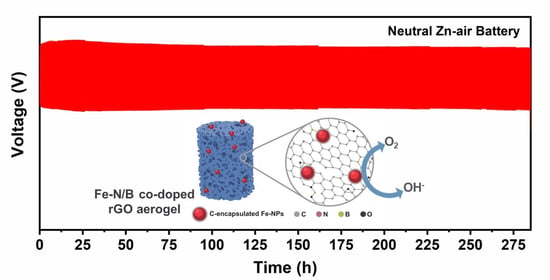
Iron-Decorated Nitrogen/Boron co-Doped Reduced Graphene Oxide Aerogel for Neutral Rechargeable Zn-Air Batteries
Abstract
1. Introduction
2. Materials and Methods
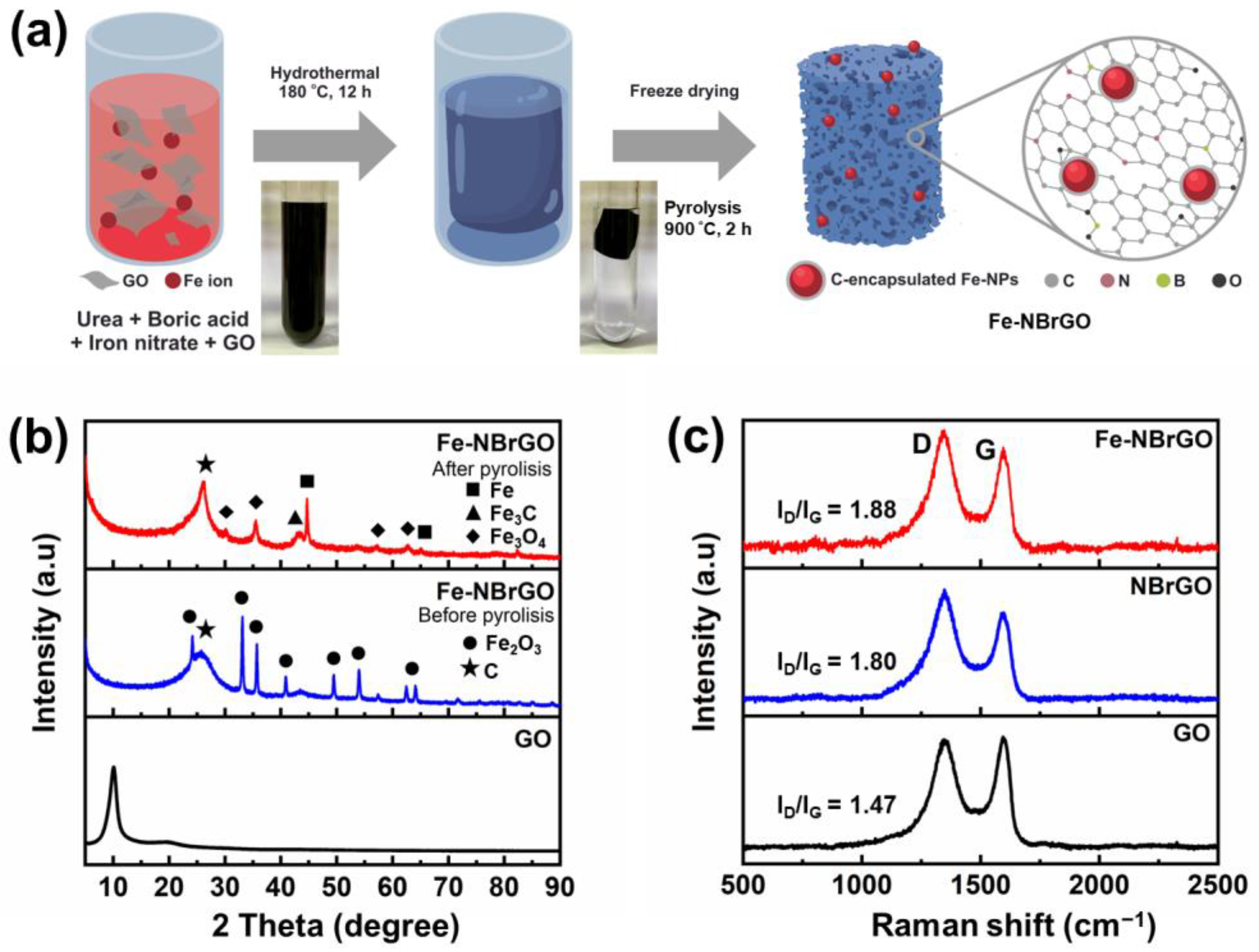
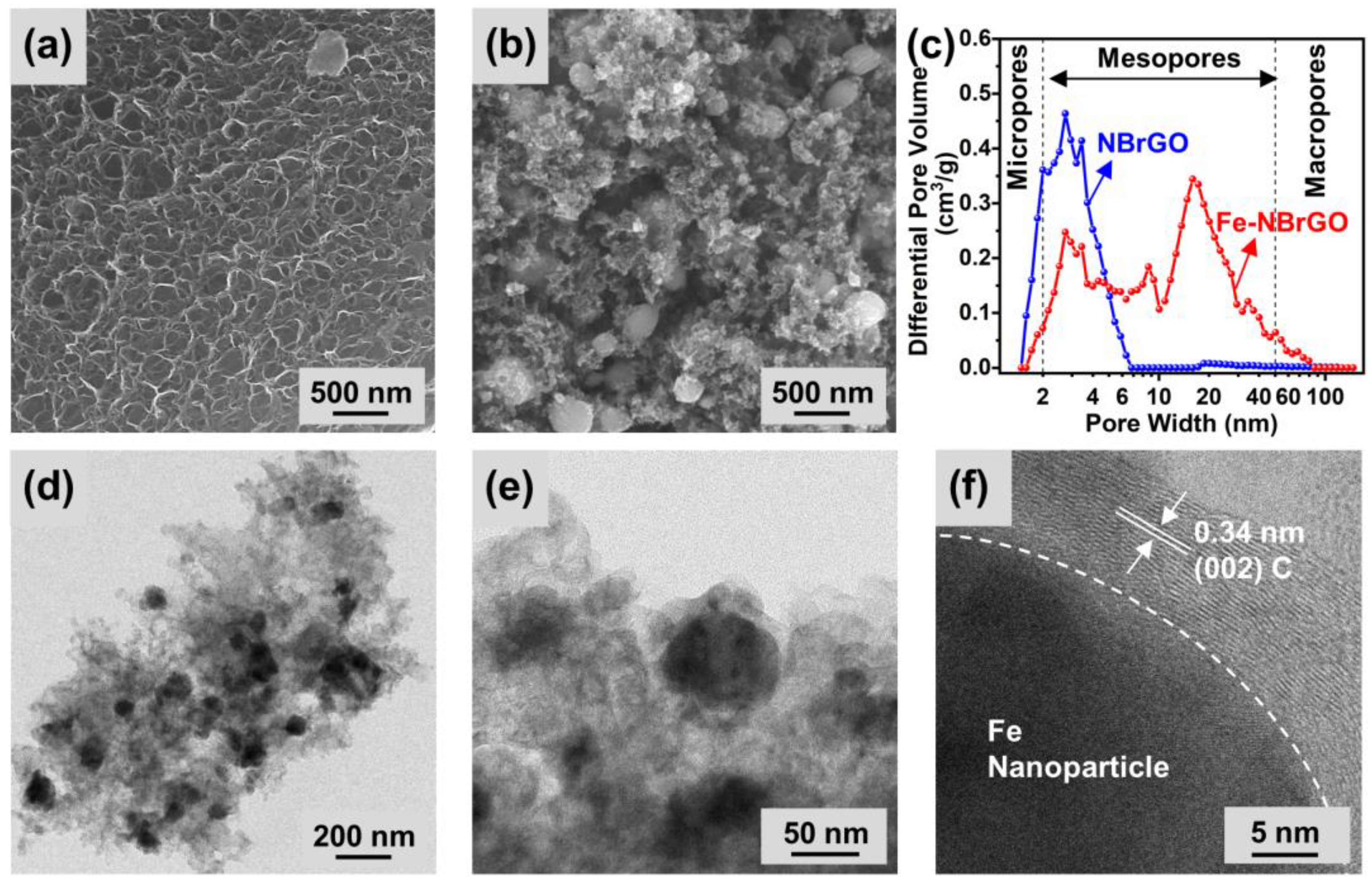
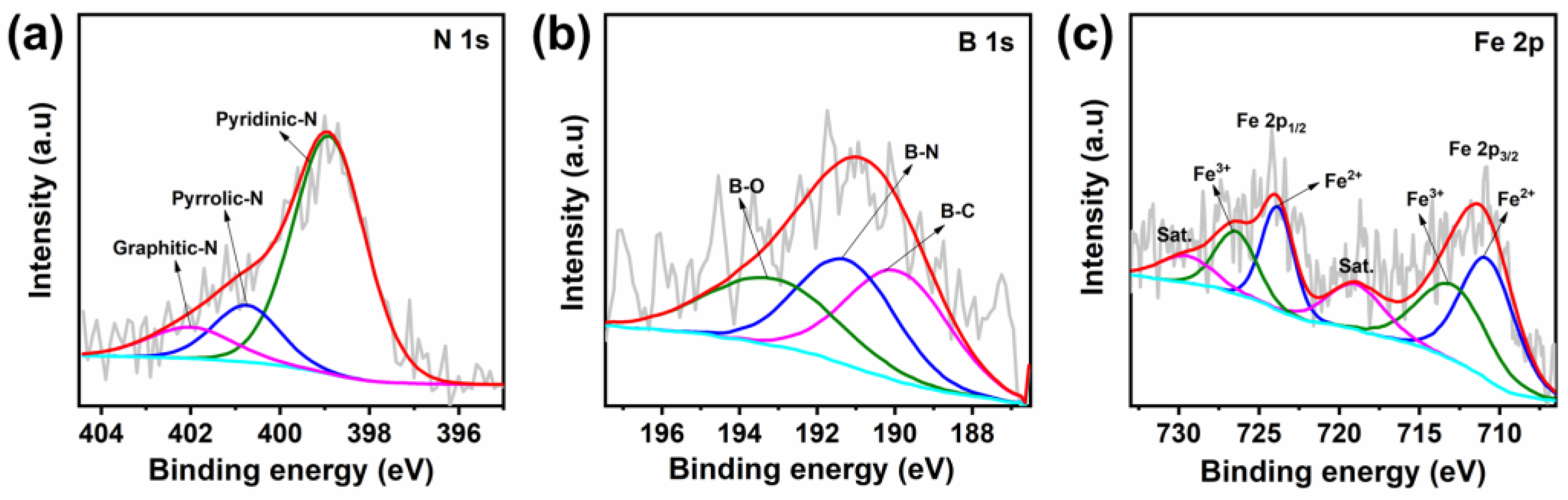
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, M.; Miao, H.; Hu, R.; Sun, Z.; Li, H. Manganese Dioxides for Oxygen Electrocatalysis in Energy Conversion and Storage Systems Over Full pH Range. J. Power Sources 2021, 494, 229779. [Google Scholar] [CrossRef]
- Qiu, Q.; Pan, Z.-Z.; Yao, P.; Yuan, J.; Xia, C.; Zhao, Y.; Li, Y. A 98.2% energy efficiency Li-O2 battery using a LaNi-0.5Co0.5O3 perovskite cathode with extremely fast oxygen reduction and evolution kinetics. Chem. Eng. J. 2023, 452, 139608. [Google Scholar] [CrossRef]
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y.C. Rechargeable Zn-air batteries: Recent trends and future perspectives. Renew. Sust. Energ. Rev. 2022, 154, 111771. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Zheng, G.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Durable rechargeable zinc-air batteries with neutral electrolyte and manganese oxide catalyst. J. Power Sources 2016, 332, 330–336. [Google Scholar] [CrossRef]
- Goh, F.T.; Liu, Z.; Hor, T.A.; Zhang, J.; Ge, X.; Zong, Y.; Yu, A.; Khoo, W. A near-neutral chloride electrolyte for electrically rechargeable zinc-air batteries. J. Electrochem. Soc. 2014, 161, A2080. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Zhou, Z.; Pan, Y.; Yu, Z.; Pei, Z.; Zhao, S.; Wei, L.; Chen, Y. Rechargeable Zinc-Air Batteries with Neutral Electrolytes: Recent Advances, Challenges, and Prospects. EnergyChem 2021, 3, 100055. [Google Scholar] [CrossRef]
- Clark, S.; Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Blázquez, J.A.; Tolchard, J.R.; Latz, A.; Horstmann, B. Towards rechargeable zinc–air batteries with aqueous chloride electrolytes. J. Mater. Chem. A 2019, 7, 11387–11399. [Google Scholar] [CrossRef]
- Prakoso, B.; Mahbub, M.A.A.; Yilmaz, M.; Khoiruddin; Wenten, I.G.; Handoko, A.D.; Sumboja, A. Recent Progress in Extending the Cycle-Life of Secondary Zn-Air Batteries. ChemNanoMat 2021, 7, 354–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.-P.; Wang, J.; Jiang, Y.; Cui, G.; Shui, L.; Yu, A.; Wang, X.; Chen, Z. Recent Progress on Flexible Zn-Air Batteries. Energy Stor. Mater. 2021, 35, 538–549. [Google Scholar] [CrossRef]
