S.; Schappacher, F.M.; Winter, M. Correlation of aging and thermal stability of commercial 18650-type lithium ion batteries. J. Power Sources 2017, 342, 382–392. [Google Scholar] [CrossRef]
- Xie, S.; Ren, L.; Yang, X.; Wang, H.; Sun, Q.; Chen, X.; He, Y. Influence of cycling aging and ambient pressure on the thermal safety features of lithium-ion battery. J. Power Sources 2020, 448, 227425. [Google Scholar] [CrossRef]
- Zhang, J.; Su, L.; Li, Z.; Sun, Y.; Wu, N. The Evolution of Lithium-Ion Cell Thermal Safety with Aging Examined in a Battery Testing Calorimeter. Batteries 2016, 2, 12. [Google Scholar] [CrossRef]
- Chen, D.; Hao, C.; Liu, T.; Han, Z.; Zhang, W. Raman Spectrum Analysis Method of Thermal Runaway Gas from Lithium-ion Batteries. Zhongguo Jiguang/Chin. J. Lasers 2022, 49, 2311001. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Guo, C.; Zhao, H. Study on thermal runaway gas release characteristics of lithium-ion battery under different state of charge. Technol. Innov. Appl. 2020, 28–31. [Google Scholar]
- Somandepalli, V.; Marr, K.; Horn, Q. Quantification of Combustion Hazards of Thermal Runaway Failures in Lithium-Ion Batteries. SAE Int. J. Altern. Powertrains 2014, 3, 98–104. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes—Impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Ribière, P.; Grugeon, S.; Morcrette, M.; Boyanov, S.; Laruelle, S.; Marlair, G. Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry. Energy Environ. Sci. 2012, 5, 5271–5280. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Wang, Q.; Duan, Q.; Li, Y.; Li, L.; Wang, Q. Experimental investigation on thermal runaway propagation of large format lithium ion battery modules with two cathodes. Int. J. Heat Mass Transf. 2021, 172, 121077. [Google Scholar] [CrossRef]
- Fleischhammer, M.; Waldmann, T.; Bisle, G.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries. J. Power Sources 2015, 274, 432–439. [Google Scholar] [CrossRef]
- Chen, F. Study on the Heat Generation Characteristics of High-Specific-Energy Lithium Ion Batteries with NCA Cathode during Aging. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Sun, J.; Li, J.; Dang, S.; Tang, N.; Zhou, T.; Li, J.; Wei, S.; Yang, K.; Fei, G. Research of toxic productions from thermal runaway processes of Li-ion battery and materials. Energy Storage Sci. Technol. 2015, 4, 7. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Lorén, A.; Mellander, B.-E. Characteristics of lithium-ion batteries during fire tests. J. Power Sources 2014, 271, 414–420. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.E. Toxic fluoride gas emissions from lithium-ion battery fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, A.; Streich, D.; He, M.; Mendez, M.; Chesneau, F.F.; Novak, P.; Berg, E.J. Decomposition of LiPF6 in high energy lithium-ion batteries studied with online electrochemical mass spectrometry. J. Electrochem. Soc. 2016, 163, A1095–A1100. [Google Scholar] [CrossRef]
- Lecocq, A.; Eshetu, G.G.; Grugeon, S.; Martin, N.; Laruelle, S.; Marlair, G. Scenario-based prediction of Li-ion batteries fire-induced toxicity. J. Power Sources 2016, 316, 197–206. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, C.; Yan, S.; Zhou, P.; Qian, X.; Yuan, M.; Liu, K. A review of fire-extinguishing agent on suppressing lithium-ion batteries fire. J. Energy Chem. 2021, 62, 262–280. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, Q.; Meng, X.; Jin, K.; Xu, J.; Sun, J.; Wang, Q. Experimental investigation on intermittent spray cooling and toxic hazards of lithium-ion battery thermal runaway. Energy Convers. Manag. 2022, 252, 115091. [Google Scholar] [CrossRef]
- ISO 13571; Life-Threatening Components of Fire-Guidelines for the Estimation of Time to Compromised Tenability in Fires. ISO: Geneva, Switzerland, 2012.
