Proof of Concept: The GREENcell—A Lithium Cell with a F-, Ni- and Co-Free Cathode and Stabilized In-Situ LiAl Alloy Anode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.1.1. Cathode Preparation
2.1.2. Anode Preparation
- Mechanical pretreatment: Pressed-in stainless steel mesh;For mechanical pretreatment, a stainless-steel mesh (1.4301, mesh size: 0.063 mm, wire diameter: 0.028–0.04 mm, thickness: 20 µm) was pressed into the Al foil with a pneumatic lever press (Quantum Design GmbH, Darmstadt, Germany) at ~2750 kg·cm−2 at room temperature. The pressing time was 15 s. In the following sections, the pressed-in stainless steel mesh will be abbreviated to the term “mesh”.
- Chemical pretreatment: SEI-precursor coating;For chemical pretreatment, the Al foil was coated with an oxide-based chemical inert SEI-precursor coating. As oxide particles, Al2O3 (ρ = 3.95 g·cm−3, Ultimate 1500, Almatis GmbH, Ludwigshafen, Germany) was used for the Al-household foil. Due to adhesion problems, Al silicate (K₂Al₆Si₆O₂₀(OH)₄, ρ = 2.82 g·cm−3, Irotec 8800, flakes, Merck KGaA, Darmstadt, Germany) was used for the Al-1050A foil in the same volumetric concentration. To start, 96 wt% oxide particles were mixed with 4 wt% PIB using the 5 wt% PIB (80 wt% B15N, 20 wt% N80) solution in toluene described above. Then, Disperbyk-161 (BYK-Chemie GmbH, Wesel, Germany) was added as a dispersing additive with 12 wt%, based on a solid fraction. Finally, toluene was added to obtain a solid content of 53–56 wt%. Afterwards, the slurry was coated on the Al foil with a doctor blade (gap size: 300 µm) and dried overnight at room temperature. In the following sections, the SEI-precursor coating will be abbreviated to the term “coating”.
2.1.3. Electrolyte Preparation
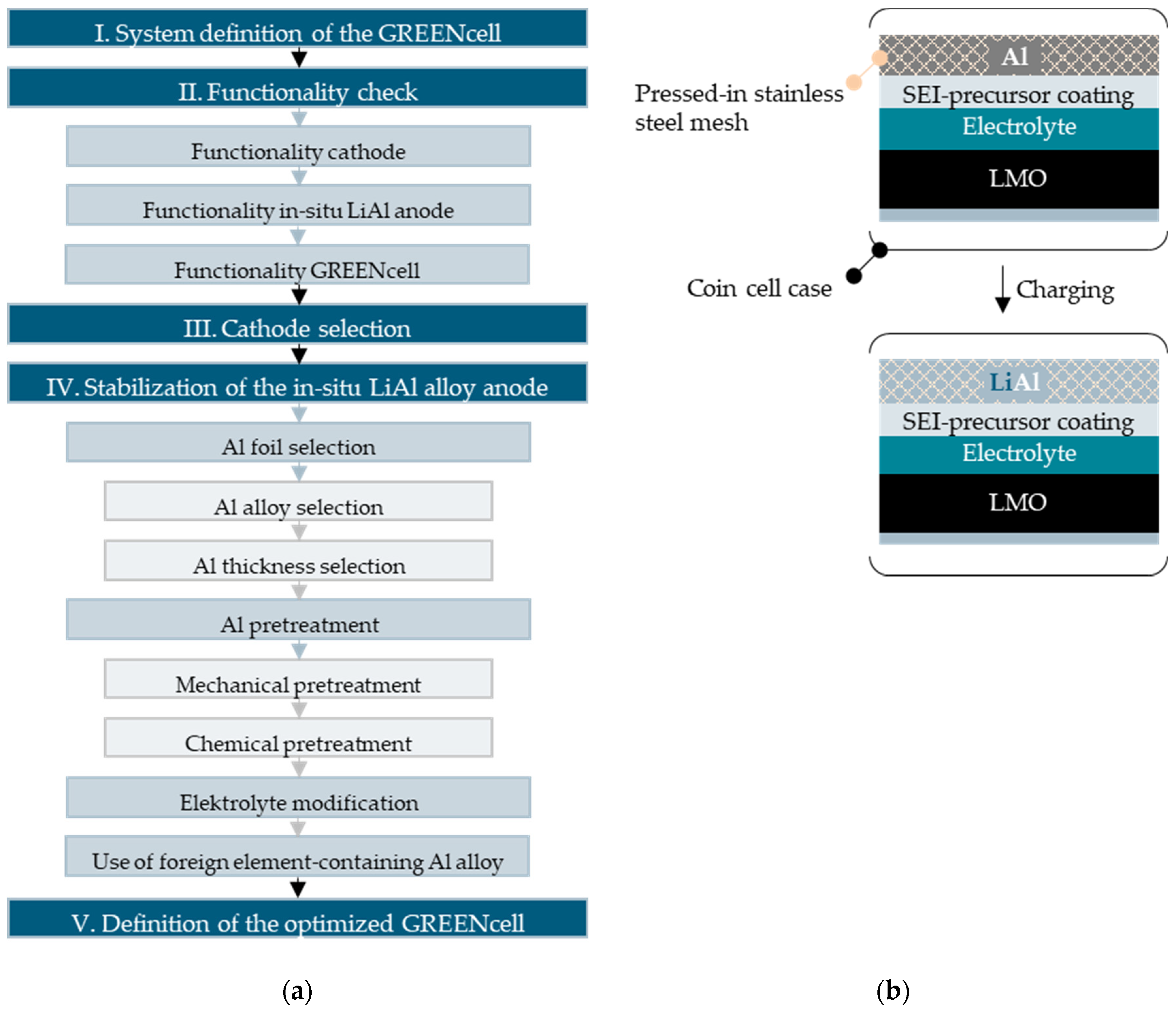
2.2. Iterative GREENcell Development Process
