Bimetallic Flower-like NiCoP Encapsulated in an N-Doped Carbon Shell with Enhanced Lithium Storage Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Synthesis
2.2. NiCoP@NC Synthesizing
2.3. Material Characterizations
2.4. Electrochemical Analyses
3. Results and Discussion
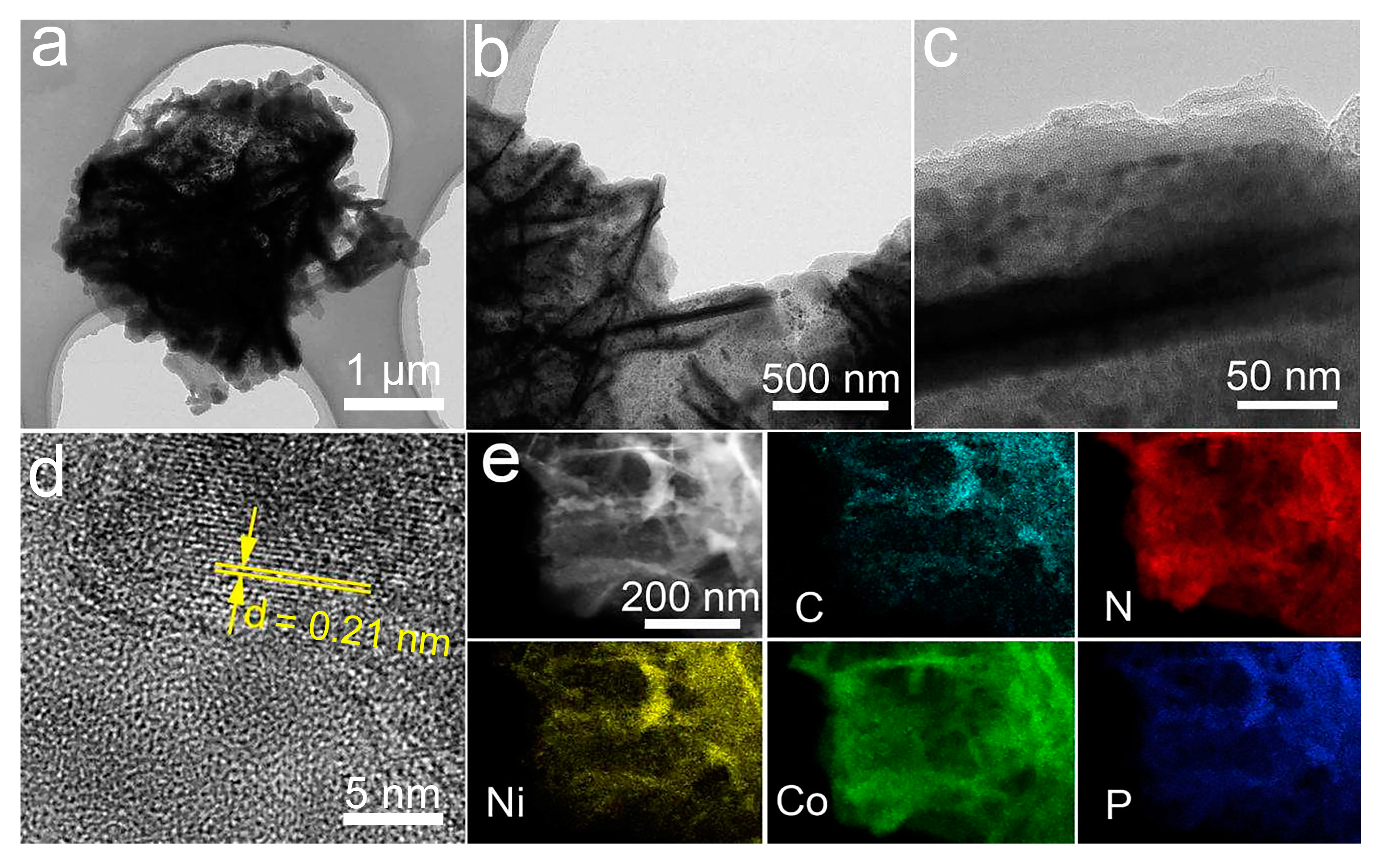
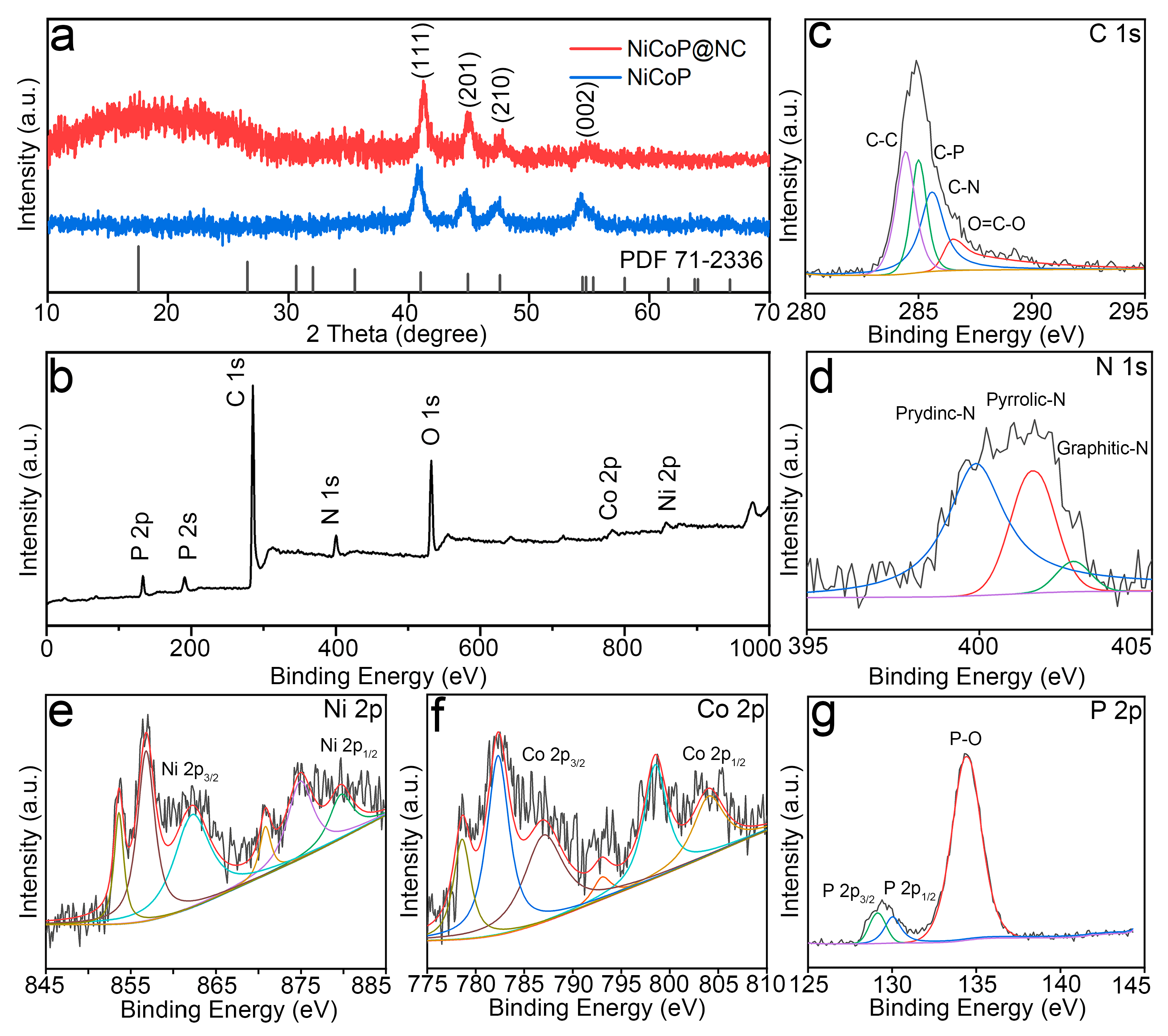
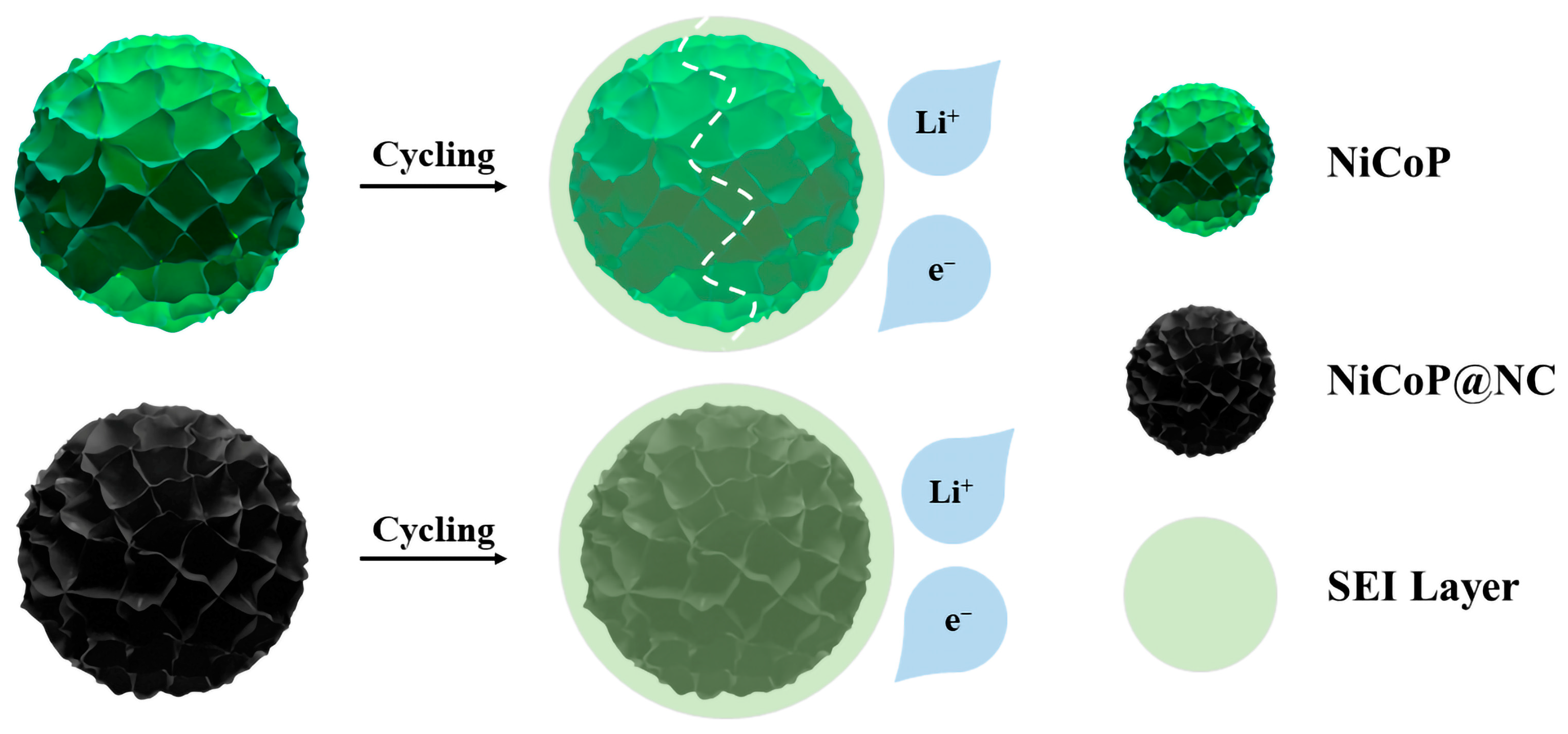
3.1. Morphology and Structure
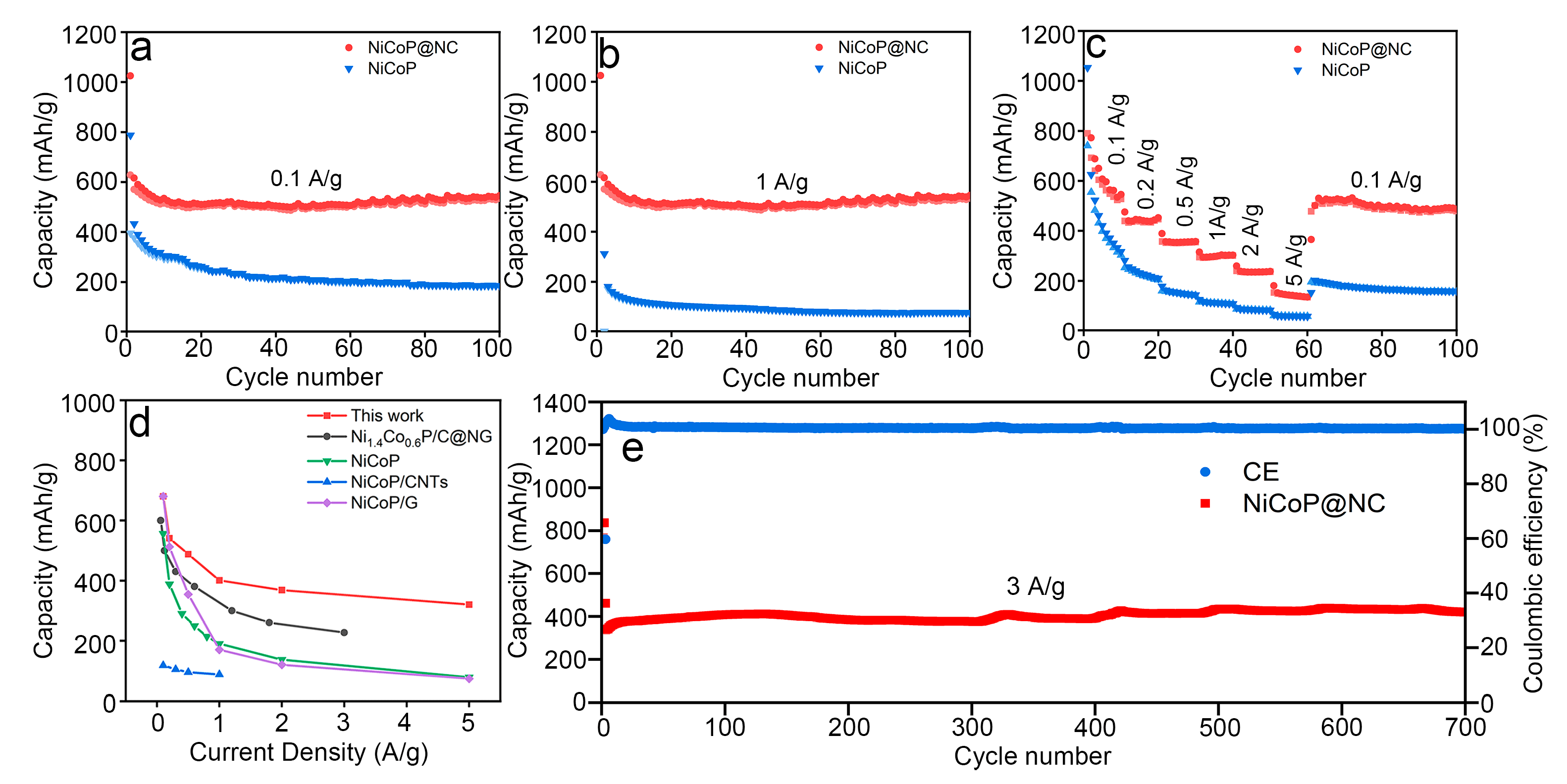
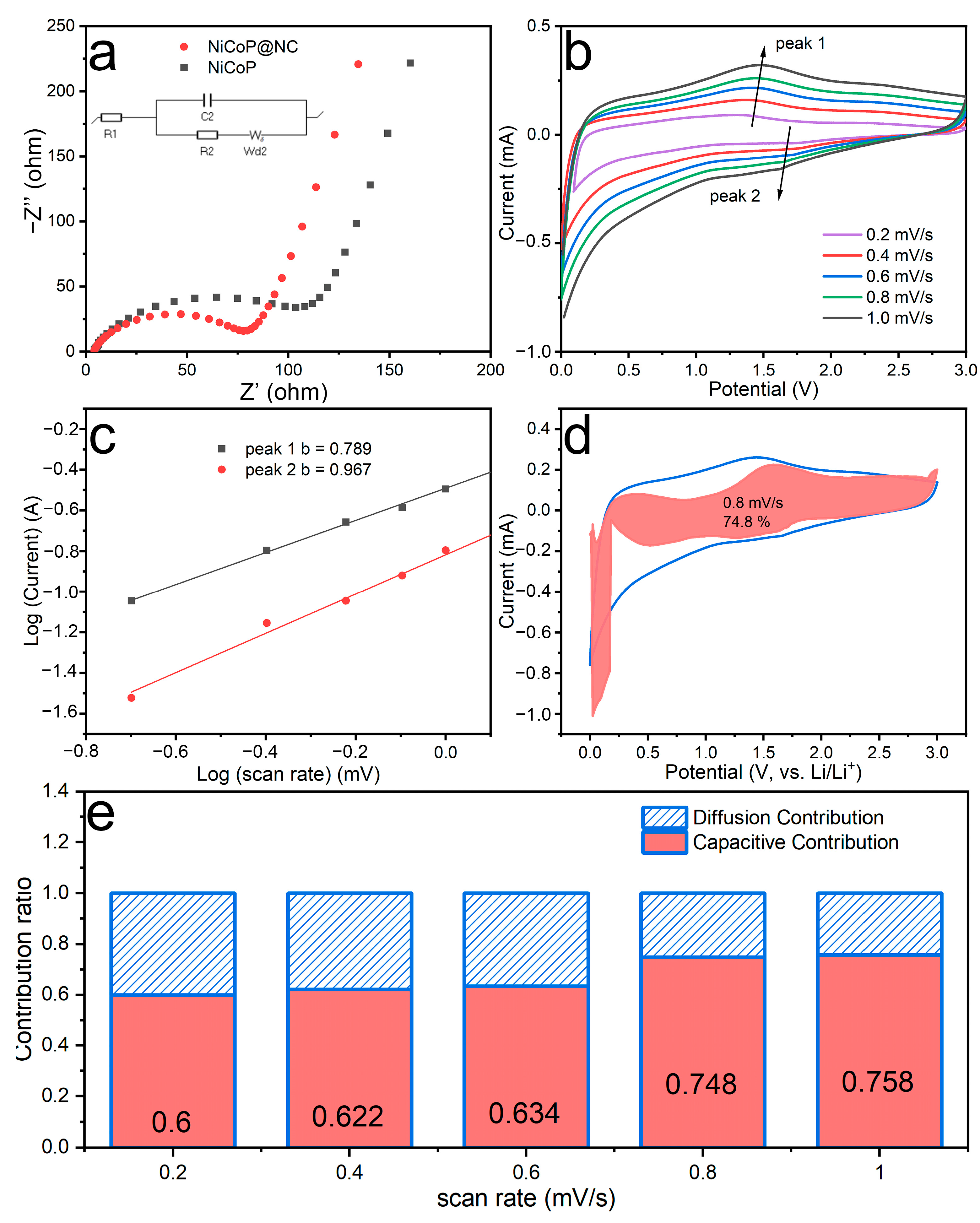
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Kim, H.-M.; Xiao, Y.; Sun, Y.-K. Nanostructured metal phosphide-based materials for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 14915–14931. [Google Scholar] [CrossRef]
- Hu, C.; Hu, Y.; Chen, A.; Duan, X.; Jiang, H.; Li, C. Atomic Interface Catalytically Synthesizing SnP/CoP Hetero-Nanocrystals within Dual-Carbon Hybrids for Ultrafast Lithium-Ion Batteries. Engineering 2022, 18, 154–160. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Zhao, L.; Hou, C.; Huang, M.; Algadi, H.; Li, D.; Xia, Q.; Wang, J.; Zhou, Z.; et al. Sandwich-like CoMoP2/MoP heterostructures coupling N, P co-doped carbon nanosheets as advanced anodes for high-performance lithium-ion batteries. Adv. Compos. Hybrid Mater. 2022, 5, 2601–2610. [Google Scholar] [CrossRef]