- Wu, W.-F.; Yan, X.; Zhan, Y. Recent progress of electrolytes and electrocatalysts in neutral aqueous zinc-air batteries. Chem. Eng. J. 2023, 451, 138608. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. Npj Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Q.; Chen, Z.; Jose, V.; Jiang, X.; Fu, G.; Lee, J.-M.; Huang, S. B, N-doped ultrathin carbon nanosheet superstructure for high-performance oxygen reduction reaction in rechargeable zinc-air battery. Carbon 2020, 164, 398–406. [Google Scholar] [CrossRef]
- Xue, Y.; Yu, D.; Dai, L.; Wang, R.; Li, D.; Roy, A.; Lu, F.; Chen, H.; Liu, Y.; Qu, J. Three-dimensional B,N-doped graphene foam as a metal-free catalyst for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2013, 15, 12220–12226. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, L.; Tian, Y.; Li, H.; Wang, K. Boron and nitrogen co-doped graphene aerogels: Facile preparation, tunable doping contents and bifunctional oxygen electrocatalysis. Carbon 2018, 137, 458–466. [Google Scholar] [CrossRef]
- Pei, Y.; Song, H.; Liu, Y.; Cheng, Y.; Li, W.; Chen, Y.; Fan, Y.; Liu, B.; Lu, S. Boron–nitrogen-doped carbon dots on multi-walled carbon nanotubes for efficient electrocatalysis of oxygen reduction reactions. J. Colloid Interface Sci. 2021, 600, 865–871. [Google Scholar] [CrossRef]
- Cheng, R.; Li, K.; Li, Z.; Jiang, M.; Wang, F.; Yang, Z.; Zhao, T.; Meng, P.; Fu, C. Rational design of boron-nitrogen coordinated active sites towards oxygen reduction reaction in aluminum-air batteries with robust integrated air cathode. J. Power Sources 2023, 556, 232476. [Google Scholar] [CrossRef]
- Hu, X.; Chen, K.; Guo, K.; Xiang, L.; Wen, Z.; Ci, S. N/B Co-doped carbon as metal-free cathode catalyst for high-performance asymmetric neutral-alkaline microbial fuel cell. Electrochim. Acta 2021, 389, 138518. [Google Scholar] [CrossRef]
- Irmawati, Y.; Balqis, F.; Destyorini, F.; Adios, C.G.; Yudianti, R.; Iskandar, F.; Sumboja, A. Cobalt Nanoparticles Encapsulated with N-Doped Bamboo-Like Carbon Nanofibers as Bifunctional Catalysts for Oxygen Reduction/Evolution Reactions in a Wide pH Range. ACS Appl. Nano Mater. 2023, 6, 2708–2718. [Google Scholar] [CrossRef]
- Rojas-Carbonell, S.; Artyushkova, K.; Serov, A.; Santoro, C.; Matanovic, I.; Atanassov, P. Effect of pH on the Activity of Platinum Group Metal-Free Catalysts in Oxygen Reduction Reaction. ACS Catal. 2018, 8, 3041–3053. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, Z.; Han, S.; Deng, L.; Yu, J.; Lin, Y.; Long, X.; Gu, M.; Yang, S. Identifying the Active Sites of a Single Atom Catalyst with pH-Universal Oxygen Reduction Reaction Activity. Cell Rep. Phys. Sci. 2020, 1, 100115. [Google Scholar] [CrossRef]
- Wei, X.; Song, S.; Wu, N.; Luo, X.; Zheng, L.; Jiao, L.; Wang, H.; Fang, Q.; Hu, L.; Gu, W.; et al. Synergistically enhanced single-atomic site Fe by Fe3C@C for boosted oxygen reduction in neutral electrolyte. Nano Energy 2021, 84, 105840. [Google Scholar] [CrossRef]