2.3. Al Thickness Calculation
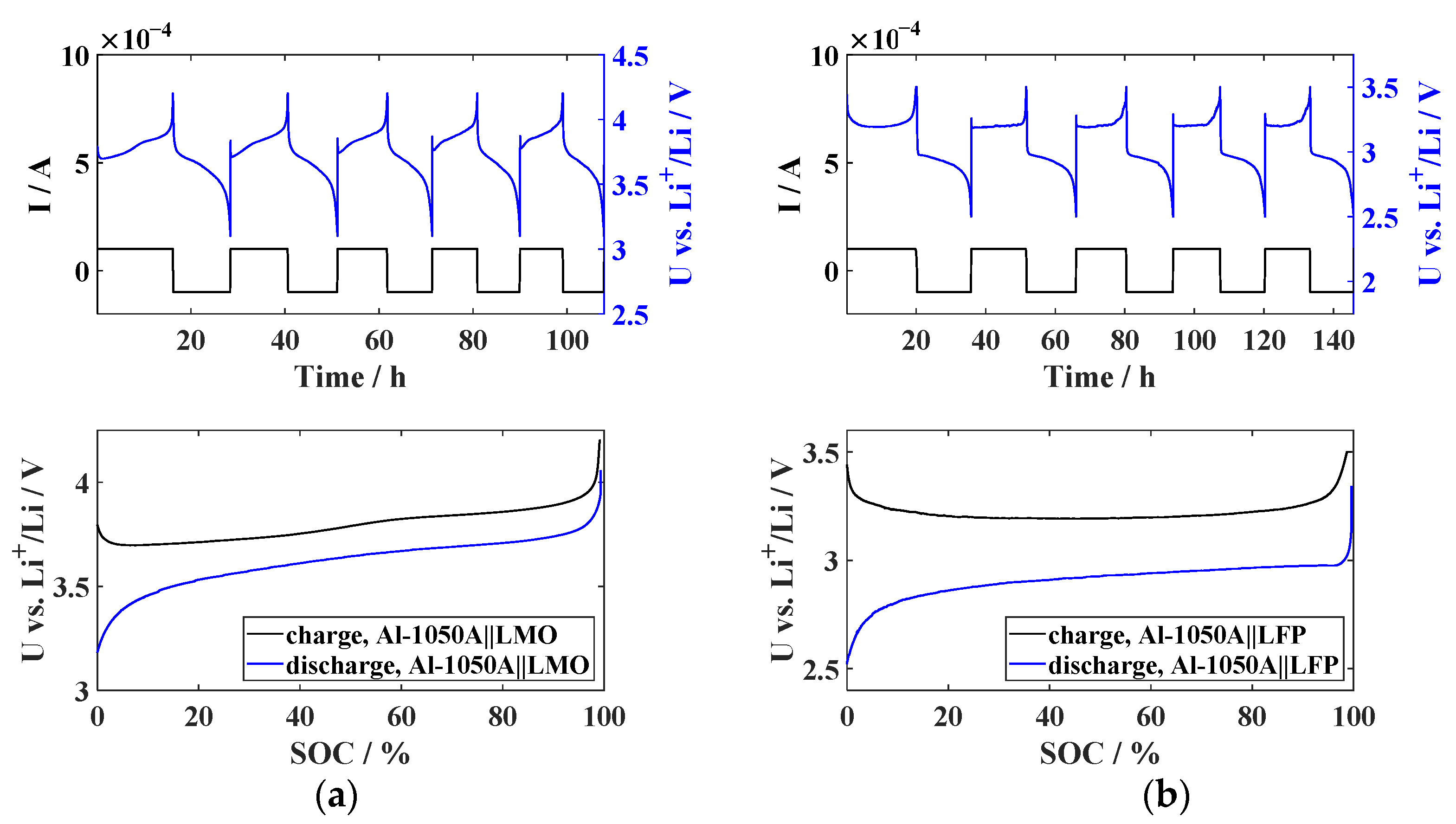
2.4. Electrochemical Characterization
3. Results and Discussion
3.1. Functionality of the GREENcell
3.1.1. Functionality of the GREENcell Cathode Materials
3.1.2. Functionality of the In-Situ LiAl Alloy Anode of the GREENcell
3.2. Cathode Selection
3.3. Methods to Improve the Cycle Stability of the In-Situ LiAl Alloy Anode
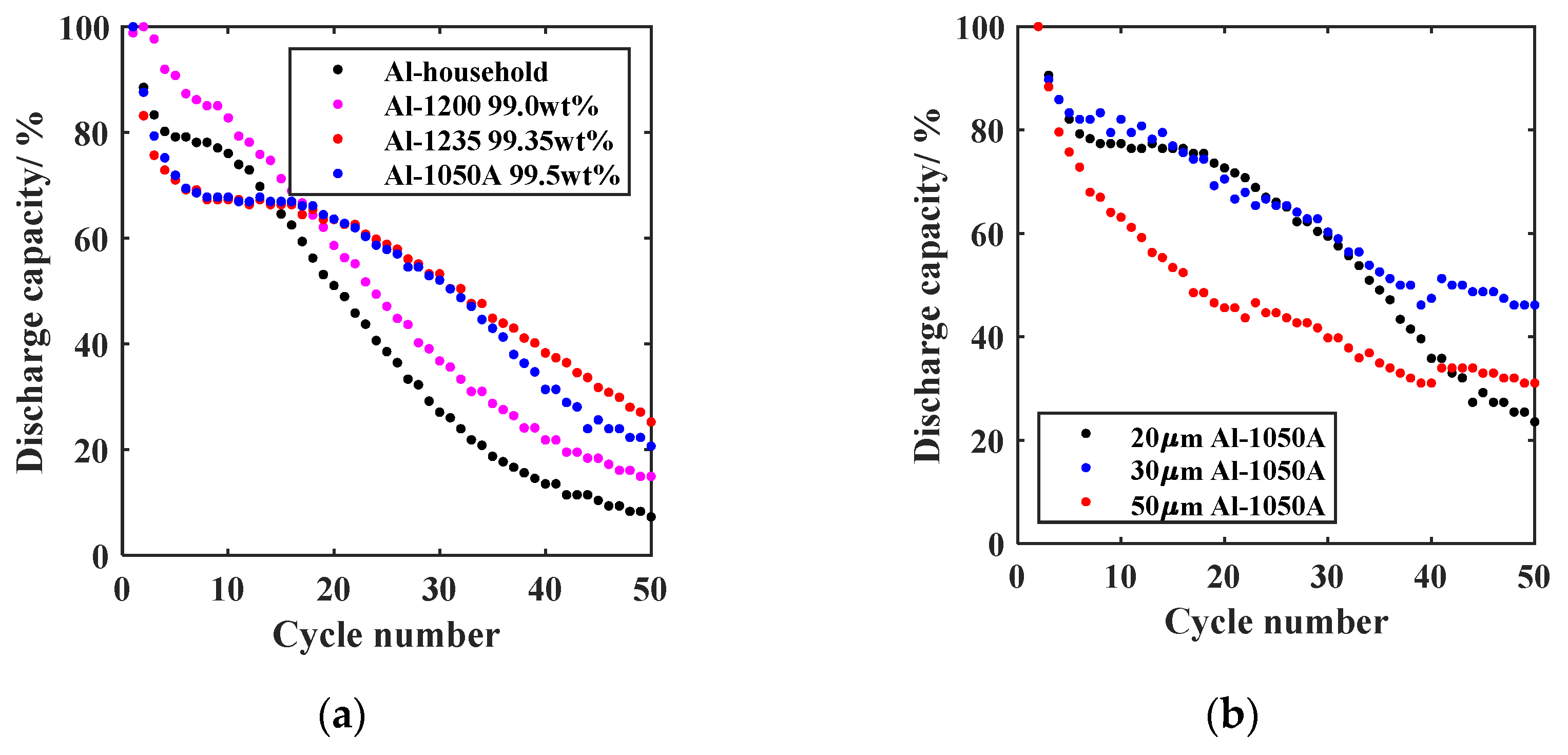
3.3.1. Al Foil Selection
- Al alloy selection;To investigate the purity influence of the Al anode on the cycling stability of the GREENcell, the relative discharge capacity curves of different Al alloys, varying in their weight fraction of Al, are shown in Figure 6a. When comparing the long-term stability, the following order is obtained concerning the capacity retention after 50 cycles: Al-household (7.3%) < Al-1200 (14.9%) < Al-1050A (23.6%) < Al-1235 (25.2%). Up to a purity level of 99.35 wt% Al, the higher Al content appears to affect long-term stability positively. This effect can be attributed to the absence of additional elements, thereby reducing the risk of parasitic side reactions that may lead to the irreversible loss of Li. The curves of Al-1050A and Al-1235 are almost congruent, indicating that purities > 99.35 wt% Al do not cause any additional improvement. Upon evaluating the formation loss during the initial five cycles, an intriguing inverse trend emerges in terms of capacity retention: Al-1235 (71.0%) < Al-1050A (71.9%) < Al-household (79.2%) < Al-1200 (90.8%). Therefore, the additional elements in the impure Al foil appear to affect the initial capacity loss positively. However, further investigations are required to validate and provide comprehensive evidence for this observation. After considering the most promising long-term stability, the decision was made to proceed with Al-1050A Al foil to perform further optimization approaches.
