Facile Synthesis of Ordered Mesoporous Orthorhombic Niobium Oxide (T-Nb2O5) for High-Rate Li-Ion Storage with Long Cycling Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Nb2O5 Nanoparticles
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
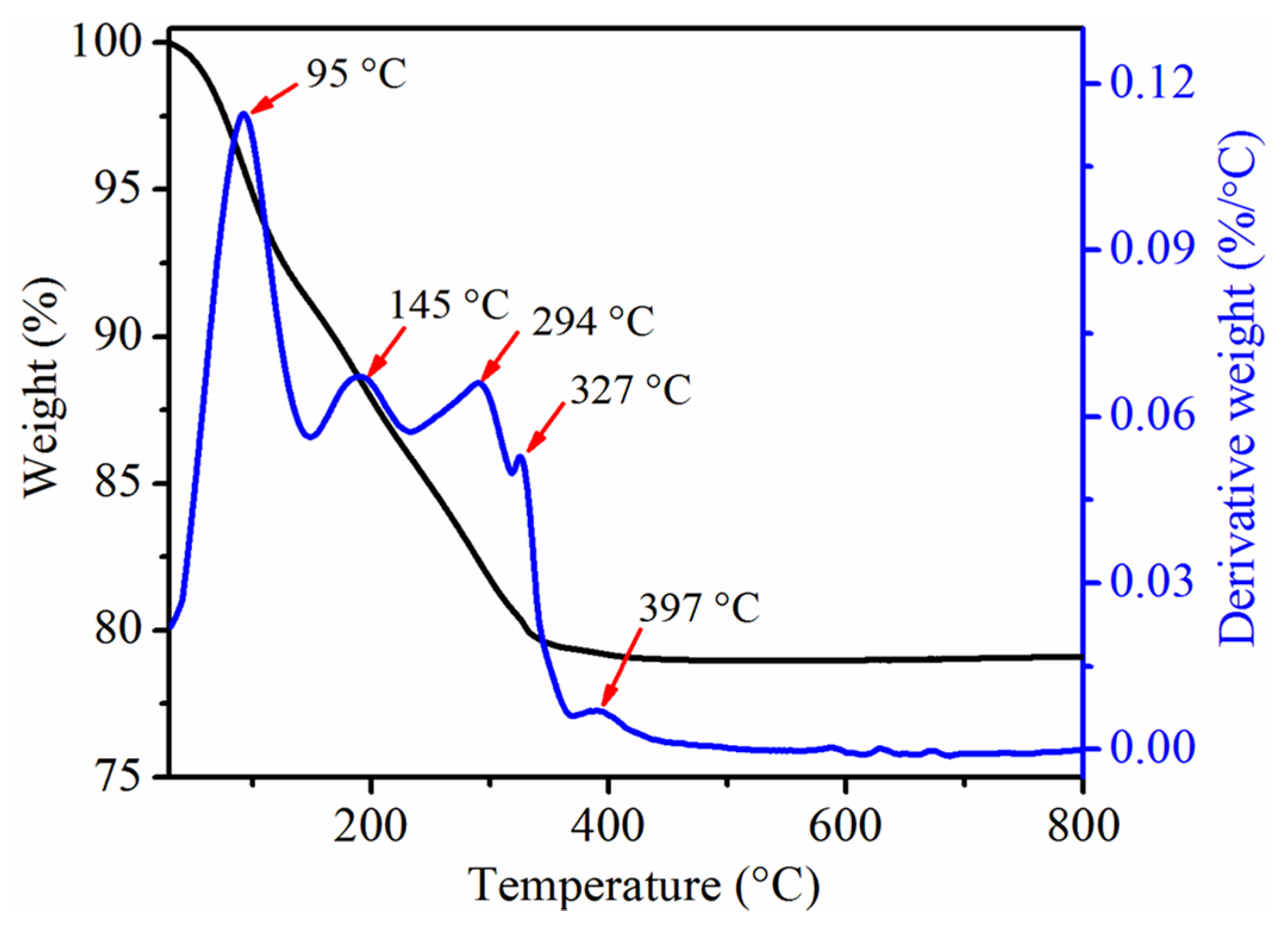
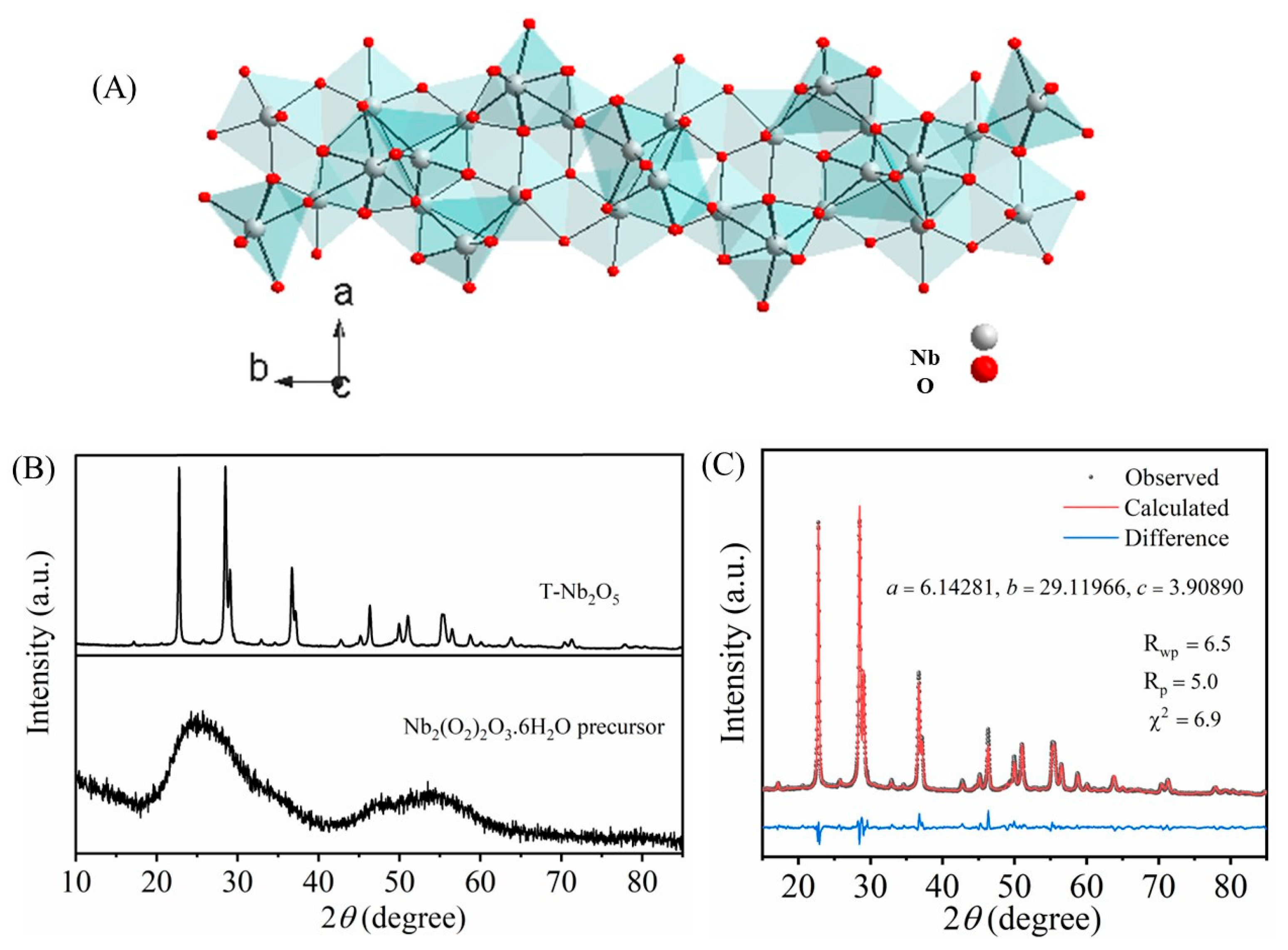
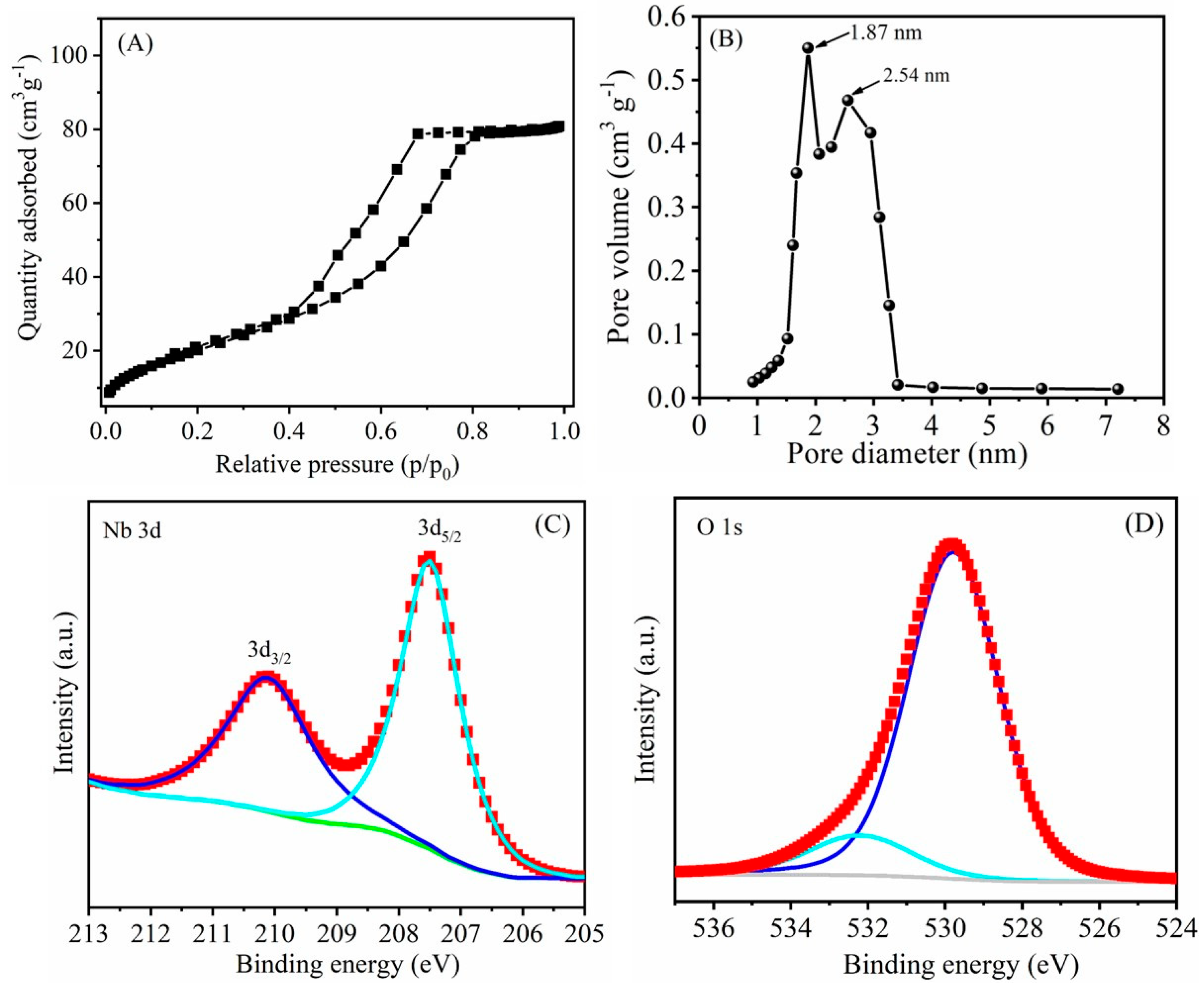
3.1. Structural Characterizations
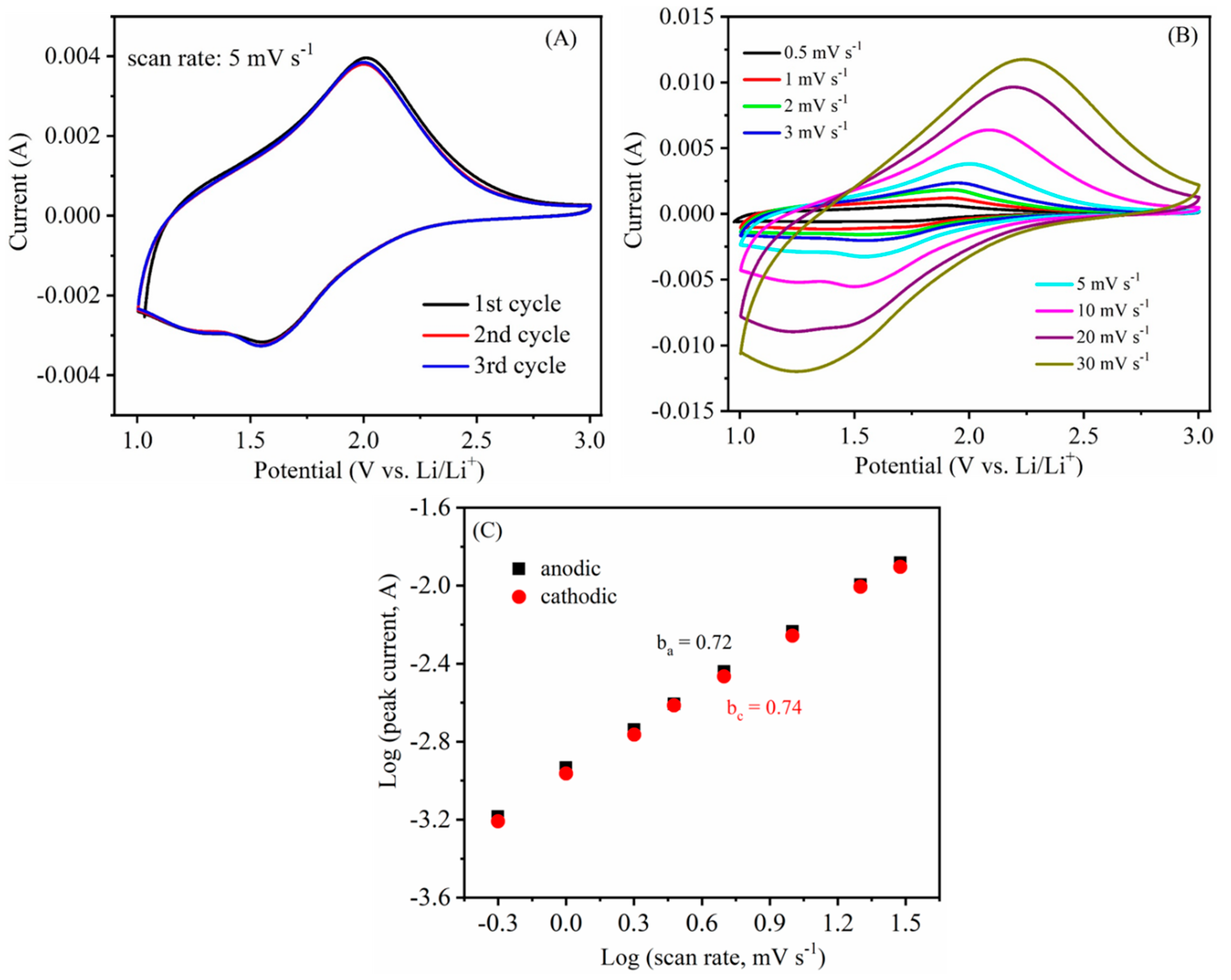
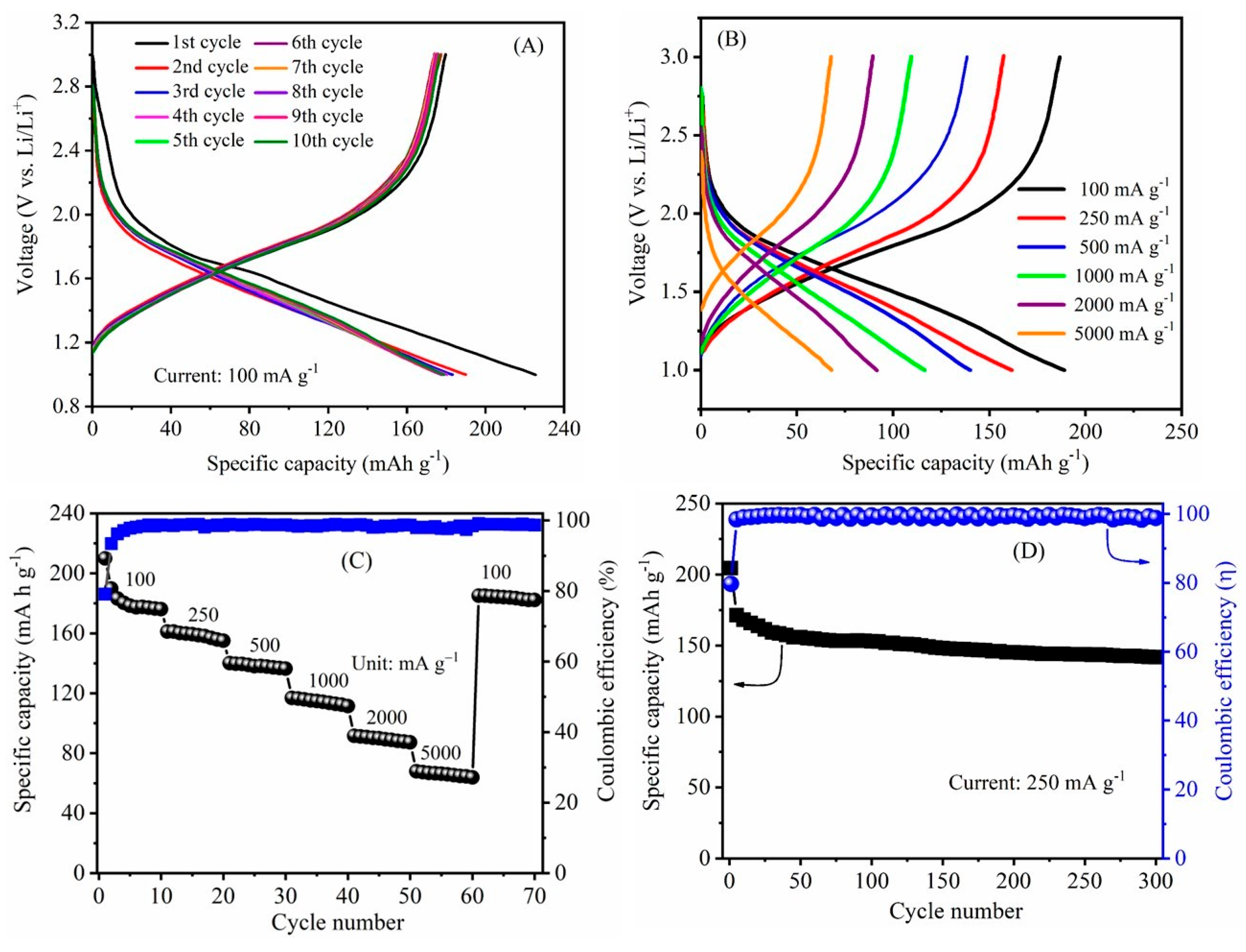
3.2. Electrochemical Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Ruic, X.; Chen, G.; Xu, W.; Zoua, G.; Luo, H. Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale 2016, 8, 8443–8465. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–30. [Google Scholar] [CrossRef]
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Environ, E.; Etacheri, V.; Marom, R.; Salitraa, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.L.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Justin, P.; Rao, G.R. Tuning the surface morphology and pseudocapacitance of MnO2 by a facile green method employing organic reducing sugars. ACS Appl. Energy Mater. 2018, 1, 3654–3664. [Google Scholar] [CrossRef]
- Konda, S.; Chidurala, S.C. Applied surface science advances the impact of hybridization on specific capacitance in hybrid NiO/V2O5@graphene composites as advanced supercapacitor electrode materials. Appl. Surf. Sci. Adv. 2022, 12, 100329. [Google Scholar]
- Shireesha, K.; Divya, V.; Pranitha, G.; Ashok, C.; Shilpa, C.C.; Himabindu, V. A systematic investigation on the effect of reducing agents towards specific capacitance of NiMg@OH/reduced graphene oxide nanocomposites. Mater. Technol. 2022, 37, 1864–1876. [Google Scholar]