- Ye, C.-W.; Xu, L. Recent advances in the design of a high performance metal–nitrogen–carbon catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2021, 9, 22218–22247. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Víctor-Román, S.; Sanahuja-Parejo, O.; Benito, A.M.; Maser, W.K. Control of the microstructure and surface chemistry of graphene aerogels via pH and time manipulation by a hydrothermal method. Nanoscale 2018, 10, 3526–3539. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Gai, H.; Chen, Z.; Sun, Z.; Huang, M. An efficient pH-universal electrocatalyst for oxygen reduction: Defect-rich graphitized carbon shell wrapped cobalt within hierarchical porous N-doped carbon aerogel. Mater. Today Energy 2020, 17, 100452. [Google Scholar] [CrossRef]
- Xue, Q.; Ding, Y.; Xue, Y.; Li, F.; Chen, P.; Chen, Y. 3D nitrogen-doped graphene aerogels as efficient electrocatalyst for the oxygen reduction reaction. Carbon 2018, 139, 137–144. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Randy, A.; Dewi, R.T.; Angelina, M.; Yudasari, N.; Rahayu, S.; Ulfah, I.M.; Maryani, F.; Cheng, Y.-W.; Liu, T.-Y. Magnetic Graphene-Based Nanosheets with Pluronic F127-Chitosan Biopolymers Encapsulated & α-Mangosteen Drugs for Breast Cancer Cells Therapy. Polymers 2022, 14, 3163. [Google Scholar] [CrossRef]
- Zheng, C.; Niu, S.; Lv, W.; Zhou, G.; Li, J.; Fan, S.; Deng, Y.; Pan, Z.; Li, B.; Kang, F.; et al. Propelling polysulfides transformation for high-rate and long-life lithium–sulfur batteries. Nano Energy 2017, 33, 306–312. [Google Scholar] [CrossRef]
- Rodríguez-Mata, V.; González-Domínguez, J.M.; Benito, A.M.; Maser, W.K.; García-Bordejé, E. Reduced Graphene Oxide Aerogels with Controlled Continuous Microchannels for Environmental Remediation. ACS Appl. Nano Mater. 2019, 2, 1210–1222. [Google Scholar] [CrossRef]
- Zhu, J.; Xiong, Z.; Zheng, J.; Luo, Z.; Zhu, G.; Xiao, C.; Meng, Z.; Li, Y.; Luo, K. Nitrogen-doped graphite encapsulated Fe/Fe3C nanoparticles and carbon black for enhanced performance towards oxygen reduction. J. Mater. Sci. Technol. 2019, 35, 2543–2551. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, J.; Chen, Y.; Li, Z.; Chen, J.; Wang, G.; Wang, R. MOF-derived porous carbon supported iron-based catalysts with optimized active sites towards oxygen reduction reaction. J. Electroanal. Chem. 2019, 847, 113191. [Google Scholar] [CrossRef]
- Tylus, U.; Jia, Q.; Strickland, K.; Ramaswamy, N.; Serov, A.; Atanassov, P.; Mukerjee, S. Elucidating Oxygen Reduction Active Sites in Pyrolyzed Metal–Nitrogen Coordinated Non-Precious-Metal Electrocatalyst Systems. J. Phys. Chem. C 2014, 118, 8999–9008. [Google Scholar] [CrossRef]