- Al thickness selection;Building on the theoretical considerations in Section 2.3, the second part of the Al selection consisted of the experimental investigation of the layer thickness influence. In addition to the selected 20 µm Al-1050A foil (23.6% capacity retention after 50 cycles), Al-1050A foils with a thickness of 30 µm and 50 µm were investigated as anodes of the GREENcell. The results are shown in Figure 6b. After 50 cycles, the relative discharge capacity of the 30 µm foil is 46.2%, and that of the 50 µm foil is 31.1%. Consequently, the 30 µm Al-1050A foil distinguishes itself from the other foils, solidifying its position as the new anode for the GREENcell. With reference to the calculation of the minimum Al thicknesses in Section 2.3 and the concept of DAU introduced by Zheng et al. [10], the experimental results indicate the need for an Al buffer. Therefore, the increased capacity drop of the 20 µm foil can be attributed to an insufficient Al reservoir, resulting in an exaggerated lithiation depth and inhomogeneities in the anode composition. Alloy inhomogeneities caused by Li deficiency are suspected to be the reason for the high-capacity loss of the 50 µm foil. Energy-dispersive X-ray spectroscopy (EDX) measurements should support this assumption in future observations. Based on this thickness dependence, the comparability of the results from Figure 6a must be viewed critically, as the foils employed in the alloy variation differ in thickness. However, the theory regarding the positive influence of Al purity on cycling stability is further supported by the fact that the Al-1200 foil, with a thickness of 25 µm, is even thicker than the Al-1235 and Al-1050A foils, measuring 20 µm.
