Abstract
Ti3C2Tx–Fe3O4–carbon nanotube composites were prepared for electrochemical energy storage in the negative electrodes of supercapacitors. The electrodes show a remarkably high areal capacitance of 6.59 F cm−2 in a neutral Na2SO4 electrolyte, which was obtained by the development of advanced nanofabrication strategies and due to the synergistic effect of the individual components. Enhanced capacitance was achieved using the in-situ synthesis method for the Fe3O4 nanoparticles. The superparamagnetic behavior of the Fe3O4 nanoparticles facilitated the fabrication of electrodes with a reduced binder content. Good mixing of the components was achieved using a celestine blue co-dispersant, which adsorbed on the inorganic components and carbon nanotubes and facilitated their co-dispersion and mixing. The capacitive behavior was optimized by the variation of the electrode composition and mass loading in a range of 30–45 mg cm−2. An asymmetric device was proposed and fabricated, which contained a Ti3C2Tx–Fe3O4–carbon nanotube negative electrode and a polypyrrole–carbon nanotube positive electrode for operation in an Na2SO4 electrolyte. The asymmetric supercapacitor device demonstrated high areal capacitance and excellent power-density characteristics in an enlarged voltage window of 1.6 V. This investigation opens a new avenue for the synthesis and design of MXene-based asymmetric supercapacitors for future energy storage devices.
1. Introduction
Advanced energy storage technology is of significance today, due to the energy crises challenge worldwide [1,2,3,4,5]. MXenes have been extensively investigated as new energy storage materials [6,7,8] since their discovery in 2011 [9]. Due to their large surface area, good conductivity and rich surface chemistry, MXenes have demonstrated great potential for the development of high performance pseudocapacitors [10,11,12,13]. As the most widely studied material among the various types of MXenes, Ti3C2Tx with the accordion-like particle shape contains alternation of Ti and C layers with a number of functional terminations, such as -O, -F and -OH [9].
Many efforts have been made to enhance the electrochemical performance of Ti3C2Tx in composites with polymers [14,15,16,17], oxides [18,19,20], hydroxides [21,22] and carbon-based materials [23,24,25]. These studies showed that combining Ti3C2Tx with other capacitive materials is a promising strategy for the development of advanced supercapacitor electrodes.
Fe-based materials, such as FeOOH, Fe2O3 and Fe3O4, exhibit high capacitance in a relatively large negative potential range [26,27,28,29,30]. Therefore, they have great potential to be considered as candidates for composites with MXene with enhanced electrochemical performance. Ma et al. [31] employed Fe2O3 nanoparticles intercalating flexible MXene hybrid paper and achieved an ultrahigh volumetric capacitance of 2607 F cm−3 as well as excellent cycling performance. In another research, a freestanding Ti3C2/FeOOH hybrid film was designed in which amorphous FeOOH quantum dots acted as pillars to prevent restacking of MXene sheets [32]. A flexible electrode was fabricated by few-layered Ti3C2Tx MXenes coupling with Fe2O3 nanorod arrays and grown on carbon cloth, which presented a remarkable areal capacitance of 725 mF cm−2 at a current density of 1 mA cm−2 [33]. It has been proven that MXene can provide iron oxides/hydroxides with efficient ion/electron transport pathways, so the electronic conductivity of the composites can be enhanced [34,35,36].
Although the tremendous efforts have been made to develop the advanced MXene electrodes with excellent electrochemical performance, very limited research paid attention to the significance of achieving high active mass loading (AML) of electrodes for supercapacitors [37,38,39,40,41,42]. For practical applications, the AML is required to be at least 10 to 20 mg cm−2 [43,44,45]. High AML is of great importance to reduce the relative mass of current collectors and other in-active components in the total mass of electrodes and devices. Designing of supercapacitors with high active mass loading will be beneficial to meet the demand of manufacturing. However, with increasing AML, the diffusion of electrolyte in the electrode and the conductivity will be drastically decreased. As the result, low capacitance and poor rate performance can be expected.
In this research, we fabricated MXene-Fe3O4-CNT anodes with high AML in the range of 30-45 mg cm−2, optimized their composition and performance and application for the fabrication of asymmetric supercapacitor devices. Fe3O4 was selected because of its high theoretical capacitance(2299 F g−1), earth abundance and low price [46]. Moreover, it exhibits advanced ferrimagnetic properties, which can be controlled by its particle size and synthetic strategy [47,48]. So Fe3O4 can impart magnetic properties to the composite materials. This offers benefits of reduced binder content in the composite electrodes and opens the gate for the fabrication of electrodes with advanced functionality. The successful synthesis of composite materials is based on the enhanced co-dispersion and improved mixing of different components, which can be attributed to the application of efficient dispersing agent celestine blue and advanced in-situ synthesis method. We analyzed the influence of electrode composition on electrochemical performance. The fabricated negative electrode showed high areal capacitance of 6.59 F cm−2 at a sweep rate of 2 mV s−1 at 40 mg cm−2 AML. Combining negative MXene-Fe3O4-carbon nanotube with positive polypyrrole (PPy)-MCNT electrode, we fabricated advanced asymmetric supercapacitor device working in enlarged voltage window of 1.6 V and showing high power-density characteristics.
2. Materials and Methods
2.1. Starting Materials
FeCl2·4H2O, FeCl3·6H2O, NH4OH, celestine blue (CB), pyrrole (Py), pyrocatechol violet (PCV), ammonium persulfate (APS), sodium sulphate (Na2SO4) and poly (vinyl butyral-co-vinyl alcohol-co-vinyl acetate) (PVBAA, 65 kDa) were ordered from Millipore Sigma company. Multiwalled carbon nanotubes (MCNT, diameter = 13 nm, length = 1–2 μm) were purchased from Bayer Corporation. Ti3C2Tx (BET surface area 5.03 m2 g−1) was supplied by Laizhou Kai Kai Ceramic Materials Co., Ltd., Weifang, China. Commercial Ni foam (surface area 2.01 m2 g−1, Vale) with 95% porosity was used as a current collector.
2.2. Synthesis of Composite Materials
In method 1, the synthesis procedure for Fe3O4 was similar to that described in a previous investigation [49]. Fe3O4 particles were precipitated from a solution containing Fe2+ and Fe3+ in a molar ratio of 1:2 at a temperature of 50 °C by adding 2 M NH4OH for pH adjustment to pH = 9. After washing, vacuum filtration and drying in a furnace overnight at 60 °C, the powders were mixed with Ti3C2Tx and MCNT in a mass ratio of 6:3:1 of Ti3C2Tx:Fe3O4:MCNT, and then dispersed in water using CB dispersant under probe sonication for 5 min.
Method 2 involved the in-situ synthesis of composites by the precipitation of Fe3O4 in the presence of co-dispersed Ti3C2Tx and MCNT. The suspensions of Ti3C2Tx and MCNT were co-dispersed by CB under probe sonication, then combined with the solution containing Fe2+ and Fe3+ in a ratio of 1:2. The pH adjustment was achieved by injection of 2 M NH4OH dropwise to 9, keeping the temperature at 50 °C. This was followed by washing, vacuum filtrating and drying processes; the powders were then ready for electrode preparation. The mass ratios between Ti3C2Tx, Fe3O4 and MCNT were 60:30:10, 55:30:15 and 55:25:20, and the obtained materials were named as 60Ti3C2Tx–30Fe3O4–10MCNT, 55Ti3C2Tx–30Fe3O4–15MCNT and 55Ti3C2Tx–25Fe3O4–20MCNT, respectively.
The synthesis of polypyrrole(PPy)–MCNT composites followed a previous work [50]. In a typical procedure, 24 µL of pyrrole monomer was injected into the well-dispersed MCNT suspension for magnetic stirring for 5 h under 4 °C. The polymerization was achieved by addition of APS as the oxidant by a mass ratio APS:Py = 1:1. PCV was applied as a dopant for the polymerization of pyrrole and as a dispersant for MCNT, and the molar ratio was PCV:Py = 1:10. The precipitate was washed, vacuum filtrated and dried at room temperature.
2.3. Fabrication of Electrodes
The obtained materials were grinded into fine powders and used for preparation of slurries in ethanol containing 3% PVBAA binder, then impregnated into a commercial nickel foam current collector. The mass of active materials of electrodes was 30–45 mg cm−2.
2.4. Characterization
The Quantum Design Magnetic Properties Measurement System was used to perform the magnetic measurements. The morphology study was performed by scanning electron spectroscopy (SEM) by JEOL JSM-7000F and transmission electron microscopy (TEM) by Talos 200X. EIS and CV were conducted by AMETEK 2273 potentiostat (Princeton). GCD was tested by Biologic AMP 300 potentiostat. For electrochemical testing, a 0.5 M Na2SO4 solution was applied as an electrolyte, and a Pt gauze and a saturated calomel electrode (SCE) were used as the counter and reference electrodes, respectively. For CV and GCD testing, the negative electrodes were tested in the potential range from −1.1 to −0.3 V. The potential range from −0.4 to +0.5 V was applied for positive electrode testing. The device was tested in a voltage window of 1.6 V. The mass- and areal-normalized capacitances (Cm and Cs, respectively) were calculated from the CV and GCD data according to the Equations (1)–(4):
where I denotes current, V is potential, m is electrode active mass, stands for scan rate, A represents the area of electrode, is the potential range, and t is the discharge time.
EIS measurements of complex impedance Z* were performed at 5 mV AC signal and components of complex capacitance C* were obtained according to the Equations (5)–(8):
Z* = Z′ − iZ″
C* = C′ − iC″
3. Results and Discussions
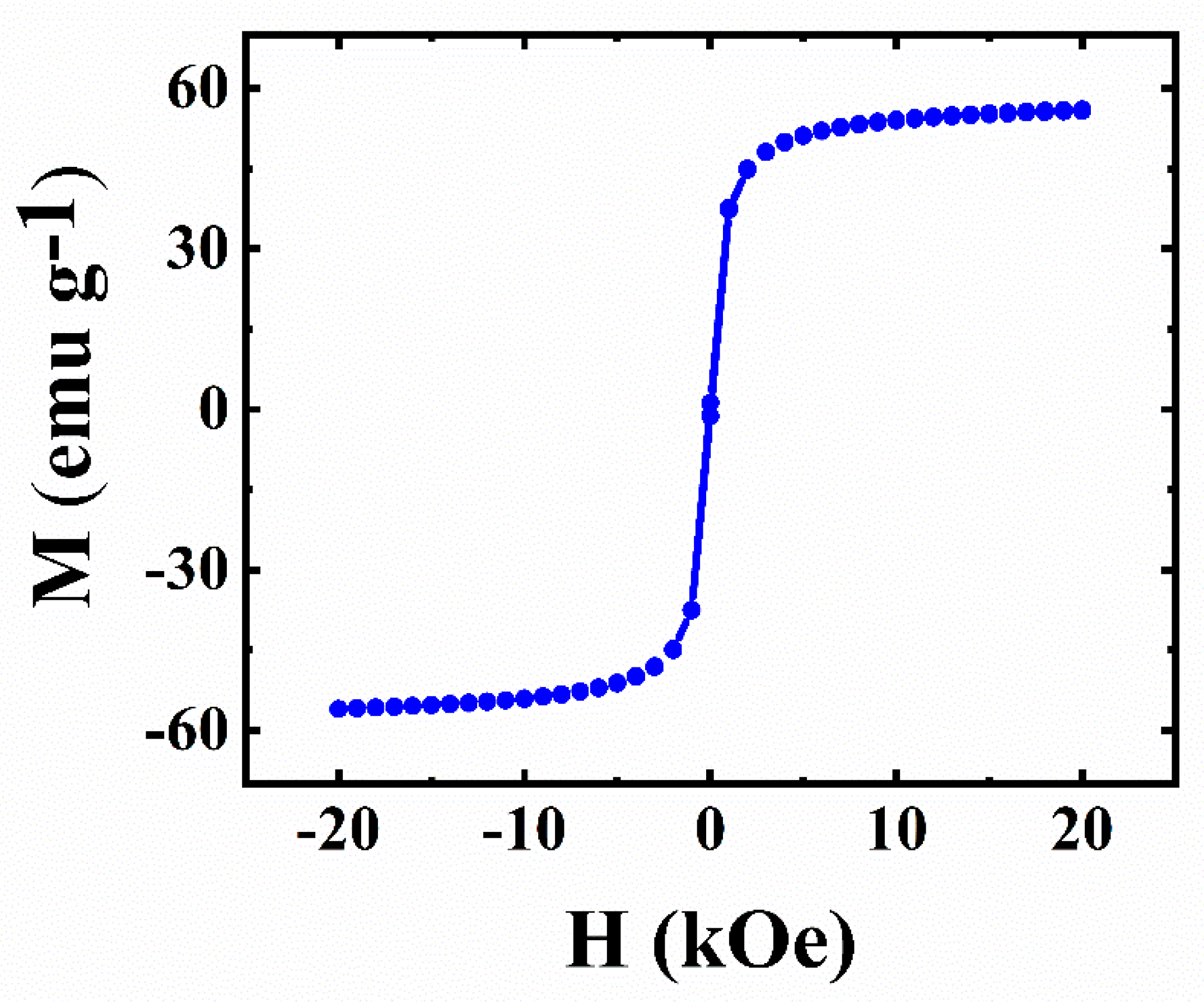
Fe3O4 is a ferrimagnetic material. Magnetic measurement of as-prepared Fe3O4 indicated its superparamagnetic behavior at room temperature (Figure 1). Such behaviour resulted from the use of small Fe3O4 particles. Magnetic particles facilitated the fabrication of the electrodes with reduced content of non-conductive binder. Moreover, magnetic particles added advanced functionality to the composite electrodes.