- Yun, Y.S.; Cho, S.Y.; Shim, J.; Kim, B.H.; Chang, S.J.; Baek, S.J.; Huh, Y.S.; Tak, Y.; Park, Y.W.; Park, S.; et al. Microporous carbon nanoplates from regenerated silk proteins for supercapacitors. Adv. Mater. 2013, 25, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A. An Outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, H.; Sano, K.; Abe, T.; Baba, M.; Kumagai, N. Charge-discharge characteristics of all-solid-state thin-filmed lithium-ion batteries using amorphous Nb2O5 negative electrodes. J. Power Sources 2007, 174, 838–842. [Google Scholar] [CrossRef]
- Arunkumar, P.; Ashish, A.G.; Babu, B.; Sarang, S.; Suresh, A.; Sharma, C.H.; Thalakulam, M.; Shaijumon, M.M. Nb2O5/graphene nanocomposites for electrochemical energy storage. RSC Adv. 2015, 5, 59997–60004. [Google Scholar] [CrossRef]
- Lübke, M.; Sumboja, A.; Johnson, I.D.; Brett, D.J.L.; Shearing, P.R.; Liu, Z.; Darr, J.A. High power nano-Nb2O5 negative electrodes for lithium-ion batteries. Electrochim. Acta 2016, 192, 363–369. [Google Scholar] [CrossRef]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nanosized transition-metaloxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 spinel anode: Fundamentals and advances in rechargeable batteries. InfoMat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Liu, L.; Zhang, Y.; Li, Z. T-Nb2O5 nanoparticles confined in carbon nanotubes with fast ion diffusion rates for lithium storage. Dalton Trans. 2021, 50, 14532–14536. [Google Scholar] [CrossRef]