3.3.2. Al Pretreatment
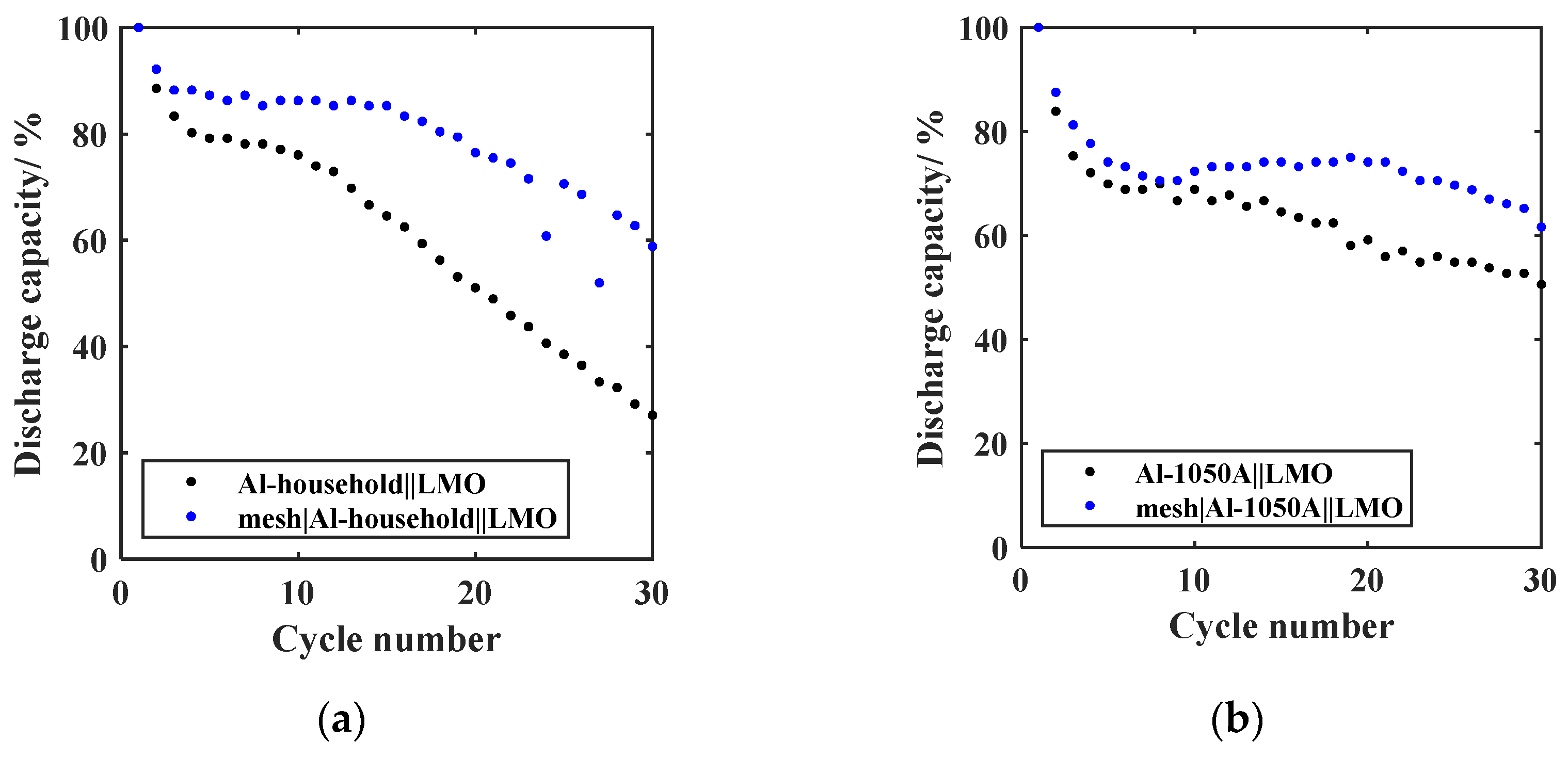
- Mechanical pretreatment;The curves of the relative discharge capacity of the GREENcells whose Al anode was mechanically pretreated with a pressed-in stainless steel mesh are shown in Figure 7 for (a) Al-household and (b) Al-1050A.After 30 cycles, the mesh|Al-household||LMO cell exhibits a relative discharge capacity of 58.8%, a notable improvement compared to the previous 27.1% for the untreated foil. Similarly, the mesh|Al-1050A||LMO cell demonstrates a relative discharge capacity of 61.6% after 30 cycles, surpassing the previous 50.5% for the untreated foil. Using the pressed-in mesh yields a significant enhancement in cycling stability for both cases, resulting in an 11.1% increase in capacity retention for Al-1050A and a 31.7% increase for Al-household. The relatively smaller impact of the mesh on the cycling stability of the 30 µm thick Al-1050A foil can be attributed to the thinner mesh thickness of only 20 µm. A mesh that matches the Al foil thickness should be used in the future. The mesh’s effectiveness confirms the experiences of Yang et al. [29] and Lu et al. [27]. The three-dimensional current density distribution and the implied homogenization of the Li deposition as LiAl alloy over the entire bulk area of the Al foil are responsible for this positive influence [27,29]. Due to the mesh pressing into the Al foil, it can also be assumed that the mechanical breaking of the Al oxide layer on the mesh bridges intensifies the positive influence. The mechanical disruption of the Al oxide layer would also explain the reduction in the formation loss after the initial five cycles of 4.2% in the case of Al-1050A and 8.1% in the case of Al-household. Mechanical stabilization of the volumetric change is an additional explanation for the improved cycling stability. The GREENcell system was therefore updated to mesh|Al-household||LMO and mesh|Al-1050A||LMO.