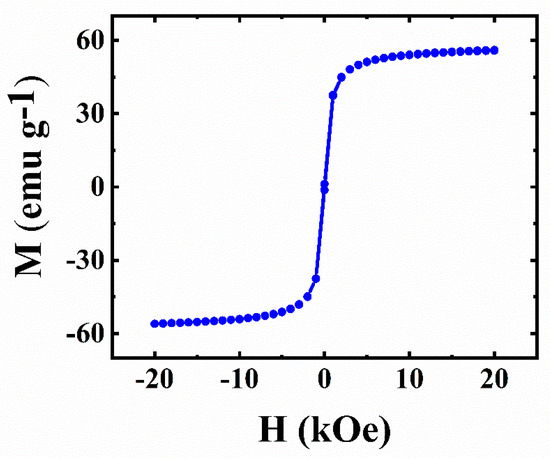
Figure 1.
Magnetization (M) versus magnetic field (H) for Fe3O4.
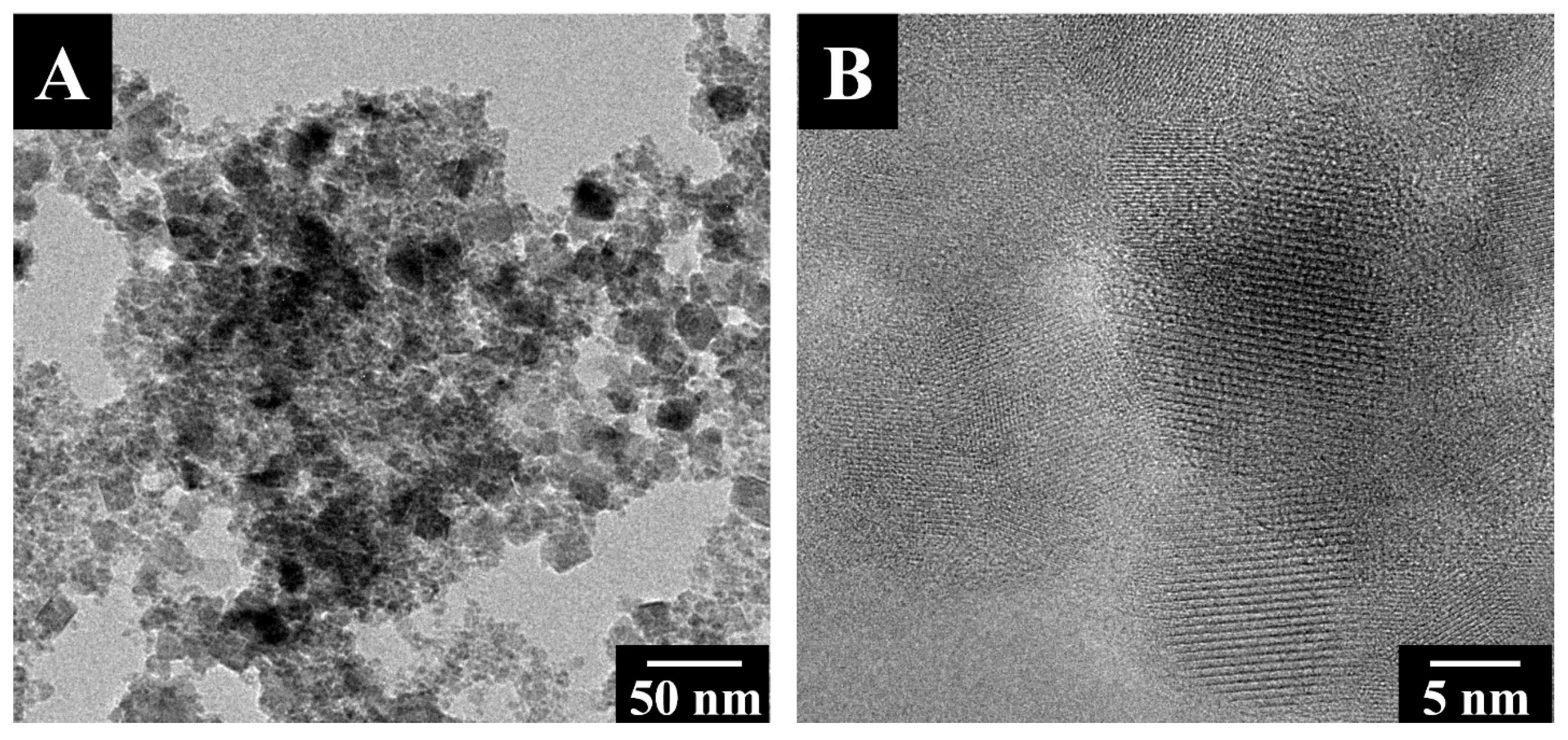
The TEM images in Figure 2 show that the typical size of the as-synthesized Fe3O4 particles was about 10–15 nm, typically smaller than the superparamagnetic critical size of ~25 nm [47]. The small particle size explains the superparamagnetic behavior of Fe3O4. It is beneficial for particle dispersion and the fabrication of composites.
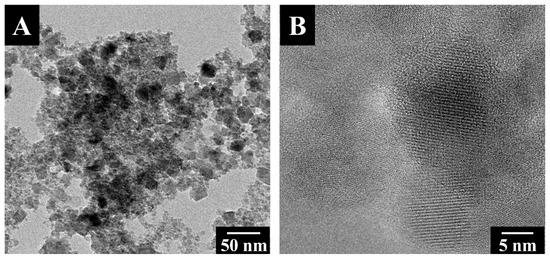
Figure 2.
TEM images of Fe3O4 at (A) low and (B) high magnifications.
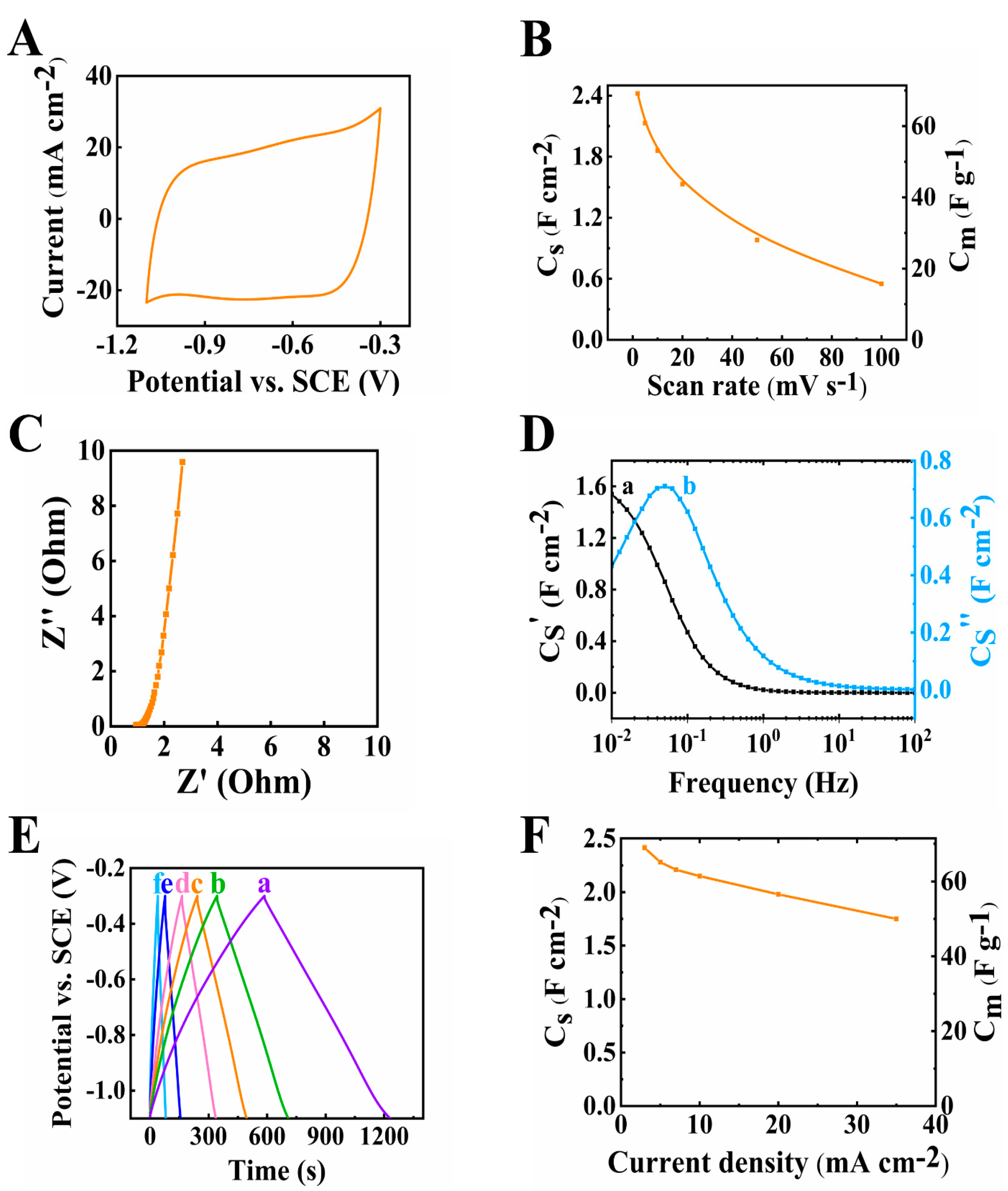
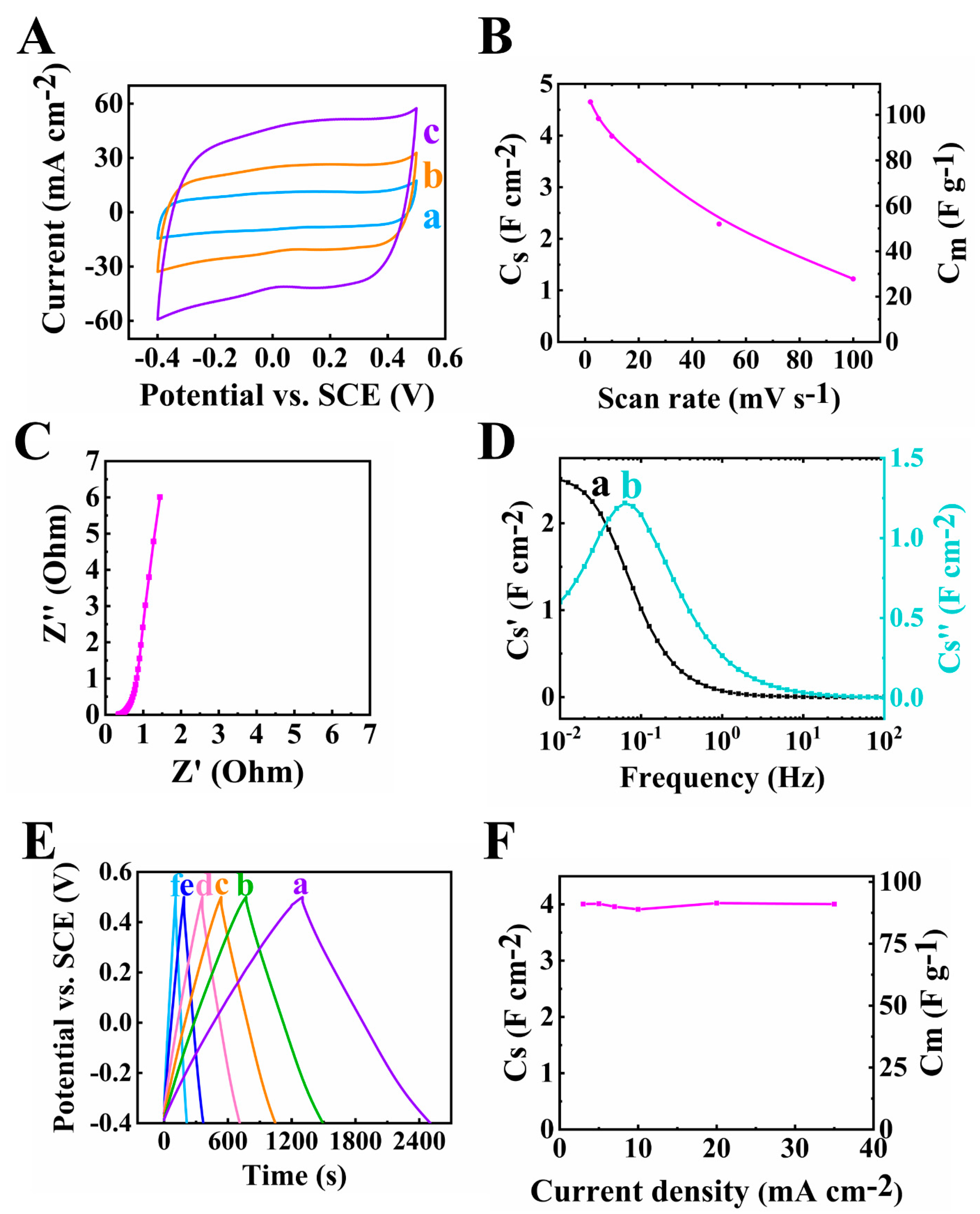
The electrode material prepared by method 1 was tested for its electrochemical performance in the potential range from −1.1 to −0.3 V. The testing results were plotted as shown in Figure 3. The CV graph in Figure 3A shows a nearly rectangular shape. The scan rate was increased from 2 to 100 mV s−1 and the capacitance was found to decrease (Figure 3B) from 2.42 to 0.55 F cm−2. The capacitance retention at a high scan rate of 100 mV s−1 (R100) was calculated to be 22.7%. The resistance of the electrode (Figure 3C), corresponding to the real part of Z*, was found to be 2.69 Ohms at a frequency of 10 mHz. The relatively low resistance achieved at 35 mg cm−2 mass loading indicated good conducting properties of the individual components. The C* dispersion of the relaxation type (Figure 3D) was observed and the relaxation frequency was 0.05 Hz, so a 20 s relaxation time constant could be calculated according to τ = 1/f from the C″ data. The charge and discharge curves were linear at 3–35 mA cm−2 current densities, showing good charge and discharge performance (Figure 3E). The capacitance was calculated as 2.42 F cm−2 at 3 mA cm−2 current density and decreased to 1.75 F cm−2 when the current density increased to 35 mA cm−2, as shown in Figure 3F.