- Yi, T.-F.; Sari, H.M.K.; Li, X.; Wang, F.; Zhu, Y.-R.; Hu, J.; Zhang, J.; Li, X. A review of niobium oxides based nanocomposites for lithium-ion batteries, sodium-ion batteries and supercapacitors. Nano Energy 2021, 85, 105955. [Google Scholar] [CrossRef]
- Liu, G.; Jin, B.; Bao, K.; Xie, H.; Guo, J.; Ji, X.; Zhang, R.; Jiang, Q. Facile synthesis of porous Nb2O5 microspheres as anodes for lithium-ion batteries. Int. J. Hydrogen Energy 2017, 42, 6065–6071. [Google Scholar] [CrossRef]
- Yang, H.; Xu, H.; Wang, L.; Zhang, L.; Huang, Y.; Hu, X. Microwave-assisted rapid synthesis of self-assembled T-Nb2O5 nanowires for high-energy hybrid supercapacitors. Chem. Eur. J. 2017, 23, 4203–4209. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Chen, H.; Ma, J.; Zhang, R. Facile synthesis of Nb2O5 nanobelts assembled from nanorods and their applications in lithium ion batteries. J. Phys. Chem. Solids 2017, 111, 8–11. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Zhang, X.; Liu, B.; Xu, L.; Zhang, Z.; Zhang, Y. 2D ultrathin nanosheet-assembled Nb2O5 microflowers for lithium ion batteries. Mater. Lett. 2018, 227, 112–115. [Google Scholar] [CrossRef]
- Song, H.; Fu, J.; Ding, K.; Huang, C.; Wu, K.; Zhang, X.; Gao, B.; Huo, K.; Peng, X.; Chu, P.K. Flexible Nb2O5 nanowires/graphene film electrode for high- performance hybrid Li-ion supercapacitors. J. Power Sources 2016, 328, 599–606. [Google Scholar] [CrossRef]
- Huang, C.; Fu, J.; Song, H.; Li, X.; Peng, X.; Gao, B.; Zhang, X.; Chu, P.K. General fabrication of mesoporous Nb2O5 nanobelts for lithium ion battery anodes. RSC Adv. 2016, 6, 90489–90493. [Google Scholar] [CrossRef]