- Liang, Z.; Xia, W.; Qu, C.; Qiu, B.; Tabassum, H.; Gao, S.; Zou, R. Edge-Abundant Porous Fe3O4 Nanoparticles Docking in Nitrogen-Rich Graphene Aerogel as Efficient and Durable Electrocatalyst for Oxygen Reduction. ChemElectroChem 2017, 4, 2442–2447. [Google Scholar] [CrossRef]
- Nugroho, A.; Wahyudhi, A.; Oktaviano, H.S.; Yudianti, R.; Hardiansyah, A.; Destyorini, F.; Irmawati, Y. Effect of Iron Loading on Controlling Fe/N−C Electrocatalyst Structure for Oxygen Reduction Reaction. ChemistrySelect 2022, 7, e202202042. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Y.; Zhu, Q.; Fan, L.; Wu, Y.; Li, Z.; Xiong, S.; Gu, F. Co/Co–N/Co-O Rooted on rGO Hybrid BCN Nanotube Arrays as Efficient Oxygen Electrocatalyst for Zn–Air Batteries. ACS Appl. Mater. Interfaces 2022, 14, 17249–17258. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Hu, C.; Sedghi, S.; Silvestre-Albero, A.; Andersson, G.G.; Sharma, A.; Pendleton, P.; Rodríguez-Reinoso, F.; Kaneko, K.; Biggs, M.J. Raman Spectroscopy Study of The Transformation of The Carbonaceous Skeleton of A Polymer-Based Nanoporous Carbon Along The Thermal Annealing Pathway. Carbon 2015, 85, 147–158. [Google Scholar] [CrossRef]
- Kang, X.; Fu, G.; Si, F.; Deng, X.; Wang, L.; Fu, X.-Z.; Luo, J.-L. Iron and boron-doped carbonized zeolitic imidazolate frameworks as efficient oxygen reduction electrocatalysts for Al-Air batteries. Int. J. Hydrogen Energy 2021, 46, 36221–36231. [Google Scholar] [CrossRef]
- Wei, X.; Luo, X.; Wang, H.; Gu, W.; Cai, W.; Lin, Y.; Zhu, C. Highly-defective Fe-N-C catalysts towards pH-Universal oxygen reduction reaction. Appl. Catal. B 2020, 263, 118347. [Google Scholar] [CrossRef]
- Wu, X.; Chen, K.; Lin, Z.; Zhang, Y.; Meng, H. Nitrogen doped graphitic carbon from biomass as non noble metal catalyst for oxygen reduction reaction. Mater. Today Energy 2019, 13, 100–108. [Google Scholar] [CrossRef]
- Tian, H.; Liang, J.; Liu, J. Nanoengineering Carbon Spheres as Nanoreactors for Sustainable Energy Applications. Adv. Mater. 2019, 31, 1903886. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, M.A.A.; Adios, C.G.; Xu, M.; Prakoso, B.; LeBeau, J.M.; Sumboja, A. Red Bean Pod Derived Heterostructure Carbon Decorated with Hollow Mixed Transition Metals as a Bifunctional Catalyst in Zn-Air Batteries. Chem. Asian J. 2021, 16, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, Z.; Leng, K.; Ma, S.; Wang, Y.; Bai, J. Biomass wood-derived efficient Fe–N–C catalysts for oxygen reduction reaction. J. Mater. Sci. 2021, 56, 12764–12774. [Google Scholar] [CrossRef]