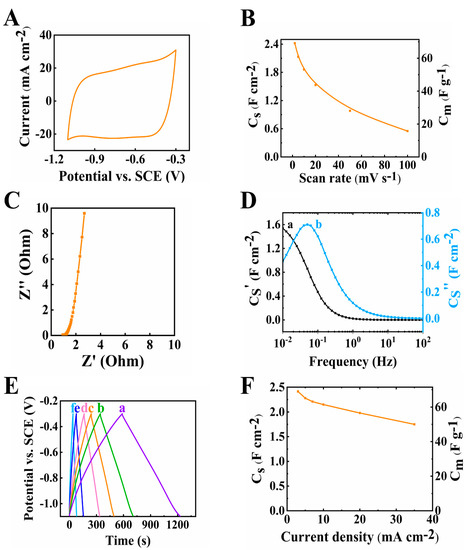
Figure 3.
(A) Cyclic voltammetry data at 10 mV s−1 scan rate, (B) capacitance from CV data, (C) Nyquist plot of Z*, (D) complex capacitance from EIS data (a) Cs’ and (b) Cs’’, (E) GCD plot at (a–f) 3, 5, 7, 10, 20 and 35 mA cm−2 and (F) capacitance from GCD data for 60Ti3C2Tx-30Fe3O4-10MCNT electrode prepared by method 1 with mass of 35 mg cm−2.
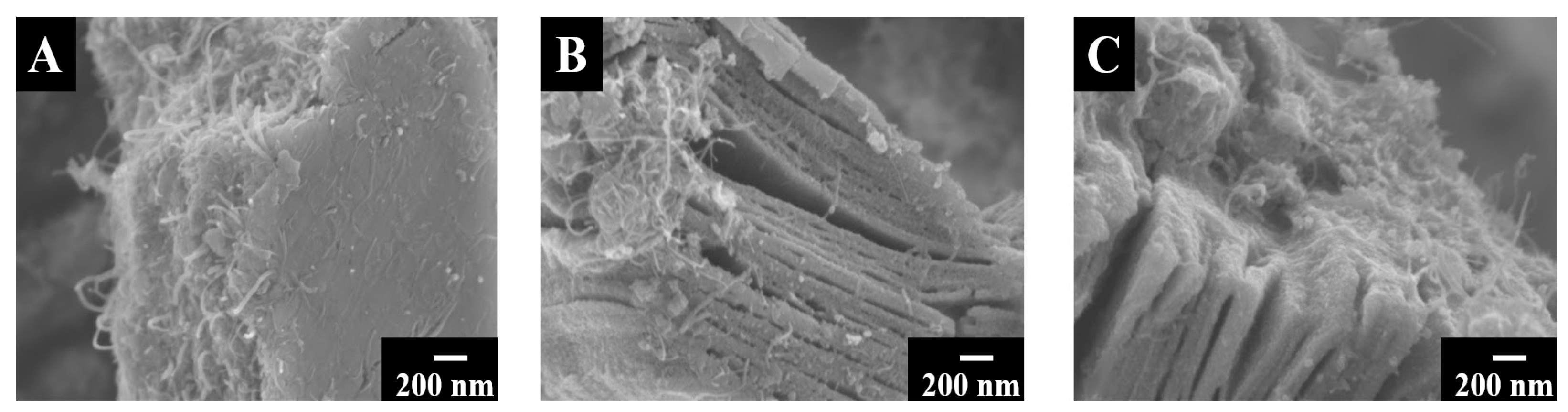
Method 2 offered the benefits of improved mixing of the components due to the in-situ synthesis of Fe3O4. It was demonstrated that the 60Ti3C2Tx–30Fe3O4–10MCNT electrodes, prepared by method 2, showed notably higher specific capacitance, compared to the electrodes of the same composition, prepared by method 1. Moreover, the 55Ti3C2Tx–30Fe3O4–15MCNT and 55Ti3C2Tx–25Fe3O4–20MCNT electrodes, were prepared by method 2 and tested for comparison. CB was applied as a co-dispersant for Ti3C2Tx and MCNT. The adsorption of the CB molecules on MCNT can be promoted by the polyaromatic structure of CB [51]. Moreover, the catechol group of CB facilitated its adsorption on Ti3C2Tx and Fe3O4 by creating bidentate chelating or bridging bonds with the Ti and Fe atoms on the material surfaces. The morphologies of the materials prepared by method 2 were studied by SEM and presented in Figure 4. It can be seen that MCNT and Fe3O4 were formed between the open layers of Ti3C2Tx and on the Ti3C2Tx surface. This resulted in improved mixing of the electrode components.

Figure 4.
SEM images of (A) 60Ti3C2Tx–30Fe3O4–10MCNT, (B) 55Ti3C2Tx–30Fe3O4–15MCNT and (C) 55Ti3C2Tx–25Fe3O4–20MCNT powders prepared by method 2.