- Tong, Z.; Yin, Y.; Huang, Q.; Lin, F. Review on quantitative evaluation methods of fire smoke toxicity. J. Saf. Environ. 2005, 05, 101–105. [Google Scholar]
- Zhang, Q.; Qu, Y.; Liu, T. A methodology study on risk analysis for thermal runaway gas toxicity of lithium batteries. J. Beijing Univ. Aeronaut. Astronaut. 2022, 1–10. [Google Scholar]
- Peng, Y.; Yang, L.; Ju, X.; Liao, B.; Ye, K.; Li, L.; Cao, B.; Ni, Y. A comprehensive investigation on the thermal and toxic hazards of large format lithium-ion batteries with LiFePO4 cathode. J. Hazard Mater. 2020, 381, 120916. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, J.; Cong, B.; Han, X.; Yin, S. Characterization and assessment of fire evolution process of electric vehicles placed in parallel. Process Saf. Environ. Prot. 2022, 166, 524–534. [Google Scholar] [CrossRef]
- Cui, Y.; Cong, B.; Liu, J.; Qiu, M.; Han, X. Characteristics and Hazards of Plug-In Hybrid Electric Vehicle Fires Caused by Lithium-Ion Battery Packs with Thermal Runaway. Front. Energy Res. 2022, 10, 878035. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J.; Roth, E.P.; Langendorf, J. Studies on the thermal breakdown of common Li-ion battery electrolyte components. J. Electrochem. Soc. 2015, 162, A2131–A2135. [Google Scholar] [CrossRef]
- Tian, G.; Yu, C.; Li, X. Study on Calculation Method of Gas Explosion Limits. Gas Heat 2006, 29–33. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Zhang, Y.; Ouyang, M. Flammability characteristics of the battery vent gas: A case of NCA and LFP lithium-ion batteries during external heating abuse. J. Energy Storage 2019, 24, 100775. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Q. Determination on explosion limit of pyrolysis gas released by lithium-ion battery and its risk analysis. J. Saf. Sci. Technol. 2016, 12, 4. [Google Scholar]
- Zhang, Q.; Niu, J.; Zhao, Z.; Wang, Q. Research on the effect of thermal runaway gas components and explosion limits of lithium-ion batteries under different charge states. J. Energy Storage 2022, 45, 103759. [Google Scholar] [CrossRef]
- Karp, M.E. Flammability Limits of Lithium-Ion Battery Thermal Runaway Vent Gas in Air and the Inerting Effects of Halon 1301. Master’s Thesis, Graduate School-New Brunswick, New Brunswick, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Wang, J.; Tong, X.; Yan, W. Lower explosion limit of the vented gases from Li-ion batteries thermal runaway in high temperature condition. J. Loss Prev. Process Ind. 2019, 63, 103992. [Google Scholar] [CrossRef]
- Ma, B.; Liu, J.; Yu, R. Study on the Flammability Limits of Lithium-Ion Battery Vent Gas under Different Initial Conditions. ACS Omega 2020, 5, 28096–28107. [Google Scholar] [CrossRef]
- Baird, A.R.; Archibald, E.J.; Marr, K.C.; Ezekoye, O.A. Explosion hazards from lithium-ion battery vent gas. J. Power Sources 2020, 446, 227257. [Google Scholar] [CrossRef]
- Niu, Z.; Jiang, X.; Xie, B.; Jin, Y. Study on Simulation and Safety Protection of Electric Vehicle Overcharge and Explosion Accident. Diangong Jishu Xuebao/Trans. China Electrotech. Soc. 2022, 37, 36–47, 57. [Google Scholar] [CrossRef]
- Kan, Q.; Chen, M.; Ren, C. Experimental study on thermal runaway gas explosion characteristics of lithium ion battery. Fire Sci. Technol. 2021, 40, 959–962. [Google Scholar]
- Cui, Y.; Liu, J.; Han, X.; Sun, S.; Cong, B. Full-scale experimental study on suppressing lithium-ion battery pack fires from electric vehicles. Fire Saf. J. 2022, 129, 103562. [Google Scholar] [CrossRef]
- Liu, Z. Research on Thermal Management System of Power Battery Coupled with Composite Phase Change Material and Liquid Cooling. Ph.D. Thesis, Nanchang University, Nanchang, China, 2022. [Google Scholar]
- Zhang, W.; Liang, Z.; Yin, X.; Ling, G. Avoiding thermal runaway propagation of lithium-ion battery modules by using hybrid phase change material and liquid cooling. Appl. Therm. Eng. 2021, 184, 116380. [Google Scholar] [CrossRef]
- Berdichevsky, E.M.; Cole, P.D.; Hebert, A.J.; Hermann, W.A.; Kelty, K.R.; Kohn, S.I.; Lyons, D.F.; Straubel, J.B.; Mendez, N.J. Mitigation of Propagation of Thermal Runaway in a Multi-Cell Battery Pack. U.S. Patent 7433794, 7 October 2008. [Google Scholar]
- Mehta, V.H.; Prilutsky, A.; Hermann, W.A. Cell Thermal Runaway Propagation Resistance Using an Internal Layer of Intumescent Material. U.S. Patent 20100075221, 24 August 2010. [Google Scholar]
- Lopez, C.F.; Jeevarajan, J.A.; Mukherjee, P.P. Experimental analysis of thermal runaway and propagation in lithium-ion battery modules. J. Electrochem. Soc. 2015, 162, A1905–A1915. [Google Scholar] [CrossRef]
- Chen, C.; Niu, H.; Li, Z.; Li, L.; Mo, S.; Huang, X. Thermal Runaway Propagation Mitigation of Lithium Ion Battery by Epoxy Resin Board. Energy Storage Sci. Technol. 2019, 8, 532–537. [Google Scholar]