- Huang, G.; Kong, Q.; Yao, W.; Wang, Q. Poly tannic acid carbon rods as anode materials for high performance lithium and sodium ion batteries. J. Colloid Interface Sci. 2023, 629 Pt A, 832–845. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Y.; Meng, X.; Cao, W.; Liu, C.; Huang, Q.; Naik, N.; Murugadoss, V.; Huang, M.; Guo, Z. The impact of electrode with carbon materials on safety performance of lithium-ion batteries: A review. Carbon 2022, 191, 448–470. [Google Scholar] [CrossRef]
- Helal, A.; Shaheen Shah, S.; Usman, M.; Khan, M.Y.; Aziz, M.A.; Mizanur Rahman, M. Potential Applications of Nickel-Based Metal-Organic Frameworks and their Derivatives. Chem. Rec. 2022, 22, e202200055. [Google Scholar] [CrossRef]
- Luan, H.; Zhao, A.; Xiao, Y.; Peng, N.; Wen, Y.; Liang, L. Modification of NiCoP nanocages anodes using epoxy-functionalized silane to improve electrochemical performance in lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2023, 34, 905. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Nsabimana, A.; Han, C.; Li, M.; Guo, L. Electrocatalytically active cobalt-based metal–organic framework with incorporated macroporous carbon composite for electrochemical applications. J. Mater. Chem. A 2015, 3, 732–738. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.T.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. -Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Shang, F.; Yu, W.; Shi, R.; Wan, S.; Zhang, H.; Wang, B.; Cao, R. Enhanced lithium storage performance guided by intricate-cavity hollow cobalt phosphide. Appl. Surf. Sci. 2021, 563, 150395. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, J.; Guan, X.; Wei, Z.; Yu, J.; Zhang, S.; Xing, Y.; Yang, P. In Situ Growth of CoP Nanosheet Arrays on Carbon Cloth as Binder-Free Electrode for High-Performance Flexible Lithium-Ion Batteries. Small 2022, 18, e2204970. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Hu, L.; Wang, Z.; Qi, R.; Yu, K.; Zhu, Z. Metal-organic frameworks-derived CoP anchored on MXene toward an efficient bifunctional electrode with enhanced lithium storage. Chem. Eng. J. 2021, 416, 129102. [Google Scholar] [CrossRef]
- Huang, C.; Ouyang, T.; Zou, Y.; Li, N.; Liu, Z.-Q. Ultrathin NiCo2Px nanosheets strongly coupled with CNTs as efficient and robust electrocatalysts for overall water splitting. J. Mater. Chem. A 2018, 6, 7420–7427. [Google Scholar] [CrossRef]
- Dang, T.; Wei, D.; Zhang, G.; Wang, L.; Li, Q.; Liu, H.; Cao, Z.; Zhang, G.; Duan, H. Homologous NiCoP/CoP hetero-nanosheets supported on N-doped carbon nanotubes for high-rate hybrid supercapacitors. Electrochim. Acta 2020, 341, 135988. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Liu, L.; Xu, H.; Wang, Y. Tunable and Specific Formation of C@NiCoP Peapods with Enhanced HER Activity and Lithium Storage Performance. Chemistry 2016, 22, 1021–1029. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Zhang, C.; Kong, F.; Tao, S.; Qian, B.; Jiang, X. Bimetal phosphide Ni1.4Co0.6P nanoparticle/carbon@ nitrogen-doped graphene network as high-performance anode materials for lithium-ion batteries. Appl. Surf. Sci. 2019, 485, 413–422. [Google Scholar] [CrossRef]