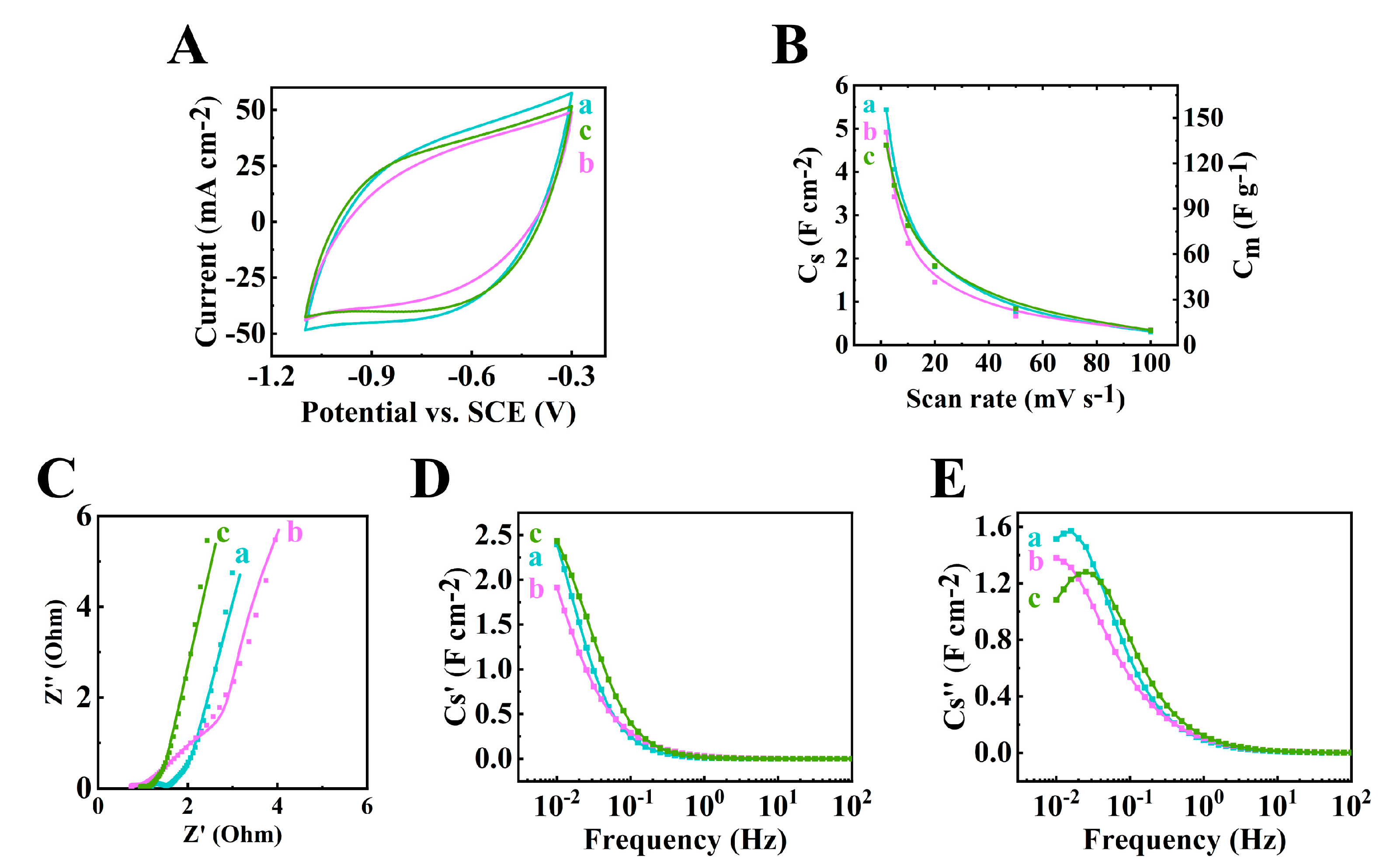
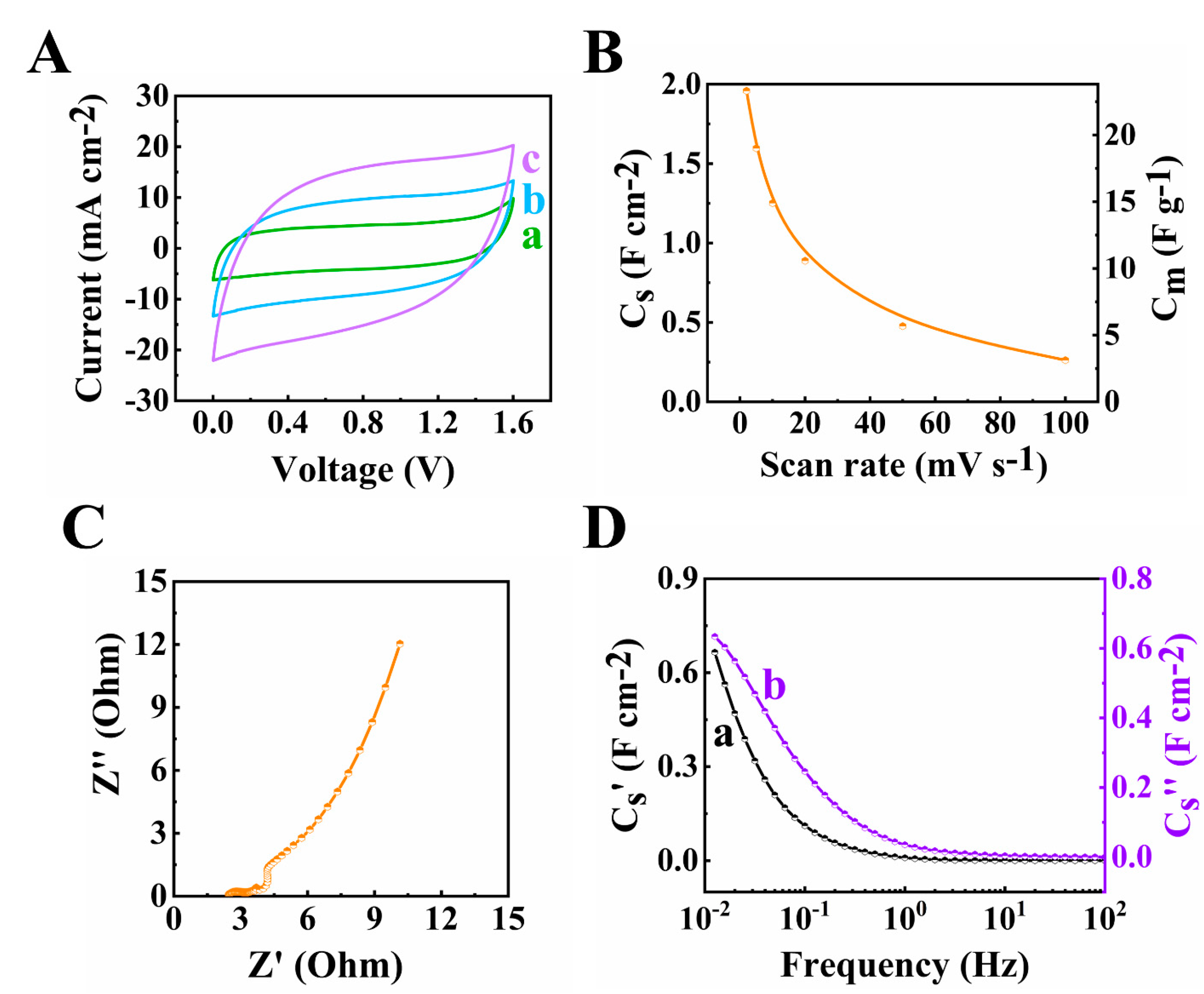
The 60Ti3C2Tx–30Fe3O4–10MCNT, 55Ti3C2Tx–30Fe3O4–15MCNT and 55Ti3C2Tx–25Fe3O4–20MCNT, electrodes with an AML of 35 mg cm−2 fabricated by method 2 were studied for their electrochemical performance. Figure 5 shows the CV and EIS results. The largest CV area was acquired for the 60Ti3C2Tx–30Fe3O4–10MCNT electrode, indicating the highest capacitance. The capacitances at 2 mV s−1 scan rate were calculated to be 5.44, 4.92 and 4.62 F cm−2 for the 60Ti3C2Tx–30Fe3O4–10MCNT, 55Ti3C2Tx–30Fe3O4–15MCNT and 55Ti3C2Tx–25Fe3O4–20MCNT electrodes, respectively. The equivalent circuit (Figure S1) developed for the high AML electrodes was used for the impedance spectroscopy [52]. The Rt values were calculated to be 0.65, 0.62 and 0.93 for the 60Ti3C2Tx–30Fe3O4–10MCNT, 55Ti3C2Tx–30Fe3O4–15MCNT and 55Ti3C2Tx–25Fe3O4–20MCNT electrodes, respectively. The relatively low impedance was achieved at high AML of 35 mg cm−2, which indicated low resistance and high capacitance. The Bode modulus are provided in Figure S2. In Figure 5D,E, the relaxation type dispersion was observed for these electrodes.
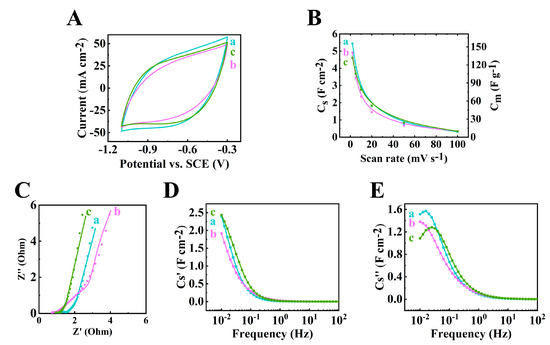
Figure 5.
(A) Cyclic voltammetry at 10 mV s−1, (B) capacitance acquired from CV measurement, (C) Nyquist plot of Z* ((●) experimental data and (–) simulation data), (D,E) components of complex capacitance: (D) Cs’ and (E) Cs’’ for (a) 60Ti3C2Tx–30Fe3O4–10MCNT, (b) 55Ti3C2Tx–30Fe3O4–15MCNT and (c) 55Ti3C2Tx–25Fe3O4–20MCNT electrodes prepared by method 2 with AML of 35 mg cm−2.
The pseudocapacitive behaviour of Ti3C2Tx is related to the oxidation state change between Ti4+/Ti3+, following the bonding and de-bonding of the oxygen group [53], as described by Equation (9). The electrochemical reaction of Fe3O4 can be attributed to the redox reaction between Fe3+/Fe2+, accompanying with the intercalation of Na+ ions [54], as shown in Equation (10).
Ti3C2OX(OH)yFz + δNa+ + δe− ↔ NaδTi3C2OX(OH)yFz
Fe3O4 + δNa+ + δe− ↔ NaδFe3O4
The contribution of surface and intercalation capacitances to the total energy storage by the composite electrodes was analyzed using the Dunn’s method [55,56,57]. A critical parameter b was applied to indicate the energy storage mechanism: for battery-type materials with diffusion-controlled capacitance, b = 0.5, and for purely double-layer capacitors with surface-controlled behaviour, b = 1 [58]. For materials with 0.5 < b < 0.8, the battery-type energy storage was dominant [59]. The b-value of as-prepared composites were determined to be 0.60, 0.56 and 0.62 for the 60Ti3C2Tx–30Fe3O4–10MCNT, 55Ti3C2Tx–30Fe3O4–15MCNT and 55Ti3C2Tx–25Fe3O4–20MCNT electrodes, respectively (Figure S3). Therefore, the composite electrodes showed a mixed energy storage mechanism with a dominant battery-type behaviour.
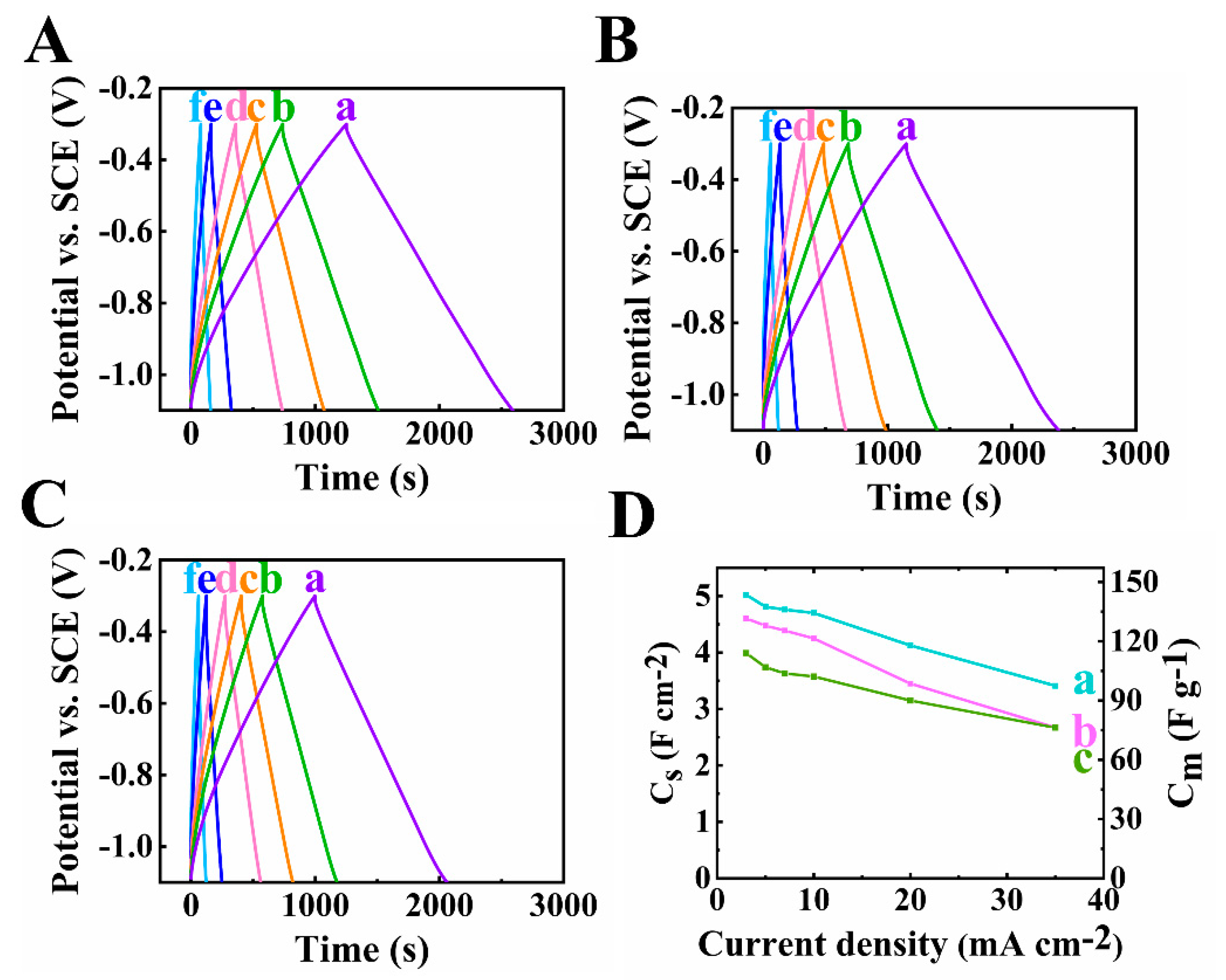
Figure 6 demonstrates the GCD results of the composite electrodes. The linear shapes of all the curves at different current densities indicated good pseudocapacitive performance. The longest charge–discharge time was obtained for the 60Ti3C2Tx–30Fe3O4–10MCNT sample, due to its highest capacitance. The decrease of capacitance was observed for all the electrodes when current was increased, as shown in Figure 6D. Based on the testing results above, the electrode of 60Ti3C2Tx–30Fe3O4–10MCNT showed the best performance with the highest capacitance and relatively low impedance at a high mass loading.