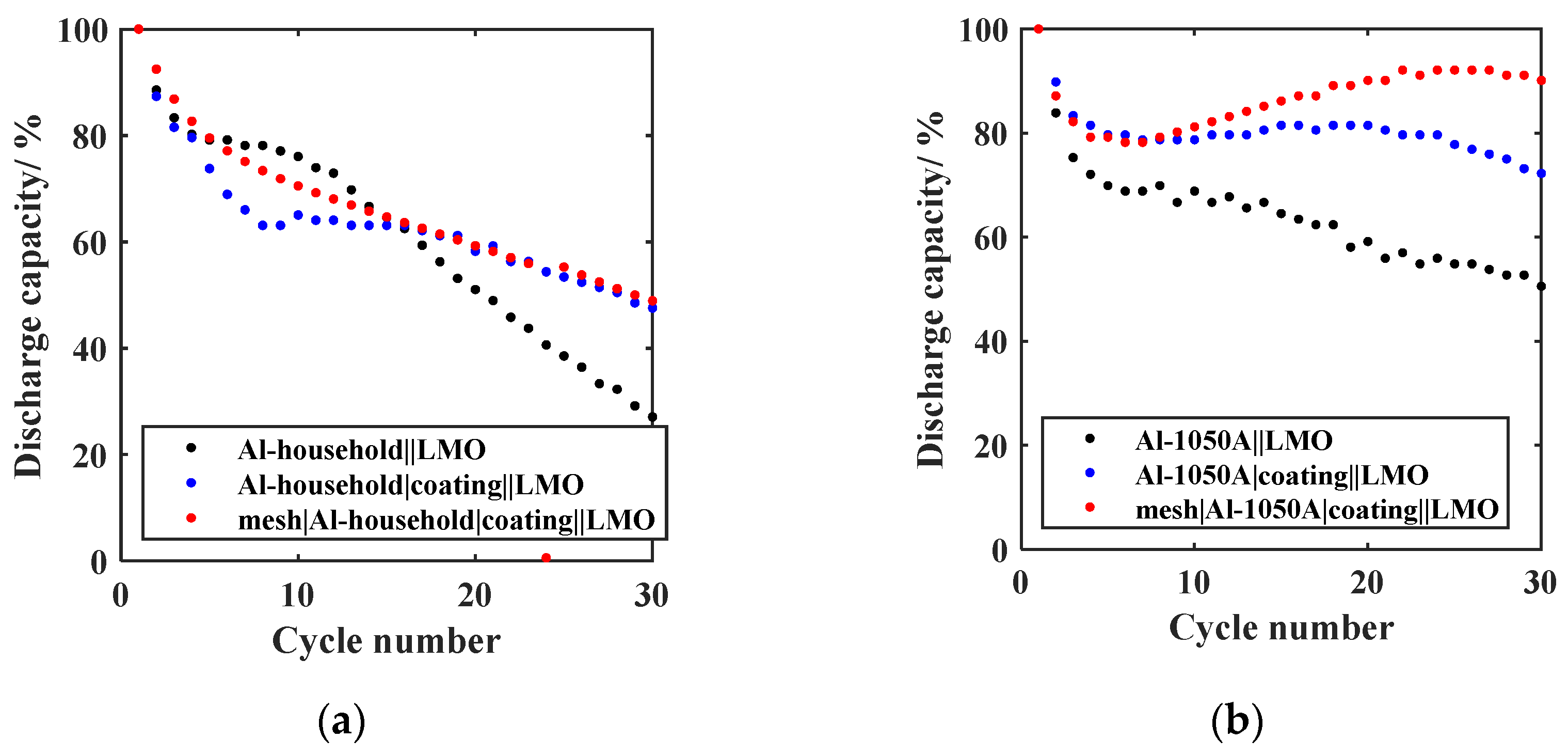
- Chemical pretreatment;The curves of the relative discharge capacity of the GREENcells, where the Al anode was chemically pretreated with a SEI-precursor coating, are shown in Figure 8 for (a) Al-household and (b) Al-1050A in red. To evaluate the isolated influence of the coating on the cycling stability, plots of the relative discharge capacity of the anode design without the mesh are shown in blue.After 30 cycles, the relative discharge capacity of the mesh|Al-household|coating||LMO cell (red) is 48.9% compared to the 27.1% for the untreated foil (black). The mesh|Al-1050A|coating||LMO cell (red) has a relative discharge capacity of 87.1% after 30 cycles compared to 50.53% for the untreated Al foil (black). This implies a further increase in capacity retention by 25.5% compared to the previous anode design mesh|Al-1050A in Figure 7b. Additionally, the formation loss after the initial five cycles can be reduced by 9.3% compared to the untreated Al-1050A and by 5.1% compared to the mesh|Al-1050A anode design. Therefore, the effectiveness of using a SEI-precursor coating on anode stability is confirmed and agrees with the results of Liang et al. [30]. Liang et al. [30] justify the positive effect of an artificially applied oxide coating with the stability increase in the SEI, improved ionic conductivity, and electrolyte distribution at the electrode/electrolyte interface.For both the Al-household foil and the Al-1050A foil, the curves with (red) and without mesh (blue) are very close. Using the mesh-coating combination, the relative discharge capacity can be increased by 1.3% for Al-household and 5.6% for Al-1050A after 30 cycles compared to just using the coating. This slight improvement in the case of the Al-household lies in the area of tolerance and does not allow a clear decision to be made. Since the goal was to define an optimal GREENcell system, the decision-making was based on the promising results of the Al-1050A foil. Thus, the new anode design was set to mesh|Al-household|coating and mesh|Al-1050A|coating. To statistically support this decision, further cells will be built in the future.