- Wang, X.-W.; Guo, H.-P.; Liang, J.; Zhang, J.-F.; Zhang, B.; Wang, J.-Z.; Luo, W.-B.; Liu, H.-K.; Dou, S.-X. An Integrated Free-Standing Flexible Electrode with Holey-Structured 2D Bimetallic Phosphide Nanosheets for Sodium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1801016. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Zhao, R.Z.; Dong, S.H.; Miao, X.G.; Zhang, Z.W.; Wang, C.X.; Yin, L.W. Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries. Energy Environ. Sci. 2019, 12, 2422–2432. [Google Scholar] [CrossRef]
- Lu, H.; Qian, R.; Yao, T.; Li, C.; Li, L.; Wang, H. Synthesis of Spherical Carbon-Coated CoP Nanoparticles for High-Performance Lithium-Ion Batteries. Energy Technol. 2021, 9, 2100605. [Google Scholar] [CrossRef]
- Han, L.; Zhang, M.T.; Wang, H.L.; Li, P.; Wei, W.R.; Shi, J.; Huang, M.H.; Shi, Z.C.; Liu, W.; Chen, S.G. Electrospun hetero-CoP/FeP embedded in porous carbon nanofibers: Enhanced Na+ kinetics and specific capacity. Nanoscale 2020, 12, 24477–24487. [Google Scholar] [CrossRef]
- Shi, S.S.; Sun, C.L.; Yin, X.P.; Shen, L.Y.; Shi, Q.H.; Zhao, K.N.; Zhao, Y.F.; Zhang, J.J. FeP Quantum Dots Confined in Carbon-Nanotube-Grafted P-Doped Carbon Octahedra for High-Rate Sodium Storage and Full-Cell Applications. Adv. Funct. Mater. 2020, 30, 1909283. [Google Scholar] [CrossRef]