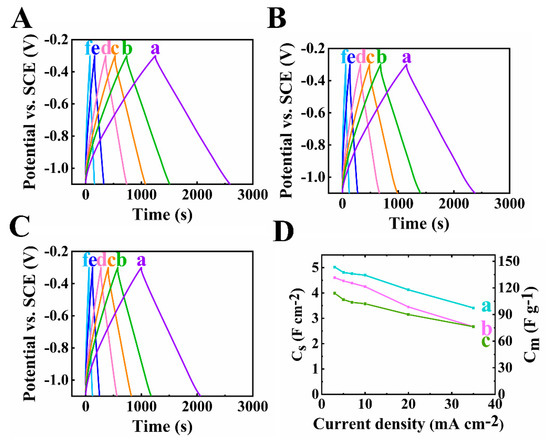
Figure 6.
(A–C) Galvanostatic charge discharge plots at scan rates of (a-f)3,5,7,10,20 and 35 mA cm−2 of (A) 60Ti3C2Tx–30Fe3O4–10MCNT, (B) 55Ti3C2Tx–30Fe3O4–15MCNT and (C) 55Ti3C2Tx–25Fe3O4–20MCNT, (D) capacitance calculated from GCD data of (a) 60Ti3C2Tx–30Fe3O4–10MCNT, (b) 55Ti3C2Tx–30Fe3O4–15MCNT and (c) 55Ti3C2Tx–25Fe3O4–20MCNT electrodes prepared by method 2 with AML of 35 mg cm−2.
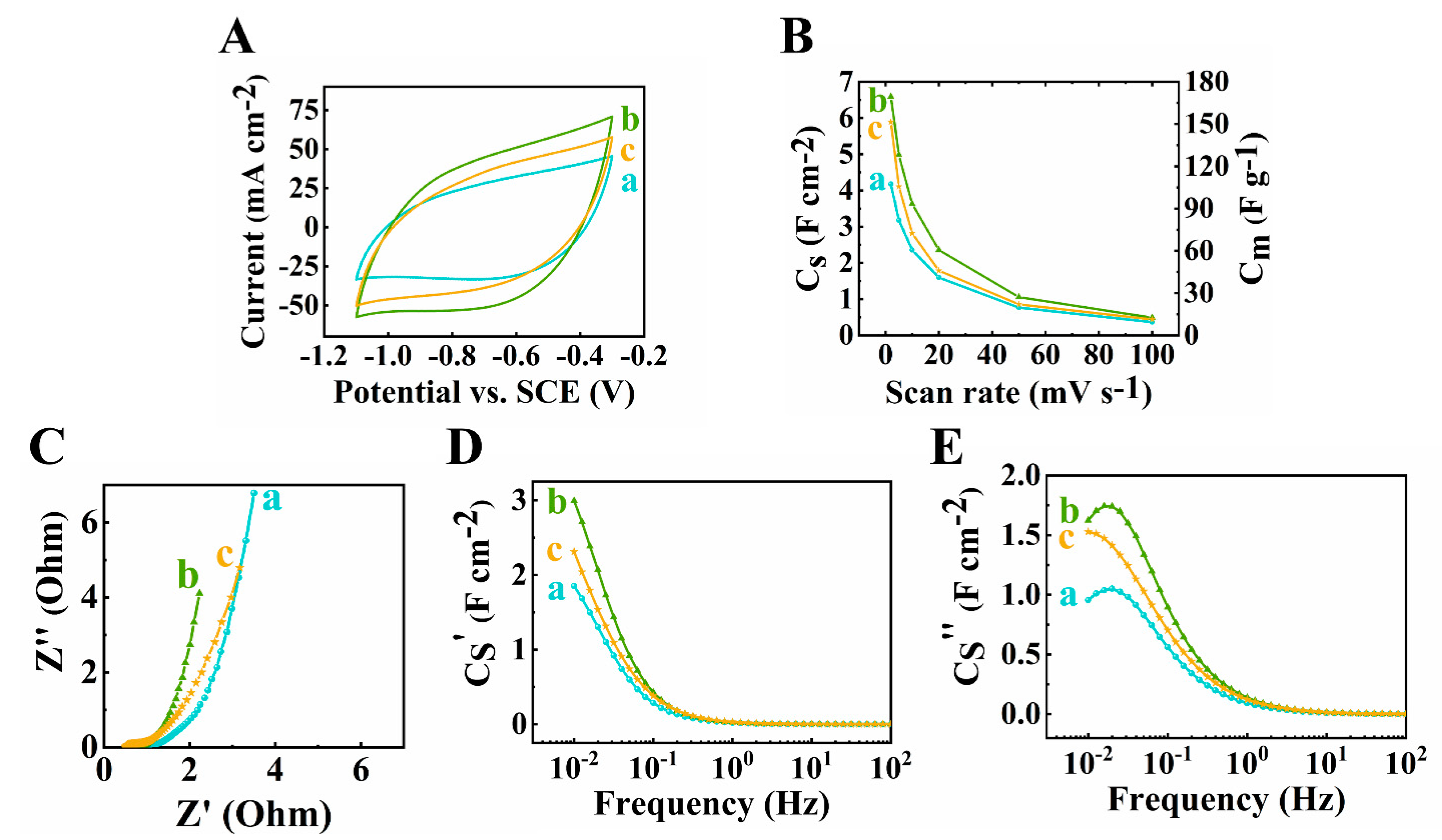
The active mass is another important factor of supercapacitor electrodes. Recent research emphasized the significance of high active mass loading of electrodes for practical applications [51]. With the increasing AML of an electrode, the performance can be drastically hindered due to the inefficient diffusion of electrolyte ions. To optimize the pseudocapacitive performance, electrodes with different AML from 30 to 45 mg cm−2 of 60Ti3C2Tx–30Fe3O4–10MCNT were fabricated and tested. The testing results were compared, as shown in Figure 5 (a) and Figure 7. As shown in Figure 7A, the largest CV area was found for the electrode at 40 mg cm−2 mass loading, indicating its highest capacitance. The areal capacitances calculated from the CV data were found to be 4.18 F cm−2, 5.44 F cm−2, 6.59 F cm−2 and 5.89 F cm−2, for mass loadings at 30, 35, 40 and 45 mg cm−2, respectively at scan rate of 2 mV s−1. From the EIS results shown in Figure 5C–E (a) and Figure 7C–E, the lowest resistance related to Z′ and the highest real capacitance C′ were achieved for the 40 mg cm−2 electrode.
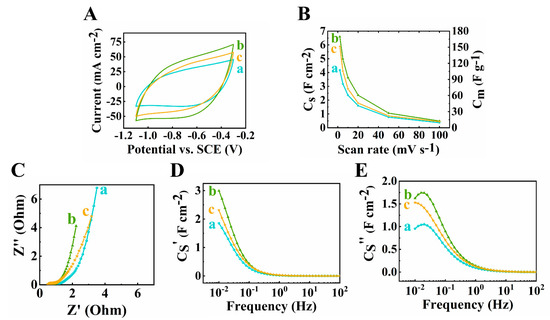
Figure 7.
(A) Cyclic voltammetry at 10 mV s−1 scan rate, (B) capacitance from CV data, (C) Nyquist plot, (D,E) complex capacitance components (D) Cs’ and (E) Cs’’ of 60Ti3C2Tx–30Fe3O4–10MCNT electrodes at (a) 30, (b) 40 and (c) 45 mg cm−2 active mass loading electrodes prepared by method 2.
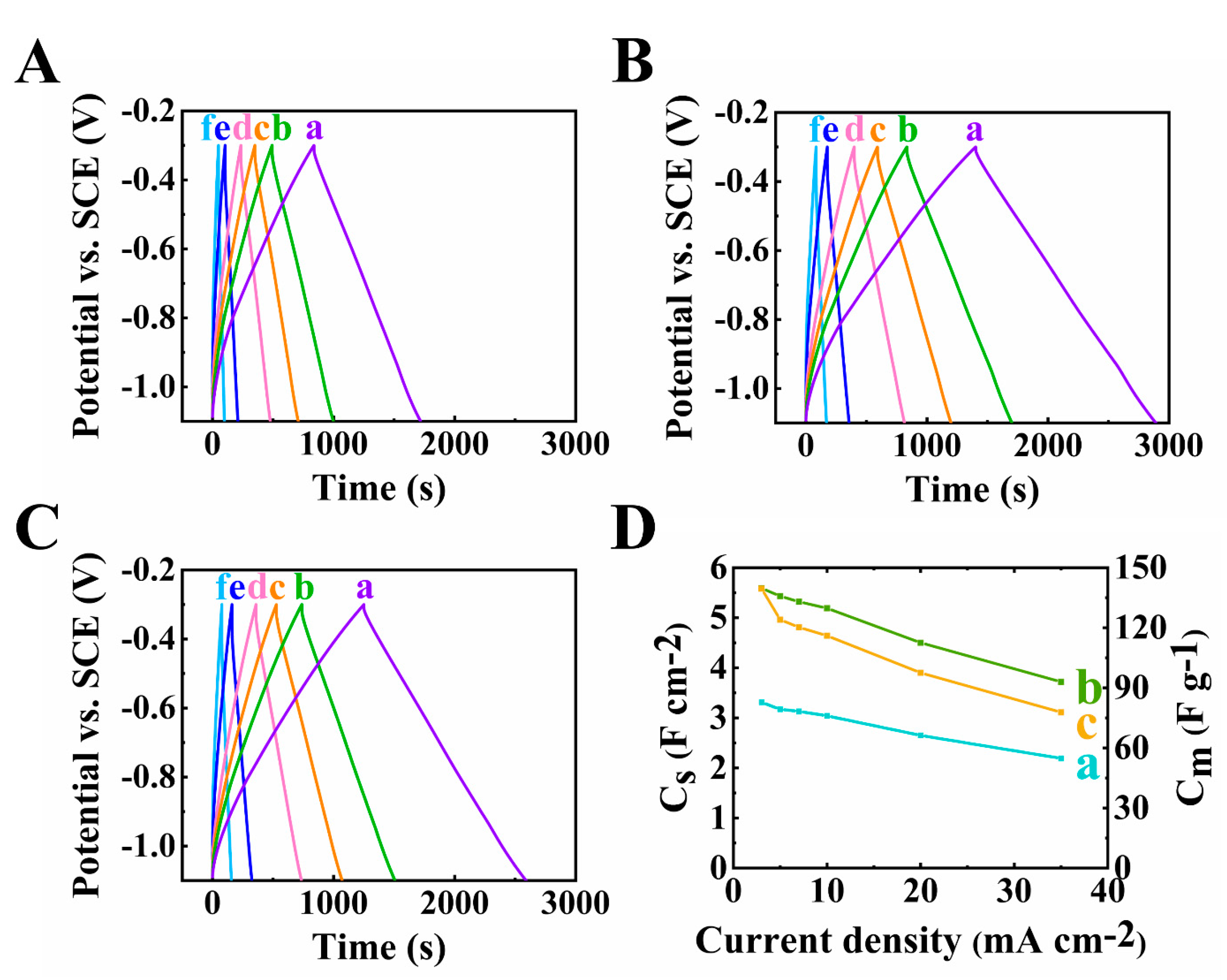
The GCD results shown in Figure 6A and Figure 8 are consistent with the CV and EIS data. Good pseudocapacitive performance was proven by the triangular-shaped curves. The longest discharge time was obtained for the electrode at 40 mg cm−2 mass loading, which is the result of its highest capacitance.