- Kong, L.; Liu, X.; Wei, J.; Wang, S.; Xu, B.B.; Long, D.; Chen, F. T-Nb2O5 nanoparticle enabled pseudocapacitance with fast Li-ion intercalation. Nanoscale 2018, 10, 14165–14170. [Google Scholar] [CrossRef]
- Lin, J.; Yuan, Y.; Su, Q.; Pan, A.; Dinesh, S.; Peng, C.; Cao, G.; Liang, S. Facile synthesis of Nb2O5/carbon nanocomposites as advanced anode materials for lithium-ion batteries. Electrochim. Acta 2018, 292, 63–71. [Google Scholar] [CrossRef]
- Wei, M.; Wei, K.; Ichihara, M.; Zhou, H. Electrochemistry communications Nb2O5 nanobelts: A lithium intercalation host with large capacity and high rate capability. Electrochem. Commun. 2008, 10, 980–983. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Jung, J.-W.; Youn, D.-Y.; Kim, C.; Yu, S.; Cho, S.-H.; Yoon, K.R.; Kim, I.-D. Mesoporous orthorhombic Nb2O5 nanofibers as pseudocapacitive electrodes with ultra-stable Li storage characteristics. J. Power Sources 2017, 360, 434–442. [Google Scholar] [CrossRef]
- Sasidharan, M.; Gunawardhana, N.; Yoshio, M.; Nakashima, K. Nb2O5 hollow nanospheres as anode material for enhanced performance in lithium ion batteries. Mater. Res. Bull. 2012, 47, 2161–2164. [Google Scholar] [CrossRef]
- Meng, J.; He, Q.; Xu, L.; Zhang, X.; Liu, F.; Wang, X.; Li, Q.; Xu, X.; Zhang, G.; Niu, C.; et al. Identification of phase control of carbon-confined Nb2O5 nanoparticles toward high-performance lithium storage. Adv. Energy Mater. 2019, 9, 1802695. [Google Scholar] [CrossRef]
- Lai, C.H.; Ashby, D.; Moz, M.; Gogotsi, Y.; Pilon, L.; Dunn, B. Designing pseudocapacitance for Nb2O5/carbide-derived carbon electrodes and hybrid devices. Langmuir 2017, 33, 9407–9415. [Google Scholar] [CrossRef]