3.3.3. Electrolyte Modification
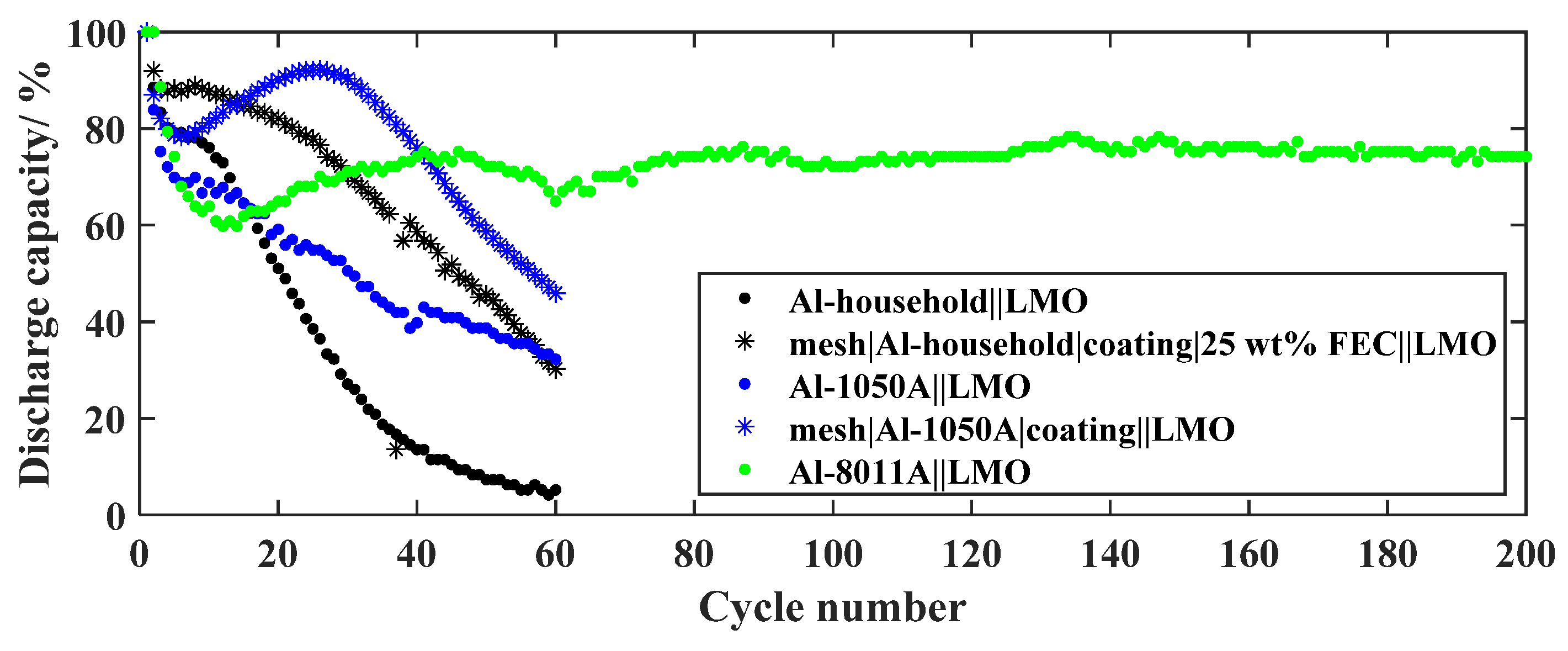
3.3.4. Foreign Element-Containing Al Alloy
3.4. Optimized In-Situ Li-Al Alloy Anode of the GREENcell
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N. A Perspective on the Sustainability of Cathode Materials used in Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2102028. [Google Scholar] [CrossRef]
- Dühnen, S.; Betz, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. Toward Green Battery Cells: Perspective on Materials and Technologies. Small Methods 2020, 4, 2000039. [Google Scholar] [CrossRef]
- Agora Verkehrswende. Lithium-Ionen-Batterien—CO2-Emissionen Durch Fertigung 2019|Statista: Zusammensetzung der Treibhausgas-Emissionen in der Herstellung von Batterien für Elektroautos nach Bestandteilen/Fertigungsschritten (in kg CO2-Äquivalenten pro kWh der Batterie; Stand: 2019). Available online: https://de.statista.com/statistik/daten/studie/1074324/umfrage/zusammensetzung-der-co2-emissionen-bei-der-herstellung-von-e-autobatterien/ (accessed on 25 June 2023).