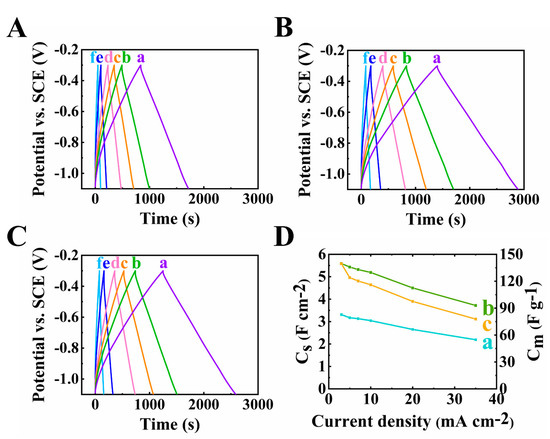
Figure 8.
(A–D) GCD plots at scan rates of (a–f)3,5,7,10,20 and 35 mA cm−2 of 60Ti3C2Tx–30Fe3O4–10MCNT electrodes at (A) 30, (B) 40 and (C) 45 mg cm−2 active mass loading and (D) capacitance from GCD data of 60Ti3C2Tx–30Fe3O4–10MCNT electrodes at (a) 30, (b) 40 and (c) 45 mg cm−2 active mass loading electrodes prepared by method 2.
Fabrication of an advanced asymmetric supercapacitor requires a high-performance positive electrode exhibiting comparable capacitance in an overlapping voltage window with the negative electrode. The PPy–MCNT electrode was tested in a −0.4–+0.5 V potential range and showed a capacitance of 4.65 F cm−2 at scan rate of 2 mV s−1. The rectangular-shaped CV curves, low impedance achieved at high mass loading and nearly linear GCD curves showed in Figure 9 confirmed the good capacitive properties of the PPy–MCNT positive electrode and as a result allowed the design of an asymmetric device.
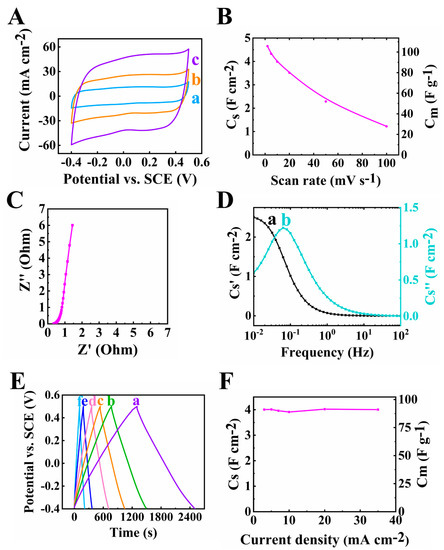
Figure 9.
(A) Cyclic voltammetry data at (a) 5, (b) 10 and (c) 20 mV s−1 scan rate, (B) capacitance from CV data, (C) Nyquist plot of Z*, (D) complex capacitance from EIS data (a) Cs′ and (b) Cs″, (E) GCD plot at (a) 3,(b) 5, (c) 7, (d) 10, (e) 20 and (f) 35 mA cm−2 and (F) capacitance from GCD data for PPy–MCNT positive electrode.
The as-assembled asymmetric supercapacitor device contained 60Ti3C2Tx–30Fe3O4–10MCNT-40 mg as a negative electrode and PPy–MCNT as a positive electrode. The charge and mass balances were achieved as described in reference [51]. Briefly, the mass of the cathode and anode should follow the charge conservation principle:
where m+ and m− are the active mass of the cathode and anode, C+ and C− represent the capacitance of the cathode and anode, and V+ and V− are the potential range of the cathode and anode, respectively.
Q = m + C + V + = m − C − V−
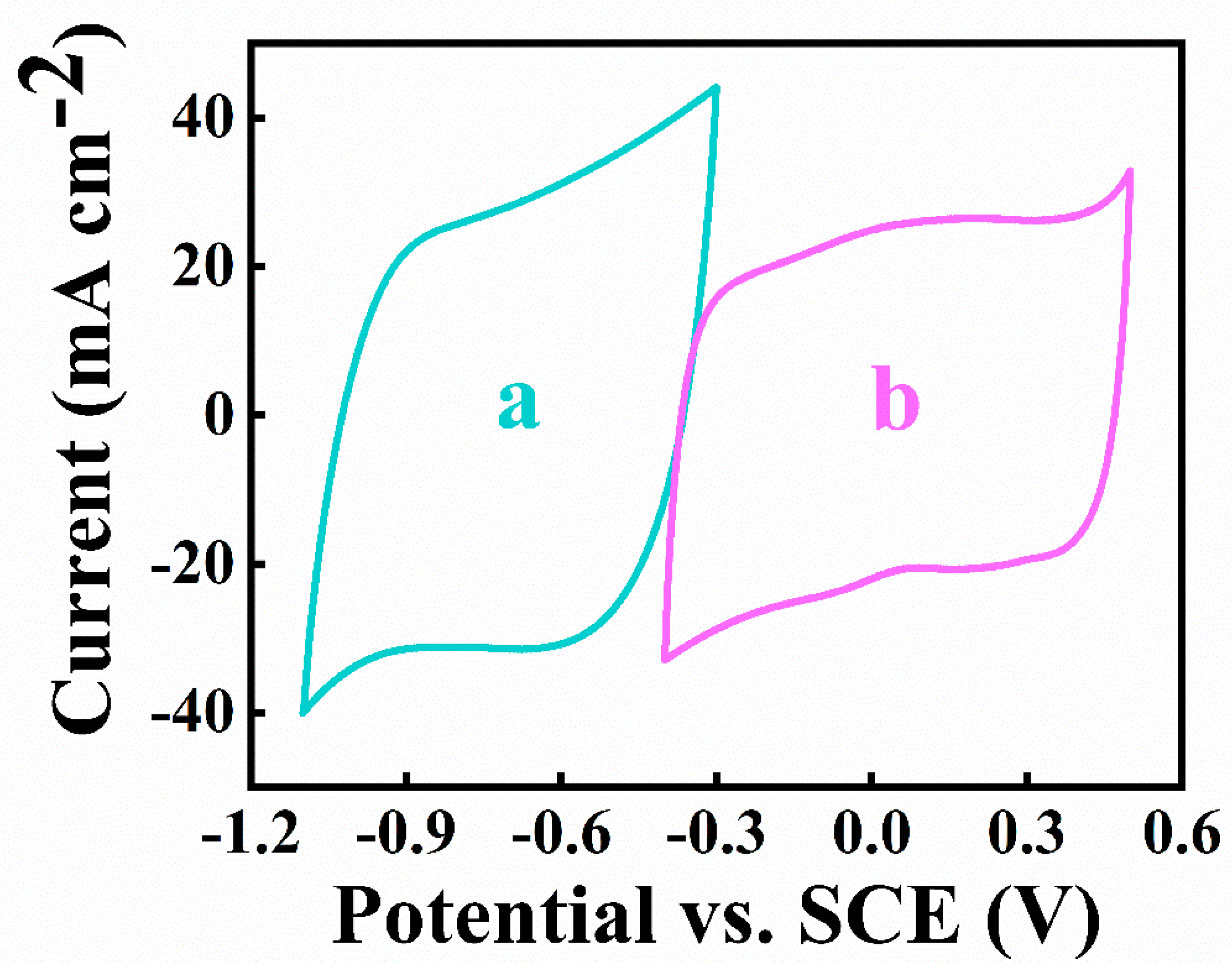
As shown in Figure 10, both the positive and negative electrodes showed good capacitive behavior in the overlapping potential windows, which is of significant importance for the successful development of an asymmetric device.
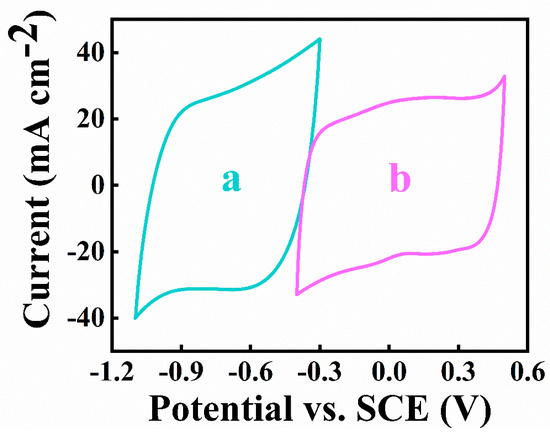
Figure 10.
CVs at 5 mV s−1 scan rate for (a)negative and (b)positive electrodes.
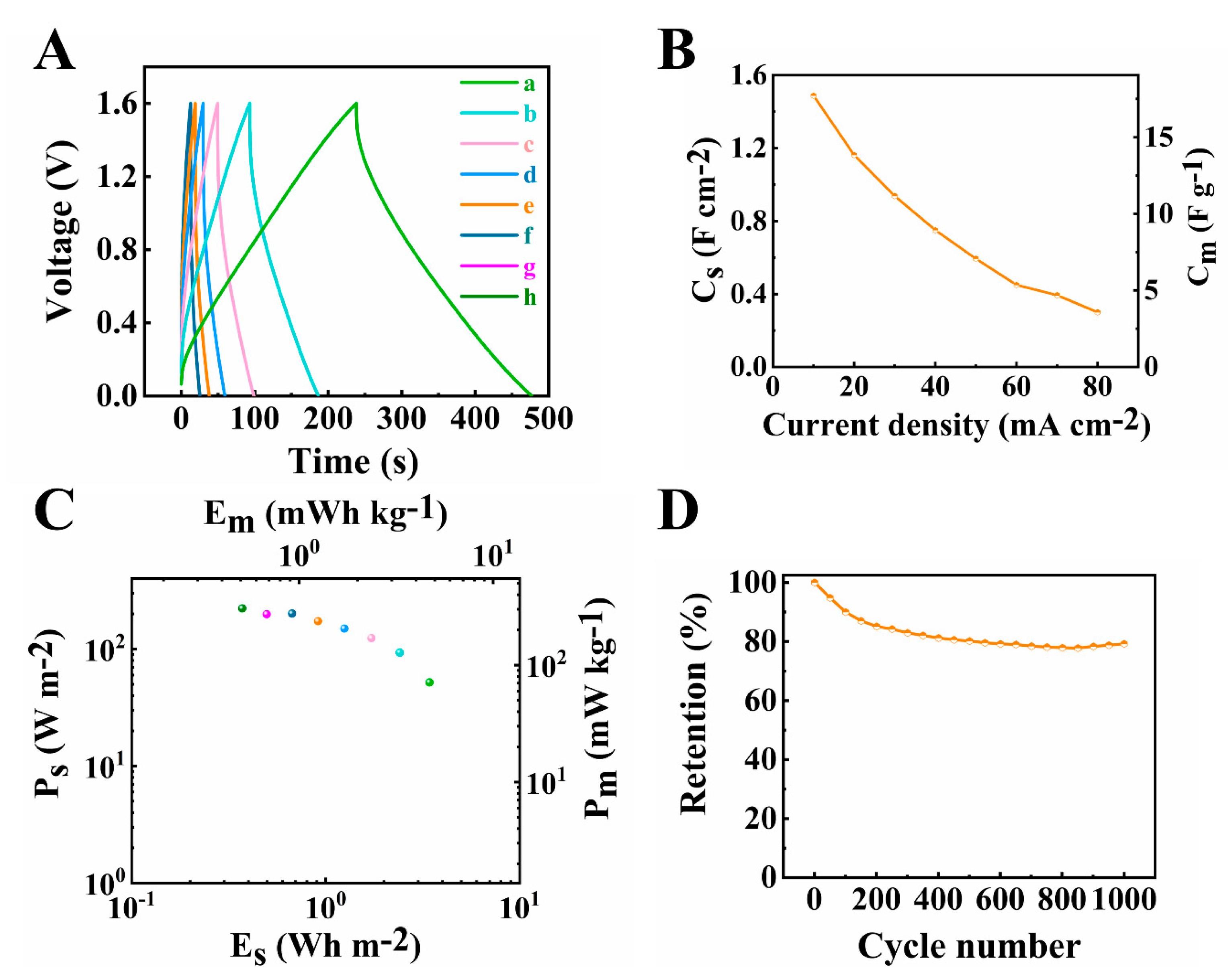
The as-fabricated asymmetric supercapacitor was tested in an enlarged voltage window of 1.6 V. An areal capacitance as high as 1.96 F cm−2 was obtained at a 2 mV s−1 scan rate from the CV (Figure 11A,B) and 1.49 F cm−2 was obtained at 10 mA cm−2 current density from the GCD (Figure 12A,B). The total resistance for the device at high active mass loading (Figure 11C) depended on the resistances, related to the ion diffusion in the pores of both the electrodes and a membrane. The Cs′ value was obtained as 0.77 F cm−2 at the frequency of 0.01 Hz (Figure 11D).