- Zheng, H.; Men, S.; Huang, X.L.; Zhou, Y.; Gao, H.C.; Huang, J.; Kang, X.W. Three-dimensional hierarchical Ni5P4 nanospheres encapsulated in graphene as high-performance anode materials of sodium ion batteries. J. Mater. Sci. 2020, 55, 9027–9036. [Google Scholar] [CrossRef]
- Bai, J.; Xi, B.J.; Mao, H.Z.; Lin, Y.; Ma, X.J.; Feng, J.K.; Xiong, S.L. One-Step Construction of N,P-Codoped Porous Carbon Sheets/CoP Hybrids with Enhanced Lithium and Potassium Storage. Adv. Mater. 2018, 30, 1802310. [Google Scholar] [CrossRef]
- Li, F.F.; Gao, J.F.; He, Z.H.; Kong, L.B. Realizing high-performance and low-cost lithium-ion capacitor by regulating kinetic matching between ternary nickel cobalt phosphate microspheres anode with ultralong-life and super-rate performance and watermelon peel biomass-derived carbon cathode. J Colloid Interface Sci. 2021, 598, 283–301. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, M.; Huang, Y.; Li, J.; Zhou, X.; Ma, G.; Ren, S. One-pot synthesis of NiCoP/CNTs composites for lithium ion batteries and hydrogen evolution reaction. Ionics 2019, 26, 1771–1778. [Google Scholar] [CrossRef]
- Wang, C.; Qian, Y.; Yang, J.; Xing, S.; Ding, X.; Yang, Q. Ternary NiCoP nanoparticles assembled on graphene for high-performance lithium-ion batteries and supercapacitors. RSC Adv. 2017, 7, 26120–26124. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. A novel strategy of fabricating high performance UV-resistant aramid fibers with simultaneously improved surface activity, thermal and mechanical properties through building polydopamine and graphene oxide bi-layer coatings. Chem. Eng. J. 2017, 310, 134–147. [Google Scholar] [CrossRef]
- Guo, H.; Chen, C.; Chen, K.; Cai, H.; Chang, X.; Liu, S.; Li, W.; Wang, Y.; Wang, C. High performance carbon-coated hollow Ni12P5 nanocrystals decorated on GNS as advanced anodes for lithium and sodium storage. J. Mater. Chem. A 2017, 5, 22316–22324. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Li, J.; Liu, P.; Huang, M.; Xiang, B. Phytic acid-derived Co2P/N-doped carbon nanofibers as flexible free-standing anode for high performance lithium/sodium ion batteries. J. Alloy. Compd. 2020, 846, 156256. [Google Scholar] [CrossRef]
- Guo, H.; Cai, H.; Li, W.; Chen, C.; Chen, K.; Zhang, Y.; Li, Y.; Wang, M.; Wang, Y. Tailored Ni2P nanoparticles supported on N-doped carbon as a superior anode material for Li-ion batteries. Inorg. Chem. Front. 2019, 6, 1881–1889. [Google Scholar] [CrossRef]
- Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. Rational Design of Three-Dimensional Graphene Encapsulated with Hollow FeP@Carbon Nanocomposite as Outstanding Anode Material for Lithium Ion and Sodium Ion Batteries. ACS Nano 2017, 11, 11602–11616. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Kopold, P.; van Aken, P.A.; Maier, J.; Yu, Y. High Performance Graphene/Ni2P Hybrid Anodes for Lithium and Sodium Storage through 3D Yolk-Shell-Like Nanostructural Design. Adv. Mater. 