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Wentker, M.; Greenwood, M.; Leker, J. A Bottom-Up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef]
- Wang, H.; Tan, H.; Luo, X.; Wang, H.; Ma, T.; Lv, M.; Song, X.; Jin, S.; Chang, X.; Li, X. The progress on aluminum-based anode materials for lithium-ion batteries. J. Mater. Chem. A 2020, 8, 25649–25662. [Google Scholar] [CrossRef]
- Chen, S.; Yang, X.; Zhang, J.; Ma, J.; Meng, Y.; Tao, K.; Li, F.; Geng, J. Aluminum–lithium alloy as a stable and reversible anode for lithium batteries. Electrochim. Acta 2021, 368, 137626. [Google Scholar] [CrossRef]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Source 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Zheng, T.; Boles, S.T. Lithium aluminum alloy anodes in Li-ion rechargeable batteries: Past developments, recent progress, and future prospects. Prog. Energy 2023, 5, 032001. [Google Scholar] [CrossRef]
- Insider Inc.; finanzen.net GmbH. Aluminium PRICE Today|Aluminium Spot Price Chart|Live Price of Aluminium per Ounce|Markets Insider. Available online: https://markets.businessinsider.com/commodities/aluminum-price?op=1 (accessed on 22 June 2023).
- Amine, K.; Liu, J.; Kang, S.; Belharouak, I.; Hyung, Y.; Vissers, D.; Henriksen, G. Improved lithium manganese oxide spinel/graphite Li-ion cells for high-power applications. J. Power Source 2004, 129, 14–19. [Google Scholar] [CrossRef]
- Zheng, T.; Kramer, D.; Mönig, R.; Boles, S.T. Aluminum Foil Anodes for Li-Ion Rechargeable Batteries: The Role of Li Solubility within β-LiAl. ACS Sustain. Chem. Eng. 2022, 10, 3203–3210. [Google Scholar] [CrossRef]
- Chen, T.; Thenuwara, A.C.; Yao, W.; Sandoval, S.E.; Wang, C.; Kang, D.H.; Majumdar, D.; Gopalaswamy, R.; McDowell, M.T. Benchmarking the Degradation Behavior of Aluminum Foil Anodes for Lithium-Ion Batteries. Batter. Supercaps 2023, 6, e202200363. [Google Scholar] [CrossRef]
- Min, Z.; Jiege, L.; Bin, Y. A Kind of Three-Dimensional Porous Lithium Metal Composite Negative Pole Material and Preparation Method and Application. Chinese Patent CN109244374A, 31 July 2018. [Google Scholar]
- Li, Q.; Zhu, S.; Lu, Y. 3D Porous Cu Current Collector/Li-Metal Composite Anode for Stable Lithium-Metal Batteries. Adv. Funct. Mater. 2017, 27, 1606422. [Google Scholar] [CrossRef]
- Ling, H.Y.; Su, Z.; Chen, H.; Hencz, L.; Zhang, M.; Tang, Y.; Zhang, S. Biomass-Derived Poly(Furfuryl Alcohol)–Protected Aluminum Anode for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1800995. [Google Scholar] [CrossRef]
- Jiang, M.; Yu, Y.; Fan, H.; Xu, H.; Zheng, Y.; Huang, Y.; Li, S.; Li, J. Full-Cell Cycling of a Self-Supporting Aluminum Foil Anode with a Phosphate Conversion Coating. ACS Appl. Mater. Interfaces 2019, 11, 15656–15661. [Google Scholar] [CrossRef]
- Salehan, S.S.; Nadirah, B.N.; Saheed, M.S.M.; Yahya, W.Z.N.; Shukur, M.F. Conductivity, structural and thermal properties of corn starch-lithium iodide nanocomposite polymer electrolyte incorporated with Al2O3. J. Polym. Res. 2021, 28, 222. [Google Scholar] [CrossRef]
- Fan, H.; Chen, B.; Li, S.; Yu, Y.; Xu, H.; Jiang, M.; Huang, Y.; Li, J. Nanocrystalline Li–Al–Mn–Si Foil as Reversible Li Host: Electronic Percolation and Electrochemical Cycling Stability. Nano Lett. 2020, 20, 896–904. [Google Scholar] [CrossRef]
- Morita, M.; Shibata, T.; Yoshimoto, N.; Ishikawa, M. Anodic behavior of aluminum in organic solutions with different electrolytic salts for lithium ion batteries. Electrochim. Acta 2002, 47, 2787–2793. [Google Scholar] [CrossRef]
- Choi, N.-S.; Yew, K.H.; Lee, K.Y.; Sung, M.; Kim, H.; Kim, S.-S. Effect of fluoroethylene carbonate additive on interfacial properties of silicon thin-film electrode. J. Power Source 2006, 161, 1254–1259. [Google Scholar] [CrossRef]
- Tahmasebi, M.H.; Kramer, D.; Geßwein, H.; Zheng, T.; Leung, K.-C.; Lo, B.T.W.; Mönig, R.; Boles, S.T. In situ formation of aluminum–silicon–lithium active materials in aluminum matrices for lithium-ion batteries. J. Mater. Chem. A 2020, 8, 4877–4888. [Google Scholar] [CrossRef]
- Fleischauer, M.D.; Obrovac, M.N.; Dahn, J.R. Al–Si Thin-Film Negative Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2008, 155, A851–A854. [Google Scholar] [CrossRef]