- Sgarbi, R.; Kumar, K.; Jaouen, F.; Zitolo, A.; Ticianelli, E.A.; Maillard, F. Oxygen reduction reaction mechanism and kinetics on M-NxCy and M@N-C active sites present in model M-N-C catalysts under alkaline and acidic conditions. J. Solid State Electrochem. 2021, 25, 45–56. [Google Scholar] [CrossRef]
- Noh, S.H.; Seo, M.H.; Kang, J.; Okajima, T.; Han, B.; Ohsaka, T. Towards a comprehensive understanding of FeCo coated with N-doped carbon as a stable bi-functional catalyst in acidic media. NPG Asia Mater. 2016, 8, e312. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Hu, X.; Bi, L.; Cai, P.; Jia, J.; Chai, G.; Wei, S.; Dai, L.; Wen, Z. Fe Vacancies Induced Surface FeO6 in Nanoarchitectures of N-Doped Graphene Protected β-FeOOH: Effective Active Sites for pH-Universal Electrocatalytic Oxygen Reduction. Adv. Funct. Mater. 2018, 28, 1803330. [Google Scholar] [CrossRef]
- Mo, Q.; Chen, N.; Deng, M.; Yang, L.; Gao, Q. Metallic Cobalt@Nitrogen-Doped Carbon Nanocomposites: Carbon-Shell Regulation toward Efficient Bi-Functional Electrocatalysis. ACS Appl. Mater. Interfaces 2017, 9, 37721–37730. [Google Scholar] [CrossRef]
- Wu, W.; Lin, F.; Yang, X.; Wang, B.; Lu, X.; Chen, Q.; Ye, F.; Zhao, S. Facile synthesis of magnetic carbon nanotubes derived from ZIF-67 and application to magnetic solid-phase extraction of profens from human serum. Talanta 2020, 207, 120284. [Google Scholar] [CrossRef]
- Chen, T.; Cao, J.; Bao, X.; Peng, Y.; Liu, L.; Fu, W. Co nanoparticles decorated with N-doped carbon nanotubes as high-efficiency catalysts with intrinsic oxidase-like property for colorimetric sensing. RSC Adv. 2021, 11, 39966–39977. [Google Scholar] [CrossRef]
- Wang, L.; Liang, K.; Deng, L.; Liu, Y.-N. Protein hydrogel networks: A unique approach to heteroatom self-doped hierarchically porous carbon structures as an efficient ORR electrocatalyst in both basic and acidic conditions. Appl. Catal. B 2019, 246, 89–99. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Xu, A.-W. Noble-Metal-Free Fe–N/C Catalyst for Highly Efficient Oxygen Reduction Reaction under Both Alkaline and Acidic Conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef]
- Jafari, M.; Gharibi, H.; Parnian, M.J.; Nasrollahpour, M.; Vafaee, M. Iron-Nanoparticle-Loaded Nitrogen-Doped Carbon Nanotube/Carbon Sheet Composites Derived from MOF as Electrocatalysts for an Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2021, 4, 459–477. [Google Scholar] [CrossRef]
- Li, C.; He, C.; Sun, F.; Wang, M.; Wang, J.; Lin, Y. Incorporation of Fe3C and Pyridinic N Active Sites with a Moderate N/C Ratio in Fe–N Mesoporous Carbon Materials for Enhanced Oxygen Reduction Reaction Activity. ACS Appl. Nano Mater. 2018, 1, 1801–1810. [Google Scholar] [CrossRef]