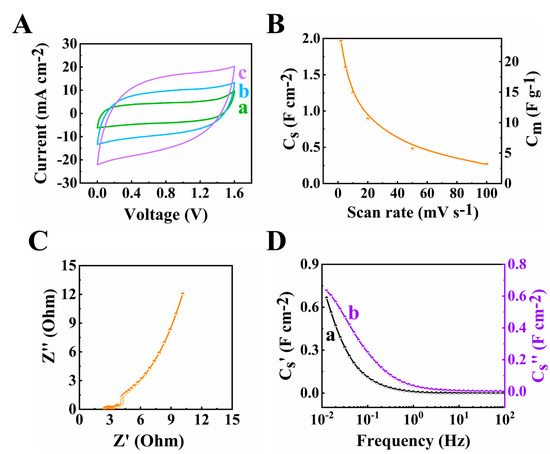
Figure 11.
(A) CVs at (a–c) 2, 5 and 10 mV s−1 scan rates, (B) capacitance acquired from CV data, (C) Nyquist plot of Z* and (D) components of complex capacitance calculated from EIS data for the asymmetric device.
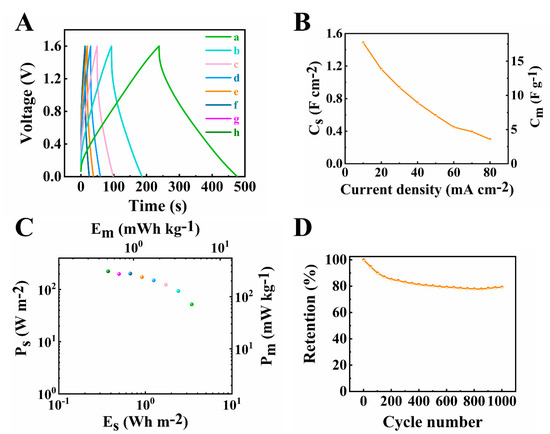
Figure 12.
(A) Galvanostatic charge–discharge results at (a–h) 10, 20, 30, 40, 50, 60, 70 and 80 mA cm−2 current densities, (B) capacitance calculated from GCD data, (C) Ragone plot derived from GCD data(corresponding to curve h-a in Figure 12A from left to right dots) and (D) capacitance retention of the asymmetric device.
The Ragone plot in Figure 12C showed that good power-energy characteristics were achieved in this investigation. The highest power density obtained from the device was 223.17 W m−2 (305.53 W kg−1) and the highest energy density was determined as 4.34 Wh m−2 (5.94 Wh kg−1). Cycling behaviour studies showed that the cell maintained 80% capacitance after 1000 CV cycles at scan rate of 50 mV s−1 (Figure 12D). It is suggested that the reduction of capacitance during cycling was not related to reduction of capacitance of the negative electrode. The investigation of cycling behavior of the negative electrode (Supplementary information, Figure S4) showed that the capacitance increased during 1000 cycles with a capacitance retention of 112%. The suggested mechanism of the capacitance increase was ascribed to microstructure changes of the electrode during cycling, as was observed in other investigations [60]. Therefore, the cycling performance of the device could be enhanced by the improvement of cycling stability of PPy–MCNT electrode.
4. Conclusions
Ti3C2Tx–Fe3O4–MCNT electrodes were prepared for applications in the anodes of asymmetric supercapacitors with high capacitance and high loading. As-prepared Fe3O4 in the Ti3C2Tx–Fe3O4–MCNT composite material showed superparamagnetic properties due to the small particle size. The use of Fe3O4 imparted magnetic properties to the composite and allowed for the fabrication of an anode with low binder content, which was the key to achieving the high loading feature. The in-situ synthesis method allowed for significant improvement in the capacitance. The capacitive behavior of the electrodes prepared by the in-situ synthesis was optimized by variation of the composition and AML. The catecholate type co-dispersant with polyaromatic structure is beneficial for adsorption on MCNT, at the same time it can form bidentate chelating or bridging bonding on the Ti3C2Tx and Fe3O4 surface. The strong adsorption and efficient co-dispersion facilitated the in-situ synthesis approach. The composite material containing 60% Ti3C2Tx, 30% Fe3O4 and 10% MCNT showed a remarkably high capacitance of 6.59 F cm−2 at a scan rate of 2 mV s−1 and low impedance at a high mass loading of 40 mg cm−2. The key factors for achieving such high capacitance were the use of the in-situ synthesis method and celestine blue as a co-dispersant, reduced binder content and optimization of the composition and mass loading. The outstanding capacitive properties of composites render them a potential candidate for negative electrodes for asymmetric supercapacitors. The advanced asymmetric supercapacitor with a negative electrode of 60Ti3C2Tx–30Fe3O4–10MCNT and a positive electrode of PPy–MCNT was fabricated, which exhibited superior electrochemical performance in an enlarged voltage window of 1.6 V. The optimal MXene-based electrode and advanced asymmetric supercapacitor showed great potential for future applications of commercial energy storage devices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/batteries9060327/s1. Figure S1. Equivalent circuit developed from high active mass loading electrodes. R-C(Q) transmission line was combined in series with solution resistance Rs. Rn elements represented electrolyte resistance. Q was related to the constant phase elements and W stood for Warburg impedance. Double layer capacitance Cdl was in parallel with the charge transfer resistance Rt. Figure S2. Bold modulus of (A) impedance and (B) phase angle plot for (a) 60Ti3C2Tx-30Fe3O4-10MCNT, (b) 55Ti3C2Tx-30Fe3O4-15MCNT and (c) 55Ti3C2Tx-25Fe3O4-20MCNT electrodes prepared by method 2 with AML of 35 mg cm−2. Figure S3. Current (i) versus scan rate (ν) dependence in a logarithmic scale used for the calculation of b value by equation i=avb for (a) 60Ti3C2Tx-30Fe3O4-10MCNT, (b) 55Ti3C2Tx-30Fe3O4-15MCNT and (c) 55Ti3C2Tx-25Fe3O4-20MCNT electrodes prepared by method 2 with AML of 35 mg cm−2. Figure S4. Cycling stability of 60Ti3C2Tx-30Fe3O4-10MCNT electrode at 40 mg cm−2 from CV at scan rate of 50 mV s−1. Table S1. Capacitance (F cm−2) and capacity (mAh g−1) for as-prepared electrodes, calculated from CV data at 2 mV s−1 scan rate. Table S2. Summary of electrochemical performance of Ti3C2Tx/Fe3O4-based composite anode for supercapacitors. References [49,61,62] are cited in Supplementary Materials.
Author Contributions
Conceptualization, W.L. and I.Z.; methodology, W.L. and I.Z.; formal analysis, W.L. and M.N.; investigation, W.L.; writing—original draft preparation, W.L. and I.Z.; writing—review and editing, W.L., R.X. and I.Z.; supervision, I.Z.; project administration, I.Z.; funding acquisition, I.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSERC Canada.
Data Availability Statement
All data are available through the manuscript.
Acknowledgments
We acknowledge Canadian Centre for Electron Microscopy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mariappan, V.K.; Krishnamoorthy, K.; Manoharan, S.; Pazhamalai, P.; Kim, S.J. Electrospun Polymer-Derived Carbyne Supercapacitor for Alternating Current Line Filtering. Small 2021, 17, 2102971. [Google Scholar] [CrossRef]
- Tian, J.; Cui, N.; Chen, P.; Guo, K.; Chen, X. High-Performance Wearable Supercapacitors Based on PANI/N-CNT@CNT Fiber with a Designed Hierarchical Core-Sheath Structure. J. Mater. Chem. A Mater. 2021, 9, 20635–20644. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; da Silva, M.I.; Toma, H.E.; Angnes, L.; Martins, P.R.; Araki, K. Trimetallic Oxides/Hydroxides as Hybrid Supercapacitor Electrode Materials: A Review. J. Mater. Chem. A Mater. 2020, 8, 10534–10570. [Google Scholar] [CrossRef]
- Yewale, M.A.; Jadhvar, A.A.; Kharade, R.B.; Kadam, R.A.; Kumar, V.; Nakate, U.T.; Shelke, P.B.; Bobade, D.H.; Teli, A.M.; Dhas, S.D.; et al. Hydrothermally Synthesized Ni3V2O8 Nanoparticles with Horny Surfaces for HER and Supercapacitor Application. Mater. Lett. 2023, 338, 134033. [Google Scholar] [CrossRef]
- Yewale, M.A.; Jadhavar, A.A.; Kadam, R.A.; Velhal, N.B.; Nakate, U.T.; Teli, A.M.; Shin, J.C.; Nguyen, L.N.; Shin, D.K.; Kaushik, N.K. Hydrothermal Synthesis of Manganese Oxide (Mn3O4) with Granule-like Morphology for Supercapacitor Application. Ceram. Int. 2022, 48, 29429–29437. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-Based Materials for Electrochemical Energy Storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Nan, J.; Guo, X.; Xiao, J.; Li, X.; Chen, W.; Wu, W.; Liu, H.; Wang, Y.; Wu, M.; Wang, G. Nanoengineering of 2D MXene-Based Materials for Energy Storage Applications. Small 2021, 17, 1902085. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liang, M.; Wang, H.; Wang, X.; Huang, Y.; Coelho, J.; Pinilla, S.; Zhang, Y.; Qi, F.; Nicolosi, V.; et al. 3D MXene Architectures for Efficient Energy Storage and Conversion. Adv. Funct. Mater. 2020, 30, 2000842. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Wang, X.; Wang, X.; Ju, Y.; Ge, S.; Lu, X.; Ding, J.; Yuan, N.; Gogotsi, Y. Low-Temperature Pseudocapacitive Energy Storage in Ti3C2Tx MXene. Energy Storage Mater. 2020, 33, 382–389. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Van Aken, K.L.; Barsoum, M.W.; Gogotsi, Y. Flexible MXene/Carbon Nanotube Composite Paper with High Volumetric Capacitance. Adv. Mater. 2015, 27, 339–345. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/Graphene Films for Ultrafast Supercapacitors with Outstanding Volumetric Capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Yan, J.; Ma, Y.; Zhang, C.; Li, X.; Liu, W.; Yao, X.; Yao, S.; Luo, S. Polypyrrole-MXene Coated Textile-Based Flexible Energy Storage Device. RSC Adv. 2018, 8, 39742–39748. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wei, D.; Zhu, J.; Niu, D.; Wang, F.; Wang, L.; Yang, L.; Yang, P.; Wang, C. Enhanced Electrochemical Performances of Organ-like Ti 3 C 2 MXenes/Polypyrrole Composites as Supercapacitors Electrode Materials. Ceram. Int. 2019, 45, 7328–7337. [Google Scholar] [CrossRef]
- Le, T.A.; Tran, N.Q.; Hong, Y.; Lee, H. Intertwined Titanium Carbide MXene within a 3 D Tangled Polypyrrole Nanowires Matrix for Enhanced Supercapacitor Performances. Chem.—A Eur. J. 2019, 25, 1037–1043. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. MXene-Polypyrrole Electrodes for Asymmetric Supercapacitors. Electrochim. Acta 2022, 406, 139843. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Yang, C.; Wang, F.; Cao, M. Composites of TiO 2 Nanoparticles Deposited on Ti 3 C 2 MXene Nanosheets with Enhanced Electrochemical Performance. J. Electrochem. Soc. 2016, 163, A785–A791. [Google Scholar] [CrossRef]
- Nagarajan, R.D.; Sundaramurthy, A.; Sundramoorthy, A.K. Synthesis and Characterization of MXene (Ti3C2Tx)/Iron Oxide Composite for Ultrasensitive Electrochemical Detection of Hydrogen Peroxide. Chemosphere 2022, 286, 131478. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, M.; Luo, W.; Dai, B.; Ren, J.; Ma, M.; Li, T.; Ma, Y. All Pseudocapacitive MXene-MnO2 Flexible Asymmetric Supercapacitor. J. Energy Storage 2022, 45, 103715. [Google Scholar] [CrossRef]