- Pligovka, A.; Zakhlebayeva, A.; Lazavenka, A. Niobium oxide nanocolumns formed via anodic alumina with modulated pore diameters. J. Phys. Conf. Ser. 2018, 987, 012006. [Google Scholar] [CrossRef]
- Pligovka, A.; Hoha, A.; Turavets, U.; Poznyak, A.; Zakharau, Y. Formation features, morphology and optical properties of nanostructures via anodizing Al/Nb on Si and glass. Mater. Today Proc. 2021, 37, A8–A15. [Google Scholar] [CrossRef]
- Hu, J.J.; Li, J.J.; Wang, K.; Xia, H.Y. Self-assembly Nb2O5 microsphere with hollow and carbon coated structure as high rate capability lithium-ion electrode materials. Electrochim. Acta 2020, 331, 135364–135373. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Rao, G.R. High electrocatalytic activity of Pt/C catalyst promoted by TT-Nb2O5 nanoparticles under acidic conditions. ChemistrySelect 2017, 2, 4204–4212. [Google Scholar] [CrossRef]
- Li, S.; Xu, Q.; Uchaker, E.; Cao, X.; Cao, G. Comparison of amorphous, pseudohexagonal and orthorhombic Nb2O5 for high-rate lithium ion insertion. CrystEngComm 2016, 18, 2532–2540. [Google Scholar] [CrossRef]
- Uekawa, N.; Kudo, T.; Mori, F.; Wu, Y.J.; Kakegawa, K. Low-temperature synthesis of niobium oxide nanoparticles from peroxo niobic acid sol. J. Colloid Interface Sci. 2003, 264, 378–384. [Google Scholar] [CrossRef]