- Chisaka, M.; Iijima, T.; Ishihara, Y.; Suzuki, Y.; Inada, R.; Sakurai, Y. Carbon catalyst codoped with boron and nitrogen for oxygen reduction reaction in acid media. Electrochim. Acta 2012, 85, 399–410. [Google Scholar] [CrossRef]
- Yuan, K.; Sfaelou, S.; Qiu, M.; Lützenkirchen-Hecht, D.; Zhuang, X.; Chen, Y.; Yuan, C.; Feng, X.; Scherf, U. Synergetic Contribution of Boron and Fe–Nx Species in Porous Carbons toward Efficient Electrocatalysts for Oxygen Reduction Reaction. ACS Energy Lett. 2018, 3, 252–260. [Google Scholar] [CrossRef]
- Cao, C.; Wei, L.; Wang, G.; Shen, J. Superiority of boron, nitrogen and iron ternary doped carbonized graphene oxide-based catalysts for oxygen reduction in microbial fuel cells. Nanoscale 2017, 9, 3537–3546. [Google Scholar] [CrossRef]
- Shi, J.; Shao, H.; Yang, F.; Li, J.; Fan, L.; Cai, W. Dual-template induced multi-scale porous Fe@FeNC oxygen reduction catalyst for high-performance electrochemical devices. Chem. Eng. J. 2022, 445, 136628. [Google Scholar] [CrossRef]
- Wang, T.; Sun, C.; Yan, Y.; Li, F. Understanding the active sites of Fe–N–C materials and their properties in the ORR catalysis system. RSC Adv. 2022, 12, 9543–9549. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, P.; Zhang, J.; Hu, Y.; Amiinu, I.S.; Wang, X.; Zhou, J.; Xia, H.; Song, Z.; Xu, Q.; et al. Carbon Nanosheets Containing Discrete Co-Nx-By-C Active Sites for Efficient Oxygen Electrocatalysis and Rechargeable Zn–Air Batteries. ACS Nano 2018, 12, 1894–1901. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, M.; Zhang, Z.; Cui, G. Cobalt-boron-oxide supported on N, P dual-doped carbon nanosheets as the trifunctional electrocatalyst and its application in rechargeable Zn-air battery and overall water-electrolysis. Electrochim. Acta 2019, 327, 134980. [Google Scholar] [CrossRef]
- Wei, C.; Sun, S.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.J. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef]
- Sumboja, A.; Prakoso, B.; Ma, Y.; Irwan, F.R.; Hutani, J.J.; Mulyadewi, A.; Mahbub, M.A.A.; Zong, Y.; Liu, Z. FeCo Nanoparticle-Loaded Nutshell-Derived Porous Carbon as Sustainable Catalyst in Al-Air Batteries. Energy Mater. Adv. 2021, 2021, 738621. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, F.; Han, L.; Xiao, J.; Zeng, X.; Zhang, C.; Dong, P.; Li, M.; Zhang, Y. Manganese Oxide/Iron Carbide Encapsulated in Nitrogen and Boron Codoped Carbon Nanowire Networks as Accelerated Alkaline Hydrogen Evolution and Oxygen Reduction Bifunctional Electrocatalysts. ACS Appl. Mater. Interfaces 2022, 14, 13280–13294. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Paul, A. Synergistic Effect of Oxygen and Nitrogen Co-doping in Metal–Organic Framework-Derived Ultramicroporous Carbon for an Exceptionally Stable Solid-State Supercapacitor via a “Proton Trap” Mechanism. Energy Fuels 2021, 35, 10262–10273. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wang, Z.; Li, P.; Zhao, J.; Zhao, H.; Li, D.; He, T.; Wei, Y.; Su, Y.; et al. Boron doping induced electronic reconfiguration of Fe-Nx sites in N-doped carbon matrix for efficient oxygen reduction reaction in both alkaline and acidic media. Int. J. Hydrogen Energy 2022, 47, 18663–18674. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Bi, Z.; Wang, Y.; Zhang, H.; Zhou, X.; Wang, Q.; Zhou, Y.; Wang, H.; Hu, G. Boron modulating electronic structure of FeN4C to initiate high-efficiency oxygen reduction reaction and high-performance zinc-air battery. J. Energy Chem. 2022, 66, 514–524. [Google Scholar] [CrossRef]
- Fajrial, A.K.; Saputro, A.G.; Agusta, M.K.; Rusydi, F.; Nugraha; Dipojono, H.K. First principles study of oxygen molecule interaction with the graphitic active sites of a boron-doped pyrolyzed Fe–N–C catalyst. Phys. Chem. Chem. Phys. 2017, 19, 23497–23504. [Google Scholar] [CrossRef]
- Mulyadewi, A.; Mahbub, M.A.A.; Irmawati, Y.; Balqis, F.; Adios, C.G.; Sumboja, A. Rechargeable Zinc–Air Batteries with Seawater Electrolyte and Cranberry Bean Shell-Derived Carbon Electrocatalyst. Energy Fuels 2022, 36, 5475–5482. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.-X.; Liu, J.-N.; Ren, D.; Li, B.-Q.; Huang, J.-Q.; Zhang, Q. Quantitative kinetic analysis on oxygen reduction reaction: A perspective. Nano Mater. Sci. 2021, 3, 313–318. [Google Scholar] [CrossRef]
- Antipin, D.; Risch, M. Calculation of the Tafel slope and reaction order of the oxygen evolution reaction between pH 12 and pH 14 for the adsorbate mechanism. Electrochem. Sci. Adv. 2022, e2100213. [Google Scholar] [CrossRef]
- Niu, H.-J.; Chen, S.-S.; Feng, J.-J.; Zhang, L.; Wang, A.-J. Assembled hollow spheres with CoFe alloyed nanocrystals encapsulated in N, P-doped carbon nanovesicles: An ultra-stable bifunctional oxygen catalyst for rechargeable Zn-air battery. J. Power Sources 2020, 475, 228594. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.; Zhang, C.; Zhang, Y.; Jiang, H.; Wang, H.; Su, G.; Huang, M.; Toghan, A. Engineering solid–liquid-gas interfaces of single-atom cobalt catalyst for enhancing the robust stability of neutral Zn-air batteries under high current density. Chem. Eng. J. 2022, 433, 133685. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B.; Ran, J.; Liu, P.; Gao, D. Fe-based species anchored on N-doped carbon nanotubes as a bifunctional electrocatalyst for acidic/neutral/alkaline Zn–air batteries. Nanotechnology 2020, 31, 265402. [Google Scholar] [CrossRef]