- Shen, B.; Huang, H.; Jiang, Y.; Xue, Y.; He, H. 3D Interweaving MXene–Graphene Network–Confined Ni–Fe Layered Double Hydroxide Nanosheets for Enhanced Hydrogen Evolution. Electrochim. Acta 2022, 407, 139913. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, J.; Zhang, W.; Ma, L.; Jiang, Z.; Wang, J.; Huang, Y. Synergistically Coupling of 3D FeNi-LDH Arrays with Ti3C2Tx-MXene Nanosheets toward Superior Symmetric Supercapacitor. Nano Energy 2022, 91, 106633. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. MXene-Carbon Nanotube Composite Electrodes for High Active Mass Asymmetric Supercapacitors. J. Mater. Chem. A Mater. 2021, 9, 10335–10344. [Google Scholar] [CrossRef]
- Yi, H.; Pang, S.; Yang, G.; Yao, X.; Li, C.; Jiang, J.; Li, Y. MXene Modified Carbon Fiber Composites with Improved Mechanical Properties Based on Electrophoretic Deposition. Mater. Res. Bull. 2022, 150, 111761. [Google Scholar] [CrossRef]
- Wen, D.; Ying, G.; Liu, L.; Li, Y.; Sun, C.; Hu, C.; Zhao, Y.; Ji, Z.; Zhang, J.; Wang, X. Direct Inkjet Printing of Flexible MXene/Graphene Composite Films for Supercapacitor Electrodes. J. Alloy. Compd. 2022, 900, 163436. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-Based Supercapacitor Electrodes: Advances and Challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Li, Y.; Hu, N. Modification of the Elemental Composition of Iron(III) Oxide as an Asymmetric Supercapacitor Anode: A Minireview. Instrum. Sci. Technol. 2020, 48, 637–666. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Wu, Q. α-Fe2O3 Based Carbon Composite as Pure Negative Electrode for Application as Supercapacitor. Eur. J. Inorg. Chem. 2019, 2019, 1301–1312. [Google Scholar] [CrossRef]
- Tufa, L.T.; Gicha, B.B.; Wu, H.; Lee, J. Fe-Based Mesoporous Nanostructures for Electrochemical Conversion and Storage of Energy. Batter. Supercaps 2021, 4, 429–444. [Google Scholar] [CrossRef]
- Ma, J.; Guo, X.; Yan, Y.; Xue, H.; Pang, H. FeOx-Based Materials for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700986. [Google Scholar] [CrossRef]
- Ma, Y.; Sheng, H.; Dou, W.; Su, Q.; Zhou, J.; Xie, E.; Lan, W. Fe2O3Nanoparticles Anchored on the Ti3C2TxMXene Paper for Flexible Supercapacitors with Ultrahigh Volumetric Capacitance. ACS Appl. Mater. Interfaces 2020, 12, 41410–41418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, H.; Zhu, C.; Lin, S.; Xu, Z.; Zhang, X. Free-Standing MXene Film Modified by Amorphous FeOOH Quantum Dots for High-Performance Asymmetric Supercapacitor. Electrochim. Acta 2019, 308, 1–8. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.L.; Wang, G.G.; Zhang, H.Y.; Zhang, B.; Li, G.Z.; Wu, Z.P.; Dang, L.Y.; Han, J.C. Few-Layered Ti3C2T: X MXenes Coupled with Fe2O3 Nanorod Arrays Grown on Carbon Cloth as Anodes for Flexible Asymmetric Supercapacitors. J. Mater. Chem. A Mater. 2019, 7, 22631–22641. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xie, Z.; Xu, X.; Yuan, Y.; Cheng, Z.; Liu, Y. A Nanoporous MXene Film Enables Flexible Supercapacitors with High Energy Storage. Nanoscale 2018, 10, 9642–9652. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Lin, S.; Li, H.; Wu, Z.; Zhu, L.; Li, C.; Wang, X.; Zhu, X.; Sun, Y. Laser Crystallized Sandwich-like MXene/Fe3O4/MXene Thin Film Electrodes for Flexible Supercapacitors. J. Power Sources 2021, 497, 229882. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene Hybrids with Ultrahigh Volumetric Capacity as an Anode Material for Lithium-Ion Batteries. J. Mater. Chem. A Mater. 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, S.; Cai, D.; Cao, J.; Wang, L.; Han, W. Planar Supercapacitor with High Areal Capacitance Based on Ti3C2/Polypyrrole Composite Film. Electrochim. Acta 2020, 330, 135277. [Google Scholar] [CrossRef]
- Wu, W.; Niu, D.; Zhu, J.; Gao, Y.; Wei, D.; Zhao, C.; Wang, C.; Wang, F.; Wang, L.; Yang, L. Hierarchical Architecture of Ti3C2@PDA/NiCo2S4 Composite Electrode as High-Performance Supercapacitors. Ceram. Int. 2019, 45, 16261–16269. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Momodu, D.Y.; Naguib, M.; Mirghni, A.A.; Masikhwa, T.M.; Khaleed, A.A.; Kebede, M.; Manyala, N. Electrochemical Performance of Two-Dimensional Ti3C2-Mn3O4 Nanocomposites and Carbonized Iron Cations for Hybrid Supercapacitor Electrodes. Electrochim. Acta 2019, 301, 487–499. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Li, H.; Lin, S.; Ding, W.; Zhu, X.; Sheng, Z.; Wang, H.; Zhu, X.; Sun, Y. 2D/2D 1T-MoS2/Ti3C2 MXene Heterostructure with Excellent Supercapacitor Performance. Adv. Funct. Mater. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, C.; Zhou, F.; Dong, Y.; Shi, X.; Nicolosi, V.; Wu, Z.S.; Bao, X. Ionic Liquid Pre-Intercalated MXene Films for Ionogel-Based Flexible Micro-Supercapacitors with High Volumetric Energy Density. J. Mater. Chem. A Mater. 2019, 7, 9478–9485. [Google Scholar] [CrossRef]
- Tian, Y.; Que, W.; Luo, Y.; Yang, C.; Yin, X.; Kong, L.B. Surface Nitrogen-Modified 2D Titanium Carbide (MXene) with High Energy Density for Aqueous Supercapacitor Applications. J. Mater. Chem. A Mater. 2019, 7, 5416–5425. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The Development of Pseudocapacitor Electrodes and Devices with High Active Mass Loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Holt, C.M.B.; Li, Z.; Tan, X.; Amirkhiz, B.S.; Xu, Z.; Olsen, B.C.; Stephenson, T.; Mitlin, D. Graphene-Nickel Cobaltite Nanocomposite Asymmetrical Supercapacitor with Commercial Level Mass Loading. Nano Res. 2012, 5, 605–617. [Google Scholar] [CrossRef]
- Liao, J.; Li, Y.; Wang, Z.; Lv, L.; Chang, L. In-Situ Preparation of Fe3O4/Graphene Nanocomposites and Their Electrochemical Performances for Supercapacitor. Mater. Chem. Phys. 2021, 258, 123995. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudžius, R.; Zarkov, A.; Kareiva, A.; Popov, A.I. Impact of Gadolinium on the Structure and Magnetic Properties of Nanocrystalline Powders of Iron Oxides Produced by the Extraction-Pyrolytic Method. Materials 2020, 13, 4147. [Google Scholar] [CrossRef]
- Liang, W.; Zhitomirsky, I. Composite Fe3O4-Mxene-Carbon Nanotube Electrodes for Supercapacitors Prepared Using the New Colloidal Method. Materials 2021, 14, 2930. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, K.; Zhitomirsky, I. Polypyrrole Coated Carbon Nanotubes for Supercapacitor Devices with Enhanced Electrochemical Performance. J. Power Sources 2014, 268, 233–239. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Fu, J.; Liu, Y.; Wu, Y.; Zhou, J.; Zhang, Z.; Xie, E. Commercial-Level Mass-Loading MnO2with Ion Diffusion Channels for High-Performance Aqueous Energy Storage Devices. J. Mater. Chem. A Mater. 2021, 9, 17945–17954. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhitomirsky, I. Surface Modification of MnO2 and Carbon Nanotubes Using Organic Dyes for Nanotechnology of Electrochemical Supercapacitors. J. Mater. Chem. A Mater. 2013, 1, 12519–12526. [Google Scholar] [CrossRef]
- Zang, X.; Wang, J.; Qin, Y.; Wang, T.; He, C.; Shao, Q.; Zhu, H.; Cao, N. Enhancing Capacitance Performance of Ti3C2Tx MXene as Electrode Materials of Supercapacitor: From Controlled Preparation to Composite Structure Construction. Nanomicro Lett. 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, J. Mechanistic Investigation of the Charge Storage Process of Pseudocapacitive Fe3O4 Nanorod Film. Electrochim. Acta 2014, 120, 52–56. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Nguyen, L.N.; Nakate, U.T.; Lingamdinne, L.P.; Koduru, J.R.; Auti, P.S.; Vattikuti, S.V.P.; Shin, D.K. Electrochemical Supercapacitor Performance of NiCo2O4 Nanoballs Structured Electrodes Prepared via Hydrothermal Route with Varying Reaction Time. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129901. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Linh, N.N.; Teli, A.M.; Shin, J.C.; Lingamdinne, L.P.; Koduru, J.R.; Shin, D.K. Mesoporous Hexagonal Nanorods of NiCo2O4 Nanoparticles via Hydrothermal Route for Supercapacitor Application. Chem. Phys. Lett. 2022, 800, 139654. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Koduru, J.R.; Velhal, N.B.; Nakate, U.T.; Jadhavar, A.A.; Sali, N.D.; Shin, D.K. Interconnected Plate-like NiCo2O4 Microstructures for Supercapacitor Application. Mater. Sci. Eng. B. Solid. State Mater. Adv. Technol. 2023, 287, 116072. [Google Scholar] [CrossRef]
- Mariappan, V.K.; Krishnamoorthy, K.; Pazhamalai, P.; Natarajan, S.; Sahoo, S.; Nardekar, S.S.; Kim, S.J. Antimonene Dendritic Nanostructures: Dual-Functional Material for High-Performance Energy Storage and Harvesting Devices. Nano Energy 2020, 77, 105248. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef]
- Liang, W. Advanced Electrode Materials and Fabrication of Supercapacitors. 2022. Available online: http://hdl.net/11375/27386 (accessed on 1 December 2022).
- Zhang, L.; Wang, Z.; Chen, W.; Yuan, R.; Zhan, K.; Zhu, M.; Yang, J.; Zhao, B. Fe3O4nanoplates Anchored on Ti3C2T: XMXene with Enhanced Pseudocapacitive and Electrocatalytic Properties. Nanoscale 2021, 13, 15343–15351. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, K.; Li, Y.; Wang, Z.; Zhan, K.; Yang, J.; Zhao, B. Nanoparticles of Fe3O4 Anchored on Ti3C2Tx MXene/RGO Aero gels as Hybrid Negative Electrodes for Advanced Supercapacitors. ACS Appl. Nano Mater. 2023, 6, 482–491. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).