- Yang, M.; Li, S.; Huang, J. Three-dimensional cross-linked Nb2O5 polymorphs derived from cellulose substances: Insights into the mechanisms of lithium storage. ACS Appl. Mater. Interfaces 2021, 13, 39501–39512. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yao, Z.; Shadike, Z.; Lei, M.; Liu, J.; Li, C. Defect-concentration-mediated T-Nb2O5 anodes for durable and fast-charging Li-ion batteries. Adv. Funct. Mater. 2021, 32, 2107060. [Google Scholar] [CrossRef]
- Rouquerol, F. Adsorption by Powders and Porous Solids; Academic Press: London, UK, 1999. [Google Scholar]
- Siekierka, A. Lithium iron manganese oxide as an adsorbent for capturing lithium ions innhybrid capacitive deionization with different electrical modes. Sep. Purif. Technol. 2019, 236, 116234. [Google Scholar] [CrossRef]
- Rozhdestvenska, L.M.; Chaban, M.O.; Dzyazko, Y.S.; Palchik, O.V.; Dzyazko, O.G. Formation of lithium-selective sorbent in nanoreactors of the support based on titanium dioxide. Appl. Nanosci. 2021, 12, 1113–1122. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, R. Green recovery of lithium from water by a smart imprinted adsorbent with photo-controlled and selective properties. Chem. Eng. J. 2019, 378, 122084. [Google Scholar] [CrossRef]
- Griffith, K.J.; Forse, A.C.; Griffin, J.M.; Grey, C.P. High-rate intercalation without nanostructuring in metastable Nb2O5 bronze phases. J. Am. Chem. Soc. 2016, 138, 8888–8899. [Google Scholar] [CrossRef]
- Luo, G.; Li, H.; Zhang, D.; Gao, L.; Lin, T. A template-free synthesis via alkaline route for Nb2O5/carbon nanotubes composite as pseudo-capacitor material with high-rate performance. Electrochim. Acta 2017, 235, 175–181. [Google Scholar] [CrossRef]