- Zhang, M.; Xiang, L.; Galluzzi, M.; Jiang, C.; Zhang, S.; Li, J.; Tang, Y. Uniform Distribution of Alloying/Dealloying Stress for High Structural Stability of an Al Anode in High-Areal-Density Lithium-Ion Batteries. Adv. Mater. 2019, 31, e1900826. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.; Hong, H.; Ellingsen, M.; Hrabe, R. Supersonic cold-sprayed Si composite alloy as anode for Li-ion batteries. Appl. Phys. Lett. 2023, 122, 023901. [Google Scholar] [CrossRef]
- Lu, Z.; Li, W.; Long, Y.; Liang, J.; Liang, Q.; Wu, S.; Tao, Y.; Weng, Z.; Lv, W.; Yang, Q. Constructing a High-Strength Solid Electrolyte Layer by In Vivo Alloying with Aluminum for an Ultrahigh-Rate Lithium Metal Anode. Adv. Funct. Mater. 2019, 30, 1907343. [Google Scholar] [CrossRef]
- Zhang, S.; Xing, Y.; Jiang, T.; Du, Z.; Li, F.; He, L.; Liu, W. A three-dimensional tin-coated nanoporous copper for lithium-ion battery anodes. J. Power Source 2011, 196, 6915–6919. [Google Scholar] [CrossRef]
- Yang, C.-P.; Yin, Y.-X.; Zhang, S.-F.; Li, N.-W.; Guo, Y.-G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Jin, X.; Huang, Z.; Kang, F. The high performances of SiO2/Al2O3-coated electrospun polyimide fibrous separator for lithium-ion battery. J. Membr. Sci. 2015, 493, 1–7. [Google Scholar] [CrossRef]
- Gu, X.; Dong, J.; Lai, C. Li-containing alloys beneficial for stabilizing lithium anode: A review. Eng. Rep. 2021, 3, e12339. [Google Scholar] [CrossRef]
- Hou, T.; Yang, G.; Rajput, N.N.; Self, J.; Park, S.-W.; Nanda, J.; Persson, K.A. The influence of FEC on the solvation structure and reduction reaction of LiPF6/EC electrolytes and its implication for solid electrolyte interphase formation. Nano Energy 2019, 64, 103881. [Google Scholar] [CrossRef]
- Tran, M.-K.; DaCosta, A.; Mevawalla, A.; Panchal, S.; Fowler, M. Comparative Study of Equivalent Circuit Models Performance in Four Common Lithium-Ion Batteries: LFP, NMC, LMO, NCA. Batteries 2021, 7, 51. [Google Scholar] [CrossRef]
- Karabelli, D.; Birke, K.P. Feasible Energy Density Pushes of Li-Metal vs. Li-Ion Cells. Appl. Sci. 2021, 11, 7592. [Google Scholar] [CrossRef]
- Flamme, B.; Yeadon, D.J.; Phadke, S.; Anouti, M. Effect of fluorinated additives or co-solvent on performances of graphite//LiMn2O4 cells cycled at high potential. J. Energy Chem. 2021, 52, 332–342. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Yin, J.; Wang, H.; Yuan, X.; Li, Q. Effect of different binders on electrochemical properties of LiFePO4/C cathode material in lithium ion batteries. Chem. Eng. J. 2014, 237, 497–502. [Google Scholar] [CrossRef]
- Rao, B.M.L.; Francis, R.W.; Christopher, H.A. Lithium-Aluminum Electrode. J. Electrochem. Soc. 1977, 124, 1490–1492. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, F.; Sheng, M.; Tong, X.; Tang, Y. A Novel and Generalized Lithium-Ion-Battery Configuration utilizing Al Foil as Both Anode and Current Collector for Enhanced Energy Density. Adv. Mater. 2017, 29, 1604219. [Google Scholar] [CrossRef]



| Anode | Cathode | Discharge Reaction | Energy Density * | Al Thickness * |
|---|---|---|---|---|
| LiAl | LMO | LiAl + 2 Li0.5Mn0.5O4 → Al + 2 LiMn2O4 [33] | 262.12 Wh·kg−1 | 19.07 µm |
| LiAl | LFP | LiAl + 2 Li0.5FePO4 → Al + 2 LiFePO4 [33] | 246.54 Wh·kg−1 | 19.39 µm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schad, K.; Welti, D.; Birke, K.P. Proof of Concept: The GREENcell—A Lithium Cell with a F-, Ni- and Co-Free Cathode and Stabilized In-Situ LiAl Alloy Anode. Batteries 2023, 9, 453. https://doi.org/10.3390/batteries9090453
Schad K, Welti D, Birke KP. Proof of Concept: The GREENcell—A Lithium Cell with a F-, Ni- and Co-Free Cathode and Stabilized In-Situ LiAl Alloy Anode. Batteries. 2023; 9(9):453. https://doi.org/10.3390/batteries9090453
Chicago/Turabian StyleSchad, Kathrin, Dominic Welti, and Kai Peter Birke. 2023. "Proof of Concept: The GREENcell—A Lithium Cell with a F-, Ni- and Co-Free Cathode and Stabilized In-Situ LiAl Alloy Anode" Batteries 9, no. 9: 453. https://doi.org/10.3390/batteries9090453
APA StyleSchad, K., Welti, D., & Birke, K. P. (2023). Proof of Concept: The GREENcell—A Lithium Cell with a F-, Ni- and Co-Free Cathode and Stabilized In-Situ LiAl Alloy Anode. Batteries, 9(9), 453. https://doi.org/10.3390/batteries9090453