2017, 29, 1604015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xue, L.-P.; Wang, N. Robustly immobilized Ni2P nanoparticles in porous carbon networks promotes high-performance sodium-ion storage. J. Alloy. Compd. 2019, 776, 912–918. [Google Scholar] [CrossRef]
- Ren, W.; Zhou, W.; Zhang, H.; Cheng, C. ALD TiO2-Coated Flower-like MoS2 Nanosheets on Carbon Cloth as Sodium Ion Battery Anode with Enhanced Cycling Stability and Rate Capability. ACS Appl. Mater. Interfaces 2017, 9, 487–495. [Google Scholar] [CrossRef]
- Ge, P.; Zhang, C.; Hou, H.; Wu, B.; Zhou, L.; Li, S.; Wu, T.; Hu, J.; Mai, L.; Ji, X. Anions induced evolution of Co3X4 (X = O, S, Se) as sodium-ion anodes: The influences of electronic structure, morphology, electrochemical property. Nano Energy 2018, 48, 617–629. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Zeng, S.; Zhao, K.; Wu, Q.; Yan, L.; Tian, H.; Jiao, Z.; Zhang, J. FeP Coated in Nitrogen/Phosphorus Co-doped Carbon Shell Nanorods Arrays as High-Rate Capable Flexible Anode for K-ion Half/Full Batteries. J. Colloid Interface Sci. 2022, 624, 670–679. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Li, W.; Yuan, F.; Wang, Q.; Zhang, D.; Wang, B.; Wu, Y.A. Spanish-dagger shaped CoP blooms decorated N-doped carbon branch anode for high-performance lithium and sodium storage. Electrochim. Acta 2021, 388, 138628. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Ding, Y.; Jin, Y.; Fang, Z. Co-Salen Complex-Derived CoP Nanoparticles Confined in N-Doped Carbon Microspheres for Stable Sodium Storage. Inorg. Chem. 2021, 60, 17151–17160. [Google Scholar] [CrossRef]
- Li, H.; Hao, S.; Tian, Z.; Zhao, Z.; Wang, X. Flexible self-supporting Ni2P@N-doped carbon anode for superior rate and durable sodium-ion storage. Electrochim. Acta 2019, 321, 134624. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; Zhao, L.; Wang, L.; Xia, Z.; Tan, W.; Jiao, Z. Bimetallic Flower-like NiCoP Encapsulated in an N-Doped Carbon Shell with Enhanced Lithium Storage Properties. Batteries 2023, 9, 361. https://doi.org/10.3390/batteries9070361
Tian H, Zhao L, Wang L, Xia Z, Tan W, Jiao Z. Bimetallic Flower-like NiCoP Encapsulated in an N-Doped Carbon Shell with Enhanced Lithium Storage Properties. Batteries. 2023; 9(7):361. https://doi.org/10.3390/batteries9070361
Chicago/Turabian StyleTian, Haoyu, Lingyu Zhao, Linlin Wang, Zijie Xia, Wenqi Tan, and Zheng Jiao. 2023. "Bimetallic Flower-like NiCoP Encapsulated in an N-Doped Carbon Shell with Enhanced Lithium Storage Properties" Batteries 9, no. 7: 361. https://doi.org/10.3390/batteries9070361
APA StyleTian, H., Zhao, L., Wang, L., Xia, Z., Tan, W., & Jiao, Z. (2023). Bimetallic Flower-like NiCoP Encapsulated in an N-Doped Carbon Shell with Enhanced Lithium Storage Properties. Batteries, 9(7), 361. https://doi.org/10.3390/batteries9070361