- Goh, F.W.T.; Liu, Z.; Ge, X.; Zong, Y.; Du, G.; Hor, T.S.A. Ag nanoparticle-modified MnO2 nanorods catalyst for use as an air electrode in zinc–air battery. Electrochim. Acta 2013, 114, 598–604. [Google Scholar] [CrossRef]
- Sabaa, H.M.; El-Khatib, K.M.; El-Kady, M.Y.; Mahmoud, S.A. Spinel structure of activated carbon supported MFe2O4 composites as an economic and efficient electrocatalyst for oxygen reduction reaction in neutral media. J. Solid State Electrochem. 2022, 26, 2749–2763. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, P.; Zhou, T.; Xu, K.; Chu, W.; Wu, C.; Xie, Y. A Bifunctional Hybrid Electrocatalyst for Oxygen Reduction and Evolution: Cobalt Oxide Nanoparticles Strongly Coupled to B,N-Decorated Graphene. Angew. Chem. Int. Ed. 2017, 56, 7121–7125. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, M.; Wang, X.; Wang, H.; Yin, Z.; Tan, X.; Li, Y. Preparing Co/N-Doped Carbon as Electrocatalyst toward Oxygen Reduction Reaction via the Ancient “Pharaoh’s Snakes” Reaction. Batteries 2022, 8, 150. [Google Scholar] [CrossRef]
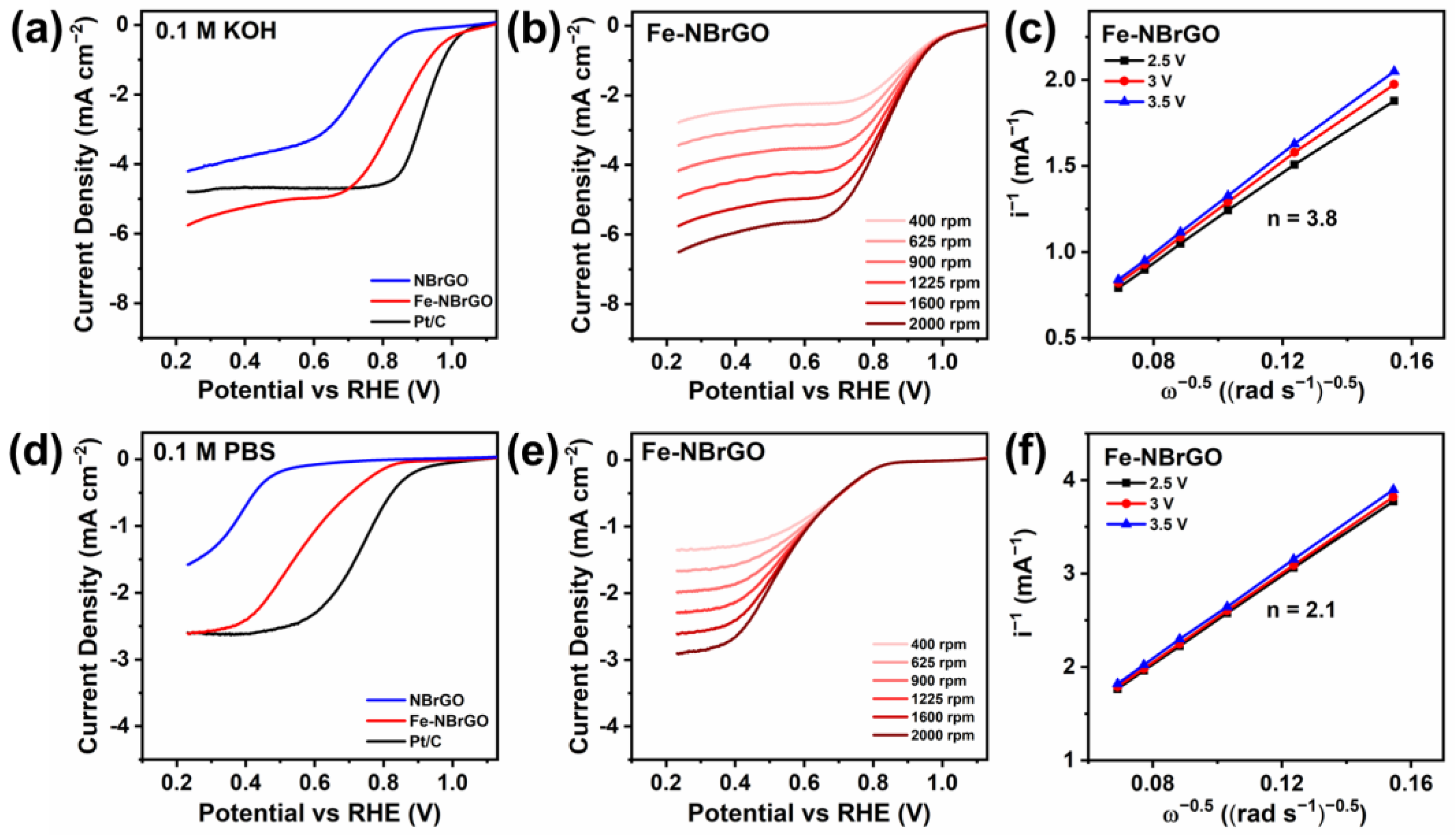
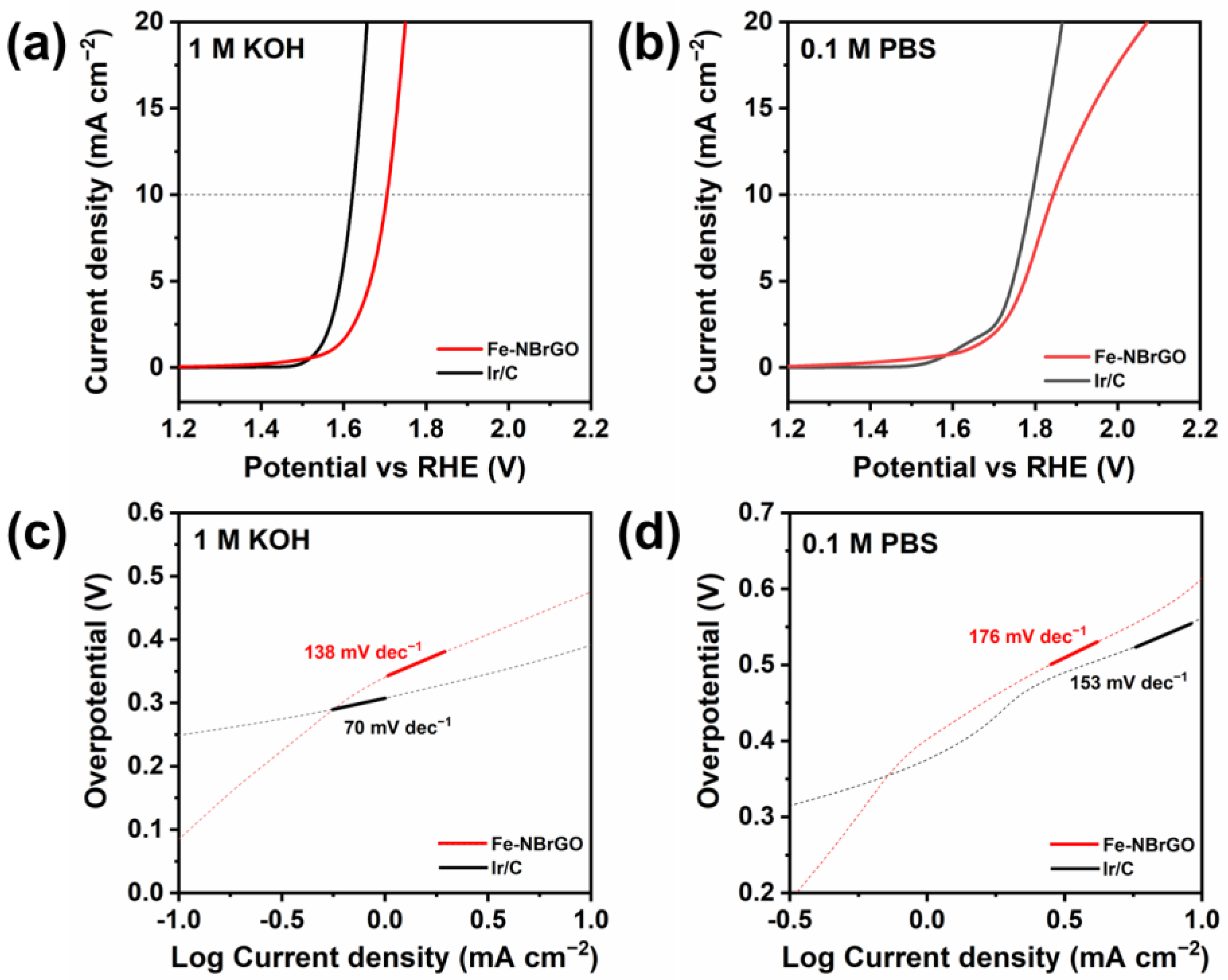
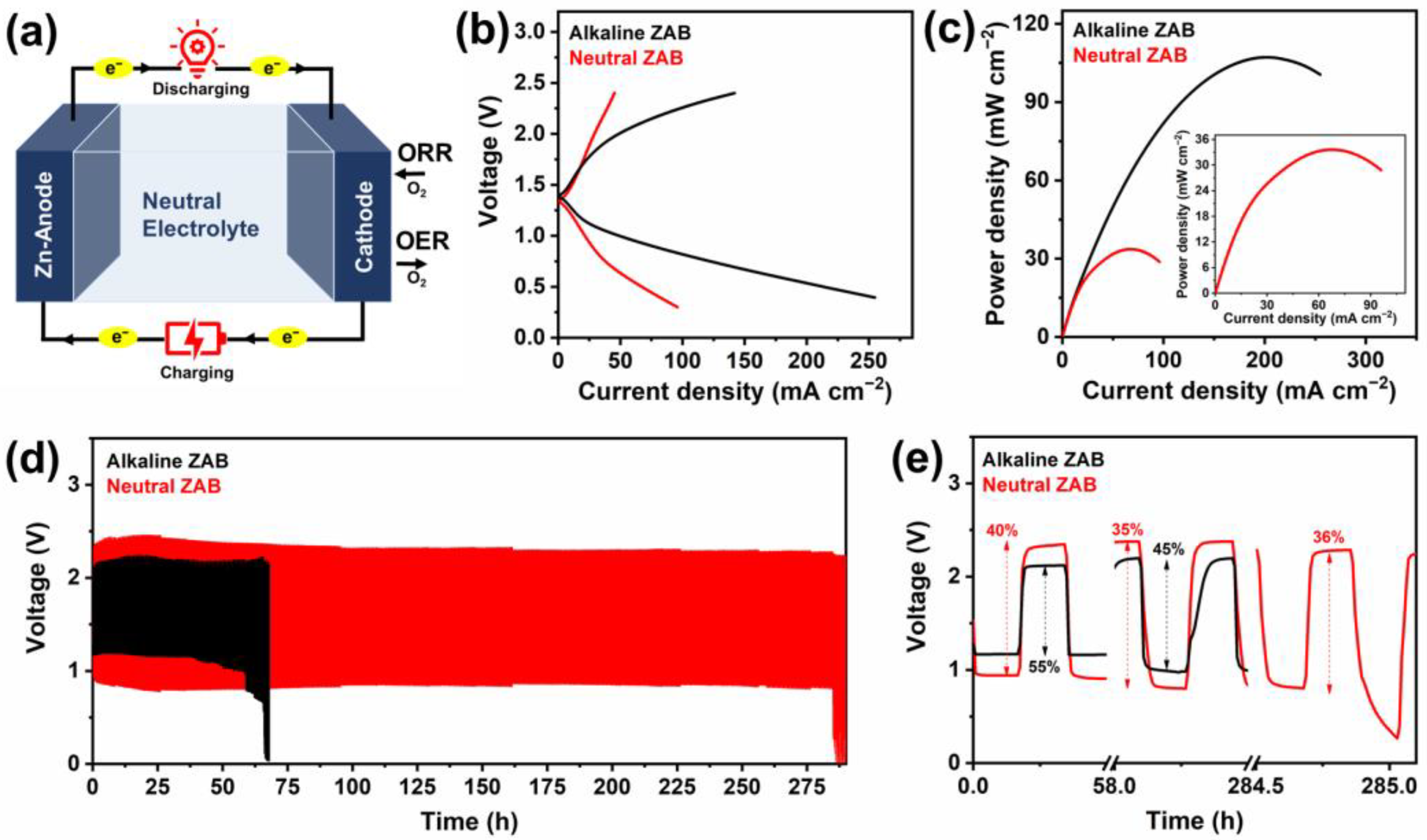
| Sample | Eonset (V vs. RHE) | E1/2 (V vs. RHE) | JL (mA cm−2) |
|---|---|---|---|
| 0.1 M KOH | |||
| NBrGO | 0.954 | 0.711 | 4.20 |
| Fe-NBrGO | 1.074 | 0.826 | 5.75 |
| 0.1 M PBS | |||
| NBrGO | 0.566 | 0.382 | 1.58 |
| Fe-NBrGO | 0.817 | 0.567 | 2.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irmawati, Y.; Balqis, F.; Persada, P.B.; Destyorini, F.; Yudianti, R.; Iskandar, F.; Sumboja, A. Iron-Decorated Nitrogen/Boron co-Doped Reduced Graphene Oxide Aerogel for Neutral Rechargeable Zn-Air Batteries. Batteries 2023, 9, 356. https://doi.org/10.3390/batteries9070356
Irmawati Y, Balqis F, Persada PB, Destyorini F, Yudianti R, Iskandar F, Sumboja A. Iron-Decorated Nitrogen/Boron co-Doped Reduced Graphene Oxide Aerogel for Neutral Rechargeable Zn-Air Batteries. Batteries. 2023; 9(7):356. https://doi.org/10.3390/batteries9070356
Chicago/Turabian StyleIrmawati, Yuyun, Falihah Balqis, Pilar Bela Persada, Fredina Destyorini, Rike Yudianti, Ferry Iskandar, and Afriyanti Sumboja. 2023. "Iron-Decorated Nitrogen/Boron co-Doped Reduced Graphene Oxide Aerogel for Neutral Rechargeable Zn-Air Batteries" Batteries 9, no. 7: 356. https://doi.org/10.3390/batteries9070356
APA StyleIrmawati, Y., Balqis, F., Persada, P. B., Destyorini, F., Yudianti, R., Iskandar, F., & Sumboja, A. (2023). Iron-Decorated Nitrogen/Boron co-Doped Reduced Graphene Oxide Aerogel for Neutral Rechargeable Zn-Air Batteries. Batteries, 9(7), 356. https://doi.org/10.3390/batteries9070356