- Qin, S. Experimental Study on Thermal Runaway of Potassium Lithium-ion Battery Based on Packaging Materials. Technol. Innov. Appl. 2017, 180–181. [Google Scholar]
- Chang, R. Study on Thermal Runaway Propagation Prevention of lithium-Ion Battery Based on Thermal Insulation Layer. Master’s Thesis, Shandong University of Technology, Zibo, China, 2022. [Google Scholar]
- Zhang, Z.; Yang, X.; Xu, C.; Wang, M. Research Progress of Aerogel Insulation Materials for Lithium-ion Power Batteries. China Build. Mater. Sci. Technol. 2023, 32, 62–68. [Google Scholar]
- Hu, Q. Study on Lithium-Ion Batteries Thermal Runaway Propagation Characteristics and Blocking Techniques. Master’s Thesis, China Ship Scientific Research Center, Wuxi, China, 2015. [Google Scholar]
- Shen, X.D.; Duan, Q.; Qin, P.; Wang, Q.; Sun, J. Experimental study on thermal runaway mitigation and heat transfer characteristics of ternary lithium-ion batteries. Energy Storage Sci. Technol. 2023, 12, 1862–1871. [Google Scholar] [CrossRef]
- Ping, P.; Dai, X.; Kong, D.; Zhang, Y.; Zhao, H.; Gao, X.; Gao, W. Experimental study on nano-encapsulated inorganic phase change material for lithium-ion battery thermal management and thermal runaway suppression. Chem. Eng. J. 2023, 463, 142401. [Google Scholar] [CrossRef]
- Wilke, S.; Schweitzer, B.; Khateeb, S.; Al-Hallaj, S. Preventing thermal runaway propagation in lithium ion battery packs using a phase change composite material: An experimental study. J. Power Sources 2017, 340, 51–59. [Google Scholar] [CrossRef]
- Zhi, W.; Jian, W. Investigation of external heating-induced failure propagation behaviors in large-size cell modules with different phase change materials. Energy 2020, 204, 117946. [Google Scholar] [CrossRef]
- Weng, J.; Xiao, C.; Ouyang, D.; Yang, X.; Chen, M.; Zhang, G.; Yuen, R.K.K.; Wang, J. Mitigation effects on thermal runaway propagation of structure-enhanced phase change material modules with flame retardant additives. Energy 2022, 239, 122087. [Google Scholar] [CrossRef]
- Dai, X.; Kong, D.; Du, J.; Zhang, Y.; Ping, P. Investigation on effect of phase change material on the thermal runaway of lithium-ion battery and exploration of flame retardancy improvement. Process Saf. Environ. Prot. 2022, 159, 232–242. [Google Scholar] [CrossRef]
- Talele, V.; Patil, M.S.; Panchal, S.; Fraser, R.; Fowler, M. Battery thermal runaway propagation time delay strategy using phase change material integrated with pyro block lining: Dual functionality battery thermal design. J. Energy Storage 2023, 65, 107253. [Google Scholar] [CrossRef]
- Weng, J.; Ouyang, D.; Yang, X.; Chen, M.; Zhang, G.; Wang, J. Alleviation of thermal runaway propagation in thermal management modules using aerogel felt coupled with flame-retarded phase change material. Energy Convers. Manag. 2019, 200, 112071. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, X.; Li, M.; Cao, B.; Liew, K.M.; Yang, L. Experimentally exploring prevention of thermal runaway propagation of large-format prismatic lithium-ion battery module. Appl. Energy 2022, 327, 120119. [Google Scholar] [CrossRef]
- Galazutdinova, Y.; Ushak, S.; Farid, M.; Al-Hallaj, S.; Grageda, M. Development of the inorganic composite phase change materials for passive thermal management of Li-ion batteries: Application. J. Power Sources 2021, 491, 229624. [Google Scholar] [CrossRef]
- Cao, J.; Ling, Z.; Lin, S.; He, Y.; Fang, X.; Zhang, Z. Thermochemical heat storage system for preventing battery thermal runaway propagation using sodium acetate trihydrate/expanded graphite. Chem. Eng. J. 2022, 433, 133536. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Zhou, T.; Yang, K.; Wei, S.; Tang, N.; Dang, N.; Li, H.; Qiu, X.; Chen, L. Toxicity, a serious concern of thermal runaway from commercial Li-ion battery. Nano Energy 2016, 27, 313–319. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, M.; Liu, J.; Cong, B.; Cui, Y. Research Progress in Thermal Runaway Vent Gas Characteristics of Li-Ion Battery. Batteries 2023, 9, 411. https://doi.org/10.3390/batteries9080411
Qiu M, Liu J, Cong B, Cui Y. Research Progress in Thermal Runaway Vent Gas Characteristics of Li-Ion Battery. Batteries. 2023; 9(8):411. https://doi.org/10.3390/batteries9080411
Chicago/Turabian StyleQiu, Mingming, Jianghong Liu, Beihua Cong, and Yan Cui. 2023. "Research Progress in Thermal Runaway Vent Gas Characteristics of Li-Ion Battery" Batteries 9, no. 8: 411. https://doi.org/10.3390/batteries9080411
APA StyleQiu, M., Liu, J., Cong, B., & Cui, Y. (2023). Research Progress in Thermal Runaway Vent Gas Characteristics of Li-Ion Battery. Batteries, 9(8), 411. https://doi.org/10.3390/batteries9080411