- Kim, J.W.; Augustyn, V.; Dunn, B. The effect of crystallinity on the rapid pseudocapacitive response of Nb2O5. Adv. Energy Mater. 2012, 2, 141–148. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, C.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Free-standing T-Nb2O5/graphene composite papers with ultrahigh gravimetric/volumetric capacitance for Li-ion intercalation pseudocapacitor. ACS Nano 2015, 9, 11200–11208. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Pham, H.D.; Mahale, K.; Nanjundan, A.K.; Dubal, D. Nanostructure-dependent electrochemical properties of Nb2O5 for long-life Li-ion batteries. ACS Appl. Eng. Mater. 2022, 1, 469–476. [Google Scholar] [CrossRef]
- Come, J.; Augustyn, V.; Kim, J.W.; Rozier, P.; Taberna, P.L.; Gogotsi, P.; Long, J.W.; Dunn, B.; Simon, P. Electrochemical kinetics of nanostructured Nb2O5 electrodes. J. Electrochem. Soc. 2014, 161, A718–A725. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Wang, L.; Gao, J.; Li, Q.; Ma, Y.; Gao, Y.; Zuo, P.; Du, C.; Yin, G. High-rate capability of three-dimensionally ordered macroporous T-Nb2O5 through Li+ intercalation pseudocapacitance. J. Power Sources 2017, 361, 80–86. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; He, X.; Xu, S.; Fang, M.; Zhao, X.; Shang, Y. Electrochemical properties of MnO2 nanorods as anode materials for lithium ion batteries. Electrochim. Acta 2014, 142, 152–156. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, W.Y.; Lee, S.; Kim, T.-S.; Lee, J.W. Graphene intercalated free-standing carbon paper coated with MnO2 for anode materials of lithium ion batteries. Electrochim. Acta 2020, 348, 136310. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Satyanarayana, M.; Karkera, G.; Pullamsetty, A.; Justin, P. Hierarchical a-MnO2 nanowires as an efficient anode material for rechargeable lithium-ion batteries. Mater. Adv. 2022, 3, 1642–1651. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Rao, G.R. Vanadium pentoxide nanochains for high-performance electrochemical supercapacitors. J. Colloid Interface Sci. 2016, 472, 210–219. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umeshbabu, E.; Velpula, D.; Karkera, G.; Satyanarayana, M.; Pasala, V.; Justin, P. Facile Synthesis of Ordered Mesoporous Orthorhombic Niobium Oxide (T-Nb2O5) for High-Rate Li-Ion Storage with Long Cycling Stability. Batteries 2023, 9, 357. https://doi.org/10.3390/batteries9070357
Umeshbabu E, Velpula D, Karkera G, Satyanarayana M, Pasala V, Justin P. Facile Synthesis of Ordered Mesoporous Orthorhombic Niobium Oxide (T-Nb2O5) for High-Rate Li-Ion Storage with Long Cycling Stability. Batteries. 2023; 9(7):357. https://doi.org/10.3390/batteries9070357
Chicago/Turabian StyleUmeshbabu, Ediga, Divya Velpula, Guruprakash Karkera, Maddukuri Satyanarayana, Vasudevarao Pasala, and P. Justin. 2023. "Facile Synthesis of Ordered Mesoporous Orthorhombic Niobium Oxide (T-Nb2O5) for High-Rate Li-Ion Storage with Long Cycling Stability" Batteries 9, no. 7: 357. https://doi.org/10.3390/batteries9070357
APA StyleUmeshbabu, E., Velpula, D., Karkera, G., Satyanarayana, M., Pasala, V., & Justin, P. (2023). Facile Synthesis of Ordered Mesoporous Orthorhombic Niobium Oxide (T-Nb2O5) for High-Rate Li-Ion Storage with Long Cycling Stability. Batteries, 9(7), 357. https://doi.org/10.3390/batteries9070357






